Abstract
Introduction:
Bronchiolitis obliterans syndrome (BOS), a subtype of chronic lung allograft dysfunction, is quite common, with up to half of all lung recipients developing BOS within five years of transplantation. Preventive efforts are aimed at alleviating known risk factors of BOS development, while the primary goal of treatment is to delay the irreversible, fibrotic airway changes and progressive loss of lung function.
Areas covered:
This narrative review will briefly discuss the updated definition, clinical presentation, pathogenesis, risk factors, and survival after BOS, while paying particular attention to the salient evidence for optimal preventive strategies and treatments based on investigations in the modern era.
Expert opinion:
Future translational research focused on further characterizing the complex interplay between immune and non-immune mechanisms mediating chronic lung rejection is the first step towards mitigating risk of allograft injury, improving early disease detection with non-invasive biomarkers, and ultimately, developing an effective, targeted therapy that can extend the life of the lung allograft.
Keywords: bronchiolitis obliterans syndrome, risk factors, treatment, lung transplantation, chronic lung allograft dysfunction
1.0. Background
Lung transplantation (LTx) is a life-saving therapy for some patients with end-stage lung disease; however, survival after LTx is shorter than that of other solid organ transplants. Although one-year survival rates for lung transplant recipients (LTxRs) have improved over the last few decades [1], long-term survival remains significantly limited, particularly due to the clinical syndrome of chronic rejection, termed chronic lung allograft dysfunction (CLAD). CLAD is the leading cause of death among recipients who survive beyond the first year of transplant [2]. In the last ten years, our understanding of CLAD has evolved significantly, which is reflected by the changes we have observed in how the LTx community defines this clinical entity. The International Society for Heart and Lung Transplantation (ISHLT) CLAD Consensus Report was recently developed in an effort to reconcile these changing definitions that collectively describe a persistent deterioration in the function of the lung allograft [3]. The current state of CLAD research is complex, and the appropriate definition is challenged by the need to account for both spirometric changes and multiple, overlapping etiologies in the post-transplant period [4]. Nevertheless, two predominant phenotypes of CLAD are well-recognized: bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS) [5].
BOS is the most common form of chronic rejection in LTxRs, with up to half of recipients developing BOS within five years of transplant [1]. Clinically, recipients with this disease experience a progressive and often unpredictable loss of lung function secondary to scarring, narrowing, and eventual obstruction of the small airways. This narrative review will briefly discuss the updated definition, clinical presentation, pathogenesis, risk factors, and survival after BOS, while paying particular attention to the salient evidence for optimal preventive strategies and treatments.
2.0. Definition
Despite many changes in the definitions of other manifestations of CLAD, the definition for BOS has remained relatively unchanged over the last two decades. Nonetheless, establishing a diagnosis of BOS first requires meeting certain CLAD criteria (Figure 1), followed by staging (Table 1). CLAD is defined as a persistent (≥20%) decline in measured forced expiratory volume in one second (FEV1) from baseline; generally, even a ≥10% decline elicits investigation into possible etiologies considering such a notable change may not reflect day-to-day variability in lung function measures. Depending on how long the decline in lung function has persisted, CLAD may be sub-classified as possible (<3 weeks), probable (≥3 weeks to 3 months), or definite (>3 months). Together, spirometric findings, total lung capacity, and chest appearance on imaging are capable of classifying CLAD by phenotype, which we have adapted and simplified in Table 2. The obstructive phenotype of CLAD, which has retained its formal designation as ‘BOS’, is still defined by a fall of forced expiratory volume in one second (FEV1) ≥ 20% from the previous baseline, with evidence of airflow limitation measured by a ratio of FEV1 to forced vital capacity (FVC) < 0.7, and an absence of opacities on chest imaging. Although a clear definition for this syndrome exists, a high level of suspicion is needed to detect it in clinical practice. It remains challenging to define the exact onset of BOS in real time, as clinicians seem to note its appearance months after the recipient has started to develop the obstructive disease [6]. Furthermore, after establishing its diagnosis, clinicians may observe changes in total lung capacity, or subsequent chest imaging, which would warrant re-classification to a mixed phenotype, or a phenotype which has not yet been defined. The definition is certainly quite complex and thus requires careful attention, prompt investigations, and early interventions for all potential causes of declining lung function.
Figure 1.
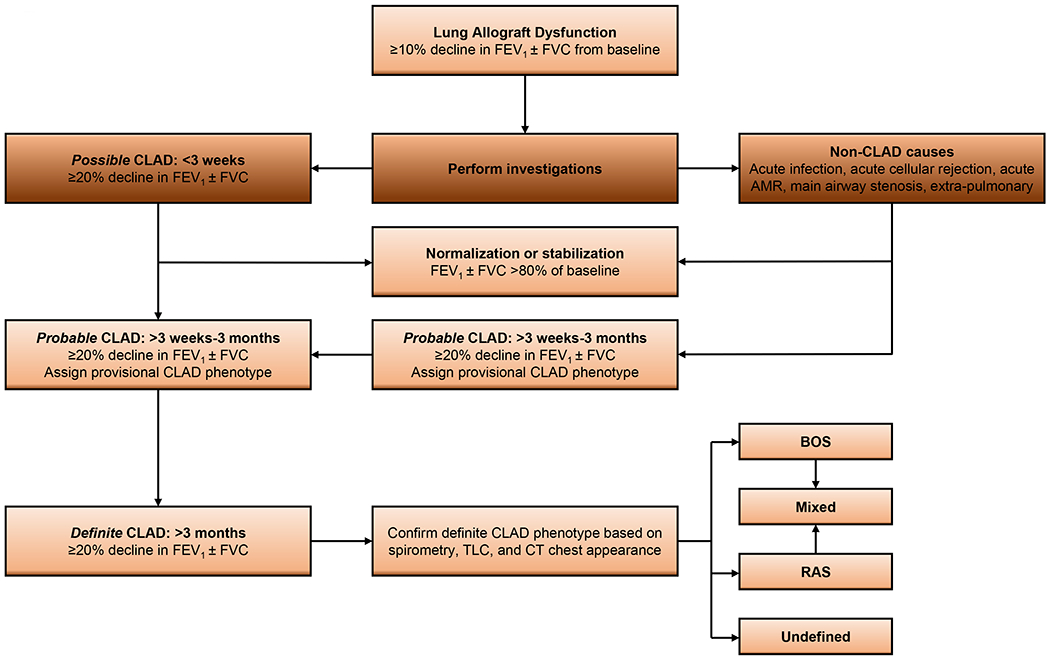
Evolution of chronic lung allograft dysfunction adapted [3].
AMR, antibody-mediated rejection; BOS, bronchiolitis obliterans syndrome; CT, computed tomography; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; RAS, restrictive allograft syndrome; TLC, total lung capacity.
Table 1.
CLAD staging, adapted [3]
| Stage | Spirometric measures |
|---|---|
| CLAD 0 | Current FEV1 >80% FEV1 baseline |
| CLAD 1 | Current FEV1 >65-80% FEV1 baseline |
| CLAD 2 | Current FEV1 >50-65% FEV1 baseline |
| CLAD 3 | Current FEV1 >35-50% FEV1 baseline |
| CLAD 4 | Current FEV1 ≤35% FEV1 baseline |
Abbreviations: CLAD, chronic lung allograft dysfunction; FEV1, forced expiratory volume in one second. Once CLAD is diagnosed, staging is performed according to the decline in FEV1 as compared with baseline. Date of CLAD onset is defined as the date at which the first value of FEV1 ≤80% of baseline is recorded when subsequent values taken ≥3 weeks (>3 months for definite CLAD) apart also fall below the threshold. The same principle holds for each stage.
Table 2.
Presently defined CLAD phenotypes, adapted [3]
| Phenotype | Obstruction (FEV1/FVC <0.7) | Restriction (TLC decline ≥10% from baseline) | CT opacities |
|---|---|---|---|
| BOS | Yes | No | No |
| RAS | No | Yes | Yes |
| Mixed | Yes | Yes | Yes |
| Undefined | Yes | No | Yes |
| Yes | Yes | No |
Abbreviations: BOS, bronchiolitis obliterans syndrome; CLAD, chronic lung allograft dysfunction; CT, computed tomography; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; RAS, restrictive allograft syndrome; TLC, total lung capacity.
3.0. Clinical Presentation
In the early stages, the clinical presentation of BOS is characterized by an insidious onset with nonspecific symptoms of dyspnea on exertion and nonproductive cough, while late stages are associated with dyspnea at rest and other signs or symptoms that may mimic bronchiectasis. Over time, subtle increases in exertional dyspnea, accompanied by a progressive decline in lung function, may be noted on examination [7]. It is unusual for BOS to present within the first few months after transplant, but nevertheless, it is vitally important to frequently monitor patients’ symptoms and results from home spirometry. Laboratory tests and formal spirometric measurements are routinely collected in the post-transplant period in an effort to detect early changes that may raise suspicion for BOS.
Several markers of early BOS have historically been studied, including bronchoalveolar lavage neutrophilia [8,9], radiographic evidence of air trapping [10], and an increase in circulating fibrocytes detected by flow cytometry [11], among others. However, these markers are marred by poor specificity and thus remain unreliable in predicting BOS development. Recent bench lab investigations have demonstrated the value in certain immune markers for detecting early allograft injury and impending failure. The development of antibodies to lung self-antigens (SAgs) (K-α1 tubulin and collagen V) [12], as well as development of circulatory exosomes with lung SAgs [13] are two such markers that may alert transplant clinicians to the possibility of underlying BOS in a LTxR. Donor-derived, cell-free DNA (cfDNA), along with select inflammatory chemokines (eg, C-X-C motif chemokine ligand 10 [CXCL10]), have also been identified in recent literature and may serve as immune markers of allograft injury. Studies have found that early detection of high levels of cfDNA and CXCL10 were associated with CLAD and worse mortality after LTx [14,15]. Tissot et al recently published a comprehensive review on the utility of varying biomarkers for predicting development of chronic rejection [16].
4.0. Pathogenesis
The predominant pathologic mechanism underlying BOS is obliteration of the small airways with advancing atherosclerotic changes in the pulmonary vasculature [17]. Early lesions of epithelial cells and subepithelial structures of the small airways leads to fibroproliferation and disruption in epithelial regeneration and tissue repair. Fibromyxoid granulation tissue in the small airway lumen leads to partial and eventually complete obstruction in cases of severe disease. Wide evidence has implicated BOS as a ‘final common pathway’ for a variety of immune-mediated and non-immune insults, as illustrated in Figure 2.
Figure 2.
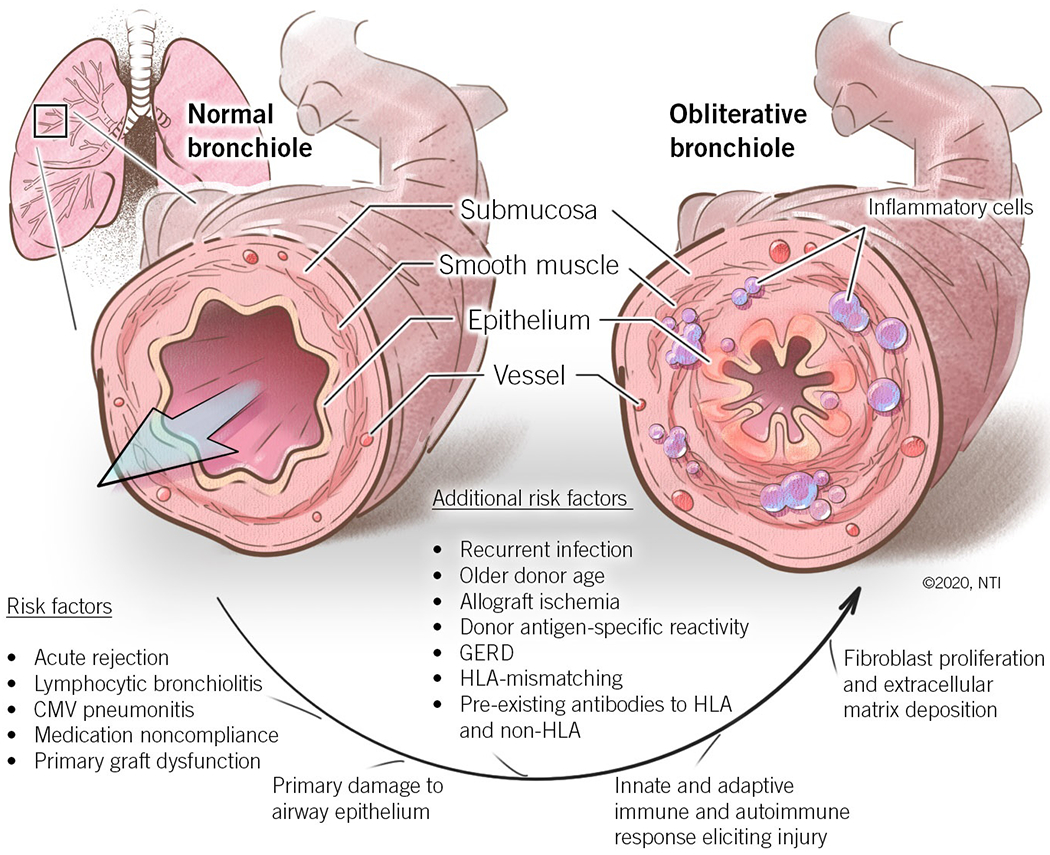
Pathogenesis of bronchiolitis obliterans syndrome.
CMV, cytomegalovirus; GERD, gastroesophageal reflux disease; HLA, human leukocyte antigen
5.0. Risk Factors
Many risk factors for the development of BOS following LTx have been proposed based on reports with varying levels of evidence. In an effort to consolidate the factors that contribute to BOS pathogenesis, the ISHLT and others historically organized them as probable and potential risk factors [18]. Since then, our understanding of these risk factors has strengthened, and in this review, we will briefly evaluate select risk factors that may have particularly important implications on BOS prevention and treatment.
5.1. Alloimmunity-induced autoimmunity
Several studies have implicated autoimmune responses to lung SAgs [13,19], along with alloimmune responses to donor human leukocyte antigens (HLAs) [12,20,21], in BOS pathogenesis. Donor-specific alloreactive T lymphocytes and increased levels of HLA class II molecules have been observed within alveolar walls and airway epithelium in LTxRs diagnosed with BOS [22]. In addition, the development of anti-HLA class I molecules has been found to precede declining pulmonary function and BOS [23,24]. There is also evidence that suggests some LTxRs have pre-existing antibodies to HLA and non-HLA molecules, which may lead to earlier post-transplant onset of BOS [25,26].
5.2. Acute rejection
Two retrospective studies in 1995 provided the first evidence supporting acute rejection as a major risk factor for BOS following LTx [27,28]. Since then, studies have illustrated the role of both minimal [29] and severe episodes of acute rejection leading to a significant risk for BOS development. In particular, episodes that manifest lymphocytic bronchiolitis lead to an increased risk of BOS and death after LTx [30].
5.3. Viral infection
Several community-acquired viral respiratory infections have been studied in the setting of LTx, with conflicting findings regarding the impact on risk of BOS. Recent data from our group demonstrated that respiratory viral infections can lead to induction of circulating exosomes with lung SAgs, and persistent infection can lead to the development of donor specific antibodies (DSA), a known risk factor for the development of BOS [31]. There is also strong evidence to suggest that cytomegalovirus (CMV) infection increases the risk of BOS in LTxRs [32]; CMV pneumonitis within the first six months post-transplant may also convey significant risk for BOS after transplant [33].
5.4. Bacterial and fungal infections
Bacterial and fungal colonization may be facilitated by the fibrotic changes associated with BOS, which are accompanied by underlying defects in host immunity. Some studies have reported a higher incidence of BOS among LTxRs with underlying infection with Aspergillus species or Pseudomonas species [34,35]. These findings have unique implications on BOS prevention, underscoring the importance of rapidly clearing infection in LTxRs.
5.5. Primary graft dysfunction (PGD)
PGD is an injury to the transplanted lung that occurs in the first 72 hours after transplant. The underlying lung injury in PGD may be related to oxidative damage, an upregulation in HLA class II molecules, or a release of circulating exosomes with lung SAgs that precedes antibody development [36], although the exact mechanism requires further study. Nevertheless, PGD has been associated with a higher risk for later development of BOS [37,38]. Of note, prolonged cold ischemic times have been implicated in higher-grade PGD and early mortality after LTx [39].
5.6. Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) is thought to play a significant role in declining lung function before transplant as well as in allograft dysfunction after transplant, including acute rejection and BOS [40–42]. Changes in foregut function (eg, GERD, esophageal dysmotility, and delayed gastric emptying) are common among LTxRs and may be influenced by pulmonary dynamics [43]. Ultimately, it is the microaspiration of gastric and bile acids that damages the airway epithelium and diminishes post-transplant lung allograft survival [44,45]. Our group is actively researching how GERD can increase the risk for development of antibodies to lung SAgs and PGD (unpublished).
5.7. Considerations
These proposed risk factors for BOS after LTx allow us to better understand the possible mechanisms leading to its pathogenesis. BOS remains one of the greatest challenges to long-term recipient and allograft survival. Addressing these risk factors with early interventions to improve survival and strengthen existing treatment strategies has become a dedicated focus of the LTx community.
6.0. Survival
BOS has a well-defined deleterious effect on long-term survival after LTx, yet studies have shown significant variability in its clinical course. Certain questions deserve greater consideration in large-scale studies, such as how the time to onset, rate of lung function decline, and post-transplant complications (eg, acute rejection) influence the poor survival outcomes associated with BOS. Early investigations at the turn of the century contained small, insufficiently sized cohorts that are now poorly representative (ie, containing majority single LTxRs) of the modern LTx era and updated classification system for CLAD [46]. A more recent investigation from Finlen Copeland and colleagues [47] included an important analysis of bilateral LTxRs that showed an early onset of BOS (occurring within two years of transplant) or an initial high grade at BOS onset (2 or 3) were associated with significantly worse survival (note, these gradings are obsolete in the present classification). In contrast, other clinical or demographic factors previously implicated as BOS risk factors, such as acute rejection or CMV pneumonitis, were not predictive of survival after the development of BOS. Although BOS is a progressive disease, the rate of progression was notably variable amongst the LTxRs in this analysis. Despite advances in the field of LTx, there is still much to learn pertaining to the prognosis of BOS after LTx, particularly in light of updated phenotypic classifications. Undoubtedly, this clinical syndrome plagues the long-term success of the procedure, but notably conveys better survival compared to restrictive and mixed phenotypes of CLAD [48].
7.0. Prevention
7.1. Addressing risk factors
One of the most important measures for preventing the development or progression of BOS is to address the above risk factors as soon as possible, even in the pretransplant period. When lung function begins to decline, transplant clinicians must respond rapidly to identify the underlying cause of airway epithelial damage. These efforts may be complicated, especially considering the number of immune and non-immune insults that contribute to BOS. Prompt preventive strategies to address the aforementioned risk factors focus on treating any viral, bacterial, or fungal infections, acute rejection episodes, and GERD symptoms.
Infection is a common complication in LTxRs and may facilitate immunological interactions that play a role in rejection pathogenesis. Thus, LTxRs are routinely vaccinated to protect against common viral infections (ie, influenza virus, varicella zoster virus); prophylactic antibacterial and antifungal regimens are also routinely administered. A growing body of evidence has established the value of antiviral prophylaxis for reducing the risk of CMV infection [49,50], which certainly makes a contribution to chronic rejection development [51].
Ischemia-reperfusion injury also promotes chronic rejection after transplant. New organ preservation techniques, which store the donor lungs in a physiological environment, have circumvented the issue of prolonged cold ischemic times and may translate into lower rates of grade 3 PGD [52]. After transplant, aggressive initial immunosuppression may eliminate early episodes of acute cellular rejection. Notably, systemic glucocorticoids are fundamental in the management of acute rejection, but remain ineffective in treating BOS [53].
Appropriate efforts to control GERD in LTxRs may necessitate surgical intervention, such as a Nissen fundoplication. Our studies have provided further evidence for previous findings [54,55] demonstrating the importance of performing a fundoplication early (within 6 months) after LTx, as it may protect against GERD-induced allograft damage in LTxRs with GERD [56]. We also demonstrated the ability of an early fundoplication to slow the decline of pulmonary function post-transplant, measured by a slower decline in FEV1. However, the impact of early surgical intervention for GERD on the occurrence of BOS requires further investigation [57–59]. Nevertheless, refluxate aspiration is a well-established risk factor for BOS, and thus reducing these aspiration events is an important preventive strategy for sustaining pulmonary function in transplant recipients [60].
7.2. Immunosuppressant therapy
Aside from making early, concerted efforts to reduce risk factors for chronic rejection in LTxRs, perhaps one of the more important preventive strategies against BOS is augmenting the maintenance immunosuppression regimen. Some evidence suggests that particular immunosuppressant agents may be more favorable than others in preventing BOS progression. Cairn et al [61] showed that substituting tacrolimus for cyclosporine may stabilize spirometric measurements in patients with BOS. Similarly, Whyte et al [62] showed that BOS progression could be slowed in patients receiving mycophenolate mofetil. Together, these studies, and others [63–65], demonstrated the potential benefit of adjusting immunosuppression and utilizing immunomodulating agents in managing chronic rejection. Notably, these substitutions have not been universally accepted, as the evidence is largely limited to case series. Nevertheless, transplant clinicians understand the close relationship between immunosuppression and allograft failure and remain aware of the potential benefit of making necessary adjustments.
7.3. Azithromycin
Given its unique anti-inflammatory and anti-microbial mechanisms, macrolide antibiotics have long been proposed as a valuable pharmacologic therapy to prevent BOS development after LTx. Some patients may receive azithromycin after LTx to combat bacterial infection, whereas in patients with underlying cystic fibrosis, it is used to combat inflammation. In early studies, azithromycin improved FEV1 in the short-term for LTxRs with established BOS [66,67]. In the first study assessing the impact of long-term administration of azithromycin (10 months), disease progression slowed in 11 LTxRs [68]. A few randomized controlled trials (RCTs) have since been conducted to evaluate azithromycin use in LTxRs with BOS. Vos et al [69] conducted the first RCT, which investigated the effect of azithromycin (250 mg, three times per week for two years) in preventing BOS in 83 LTxRs. BOS developed in 12% of patients receiving azithromycin compared to 44% of patients on placebo. Gan et al [70] conducted a double-blind, placebo-controlled trial comparing LTxRs initially treated with azithromycin (250 mg, alternate days) against recipients started on placebo, followed by azithromycin after three months; treatment with azithromycin similarly slowed down the progression of BOS. Ruttens et al [71] published a particularly valuable study in view of the updated classification system for CLAD, evaluating long-term effects of the azithromycin prophylaxis on CLAD-free survival, finding prolonged freedom from CLAD, improved pulmonary function, and better functional exercise capacity in patients receiving azithromycin versus placebo. Finally, Li et al conclusively demonstrated its benefit on improving not only risk of CLAD, but also improved survival after LTx in a large cohort from 12 years of recipient data [72]. Based on the accumulated data from these clinical experiences, azithromycin prophylaxis is established as an effective preventive measure, with the added advantage of being low cost, safe, and highly feasible to administer.
7.4. Statins
Statins (3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) have notable anti-inflammatory and immunomodulatory effects that have been leveraged in an attempt to prevent CLAD in LTxRs. Szczepanik et al [73] conducted a pivotal study in 130 LTxRs receiving statin therapy for a minimum of six months. At three years post-transplant, patients receiving statins experienced a significant survival benefit compared to those patients in the control group, but there was no difference in time to CLAD development. From the results of this study, statins are certainly a less-established therapy for preventing chronic rejection after LTx and may be unreliable to this end. Yet, their survival benefit warrants greater consideration.
7.5. Transition to further treatment
Ultimately, it is not uncommon for BOS to progress in some LTxRs despite the best efforts to employ these preventive strategies. In these scenarios, clinicians must consider the next step in definitive treatment for patients with refractory disease. Preventive strategies and the established treatments (below) for BOS are summarized in Figure 3.
Figure 3.
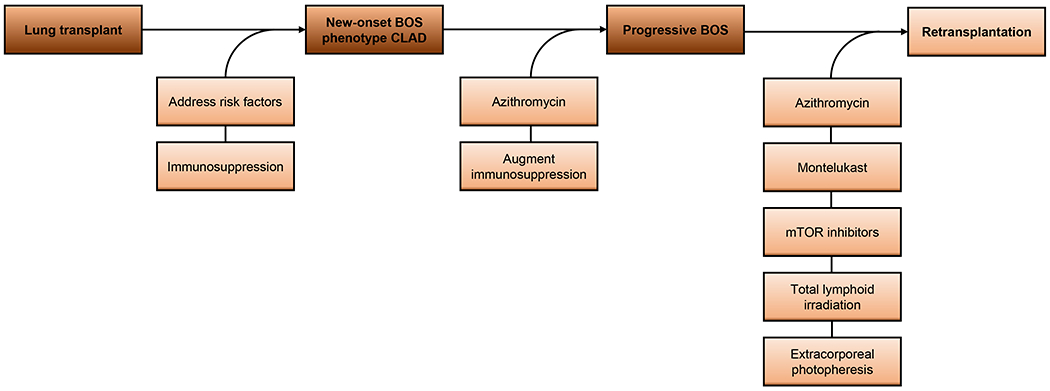
Preventive strategies and established treatments for bronchiolitis obliterans syndrome after lung transplantation.
8.0. Treatments
8.1. Montelukast
In patients with a progressive decline in FEV1 despite prophylaxis with azithromycin and optimization of maintenance immunosuppression regimens, leukotriene receptor antagonists, such as montelukast, have been used successfully as a salvage therapy to prevent further decline in pulmonary function. In an early pilot study, montelukast was administered and compared between two groups of LTxRs with BOS who were receiving concurrent azithromycin [74]. The rate of FEV1 decline did not change in the recipients who did not receive montelukast, whereas a decrease in the rate of FEV1 decline was observed in the study group. This study demonstrated the promise of using montelukast for patients with progressing BOS that remains refractory to azithromycin therapy. Ruttens et al [75] have since conducted an RCT that showed no survival benefit with montelukast compared to placebo, although it did slow the rate of FEV1 decline in recipients with late-onset stage 1 BOS, corroborating the results of previous investigations. Oral montelukast has also been used in combination with inhaled fluticasone and azithromycin as a triple therapy with some success in slowing lung function decline. Vos et al [76] performed a recent single-center investigation, stratifying 153 patients (N=115 with BOS) with CLAD according to the updated phenotypic definitions, and found that montelukast significantly attenuated the rate of FEV1 decline after 3 and 6 months. Both progression-free and overall survival after CLAD onset was improved in those patients whose FEV1 improved or stabilized, demonstrating its promise.
8.2. mTOR inhibitors
Sirolimus and everolimus are both mTOR inhibitors that are routinely added to immunosuppressive regimens after kidney transplantation, particularly because of their relative lack of nephrotoxic effects [77]. However, mTOR inhibitors have controversial benefits in LTxRs, specifically related to preventing BOS. Given the potent anti-fibrotic effects of mTOR inhibitors, these agents may alter the progression of BOS after LTx. In a retrospective investigation involving 65 LTxRs, everolimus was associated with improved renal function in patients who developed renal insufficiency secondary to calcineurin inhibitor nephrotoxicity, but its effects on preventing BOS were inconclusive [78]. Ultimately, special precautions should be taken when administering these agents. Sirolimus and everolimus are both contraindicated within the first 90 days after LTx because of historical associations with severe wound-healing complications involving the bronchial anastomosis [79]; however, recent evidence conflicts with this guidance [80]. Additionally, mTOR inhibitors are associated with bone marrow suppression. Overall, the effect on BOS is not known, and for this reason, mTOR inhibitors are not considered the pharmacologic agent of choice for preventing BOS or slowing disease progression.
8.3. Total lymphoid irradiation
Total lymphoid irradiation (TLI) is a treatment that is employed for LTxRs who present with refractory allograft rejection unresponsive to immunosuppressive therapy. Although traditionally used to control rejection in renal and heart transplant recipients, its efficacy in treating chronic rejection has been subject to considerable investigation over the last two decades. In 2005, Fisher et al [81] were among some of the first to study the safety and efficacy of TLI in a small cohort of 37 LTxRs with progressive BOS (majority grade 2 and 3). TLI was generally well-tolerated in 27 of 37 recipients and substantially slowed the rate of decline of lung function. In 2009, Verleden et al [82] evaluated the impact of TLI on six LTxRs with progressive BOS despite azithromycin treatment. A significant decrease in the rate of decline in lung function was noted in all six patients after TLI treatment. Importantly, two patients required subsequent retransplantation within 6 and 19 months after TLI, and three patients ultimately died due to BOS after 3.5, 11, 26 months after TLI. These findings suggest there may be a worthwhile benefit for TLI in patients with refractory BOS yet illustrate the somber reality of poor long-term survival after chronic rejection. Most recently, Lebeer et al [83] performed a small, retrospective study with 14 years of experience using TLI in LTxRs suffering from progressive BOS at a single center. Treatment with TLI resulted in significant attenuation of lung function decline, especially in those who were rapidly declining, with minimal side effects or adverse events. Notably, this treatment allowed bridging to retransplantation in five patients. Overall, although the evidence for TLI depends heavily on the experience of small, observational, single-center studies, this therapeutic modality has a role in treating progressive, refractory BOS.
8.4. Extracorporeal photopheresis
Extracorporeal photopheresis (ECP) is another treatment modality used to reduce the rate of decline in lung function in LTxRs with progressive BOS. In ECP, peripheral blood lymphocytes are first collected, exposed to a photosensitizing agent (8-methoxypsoralen), irradiated with ultraviolet A radiation, and then reinfused. The combination of 8-methoxypsoralen and ultraviolet A radiation is thought to promote the apoptosis of abnormal (treated) lymphocytes, which ultimately promotes immune tolerance and production of antigen-specific regulatory lymphocytes [84]. Data supporting the use of ECP for progressive BOS after LTx is extensive, and studies have strongly associated ECP with a reduction in the rate of lung function decline [85–88]. Baskaran et al [89] also demonstrated the capacity for ECP to reduce levels of circulating donor specific antibodies, pro-inflammatory cytokines, and antibodies to lung-associated SAgs, which likely contributes to the benefit of ECP in reducing lung function decline. Karnes et al [90] recently investigated the factors associated with the response to ECP in LTxRs with BOS. This study found that response to ECP was influenced by the extent of decline and the relationship between FEV1 and time before initiating ECP, which underscores the critical importance of earlier BOS detection and timely treatment. Transplant clinicians should strongly consider ECP in LTxRs with progressive or refractory BOS.
8.5. Retransplantation
Retransplantation is relatively uncommon in LTxRs, accounting for less than 5% of indications according to the ISHLT [1]. However, retransplant may be pursued for patients with CLAD (obstructive or restrictive phenotypes) that remains refractory to all other treatment modalities. Although retransplantation can be controversial given the shortages in the current donor pool, its benefit has been demonstrated in carefully selected candidates. At our center, we evaluated survival in 29 patients who were retransplanted for CLAD between March 2010 and May 2016. One and five-year survival rates were 89% and 64%, which was comparable to one and five-year survival rates of 89% and 58% observed in 391 primary LTxRs performed in the same period [91]. However, we did observe higher rates of cardiopulmonary bypass, re-exploration for bleeding, and post-retransplant extracorporeal membrane oxygenation retransplanted recipients. An important multi-center collaboration conducted by Verleden et al [92] evaluated the effect of CLAD phenotype on survival after retransplantation for CLAD. BOS constituted the majority of indications for retransplant (66% versus 34% restrictive phenotype). Notably, patients with restrictive phenotype were more likely to redevelop CLAD, and survival was worse compared to patients retransplanted for BOS. In sum, retransplantation seems to be a viable last treatment option for progressive BOS, but critical attention should be paid to patients retransplanted for RAS.
9.0. Expert Opinion
9.1. Future in focus
Although the LTx procedure may be the only life-prolonging treatment modality in some patients with end-stage lung disease, its success hinges on attenuating the complex, immune cascades that silently mar the integrity of the allograft. Understanding these mechanisms is the first step towards treating allograft rejection, which we have illustrated as an all-too-common fate after LTx. In this article, we consolidated evidence for select risk factors and associated treatments of chronic rejection after LTx, diagnosed clinically as BOS. Yet, the expertise of our thoracic immunology laboratory is in characterizing these mechanisms that synergistically mediate rejection immunopathogenesis at the molecular level. These efforts to understand what we are observing after LTx – namely, a mixed anti-donor response between de novo-developed DSA, autoantibodies, and allograft insult promoting exosome release – may serve as the most exciting development in our field for decades to come. For years, our community has valiantly focused on optimizing gross surgical technique, perioperative management, and post-transplant immunosuppression – factors which certainly promote the safety, sustainability, and survivability of the transplant procedure. Without these successes, our community would not be ready to address the greatest limitation after LTx, which is chronic rejection. And now that we are beginning to understand how chronic rejection is mediated not only through allograft-targeting immune responses, but also immune responses to tissue restricted SAgs (autoimmunity), we can begin investigating the factors that mediate these responses, which may ultimately precede a therapy that extends the life of the allograft.
9.2. Alloimmunity and autoimmunity
During and after LTx, the donor lungs are subjected to injuries that increase the antigenicity of the transplanted organ. Our laboratory has focused on studying these injuries and their role in the development of inflammatory mediators that predispose chronic rejection. While alloimmunity directed against donor HLA have been implicated in rejection, lung-restricted autoimmunity has emerged as potentially the final common terminal pathway leading to chronic rejection from a variety of risk factors that promote donor lung insult. Repeated cycles of injury and repair in LTxRs exposed to these risk factors may even expand lung-restricted autoimmunity. For these reasons, the studies which we have published in the last year implicating ischemia-reperfusion (ie, primary graft dysfunction), gastroesophageal reflux, microbial infection, and stress-induced exosome release [31,93–95] may supply even more evidence towards the importance of mitigating risk of allograft injury. We have since conducted proteomic analyses which revealed protein signatures in circulating extracellular vesicles that uniquely belong to LTxRs with acute rejection, BOS, and respiratory viral infection, offering new insight into the immunological mechanisms of allograft rejection [96].
9.3. Exosomes as a biomarker
Recognizing that immune responses underlie the development of chronic rejection after LTx, the community has long sought to identify immune biomarkers of rejection. We briefly reviewed some of the markers that have been measured previously (see section 3.0), which may still require refinements to improve specificity. However, recently, our laboratory published definitive evidence of a highly specific and sensitive biomarker for impending BOS in LTxRs. We have demonstrated the propensity of circulating exosomes containing lung SAgs isolated from LTxRs at both six and 12 months prior to diagnosis of BOS to serve as a non-invasive biomarker of chronic rejection, with 100% specificity and 90% sensitivity [97]. This finding may allow the development of strategies for prevention and/or early treatment of LTxRs at risk for developing BOS if detected early. Management practices can consist of removal of the circulating exosomes with lung SAgs by plasmapheresis to reduce the development of immune responses and increase the post-transplant freedom from CLAD. Likewise, instituting treatment with extracorporeal photophoresis based on the early detection of circulating exosomes may protect recipients from lung damage that may have otherwise persisted silently until later onset of symptoms supported BOS diagnosis.
9.4. Targeted therapies
Having long demonstrated effective immunosuppression in kidney transplant recipients, without the nephrotoxicity associated with calcineurin inhibitors, the LTx community has proposed belatacept as a potential targeted therapeutic that may offer value in LTxRs. Belatacept is a fusion protein composed of the Fc fragment of human IgG1 linked with the extracellular domain of cytotoxic T-lymphocyte associated antigen 4 (CTLA4), which selectively binds CD80 and CD86, thereby blocking co-stimulatory signals necessary for T lymphocyte activation. Although it appears to be in the incipient stages of investigation in LTx, belatacept may reduce the development of de novo-developed DSA after transplant, and thus we eagerly await the results of ongoing clinical trials studying allograft function and survival in cardiothoracic transplant recipients whose maintenance immunosuppression included belatacept.
Furthermore, lung allograft rejection is associated with the production of a number of cytokines. Recent studies in human LTxRs have confirmed the relationship between interleukin (IL)-17A, and the development of BOS and its risk factors, such as airway epithelial injury [98]. Jordan et al [99] recently detailed the critical role of IL-6 transcriptional dysregulation in the perpetuation of immune and inflammatory responses within the allograft. Thus, IL-6 and IL-17A represent two pro-inflammatory mediators that may serve as targets for future therapeutics aiming to prevent the cytokine secretion and proliferation that promotes lung injury after transplant. Under the reign of the ongoing Coronavirus Disease 2019 pandemic, we have observed the benefits of tocilizumab (IL-6 inhibitor) in protecting solid organ transplant recipients from viral-induced cytokine storms. Perhaps, our present-day observations will inspire future use of IL-6 inhibitors in LTxRs, as available evidence seems to illustrate promise in their ability to ameliorate lung damage.
Antibody-mediated rejection (AMR) is another known predictor for the development of CLAD (obstructive or restrictive phenotypes). Higher pre-transplant DSA levels have been associated with higher AMR risk after transplant, and thus a reduction of DSA levels prior to transplant may reduce the risk of DSA-mediated allograft injury at the time of transplant and reperfusion. Plasma cells are a major source of pathogenic alloantibodies, and thus plasma cell targeted therapies for reducing the risk of developing AMR has been the subject of growing investigation, particularly in kidney transplant recipients. Immunoproteasome inhibitors (PIs) have been evaluated for their anti-humoral responses, and recent advances to second-generation PIs have brought attention to their potential utility in depleting plasma cells and antibody production. Whether these agents find similar success in LTxRs is yet to be determined but appears to be an area of future investigation.
10.0. Conclusions
BOS is the primary cause of mortality after the first year following LTx, as well as the representative form of chronic lung rejection. Studies in the modern era of LTx have validated several preventive and treatment strategies for BOS, yet these approaches only serve to slow the decline observed in lung function. Although a daunting challenge, the results from our investigations, among countless others, have helped orchestrate a new era in transplant medicine, emphasizing the development of targeted therapies against known pathophysiologic mechanisms of allograft injury and rejection.
Article highlights.
Bronchiolitis obliterans syndrome (BOS) is a subtype of chronic lung allograft dysfunction (CLAD) that affects a majority of lung transplant recipients and significantly impairs the long-term survivability of the procedure.
BOS is mediated by several risk factors that elicit a mixed anti-donor response between de novo-developed donor specific antibodies, autoantibodies, and non-immune related allograft insults which promote exosome release.
Prompt preventive strategies to address the above risk factors focus on treating any viral, bacterial, or fungal infections, acute rejection episodes, and GERD symptoms, among others.
Investigations conducted in lieu of the updated CLAD classification system have established the value of pharmacologic therapy (ie, azithromycin and montelukast) and other notable interventions in preventing or treating BOS phenotype CLAD.
Non-invasive, immune biomarkers have improved the early detection of allograft dysfunction and impending rejection.
Targeted therapeutics against known pathophysiologic mechanisms of allograft injury and rejection may demonstrate great promise in improving freedom from CLAD and overall survival after transplant.
Acknowledgements
The authors would like to thank Kristine Nally and Billie Glasscock for their assistance in editing and submitting this manuscript.
Funding
This paper was not funded. The author T. Mohanakumar is supported by NIH R01 HL056643.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018October;37(10):1169–83. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016October;35(10):1170–84. [DOI] [PubMed] [Google Scholar]
- 3.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019May;38(5):493–503. [DOI] [PubMed] [Google Scholar]; **This study is considerably important, as it provides the most updated classification system for CLAD and its associated phenotypes.
- 4.Kotecha S, Paraskeva MA, Levin K, Snell GI. An update on chronic lung allograft dysfunction. Annals of translational medicine. 2020March;8(6):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato M Bronchiolitis obliterans syndrome and restrictive allograft syndrome after lung transplantation: why are there two distinct forms of chronic lung allograft dysfunction? Annals of translational medicine. 2020March;8(6):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian D, Huang H, Wen HY. Noninvasive methods for detection of chronic lung allograft dysfunction in lung transplantation. Transplant Rev (Orlando). 2020July;34(3):100547. [DOI] [PubMed] [Google Scholar]
- 7.Cebria IIMA, Vos R, Verleden GM, Gosselink R, Langer D. Evolution of Functional Exercise Capacity in Lung Transplant Patients With and Without Bronchiolitis Obliterans Syndrome: A Longitudinal Case-Control Study. Arch Bronconeumol. 2019May;55(5):239–45. [DOI] [PubMed] [Google Scholar]
- 8.Neurohr C, Huppmann P, Thum D, et al. Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int. 2010September;23(9):887–96. [DOI] [PubMed] [Google Scholar]
- 9.Vos R, Vanaudenaerde BM, Verleden SE, et al. Bronchoalveolar lavage neutrophilia in acute lung allograft rejection and lymphocytic bronchiolitis. J Heart Lung Transplant. 2010November;29(11):1259–69. [DOI] [PubMed] [Google Scholar]
- 10.Konen E, Gutierrez C, Chaparro C, et al. Bronchiolitis obliterans syndrome in lung transplant recipients: can thin-section CT findings predict disease before its clinical appearance? Radiology. 2004May;231(2):467–73. [DOI] [PubMed] [Google Scholar]
- 11.LaPar DJ, Burdick MD, Emaminia A, et al. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Ann Thorac Surg. 2011August;92(2):470–7; discussion 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011June;30(6):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T. Circulating Exosomes with Distinct Properties during Chronic Lung Allograft Rejection. J Immunol. 2018April15;200(8):2535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agbor-Enoh S, Wang Y, Tunc I, et al. Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine. 2019February;40:541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JYC, Verleden SE, Zarinsefat A, et al. Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival. J Clin Med. 2019February13;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tissot A, Danger R, Claustre J, Magnan A, Brouard S. Early Identification of Chronic Lung Allograft Dysfunction: The Need of Biomarkers. Front Immunol. 2019;10:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study is important, as it reviews all non-invasive biomarkers for CLAD in the modern era.
- 17.Krishna R, Anjum F, Oliver TI. Bronchiolitis Obliterans (Obliterative Bronchiolitis, Constrictive Bronchiolitis). StatPearls. Treasure Island (FL) 2020. [Google Scholar]
- 18.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21 (3):297–310. [DOI] [PubMed] [Google Scholar]
- 19.Gunasekaran M, Xu Z, Nayak DK, et al. Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection. Am J Transplant. 2017February;17(2):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study is important, as it implicates circulating exosomes in the immune pathogenesis of BOS; exosomes have since been identified as a highly reliable biomarker for impending allograft rejection.
- 20.Nayak DK, Zhou F, Xu M, et al. Zbtb7a induction in alveolar macrophages is implicated in anti-HLA-mediated lung allograft rejection. Sci Transl Med. 2017July12;9(398). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009January1;182(1):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao U, Sharma M, Mohanakumar T, Ahn C, Gao A, Kaza V. Prevalence of antibodies to lung self-antigens (Kalpha1 tubulin and collagen V) and donor specific antibodies to HLA in lung transplant recipients and implications for lung transplant outcomes: Single center experience. Transpl Immunol. 2019June;54:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tikkanen JM, Singer LG, Kim SJ, et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2016September1;194(5):596–606. [DOI] [PubMed] [Google Scholar]
- 24.Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014December;33(12):1288–94. [DOI] [PubMed] [Google Scholar]
- 25.Lyu DM, Grazia TJ, Benson AB, Cagle LR, Freed BM, Zamora MR. Pre-transplant presence of antibodies to MICA and HLA class I or II are associated with an earlier onset of bronchiolitis obliterans syndrome in lung transplant recipients. Clin Transpl. 2012:237–46. [PubMed] [Google Scholar]
- 26.Fernandez R, Chiu S, Raparia K, et al. Humoral Human Lung Allograft Rejection by Tissue-Restricted Non-HLA Antibodies. Ann Thorac Surg. 2016October;102(4):e339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bando K, Paradis IL, Similo S, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110(1):4–13. [DOI] [PubMed] [Google Scholar]
- 28.Keller CA, Cagle PT, Brown RW, Noon G, Frost AE. Bronchiolitis obliterans in recipients of single, double, and heart-lung transplantation. Chest. 1995April;107(4):973–80. [DOI] [PubMed] [Google Scholar]
- 29.Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005November27;80(10):1406–13. [DOI] [PubMed] [Google Scholar]
- 30.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008May1;177(9):1033–40. [DOI] [PubMed] [Google Scholar]
- 31.Gunasekaran M, Bansal S, Ravichandran R, et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant. 2020April;39(4):379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am J Transplant. 2015December;15(12):3024–40. [DOI] [PubMed] [Google Scholar]
- 33.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010June15;181(12):1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009August;9(8):1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008March15;85(5):771–4. [DOI] [PubMed] [Google Scholar]
- 36.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008July;86(1):189–95; discussion 96-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007March1;175(5):507–13. [DOI] [PubMed] [Google Scholar]
- 38.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007October;26(10):1004–11. [DOI] [PubMed] [Google Scholar]
- 39.Leiva-Juarez MM, Urso A, Arango Tomas E, et al. Extended post-ex vivo lung perfusion cold preservation predicts primary graft dysfunction and mortality: Results from a multicentric study. J Heart Lung Transplant. 2020 May 16. [DOI] [PubMed] [Google Scholar]
- 40.Shah N, Force SD, Mitchell PO, et al. Gastroesophageal reflux disease is associated with an increased rate of acute rejection in lung transplant allografts. Transplant Proc. 2010September;42(7):2702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King BJ, Iyer H, Leidi AA, Carby MR. Gastroesophageal reflux in bronchiolitis obliterans syndrome: a new perspective. J Heart Lung Transplant. 2009September;28(9):870–5. [DOI] [PubMed] [Google Scholar]
- 42.Bobadilla JL, Jankowska-Gan E, Xu Q, et al. Reflux-induced collagen type v sensitization: potential mediator of bronchiolitis obliterans syndrome. Chest. 2010August;138(2):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuda T, Mittal SK, Kovacs B, et al. Foregut function before and after lung transplant. J Thorac Cardiovasc Surg. 2019August;158(2):619–29. [DOI] [PubMed] [Google Scholar]
- 44.D'Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005May;129(5):1144–52. [DOI] [PubMed] [Google Scholar]
- 45.Griffin SM, Robertson AG, Bredenoord AJ, et al. Aspiration and allograft injury secondary to gastroesophageal reflux occur in the immediate post-lung transplantation period (prospective clinical trial). Ann Surg. 2013November;258(5):705–11; discussion 11-2. [DOI] [PubMed] [Google Scholar]
- 46.Heng D, Sharples LD, McNeil K. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–63. [PubMed] [Google Scholar]
- 47.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010September15;182(6):784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leuschner G, Lauseker M, Howanietz AS, et al. Longitudinal lung function measurements in single lung transplant recipients with chronic lung allograft dysfunction. J Heart Lung Transplant. 2020August26. [DOI] [PubMed] [Google Scholar]
- 49.Finlen Copeland CA, Davis WA, Snyder LD, et al. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J Heart Lung Transplant. 2011September;30(9):990–6. [DOI] [PubMed] [Google Scholar]
- 50.Schulz U, Solidoro P, Muller V, et al. CMV Immunoglobulins for the Treatment of CMV Infections in Thoracic Transplant Recipients. Transplantation. 2016March;100Suppl 3:S5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clausen ES, Zaffiri L. Infection prophylaxis and management of viral infection. Annals of translational medicine. 2020March;8(6):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018May;6(5):357–67. [DOI] [PubMed] [Google Scholar]
- 53.Whitford H, Walters EH, Levvey B, et al. Addition of inhaled corticosteroids to systemic immunosuppression after lung transplantation: a double-blind, placebo-controlled trial. Transplantation. 2002June15;73(11):1793–9. [DOI] [PubMed] [Google Scholar]
- 54.Davis RD Jr., Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003March;125(3):533–42. [DOI] [PubMed] [Google Scholar]
- 55.Cantu E 3rd, Appel JZ 3rd, Hartwig MG, et al. J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004October;78(4):1142–51; discussion 42-51. [DOI] [PubMed] [Google Scholar]
- 56.Biswas Roy S, Elnahas S, Serrone R, et al. Early fundoplication is associated with slower decline in lung function after lung transplantation in patients with gastroesophageal reflux disease. J Thorac Cardiovasc Surg. 2018June;155(6):2762–71 e1. [DOI] [PubMed] [Google Scholar]
- 57.Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg. 2011August;92(2):462–8; discussion; 68-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gulack BC, Meza JM, Lin SS, Hartwig MG, Davis RD. Reflux and allograft dysfunction: is there a connection? Thorac Surg Clin. 2015;25(1):97–105. [DOI] [PubMed] [Google Scholar]
- 59.Verleden GM, Vos R, Vanaudenaerde B, et al. Current views on chronic rejection after lung transplantation. Transpl Int. 2015October;28(10):1131–9. [DOI] [PubMed] [Google Scholar]
- 60.Davidson JR, Franklin D, Kumar S, et al. Fundoplication to preserve allograft function after lung transplant: Systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2019November24. [DOI] [PubMed] [Google Scholar]
- 61.Cairn J, Yek T, Banner NR, Khaghani A, Hodson ME, Yacoub M. Time-related changes in pulmonary function after conversion to tacrolimus in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003January;22(1):50–7. [DOI] [PubMed] [Google Scholar]
- 62.Whyte RI, Rossi SJ, Mulligan MS, et al. Mycophenolate mofetil for obliterative bronchiolitis syndrome after lung transplantation. Ann Thorac Surg. 1997October;64(4):945–8. [DOI] [PubMed] [Google Scholar]
- 63.Roman A, Bravo C, Monforte V, Reyes L, Canela M, Morell F. Preliminary results of rescue therapy with tacrolimus and mycophenolate mofetil in lung transplanted patients with bronchiolitis obliterans. Transplant Proc. 2002February;34(1):146–7. [DOI] [PubMed] [Google Scholar]
- 64.Revell MP, Lewis ME, Llewellyn-Jones CG, Wilson IC, Bonser RS. Conservation of small-airway function by tacrolimus/cyclosporine conversion in the management of bronchiolitis obliterans following lung transplantation. J Heart Lung Transplant. 2000December;19(12):1219–23. [DOI] [PubMed] [Google Scholar]
- 65.Verleden GM, Dupont LJ, Van Raemdonck D, Vanhaecke J. Effect of switching from cyclosporine to tacrolimus on exhaled nitric oxide and pulmonary function in patients with chronic rejection after lung transplantation. J Heart Lung Transplant. 2003August;22(8):908–13. [DOI] [PubMed] [Google Scholar]
- 66.Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004May15;77(9):1465–7. [DOI] [PubMed] [Google Scholar]
- 67.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005September15;172(6):772–5. [DOI] [PubMed] [Google Scholar]
- 68.Shitrit D, Bendayan D, Gidon S, Saute M, Bakal I, Kramer MR. Long-term azithromycin use for treatment of bronchiolitis obliterans syndrome in lung transplant recipients. J Heart Lung Transplant. 2005September;24(9):1440–3. [DOI] [PubMed] [Google Scholar]
- 69.Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J. 2011January;37(1):164–72. [DOI] [PubMed] [Google Scholar]
- 70.Gan CT, Ward C, Meachery G, Lordan JL, Fisher AJ, Corris PA. Long-term effect of azithromycin in bronchiolitis obliterans syndrome. BMJ Open Respir Res. 2019;6(1):e000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruttens D, Verleden SE, Vandermeulen E, et al. Prophylactic Azithromycin Therapy After Lung Transplantation: Post hoc Analysis of a Randomized Controlled Trial. Am J Transplant. 2016January;16(1):254–61. [DOI] [PubMed] [Google Scholar]; *This study is important, as it provides considerable support for use of azithromycin (in the modern era) in preventing BOS development.
- 72.li D, Duan Q, Weinkauf J, Laing B, Nagendran J, Halloran K Azithromycin prophylaxis after lung transplant is associated with improved overall survival. J Heart and Lung Transplantation. 2020. [DOI] [PubMed] [Google Scholar]
- 73.Szczepanik A, Hulbert A, Lee HJ, Benedetti C, Snyder L, Byrns J. Effect of HMG CoA reductase inhibitors on the development of chronic lung allograft dysfunction. Clin Transplant. 2018January;32(1). [DOI] [PubMed] [Google Scholar]
- 74.Verleden GM, Verleden SE, Vos R, et al. Montelukast for bronchiolitis obliterans syndrome after lung transplantation: a pilot study. Transpl Int. 2011July;24(7):651–6. [DOI] [PubMed] [Google Scholar]
- 75.Ruttens D, Verleden SE, Demeyer H, et al. Montelukast for bronchiolitis obliterans syndrome after lung transplantation: A randomized controlled trial. PLoS One. 2018;13(4):e0193564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vos R, Eynde RV, Ruttens D, et al. Montelukast in chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2019May;38(5):516–27. [DOI] [PubMed] [Google Scholar]; *This study is important, as it provides considerable support for use of montelukast (in the modern era) in preventing BOS progression.
- 77.Moes DJ, Guchelaar HJ, de Fijter JW. Sirolimus and everolimus in kidney transplantation. Drug Discov Today. 2015October;20(10):1243–9. [DOI] [PubMed] [Google Scholar]
- 78.Roman A, Ussetti P, Zurbano F, et al. A retrospective 12-month study of conversion to everolimus in lung transplant recipients. Transplant Proc. 2011September;43(7):2693–8. [DOI] [PubMed] [Google Scholar]
- 79.Groetzner J, Kur F, Spelsberg F, et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. J Heart Lung Transplant. 2004May;23(5):632–8. [DOI] [PubMed] [Google Scholar]
- 80.Wojarski J, Zeglen S, Ochman M, Karolak W. Early Sirolimus-Based Immunosuppression is Safe for Lung Transplantation Patients: Retrospective, Single Arm, Exploratory Study. Ann Transplant. 2018August23;23:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisher AJ, Rutherford RM, Bozzino J, Parry G, Dark JH, Corris PA. The safety and efficacy of total lymphoid irradiation in progressive bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2005March;5(3):537–43. [DOI] [PubMed] [Google Scholar]
- 82.Verleden GM, Lievens Y, Dupont LJ, et al. Efficacy of total lymphoid irradiation in azithromycin nonresponsive chronic allograft rejection after lung transplantation. Transplant Proc. 2009June;41(5):1816–20. [DOI] [PubMed] [Google Scholar]
- 83.Lebeer M, Kaes J, Lambrech M, et al. Total lymphoid irradiation in progressive bronchiolitis obliterans syndrome after lung transplantation: a single-center experience and review of literature. Transpl Int. 2020February;33(2):216–28. [DOI] [PubMed] [Google Scholar]
- 84.Xia CQ, Campbell KA, Clare-Salzler MJ. Extracorporeal photopheresis-induced immune tolerance: a focus on modulation of antigen-presenting cells and induction of regulatory T cells by apoptotic cells. Current opinion in organ transplantation. 2009August;14(4):338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benden C, Speich R, Hofbauer GF, et al. Extracorporeal photopheresis after lung transplantation: a 10-year single-center experience. Transplantation. 2008December15;86(11):1625–7. [DOI] [PubMed] [Google Scholar]
- 86.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010April;29(4):424–31. [DOI] [PubMed] [Google Scholar]
- 87.Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2012September;31(9):950–7. [DOI] [PubMed] [Google Scholar]
- 88.Del Fante C, Scudeller L, Oggionni T, et al. Long-Term Off-Line Extracorporeal Photochemotherapy in Patients with Chronic Lung Allograft Rejection Not Responsive to Conventional Treatment: A 10-Year Single-Centre Analysis. Respiration. 2015;90(2):118–28. [DOI] [PubMed] [Google Scholar]
- 89.Baskaran G, Tiriveedhi V, Ramachandran S, et al. Efficacy of extracorporeal photopheresis in clearance of antibodies to donor-specific and lung-specific antigens in lung transplant recipients. J Heart Lung Transplant. 2014September;33(9):950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karnes HE, Schindler EI, Morrell M, et al. Factors Associated With Mortality and Response to Extracorporeal Photopheresis in Lung Allograft Recipients With Bronchiolitis Obliterans Syndrome. Transplantation. 2019May;103(5):1036–42. [DOI] [PubMed] [Google Scholar]
- 91.Biswas Roy S, Panchanathan R, Walia R, et al. Lung Retransplantation for Chronic Rejection: A Single-Center Experience. Ann Thorac Surg. 2018January;105(1):221–27. [DOI] [PubMed] [Google Scholar]
- 92.Verleden SE, Todd JL, Sato M, et al. Impact of CLAD Phenotype on Survival After Lung Retransplantation: A Multicenter Study. Am J Transplant. 2015August;15(8):2223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohanakumar T, Sharma M, Bansal S, Ravichandran R, Smith MA, Bremner RM. A novel mechanism for immune regulation after human lung transplantation. J Thorac Cardiovasc Surg. 2019May;157(5):2096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sureshbabu A, Fleming T, Mohanakumar T. Autoantibodies in lung transplantation. Transpl Int. 2020January;33(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rahman M, Sureshbabu A, Tokman S, Mohanakumar T. Chronic Lung Allograft Dysfunction: Immune Responses Induced by Circulating Exosomes with Lung-Associated Self-Antigens. Crit Rev Immunol. 2019;39(2):123–34. [DOI] [PubMed] [Google Scholar]
- 96.Bansal S, McGilvrey M, Garcia-Mansfield K, et al. Global Proteomics Analysis of Circulating Extracellular Vesicles Isolated from Lung Transplant Recipients. ACS Omega. 2020June23;5(24):14360–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma M, Gunasekaran M, Ravichandran R, Fisher C, Limaye A, Hu C, McDyer J, Kaza V, Bharat A, Tokman S, Omar A, Aujuna A, Walia R, Bremner R, Smith M, Hachem R, Mohanakumar T Circulating exosomes with lung self-antigens as a biomarker for chronic lung allograft dysfunction: A retrospective analysis. Journal Heart and Lung Transplantation. 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang R, Fang H, Chen R, Ochando JC, Ding Y, Xu J. IL-17A Is Critical for CD8+ T Effector Response in Airway Epithelial Injury After Transplantation. Transplantation. 2018December;102(12):e483–e93. [DOI] [PubMed] [Google Scholar]
- 99.Jordan SC, Ammerman N, Choi J, et al. Interleukin 6: An Important Mediator of Allograft Injury. Transplantation. 2020March30. [DOI] [PubMed] [Google Scholar]


