Central Illustration
Key Words: coronavirus disease 2019, disparities, long COVID, postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection, vulnerable population
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; AKI, acute kidney injury; COVID-19, coronavirus disease-2019; PASC, postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection; PIMS, pediatric inflammatory multisystem syndrome; POTS, postural tachycardia syndrome; RRT, renal replacement therapy; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Highlights
-
•
Long-term manifestation of PASC are experienced by 33% to 98% of patients who have recovered from initial COVID-19 illness.
-
•
This review summarizes and synthesizes the emerging evidence about multisystem manifestations of PASC.
-
•
Evidence points to disproportionate impact on racial/ethnic minorities, older patients, patients with preexisting conditions, and rural residents.
-
•
Continued research is needed to better understand, anticipate, and mitigate the long-term effects of PASC on individual and population health.
Summary
The vast majority of patients (>99%) with severe acute respiratory syndrome coronavirus 2 survive immediate infection but remain at risk for persistent and/or delayed multisystem. This review of published reports through May 31, 2021, found that manifestations of postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) affect between 33% and 98% of coronavirus disease 2019 survivors and comprise a wide range of symptoms and complications in the pulmonary, cardiovascular, neurologic, psychiatric, gastrointestinal, renal, endocrine, and musculoskeletal systems in both adult and pediatric populations. Additional complications are likely to emerge and be identified over time. Although data on PASC risk factors and vulnerable populations are scarce, evidence points to a disproportionate impact on racial/ethnic minorities, older patients, patients with preexisting conditions, and rural residents. Concerted efforts by researchers, health systems, public health agencies, payers, and governments are urgently needed to better understand and mitigate the long-term effects of PASC on individual and population health.
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, has created unprecedented challenges for public health and health care infrastructures around the world. As of June 18, 2021, there have been more than 177 million cases of COVID-19 worldwide, including 33 million in the United States, resulting in more than 3.8 million deaths globally and 600,000 deaths in the United States (1). Despite ongoing vaccination efforts, COVID-19 continues to spread around the world, driven by emergent variant strains and relaxation of prevention/mitigation strategies (2,3). With >99% of patients surviving the acute infectious period (4) and data on the long-term sequelae of COVID-19 disease beginning to emerge, there is an urgent need to better understand the lasting effects of COVID-19 on survivors (5). These long-term complications, collectively referred to as the postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) (6) and more colloquially as “long COVID” or “long haulers,” span multiple systems and may have significant effects on health, function status, and quality of life.
In this review, we summarize and synthesize the emerging evidence about symptoms and conditions comprising PASC, characterize what is known about the frequency and timing of their occurrence, and seek to identify individuals at highest risk. In the context of constrained resources and structural disparities within the US health care system, this review has several key objectives. First, it will help patients, clinicians, and health systems understand the epidemiology of PASC to inform timely evidence-based screening, diagnosis, and treatment and anticipate the resources required to care for patients with increasing burden of chronic health conditions. Second, it will be informative for payers as they anticipate resource use, costs of care, and consider optimal disease management, risk mitigation, and payment models. Lastly, it may help federal and state governments and public health agencies to coordinate their responses and ameliorate disparities in health care access, utilization, and health outcomes.
Methods
We conducted a comprehensive database search for studies published between December 1, 2019, and March 4, 2021, excluding animal and in vitro studies. During the revision process, we conducted a second scan for pertinent published reports published through May 31, 2021. Searched databases included Ovid MEDLINE and Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus.
The search strategy was designed and conducted by an experienced librarian (L.C.H.) with input from the study’s principal investigators (D.H.J. and R.G.M.). We focused exclusively on PASC, defined as any symptoms that began or persisted after 28 days of laboratory-confirmed COVID-19. Controlled vocabulary supplemented with key words was used to search for studies describing long-term complications of COVID-19. The full search strategy is available in the Supplemental Appendix. Ethical approval was not obtained because this review did not involve human subjects research.
The initial search identified 3,142 unique papers. These were screened using title and abstract information by 2 investigators (D.H.J. and R.G.M.), resulting in 293 papers reviewed in full length by the study team (D.H.J., D.J.R., and B.J.G.). The study team reviewed the papers for any symptoms that persisted or may persist after 28 days of laboratory-confirmed or suspected COVID-19 case and also made note of any case studies reporting on fewer than 5 participants. The study team excluded non-English papers. After comprehensive review, 143 papers were deemed relevant. PASC complications were summarized by body system. Data on at-risk populations (defined by age, sex, race/ethnicity, income, or geography) was abstracted when available.
Results
Data on PASC is continuing to emerge and reflects complications and symptoms experienced by patients up to approximately 1 year of observation following first COVID-19 infection. There was marked heterogeneity among studies in terms of time frame and duration of observation, definitions of complications, and means of their ascertainment, precluding data synthesis and estimation of the incidence of reported complications. Most recent estimates of symptom burden obtained using a mobile application available to patients in the United Kingdom, United States, and Sweden suggest that up to 13% of patients who recovered from acute COVID-19 disease experience persistent symptoms 1 to 2 months after initial diagnosis, 4.5% are symptomatic for longer than 2 months, and 2.6% have symptoms lasting 3 months or longer (7). Other studies reported similar findings: 33% to 98% of patients can experience at least 1 new or persistent symptom months after recovery from the acute infection (8, 9, 10). The most commonly reported symptoms are fatigue (28.3%-98%), headache (91.2%), dyspnea (13.5%-88%), cough (10%-13%), chest pain (5%-42.7%), anxiety/depression (14.6%-23%), and olfactory/gustatory deficits (13.1%-67.5%) (7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19). Other symptoms are reported less frequently, including palpitations/tachycardia (11.2%) (7,9,12), concentration or memory deficits (23%) (9,20), tinnitus or earache (3.6%) (9), and sensory neuropathy (2.0%) (7). Most symptoms are more frequently reported by women and older individuals (7).
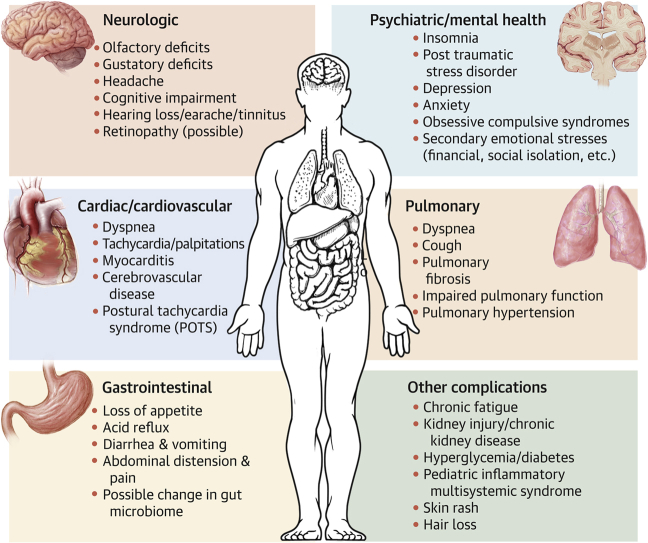
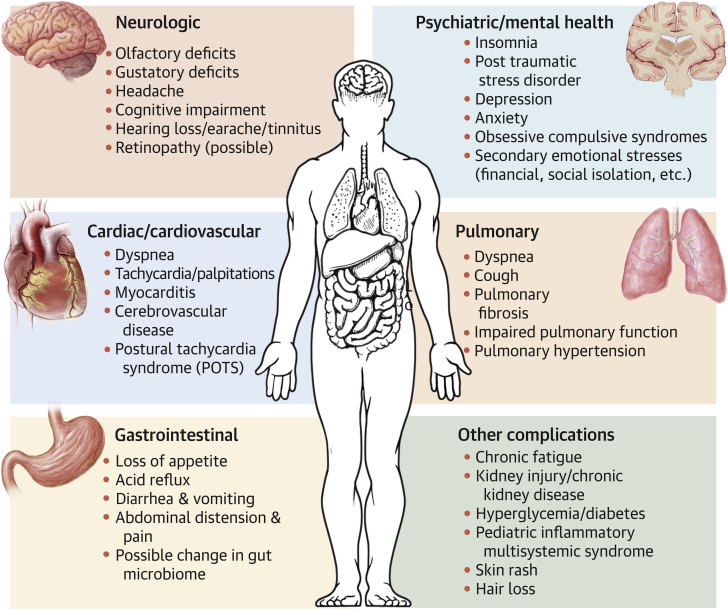
In the following sections, we summarize the most frequently reported complications and disorders described in the published reports as persisting or developing at least 4 weeks (28 days) after initial diagnosis of the acute COVID-19 infection. Complications are organized by body system, though most patients experience more than 1 manifestation of PASC. The Central Illustration provides a graphical summary of PASC manifestations. It is likely that new long-term sequelae will emerge in the coming years, calling for continued vigilance, close monitoring, and longitudinal tracking of patients who survived both symptomatic and asymptomatic infections with COVID-19.
Central Illustration.
Multi-System Manifestation of PASC
Postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) is an emerging multisystemic condition that manifests subsequent to an acute infection of severe acute respiratory syndrome-coronavirus-2. Conditions and symptoms characterized in the published reports and developing or persisting beyond 28 days of the initial coronavirus disease-2019 are summarized in this figure by body systems. POTS = postural tachycardia syndrome.
Pulmonary Complications
Persistent dyspnea is among the most common symptoms reported by patients recovering from COVID-19, is experienced by up to 88% of survivors, and can take 3 months or longer to resolve (8, 10,11,13, 14, 15, 16). Several studies have identified radiographic evidence of interstitial infiltrates, and pulmonary function tests demonstrate restrictive functional deficits that persist months after recovery from acute COVID-19 disease. In a comprehensive evaluation of patients after severe (requiring hospitalization) COVID-19 6 months after the initial infection, 22% to 56% of patients had persistent oxygen diffusion abnormalities on pulmonary function testing that corresponded to pulmonary interstitial changes (eg, ground glass opacities and irregular pleural lines) on chest computed tomography (9). Persistent hypoxia may also occur, with 6.6% of patients (32 of 488) hospitalized with COVID-19 in 1 study reporting oxygen use 60 days after discharge (10). Other studies found that up to 55% of patients with severe COVID-19 requiring hospitalization have evidence of ground glass opacities and 39.6% have evidence of fibrous stripes 1 week after discharge (21), which may persist long term. Three months after discharge, evidence of fibrosis can be detected on chest computed tomography of 25% to 65% of patients, depending on the severity of the initial disease, especially for those who required mechanical ventilation (22,23). Pulmonary function tests also reveal restrictive lung disease with diminished inspiratory and forced vital capacities (24,25). In 1 study, 81% and 24.1% of patients with COVID-19 who underwent pulmonary function testing 2 weeks after discharge had diminished inspiratory and forced vital capacities, respectively (25). This diminished performance was also observed in a study of patients with COVID-19 reassessed 1 month after symptom onset, where at least 50% of patients had abnormal pulmonary function tests (26).
Additional research is needed to better understand the etiology and natural history of patient-reported dyspnea, as well as how to provide symptom relief and improve patients’ functional capacity and quality of life. It is likely that dyspnea stems from a combination of pulmonary, cardiac, and neuromuscular pathology, as symptom burden often does not consistently correlate with objective radiographic or pulmonary function deficits (9,27). Indeed, whereas in most studies objective measures of pulmonary function and radiographic abnormalities returned to normal within 48 days of initial infection, patients frequently continued to endorse dyspnea (33%), cough (33%), and fatigue (45%), suggesting multifactorial impairment that warrants closer examination and patient follow-up (21,28,29). Interventions such as pulmonary rehabilitation have been suggested to improve symptom burden in patients experiencing PASC-related dyspnea (30,31).
Cardiovascular Complications
The most commonly endorsed cardiovascular symptoms that persist after recovery from COVID-19 are dyspnea (reported by up to 88% of patients and discussed in the preceding text) (8,10,11,13, 14, 15, 16), chest pain (reported by up to 43%) (8, 9, 10, 11, 12,16), and tachycardia/palpitations (reported by up to 11%) (7,9,12). Although self-limited for some, the time frame to symptom resolution for others remains unknown because many patients described in the published reports were still symptomatic at the time of ascertainment up to 6 months after the initial infection (9).
Some cardiac symptoms appear to be driven, at least in part, by myocardial injury. Myocarditis is a known complication of many acute viral infections and can range from fulminant heart failure and cardiogenic shock to minimal symptoms with gradual complete functional and structural resolution (32). Concerns about myocardial injury were raised by early case reports and studies demonstrating mild left ventricular dysfunction in up to 78% of patients recovered from acute COVID-19 (33,34). However, recent evidence suggests that this may not be as common as was previously thought. A prospective case control study that followed 149 patients for 6 months after mild COVID-19 infection did not find evidence of excess cardiovascular risk in COVID-19 survivors than in patients who had no history of the disease (35). However, these patients had mild COVID-19 infection, and myocardial injury may manifest after more severe disease; additional studies will be needed to better delineate the association between COVID-19 infection and long-term myocardial dysfunction.
Other studies have raised the possibility of cardiac injury associated with strenuous physical activity among COVID-19 survivors. In a study of 789 athletes in major North American professional sports leagues (58.3% with symptomatic COVID-19 illness, 41.7% asymptomatic or mildly symptomatic) who underwent cardiac testing prior to resuming play, 3.8% had abnormal cardiac testing (0.8% had elevated troponin levels, 1.3% had abnormal electrocardiogram, and 2.5% had abnormal echocardiogram findings) and 0.6% had cardiac magnetic resonance evidence of myocarditis (36). Other studies found similar rates of myocarditis or other serious cardiovascular complications among recovering athletes (37,38). Myocardial injury may persist for at least 2 months after COVID-19 diagnosis, although not necessarily meeting all diagnostic criteria for myocarditis (33,34,39). It will be important to monitor the natural history of any identified myocardial dysfunction closely. Nevertheless, current evidence from other viral myocarditis suggests that patients who have recovered from acute COVID-19 infection may be at increased risk for cardiac injury on returning to strenuous physical activity and several groups have provided guidance on safe return to play for both professional and recreational athletes (40, 41, 42, 43).
The combination of several commonly reported PASC symptoms (eg, dyspnea, palpitations, chest discomfort) in conjunction with orthostatic tachycardia may be a manifestation of postural tachycardia syndrome (POTS) (44). Several case studies have linked new diagnoses of POTS with prior COVID-19 infection (45, 46, 47), and additional surveillance will be necessary to characterize the frequency and persistence of these symptoms. Whether and how COVID-19 infection directly causes POTS is still unknown.
Acute complications experienced during COVID-19 infection can lead to long-term cardiovascular and cerebrovascular morbidity and related disability among survivors. Up to 25% of patients experience an acute coronary artery disease event during the acute illness and may face increased risk of ischemic cardiomyopathy and heart failure in the long term. Similarly, approximately 5% of patients with COVID-19 experience acute stroke (48, 49, 50, 51) and may develop prolonged or permanent neurological deficits as a result. Finally, thromboembolic events during acute illness can lead to persistent pathology after recovery from COVID-19 and the acute thrombotic event. In a retrospective study of patients hospitalized with COVID-19, 2.5% of patients followed for up to 30 days after discharge experienced some form of thrombotic event (segmental pulmonary embolism, intracardiac thrombus, thrombosed arteriovenous fistula, and/or ischemic stroke) (52). The incidence of venous thromboembolism is 0.48% to 0.6% (52,53) and is most likely in patients who had required intensive care unit–level care (53). Thromboembolic events in COVID-19 survivors outside of the acute infectious period have not been reported. Further follow-up will be needed to gauge the prevalence and severity of postthrombotic complications including chronic thromboembolic pulmonary hypertension, which can present with persistent or progressive dyspnea typically 3 months to 2 years after initial diagnosis.
Neurologic Complications
Sensory dysfunction
Olfactory and gustatory deficits are among the most prevalent and specific symptoms of COVID-19 infection (11,54), potentially caused by cross-reactivity of SARS-CoV-2 with the angiotensin-converting enzyme 2 (ACE2) receptor resulting in disruption of the olfactory epithelium (55,56). There is marked heterogeneity among studies in the modality and timing of olfactory and gustatory dysfunction ascertainment, with 4% to 53% of patients reporting symptoms that persist beyond 4 weeks of acute infection (56, 57, 58, 59, 60, 61, 62, 63). Deficits can occur together or in isolation. Some of the clinical heterogeneity among patients may be explained by different variants of SARS-CoV-2 (64) and ACE2 sequence variant between European and Asian populations (65). Patients with underlying cardiovascular disease appear to have a higher risk of persistent olfactory and gustatory deficits (63), though the association between olfactory and gustatory dysfunction and multimorbidity in general has not been examined.
To prevent long-term morbidity from olfactory and gustatory deficits, some have suggested olfactory training, which has small to moderate benefit but minimal risk of harm (66). Oral but not topical steroids were demonstrated to improve olfactory function, but usage should be delayed until day 20 to reduce risks of long-term morbidity (67).
Less frequently reported sensory deficits include hearing loss, earache, and tinnitus. A Manchester, United Kingdom, study reported that 13.2% of 138 patients hospitalized with severe COVID-19 experienced change in hearing and/or tinnitus 8 weeks after discharge (68). Men, older patients, and patients with comorbidities were most frequently affected. There is also evidence of ophthalmologic complications, specifically alterations in retinal microvasculature that may predispose patients to longer-term retinal vascular complications (69). Whether patients who are already at increased risk for retinal disease, such as those with diabetes, are more severely affected is unknown, but these patients may benefit from closer monitoring.
Headache
Headache, particularly with migraine-like features, is another frequent characteristic of PASC. It is hypothesized to be driven by localized cytokine-driven neuroinflammation (70, 71, 72, 73, 74, 75, 76) or direct viral invasion of cerebral circulation through the ACE2 receptor in the meningeal endothelium, triggering trigeminovascular neuron sensitization (77). Trigeminovascular sensitization may also be precipitated by systemic inflammation and the resultant inflammatory peptides that stimulate trigeminal terminals (77). Headache persisting more than 4 weeks after the initial infection is frequently reported, though precise estimates vary widely among studies. Overall, between 17% and 91% of patients report headaches, with up to 25% experiencing migraine-like severe pain (7,14,15,77, 78, 79). There does not appear to be a correlation between the severity of the initial COVID-19 infection and the likelihood or severity of headache (77). Headaches are reported more frequently by younger patients, women, and patients with prior history of headache disorders (77).
Cognitive impairment
Persistent cognitive decline is a potential complication of any critical illness (80). Cognitive deficits among COVID-19 survivors, colloquially described as “brain fog,” may manifest as perceived difficulties in concentration, memory, receptive language, and/or executive functioning (81). In 1 study, up to 21% of patients aged ≥40 years who tested positive for SARS-CoV-2 endorse cognitive impairment after 6 months of follow-up and found that those who tested positive for SARS-CoV-2 were 18× more likely to experience cognitive decline (9,13,20).
Several proposed mechanisms may underlie the neurocognitive deficits that arise during and after COVID-19 infection. SARS-CoV-2 may accelerate neuroinflammatory responses, synaptic pruning, and neuronal loss, which are the structural basis of Alzheimer’s disease (82). The expression of ACE2 in glutamatergic and GABAergic neurons are additional potential pathways by which SARS-CoV-2 may disrupt neurotransmitter balance, promote loss of neurons, and damage cerebral tissue (83, 84, 85). Some studies have also suggested that olfactory and gustatory deficits may indicate neuroinflammation induced by COVID-19, which may herald deeper neurodegenerative diseases such as parkinsonism, as up to 90% of patients with early Parkinson disease exhibit sensory deficits (86). This reinforces the importance of long-term surveillance of COVID-19 survivors for sequelae that may emerge years or decades later, including Parkinson disease and Alzheimer disease.
Other neuropathic symptoms
Symptoms suggestive of peripheral neuropathy such as numbness, tingling, and a pins-and-needles sensation affect 2% of COVID-19 survivors (11). In 1 report, a previously healthy 46-year-old man developed bilateral leg pain and hypoesthesia 53 days after COVID-19 infection (87). The patient experienced painful sensory symptoms followed by precipitous lower motor neuron weakness affecting all limbs, face, and respiratory muscles (87). However, with a single reported event, it remains unclear whether it is etiologically related to COVID-19. Neuroinflammation (88) and demyelination induced by SARS-CoV-2 in the brain and spinal cord (89) have similarities to those observed in multiple sclerosis (90) and may explain some patient-reported neuropathic symptoms. Whether COVID-19 increases the risk of subsequent multiple sclerosis or other central or peripheral nervous system disorders remains to be seen.
Psychiatric Complications
Psychiatric complications, including posttraumatic stress disorder, anxiety, depression, insomnia, and obsessive-compulsive symptoms are reported by 35% to 56% of patients who have recovered from COVID-19 (79,91,92). In a study of 62,354 patients diagnosed with COVID-19, psychiatric assessment conducted between 14 and 90 days after initial diagnosis detected mental illness in 5.8% of survivors, nearly double the rate among survivors of other infections such as influenza (2.5%-3.4%) (92). Patients with prior history of mental health conditions, younger patients, and women are most likely to experience new psychiatric symptoms (91). Patients with severe COVID-19 are also more likely to experience these complications, with 1 study reporting new mental health symptoms in 56% of patients who were previously hospitalized (91). These are likely conservative underestimates, as psychiatric symptoms related to PASC may be misattributed to isolated anxiety, depression, adjustment disorder, or other mental health conditions and thus may be difficult to distinguish from PASC symptoms.
In addition, the COVID-19 pandemic has had substantial indirect effects on the mental health of patients, caregivers, and society. Stresses incurred by job loss (93), financial instability (94, 95, 96, 97, 98), social isolation (98), and fear of contracting the infection (99) were felt acutely and will have lasting effects. In the United States, as in many countries around the world, stressors related to COVID-19 are likely to be compounded by social unrest (100,101), reinforcing the importance of engaging and supporting the most vulnerable members of society (102,103). The impacts of concurrent civil unrest and the COVID-19 pandemic were examined in Hong Kong, where stress from social unrest and COVID-19 was positively correlated with the prevalence of anxiety and depression (104). When comparing people with high levels of stress secondary to unrest and COVID-19 to people with low levels of stress secondary to unrest and low COVID-19 stress, there is a higher prevalence of anxiety (adjusted odds ratio = 13.1) and depression (adjusted odds ratio =3.4) among those reporting higher levels of stress, particularly among individuals of lower socioeconomic status (104).
Gastrointestinal Complications
Up to 44% of patients hospitalized for COVID-19 reported gastrointestinal symptoms 90 days after discharge (105). The most common gastrointestinal symptoms recorded are loss of appetite (8%-24%), nausea (18%), acid reflux (18%), and diarrhea (5%-15%). Other persistent symptoms include abdominal distension (14%), belching (10%), vomiting (9%), abdominal pain (7%), and bloody stools (2%) (9,105). Some symptoms, such as loss of appetite, diarrhea, and vomiting persisted 6 months after discharge (9). Persistent gastrointestinal symptoms may be driven by longer presence of the virus in the gut, with studies demonstrating detectible SARS-CoV-2 RNA in fecal material for a mean duration of 28 days after symptom onset and persisting a mean of 11 days after a negative respiratory test (81,106,107). Other studies suggest that COVID-19 alters the gut microbiome by increasing opportunistic infectious organisms and depletion of beneficial organisms (81,108,109). More studies of the long-term effects of COVID-19 on the gastrointestinal system are needed to better understand the pathogenesis, epidemiology, and natural history of emerging gastrointestinal complications.
Kidney Complications
Acute kidney injury (AKI) is a common complication of acute COVID-19 disease (110,111), affecting up to 36.6% of patients who were hospitalized (112). In another studies, 22.4% of patients who were hospitalized developed stage 2 AKI, 33.1% developed stage 3 AKI, and 14.3% (of all patients) required renal replacement therapy (RRT) (112). Kidney function does recover among most survivors, even among those with stage 3 AKI (113,114), but the long-term effects on kidney function and risk of future chronic kidney disease are not known. Patients at highest risk for AKI are older, Black, and have diabetes and/or hypertension (112,115). These are the same populations at highest risk for severe COVID-19 and chronic kidney disease, reinforcing the importance of closely monitoring kidney function and developing interventions to prevent the progression of AKI to more severe chronic kidney disease. Among patients requiring RRT in the hospital, 20% to 34% remain dependent on RRT after hospital discharge, and among those still alive more than 60 days after discharge, 56.5% remained RRT-dependent (115,116). Thus, patients with history of AKI during acute COVID-19 may benefit from close monitoring of their kidney function and proactive engagement to reduce exposure to nephrotoxins and other risk factors for progressive chronic kidney disease.
Endocrine Complications
Not only is diabetes mellitus a major risk factor for severe COVID-19 disease and mortality (117), but also acute COVID-19 infection can cause hyperglycemia and new onset diabetes among patients without preexisting history of the disease (118). The ACE2 receptor is strongly expressed in pancreatic endocrine tissue (119), predisposing patients to islet cell injury and diabetes, though not acute pancreatitis. New onset insulin-requiring diabetes in patients with COVID-19 has been reported in multiple studies and appears to persist after recovery from acute infection (118,120, 121, 122, 123). Patients may present with severe hyperglycemia, including hyperosmolarity or ketoacidosis, or with milder hyperglycemia (124, 125, 126, 127). Patients with more severe manifestations of acute COVID-19 appear to be at higher risk, with noted correlation between hypoxia and hyperglycemia among hospitalized patients without preexisting diabetes and prior to administration of glucocorticoid therapy (128). The natural history of COVID-19-induced diabetes, and optimal ways to treat these patients, will need to be determined.
COVID-19 survivors may also experience detrimental effects on bone and muscle health. Steroid therapy, critical illness, and decreased mobility all contribute to bone loss (129) and sarcopenia (130). These effects would be most pronounced in older, frailer patients who may therefore benefit from close monitoring of their functional status, fall risk, and osteoporosis/bone health.
Musculoskeletal and Other Systemic Complications
Chronic fatigue is the most common symptom that persists long after recovery from acute COVID-19, reported by nearly all survivors (up to 97.7% in 1 population-based study) (7). Symptoms frequently last 60 to 70 days after initial diagnosis or even longer (8,131,132). Most patients experiencing fatigue also endorse other symptoms, including dyspnea, joint pain, and chest pain. Fatigue has been reported in children (133) as well as adults and is more common in women and patients with underlying history of depression or anxiety (131). The persistence and severity of fatigue does not appear to be related to the severity of acute COVID-19 disease, with no apparent correlation with need for hospitalization, supplemental oxygen use, intensive care unit–level care, or any laboratory markers of inflammation (131). Some studies have suggested that chronic fatigue associated with PASC may be caused by myalgia encephalomyelitis/chronic fatigue syndrome (134, 135, 136). However, not enough data have been collected to make a clear determination of the rates, timing, or persistence of myalgia encephalomyelitis/chronic fatigue syndrome and its etiologic association with COVID-19.
Dermatological complications are reported starting some 7.9 days after initial diagnosis and can last more than 6 months (9,137). The most common symptoms are hair loss, reported in up to 25% of survivors (9,15), and skin rash (ie, hives, pernio lesions, “COVID toes,” chilblains), reported in up to 7% (9,138). Although these symptoms generally appear to dissipate over time, there are reports of lasting beyond 6 months after acute infection (9).
Pediatric Considerations
PASC is observed in children as well as in adults, though it does not appear to be as prevalent (133,139). The most common symptoms are insomnia (18.6%), fatigue (10.9%), muscle pain (10.1%), headache (10.1%), and lack of concentration (10.1%) (139). These symptoms were reported in children who were either asymptomatic or symptomatic with COVID-19. In a study of children who had tested positive for SARS-CoV-2 at least 30 days before, 66% of 30 patients had at least 1 symptom between 60 and 120 days after their initial infection and 27% of 68% had symptoms 120 days and beyond (139).
Children are also at risk for pediatric inflammatory multisystem syndrome (PIMS), also known as multisystem inflammatory syndrome in children, a Kawasaki-like disease with toxic shock syndrome and myocarditis (140, 141, 142, 143, 144). Symptoms can begin days to weeks after recovery from acute infection, with reports of fatigue, fever, gastrointestinal symptoms (ie, abdominal pain, diarrhea, vomiting), dyspnea, headache, Kawasaki-like disease, and toxic shock syndrome (144,145). One study found that 2.3% of children with COVID-19 also had PIMS (139), and another has found a 30-fold increase in PIMS during the COVID-19 pandemic (141). A recent meta-analysis found that 91% of those with COVID-19–associated PIMS eventually recover, and 3.5% die (146). In contrast to traditional Kawasaki disease, patients with PIMS are often older and present with predominantly gastrointestinal symptoms, meningeal signs, myocarditis, and elevated ferritin levels (146). PIMS has been described more frequently among patients of Afro-Caribbean descent, with no cases detected in Asian countries yet, even though that is where Kawasaki disease is most commonly reported (147).
Discussion
As of July 22, 2021, more than 1 in 10 Americans is a survivor of COVID-19 and is therefore at risk for a wide range of symptoms and disorders that are direct consequence of the acute infection. Manifestations of PASC are heterogenous and evolving and it will be important for health systems, researchers, and public health agencies to closely monitor the history of COVID-19 survivorship in diverse populations to ensure complete and accurate understanding of this highly prevalent condition. It is also essential to identify patients and populations most susceptible to PASC, characterize the different phenotypes of PASC symptom burden and pathophysiology, and ensure that the health care system, payers, and public health agencies can adequately care for people experiencing long-term morbidity and disability caused by this disease.
While there is substantial heterogeneity within and across studies, older patients and women appear to be more frequently and severely affected than younger patients and men. Individuals with preexisting health conditions also have an increased risk of PASC. Although it is too early to establish a causal link between PASC and preexisting conditions, patients with serious or multiple chronic health conditions are more likely to experience severe COVID-19, which, in turn increases their risk for many PASC symptoms, particularly for cardiac and pulmonary symptoms (9,13,148). Many of these patients are already clinically complex and therefore may require additional support from multidisciplinary and multispecialty clinical teams to support the additional disease burden and functional impairments posed by PASC.
Racial and ethnic minorities are disproportionately affected by COVID-19 and thus are at highest risk for PASC-related morbidity, disability, and mortality (149). Communities of color are more likely to be exposed to COVID-19, more likely to be not receive COVID-19 diagnoses, more likely to experience severe illness, and more likely to ultimately die from COVID-19 (150, 151, 152, 153, 154, 155). This heightened risk stems from multiple structural, socioeconomic, and individual-level factors (156). Non-White Americans more frequently live in densely populated urban areas, are part of multigenerational households, rely on public transportation, and work in professions unamenable to remote work and without options for paid sick leave (100,157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170). Patients who belong to a racial/ethnic minority have higher prevalence of chronic health conditions that predispose them to severe COVID-19 (eg, obesity, hypertension, diabetes) (171,172) and face multiple barriers to health care including lack of insurance, language, competing familial/financial commitments, discrimination, and distrust of medical institutions (173,174). Whereas contemporary evidence has not explicitly assessed racial/ethnic disparities in PASC incidence and severity, the same structural barriers to health that result in higher incidence and severity of acute COVID-19 are likely to exacerbate PASC-related morbidity and mortality. It will therefore be important for future studies of PASC to specifically examine the impacts on racial/ethnic minorities as well as other disadvantaged groups such as rural populations; individuals who are lesbian, gay, bisexual, transgender, questioning (queer), and others; and people with disabilities. Similarly, as health and public health systems respond to the emerging epidemic of PASC complications, we need to be mindful to eliminate, not perpetuate, the inequities exposed by acute COVID-19 disease.
Rural residents may also be at increased risk for PASC. The population-adjusted rate of COVID-19 is higher in rural than urban areas (175,176). Several factors predispose rural populations to COVID-19, including lower rates of compliance with social distancing and masking guidelines (177, 178, 179) and employment in industries unamenable to telework (ie, agriculture, manufacturing, service industries) (180,181). Rural residents also have a higher prevalence of chronic health conditions that put them at risk for severe COVID-19 and, indirectly, PASC. The population-level impact of PASC in rural areas may be substantial, as rural areas often lack access to primary and specialty care that may be necessary to meet the demand posed by PASC-exacerbated multimorbidity and clinical complexity (182,183). Although we have outlined those populations who are more likely to be susceptible to PASC, it is noted that PASC can occur in patients who have received COVID-19 diagnoses, regardless of initial disease severity. Figure 1 summarizes those who are at heightened risk of PASC.
Figure 1.
Patients at Highest Risk for PASC
Whereas data on risk factors for postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) are scarce, early published reports suggests several clinical and sociodemographic risk factors. COVID-19 = coronavirus disease-2019.
As evidence regarding the multisystemic nature of PASC is starting to emerge, more research is needed capture the full impact of PASC. In particular, there are scarce data about the long-term persistent complications in the endocrine (outside of diabetes), gynecologic, obstetric, and rheumatologic systems. Some case reports on potential sexual dysfunction (eg, erectile dysfunction, anorgasmia) have been reported, but there is yet to be conclusive data on whether these conditions persist more than 4 weeks (184,185). Emerging data regarding POTS, myalgia encephalomyelitis/chronic fatigue syndrome, gastrointestinal complications, and others will need to be substantiated with greater follow-up of patients over time. We anticipate that information about complications in these systems will emerge over time, underscoring the importance of COVID-19 registries and other population-based surveillance infrastructures.
For example, based on published reports from the 2003 SARS pandemic, thyroid axis dysregulation may be a long-term feature of COVID-19 disease (186). In a study of 61 SARS survivors, 6.7% had persistent new onset of biochemical hypothyroidism requiring replacement therapy 3 months after recovery of the acute infection (187). The majority of these patients (75%) had central hypothyroidism, reflecting hypothalamic-pituitary dysfunction, whereas 25% had primary hypothyroidism with evidence of underlying autoimmunity, potentially suggesting higher risk for patients with autoimmune thyroid disease. Hypothalamic-pituitary dysfunction can also contribute to adrenal insufficiency. Both primary and central adrenal insufficiency in patients with SARS have been described and may affect patients with SARS-CoV-2 as well (186). Central adrenal insufficiency may also be worsened by glucocorticoid therapy received for the treatment of severe COVID-19 disease. Impacts of COVID-19 on the hypothalamic-pituitary axis have not yet been examined but need to be considered when caring for patients who have recovered from COVID-19.
Diagnosis and management of PASC
PASC is a multisystemic disease, and all health care providers need to be aware of its potential manifestations and effects on the health and well-being of their patients. Multidisciplinary long COVID clinics are being increasingly introduced by academic medical centers around the country (188, 189, 190, 191, 192, 193, 194), building on models of cancer survivorship programs. In most areas of the country, however, such programs will not be available and primary care providers do and will continue to play a central role in the diagnosis and management of PASC and ensuring that all patients have equitable access to timely, evidence-based care (195). Because data on PASC is only beginning to emerge, we recommend all survivors of COVID-19 establish care with a primary care provider and seek timely consultation for any new or persistent symptoms.
Several professional societies have issued guidance related to the diagnosis and management of PASC and its heterogeneous manifestations. These societies include the American Autonomic Society (196), Infectious Diseases Society of America (197), and the United Kingdom’s National Health Service (198), British Thoracic Society (199), and National Institute for Health and Care Excellence (200). They recommend timely follow-up appointments, especially for patients with severe COVID-19 manifestation, providing/offering rehabilitation services, managing workloads, and working across specialties to ensure resource availability. We anticipate that these guidelines will continue to evolve as more evidence emerges, and additional guidelines will be developed to address other commonly occurring PASC complications.
Although there is insufficient data to recommend an optimal time frame for evaluation, it is reasonable to assess patients at least 4 weeks after initial recovery to screen for symptoms and conditions associated with PASC and determine an individualized care plan. For young and otherwise healthy patients, the initial encounter can be a telephone or virtual touchpoint with the primary care team to identify whether any symptoms are present that may benefit from an evaluation. However, older patients, patients with chronic health conditions, and patients who required emergency department– or hospital-level care for COVID-19 should be directly evaluated by a health care provider because of the high probability that some symptoms associated with PASC will be present. Patients who had a complicated course of illness should have close outpatient follow-up scheduled at the time of discharge and be seen sooner than 1 month after recovery.
At the time of the initial appointment, we recommend that all patients be screened for depression, anxiety, insomnia, and functional impairment (ie, limitations in activities of daily living and instrumental activities of daily living). For other PASC complications, current published reports suggests that diagnostic testing be informed by the patient’s symptoms, though this recommendation will likely change as more data emerge and clinical practice guidelines are developed. Patients endorsing dyspnea should be screened for anemia (complete blood count), kidney and/or liver dysfunction (complete metabolic panel, including albumin), hypothyroidism (thyroid stimulating hormone and free thyroxine). Patients with dyspnea should also be screened for cardiovascular disease, myocardial injury, and heart failure (12-lead electrocardiogram, chest x-ray, brain natriuretic peptide, and troponin). Additional testing, such as chest computed tomography, echocardiogram, pulmonary function testing, cardiac stress testing, and others would be informed by these preliminary results. Importantly, this care needs to be covered by health insurance akin to other preventive services, ideally with no cost-sharing obligations to the patient that would hinder timely access to care.
Potential mitigation strategies
Reassuringly, several recent reports described improvements in PASC symptoms after receiving either the Pfizer-BioNTech or Oxford-AstraZeneca COVID-19 vaccine (201,202). Although more population-based research will be necessary to verify these findings, this can strengthen public health campaigns encouraging vaccination. At the time of manuscript submission, nearly 176 million Americans (53% of the population) have received at least 1 dose of any COVID-19 vaccine and over 148 million (45% of the population) have been fully vaccinated (203). Vaccination rates are lower among some subgroups already at high risk for both COVID-19 infection and PASC, specifically racial/ethnic minorities and rural residents (204, 205, 206), such that greater attention needs to be paid to improving access to vaccination in these communities. This can be achieved through targeted culturally adapted outreach efforts and mobile vaccination clinics, which can double as screening and education centers about PASC.
Public and private payers have an opportunity to help prevent PASC, mitigate its effects, and help understand its burden. To prevent PASC, payers can ensure timely access to medical care for patients with and recovering from acute COVID-19 by encouraging and eliminating/reducing out-of-pocket cost-sharing for primary, specialty, and rehabilitation care for COVID-19 and subsequent recovery. To mitigate PASC effects, payers can improve access to essential services such as physical therapy, mental health counseling, and care management (207). Because payers are uniquely informed about the services and diagnoses patients receive (because they pay for them), they can directly reach out to patients and their health care providers about the need for postrecovery general health exams and cover them at no cost to patients. Importantly, PASC should not be considered a preexisting condition for the purpose of health insurance coverage or cost decisions. Early diagnosis and management of PASC complications is likely to be cost-effective in the long term, though research about optimal monitoring, diagnosis, and management of PASC is urgently needed. Payers can maintain registries of enrollees with PASC and the diagnoses for which they seek care, as this can help delineate the epidemiology of PASC across diverse populations and identify any rare conditions that may develop as part of PASC now and in the future (208).
Federal and local governments need to prepare for the long-term medical and fiscal impacts of PASC on the health care and public health systems as well as the general productivity of upcoming generations. Federal and state governments must work with hospitals and health systems to train and allocate medical staff and other resources to plan for the increased demand for screening and management. This is especially important for rural and socioeconomically deprived areas, where access to medical care may be limited.
Conclusions
Although the end of the COVID-19 pandemic may be in sight with successful vaccination efforts, the fight against its long-term complications is just beginning. PASC affects children and adults irrespective of the severity of the COVID-19 infection itself, though it is more common among patients with more severe COVID-19. It can cause a wide range of complications in the pulmonary, cardiovascular, neurologic, psychiatric, gastrointestinal, renal, endocrine, and musculoskeletal systems that may persist months or years beyond the initial infection. Racial and ethnic minorities, rural residents, older patients, and patients with preexisting conditions may be more likely to develop PASC, though more population-level data on PASC are urgently needed. A concerted approach between health care systems, payers, public health agencies, and governments will be necessary to understand, prevent, and mitigate the long-term impact of PASC on the nation’s health.
Funding Support and Author Disclosures
This effort was funded by the National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant K23DK114497 [Dr McCoy]) and the Mayo Clinic Research Pipeline K2R Program Award (Dr McCoy). Study contents are the sole responsibility of the authors and do not necessarily represent the official views of National Institutes of Health. Dr McCoy has received support from the National Institute of Diabetes and Digestive and Kidney Diseases (grants R03DK127010 and P30DK11024) and AARP (Quality Measure Innovation Grant) in the past 36 months. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Andrea M. Li, BSc (Eastern Virginia Medical School), for her assistance in editing the manuscript.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For the supplemental list of search strategies, please see the online version of this paper.
Appendix
References
- 1.COVID-19 United States cases by county. Coronavirus Resources Center. Johns Hopkins University; 2021. https://coronavirus.jhu.edu/ Accessed June 18, 2021. [Google Scholar]
- 2.Bosman J, Smith M. Covid-19: U.S. vaccinations increase, but virus continues to spread. New York Times. July 2, 2021. Accessed July 22, 2021. https://www.nytimes.com/live/2021/03/19/world/covid-vaccine-coronavirus-cases
- 3.Borchering R.K., Viboud C., Howerton E. Modeling of future COVID-19 cases, hospitalizations, and deaths, by vaccination rates and nonpharmaceutical intervention scenarios—United States, April-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(19):719–724. doi: 10.15585/mmwr.mm7019e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis J.P.A. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull World Health Organ. 2021;99(1):19–33f. doi: 10.2471/BLT.20.265892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raveendran A.V., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15(3):869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins F.S. NIH launches new initiative to study “Long COVID.” February 23, 2021. https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid Accessed May 1, 2021.
- 7.Sudre C.H., Murray B., Varsavsky T. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Huang L., Wang Y. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menni C., Valdes A.M., Freidin M.B. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Q., Xu M., Li J. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpin S.J., McIvor C., Whyatt G. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 14.Dennis A., Wamil M., Alberts J. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Leon S., Wegman-Ostrosky T., Perelman C. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Preprint. Posted online March 1, 2021. Res Sq. 2021;rs.3:rs-266574. doi: 10.21023/rs.3.rs-266574/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cares-Marambio K., Montenegro-Jiménez Y., Torres-Castro R. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Chron Respir Dis. 2021;18 doi: 10.1177/14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehme M., Braillard O., Alcoba G., COVICARE Team COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021;174(5):723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandal S., Barnett J., Brill S.E. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logue J.K., Franko N.M., McCulloch D.J. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Brutto O.H., Wu S., Mera R.M., Costa A.F., Recalde B.Y., Issa N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: a longitudinal prospective study nested to a population cohort. Eur J Neurol. 2021;28:3245–3253. doi: 10.1111/ene.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D., Zhang W., Pan F. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020;21(1):125. doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y.-M., Shang Y.-M., Song W.-B. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah A.S., Wong A.W., Hague C.J. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76(4):402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 24.Crameri G.A.G., Bielecki M., Zust R., Buehrer T.W., Stanga Z., Deuel J.W. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 2020;25(36):2001542. doi: 10.2807/1560-7917.ES.2020.25.36.2001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv D., Chen X., Wang X. Pulmonary function of patients with 2019 novel coronavirus induced-pneumonia: a retrospective cohort study. Ann Palliat Med. 2020;9:3447–3452. doi: 10.21037/apm-20-1688. [DOI] [PubMed] [Google Scholar]
- 26.Frija-Masson J., Debray M.-P., Gilbert M. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56(2):2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie S., Han S., Ouyang H., Zhang Z. Coronavirus disease 2019-related dyspnea cases difficult to interpret using chest computed tomography. Respir Med. 2020;167:105951. doi: 10.1016/j.rmed.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daher A., Balfanz P., Cornelissen C. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogliani P., Calzetta L., Coppola A. Are there pulmonary sequelae in patients recovering from COVID-19? Respir Res. 2020;21(1):286. doi: 10.1186/s12931-020-01550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. Management of postacute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 31.Yang L.L., Yang T. Pulmonary rehabilitation for patients with coronavirus disease 2019 (COVID-19) Chronic Dis Transl Med. 2020;6(2):79–86. doi: 10.1016/j.cdtm.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker R.C. Anticipating the long-term cardiovascular effects of COVID-19. J Thromb Thrombolysis. 2020;50(3):512–524. doi: 10.1007/s11239-020-02266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L., Zhao P., Tang D. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joy G., Artico J., Kurdi H. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. J Am Coll Cardiol Img. Published online May 5, 2021 doi: 10.1016/j.jcmg.2021.04.011. [DOI] [Google Scholar]
- 36.Martinez M.W., Tucker A.M., Bloom O.J. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6(7):745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starekova J., Bluemke D.A., Bradham W.S. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark D.E., Parikh A., Dendy J.M. COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR) Circulation. 2021;143(6):609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sardari A., Tabarsi P., Borhany H., Mohiaddin R., Houshmand G. Myocarditis detected after COVID-19 recovery. Eur Heart J Cardiovasc Imaging. 2021;22(1):131–132. doi: 10.1093/ehjci/jeaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliott N., Martin R., Heron N., Elliott J., Grimstead D., Biswas A. Infographic. Graduated return to play guidance following COVID-19 infection. Br J Sports Med. 2020;54(19):1174–1175. doi: 10.1136/bjsports-2020-102637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzl J.D., McElheny K., Robinson J.N., Scott D.A., Sutton K.M., Toresdahl B.G. Considerations for return to exercise following mild-to-moderate COVID-19 in the recreational athlete. HSS J. 2020;16(suppl 1):1–6. doi: 10.1007/s11420-020-09777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelan D., Kim J.H., Elliott M.D. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. J Am Coll Cardiol Img. 2020;13(12):2635–2652. doi: 10.1016/j.jcmg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J.H., Levine B.D., Phelan D. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219–227. doi: 10.1001/jamacardio.2020.5890. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein D.S. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18(4):508–509. doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanjwal K., Jamal S., Kichloo A., Grubb B.P. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag. 2020;11(11):4302–4304. doi: 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miglis M.G., Prieto T., Shaik R., Muppidi S., Sinn D.-I., Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30(5):449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umapathi T., Poh M.Q.W., Fan B.E., Li K.F.C., George J., Tan J.Y.L. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin Auton Res. 2020;30(6):571–573. doi: 10.1007/s10286-020-00733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qureshi A.I., Baskett W.I., Huang W. Acute ischemic stroke and COVID-19. Stroke. 2021;52(3):905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16(2):137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Modin D., Claggett B., Sindet-Pedersen C. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patell R., Bogue T., Koshy A. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(1):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts L.N., Whyte M.B., Georgiou L. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paderno A., Schreiber A., Grammatica A. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020;10(8):955–962. doi: 10.1002/alr.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 56.Vaira L.A., Hopkins C., Petrocelli M. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. 2020;134(8):703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 58.Panda S., Mohamed A., Sikka K. Otolaryngologic manifestation and long-term outcome in mild COVID-19: experience from a tertiary care centre in India. Indian J Otolaryngol Head Neck Surg. 2020;73(1):1–6. doi: 10.1007/s12070-020-02217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meini S., Suardi L.R., Busoni M., Roberts A.T., Fortini A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020;277:3519–3523. doi: 10.1007/s00405-020-06102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coelho D.H., Kons Z.A., Costanzo R.M., Reiter E.R. Subjective changes in smell and taste during the COVID-19 pandemic: a national survey-preliminary results. Otolaryngol Head Neck Surg. 2020;163(2):302–306. doi: 10.1177/0194599820929957. [DOI] [PubMed] [Google Scholar]
- 61.Reiter E.R., Coelho D.H., Kons Z.A., Costanzo R.M. Subjective smell and taste changes during the COVID-19 pandemic: short term recovery. Am J Otolaryngol. 2020;41(6):102639. doi: 10.1016/j.amjoto.2020.102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan C.H., Prajapati D.P., Ritter M.L., DeConde A.S. Persistent smell loss following undetectable SARS-CoV-2. Otolaryngol Head Neck Surg. 2020;163(5):923–925. doi: 10.1177/0194599820934769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lv H., Zhang W., Zhu Z. Prevalence and recovery time of olfactory and gustatory dysfunction in hospitalized patients with COVID-19 in Wuhan, China. Int J Infect Dis. 2020;100:507–512. doi: 10.1016/j.ijid.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao Y., Li L., Feng Z. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heilmann S., Huettenbrink K.B., Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. 2004;18(1):29–33. [PubMed] [Google Scholar]
- 67.Sorokowska A., Drechsler E., Karwowski M., Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017;55(1):17–26. doi: 10.4193/Rhino16.195. [DOI] [PubMed] [Google Scholar]
- 68.Munro K.J., Uus K., Almufarrij I., Chaudhuri N., Yioe V. Persistent self-reported changes in hearing and tinnitus in post-hospitalisation COVID-19 cases. Int J Audiol. 2020;59(12):889–890. doi: 10.1080/14992027.2020.1798519. [DOI] [PubMed] [Google Scholar]
- 69.Abrishami M., Emamverdian Z., Shoeibi N. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol. 2021;56(1):24–30. doi: 10.1016/j.jcjo.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D'Alessandro A., Thomas T., Dzieciatkowska M. Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level. J Proteome Res. 2020;19(11):4417–4427. doi: 10.1021/acs.jproteome.0c00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y.Q., Liu Z., Liu Z.H. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13(1):141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarchielli P., Alberti A., Baldi A. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46(2):200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 76.Han J.S., Adwanikar H., Li Z., Ji G., Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain. 2010;6:10. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caronna E., Ballve A., Llaurado A. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–1421. doi: 10.1177/0333102420965157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goërtz Y.M.J., Van Herck M., Delbressine J.M. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542–2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poyraz B.C., Poyraz C.A., Olgun Y. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2020;295:113604. doi: 10.1016/j.psychres.2020.113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakusic A., Rabinstein A.A. Cognitive outcomes after critical illness. Curr Opin Crit Care. 2018;24(5):410–414. doi: 10.1097/MCC.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 81.Nalbandian A., Sehgal K., Gupta A. Postacute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heneka M.T., Carson M.J., El Khoury J. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barrantes F.J. Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem Neurosci. 2020;11(18):2793–2803. doi: 10.1021/acschemneuro.0c00434. [DOI] [PubMed] [Google Scholar]
- 84.Lukiw W.J., Pogue A., Hill J.M. SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. Published online August 25, 2020 doi: 10.1007/s10571-020-00947-71-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 2020;15(1):40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ansari K.A., Johnson A. Olfactory function in patients with Parkinson's disease. J Chronic Dis. 1975;28(9):493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- 87.Raahimi M.M., Kane A., Moore C.E., Alareed A.W. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of “long COVID-19 syndrome”? BMJ Case Rep. 2021;14(1) doi: 10.1136/bcr-2020-240178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kempuraj D., Selvakumar G.P., Ahmed M.E. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020;26(5-6):402–414. doi: 10.1177/1073858420941476. [DOI] [PubMed] [Google Scholar]
- 89.Zanin L., Saraceno G., Panciani P.P. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45:102377. doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mazza M.G., De Lorenzo R., Conte C. COVID-19 BioB Outpatient Clinic Study Group. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cable N. COVID-19 pandemic: urgent needs to support and monitor long-term effects of mental strain on people. Am J Public Health. 2020;110(11):1595–1596. doi: 10.2105/AJPH.2020.305938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hertz-Palmor N., Moore T., Gothelf D. Association among income loss, financial strain and depressive symptoms during COVID-19: evidence from two longitudinal studies. J Affect Disord. 2021;291:1–8. doi: 10.1016/j.jad.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shanahan L., Steinhoff A., Bechtiger L. Emotional distress in young adults during the COVID-19 pandemic: evidence of risk and resilience from a longitudinal cohort study. Psychol Med. Published online June 23, 2020 doi: 10.1017/S003329172000241X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Connor R.C., Wetherall K., Cleare S. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br J Psychiatry. Published October 21, 2020 doi: 10.1192/bjp.2020.212. doi:v10.1192/bjp.2020,212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pierce M., Hope H., Ford T. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7(10):883–892. doi: 10.1016/S2215-0366(20)30308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kikuchi H., Machida M., Nakamura I. Changes in psychological distress during the COVID-19 pandemic in Japan: a longitudinal study. J Epidemiol. 2020;30(11):522–528. doi: 10.2188/jea.JE20200271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holingue C., Kalb L.G., Riehm K.E. Mental distress in the United States at the beginning of the COVID-19 pandemic. Am J Public Health. 2020;110(11):1628–1634. doi: 10.2105/AJPH.2020.305857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galea S., Abdalla S.M. COVID-19 pandemic, unemployment, and civil unrest: underlying deep racial and socioeconomic divides. JAMA. 2020;324(3):227–228. doi: 10.1001/jama.2020.11132. [DOI] [PubMed] [Google Scholar]
- 101.Fowers A, Wan W. Depression and anxiety spiked among black Americans after George Floyd’s death. Washington Post. June 12, 2020. Accessed May 1, 2021. https://www.washingtonpost.com/health/2020/06/12/mental-health-george-floyd-census/
- 102.Kessler R.C., Rose S., Koenen K.C. How well can post-traumatic stress disorder be predicted from pre-trauma risk factors? An exploratory study in the WHO World Mental Health Surveys. World Psychiatry. 2014;13:265–274. doi: 10.1002/wps.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forneris C.A., Gartlehner G., Brownley K.A. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44(6):635–650. doi: 10.1016/j.amepre.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Hou W.K., Lee T.M., Liang L. Civil unrest, COVID-19 stressors, anxiety, and depression in the acute phase of the pandemic: a population-based study in Hong Kong. Soc Psychiatry Psychiatr Epidemiol. Published online February 16, 2021 doi: 10.1007/s00127-021-02037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weng J., Li Y., Li J. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6(5):344–346. doi: 10.1016/S2468-1253(21)00076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheung K.S., Hung I.F.N., Chan P.P.Y. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Y., Guo C., Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zuo T., Zhang F., Lui G.C.Y. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Donati Zeppa S., Agostini D., Piccoli G., Stocchi V., Sestili P. Gut microbiota status in COVID-19: an unrecognized player? Front Cell Infect Microbiol. 2020;10:576551. doi: 10.3389/fcimb.2020.576551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirsch J.S., Ng J.H., Ross D.W., Northwell COVID-19 Research Consortium. Northwell Nephrology COVID-19 Research Consortium Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stevens J.S., King K.L., Robbins-Juarez S.Y. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS One. 2021;15(12) doi: 10.1371/journal.pone.0244131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilbers T.J., Koning M.V. Renal replacement therapy in critically ill patients with COVID-19: a retrospective study investigating mortality, renal recovery and filter lifetime. J Crit Care. 2020;60:103–105. doi: 10.1016/j.jcrc.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bowe B., Cai M., Xie Y., Gibson A.K., Maddukuri G., Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta S., Coca S.G., Chan L., STOP-COVID Investigators AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021;32(1):161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hartmann-Boyce J., Morris E., Goyder C. Diabetes and COVID-19: risks, management, and learnings from other national disasters. Diabetes Care. 2020;43(8):1695–1703. doi: 10.2337/dc20-1192. [DOI] [PubMed] [Google Scholar]
- 118.Rubino F., Amiel S.A., Zimmet P. New-onset diabetes in Covid-19. N Engl J Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aabdi M., Aarab A., Es-Saad O., Malki K., Bkiyar H., Housni B. New-onset diabetes in children during COVID-19: clinical case report. Case Rep Endocrinol. 2021;2021:6654019. doi: 10.1155/2021/6654019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nassar M., Nso N., Baraka B. The association between COVID-19 and type 1 diabetes mellitus: a systematic review. Diabetes Metab Syndr. 2021;15(1):447–454. doi: 10.1016/j.dsx.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cariou B., Pichelin M., Goronflot T., CORONADO Investigators Phenotypic characteristics and prognosis of newly diagnosed diabetes in hospitalized patients with COVID-19: results from the CORONADO study. Diabetes Res Clin Pract. 2021;175:108695. doi: 10.1016/j.diabres.2021.108695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alsadhan I., Alruwashid S., Alhamad M. Diabetic ketoacidosis precipitated by coronavirus disease 2019 infection: case series. Curr Ther Res Clin Exp. 2020;93:100609. doi: 10.1016/j.curtheres.2020.100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Siddiqui R.S., Zirkiyeva M., Saliaj M. Onset of ketosis-prone diabetes in the setting of COVID-19 infection. Cureus. 2020;12(10) doi: 10.7759/cureus.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pasquel F.J., Messler J., Booth R. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 129.Orford N.R., Bailey M., Bellomo R. The association of time and medications with changes in bone mineral density in the 2 years after critical illness. Crit Care. 2017;21(1):69. doi: 10.1186/s13054-017-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. 2020;42(6):1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Townsend L., Dyer A.H., Jones K. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mizrahi B., Shilo S., Rossman H. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun. 2020;11(1):6208. doi: 10.1038/s41467-020-20053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ludvigsson J.F. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110(3):914–921. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perrin R., Riste L., Hann M., Walther A., Mukherjee A., Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses. 2020;144:110055. doi: 10.1016/j.mehy.2020.110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021;146:110469. doi: 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Komaroff A.L., Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med (Lausanne) 2021;7:606824. doi: 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mirza F.N., Malik A.A., Omer S.B., Sethi A. Dermatologic manifestations of COVID-19: a comprehensive systematic review. Int J Dermatol. 2021;60(4):418–450. doi: 10.1111/ijd.15168. [DOI] [PubMed] [Google Scholar]
- 138.McMahon D.E., Gallman A.E., Hruza G.J. Long COVID in the skin: a registry analysis of COVID-19 dermatological duration. Lancet Infect Dis. 2021;21(3):313–314. doi: 10.1016/S1473-3099(20)30986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buonsenso D., Munblit D., De Rose C. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 143.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Almoosa Z.A., Al Ameer H.H., AlKadhem S.M., Busaleh F., AlMuhanna F.A., Kattih O. Multisystem inflammatory syndrome in children, the real disease of COVID-19 in pediatrics: a multicenter case series from Al-Ahsa, Saudi Arabia. Cureus. 2020;12(10) doi: 10.7759/cureus.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed M., Advani S., Zoretic S. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang L., Tang K., Levin M. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Henderson L.A., Canna S.W., Friedman K.G. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72(11):1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arnold D.T., Hamilton F.W., Milne A. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ayoubkhani D., Khunti K., Nafilyan V. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adegunsoye A., Ventura I.B., Liarski V.M. Association of Black race with outcomes in COVID-19 disease: a retrospective cohort study. Ann Am Thorac Soc. 2020;17(10):1336–1339. doi: 10.1513/AnnalsATS.202006-583RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bandi S., Nevid M.Z., Mahdavinia M. African American children are at higher risk of COVID-19 infection. Pediatr Allergy Immunol. 2020;31(7):861–864. doi: 10.1111/pai.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jehi L., Ji X., Milinovich A. Individualizing risk prediction for positive coronavirus disease 2019 testing: results from 11,672 patients. Chest. 2020;158(4):1364–1375. doi: 10.1016/j.chest.2020.05.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martinez D.A., Hinson J.S., Klein E.Y. SARS-CoV-2 positivity rate for Latinos in the Baltimore–Washington, DC region. JAMA. 2020;324(4):392–395. doi: 10.1001/jama.2020.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fernandes D.M., Oliveira C.R., Guerguis S. Tri-State Pediatric COVID-19 Research Consortium. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2021;230:23–31.e10. doi: 10.1016/j.jpeds.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zalla L.C., Martin C.L., Edwards J.K., Gartner D.R., Noppert G.A. A geography of risk: structural racism and COVID-19 mortality in the United States. Am J Epidemiol. 2021;190:1439–1446. doi: 10.1093/aje/kwab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Katikireddi S.V., Lal S., Carrol E.D. Unequal impact of the COVID-19 crisis on minority ethnic groups: a framework for understanding and addressing inequalities. J Epidemiol Community Health. 2021;75:970–974. doi: 10.1136/jech-2020-216061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Millett G.A., Jones A.T., Benkeser D. Assessing differential impacts of COVID-19 on Black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lewis N.M., Friedrich M., Wagstaff S. Disparities in COVID-19 incidence, hospitalizations, and testing, by area-level deprivation—Utah, March 3–July 9, 2020. MMWR Morb Mortality Wkly Rep. 2020;69(38):1369–1373. doi: 10.15585/mmwr.mm6938a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am J Prev Med. 2020;59(3):317–325. doi: 10.1016/j.amepre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Okoh A.K., Sossou C., Dangayach N.S. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19(1):93. doi: 10.1186/s12939-020-01208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wallace M., Hagan L., Curran K.G. COVID-19 in correctional and detention facilities—United States, February-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):587–590. doi: 10.15585/mmwr.mm6919e1. [DOI] [PubMed] [Google Scholar]
- 162.Wallace M., Marlow M., Simonson S. Public health response to COVID-19 cases in correctional and detention facilities—Louisiana, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):594–598. doi: 10.15585/mmwr.mm6919e3. [DOI] [PubMed] [Google Scholar]
- 163.Tobolowsky F.A., Gonzales E., Self J.L. COVID-19 Outbreak among three affiliated homeless service sites—King County, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(17):523–526. doi: 10.15585/mmwr.mm6917e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McMichael T.M., Clark S., Pogosjans S. COVID-19 in a long-term care facility—King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):339–342. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiménez M.C., Cowger T.L., Simon L.E., Behn M., Cassarino N., Bassett M.T. Epidemiology of COVID-19 among incarcerated individuals and staff in Massachusetts jails and prisons. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Henry M, Watt R, Mahathey A, et al; Abt Associates. The 2019 Annual Homeless Assessment Report (AHAR) to Congress. Office of Community Planning and Development. United States Department of Housing and Urban Development. 2020. Accessed May 1, 2021. https://www.huduser.gov/portal/sites/default/files/pdf/2019-AHAR-Part-1.pdf
- 167.Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med. 2020;63(9):817–820. doi: 10.1002/ajim.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bui D.P., McCaffrey K., Friedrichs M. Racial and ethnic disparities among COVID-19 cases in workplace outbreaks by industry sector—Utah, March 6-June 5, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1133–1138. doi: 10.15585/mmwr.mm6933e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waltenburg M.A., Victoroff T., Rose C.E. Update: COVID-19 among workers in meat and poultry processing facilities—United States, April-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):887–892. doi: 10.15585/mmwr.mm6927e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Selden T.M., Berdahl T.A. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff (Millwood) 2020;39(9):1624–1632. doi: 10.1377/hlthaff.2020.00897. [DOI] [PubMed] [Google Scholar]
- 171.Hsu H.E., Ashe E.M., Silverstein M. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an urban safety-net medical center—Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):864–869. doi: 10.15585/mmwr.mm6927a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davis J., Penha J., Mbowe O., Taira D.A. Prevalence of single and multiple leading causes of death by race/ethnicity among US adults aged 60 to 79 years. Prev Chronic Dis. 2017;14:E101. doi: 10.5888/pcd14.160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Care Without Coverage: Too Little, Too Late. The National Academies Press; 2002. [PubMed] [Google Scholar]
- 174.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 175.Huang Q., Jackson S., Derakhshan S. Urban-rural differences in COVID-19 exposures and outcomes in the South: a preliminary analysis of South Carolina. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.COVID-19 stats: COVID-19 incidence, by urban-rural classification—United States, January 22-October 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(46):1753. doi: 10.15585/mmwr.mm6946a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gigliotti P., Martin E.G. Predictors of state-level stay-at-home orders in the United States and their association with mobility of residents. J Public Health Manag Pract. 2020;26(6):622–631. doi: 10.1097/PHH.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 178.Greiner B., Ottwell R., Vassar M., Hartwell M. Public interest in preventive measures of coronavirus disease 2019 associated with timely issuance of statewide stay-at-home orders. Disaster Med Public Health Prep. 2020;14(6):765–768. doi: 10.1017/dmp.2020.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grossman G., Kim S., Rexer J.M., Thirumurthy H. Political partisanship influences behavioral responses to governors' recommendations for COVID-19 prevention in the United States. Proc Natl Acad Sci U S A. 2020;117(39):24144–24153. doi: 10.1073/pnas.2007835117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Solis J., Franco-Paredes C., Henao-Martínez A.F., Krsak M., Zimmer S.M. Structural vulnerability in the U.S. revealed in three waves of COVID-19. Am J Trop Med Hyg. 2020;103(1):25–27. doi: 10.4269/ajtmh.20-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dobis E., McGranahan D. Rural residents appear to be more vulnerable to serious infection or death from coronavirus COVID-19. Amber Waves. Economic Research Service. U.S. Department of Agriculture; February 1, 2021. https://www.ers.usda.gov/amber-waves/2021/february/rural-residents-appear-to-be-more-vulnerable-to-serious-infection-or-death-from-coronavirus-covid-19/ Accessed May 1, 2021.
- 182.Peters D.J. Community susceptibility and resiliency to COVID-19 across the rural-urban continuum in the United States. J Rural Health. 2020;36(3):446–456. doi: 10.1111/jrh.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Henning-Smith C. The unique impact of COVID-19 on older adults in rural areas. J Aging Soc Policy. 2020;32(4-5):396–402. doi: 10.1080/08959420.2020.1770036. [DOI] [PubMed] [Google Scholar]
- 184.Shoar S., Khavandi S., Tabibzadeh E. A late COVID-19 complication: male sexual dysfunction. Prehosp Disaster Med. 2020;35(6):688–689. doi: 10.1017/S1049023X20001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kresch E., Achua J., Saltzman R. COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health. 2021;39(3):466–469. doi: 10.5534/wjmh.210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Agarwal S., Agarwal S.K. Endocrine changes in SARS-CoV-2 patients and lessons from SARS-CoV. Postgrad Med J. 2020;96(1137):412–416. doi: 10.1136/postgradmedj-2020-137934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leow M.K.-S., Kwek D.S.-K., Ng A.W.-K., Ong K.-C., Kaw G.J.-L., Lee L.S.-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63(2):197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Austin J. Mayo Clinic's COVID-19 “rehab” program helps patients with long-lasting symptoms. KARE11; 2021. Accessed May 1, 2021. https://www.kare11.com/article/news/health/coronavirus/covid-long-hauler-mayo-clinic-rehab-program-rochester/89-86a7f6ad-b8d7-4a20-bd96-b8b0ec76f49f
- 189.Novak S. “Long COVID” can last months. New St. Luke’s clinic will aid “long-hauler” recovery. Advance Local Media; 2021. Accessed May 1, 2021. https://www.lehighvalleylive.com/coronavirus/2021/04/long-covid-can-last-months-new-st-lukes-clinic-will-aid-long-hauler-recovery.html
- 190.Reed J. Inside a long Covid clinic: “I want to play with my kids again.” BBC; 2021. Accessed May 1, 2021. https://www.bbc.com/news/health-56879203
- 191.Mattison S. Post-COVID clinic on Oahu has helped dozens of long haul patients. KHON; 2021. Accessed May 1, 2021. https://www.khon2.com/coronavirus/post-covid-clinic-on-oahu-has-helped-dozens-of-long-haul-patients/
- 192.Hurley B. Clinics, treatments for COVID-19 long haulers to be offered soon in the Valley. Valley News Live; 2021. Accessed May 1, 2021. https://www.valleynewslive.com/2021/04/22/clinics-treatments-for-covid-9-long-haulers-to-be-offered-soon-in-the-valley/
- 193.Riemer E. Specialized Boston clinics treat long-haul COVID-19 patients. WCVB; 2021. Accessed May 1, 2021. https://www.wcvb.com/article/specialized-boston-clinics-treat-long-haul-covid-19-patients/36027344
- 194.Carbajal E. 33 hospitals, health systems that have launched post-COVID-19 clinics. Becker’s Hospital Review. Becker’s Healthcare, 2021. Accessed May 1, 2021. https://www.beckershospitalreview.com/patient-safety-outcomes/13-hospitals-health-systems-that-have-launched-post-covid-19-clinics.html
- 195.Berger Z., Altiery D.E.J.V., Assoumou S.A., Greenhalgh T. Long COVID and health inequities: the role of primary care. Milbank Q. 2021;99(2):519–541. doi: 10.1111/1468-0009.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Raj S.R., Arnold A.C., Barboi A. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res. 2021;31(3):365–368. doi: 10.1007/s10286-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Post COVID/long COVID. COVID-19 Real-Time Learning Network. Infectious Diseases Society of America; 2021. Accessed May 1, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations--complications/post-covid-syndrome/
- 198.Parkin A., Davison J., Tarrant R. A multidisciplinary NHS COVID-19 service to manage post-COVID-19 syndrome in the community. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.George P.M., Barratt S., Desai S.R. British Thoracic Society; 2020. British Thoracic Society Guidance on Respiratory Follow Up of Patients with a Clinico-Radiological Diagnosis of COVID-19 Pneumonia. [Google Scholar]
- 200.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; 2020. Clinical Guidelines. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. [PubMed] [Google Scholar]
- 201.Arnold D., Milne A., Samms E., Stadon L., Maskell N., Hamilton F. Are vaccines safe in patients with Long COVID? A prospective observational study. Preprint. Posted online March 14, 2021. medRxiv. 2021 doi: 10.1101/2021.03.11.21253225. [DOI] [Google Scholar]
- 202.Guarino B. Some long-haul covid-19 patients say their symptoms are subsiding after getting vaccines. Washington Post; 2021. Accessed May 1, 2021. https://www.washingtonpost.com/health/long-haul-covid-vaccine/2021/03/16/6effcb28-859e-11eb-82bc-e58213caa38e_story.html
- 203.COVID-19 vaccinations in the United States. COVID Data Tracker. Centers for Disease Control and Prevention; 2021. Accessed May 1, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 204.Razai M.S., Osama T., McKechnie D.G.J., Majeed A. Covid-19 vaccine hesitancy among ethnic minority groups. BMJ. 2021;372:n513. doi: 10.1136/bmj.n513. [DOI] [PubMed] [Google Scholar]
- 205.MacKenna B., Curtis H.J., Morton C.E. Trends, regional variation, and clinical characteristics of COVID-19 vaccine recipients: a retrospective cohort study in 23.4 million patients using OpenSAFELY. Preprint. Posted online January 26, 2021. medRxiv. 2021 doi: 10.1101/2021.01.25.21250356. [DOI] [Google Scholar]
- 206.Khan M.S., Ali S.A.M., Adelaine A., Karan A. Rethinking vaccine hesitancy among minority groups. Lancet. 2021;397(10288):1863–1865. doi: 10.1016/S0140-6736(21)00938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jiang D.H., McCoy R.G. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036–3039. doi: 10.1007/s11606-020-06042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yelin D., Wirtheim E., Vetter P. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20(10):1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.