Abstract
The long terminal repeat (LTR)-containing retrotransposon Tf1 propagates within the fission yeast Schizosaccharomyces pombe as the result of several mechanisms that are typical of both retrotransposons and retroviruses. To identify host factors that contribute to the transposition process, we mutagenized cultures of S. pombe and screened them for strains that were unable to support Tf1 transposition. One such strain contained a mutation in a gene we named nup124. The product of this gene contains 11 FXFG repeats and is a component of the nuclear pore complex. In addition to the reduced levels of Tf1 transposition, the nup124-1 allele caused a significant reduction in the nuclear localization of Tf1 Gag. Surprisingly, the mutation in nup124-1 did not cause any reduction in the growth rate, the nuclear localization of specific nuclear localization signal-containing proteins, or the cytoplasmic localization of poly(A) mRNA. A two-hybrid analysis and an in vitro precipitation assay both identified an interaction between Tf1 Gag and the N terminus of Nup124p. These results provide evidence for an unusual mechanism of nuclear import that relies on a direct interaction between a nuclear pore factor and Tf1 Gag.
Retroviruses and long terminal repeat (LTR)-containing retrotransposons possess similar methods of propagation that include the conversion of their mRNA into cDNA by reverse transcriptase (RT) and the insertion of this double-stranded DNA into the host genome by integrase (IN). Because reverse transcription occurs in the cytoplasm, the preintegration complexes (PIC) of cDNA and IN must be transported into the nucleus for integration to occur. Nuclear pore complexes (NPC) are assemblies of more than 50 proteins that provide the means for selective passage of proteins and nucleic acids between the cytoplasm and the nucleus (55, 58). Although significant progress has been made describing the families of transport factors that deliver nuclear localization sequence (NLS)-containing proteins to the nucleus (13, 21, 51, 53, 65), the current models of nuclear import do not directly address how macromolecules as large as virus complexes pass through the nuclear pore.
The length and diameter of the transport channel have been measured by testing nucleoplasmin-coated gold particles of various sizes for the ability to pass through the nuclear pore. The maximum diameter of the channel was found to be 20 to 25 nm, and this passage extends approximately 50 nm across the nuclear envelope (15, 46). Large macromolecular substrates that must in some form pass through the NPCs in intact nuclear envelopes include the 50-nm virus particles of simian virus 40 (SV40) (24, 49, 70), the 90-nm particles of adenovirus (24, 60), and the 160S PIC of human immunodeficiency virus (HIV). Although the adenovirus particles attach to the NPC and disassemble before the transport of the DNA-protein VII complex, SV40 appears to pass through the NPC as virion particles (25, 49, 70). The nuclear import of the HIV PIC reflects not only the presence of NLS activity in matrix and IN but also the unusual β-karyopherin-like properties of Vpr (8, 17, 18, 28, 30, 54, 61, 62). Recent evidence suggests that the import of the HIV PIC in nondividing cells relies on an interaction between Vpr and specific nuclear pore factors that contain FXFG motifs (16, 61).
It is interesting that the passage of retroviruses through the NPCs is not thought to be important in dividing cells, because retroviruses may readily access the host genome after breakdown of the nuclear envelope. Nevertheless, the IN of avian sarcoma virus possesses efficient NLS activity that contributes to virus replication (34, 35). In addition, the retrotransposons of Saccharomyces cerevisiae and Schizosaccharomyces pombe must travel through NPCs to access the nucleus because the nuclear envelopes of these organisms do not breakdown during mitosis.
Although these examples of large transport substrates indicate that multiple mechanisms may exist to allow their import, little is known about the principal activities required for these mechanisms. Because LTR-containing retrotransposons produce large virus-like particles (VLPs) and because these transposons propagate in yeast, the genetic analysis of transposition may reveal whether specialized host activities are required for the nuclear import of large transposon complexes. In addition, the extensive similarity between retroviruses and LTR-containing retrotransposons suggests that any information relevant to the import of transposon complexes may lead to a better understanding of retrovirus import. Recent studies of Ty1 transposition in S. cerevisiae revealed that IN contains a bipartite NLS that is required for Ty1 transposition (33, 47). Although these results constitute important first steps in the understanding of the nuclear transport of retrotransposon proteins, little is known about what components of the NPC contribute to Ty1 import.
The fission yeast S. pombe contains Tf1, an active LTR-retrotransposon that expresses functional copies of Gag, protease (PR), reverse transcriptase (RT), and integrase (IN) proteins (39, 41). The transposition activity of a neo-marked copy of Tf1 can be monitored in vivo, and as many as 4% of the cells induced for transposition receive Tf1 insertions (40). Although Tf1 possesses an unusual self-priming mechanism for the initiation of reverse transcription (36–38), the other aspects of its reverse transcription and integration appear to model the general properties of LTR retroelements (2, 3, 39–41). The results of sucrose gradient fractionation and blotting techniques indicate that extracts of S. pombe cells induced for transposition contain large Tf1 particles composed of Gag, RT, IN, Tf1 mRNA, and Tf1 cDNA (3, 37, 40).
To search for host factors that contribute to Tf1 function, we mutagenized cultures of S. pombe and screened for strains that were unable to support Tf1 transposition. We identified a mutation in a host gene that lowered Tf1 transposition by 12-fold without reducing the levels of reverse transcription. Immunofluorescence microscopy indicated that this mutation also caused a significant reduction in the nuclear localization of Tf1 Gag. We named this host gene nup124 (nuclear pore factor of 124 kDa) because this protein localized to the nuclear envelope and because the hypothetical coding sequence included 11 copies of the FXFG repeat that is present in a large family of nuclear pore factors. The mutant allele of nup124 caused no alterations in growth rates or in the nuclear localization of other proteins examined. The results of two-hybrid analyses and glutathione S-transferase (GST) precipitation assays detected an interaction between Tf1 Gag and Nup124p. This evidence indicates that Nup124p possesses a specialized activity required for the nuclear import of Tf1 complexes.
MATERIALS AND METHODS
Media and growth of S. pombe strains.
The S. pombe minimal liquid and plate media were composed of EMM (2). For growth rate determinations, overnight cultures in EMM complete plus vitamin B1 were diluted to an optical density at 600 nm (OD600) of 0.05 in fresh medium (50 ml). Growth of all S. pombe strains was conducted at 32°C unless otherwise stated. To examine temperature sensitivity, strains were grown on plates incubated at 16, 20, 25, 37, and 42°C. The yeast strains used in this study are listed in Table 1.
TABLE 1.
Yeast strains
| Strain | Genotype | Strain/Plasmid | Source or reference |
|---|---|---|---|
| 461 | h+ ade6-210 hist3-1Δ ura4− Δ 18 leu1-32 | Parent/no plasmid | 52 |
| 912 | h− ura4-294 leu1-32 | Parent/no plasmid | J. Boeke 21X52 |
| 965 | h− ura4-294 leu1-32 | 912/pSP1 | This study |
| 972 | h− ura4-294 leu1-32 | 912/pSp2 | 41 |
| 1282 | h− ura4-294 leu1-32 | 912/pHL449-1 | 37 |
| 1836 | h− ura4-294 leu1-32 | 912/pHL490-80 | 3 |
| 1858 | h+ ura4-D18 leu1-32 ade6-m210 | 1605/pHL449-1 | This study |
| 1554 | h− ura4-294 leu1-32 | 912/pHL476-3 | 37 |
| 4990 | h− ura4-294 leu1-32 | 1836/pSP1 | This study |
| 4992 | h− ura4-294 leu1-32 | 1554/pSP1 | This study |
| 5533 | h− ura4-294 leu1-32 | 1282/pSP1 | This study |
| 5750 | h− ura4-294 leu1-32 nup124-1 | Parent/no plasmid | This study |
| 5754 | h− ura4-294 leu1-32 nup124-1 | 5750/pHL449-1 | This study |
| 5895 | h− ura4-294 leu1-32 | 912/pHL1276 | This study |
| 6061 | h− ura4-294 leu1-32 nup124-1 | 5754/pSP1 | This study |
| 6106 | h− ura4-294 leu1-32 nup124-1 | 5754/pHL1338-3 | This study |
| 6110 | h− ura4-294 leu1-32 nup124-1 | 5754/pHL1288 | This study |
| 6136 | h− ura4-294 leu1-32 pHL1342:leu+ nup124-1 | Stable leu+ integrant of pHL1342 in 5750 | This study |
| 6404 | h− ura4-294 leu1-32 | 1282/pHL1343-1 | This study |
| 6405 | h− ura4-294 leu1-32 | 1282/pHL1344-1 | This study |
| 6406 | h− ura4-294 leu1-32 nup124-1 | 5754/pHL1343-1 | This study |
| 6407 | h− ura4-294 leu1-32 nup124-1 | 5754/pHL1344-1 | This study |
| 6569 | h− ura4-294 leu1-32 nup124-1 | 5750/pHL1276 | This study |
| 6565 | h− ura4-294 leu1-32 nup124-1 | 5750/pFL20 | This study |
| 6566 | h− ura4-294 leu1-32 | 912/pFL20 | This study |
| 6576 | h− ura4-294 leu1-32 | 912/1587-18 | This study |
| 6577 | h− ura4-294 leu1-32 | 912/1587-18 (independent transformant of 6576) | This study |
| 6620 | h− ura4-294 leu1-32 nup124-1 | 6136/pHL449-1 | This study |
| 6876 | h+ ade6-210 his3-1 Δ ura4-Δ18 leu1-32+ nup124::pk1(3) GFP SV40 poly(A) ura4+ | Integration of the pk1(3) GFP SV40 poly(A) ura4+ tag at the C-terminal end of Nup124 in strain 461 | This study |
| EGY48 | Matα ura3 his3 trp1 3LexAop-leu2 | S. cerevisiae strain used as a two-hybrid host | 20 |
Plasmid constructions.
Many DNA fragments used to create plasmids for this study were generated by PCR. To avoid complications due to the inadvertent creation of mutations by the polymerases, we used the high-fidelity enzymes Turbo Pfu (Stratagene) and Deep Vent (New England Biolabs). In addition, the plasmids that were generated with PCR products were created in duplicate from independent PCRs and the properties of each plasmid were studied in parallel.
The HA-tagged allele of nup124 included a double copy of the HA epitope that was inserted into a BclI site introduced at the N terminus of the coding sequence in plasmid pHL1587-18. To create a FLAG-tagged version of Gag, the sequence encoding 10 amino acids of Tf1 Gag in pHL1258 was replaced by creating a NaeI restriction site near the sequence encoding the C terminus of Gag. The NaeI site was created within a product of fusion PCR by using oligonucleotides HL211 and HL212 for the NaeI site. Using two complementary oligonucleotides, HL220 and HL221, a 24-bp DNA fragment encoding the FLAG epitope was cloned into the newly created NaeI site to generate pHL1276.
A mutation in Xenopus nucleoplasmin within the bipartite NLS amino acids was created by fusion in the context of the green fluorescent protein (GFP)-nucleoplasmin fusion protein. A 1.3-kb BamHI-MscI fragment encoding the wild-type nucleoplasmin NLS, KKAGQAKKKK (pREP-GFP-Nucleoplasmin, [Table 2]), was replaced by a similar fragment containing the mutated NLS KKAGQANNKK to create pHL1769 (Table 2). The 1.3-kb region generated by PCR containing the mutation was confirmed by sequence analysis. This mutated version of the nucleoplasmin NLS has been previously reported to inhibit nuclear import (57).
TABLE 2.
Plasmids
| Plasmid | Description | Source or reference |
|---|---|---|
| pAF1 | Contains the S. pombe his3 gene | 52 |
| pSP1 | S. pombe ars1 vector with the S. cerevisiae LEU2 | 10 |
| pSP2 | S. pombe ars1 vector with the S. cerevisiae URA3 | 10 |
| pREP3 | leu-1 selectable multicopy plasmid | 45 |
| pFL20 | S. pombe vector with ARS and stabilization fragment | 44 |
| pGH54 | Used to make a neo probe | 31 |
| pJK148 | Integrating vector containing complete sequence of the leu1 gene of S. pombe | 32 |
| pHL449-1 | neoAI-marked version of Tf1 in a ura4 selectable plasmid | 37 |
| pHL476-3 | Tf1-neoAI with frameshift in IN | 37 |
| pHL481 | Integration vector | This report |
| pHL490-80 | Tf1-neoAI with frameshift in PR | 3 |
| pHL1258 | 449-1 with a unique NgoMI site in Gag used to insert the FLAG epitope | This report |
| pHL1276 | pHL1258 with a FLAG sequence inserted into the NgoMI site | This report |
| pHL1288 | Genomic library plasmid | This report |
| pHL1335 | Genomic library DNA insert in pHL1288 suppressing the nup124-1 defect | This report |
| pHL1336 | Genomic library DNA insert in pHL1288 suppressing the nup124-1 defect | This report |
| pHL1337 | Genomic library DNA insert in pHL1288 suppressing the nup124-1 defect | This report |
| pHL1338-3 | Genomic library DNA insert in pHL1288 suppressing the nup124-1 defect | This report |
| pHL1339-2 | Genomic library DNA insert in pHL1288 suppressing the nup124-1 defect | This report |
| pHL1342 | pHL481containing a 4.4-kb SpeI-BamHI nup124 fragment from pHL1335 | This report |
| pHL1343-1 | pHL 1338-3 with a framshift mutation of nup124 | This report |
| pHL1344-1 | pHL1338-3 with a 3.97-kb deletion in nup124 | This report |
| pHL1346 | pHL1338-3 with a 4.22-kb deletion in nup124 | This report |
| pHL1469-2 | PCR-generated BclI site at the ATG of nup124 ORF in pHL1339-2 | This report |
| pHL1470-6 | PCR-generated BaclI site at the ATG of nup124 ORF in pHL1339-2 | This report |
| pHL1471-2 | pHL1469-2 amplified in a dam bacterial strain, DM1 | This report |
| pHL1472-6 | pHL1470-6 amplified in a dam bacterial strain, DM1 | This report |
| pHL1572 | pHL1472-6 containing a his3 gene in the place of the nup124 ORF | This report |
| pHL1587-18 | HA epitope inserted into the nup124 coding sequence in pHL1471-2 | This report |
| pGEX-6P-1 | GST fusion expression plasmid | Pharmacia |
| pHL1613-4 | pGEX-6P-1 containing full-length Gag | This report |
| pHL1623-1 | pGEX-6P-1 containing N-terminal portion of nup124 | This report |
| pHL1622-1 | pGEX-6P-1 containing C-terminal portion of nup124 | This report |
| pHL1678 | PCR-generated nup124-1 mutation, cytosine to thymine in pHL1338-3 | This report |
| pREP-GFP-Nucleoplasmin | Leu2 selectable marker, nmt1 promoter drives transcription of GFP fusion to Xenopus nucleoplasmin which contains a bipartite NLS sequence KKAGQAKKKK | 4 |
| pHL1769 | Nucleoplasmin NLS, KKAGQAKKKK in pREP-GFP-Nucleoplasmin, was replaced by a similar fragment containing the mutated NLS KKAGQANNKK | This report |
| pSGP502-SV40 | Leu1 selectable marker, nmt1 promoter drives transcription of GFP fused to lacZ; contains SV40 large T antigen NLS sequence MAPKKKRKV | S. G. Pasion and S. Forsburg |
| pSGP502-SV40mut | pSGP502-SV40 in which the SV40 large T antigen NLS sequence MAPKKKRKV was mutated to MAPKEKDKV | S. G. Pasion and S. Forsburg |
| pCS2-KSu | Contains pk1(3) GFP SV40 poly(A) ura4 used as template to generate by PCR an integration tag nup124::pk1(3) GFP SV40 poly(A) ura4+ | C. Troxell and J. R. McIntosh |
A mutation from cytosine to thymine in the wild-type nup124 sequence was created by fusion PCR to re-create the nup124-1 allele. A 2.6-kb AvrII-NcoI fragment containing the wild-type nup124 in pHL1338-3 (Table 2) was replaced by a fusion PCR fragment containing the mutated nup124-1 recreated sequence to form pHL1678 (Table 2). The sequence generated by fusion PCR containing the mutation was confirmed.
Integration of GFP into the C terminal of the nup124+ product by homologous integration gene tagging.
A construct designed to contain the 3′ end of the nup124 open reading frame (ORF), pK1 antigen (three repeats), GFP gene, SV40 poly(A) signal and ura4+ marker followed by sequence after the nup124 stop codon was generated by PCR with HL613 and HL614 (Table 3) as primers and pCS2pkSu (Table 2) as template. The PCR-generated fragment was used to transform S. pombe 461 (Table 3). Stable ura4+ transformants were microscopically examined for GFP fluorescence. Integration of the nup124-3pk1-GFP-ura4+ construct into the nup locus in single copy was verified by Southern blot analysis. Strains carrying the genomically tagged nup124 were then processed for immunoelectron microscopy.
TABLE 3.
Oligonucleotides
| Oligonucleotide | Sequence (5′-3′) | Use |
|---|---|---|
| HL446 | atgtgtaggttaagtgatcaaggcgtaatcaggcacatcatatggg taggcgtaatcaggcacatcatatggg tacatcaatacaaactcatcgacaactac |
PCR with HA epitope |
| HL211 | tgaaacatttgttttctttgggccggcaaacttattgtttttccaattg | Bottom-strand oligonucleotide creates a unique NgoMI restriction site in gag in pHL1258 |
| HL212 | caattggaaaaacaataagtttgccggcccaaagaaaacaaatgtttca | Top strand oligonucleotide creates a unit NgoMI restriction site in gag in pHL1258 |
| HL220 | gactacaaggacgacgatgacaag | To generate a 24-bp DNA fragment encoding the FLAG epitope of pHL1276 |
| HL221 | cttgtcatcgtcgtccttgtagtc | To generate a 24-bp DNA fragment encoding the FLAG epitope in pHL1276 |
| HL199 | gcaactagttcagatcttagtcgaccgatgcataaggatccctgagct | 5′-flanking oligonucleotide of polylinker sequence in library vector pHL1288 |
| HL200 | cagggatccttatgcatcggtcgactaagatctgaactagttgctgca | 3′-flanking oligonucleotide of polylinker sequence in library vector pHL1288 |
| HL417 | ctgcagcaactagttcagatcttagtcgaccgatgtataaggatccctgagctc | 5′-flanking oligonucleotide in nup124 mutating ATG to a BclI site |
| HL340 | gtcgatgagtttgtattgatcactcctgtttcaaaaaatac | 3′-flanking fusion oligonucleotide for overlapping the pair HL417-HL418 with BclI mutation |
| HL418 | ctagattcctaacctcgaggtataatcttgcttttgctttggc | 3′-flanking oligonucleotide in nup124 mutating ATG to a BclI site |
| HL420 | gtattttttgaaacaggagtgatcaatacaaactcatcgac | 5′-flanking oligonucleotide for overlapping the pair HL417-HL418 with BclI mutation |
| HL447 | catcgtaaggatccatttgtcttccaccaatttttgaatt | PCR generation of the 3′ flank of S. pombe his3 gene with pHL1570 as template |
| HL440 | gtatatacggccgtctatgcaaagctaacgaatct | PCR generation of the 5′ flank of S. pombe his3 gene with pHL1570 as template |
| HL495 | tttggcagatctctttcaacgttt | PCR oligonucleotide 5′ flank for generation of Gag in GST vector |
| HL496 | gctttaatcagaggatccatgaaaaactcatcacagaaaagaatt | PCR oligonucleotide 3′ flank for generation of Gag in GST vector |
| HL520 | agttagcacaaggataccctcgagtcaataacgtcttttcttgtattttgta | PCR oligonucleotide 5′ flank for generation of an N-terminal portion of Nup124p in GST vector |
| HL510× | catgctttaatcagaggatccagaattattcgcgttagtaatcggagccat | PCR oligonucleotide 3′ flank for generation of an N-terminal portion of Nup124p in GST vector |
| HL521 | agttagcacaaggataccgtcgactcacaaacgattagtttgcacatcagc | PCR oligonucleotide 5′ flank for generation of C-terminal section of Nup124p in GST vector |
| HL501 | catgctttaatcagaggatccaaggagaatgagcccaagccaacg | PCR oligonucleotide 3′ flank for generation of C-terminal section of Nup124p in GST vector |
| HL339 | gaattcatgcctcctgtttcaaaaaatac | 5′ flank for 779–1073 nucleotide synthesis of nup124 segment 1 to generate LexA fusions |
| HL340 | ctcgaggtataacttgcttttgctttgg | 3′ flank for 779–1073 nucleotide synthesis of nup124 segment 1 to generate LexA fusions |
| HL341 | gaattcagaattattcgcgttagtaatcgg | 5′ flank for 1073–1718 nucleotide synthesis of nup124 segment 2 to generate LexA fusions |
| HL342 | ctcgagtcatgcattgactaatacaggcttc | 3′ flank for 1073–1718 nucleotide synthesis of nup124 segment 2 to generate LexA fusions |
| HL343 | gaattcgaagttcagacagattccaacc | 5′ flank for 1718–2363 nucleotide synthesis of nup124 segment 3 to generate LexA fusions |
| HL344 | ctcgagtcacaaacgattagtttgcacaatca | 3′ flank for 1718–2363 nucleotide synthesis of nup124 segment 3 to generate LexA fusions |
| HL345 | gaattcaaggagaatgagcccaagcc | 5′ flank for 2363–3008 nucleotide synthesis of nup124 segment 4 to generate LexA fusions |
| HL346 | ctcgagtcacttggtaaaattaaatgaaaactg | 3′ flank for 2363–3008 nucleotide synthesis of nup124 segment 4 to generate LexA fusions |
| HL347 | gaattcccaaatacggatgccaaaacc | 5′ flank for 3008–3653 nucleotide synthesis of nup124 segment 5 to generate LexA fusions |
| HL348 | ctcgagtcaggtagtctttgaagttgcagac | 3′ flank for 3008–3653 nucleotide synthesis of nup124 segment 5 to generate LexA fusions |
| HL349 | gaattctctgaaggaaccgctccagc | 5′ flank for 3653–4306 nucleotide synthesis of nup124 segment 6 to generate LexA fusions |
| HL350 | ctcgagttaacgttttcttcgacttcgg | 3′ flank 3653–4306 nucleotide synthesis of nup124 segment 6 to generate LexA fusions |
| HL414 | gaattcatgaaaaaactcatcacagaaaaggattcgaatggaaatgg | 5′ flank for Gag to generate B42 activation domain fusions |
| HL355 | ctcgagtcaataacgtcttttcttgtattttg | 3′ flank for Gag to generate B42 activation domain fusions |
| HL613 | ggtattcaattcaatttgggctcttcta actcacaaacaaatgcgcccccgggccg taaaattgctgtgccccgaagtcgaa gaaaacgttccggaggaggtggaggagagctcatgggtattcctaac |
5′-flanking PCR oligonucleotide for generating nup124::pk1(3) GFP SV40 poly(A) ura4+ integration tag |
| HL614 | taaaagcgtccgtatgtgtaaacaaaaatgtaataattatt ccttagcaattaatttaaaggacaaagtctatctaaac tatcatatgagaaagtctttgctgatatgcctt |
3′-flanking PCR oligonucleotide for generating nup124::pk1(3) GFP SV40 poly(A) ura4+ integration tag |
Immunoelectron microscopy.
Cells were prepared for electron microscopy by freeze-substitution fixation after rapid freezing by previously published methods (12). The anti-GFP antibodies (the kind gift of Jason Kahana and Pam Silver) were diluted 200-fold in blocking buffer, and sections mounted on grids were floated overnight on a 20-μl drop of this solution (12, 67). The grids were then rinsed in buffered saline and treated with goat anti-rabbit immunoglobulin labeled with 10-nm-diameter colloidal gold (12, 67).
Molecular and genetic techniques.
Strains to be tested for transposition and cDNA recombination were treated as previously described (2, 11).
The levels of Tf1 cDNA and protein in cells induced for transposition were measured by DNA blot analysis and Western analysis, respectively (2).
Mutagenesis of S. pombe YHL1858.
The parent strain YHL1858 was subjected to mutagenesis with ethyl methanesulfonate (EMS) as described by Moreno et al. (48). The mutagenized culture exhibited 16% viability compared to the cells not treated with EMS. The same preparation of cells was plated on EMM − ura medium to generate colonies of cells that contained the Tf1-neoAI plasmid. We screened 2,500 of these colonies for reduced transposition activity by the Tf1 patch assay.
Construction of an S. pombe genomic library.
Genomic DNA was isolated from the wild-type strain 972 in 500 ml of YES medium. The DNA was subjected to partial digestion with Sau3A after having established the optimal conditions for the fractionation of a population corresponding to 4 to 6 kb. Sau3A created 3′ overhang ends that were partially filled in with dATP and dGTP by using Klenow enzyme. The resulting DNA was inserted into the vector pHL1288.
The polylinker and the nmt1 sequence of the multicopy plasmid pREP3 (45) were excised with PstI and SacI and replaced with a 48-base polylinker fragment composed of HL199 and HL200, GCAACTAGTTCAGATCTTAGTCGACCGATGTATAAGGATCCCTGAGCT, containing the restriction sites PstI, SpeI, BglII, SalI, BamHI, and SacI. pHL1288 was linearized with SalI followed by partial filling-in of the first two nucleotides with dCTP and dTTP by using Klenow. The genomic DNA digested with Sau3A was ligated into pHL1288 overnight at 16°C.
Strain YHL5754 (nup124-1) was transformed by treatment with lithium acetate and library DNA in amounts optimized to yield 600 to 800 colonies/plate (48). The colonies were then put through the cDNA recombination assay to identify clones able to rescue the recombination defect of the strain with nup124-1. A total of 54,155 colonies tested in the screen yielded five plasmids that complemented both the transposition and recombination defects of YHL5754. Plasmid DNA was isolated by extraction from the strains that suppressed the nup124-1 defect (2).
Deletion of the nup124 sequence in the suppressor plasmid and creation of a frame shift.
A deletion in the nup124 sequence of the suppressor plasmid pHL1338-3 was created by digestion with SnaBI and SmaI followed by ligation. A frameshift mutation was created 375 bases downstream from the start of translation of nup124 in the suppressor plasmid by linearizing pHL1338-3 with AvrII. The 5′ overhangs were filled with Klenow, and the resulting blunt ends were ligated to create pHL1343.
To insert a suppressing fragment of nup124 back into the genome, an integration vector, pHL481, was created by removing the 742-nucleotide ClaI fragment from pJK148 (32). This plasmid, contained a multicloning site and the complete sequence of the leu1 gene of S. pombe. A 4.4-kb SpeI-BamHI fragment from pHL1338-3 with nup124 was inserted into pHL481 digested with XbaI and BamHI to produce pHL1342. To integrate this plasmid, pHL1342 was digested with AgeI and transformed into the wild-type strain YHL912 and the nup124-1 mutant strain YHL5750. Stable Leu+ transformants were selected, and stable integrants were identified. DNA blot analysis of YHL6136 indicated that the integration of pHL1342 occurred at the genomic location of nup124.
Construction of a strain with nup124 deleted.
To delete the entire ORF of nup124 and replace it with his3, we used strains and plasmid DNA as described by Ohi et al. (52). A BglII-NaeI fragment with the his3 gene of S. pombe was generated by PCR with oligonucleotides HL447 and HL440 with pAf1 as template. DNA from pHL1472-6 was digested with BclI and SmaI to remove the 3.47-kb nup124 ORF, and the remaining DNA was ligated to the BglII-NaeI fragment of the his3 sequence to create pHL1572. The 3.6-kb BglII-Ecl136II fragment was used to transform a homozygous wild-type his3 diploid. Strains were identified by DNA blot hybridization in which the nup124-1 allele had been replaced by the integration of a Δnup124::HIS3-disrupted copy.
Immunofluorescence microscopy.
FLAG-tagged Gag was localized by incubating mutant and wild-type cells induced to overexpress the FLAG-tagged Gag by the absence of vitamin B1. A total of 5 OD600 units of stationary-phase cells (OD600 = 10 to 11) were harvested and processed essentially as described previously (5). The cells were reacted with a 1:1,000 dilution of primary antibody, anti-FLAG M2 monoclonal antibody (no. IB13025; Eastman Kodak, New Haven, Conn.), at room temperature in a humidified chamber overnight. After five washes, these cells were incubated with a secondary antibody consisting of Oregon Green 488 goat anti-mouse immunoglobulin G (Molecular Probes, Eugene, Oreg.) at a 1:500 dilution for 2 h in the dark. Cells were mounted prior to visualization with 1 mg of p-phenylenediamine (Sigma P-1519) per ml, 1 μg of 4′,6-diamidino-2-phenylindole (DAPI) per ml in 50% glycerol was added, and the coverslips were sealed with clear nail polish.
The localization of HA-tagged Nup124p was determined by the same method used to visualize the FLAG-Gag protein. A total of 5 OD600 units of cells grown in EMM − leu dropout medium were harvested at an OD600 of 0.25. They were fixed for 75 min and treated with Zymolyase 100T (Seikagaku Corp.) for 1 h. The primary antibody was a 1:5,000 dilution of the monoclonal antibody HA.11 (MMS-101P; BAbCO), and the secondary antibody was a 1:1,000 dilution of Oregon Green 488 goat anti-mouse immunoglobulin G.
To localize the SV40 NLS-GFP-LacZ protein, nup124-1 and wild-type strains were transformed with pSGP502-SV40 and pSGP502-SV40mut plasmids (kindly supplied by Sally G. Pasion, Salk Institute, San Diego, Calif.) (Table 2). To induce the overexpression of the GFP-LacZ fusion proteins, the strains were grown in the absence of vitamin B1. A total of 5 OD600 units of log-phase cells (OD600, 0.6 to 0.8) were harvested and fixed for 2 min in 2% (vol/vol) formaldehyde–0.05% (vol/vol) glutaraldehyde (G5882; Sigma) in phosphate-buffered saline. After two washes with phosphate-buffered saline, the cells were allowed to adhere for 40 min at room temperature to slides coated with 1.0% polyethylenimine. The slides were rinsed in distilled water and air dried. The cells were next mounted with a solution containing p-phenylenediamine (1 mg/ml) and DAPI (1 mg/ml) in 50% glycerol before the coverslips were sealed with clear nail polish.
To study the localization of the GFP-nucleoplasmin protein, nup124-1 and wild-type strains were transformed with the pREP-GFP-Nucleoplasmin plasmid (kindly supplied by Tokio Tani and Yasumi Ohshima, Kyushu University, Kyushu, Japan) and pHL1769 (Table 2). After the cells were induced for Tf1 expression, 5 OD600 units of log-phase cells (OD600 of 0.6 to 0.8) were harvested and directly incubated for 40 min in the dark at room temperature in Hoechst 33342 (bis-benzimidine; B2261 [Sigma] at 25 mg/ml in 50% glycerol while adhering to slides coated with 1.0% polyethylenimine. The slides were then rinsed in distilled water and air dried. The cells were mounted with 12.5 μg of Hoechst 33342 per ml in 50% glycerol before the coverslips were sealed with clear nail polish.
The in situ hybridization assay to detect poly(A) mRNA was conducted as previously published (27).
Cells were observed and photographed with a Zeiss Axiophot fluorescence microscope equipped with a 100× objective. Unless otherwise mentioned, Kodak Ektachrome P1600 film was used to capture the images.
Two-hybrid analysis.
To screen for the interactions between Tf1 Gag and the Nup124 protein, the yeast interaction trap two-hybrid system was used (20, 26). DNA segments encoding full-length Gag as well as six segments of Nup124p (see Fig. 8) were amplified by PCR and cloned into activation domain (AD) and binding-domain (BD) plasmid vectors. To clone these fragments into the two-hybrid vectors, primers for each PCR product were designed to create EcoRI and XhoI restriction sites at the 5′ and 3′ ends, respectively. Duplicates of each PCR product were cloned into the DNA binding-domain plasmid, pEG202, and activation domain plasmid, pJG4-5 (20). The various combinations of plasmids were transformed into yeast strain EGY48 (20), which has the upstream activating sequences of the chromosomal LEU2 gene replaced with by LexA operators. Potential interactions were scored by printing patches from galactose plates containing leucine to galactose plates lacking leucine. The plates were monitored on a daily basis for up to 5 days. The growth of test interaction patches was compared to that demonstrated by a known interaction consisting of p53 fused to LexA and SV40 large T antigen fused to B42.
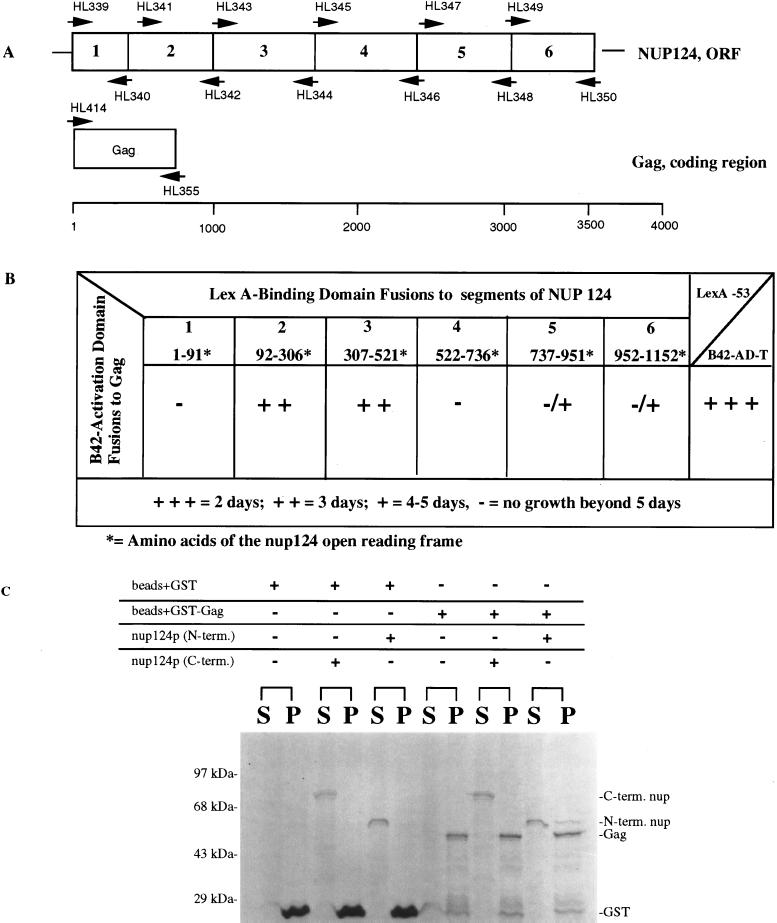
FIG. 8.
Analysis of interactions between Gag and Nup124p. (A) Segments 1 through 6 of Nup124p correspond to the following amino acids of Nup124p: 1, 1 to 91; 2, 92 to 306; 3, 307 to 521; 4, 522 to 736; 5, 737 to 951; 6, 952 to 1152. Corresponding nucleotide sequence were fused to the transcription activation domain B42. Numbers above and below the arrowheads indicate the primers used to generate the PCR products that were inserted into the two-hybrid vectors. (B) Summary of two-hybrid interactions between segments 1 to 6 of Nup124p expressed as fusions to the LexA binding domain and the Tf1 Gag expressed as a fusion to the B42 activation domain. Potential interactions were scored as growth on SC medium containing galactose and lacking leucine. All fusions were tested for intrinsic or nonspecific activation. The AD plasmids were cotransformed with LexA fused to the Bicoid protein to test for specificity of interaction with each of the BD fusions, while all the BD plasmids were cotransformed with an empty AD to test for intrinsic activation. The BD fusion with the Gag protein resulted in significant intrinsic activation and therefore could not be used in this study. All other fusions used did not intrinsically or nonspecifically activate expression of the LEU2 reporter. Additionally, a positive control was used in these two-hybrid experiments based on the strong interaction of murine p53 and SV40 large T antigen. Plasmids containing p53 fused to LexA and SV40 large T antigen fused to B42 (Clontech) were cotransformed into EGY48 and tested for growth on medium lacking leucine. Strains containing these fusion proteins showed visible growth in approximately 1 to 2 days. (C) GST precipitation analysis of interactions between purified samples of Gag and two portions of Nup124p. The N-terminal and C-terminal portions of Nup124p as well as GST and GST-Gag were purified from bacteria. The GST and GST-Gag proteins were coupled to glutathione-Sepharose and combined with either the C-terminal or N-terminal fragments of Nup124p. The samples were incubated at room temperature for 45 min and washed three times in binding buffer. The beads and the supernatant were combined with 2× sample-loading buffer and loaded in equal proportions onto an SDS–10% polyacrylamide gel that was stained with Coomassie blue. The brackets over S and P indicate the pairs of supernatant and pellet fractions from separate binding reactions. The components of each binding reaction are indicated by plus signs above each bracket. The positions of molecular mass standards are indicated on the left of the panel.
GST precipitation.
The Gag of Tf1 and the two halves of Nup124p were expressed as C-terminal GST fusions in the BLR (Novagen) strain of bacteria. A PCR product encoding Gag was generated with oligonucleotides that produced a BamHI site (HL495) at the beginning of the Gag gene and a XhoI site (HL496) at the predicted end of the Gag gene. This product was inserted into the BamHI and XhoI sites of pGEX-6P-1 (Pharmacia Biotech) to produce pHL1613-4. A PCR product encoding an N-terminal portion of Nup124p that corresponded to sections 2 and 3 in Fig. 8 was created with a BamHI site at its beginning (HL520) and a SalI site (HL510×) at its 3′ end. This product was inserted into the BamHI and SalI sites of pGEX-6P-1 to create pHL1623-1. Similarly, a PCR product encoding the C-terminal section of Nup124p (see Fig. 8, sections 4, 5, and 6) was created with a BamHI site (HL521) at the 5′ end and a XhoI site (HL501) at the 3′ end. Insertion of this product into the BamHI and XhoI sites of pGEX-6P-1 produced pHL1622-1. The GST-Nup124p proteins were cleaved with PreScission protease (Pharmacia Biotech) and eluted from the glutathione-Sepharose 4B beads whereas the GST-Gag proteins were purified on the glutathione-Sepharose 4B beads and used directly in precipitation experiments.
The pull-down experiments were performed, as described by Rexach and Blobel (56), in binding buffer that contained 20 mM HEPES (pH 6.8), 150 mM potassium acetate, 2 mM magnesium acetate, 2 mM dithiothreitol, 0.1 mM Tween 20, and 0.1% Casamino Acids. After the beads with GST-Gag were washed in binding buffer and the Nup124p proteins were dialyzed in binding buffer, approximately 1 μg of the Nup124p proteins was added in 15 μl to 15 μl of resin bed with 1 μg of GST-Gag. The sample was rotated end over end at room temperature for 45 min, and after three washes with 250 μl of binding buffer, the beads and the supernatant fractions were combined with sample loading buffer and loaded onto a sodium dodecyl sulfate–10% polyacrylamine gel.
RESULTS
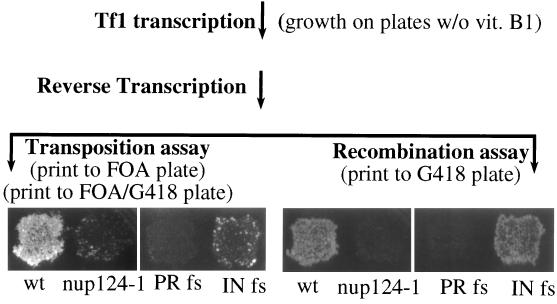
To identify genes in S. pombe that contribute to the function of Tf1, we mutagenized cultures with EMS and screened the strains for reduced levels of transposition. Tf1 activity in individual colonies was monitored by a previously described assay that detected the insertion of neo-marked Tf1 elements into the genome of S. pombe (40). To measure transposition, a plasmid-encoded copy of Tf1 that included a bacterial neomycin resistance gene (Tf1-neoAI) was induced for transposition by activating Tf1 transcription. After first selecting against cells that retained the Tf1-neoAI plasmid, we identified cells with genomic inserts of Tf1-neo by virtue of the resistance to G418 provided by the neo gene. Figure 1 contains the results of a transposition assay and shows that a patch of cells that initially contained wild-type Tf1-neoAI produced confluent growth on a plate that contained G418 whereas a similar patch of cells that contained Tf1-neoAI with a frameshift in IN showed significantly less transposition.
FIG. 1.
Transposition and cDNA recombination assays of Tf1. The genetic manipulations and replica printing required for the transposition and recombination assays are indicated in parentheses. The ability of the reverse transcripts in both assays to produce G418 resistance is shown. Although wild-type (wt) Tf1-neoAI produced G418 resistance in both assays, a mutation that blocked integrase expression, IN fs, greatly reduced growth on the transposition plates without significantly reducing growth on the recombination plates. PR fs is a strain with a frameshift mutation in Tf1 that blocks the expression of PR, RT, and IN. FOA- 5-fluoroorotic acid.
The strains that appeared to have significantly lower transposition activity were examined for several trivial causes of reduced growth on the plates that contained G418. Each candidate suspected of possessing transposition defects was tested for reduced function of the nmt1 promoter as fused to lacZ. We also retransformed each candidate with a fresh copy of the Tf1-neoAI plasmid to identify which strains were defective for transposition simply due to mutations in the assay plasmid. In addition, we tested strains with a version of Tf1 that contained arg3 as a transposition marker. In this way, we could exclude candidates that showed low growth on the G418/5-fluoroorotic acid (FOA) plates due to alterations specific to the metabolism of G418. One strain that exhibited a genuine reduction in transposition was crossed with a wild-type strain. The spores of 14 tetrads exhibited a 2:2 segregation of the transposition defect, which indicated that the reduced Tf1 activity was due to a mutation in a single gene. For reasons described below, we named this gene nup124.
The transposition activity of cells that contained the nup124-1 mutation is shown in Fig. 1. To measure the magnitude of this defect we subjected strains to a quantitative-transposition assay that was developed previously (2, 43). The results of the quantitative-transposition assay showed that the strain with the nup124 mutation produced 12-fold-fewer transposition events than did the wild-type strain (described in Materials and Methods).
After the mutagenized strains were tested for defects in transposition activity, they were screened by a previously described assay that detected homologous recombination between Tf1 plasmid sequences and copies of Tf1 cDNA. The occurrence of wild-type levels of this recombination indicates that normal levels of Tf1 cDNA are present in the nucleus (2). The homologous-recombination assay was performed with the same Tf1-neoAI plasmid that was used for the transposition assays. The presence of an artificial intron (AI) disrupted the neo reading frame, and because the intron orientation was inverted relative to neo, the intron could not be spliced from the neo transcript. However, strains induced for Tf1 expression become resistant to G418 because the intron was in the appropriate orientation to be spliced from the Tf1 mRNA. We found that if colonies induced for Tf1 expression were replica printed directly to plates that contained G418, significant levels of G418 resistance occurred that could be attributed to two equally efficient processes (2). The reverse transcripts of the spliced Tf1 mRNA, if present in the nucleus, could homologously recombine with the Tf1-neoAI plasmid and generate a G418-resistant version of the plasmid. The cDNA also was able to serve as the substrate for conventional transposition events. Although these processes occurred with about equal proportions in wild-type strains, we could use this method to measure the levels of homologous cDNA recombination in strains that were known to be defective for transposition (2). The feature of the transposition assay that masks the detection of cDNA recombination was growth on a medium that selects against the Tf1-neoAI plasmid.
The recombination assay in Fig. 1 shows that a wild-type copy of the Tf1-neoAI plasmid produced confluent growth on agar medium that contained G418. The confluent growth produced by a version of Tf1-neoAI that lacked IN due to a frameshift mutation and the lack of G418 resistance due to a frameshift just upstream of RT (PR fs) are demonstrations used to indicate that the homologous-recombination assay detects products of reverse transcription even in the absence of IN activity (2). Figure 1 also shows that the mutation in nup124 that was responsible for low transposition activity caused a dramatic reduction in the homologous recombination of Tf1 cDNA. The magnitude of the recombination defect was determined by subjecting the strains to a quantitative version of the homologous-recombination assay (2). The strain with the nup124-1 mutation produced 40-fold-lower levels of cDNA recombination than did the wild-type strain (see Materials and Methods).
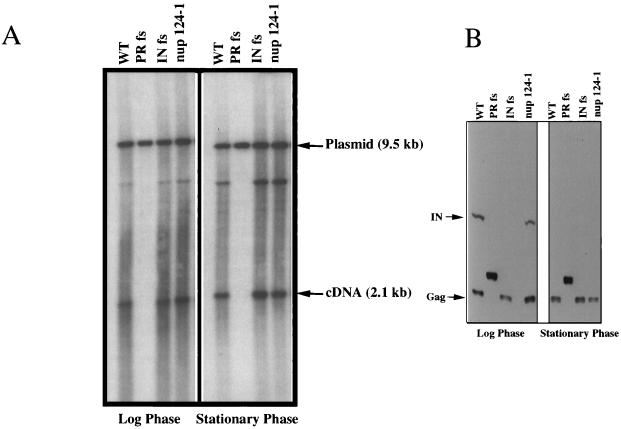
The results of the recombination assays suggested either that the levels of Tf1 reverse transcripts were reduced by the nup124-1 mutation or that normal levels of cDNA were produced but did not accumulate in the nucleus. We used a previously published technique to measure directly the accumulated levels of Tf1 cDNA in cells (2, 38). Liquid cultures of cells induced for Tf1 expression were extracted for total DNA, which was then digested with BstXI and subjected to DNA blot analysis. DNA was extracted from cultures that were in log-phase growth as well as from cells that had reached stationary phase. The results showed that the wild-type Tf1-neoAI plasmid produced the same amount of a 2.1-kb fragment of cDNA as did the strain with the mutation in nup124-1 (Fig. 2A). A 9.5-kb band resulted from the BstXI digestion of the Tf1-neoAI plasmid, and this served as an internal control for levels of DNA loaded in each lane.
FIG. 2.
Levels of Tf1 cDNA and proteins are not altered by the mutation in nup124-1. (A) Tf1 cDNA production in wild-type and nup124-1 cells at the logarithmic and stationary phases of growth. A DNA blot containing nucleic acid extracted from wild-type (WT) (YHL1282), PR frameshift (YHL1836), INT frameshift (YHL1554), and the nup124-1 mutant (YHL5754), is shown. Genomic DNA from the logarithmic and stationary phases of growth on medium without vitamin B1 was digested with BstXI and loaded onto a 0.6% agarose gel, which was transferred to a filter and probed with a 1.0-kb neo fragment. (B) Tf1 proteins in wild-type and nup124-1 cells at the logarithmic and stationary phases of growth. An immunoblot of extracts from the cells used for the Tf1 cDNA determination in panel A is shown. The filter was probed with both anti-Gag and anti-IN antisera. The arrows marked IN and Gag show the positions of the IN and Gag proteins.
Another possibility we considered was that the nup124-1 mutation indirectly caused a reduction in the level of one or all of the Tf1 proteins. An immunoblot of proteins extracted from cultures harvested in both stationary and exponential phases showed that the levels of Gag and IN proteins in cells with the nup124-1 defect were indistinguishable from those in wild-type cells (Fig. 2B). Since IN is the last protein encoded by the single ORF of Tf1, the presence of normal levels of IN indicated that all Tf1 proteins were translated with wild-type efficiency.
Isolation and sequence of the nup124 gene.
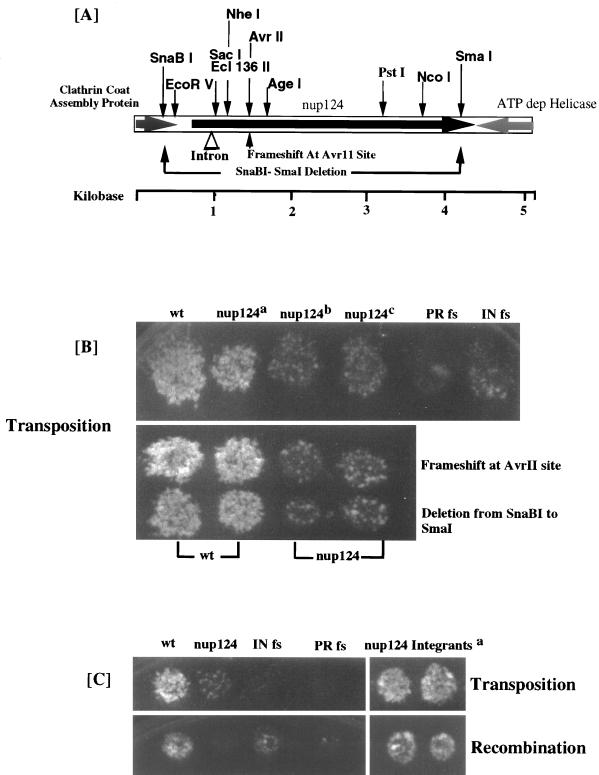
To isolate a wild-type copy of the nup124 gene and determine its sequence, we transformed a genomic library of plasmids into a strain with the nup124-1 allele and screened for complementation of the transposition defect. The strains with high levels of transposition activity were all found to contain the same fragment of genomic DNA. Both orientations of the insert were isolated. Furthermore, these plasmids complemented both the transposition and cDNA recombination defects caused by the nup124-1 mutation. The sequence of the entire 5,075-bp genomic fragment was available from the S. pombe genome project.
Figure 3A is a diagram of the fragment that included the significant ORFs and the restriction sites used to determine that each of the complementing plasmids contained the same insert. The sequence began with 106 codons of the ORF that corresponded to the last 64% of a gene for a clathrin coat assembly protein. The end of the fragment encoded the last 20% of a 754-amino-acid protein with sequence similarity to ATP-dependent RNA helicases. The center of the fragment contains the entire gene (encoding 1,159 amino acids) for a hypothetical protein of 124 kDa. When a deletion was made from the SnaBI site to the SmaI site, the complete coding sequence of the central ORF was removed and the resulting plasmid no longer complemented the transposition or recombination defect of the nup124-1 mutation (Fig. 3B). We also found that a frameshift mutation generated near the beginning of the central ORF in the AvrII site destroyed the complementation activity of the plasmid (Fig. 3B). These data indicated that the central ORF, encoding 1,159 amino acids, was the source of the complementation. To test whether this suppressor gene was allelic to nup124, we subcloned the entire fragment of genomic sequence shown in Figure 3A into an integration vector that contained the leu1 gene of S. pombe. The resulting plasmid was integrated at the genomic site of the complementing ORF in a haploid strain that contained the nup124-1 allele. The structure and position of the integrated plasmid were confirmed by DNA blot analysis. The single-copy integrated fragment was found to retain its ability to complement the nup124-1 defects in transposition and homologous recombination (Fig. 3C). The resulting strain was mated with a haploid that possessed a wild-type copy of nup124, and 20 tetrads were dissected. All four spores of these tetrads possessed wild-type transposition and cDNA recombination activity. Taken together, these data indicate that the central ORF from the complementing fragment was allelic with nup124.
FIG. 3.
Isolation and sequence of the nup124 gene. (A) Restriction fragment of the genomic sequence that complemented the nup124-1 defect, with the locations of the ORFs indicated by large arrows. The C-terminal sections of the ATP-dependent helicase and the clathrin assembly protein are shown with shaded arrows, and the nup124 ORF is shown with a black arrow. Also shown are the positions of restriction sites including AvrII, SnaBI, and SmaI, the sites used to disrupt the nup124 ORF. (B) All strains were assayed for transposition activity. The strains represented in the upper panel are as follows (from left to right); wild type (wt) (YHL5533), nup124a (YHL6106, a nup124-1 strain expressing the entire complementing fragment, nup124b (YHL6061, a strain with an empty vector, pSP1), nup124c (YHL6110, a strain with the empty library vector pHL1288); a wild-type strain containing Tf1 PR fs (YHL4990); and a wild-type strain containing Tf1 IN fs (YHL4992). The strains in the top row of the lower panel are, from left to right, two transformants of a wild-type strain with the complementing fragment that contained the frameshift mutation at the AvrII site (YHL6404) and two transformants of a strain with the nup124-1 mutation and the plasmid with the complementing fragment and the frameshift mutation at the AvrII site (YHL6406). The strains in the lower row of the bottom panel are two transformants of a wild-type strain with the plasmid copy of the complementing fragment that contained the SnaBI-SmaI deletion (YHL6405) and two transformants of a strain with the nup124-1 mutation and the plasmid that contained the SnaBI-SmaI deletion (YHL6407). (C) The strain with the nup124-1 allele was transformed with an integrating plasmid containing the entire complementing sequence. A stable transformant (YHL6136), shown to contain an integration of the suppressor sequence into the nup124-1 loci, was transformed with the Tf1-neoAI plasmid pHL449 (YHL6620) and assayed for transposition (upper panel) and recombination (lower panel). aTwo independent transformants are shown. Also represented in both top and bottom sections are (from left to right) strains with wild-type (wt) and nup124-1 alleles (YHL1282 and YHL5754, respectively) and the standard control strains consisting of Tf1 IN fs (YHL1554) and Tf1 PR fs (YHL1836).
The predicted amino acid sequence encoded by nup124 was analyzed by TFASTA, and a low but measurable level of similarity to several nuclear pore factors was found. Further examination of the alignments revealed that the nuclear pore factors all possessed FXFG motifs, and it was primarily these sequences that aligned with nup124. The predicted amino acid sequence of the nup124 product is shown in Fig. 4 and the positions of 11 repeats of FXFG are shown. The FXFG is one type of the FG repeat motifs found in nuclear pore factors (65).
FIG. 4.
Amino acid sequence of the Nup124p (Q09904) protein as predicted with annotation software that identified a small intron marked here with an asterisk. The 11 FXFG repeats at the C-terminal end are underlined. The mutation site of the nup124-1 allele is indicated by a short vertical line before the Q where the codon CAG coding for Q is mutated to a Stop codon, TAG.
The mutation in the nup124-1 allele introduced a stop codon that truncated the protein between the second and third FXFG repeats.
To determine the nature of the defect in the protein expressed by nup124-1, we used PCR to produce four overlapping regions of nup124, using as the template genomic DNA from a strain of S. pombe that contained the nup124-1 allele. Two independent PCR products of each region were cloned and sequenced. The sequences of the cloned PCR products were compared to the sequence of the wild-type nup124 isolated from the S. pombe library. A single-nucleotide substitution was found that converted codon 722 into a termination codon. The result of this nonsense mutation was predicted to remove 9 of the 11 FXFG repeats (Fig. 4). To test whether this single-nucleotide substitution was indeed the cause of the transposition defect, we regenerated the mutation in the context of the original plasmid with nup124, which complemented the genomic nup124-1 allele. We found that the single-nucleotide substitution did inactivate the ability of the plasmid to complement the transposition defect caused by nup124-1 (results not shown).
Nup124p is a nuclear pore factor.
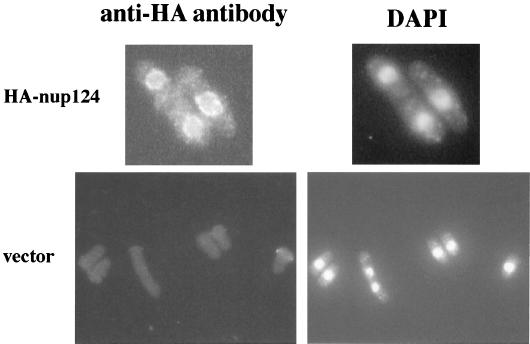
To test whether Nup124p was a component of nuclear pores, we determined its cellular localization by indirect-immunofluorescence microscopy. A double HA tag was fused to the N terminus of nup124 as expressed from its own promoter on the same plasmid that complemented the nup124-1 mutation. The addition of the HA tag did not reduce the ability of nup124 to complement the transposition or cDNA recombination defects caused by nup124-1. Cells were fixed and prepared for visualization with an anti-HA monoclonal antibody. The fluorescence image showed punctate foci that encircled the position of the nucleus as visualized by DAPI staining (Fig. 5). This signal was specific for the HA-tagged nup124 since the control strain that contained the vector without HA-tagged nup124 produced no fluorescence signal. The punctate localization of HA-tagged Nup124p around the edge of the nucleus is typical of the signals produced by antibodies that recognize nuclear pore proteins.
FIG. 5.
Cellular location of Nup124p. Two strains, YHL965 (bottom, vector without nup124) and YHL6576 (top, plasmid with HA-tagged nup124) were grown in EMM − leu dropout medium and prepared for immunofluorescence microscopy. The left two panels show the FITC signal produced by the anti-HA antibody, and the right two panels contain images of the DAPI signals.
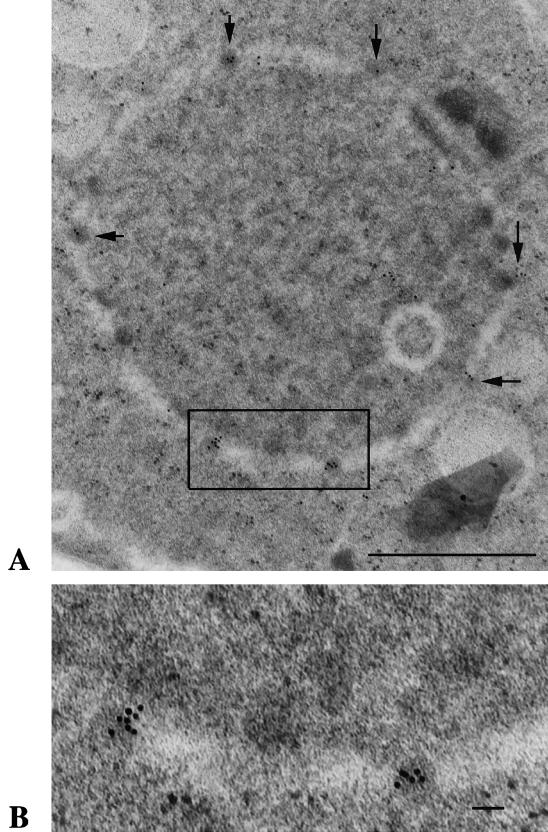
Although the appearance of HA-Nup124p at the nuclear rim was consistent with a localization at the NPCs, the resolution of light microscopy did not allow us to determine whether Nup124p was specifically a component of the NPCs. For this purpose, we examined the localization of Nup124p at high resolution by immunological electron microscopy. We constructed a strain that expressed GFP fused to the C terminus of Nup124p. The fusion protein was expressed by the nup124 promoter from its genomic location. Figure 6 shows a thin section that was treated with the anti-GFP antibody and goat anti-rabbit immunoglobulin labeled with 10-nm-diameter colloidal gold. A significant number of the gold particles localized to the nuclear pore structures. The positions of gold particles on 21 sections were examined. The density of gold particles directly associated with nuclear pore structures was determined, and the average value was found to be 24 gold particles per μm2 of NPC surface. Compared to the density of particles specifically associated with nuclear pore structures (252 gold particles; 24 particles/μm3) the gold particles were 18 times less likely to be found in the nucleoplasm (180 particles; 1.3 particles/μm3) and 40-fold less likely to be found in the cytoplasm (169 particles; 0.6 particle/μm3). The large numbers of gold particles associated with the nuclear pore structures indicated that Nup124p is a component of the NPC.
FIG. 6.
Immunoelectron microscopy demonstrating the presence of a Nup124-GFP fusion protein within nuclear pores. Strain YHL6876 expressed a single-copy allele of GFP fused to the end of Nup124p. Cells were grown in EMM and processed for immunoelectron microscopy with an antibody specific for GFP. (A) A thin section of a cell shows a ring of reduced density that indicates the position of the nuclear envelope. The dark structures embedded in the ring are the nuclear pore. A high concentration of gold particles are associated with the nuclear pores (arrows). The square indicated the region shown at higher magnification in panel B. Bar, 1.0 μm. (B) The inset from panel A was enlarged to allow inspection of the gold particles associated with two nuclear pores. Bar, 0.1 μm.
The nup124-1 mutation reduced the nuclear localization of Gag.
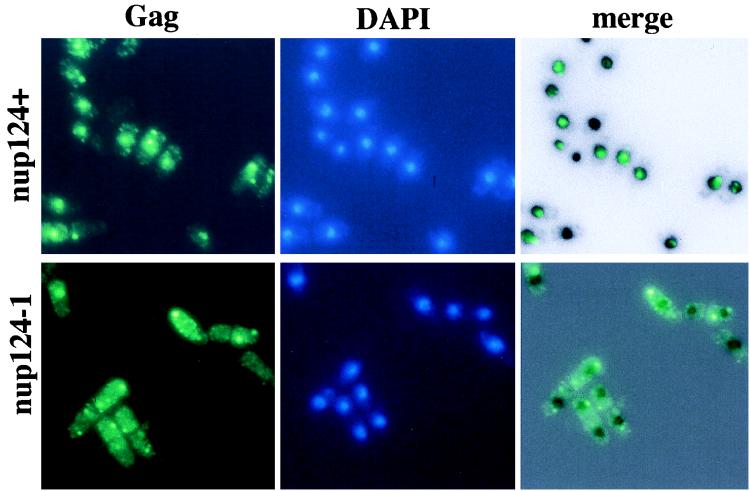
To pursue the possibility that the mutation in nup124 reduced the nuclear localization of Tf1 complexes, we surveyed Tf1 Gag and IN for their tendency to localize to the nucleus of wild-type cells grown at 32°C, the same temperature used during the transposition assays. Because levels of Gag are substantially greater than those of IN, we found it much more reliable to monitor the localization of Gag. A FLAG epitope was inserted near the C terminus of Gag, and the resulting transposon, Tf1(FLAG)-neoAI, possessed wild-type levels of transposition and homologous recombination activity (data not shown). Wild-type cells that were induced for the expression of Tf1(FLAG)-neoAI were grown to saturation and prepared for immunofluorescence microscopy with the M2 anti-flag monoclonal antibody (Kodak). We found that the majority of the wild-type cells produced a single strong focused signal (Fig. 7). The fluorescence image produced by the anti-FLAG antibody was merged with an inverted black-and-white image of the nucleus generated by DAPI staining. The merged image showed that the majority of the FLAG-based signal was entirely overlapped by the nucleus. We found that the anti-FLAG signals were specific for Tf1 since no signal was observed from cells that did not include Tf1(FLAG)-neoAI (results not shown). In sharp contrast to the wild-type cells, the strain with the mutation in nup124 showed almost no FLAG signal within the nucleus (Fig. 7). Table 4 contains a compilation of localization data that includes 99 nuclei from wild-type cells and 79 nuclei from cells with the nup124-1 mutation. Only the nuclei from cells that produced a FLAG signal were tabulated for localization. These data indicate that Gag in the wild-type cells localized to the nucleus while the mutation in nup124 reduced the number of nuclei with nuclear localization of Gag by 7.3-fold. Interestingly, the 7.3-fold drop was associated with a 4.6-fold increase in the number of nuclei that not only lacked Gag but also appeared to be directly attached to aggregates of cytoplasmic Gag.
FIG. 7.
Immunofluorescence of Tf1 FLAG-Gag in wild-type cells. (Top) Strain YHL5895 contained a wild-type allele of nup124 and the Tf1-neoAI FLAG-Gag plasmid, pHL1276. The green signal is specific for the FLAG-Gag protein, and the blue signal is produced by DAPI and indicates the position of the nucleus. The panel on the right is a merge of the FLAG-Gag signal produced by YHL5895 with an inverted black-and-white image of its DAPI stain. The merge was generated with Adobe Photoshop 4.0 with the screen function set at 65% opacity. (Bottom) Same experiment as in the top three panels, except that strain YHL6565 (bottom) contained the nup124-1 allele.
TABLE 4.
Localization of transport substrates
| Transport substrate | Genotype of strain | Transport function studied | No. of nuclei observed
|
|
|---|---|---|---|---|
| Counteda | % Exhibiting nuclear localization | |||
| FLAG-tagged Gag | nup124+ | Import | 99a | 74 |
| FLAG-tagged Gag | nup124-1 | Import | 79a | 10 |
| SV40-NLS-GFP-LacZ | nup124+ | Import | 122b | 99 |
| SV40-NLS (Mut)-GFP-LacZ | nup124+ | Import | 66b | 0 |
| SV40-NLS-GFP-LacZ | nup124-1 | Import | 117b | 96 |
| SV40-NLS (Mut)-GFP-LacZ | nup124-1 | Import | 54b | 0 |
| Nucleoplasmin-NLS-GFP | nup124+ | Import | 88b | 98 |
| Nucleoplasmin-NLS (Mut)-GFP | nup124+ | Import | 89b | 0 |
| Nucleoplasmin-NLS-GFP | nup124-1 | Import | 89b | 98 |
| Nucleoplasmin-NLS (Mut)-GFP | nup124-1 | Import | 90b | 0 |
| Poly(A) mRNA | nup124+ | Export | 123c | 0.81 |
| Poly(A) mRNA | nup124-1 | Export | 153c | 1.96 |
| Poly(A) mRNA | rae1-1 | Export | 87c | 94 |
Only nuclei of cells displaying a Gag-specific FITC generated immunofluorescence (a), GFP-specific fluorescence (b), or FITC-conjugated anti-deoxygenin-generated immunofluorescence (c) were tabulated.
The nup124-1 allele does not cause a general defect in the function of the NPC.
One fundamental question about the nuclear import of Tf1 Gag was how a mutation in a nuclear pore factor could cause a significant defect in the import of a particular protein without resulting in a general loss of import function and viability. We tested whether cells with the nup124-1 allele had reduced growth. The doubling time of these strains at 32°C was measured in liquid cultures that contained EMM plus a complete mixture of supplements. The nup124-1 mutation did not cause any reduction in growth rate (results not shown). In addition, the colony sizes of cells with the nup124-1 mutation were tested after growth at 25, 32, and 37°C. The mutant cells formed the same-sized colonies as the wild-type cells at all three temperatures (results not shown).
The wild-type growth rate of cells with the nup124-1 mutation suggested that the mutation did not cause a defect in the bulk import of proteins into the nucleus. To determine directly whether nuclear import was altered, we asked whether the nup124-1 allele reduced the nuclear localization of specific proteins in cells grown at 32°C. To test whether the mutation in nup124-1 altered the nuclear import of a substrate with a canonical monopartite NLS, we monitored the cellular localization of a fusion protein that included the SV40 NLS, GFP, and β-galactosidase. Fluorescence micrographs showed that the GFP fusion localized to the nuclei of wild-type cells (results not shown). This nuclear localization was due to the function of the SV40 NLS, since amino acid substitutions in the NLS resulted in a cytoplasmic localization. We found that the cells with the nup124-1 allele showed the same localization of the GFP fusion protein in the nucleus as did the wild-type cells (results not shown). The effect of the nup124-1 allele on the nuclear localization of the NLS-GFP-β-galactosidase was evaluated for a large number of nuclei, and this tabulation (Table 4) confirmed the finding that no change in nuclear localization resulted from the nup124-1 mutation.
To evaluate the effect of the nup124-1 allele on the nuclear localization of a protein with a bipartite NLS, we examined the behavior of GFP fused to nucleoplasmin, a protein of Xenopus (57). In wild-type cells, GFP-nucleoplasmin localized to the nucleus (Table 4). This nuclear localization was dependent on the function of the nucleoplasmin NLS since amino acid substitutions in this sequence resulted in cytoplasmic localization (Table 4). In addition to causing a cytoplasmic localization, the altered nucleoplasmin NLS generated punctate fluorescence that may have resulted from aggregation (results not shown). The localization of GFP-nucleoplasmin in cells with the nup124-1 mutation was nuclear and appeared indistinguishable from the pattern seen in wild-type cells (Table 4). Here, too, the nuclear localization in cells with the nup124-1 mutation was dependent on the function of the nucleoplasmin NLS. These data clearly show that the nup124-1 allele did not reduce the nuclear localization of GFP-nucleoplasmin.
In addition to alterations in the nuclear import of proteins, mutations in proteins of the NPC reduce the nuclear export of poly(A) mRNA. In fact, mutations in several nuclear pore factors of S. cerevisiae cause obvious defects in the export of mRNA without noticeably lowering the import of proteins (65). We therefore tested the possibility that the nup124-1 allele generated a defect in NPC function that could be observed as the accumulation of poly(A) mRNA in the nucleus. To visualize the localization of mRNA, cells grown at 32°C were fixed and treated with deoxygenin-labeled oligo(dT) and fluroescein isothiocyanate (FITC)-conjugated anti-deoxygenin antisera. Cells that contained the nup124-1 mutation did not accumulate poly(A) mRNA in the nucleus and were indistinguishable from wild-type cells (Table 4). As an example of cells that do accumulate poly(A) mRNA in the nucleus, we included a strain with a mutation in the nuclear pore protein Rae1p. This rae1-1 allele causes a defect in the nuclear export of poly(A) mRNA when cells are shifted to the nonpermissive temperature of 35°C (7, 68). Taken together, the unaltered localizations of poly(A) mRNA, SV40 NLS-GFP-LacZ, and GFP-nucleoplasmin in cells with the nup124-1 allele indicated that this mutation did not cause a general defect in the transport of material in and out of the nucleus.
To ask whether the defect in nup124 caused any gross alteration in the positioning or distribution of the NPCs within the nuclear envelope, we treated cells with the FXFG-specific antibody MAb414 and examined them by immunofluorescence. Cells with the nup124-1 allele showed a nuclear-rim pattern that was typical of proteins in the NPC, and this staining was indistinguishable from the pattern observed for wild-type cells (results not shown).
nup124 is not an essential gene.
To test whether nup124 is required for viability, we deleted just the ORF of nup124 from one allele of a diploid strain and replaced it with the his3 gene. The structure and position of the deletion were confirmed by using DNA blots that were probed separately with his3 and nup124 sequences. Each of 48 tetrads produced two viable spores and two dead spores (results not shown). Although this result suggested that nup124 was essential for viability, we tested the possibility that Nup124p protein was important only for spore germination and not for vegetative growth. The diploid that contained a deletion in one allele of nup124 was transformed with a plasmid that contained a wild-type copy of nup124. Spores derived from this strain were germinated on medium that required the presence of the plasmid for growth. Among the resulting colonies were haploid cells that contained the chromosomal deletion of nup124 but retained the copy of nup124 in the plasmid. Subsequent growth on rich medium demonstrated that cells possessed viability even after loss of the plasmid that carried the only remaining copy of nup124 (results not shown). The absence of nup124 coding sequence in this strain was verified by DNA blot analysis (results not shown). These results indicated that nup124 is not required for vegetative growth and, as a result, may be important for spore germination.
Nup124p interacts directly with Tf1 Gag.
The possibility existed that the nup124-1 allele did not directly cause the defect in nuclear localization of Gag but instead caused a reduction in import of another protein that, in turn, was required for Gag import. However, the following observation suggested that Nup124p contributed directly to the nuclear localization of Gag. We noted that the nup124-1 allele caused a substantial reduction in colony size in cells that were subjected to multiple cycles of Tf1 induction (results not shown). A single cycle of growth on induction medium, as was typical of the transposition assay, did not result in a reduction in growth or viability. This genetic interaction of Tf1 expression with the defect in Nup124p may have resulted from the overaccumulation of Gag and its association with Nup124p. Additional experiments were developed to test directly for an interaction between Gag and Nup124p.
We used the two-hybrid system of S. cerevisiae to identify sequences within Nup124p that may interact with Gag. The coding sequence of nup124 was divided into six segments that were individually fused to the DNA BD of LexA. The Gag protein was fused to a transcriptional AD, B42 and tested for interactions with each of the six segments of Nup124p (Fig. 8A). Segments 2 and 3 of nup124 interacted strongly with Gag, as indicated by expression of a reporter gene that allowed growth on medium lacking leucine (Fig. 8B). In addition, segments 5 and 6 showed weak interaction with Gag. All the interactions observed were specific in that they required the expression of both fusion proteins and that the fusion proteins include the Nup124p and Gag sequences.
The results of the two-hybrid analysis indicated that Gag may interact with segments of nup124. However, it was possible that the interaction was mediated by additional factors. We asked whether Gag could interact directly with regions of nup124 by expressing the proteins in bacteria as GST fusions and subjecting them to precipitation analysis. An N-terminal domain (segments 2 and 3) and a C-terminal domain (segments 4 to 6) of Nup124p, as well as the intact Gag, were fused to the C terminus of GST and expressed in bacteria. All three fusion proteins were purified from bacterial extracts by using glutathione-Sepharose, and the GST domains were cleaved off the two segments of Nup124p. Gag bound to the Sepharose beads was mixed separately with the N-terminal and C-terminal domains of Nup124p, and after an incubation of 45 min, the beads were pelleted and washed. Figure 8C shows that a fraction of the N-terminal domain of Nup124p did pellet with GST-Gag but not with GST alone attached to beads. This direct interaction was specific in that the C-terminal domain of Nup124p did not precipitate with the GST-Gag. The coprecipitation of Gag with segments 2 and 3 of Nup124p coincided with the observation that the strongest interaction detected by two-hybrid analysis was between Gag and segments 2 and 3 of Nup124p.
DISCUSSION
Nup124p is a nuclear pore factor required for nuclear localization of Tf1.
The nup124 gene was predicted to encode a protein of 124 kDa with 11 copies of the FXFG repeats in a family of nuclear pore factors. BLAST-based alignments confirmed that Nup124p was most closely related to a large class of nuclear pore factors that possess variable numbers and arrangements of FXFG repeats. The nuclear pore proteins with FXFG repeats encoded in the genome of S. cerevisiae include Nup1p, Nup2p, Nsp1p, and Nup159p. Some mammalian nuclear pore factors with FXFG repeats are Nup153p, Nup358p, Nup62, and Nup214p. The results of in vitro binding experiments led to the current model that the FXFG repeats of the factors in the NPC serve as docking sites for karyopherin proteins associated with transport cargo (1, 56, 59, 71). A related class of nuclear pore proteins possesses repeats of GLFG and also participates in nuclear transport. Members of this class of factors bind karyopherin complexes in vitro and appear to participate in nuclear export (65).
That nup124 encoded a component of the NPC was indicated by the nuclear-rim signal produced by the HA-Nup124p protein in immunolocalization experiments and by the results of immunoelectron microscopy studies. The identification of Nup124p as a nuclear pore factor supports the evidence that nup124-1 caused a defect in the transport of FLAG-Gag to the nucleus. The immunofluorescence of cells treated with anti-FLAG antibodies revealed that the nup124-1 allele caused a 7.3-fold drop in the number of nuclei with high levels of FLAG-Gag. The drop in the number of nuclei with significant concentrations of FLAG-Gag correlated well with the 4.6-fold increase in the number of cells with large aggregates of FLAG-Gag attached to the outside of the nuclear envelope.
Although we have no direct evidence that the nuclear import of Gag is required for transposition, the presence of Gag in the nucleus supports this possibility. The capsid proteins of many viruses form complexes with viral RNA or DNA that are imported into the nucleus. For example, the matrix protein of HIV is a component of the preintegration complex and possesses NLS activity that may contribute to the infectivity of nondividing cells (9, 18, 19). The behavior of Tf1 proteins in sucrose gradients indicated that in cells grown to stationary phase, the bulk of Gag and IN are coassembled into VLPs that also contain cDNA (40). Therefore, the results of the sucrose gradient fractionation and the immunolocalization of Gag indicate that these components are probably present together in the nucleus as VLPs. Unfortunately, we have been unable, by immunofluorescence microscopy, to detect Tf1 IN in wild-type cells that were grown to stationary phase. The level of IN in stationary-phase cells is very low due to a regulated degradation process that lowers the amount of IN by more than 20-fold (3). The results of the cDNA recombination assays indicated that the nup124-1 mutation also disrupted the nuclear import of the Tf1 reverse transcripts. If the cDNA is imported into the nucleus as a component of the preintegration complex, as suggested above, the mislocalization of this complex could greatly reduce the potential for homologous recombination between cDNA and plasmid sequences of Tf1.
The nuclear import of Tf1 is specifically inhibited by the nup124-1 allele.
Because most proteins larger than 40 kDa are thought to require active transport through the NPC before they can accumulate in the nucleus, we expected that a mutation in an individual pore factor could lead to defects in the import of many cellular proteins. Nevertheless, the wild-type growth of strains with the nup124-1 allele indicated that the bulk of nuclear import was unaffected by the mutation. We also examined the nuclear import of two proteins that possessed two different types of NLSs. We found that the nup124-1 mutation did not reduce the nuclear localization of SV40 NLS-GFP-LacZ or GFP-nucleoplasmin. These result indicated that the karyopherin functions required for the transport of proteins with classical or bipartite NLSs were unaffected by the nup124-1 allele.
Two other properties of the NPC that were investigated were the ability to export poly(A) mRNA and the distribution of the NPCs within the nuclear envelope. These characteristics were examined in cells with the nup124-1 mutation, because a number of strains of S. cerevisiae with defects in nuclear pore proteins show increased levels of poly(A) mRNA in the nucleus as well as clustering of NPCs in the nuclear envelope (6, 14, 22, 27, 42, 63, 64). The observation that the nup124-1 mutation did not visibly alter the export of mRNA from the nucleus or the gross distribution of the NPCs in the nuclear envelope provided further evidence that this mutation did not reduce the ability of the pore complexes to transport material in and out of the nucleus. In addition, these results indicate that Nup124p possesses an activity required for the nuclear import of Tf1 that does not appear to be required for the overall function of the NPCs.
The defect in the transport of Tf1 material might not have been directly due to a lack of Nup124p function but, instead, might have been caused by the mislocalization of another protein that contributed to Tf1 import. The results of two-hybrid analysis revealed strong interactions between Gag and amino acid residues 92 to 521 of Nup124p (Fig. 8). These results correlated well with results of experiments that showed that, as purified proteins from bacteria, the N-terminal half of Nup124p bound and coprecipitated with Gag fused to GST. The detection of this interaction by direct binding in vitro and by two-hybrid analysis in vivo suggested that Nup124p contributes directly to the nuclear import of Gag.
The mutation in the nup124-1 allele was found to be a nonsense mutation that shortened the ORF by 32% and as a result removed 9 of the 11 copies of the FXFG repeats. Therefore, the nup124-1 mutation left intact the coding sequence for the portion of the protein that interacted with Gag in the two-hybrid and GST precipitation analyses. The presence of the interaction domain in the defective form of Nup124-1 suggests that the drop in the nuclear localization of Gag due to the mutation in nup124-1 was not the result of reduced binding of Gag caused by an altered conformation of the binding surface of Nup124p. The accumulation of Gag outside the nucleus in cells with the nup124-1 allele suggests the possibility that the N-terminal domain of Nup124p was present in the NPC and bound Gag, but the absence of the C-terminal portion of Nup124p may have inhibited the release of Gag and its subsequent import. Alternatively, the C-terminal domain of Nup124p may contribute to the binding of Gag, and although this interaction was not detected by our binding assays, it may be important for binding in vivo. Another possibility is that the truncation of Nup124p caused a change in the localization of the protein. For example, the C-terminal truncation of Nup124p may remove structures necessary for its interaction with the NPC. As a result, Gag would not be able to complete its process of nuclear import.
If FXFG domains play an important role in docking the substrates of nuclear transport, why did the nup124-1 mutation block the nuclear import of Gag but not of other proteins? The answer to the specificity of the import defect may lie in the interaction between Tf1 Gag and Nup124p. This particular interaction may play a central role in the docking of Tf1 VLPs to the NPC. The import of cellular proteins may not require interactions with specific nuclear pore proteins, but instead, may occur via proteins with redundant function. Evidence that FXFG and GLFG motifs possess redundant functions exists for Nsp1p (FXFG protein), Nup49p (GLFG protein), Nup57p (GLFG protein), and Nup145p (GLFG protein) of S. cerevisiae. Although all four of these proteins are essential for viability (23, 50, 66, 69), deletion of the FXFG or GLGF repeats in these proteins does not reduce viability (23, 29, 50, 63, 66). As a result of these observations, the essential functions that mapped to the nonrepeat portions of the pore proteins are thought to be structural.
We propose that Tf1 evolved an import strategy that relies on a specific interaction between Gag and the N terminus of Nup124p. We predict that the interaction between Gag and Nup124p is required for import of Gag. Future experiments will be conducted to test the impact of mutations in the N-terminal domain of Nup124p on the localization of Gag in the nucleus.
Although little is known about the nuclear pore proteins that are directly responsible for the import of large viral complexes, recent results indicate that the Vpr protein of HIV-1 plays a unique role in the nuclear import of the HIV-1 proteins (16, 30, 54, 61). Although the matrix (MA) and IN proteins of HIV carry conventional NLSs and probably use the karyopherin α/β pathway for nuclear import (17, 18), Vpr has karyophilic properties that contribute significantly to the HIV infection of nondividing cells (18, 61). Vpr localizes at the nuclear envelope of yeast and human cells, and this behavior is thought to lead to the import of preintegration complexes (16, 61). Evidence for this model includes the finding that Vpr is a component of the preintegration complex and that Vpr also interacts with FXFG repeat nucleoporins (19, 28, 61). Recently, Vpr was found to interact directly with Pom121, a nuclear pore factor that contains FXFG repeats (16). This result suggests that Vpr may contribute to the import of HIV preintegration complex by causing a direct interaction between the preintegration complex and FXFG-containing proteins in the NPC. Consistent with this model is the finding that Vpr uses an import pathway distinct from classical NLS or M9 substrates (30). This specialized role of Vpr in the nuclear import of HIV is surprisingly similar to the importance of nup124 for the import of Tf1 Gag and suggests that large viral complexes may require direct contacts with FXFG proteins to successfully navigate through the NPC. Nevertheless, our result that the nuclear import of a VLP can be inhibited without affecting the import of other proteins suggests that the reduction of nuclear import may prove to be an effective strategy for antiviral therapies.
ACKNOWLEDGMENTS
Portions of this work was supported by National Institutes of Health grant RR-0592 to J. Richard McIntosh.
Cindy Troxell and Richard McIntosh kindly provided pCS2pkSu before publication. We thank Shelley Sazer and Susan Forsburg for many helpful suggestions. We are grateful to Ravi Dhar and William Whalen for providing the strain with the rae1-1 allele and for guidance with the analysis of poly(A) mRNA. Sally Pasion and Susan Forsburg generously provided SV40-NLS-GFP-lacZ plasmids in advance of publication. We also thank Mary Dasso for reading the manuscript and providing helpful suggestions.
REFERENCES
- 1.Aitchison J D, Blobel G, Route M P. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- 2.Atwood A, Choi J, Levin H L. The application of a homologous recombination assay revealed amino acid residues in a long terminal repeat retrotransposon that were critical for integration. J Virol. 1998;72:1324–1333. doi: 10.1128/jvi.72.2.1324-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwood A, Lin J, Levin H. The retrotransposon Tf1 assembles virus-like particles with excess Gag relative to integrase because of a regulated degradation process. Mol Cell Biol. 1996;16:338–346. doi: 10.1128/mcb.16.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azad A K, Tani T, Shiki N, Tsuneyoshi S, Urushiyama S, Ohshima Y. Isolation and molecular characterization of mRNA transport mutants in Schizosaccharomyces pombe. Mol Biol Cell. 1997;8:825–841. doi: 10.1091/mbc.8.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkower C, Loayza D, Michaelis S. Metabolic instability and constitutive endocytosis of STE6, the a-factor transporter of Saccharomyces cerevisiae. Mol Biol Cell. 1994;5:1185–1198. doi: 10.1091/mbc.5.11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogerd A M, Hoffman J A, Amberg D C, Fink G R, Davis L I. nup1 mutants exhibit pleiotropic defects in nuclear pore complex function. J Cell Biol. 1994;127:319–332. doi: 10.1083/jcb.127.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown J A, Bharathi A, Ghosh A, Whalen W, Fitzgerald E, Dhar R. A mutation in the Schizosaccharomyces pombe rae1 gene causes defects in poly(A)+ RNA export and in the cytoskeleton. J Biol Chem. 1995;270:7411–7419. doi: 10.1074/jbc.270.13.7411. [DOI] [PubMed] [Google Scholar]
- 8.Bukrinsky M I, Haggerty S, Demsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cottarel G, Beach D, Deuschle U. Two new multi-purpose multicopy Schizosaccharomyces pombe shuttle vectors, pSP1 and pSP2. Curr Genet. 1993;23:547–548. doi: 10.1007/BF00312650. [DOI] [PubMed] [Google Scholar]
- 11.Dang V D, Benedik M J, Ekwall K, Choi J, Allshire R C, Levin H L. A new member of the sin3 family of corepressors is essential for cell viability and required for retroelement propagation in fission yeast. Mol Cell Biol. 1999;19:2351–2365. doi: 10.1128/mcb.19.3.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding R, West R R, Morphew D M, Oakley B R, McIntosh J R. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 14.Fabre E, Boelens W C, Wimmer C, Mattaj I W, Hurt E C. Nup145p is required for nuclear export of mRNA and binds homopolymeric RNA in vitro via a novel conserved motif. Cell. 1994;78:275–289. doi: 10.1016/0092-8674(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 15.Feldherr C M, Kallenback E, Schulz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984;99:2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouchier R, Meyer B, Simon J, Fischer U, Albright A, González-Scarano F, Malim M. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virology. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 20.Golemis E, Gyuris J, Brent R, editors. Interaction trap/two-hybrid system to identify interacting proteins. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 21.Gorlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 22.Gorsch L C, Dockendorff T C, Cole C N. A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J Cell Biol. 1995;129:939–955. doi: 10.1083/jcb.129.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandi P, Schlaich N, Tekotte H, Hurt E C. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein Nup57p. EMBO J. 1995;14:76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greber U F, Kasamatsu H. Nuclear targeting of SV40 and adenovirus. Trends Cell Biol. 1996;6:189–195. doi: 10.1016/0962-8924(96)10016-7. [DOI] [PubMed] [Google Scholar]
- 25.Greber U F, Suomalainen M, Stidwell R P, Boucke K, Ebersold M W, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyuris J, Golmis E, Chertkov H, Brent R. Cdi1, a human G1- and S-phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 27.Heath C V, Copeland C S, Amberg D C, Del Priore V, Snyder M, Cole C N. Nuclear pore complex clustering and nuclear accumulation of poly(A)+ RNA associated with mutation of the Saccharomyces cerevisiae RAT2/NUP120 gene. J Cell Biol. 1995;131:1677–1697. doi: 10.1083/jcb.131.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinzinger N K, Bukinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iovine M K, Watkins J L, Wente S R. The GLFG repetitive region of the nucleoporin Nup116p interacts with Kap95p, an essential yeast nuclear import factor. J Cell Biol. 1995;131:1699–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins Y, McEntee M, Weis K, Greene W C. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways J. Cell Biol. 1998;143:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joyce C M, Grindley N. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984;158:636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeney J B, Boeke J D. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics. 1994;136:849–856. doi: 10.1093/genetics/136.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenna M A, Brachmann C B, Devine S E, Boeke J D. Invading the yeast nucleus: a nuclear localization signal at the C terminus of Ty1 integrase is required for transposition in vivo. Mol Cell Biol. 1998;18:1115–1124. doi: 10.1128/mcb.18.2.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kukolj G, Jones K S, Skalka A M. Subcellular localization of avian sarcoma virus and human immunodeficiency virus type 1 integrases. J Virol. 1997;71:843–847. doi: 10.1128/jvi.71.1.843-847.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukolj G, Katz R A, Skalka A M. Characterization of the nuclear localization signal in the avian sarcoma virus integrase. Gene. 1998;223:157–163. doi: 10.1016/s0378-1119(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 36.Levin H L. It’s prime for reverse transcriptase. Cell. 1997;88:5–8. doi: 10.1016/s0092-8674(00)81851-6. [DOI] [PubMed] [Google Scholar]
- 37.Levin H L. A novel mechanism of self-primed reverse transcription defines a new family of retroelements. Mol Cell Biol. 1995;15:3310–3317. doi: 10.1128/mcb.15.6.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin H L. An unusual mechanism of self-primed reverse transcription requires the RNase H domain of reverse transcriptase to cleave an RNA duplex. Mol Cell Biol. 1996;16:5645–5654. doi: 10.1128/mcb.16.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin H L, Boeke J D. Demonstration of retrotransposition of the Tf1 element in fission yeast. EMBO J. 1992;11:1145–1153. doi: 10.1002/j.1460-2075.1992.tb05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin H L, Weaver D C, Boeke J D. Novel gene expression mechanism in a fission yeast retroelement: Tf1 proteins are derived from a single primary translation product. EMBO J. 1993;12:4885–4895. doi: 10.1002/j.1460-2075.1993.tb06178.x. . (Erratum, 13:1494, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin H L, Weaver D C, Boeke J D. Two related families of retrotransposons from Schizosaccharomyces pombe. Mol Cell Biol. 1990;10:6791–6798. doi: 10.1128/mcb.10.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li O, Heath C V, Amberg D C, Dockendorff T C, Copeland C S, Snyder M, Cole C N. Mutation or deletion of the Saccharomyces cerevisiae RAT3/NUP133 gene causes temperature-dependent nuclear accumulation of poly(A)+ RNA and constitutive clustering of nuclear pore complex. Mol Biol Cell. 1995;6:401–417. doi: 10.1091/mbc.6.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin J H, Levin H L. A complex structure in the mRNA of Tf1 is recognized and cleaved to generate the primer of reverse transcription. Genes Dev. 1997;11:270–285. doi: 10.1101/gad.11.2.270. [DOI] [PubMed] [Google Scholar]
- 44.Losson R, Lacroute F. Plasmids carrying the yeast OMP decarboxylase structural and regulatory genes: transcription regulation in a foreign environment. Cell. 1983;32:371–377. doi: 10.1016/0092-8674(83)90456-7. [DOI] [PubMed] [Google Scholar]
- 45.Maundrell K. Thiamine-repressible expression vector pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 46.Mehlin H, Dandeholt B, Skoglund U. Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell. 1992;69:605–613. doi: 10.1016/0092-8674(92)90224-z. [DOI] [PubMed] [Google Scholar]
- 47.Moore S P, Rinckel L A, Garfinkel D J. A Ty1 integrase nuclear localization signal required for retrotransposition. Mol Cell Biol. 1998;18:1105–1114. doi: 10.1128/mcb.18.2.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi A, Clever J, Yamada M, Li P P, Kasamatsu H. Association with capsid proteins promotes nuclear targeting of simian virus 40 DNA. Proc Natl Acad Sci USA. 1996;93:96–100. doi: 10.1073/pnas.93.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nehrbass U, Kern H, Mutvei A, Horstmann H, Marshallsay B, Hurt E C. NSP1: a yeast nuclear envelope protein localized at the nuclear pores exerts its essential function by its carboxy-terminal domain. Cell. 1990;61:979–989. doi: 10.1016/0092-8674(90)90063-k. [DOI] [PubMed] [Google Scholar]
- 51.Nigg E A. Nucleoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 52.Ohi R, Feoktistova A, Gould K L. Construction of vectors and a genomic library for use with his3-deficient strains of Schizosaccharomyces pombe. Gene. 1996;174:315–318. doi: 10.1016/0378-1119(96)00085-6. [DOI] [PubMed] [Google Scholar]
- 53.Ohno M, Fornerod M, Mattaj I W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- 54.Popov S, Rexach M, Zybarth G, Reiling N, Lee M A, Ratner L, Lane C M, Moore M S, Blobel G, Burkrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reichelt R, Holzenburg A, Buhle E L, Jarnik M, Engel A, Aebi U. Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J Cell Biol. 1990;110:883. doi: 10.1083/jcb.110.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 57.Robbins J, Dilworth S M, Laskey R A, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 58.Rout M P, Blobel G. Isolation of the yeast nuclear pore complex. J Cell Biol. 1993;123:771–783. doi: 10.1083/jcb.123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rout M P, Blobel G, Aitchison J D. A distinct nuclear import pathway used in ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 60.Stevenson M. Portals of entry: uncovering HIV transport pathways. Trends Cell Biol. 1996;6:9–15. doi: 10.1016/0962-8924(96)81032-4. [DOI] [PubMed] [Google Scholar]
- 61.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wente S R, Blobel G. NUP145 encodes a novel yeast glycine-leucine-phenylalanine-glycine (GLFG) nucleoporin required for nuclear envelope structure. J Cell Biol. 1994;125:955–969. doi: 10.1083/jcb.125.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wente S R, Blobel G. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J Cell Biol. 1993;123:275–284. doi: 10.1083/jcb.123.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wente S R, Gasser S M, Caplan A J. The nucleus and nucleocytoplasmic transport in Saccharomyces cerevisiae. In: Pringle J, Broach J, Jones E, editors. The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 471–546. [Google Scholar]
- 66.Wente S R, Rout M P, Blobel G. A new family of yeast nuclear pore complex proteins. J Cell Biol. 1992;119:705–723. doi: 10.1083/jcb.119.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West R R, Vaisberg E V, Ding R, Nurse P, McIntosh J R. cut11(+): A gene required for cell cycle-dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:2839–2855. doi: 10.1091/mbc.9.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whalen W A, Bharathi A, Danielewicz D, Dhar R. Advancement through mitosis require rae1 gene function in fission yeast. Yeast. 1997;13:1167–1179. doi: 10.1002/(SICI)1097-0061(19970930)13:12<1167::AID-YEA154>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 69.Wimmer C, Doye V, Grandi P, Nehrbass U, Hurt E C. A new subclass of nucleoporins that functionally interact with nuclear pore protein NSP1. EMBO J. 1992;11:5051–5061. doi: 10.1002/j.1460-2075.1992.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamada M, Kasamatsu H. Role of nuclear pore complex in simian virus 40 nuclear targeting. J Virol. 1993;67:119–130. doi: 10.1128/jvi.67.1.119-130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yaseen N R, Blobel G. Cloning and characterization of human karyopherin beta3. Proc Natl Acad Sci USA. 1997;94:4451–4456. doi: 10.1073/pnas.94.9.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]