Abstract
Attention-deficit hyperactivity disorder (ADHD) is common in patients with (ADHD+PAE) and without (ADHD-PAE) prenatal alcohol exposure (PAE). Many patients diagnosed with idiopathic ADHD actually have covert PAE, a treatment-relevant distinction. To improve differential diagnosis, we sought to identify brain differences between ADHD+PAE and ADHD-PAE using neurobehavioral, magnetic resonance spectroscopy, and diffusion tensor imaging metrics that had shown promise in past research. Children 8–13 were recruited in three groups: 23 ADHD+PAE, 19 familial ADHD-PAE, and 28 typically developing controls (TD). Neurobehavioral instruments included the Conners 3 Parent Behavior Rating Scale and the Delis-Kaplan Executive Function System (D-KEFS). Two dimensional magnetic resonance spectroscopic imaging was acquired from supraventricular white matter to measure N-acetylaspartate compounds, glutamate, creatine + phosphocreatine (creatine), and choline-compounds (choline). Whole brain diffusion tensor imaging was acquired and used to to calculate fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity from the same superventricular white matter regions that produced magnetic resonance spectroscopy data. The Conners 3 Parent Hyperactivity/Impulsivity Score, glutamate, mean diffusivity, axial diffusivity, and radial diffusivity were all higher in ADHD+PAE than ADHD-PAE. Glutamate was lower in ADHD-PAE than TD. Within ADHD+PAE, inferior performance on the D-KEFS Tower Test correlated with higher neurometabolite levels. These findings suggest white matter differences between the PAE and familial etiologies of ADHD. Abnormalities detected by magnetic resonance spectroscopy and diffusion tensor imaging co-localize in supraventricular white matter and are relevant to executive function symptoms of ADHD.
Keywords: Fetal alcohol spectrum disorder, Attention-deficit hyperactivity disorder, White matter, Magnetic resonance spectroscopy, Diffusion tensor imaging, Tower test
Introduction
Attention-deficit hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders diagnosed in children and has been demonstrated to have multiple etiological risk factors, diverse symptomatology, and divergent long-term outcomes (Luo et al. 2019). According to the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM 5) (American Psychiatric Association 2013), ADHD in children is characterized by age inappropriate behavior presenting in three ways: ADHD combined presentation is the most common type of ADHD and is characterized by impulsive and hyperactive behaviors as well as inattention and distractibility; ADHD impulsive/hyperactive presentation is the least common type of ADHD and is characterized by impulsive and hyperactive behaviors without inattention and distractibility; and ADHD inattentive and distractible presentation is characterized predominately by inattention and distractibility without hyperactivity. Neurocognitive impairments are a core part of ADHD symptomatology including problems in sustained attention, executive function, working memory, and self-regulation. Evidence suggests that ADHD is a genetic brain-based biological disorder but that etiological heterogeneity, including environmental factors, are likely influences that also may be reflected in differences in neural correlates. Comparing neural substrates of familial and non-familial ADHD could facilitate the development of more tailored interventions that target underlying neural anomalies characteristic of these subgroups.
Confounding the literature attributing ADHD to familial transmission is growing evidence that many children diagnosed with ADHD may, in fact, exhibit symptoms of ADHD secondary to prenatal alcohol exposure (PAE). PAE presents a significant public health concern, with fetal alcohol spectrum disorders (FASDs) affecting 5–20% of children glob-ally (Lange et al. 2017; May et al. 2018). ADHD is very common in PAE (Mattson et al. 2013; O’Connor 2014), but, in diagnosing ADHD, underlying PAE often goes unrecognized. About 95% of children with PAE exhibit ADHD-like symptoms (Fryer et al. 2007) such as hyperactivity, impulsivity, distractibility, and impaired executive function (Glass et al. 2014; Rasmussen 2005; Streissguth and O’Malley 2000) and the majority are diagnosed with ADHD (O’Connor 2014). ADHD with PAE (ADHD + PAE), however, may embody a distinct subtype of ADHD with earlier onset and unique presentation (Coles et al. 1997; Mattson et al. 2013) compared with ADHD without PAE (ADHD-PAE). Stimulants are frequently used to treat ADHD +PAE (O’Malley and Nanson 2002), but there is limited evidence of efficacy (Doig et al. 2008; Frankel et al. 2006; Oesterheld et al. 1998; O’Malley and Nanson 2002; Peadon et al. 2009; Snyder et al. 1997). Conversely, occult PAE is well represented in clinical ADHD populations (Coles 2001), but few studies of ostensible ADHD-PAE screen for PAE and unintended mixing of PAE and non-PAE subjects in studies of ADHD may hinder efforts to achieve statistically significant results. Moreover, findings ascribed to idiopathic ADHD may in reality stem from PAE. Thus, there are practical clinical and research needs to distinguish ADHD+PAE from ADHD-PAE. Differential diagnosis of ADHD+PAE versus ADHD-PAE can be challenging. The PAE key criterion of maternal alcohol consumption during pregnancy often cannot be established as many children with PAE are adopted or in foster care with the birth mother unavailable (Chernoff et al. 1994). Further, birth mothers are understandably reluctant to admit alcohol consumption while pregnant (CDC 2004). Moreover, many children with PAE lack the characteristic (but not essential) facial stigmata of the condition (Mattson et al. 2011, 2013). The use of neurobehavior assessment together with neuroimaging to improve our understanding of the neurobiological bases of ADHD in both PAE and non-PAE etiologies may contribute to more refined differential diagnosis and thereby to treatment innovation.
Neurobehaviorally, differing primary and secondary symptom profiles hint at distinct brain bases for the two etiologies. Inattention and hyperactivity/impulsivity, as assessed, by the Conners Global Index (Conners et al. 2011), are the primary symptoms of ADHD and their proportions vary in ADHD+PAE versus ADHD-PAE (Brown et al. 1991; Coles et al. 1997; O’Malley and Nanson 2002; Raldiris et al. 2018; Roebuck et al. 1999). Secondary symptoms of both ADHD (Krieger and Amador-Campos 2018, Mueller et al. 2017) and PAE (Glass et al. 2013, 2014; Mattson et al. 2013) prominently include disturbances in executive function. Building on earlier efforts, Mattson et al. (2010, 2013) and Nguyen et al. (2014) found that multiple subtests of the Delis-Kaplan Executive Function System (D-KEFS) executive-function battery (Delis et al. 2001) revealed more severe deficits in ADHD+PAE than in ADHD-PAE. Neurobehavioral metrics that differ between ADHD+PAE and ADHD-PAE, moreover, may have brain physiological reflexes, possibly ones detectable by neuroimaging.
Proton magnetic resonance spectroscopy of the brain has demonstrated effects of ADHD and PAE. The ADHD magnetic resonance spectroscopy literature (reviewed in O’Neill et al. 2013; Perlov et al. 2009) is replete with findings involving levels of N-acetylaspartate compounds, glutamate and choline-containing compounds in various cortical, subcortical, and white matter regions. A few findings involve creatine + phosphocreatine and many more are expressed as ratios to creatine, implying that creatine disturbances may attend ADHD as well. Levels of all these metabolites are related to brain energy metabolism, while N-acetylaspartate may particularly reflect neuronal density and activity, glutamate excitatory neurotransmission, and choline membrane metabolism (Rae 2014). We are aware, however, of no magnetic resonance spectroscopy study of ADHD that screened the study sample for possible PAE. Indeed, few ADHD neuroimaging investigations of any kind conduct such screening, despite the likely prevalence of covert FASD patients in ADHD clinical populations. Magnetic resonance spectroscopy studies of FASD have also yielded evidence for involvement of N-acetylaspartate (Cortese et al. 2006; du Plessis et al. 2014; Fagerlund et al. 2006), glutamate (du Plessis et al. 2014; Howells et al. 2016), and/or choline (Astley et al. 1995, 2009; Cortese et al. 2006; du Plessis et al. 2014; Fagerlund et al. 2006; Gonçalves et al. 2009). Some of these results were also given relative to creatine. Typically, these studies did not explicitly account for ADHD. There are additional reasons to further examine glutamate and choline in ADHD and FASD. There are both hyperglutamatergic (MacMaster et al. 2003) and hypoglutamatergic (Carlsson 2000, 2001; O’Neill et al. 2013) models of ADHD which link the condition to cell energy metabolism (Russell et al. 2006; Todd and Botteron 2001), but it is not established whether putative glutamatergic abnormalities prevail in both ADHD+PAE and ADHD-PAE etiologies. Recent rodent (Ryan et al. 2008; Schneider and Thomas 2016; Thomas et al. 2000, 2004, 2007, 2009, 2012) and human (Coles et al. 2015; Kable et al. 2015; Keen et al. 2010; Nguyen et al. 2016; Wozniak et al. 2020) trials of prospective cholinotherapies for FASD have rekindled interest in choline. In a pilot study (O’Neill et al. 2019), we found lower choline in the anterior corona radiata in ADHD+PAE than ADHD-PAE.
Diffusion tensor imaging is a neuroimaging modality that has demonstrated effects of ADHD and FASD. There are findings involving each of the four commonly employed diffusion tensor imaging metrics fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity in both ADHD (Aoki et al. 2018; Hamilton et al. 2008; Lawrence et al. 2013; Svatkova et al. 2016; Tamm et al. 2012) and FASD (Green et al. 2013; Ma et al. 2005; Treit et al. 2017; Wozniak et al. 2006). Again, ADHD and PAE have rarely been analyzed separately. Our pilot (O’Neill et al. 2019) found low fractional anisotropy in ADHD+PAE compared with ADHD-PAE in the corona radiata. Low fractional anisotropy may imply lower myelin (Budde et al 2007; Mädler et al. 2008; Song et al. 2003, 2005), thinner axons, lower axon density and/or diameter, frayed alignment of fibers (Friedrich et al. 2020; Kelley et al. 2016; Kochunov et al. 2012), or overabundance of crossing fibers (Sherbaf et al. 2019). These findings encourage testing more widely in white matter. Notably, the fractional anisotropy and choline findings of O’Neill et al. (2019) occurred in anatomically overlapping volumes. A need persists for more studies examining diffusion tensor imaging and magnetic resonance spectroscopy in the same regions-of-interest in order to provide converging or contrasting evidence for prospective models of pathology.
The present study assessed ADHD core and executive-function symptoms using the Conners and the D-KEFS assessments in a sample of ADHD+PAE, ADHD-PAE, and typically developing (TD) children. Magnetic resonance spectroscopy and diffusion tensor imaging were acquired from a wide, overlapping section of bilateral white matter in the frontal lobes (“supraventricular white matter”). Looking forward to possible future clinical applications, this region was chosen because it typically yields high-quality magnetic resonance spectroscopy and diffusion tensor imaging data and is readily located by MR technologists. Additionally, our pilot study (O’Neill et al. 2019) identified choline and fractional anisotropy differences between ADHD+PAE and ADHD-PAE in part of this region (corona radiata) and we were interested to see if such effects existed more broadly in white matter. More rigorous techniques were applied than in the pilot study. These included prospective design, systematic assessment (rather than simple screening) of PAE in all participants, equally thorough assessment of ADHD, restriction of ADHD-PAE participants to only those with familial ADHD, 3 T (rather than 1.5 T) magnetic field strength, shorter magnetic resonance spectroscopy echo-time, smaller magnetic resonance spectroscopy and diffusion tensor imaging voxels, and more diffusion tensor imaging gradient directions. The goals were (1) to identify core ADHD and executive-function symptoms that differ between ADHD+PAE and ADHD-PAE, (2) to identify magnetic resonance spectroscopy and diffusion tensor imaging signs of potential brain differences between ADHD+PAE and ADHD-PAE, and (3) to identify possible correlations of magnetic resonance spectroscopy and diffusion tensor imaging metrics with neurobehavioral assessment. These may point to brain differences between the ADHD+ PAE and ADHD-PAE etiologies.
Subjects and Methods
Subjects
Participants (Table 1) comprised 23 children with ADHD+PAE, 19 with familial ADHD-PAE, and 28 TD control children with neither ADHD nor PAE. These numbers reflect studies that passed magnetic resonance spectroscopy and diffusion tensor imaging quality control (described below). All children were assessed by clinicians who were blind to the child’s prenatal alcohol exposure history. Children in the ADHD groups were required to meet DSM-5 criteria for ADHD, any subtype, according to the computerized clinician administered Schedule for Affective Disorders and Schizophrenia for School-Aged Children Parent Version (K-SADS), (Kaufman et al. 1997; Townsend et al. 2020). Children in the ADHD+PAE group had to meet criteria for fetal alcohol syndrome, partial fetal alcohol syndrome, or alcohol-related neurodevelopmental disorder using the modified Institute of Medicine criteria (Hoyme et al. 2016). This system examines four key diagnostic features: growth retardation; the FASD facial phenotype, including short palpebral fissures, flat philtrum, and flat upper vermillion border; neurodevelopmental dysfunction; and gestational alcohol exposure. Alcohol exposure was assessed using the Health Interview for Women or the Health Interview for Adoptive and Foster Parents (Quattlebaum and O’Connor 2013). Other teratogens (Table 1) were assessed on both the interviews. Criteria for alcohol exposure included more than 6 drinks/week for 2 weeks or more and/or more than 3 drinks on 2 or more occasions including the time periods prior to and following pregnancy recognition. These criteria are based on findings that 1 drink/day (or more than 6 drinks/week) is an adequate measure of exposure for FASD and on epidemiologic studies demonstrating adverse fetal effects of episodic drinking of 3 or more drinks per occasion (Hoyme et al. 2016).
Table 1.
Demographic characteristics of the sample, listed as number or mean (standard deviation)
| Variable | ADHD+PAE | ADHD-PAE | TD | Three-group p |
|---|---|---|---|---|
| Number | 23 | 19 | 28 | – |
| Gender (number male) | 16 | 12 | 13 | 0.222 |
| Age (years) | 9.7 (1.5)a,b | 10.7 (0.9) | 11.1 (1.5) | 0.002 |
| Mother’s education (years) | 15.7 (1.9) | 17.2 (3.2) | 17.6 (4.2) | 0.185 |
| WASI-II Full-Scale IQ | 94.9 (12.5)a | 107.5 (11.2)c | 116.7 (15.4) | < 0.001 |
| Current Psychotropics | ||||
| None | 10 | 14 | 28 | – |
| Stimulants | 10 | 4 | 0 | – |
| Antidepressants | 1 | 3 | 0 | – |
| Mood stabilizers | 1 | 1 | 0 | – |
| Antipsychotics | 4 | 1 | 0 | – |
| Noradgrenergics | 4 | 1 | 0 | – |
| Other | 0 | 0 | 0 | – |
| Exposure to Other Teratogens | ||||
| Tobacco | 1 | 1 | 3 | – |
| Marijuana | 4 | 1 | 0 | – |
| Methamphetamine | 4 | 0 | 0 | – |
| Cocaine | 0 | 0 | 0 | – |
| Barbiturates | 1 | 0 | 0 | – |
| Opioids | 0 | 0 | 0 | – |
| Hallucinogens | 0 | 0 | 0 | – |
| Anticonvulsants | 0 | 0 | 1 | – |
| Antibiotics | 0 | 2 | 0 | – |
| OTC painkillers (aspirin, acetaminophen, ibuprofen) | 2 | 1 | 2 | – |
p < 0.01 for comparison between ADHD+PAE and ADHD-PAE
p < 0.01 for comparison between ADHD+PAE and TD
p < 0.05 for comparison between ADHD-PAE and TD
Additional criteria for ADHD-PAE were (1) at least one first-degree family member (e.g., biological parent or sibling) with diagnosed ADHD and (2) two or fewer standard drinks throughout gestation. The inclusion criteria for the TD group were (1) no current or lifetime Axis I mental disorder by K-SADS and (2) two or fewer standard drinks throughout gestation.
Participants from all groups were excluded for any of the following reasons: (1) Full Scale IQ less than 70; (2) known genetic syndrome associated with ADHD including fragile X, tuberous sclerosis, or generalized resistance to thyroid hormone; (3) pervasive developmental disorder; (4) serious medical or neurologic illness likely to influence brain function, (e.g., seizures and closed-head trauma); (5) gestation less than 34 weeks; (6) claustrophobia; (7) ferromagnetic metal or other contraindication to MRI; (8) English not primary language in the home; or (9) unable to comply with study procedures.
Children were recruited from community organizations, FASD parent organizations, national websites, other pediatric research studies, or physician referrals from local child psychiatry or pediatric clinics. Participants were screened for ADHD, PAE, and exclusionary criteria using a brief telephone questionnaire. Eligible participants were seen for two sessions. The first session included demographic and medical history, diagnostic and clinical interviews, neurocognitive testing, and mock MRI scanning. During the second session, children underwent MRI scanning. Children taking stimulants were asked to be off-medication for at least 24 h prior to each session. The UCLA Institutional Review Board approved all procedures. All parents gave informed consent; all children gave assent and were compensated for their time.
Neurobehavioral Assessments
Full Scale IQ was estimated with the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) (Wechsler 2011). Parent ratings on the Conners 3 Parent Behavior Rating Scale (Conners et al. 2011) were used to evaluate inattentive and hyperactive/impulsive symptoms of ADHD. The corresponding Teacher Rating Scales were not used due to low teacher response rate. The Delis Kaplan Executive Function System (D-KEFS) (Delis et al. 2001) was used to assess executive functions. The specific tests chosen for analysis were among those for which Nguyen et al. (2014) had found effects of ADHD or of PAE. These included Color-Word Inhibition, the Trail Making Test, Verbal Fluency, and the Tower Test. Of the numerous metrics provided by each of these tests, analysis was restricted to the base metrics and, in a few cases, to ancillary metrics for which Nguyen et al. (2014) had observed effects. In particular, for Color-Word Interference we examined Conditions 1–4: Color Naming, Word Recognition, Inhibition, and Inhibition Switching, as well as Condition 3 Inhibition Total Errors (Nguyen et al. 2014). For the Trail Making Test, we examined scores for Conditions 1–4: Visual Scanning, Number Sequence, Letter Sequence, and Number-Letter Switching, as well as Condition 4 Number-Letter Switching Set-Loss Errors (Nguyen et al. 2014). For Verbal Fluency, we examined Total Correct for Letter Fluency, Category Fluency, and Category Switching. For the Tower Test, we examined Total Achievement Score, Total Rule Violations, and Mean First Move Time (Nguyen et al. 2014).
Magnetic Resonance Acquisition
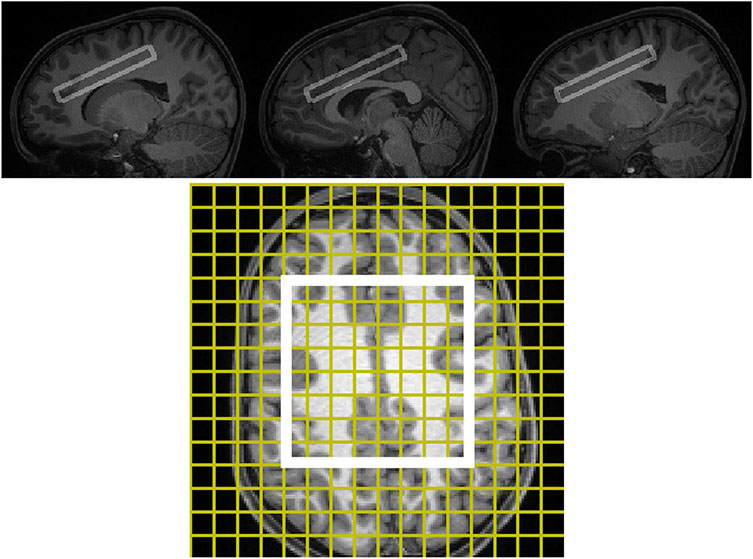
Prior to MRI, participants underwent extensive desensitization to the MRI scanner and training in keeping the head and body still. After training, acquisition of magnetic resonance spectroscopy and diffusion tensor imaging was performed using a 3 Tesla Siemens Prisma-fit MRI scanner in a single session lasting ~ 90 min. Participants watched a children’s movie of their choice via mirror back-projection during acquisition. They were monitored by real-time video and encouraged to hold still when motion was detected. A 32-channel phased-array head receiver coil was used with a body transmitter coil. Magnetization-prepared rapid gradient-echo T1-weighted imaging and diffusion tensor imaging were acquired with custom pulse-sequences and parameters prepared at the University of Minnesota for the Human Connectome Project. Magnetization-prepared rapid gradient-echo 3-dimensional T1-weighted imaging was performed using a sagittal multi-echo method that included real-time motion correction (TR/TEs/TI = 2500/1.81, 3.6, 5.39, 7.18/1000 ms; flip 8°; voxel dimensions 0.8 × 0.8 × 0.8 mm3). This acquisition produced a T1-weighted 3-dimensional volume image that was used for positioning of subsequent imaging, tissue-segmentation, and radiologic review. Diffusion tensor imaging was performed using multiband accelerated multislice spin-echo echo-planar imaging (TR/TE = 3230/89.2 ms; b = 0, 1500, 3000 s/mm2; 92 slices; voxels 1.5 × 1.5 × 1.5 mm; multiband factor 4; genu-splenium parallel slicing; whole-brain coverage with 98 and 99 alternating echo-planar phase-encode directions). Water-suppressed proton magnetic resonance spectroscopic imaging was acquired as 2-dimensional magnetic resonance spectroscopic imaging with a stimulated-echo acquisition mode pulse-sequence provided by Siemens with TR/TE/TM = 2000/20/10 ms, voxel dimensions 10 × 10 × 10 mm3, and 4 excitations. A non-water-suppressed acquisition using 1 excitation was acquired from an identical volumetric prescription. The non-water-suppressed data were used for offline quality control, and quantitation of metabolites. The prescription (Fig. 1) consisted of a coronal-oblique 16 × 16 voxel-array oriented tangent to the dorsum of the corpus callosum as seen in the sagittal plane. The 8 × 8 (or slightly different, depending on anatomy) subarray in the center of the slab constituted the “excitation box” from which usable magnetic resonance spectra were recorded. Rostro-caudally this box extended approximately from pregenual anterior cingulate to premotor cortex. Lateral-mesially it straddled the longitudinal fissure symmetrically and extended to lateral cortices (e.g., middle frontal cortex). Figure 2 shows a representative data. T1-weighted MRI, diffusion tensor imaging, and magnetic resonance spectroscopy data were examined immediately after acquisition and those contaminated by excessive motion were repeated.
Fig. 1.
STimulated Excitation Acquisition Mode (TR/TE/TM = 2000/20/10 ms) magnetic resonance spectroscopic imaging for a representative participant in register with T1-weighted MRI in sagittal (upper frame) section. In the lower frame, the T1-weighted MRI has been resliced to match the orientation of the magnetic resonance spectroscopic imaging slab. The yellow lines in the lower frame demarcate the 16 × 16 two-dimensional array of 10 × 10 × 10 mm3 spectroscopic imaging voxels. The white box in the lower frame indicates the 8 × 8 subarray from which usable spectra are acquired. The upper frame illustrates three sagittal T1w MRI sections that intersect with the excitation box and include the left frontal white matter, right frontal white matter, and midline frontal gray matter. Together the upper and lower frames illustrate that the magnetic resonance spectroscopic imaging sampling volume includes a substantial amount of frontal supraventricular white matter
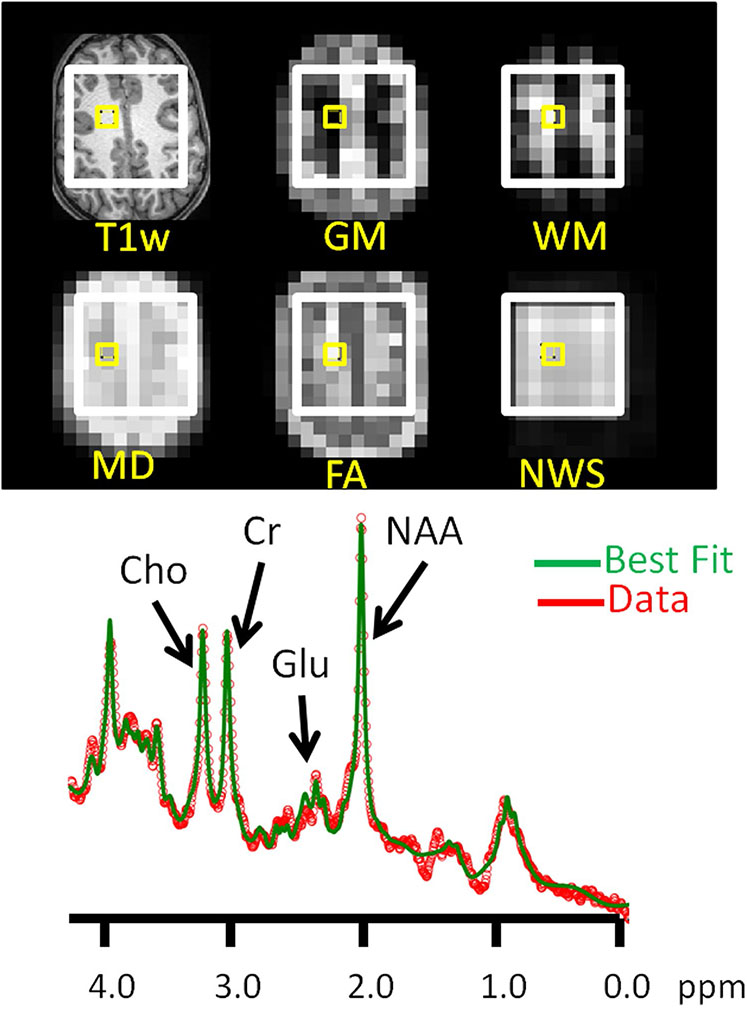
Fig. 2.
STimulated Excitation Acquisition Mode (TR/TE/TM = 2000/20/10 ms) magnetic resonance spectroscopic imaging data for a sample voxel in supraventricular white matter. (Upper frame) The yellow box identifies a voxel on slices of T1w MRI and (resampled to magnetic resonance spectroscopic imaging resolution) gray matter (GM), white matter (WM), mean diffusivity (MD), and fractional anisotropy (FA) volumes, as well as the non-water-suppressed (NWS) magnetic resonance spectroscopic image. (Lower frame) Measured (red) and best fit (green) water-supressed magnetic resonance spectrum from the voxel identified in the images. The abscissa marks chemical shift in parts per million (ppm). Labelled are principal resonances for N-acetyaspartate compounds (NAA), glutamate (Glu), total creatine (= creatine + phosphocreatine), and choline-compounds (Cho). Note that relatively tight fit of these signals achieved with the SVFit2016 software package (Alger et al. 2016)
MRI, Magnetic Resonance Spectroscopy, and Diffusion Tensor Imaging Co-analysis
Post-processing was performed blind to participant diagnosis. Offline magnetic resonance spectroscopy and diffusion tensor imaging data were reviewed by experts (JON, JRA). Studies showing poor quality data (e.g., excess head motion, inaccurately positioned magnetic resonance spectroscopy slabs) were excluded.
Each 3-dimensional T1-weighted volume image was tissue-segmented into gray matter, white matter, and cerebrospinal fluid subvolumes using the BET and FAST tools of the FMRIB Software Library (FSL v5.0, https://fsl.fmrib.ox.ac.uk/fsl). These operations were performed without spatial transform, so the resulting tissue-segment subvolumes are aligned with the 3D T1w volume (“native space”).
Magnetic resonance spectroscopic imaging spatial reconstruction and combination of data from the 32 receive channels were performed by the manufacturer’s software. This yielded a 16 × 16 grid of “voxel spectra” for each water-suppressed and non-water-suppressed study. Further processing was done with SVFit2016 (Alger et al. 2016) together with a series of purpose-written software tools. Operations performed by SVFit2016 included time-domain filtering and non-linear least-squares spectral fitting to determine neurometabolite levels for each magnetic resonance spectroscopic imaging voxel within the excitation box (exclusive of box edges). SVFit2016 was written in the Interactive Data Language and uses the Levenberg–Marquardt implementation of the Gauss-Newton method to fit spectra in the frequency domain. The specific fitting routine is a modified version of MPFIT (Markwardt 2009, http://purl.com/net/mpfit). Fits for non-water-suppressed spectral arrays used a model spectrum that included only a single water signal. Fits for water-suppressed spectra included models of spectra for lactate, N-acetylaspartate, N-acetylaspartylglutamate, glutamate, glutamine, γ-aminobutyric acid, creatine, phosphocreatine, choline-compounds, inositol compounds, numerous low-level neurometabolites, residual water, lipids, and macromolecules. Model spectra were simulated in Versatile Simulation, Pulses and Analysis software (Young et al. 1998a, b; Soher et al. 1998; https://scion.duhs.duke.edu/vespa/project). Following fitting, the N-acetylaspartate and N-acetylaspartylglutamate signals were summed to form total N-acetylaspartate. Similarly, creatine and phosphocreatine were summed to total creatine. Fit quality for all spectra was reviewed by two experts (JRA, JON). Poor fits determined by visual inspection were resubmitted for fitting with different starting estimates of various parameters. Voxel spectra that showed poor fit quality after multiple retries were not included in further analyses. Spectra with signal-to-noise ratio less than 5 in the creatine spectral region were excluded, as were spectra with voxel static magnetic field inhomogeneity greater than 0.045 parts-per-million.
Diffusion tensor imaging was post-processed, blind to diagnosis, using purpose-written Interactive Data Language software that calculates the diffusion tensor, fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity within each voxel using the formalism of Basser et al. (1994).
Spatial co-ordinates of the T1-weighted imaging, diffusion tensor imaging, and magnetic resonance spectroscopy studies were read from the corresponding file headers. This, together with the tissue-segmented subvolumes produced by FSL enabled co-alignment of gray matter, white matter, and CSF segment volumes with the magnetic resonance spectroscopic imaging voxels. In co-alignment, the tissue-segmented subvolumes were resampled in order to estimate the tissue content of each magnetic resonance spectroscopic imaging voxel (as shown in Fig. 2). These data were used to implement CSF-correction of metabolite levels and to select specific magnetic resonance spectroscopic imaging voxel spectra for further analysis. Similar procedures were used to resample the above-described fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity volumes to the magnetic resonance spectroscopic imaging spatial grid. This co-alignment provided estimates of the values of fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity in each magnetic resonance spectroscopic imaging voxel and permitted study of the relationships between neurometabolite levels and diffusion tensor imaging metrics. Magnetic resonance spectroscopic imaging voxels from left and right hemispheres that contained 60% or more white matter and passed the above described quality control procedures were selected for further analysis. Each participant produced between 2 and 18 quality-control pass spectroscopic imaging voxels. Figure 2 shows a representative example of a quality-control pass spectrum. Group-mean (standard deviation) numbers of voxels per subject were 18 (2.7) for ADHD+PAE, 18.7 (3.4) for ADHD-PAE, and 19.9 (2.8) for TD. Volume percent white matter was 82% (3%) for ADHD+PAE, 84% (4%) for ADHD-PAE, and 83% (4%) for TD. Cross-voxel averaging was performed for each participant to yield a single value for each neurometabolite level and diffusion tensor imaging metric for that participant’s left + right supraventricular white matter.
Statistical Analysis
Table 1 reveals that the three participant groups were not well age-matched. Accordingly, each magnetic resonance spectroscopy and diffusion tensor imaging metric was adjusted for participant age prior to statistical analyses. This was accomplished by linear least-squares fitting of each metric as a function of age within each group, followed by extrapolation to the common age of 128 months. These “age-extrapolated” neuroimaging metrics were used throughout the results reported below. Scaled scores (already normed for age) were used for all neurobehavioral metrics.
Non-parametric statistics were used throughout since these are more conservative, less sensitive to outliers, and appropriate for both normally and non-normally distributed data. Preliminary histogram examination and Shapiro-Wilks testing revealed that several metrics were non-normally distributed. Criterion for statistical significance for all tests was p < 0.05.
To deal with multiple comparisons in pursuit of goal (1), Kruskal-Wallis omnibus testing was performed across all three participant groups. For those neurobehavioral metrics showing a significant or trend (p < 0.10) main effect of group, this was followed by pair-wise protected post hoc Mann-Whitney U-Tests to determine which group-pairs (ADHD+PAE versus ADHD-PAE, ADHD+PAE versus TD, ADHD-PAE versus TD) manifested significant between-group differences.
For goal (2), an identical procedure was followed for the age-extrapolated magnetic resonance spectroscopy and diffusion tensor imaging metrics.
For the first part of goal (3)—within-group correlations between neurobehavioral and neuroimaging metrics, to deal with multiple comparisons, multivariate analysis of covariance was performed on the rank-transformed neurobehavioral and neuroimaging metrics within each participant group. For those neurobehavioral metrics having a significant or trend effect on the ensemble of magnetic resonance spectroscopy or diffusion tensor imaging metrics, protected posthoc Spearman correlations were performed between the neurobehavioral metric in question and each of the individual neuroimaging metrics. For the second part of goal (3)—within-group correlations between magnetic resonance spectroscopy and diffusion tensor imaging metrics, a similar procedure was followed, whereby the effects of each diffusion tensor imaging metric on each magnetic resonance spectroscopy metric were determined.
Results
Group Demographics
χ2-testing found no significant difference in number of boys and girls between the groups (χ2(2) = 3.0, p > 0.10; Table 1). Age differed significantly between the groups (Kruskal-Wallis H(2) = 13.8, p = 0.001). Post hoc testing confirmed that ADHD+PAE were significantly younger than ADHD-PAE (Mann-Whitney U = 107, p = 0.005) and TD (U = 147.5, p < 0.0005), but ADHD-PAE did not differ significantly in age from TD (U = 200, p > 0.10). As indicated above, all neuroimaging metrics were age-extrapolated to account for these imbalances. Years of mother’s education (a proxy for socioeconomic status) did not differ significantly between the groups (H(2) = 3.3, p > 0.10). As is common in studies of FASD, Full Scale IQ differed significantly between the groups (H(2) = 22.9, p < 0.0005). IQ was lower in ADHD+PAE than in ADHD-PAE (U = 105, p < 0.005) and TD (U = 86, p < 0.0005), and was lower in ADHD-PAE than in TD (U = 161, p < 0.05). No adjustment was made for IQ imbalances as it is not conventional to do so; low IQ is a frequent symptom of ADHD (Kuntsi et al. 2004) and of PAE (Coles et al. 1991) and to correct for such could inadvertently remove the effects of illness.
Between-Group Differences in Neurobehavioral Metrics
Kruskal-Wallis testing (Table 2) revealed significant or trend effects of group on Conners Inattention (H(2) = 42.5, p < 0.0005), Conners Hyperactivity/Impulsivity (H(2) = 46.5, p < 0.0005), Color-Word Interference Word Recognition (H(2) = 2.9, p < 0.10 trend), Trail Making Test Letter Sequence (H(2) = 12.3, p < 0.005), Trail Making Test Switching Set-Loss Errors (H(2) = 7.1, p < 0.01), Verbal Fluency Letter Fluency Total Correct (H(2) = 6.6, p < 0.05), Verbal Fluency Category Switching (H(2) = 3.3, p < 0.10 trend), Tower Test Total Achievement Score (H(2) = 8.1, p < 0.05), and Tower Test Total Rule Violations (H(2) = 15.1, p = 0.001). No other scores manifested a significant omnibus effect of group (all p > 0.10).
Table 2.
Neurobehavioral characteristics of the sample, listed as mean (standard deviation)
| Metric | ADHD+PAE | ADHD-PAE | TD | Three-group p |
|---|---|---|---|---|
| Conners Parent Metrics | ||||
| Inattention | 80.1 (13.0)a | 81.7 (8.8)b | 47.6 (10.3) | < 0.001 |
| Hyperactivity/Impulsivity | 86.4 (5.1)a,c | 77.0 (13.0)b | 39.4 (10.4) | < 0.001 |
| Delis Kaplan Executive Function System Metrics | ||||
| Color Word Interference | ||||
| Color Naming | 8.6 (2.9) | 9.7 (3.6) | 10.8 (3.0) | 0.131 |
| Word Recognition | 9.8 (2.8) | 11.2 (1.7) | 11.0 (2.6) | 0.069 |
| Inhibition | 10.0 (3.1) | 10.3 (3.1) | 11.2 (3.1) | 0.365 |
| Inhibition/Switching | 8.7 (3.5) | 9.6 (3.8) | 10.9 (2.7) | 0.294 |
| Inhibition Total Errors | 8.8 (3.3) | 9.8 (3.2) | 10.6 (2.6) | 0.113 |
| Trail Making Test | ||||
| Visual Scanning | 9.6 (3.3) | 8.6 (3.9) | 10.9 (2.3) | 0.240 |
| Number Sequence | 9.7 (2.9) | 10.5 (3.7) | 11.5 (2.9) | 0.232 |
| Letter Sequence | 6.7 (3.9)a | 8.7 (4.3) | 10.6 (3.9) | 0.002 |
| Number-Letter Switching | 7.2 (4.0) | 8.5 (4.3) | 10.1 (4.1) | 0.298 |
| Switching Set Loss Errors | 1.6 (1.8)d | 0.8 (1.2) | 0.5 (1.0) | 0.029 |
| Verbal Fluency | ||||
| Letter Fluency | 8.9 (3.1)e | 10.2 (3.2) | 11.5 (3.6) | 0.037 |
| Category Fluency | 10.6 (3.2) | 11.4 (3.9) | 12.9 (3.7) | 0.621 |
| Category Switching | 8.1 (2.8)a | 9.8 (3.7) | 11.8 (2.7) | 0.071 |
| Tower Test | ||||
| Total Achievement Score | 9.0 (3.3)e | 9.7 (2.3)f | 10.9 (2.9) | 0.017 |
| Total Rule Violations | 5.7 (6.4)a | 5.2 (7.3)g | 1.3 (1.8) | 0.001 |
| Mean First Move Time | 12.7 (1.4) | 12.0 (1.6) | 12.2 (1.9) | 0.377 |
p < 0.05 for comparison between ADHD+PAE and ADHD-PAE
p < 0.001, 0.01, 0.05 for comparison between ADHD+PAE and TD
p < 0.001, 0.01, 0.05 for comparison between ADHD-PAE and TD
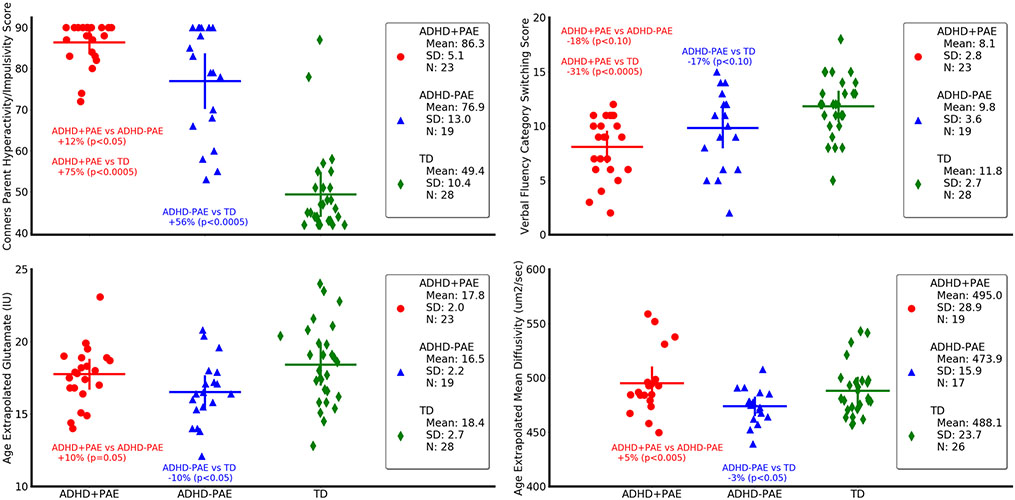
In protected post hoc tests (Table 2), Conners Hyperactivity/Impulsivity Index was 12.2% higher in ADHD+PAE than ADHD-PAE (U = 133.5, p < 0.05; Fig. 3) but no other neurobehavioral metric (i.e., none of the other scores) distinguished ADHD+PAE from ADHD-PAE (all p > 0.10). Conners Hyperactivity/Impulsivity Index was 74.8% higher in ADHD+PAE than TD (U = 10, p < 0.0005; Fig. 3). The Conners Inattention Index was 68.2% higher in ADHD+PAE than TD (U = 36, p < 0.0005; Fig. 3) and 71.7% higher in ADHD-PAE than TD (U = 27, p < 0.0005; Fig. 3). Several executive function measures signaled worse performance in ADHD+PAE than in TD: ADHD+PAE scored 37.1% lower on Trail Making Test Letter Sequence (U = 129.5, p < 0.0005), 213% higher on Trail Making Test Switching Set-Loss Errors (U = 200, p < 0.01), 22.7% lower on Verbal Fluency Letter Fluency (U = 188.5, p < 0.05), 31.6% lower on Verbal Fluency Category Switching (U = 104.5, p < 0.0005; Fig. 3), 17% lower on Tower Test Total Achievement Score (U = 198.5, p < 0.05), and 355.7% higher on Tower Test Total Rule Violations (U = 133, p < 0.0005). ADHD-PAE exhibited inferior performance to TD on two Tower Test metrics: 11.1% lower Total Achievement Score and (U = 145, p < 0.01) and 316.8% higher Total Rule Violations (U = 121, p < 0.01). Thus, while only the Conners Hyperactivity/Impulsivity Index distinguished ADHD+PAE from ADHD-PAE in head-to-head comparison, compared with TD, executive function deficits were more severe and diverse in children with ADHD+PAE than in children with ADHD-PAE.
Fig. 3.
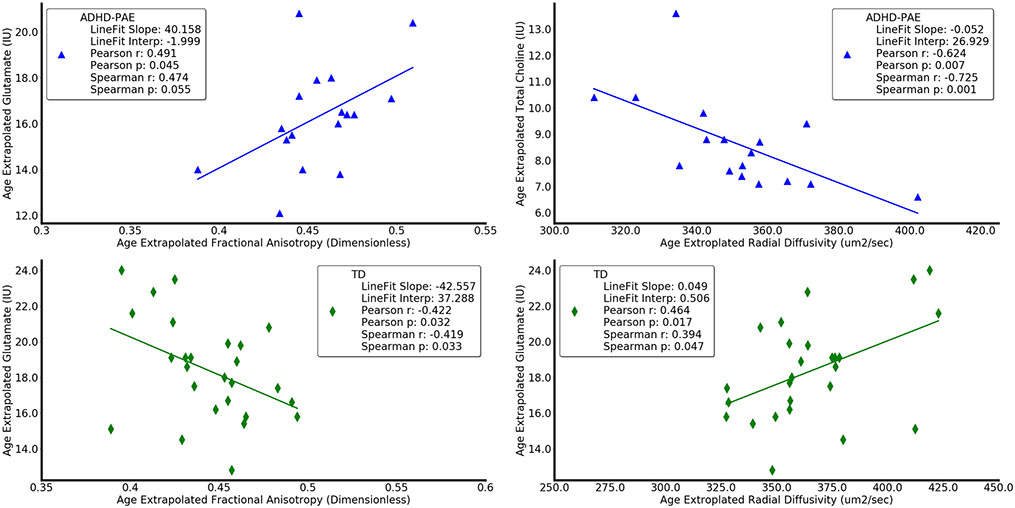
Neurobehavioral (upper panels) and neuroimaging (lower panels) metrics differ in children with attention deficit-hyperactivity disorder with prenatal alcohol exposure (ADHD+PAE; red circles) from those with familial attention deficit-hyperactivity disorder without prenatal alcohol exposure (ADHD-PAE; blue triangles), and typically developing controls (TD; green diamonds). Means and standard deviations are depicted by horizontal and vertical lines, respectively. In ADHD+PAE, the Conners Hyperactivity/Impulsivity Score was 12.2% higher than in ADHD-PAE (p < 0.05; post hoc protected Mann-Whitney U-test following omnibus Kruskal-Wallis test) and 74.8% higher than in TD (p < 0.005). The Conners Hyperactivity/Impulsivity Sore was also 55.8% higher in ADHD-PAE than in TD (p < 0.005). The Verbal Fluency Category Switching Score from the Delis-Kaplan Executive Function System significantly distinguished ADHD+PAE from TD (− 31,6%, p < 0,.0005), but only distinguished ADHD+PAE from ADHD-PAE (− 17.8%, p < 0.10 trend) and ADHD-PAE from TD (− 16.7%, p < 0.10) at trend level. Glutamate in supraventricular white matter was 9.6% higher (p = 0.05) in ADHD+PAE than in ADHD-PAE and 10.3% lower (p < 0.05) in ADHD-PAE than in TD. Mean diffusivity (MD) of supraventricular white matter was 5.4% higher (p < 0.005) in ADHD+PAE than in ADHD-PAE. Similar results (not shown) were observed for axial diffusivity (2.8%, p < 0.05) and radial diffusivity (6.1%, p < 0.05). Also, mean diffusivity was 2.9% lower in ADHD-PAE than in TD (p < 0.05). Although there is overlap between groups for all metrics, these findings suggest brain differences between the PAE and familial etiologies of ADHD. All metrics are adjusted for participant age. IU (Institutional Units)
Between-Group Differences in Neuroimaging Metrics
Kruskal-Wallis testing (Table 3) revealed significant or trend effects of group on glutamate (H(2) = 6.4, p < 0.05), mean diffusivity (H(2) = 6.8, p < 0.05), axial diffusivity (H(2) = 4.7, p < 0.10 trend), and radial diffusivity (H(2) = 5.7, p < 0.10). No other neuroimaging metrics (including magnetic resonance spectroscopy measures other than glutamate) manifested a significant omnibus effect of group (all p > 0.10).
Table 3.
Group comparisons of MRS neurometabolite levels and DTI indices in supraventricular white matter, listed as mean (standard deviation) for each group
| Metric | ADHD+PAE | ADHD-PAE | TD | Three-group p |
|---|---|---|---|---|
| Magnetic Resonance Spectroscopy Neurometabolite Metrics | ||||
| N-acetylaspartate-compounds [IU] | 33.9 (1.8) | 34.5 (1.9) | 34.4 (2.8) | 0.600 |
| Glutamate [IU] | 17.8 (2.0)a | 16.5 (2.3)b | 18.4 (2.7) | 0.041 |
| Total creatine (creatine + phosphocreatine) [IU] | 18.4 (1.0) | 17.8 (1.2) | 18.2 (1.5) | 0.186 |
| Choline-compounds [IU] | 9.1 (1.9) | 8.6 (1.6) | 8.5 (1.4) | 0.408 |
| Diffusion Tensor Imaging Metrics | ||||
| Fractional anisotropy [dimensionless] | 0.44 (0.03) | 0.46 (0.03) | 0.44 (0.03) | 0.257 |
| Mean diffusivity [μm2/s] | 495 (29.7)a | 474 (16.4)b | 488 (24.1) | 0.034 |
| Axial diffusivity [μm2/s] | 370 (15.5)a | 360 (9.0) | 366 (12.2) | 0.097 |
| Radial diffusivity [μm2/s] | 372 (31.3)a | 351 (20.7) | 366 (26.8) | 0.057 |
p ≤ 0.05 for comparison between ADHD+PAE and ADHD-PAE
p ≤ 0.05 for comparison between ADHD-PAE and TD
In protected post hoc tests (Table 3), glutamate was 9.6% higher in ADHD+PAE than ADHD-PAE (U = 142, p = 0.05; Fig. 3); mean diffusivity was 4.5% higher (U = 84, p < 0.05; Fig. 3). Axial diffusivity was 2.8% higher (U = 97, p < 0.05), and radial diffusivity was 6.1% higher (U = 90, p < 0.05). There were no significant differences between ADHD+PAE and TD in neuroimaging metrics. For ADHD-PAE versus TD, glutamate was 10.3% lower (U = 158, p < 0.05; Fig. 3) and mean diffusivity was 2.9% lower (U = 142, p = 0.05; Fig. 3). Thus, a handful of neuroimaging metrics distinguished ADHD+PAE from ADHD-PAE or ADHD-PAE from TD.
Within-Group Correlations Between Neurobehavioral and Neuroimaging Metrics
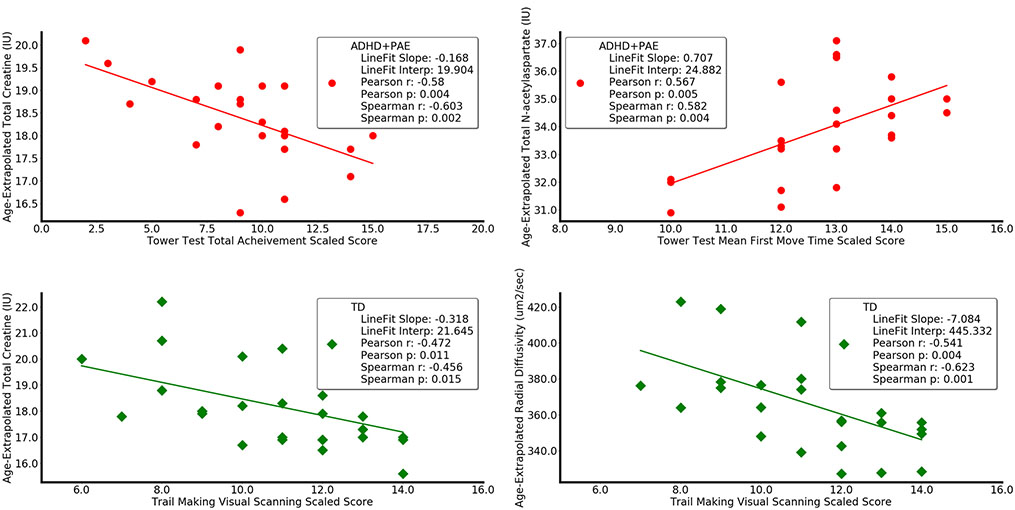
Within ADHD+PAE, omnibus multivariate analysis of covariance of rank-transformed data found significant relationships between the Tower Test Total Achievement Score and magnetic resonance spectroscopy metrics (F(4.18) = 4.4, p < 0.05) and between the Tower Test Mean First Move Time and magnetic resonance spectroscopy metrics (F(4.18) = 3.2, p < 0.05) and trend relationships between Color-Word Interference Color Naming and diffusion tensor imaging metrics (F(4.13) = 2.4, p < 0.10), between Trail Making Test Number-Letter Switching and diffusion tensor imaging metrics (F(4.14) = 2.9, p < 0.10), and between Trail Making Test Number-Letter Switching Set-Loss Errors and diffusion tensor imaging metrics (F(4.14) = 3.0, p < 0.10). Significant protected post-hoc Spearman correlations (Table 4) within ADHD+PAE included those between Trail Making Test Letter Sequence and N-acetylaspartate (r = 0.46, p < 0.05), Trail Making Test Number-Letter Switching and fractional anisotropy (r = 0.55, p < 0.05), Trail Making Test Number-Letter Switching and radial diffusivity (r = − 0.50, p < 0.05), Tower Test Total Achievement Score and glutamate (r = − 0.41, p < 0.05), Tower Test Total Achievement Score and creatine (r = − 0.60, p < 0.005; Fig. 4), Tower Test Total Achievement Score and choline (r = − 0.42, p < 0.05), Tower Test Mean First Move Time and N-acetylaspartate (r = 0.59, p < 0.005), and Tower Test Mean First Move Time and glutamate (r = 0.49, p < 0.05).
Table 4.
For each group, significant correlations between neurobehavioral and neuroimaging measures
| Participant group | Neurobehavioral measure | Neuroimaging metric |
Correlationr | Correlation p |
|---|---|---|---|---|
| ADHD+PAE | Trail Making Test Letter Sequence | NAA | + 0.46 | 0.028 |
| Trail Making Test Number-Letter Switching | FA | + 0.55 | 0.015 | |
| Trail Making Test Number-Letter Switching | RD | − 0.50 | 0.030 | |
| Tower Test Total Achievement Score | Glu | − 0.41 | 0.050 | |
| Tower Test Total Achievement Score | Cr | − 0.60 | 0.003 | |
| Tower Test Total Achievement Score | Cho | − 0.42 | 0.044 | |
| Tower Test Mean First Move Time | NAA | + 0.59 | 0.003 | |
| Tower Test Mean First Move Time | Glu | + 0.49 | 0.018 | |
| TD | Trail Making Test Visual Scanning | Cr | − 0,45 | 0.017 |
| Trail Making Test Visual Scanning | FA | + 0.53 | 0.005 | |
| Trail Making Test Visual Scanning | MD | − 0.52 | 0.006 | |
| Trail Making Test Visual Scanning | RD | − 0.62 | 0.001 |
Magnetic Resonance Spectroscopy neurometabolite metrics: N-acetylaspartate plus N-acetylaspartateglutamate (NAA), glutamate (Glu), creatine + phosphocreatine (Cr), Choline- compounds (Cho). Diffusion Tensor Imaging metrics: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD)
Fig. 4.
Selected correlation analysis (including linear regression and Pearson and Spearman correlation coefficients with p-values) between neurobehavioral and neuroimaging metrics in children with ADHD+PAE (red circles, upper) and in TD (green diamonds, lower). In ADHD+PAE, the Tower Test Total Achievement Score decreased with increasing creatine in supraventricular white matter (r = − 0.60, p < 0.005). Similar results (not shown) were observed for glutamate (r = − 0.41, p = 0.05) and choline (r = − 0.42, p < 0.05). Higher Tower Test Mean First Move Time (indicating inferior performance) accompanied higher levels of N-acetylaspartate (r = 0.59, p < 0.05). A similar result (not shown) was observed for glutamate (r = 0.49, p < 0.05). In TD, Trail Making Test Visual Scanning Score decreased (i.e., worse performance) for increasing creatine (r = 0.59, p < 0.05) and for increasing radial diffusivity (r = − 0.62, p = 0.001). A complementary opposite result (not shown) was observed for fractional anisotropy (r = 0.53, p = 0.005). All metrics are adjusted for participant age
Within ADHD-PAE, there were no significant or trend multivariate analysis of covariance relationships between neurobehavioral and neuroimaging metrics.
Within TD, omnibus multivariate analysis of covariance of rank-transformed data found significant relationships between Trail Making Test Visual Scanning and magnetic resonance spectroscopy metrics (F(4.23) = 3.6, p < 0.05) and diffusion tensor imaging metrics (F(4.21) = 4.7, p < 0.01) and between Tower Test Mean First Move Time and diffusion tensor imaging metrics (F(4.21) = 7.9, p < 0.0005). Significant protected post hoc Spearman correlations within TD (Table 4) included those between Trail Making Test Visual Scanning and creatine (r = − 0.45, p < 0.05), fractional anisotropy (r = 0.53, p = 0.005), mean diffusivity (r = − 0.52, p < 0.005), and radial diffusivity (r = − 0.62, p = 0.001).
An analysis was performed to determine whether outliers potentially have impact on the correlation results presented in Table 4. The analysis calculated Cook’s distance for each data point that contributed to each of the results presented in Table 4. Cook’s distance is a commonly used estimate of the influence a particular data point has in a regression analysis. For all data points, Cook’s distance was less than 1, the traditionally used criterion for formally identifying the presence of an outlier that may be responsible for an incorrectly significant correlation. Furthermore, several of the correlations plotted in Fig. 4 illustrate there is a unlikely impact of non-normal distributions. Pearson and Spearman statistics are nearly equivalent.
Within-Group Correlations Between Magnetic Resonance Spectroscopy and Diffusion Tensor Imaging Metrics in Identical Anatomic Volumes
Within ADHD+PAE (Table 5), omnibus multivariate analysis of covariance of rank-transformed data found a significant relationship between magnetic resonance spectroscopy creatine and diffusion tensor imaging metrics (F(4.14) = 6.9, p = 0.001). There was a significant protected post-hoc Spearman correlation (Table 5) within ADHD+PAE between axial diffusivity and creatine (r = 0.68, p = 0.001).
Table 5.
Significant correlations between magnetic resonance spectroscopy neurometabolite levels and diffusion tensor imaging metrics in supraventricular white matter
| Participant group | Magnetic Resonance Spectroscopy Neurometabolite metric |
Diffusion Tensor Imaging Metric | Correlation r | Correlation p |
|---|---|---|---|---|
| ADHD+PAE | Total creatine | axial diffusivity | + 0.68 | 0.001 |
| ADHD-PAE | Glutamate | fractional anisotropy | + 0.49 | 0.047 |
| ADHD-PAE | Choline-compounds | fractional anisotropy | + 0.75 | 0.001 |
| ADHD-PAE | Choline-compounds | mean diffusivity | − 0.57 | 0.017 |
| ADHD-PAE | Choline-compounds | radial diffusivity | − 0.71 | 0.001 |
| TD | Glutamate | fractional anisotropy | − 0.42 | 0.033 |
| TD | Glutamate | radial diffusivity | + 0.39 | 0.046 |
Within ADHD-PAE, omnibus multivariate analysis of covariance found significant relationships between magnetic resonance spectroscopy glutamate and diffusion tensor imaging metrics (F(4.12) = 7.0, p < 0.005) and between choline and diffusion tensor imaging metrics (F(4.12) = 4.4, p < 0.05). There were significant protected post hoc Spearman correlations within ADHD-PAE between fractional anisotropy and glutamate (r = 0.49, p < 0.05; Fig. 5), fractional anisotropy and choline (r = 0.75, p = 0.001), mean diffusivity and choline (r = − 0.57, p < 0.05), and radial diffusivity and choline (r = − 0.71, p < 0.05; Fig. 5).
Fig. 5.
Selected correlations (including linear regression and Pearson and Spearman correlation coefficients and p-values between diffusion tensor imaging and magnetic resonance spectroscopy metrics in children with ADHD-PAE (blue triangles, upper panel) and in TD (green diamonds, lower panel). In ADHD-PAE, fractional anisotropy in supraventricular white matter increased with increasing glutamate (r = 0.49, p < 0.05). A similar relationship (not shown) was observed for fractional anisotropy and choline (r = 0.75, p = 0.001). In TD, in contrast, fractional anisotropy decreased with increasing glutamate (r = − 0.42, p < 0.05) and (not shown) with increasing creatine (r = − 0.49, p < 0.05). Radial diffusivity in ADHD+PAE decreased with increasing choline (r = − 0.71, p = 0.001). A similar relationship was observed for mean diffusivity and choline (r = − 0.57, p < 0.05). In TD, in contrast, radial diffusivity increased with increasing glutamate (r = 0.39, p < 0.05)
Within TD, omnibus multivariate analysis of covariance found a significant relationship between magnetic resonance spectroscopy glutamate and diffusion tensor imaging metrics (F(4.21) = 3.0, p < 0.05). There were significant protected post hoc Spearman correlations within TD between fractional anisotropy and glutamate (r = − 0.42, p < 0.05; Fig. 5) and between radial diffusivity and glutamate (r = 0.39, p < 0.05; Fig. 5).
A Cook’s distance analysis (see above) indicated it is unlikely that outliers are responsible for the significant correlations reported in Table 5. Furthermore, presentation of equivalent Spearman and Pearson results in Fig. 5 indicate the absence problematic non-normal distributions.
Discussion
Summary of Major Findings
This study combined ADHD and PAE targeted neurobehavioral assessment in combination with advanced magnetic resonance spectroscopic imaging and diffusion tensor imaging to differentiate ADHD+PAE from ADHD-PAE. In recent pilot work (O’Neill et al. 2019), we had found between-group magnetic resonance spectroscopy and diffusion tensor imaging differences in the anterior corona radiata. Here, we studied supraventricular white matter, a region that is larger and less precisely defined, but that would be more easily located by MRI technologists and would yield higher data quality in a routine MRI examination. The principal findings were as follows: (1) The Conners Hyperactivity/Impulsivity Index was greater in ADHD+PAE than ADHD-PAE and (2) supraventricular white matter glutamate was higher in ADHD+PAE than ADHD-PAE and was lower in ADHD-PAE than TD. In the same volume of supraventricular white matter, mean diffusivity, axial diffusivity, and radial diffusivity were higher in ADHD+PAE than in ADHD-PAE; and (3) Within ADHD+PAE, Trail Making Test Number-Letter Switching performance correlated with fractional anisotropy and radial diffusivity while, two scores on the Tower Test correlated with N-acetylaspartate, glutamate, and/or choline. Also, differing relationships between diffusion tensor imaging and magnetic resonance spectroscopy metrics in supraventricular white matter were obtained in the three groups. Taken together, these findings suggest the following: (1) There is subtly diverse and perhaps more severe white matter pathology in ADHD+PAE in comparison with ADHD-PAE. (2) The combination of diffusion tensor imaging, magnetic resonance spectroscopy and neurobehavioral assesstment offers potential to objectively distinguish ADHD+PAE from ADHD-PAE. (3) White matter abnormalities may underlie neurobehavioral symptoms in ADHD+PAE and ADHD-PAE.
Between-Group Differences in Neurobehavioral Metrics
The first major finding was that Conners Hyperactivity/Impulsivity was greater in ADHD+PAE than ADHD-PAE (Table 2; Fig. 3). As anticipated given participant selection criteria, inattention and hyperactivity/impulsivity were higher in both ADHD+PAE and ADHD-PAE than in TD. Our findings support prior notions that ADHD+PAE and ADHD-PAE differ in the relative expression of inattentive versus hyperactive/impulsive symptoms (Brown et al. 1991; Coles et al. 1997; O’Malley and Nanson 2002; Raldiris et al. 2018; Roebuck et al. 1999). While most previous publications favored greater inattention in ADHD+PAE, we, like Raldiris et al. (2018), found greater hyperactivity/impulsivity. Given this variability in results, future researchers should take care in generalizing across samples. This study restricted the ADHD-PAE sample to familial ADHD and, to our knowledge, this is the first formal comparison of ADHD+PAE to familial ADHD-PAE. Since inattention may be more heritable than hyperactivity in ADHD (Kuntsi et al. 2014), it is reasonable to anticipate lower severity of hyperactive symptoms in a familial ADHD-PAE than a non-familial ADHD+PAE sample. Further enquiry may delineate the circumstances under which inattentive versus hyperactive symptoms prevail in ADHD subpopulations.
In contrast to some prior investigations (Mattson et al. 2010, 2013; Nguyen et al. 2014), no executive function metrics differed significantly between ADHD+PAE and ADHD-PAE. This may have been due to the use of familial ADHD-PAE in our study. On the other hand, some executive function metrics were trendwise worse in ADHD+PAE (e.g., Verbal Fluency Category Switching, Fig. 3) and performance was worse for ADHD+PAE than for TD on six executive function metrics (Trails Letter Sequence and Number-Letter Switching, Verbal Fluency Letter Fluency and Category Switching, and Tower Test Total Achievement Score and Total Rule Violations) compared with only two (Tower Test Total Achievement Score and Total Rule Violations) for ADHD-PAE. In these ways, present findings are in harmony with the conclusion of earlier authors that executive functions are more impaired in ADHD+PAE than in ADHD-PAE. With continued exploration of different measures of executive functions, we may be better able to define independent neurobehavioral profiles for these two conditions.
Between-Group Differences in Neuroimaging Metrics
The second major finding was that glutamate, mean diffusivity, axial diffusivity, and radial diffusivity were higher in ADHD+PAE than ADHD-PAE (Table 3). Furthermore, glutamate and mean diffusivity were lower in ADHD-PAE than TD. The differences were small (< 10%), but their existence hints that combinations of neuroimaging and neurobehavioral metrics, and perhaps with further metrics (e.g., other types of neuroimaging and/or neuroimaging of other brain regions), could eventually yield an objective approach to differential diagnosis of these two ADHD etiologies without the aid of maternal informants or facial stigmata. Neurobiologically, these measures point towards distinct pathology in supraventricular white matter in ADHD+PAE versus ADHD-PAE.
Glutamate levels presented in the order ADHD-PAE < ADHD+PAE ≤ TD (Fig. 3). Low glutamate in ADHD-PAE is consistent with previous magnetic resonance spectroscopy studies (reviewed in O’Neill et al. 2013; Perlov et al. 2009) showing diminished glutamatergic compounds in various brain regions in idiopathic ADHD in children (Hai et al. 2017) and adults (Maltezos et al. 2014; Naaijen 2018; Perlov et al. 2007), although other investigations found elevated levels (Courvoisie et al. 2004; MacMaster et al. 2003; Moore et al. 2006) or no difference from controls (Endres et al. 2015; Soliva et al. 2010; Tafazoli et al. 2013). Variant results across these studies may stem from expression of results as glutamate + glutamine and/or as ratios to creatine in some reports; also, unlike our study, these earlier studies typically did not account for PAE or for familial versus non-familial ADHD. Low glutamate in ADHD-PAE is also consistent with hypoglutamatergic models of ADHD (Carlsson 2000, 2001; O’Neill et al. 2013). These theories focus on cortex, where glutamate is present in relatively high concentration, but this present study illustrates that magnetic resonance spectroscopy also readily measures glutamate alteration in white matter (e.g., Srinavasan et al. 2005) where, for example, it undergoes vesicular release from axons (Doyle et al. 2018; Kukley et al. 2007; Ziskin et al. 2007). Our results suggest that hypoglutamatergia exists in white matter specifically in ADHD-PAE. Hypoglutamatergic models interface with theories of brain-energy deficiency in ADHD (Russell et al. 2006; Todd and Botteron 2001). One of these models (Russell et al. 2006) postulates, in part, that expression of genes related to glutamate transporters, metabotropic glutamate receptors, or oligodendrocytic myelin synthesis, is altered in ADHD. This impairs myelination and cellular energy production leading to ADHD symptoms.
Mean diffusivity (Fig. 3), axial diffusivity, and radial diffusivity presented in the order ADHD-PAE ≤ TD < ADHD+PAE. Mean diffusivity represents average water diffusion across all directions in the sampled supraventricular white matter, while axial diffusivity and radial diffusivity measure diffusion parallel, respectively, perpendicular to the principal fiber direction. Mean diffusivity decreases with maturation as myelin develops and intracellular space fills in (Beaulieu 2002; Engelbrecht et al. 2002); mean diffusivity is higher for developmental or acquired defects (Neil et al. 2002). Axial diffusivity is higher near axonal damage and fragmentation (Concha 2014). Radial diffusivity is influenced by axon packing, axon diameter, and inflammation (Wheeler-Kingshott and Cercignani 2009); but is more strongly associated with myelin integrity (Aung et al. 2013; Concha 2014; Song et al. 2002). Elevated radial diffusivity is associated with demyelination (Janve et al. 2013; Schmierer 2007, 2008; Wang et al. 2015) and dysmyelination (Harsan et al. 2006; Song et al. 2002) and is present in normal appearing but injured white matter (Budde et al. 2007). There are (more frequent) findings of above-normal and (less common) findings of below-normal mean diffusivity, axial diffusivity, and radial diffusivity in idiopathic ADHD (Adisetiyo et al. 2014; Aoki et al. 2018; Chen et al. 2015; Lei et al. 2014; Nagel et al. 2011; Onnink et al. 2015; Svatkova et al. 2016; Tamm et al. 2012; van Ewijk et al. 2014). The possible covert PAE in such samples is scarcely accounted for. In FASD, mainly mean diffusivity has been measured with elevated values being the prevalent finding (Fryer et al. 2009; Lebel et al. 2008; Ma et al. 2005; Wozniak et al. 2006; Wozniak and Muetzel 2011). Cross-sectionally, abnormalities in axial diffusivity and mean diffusivity have been found in newborns (Donald et al. 2015; Taylor et al. 2015), while children over 5 years old demonstrated elevated mean diffusivity and radial diffusivity (Sherbaf et al. 2019), indicating axonal damage in newborns and oligodendrocyte damage in older children (Sherbaf et al. 2019). Our present results in ADHD+PAE are consistent with the foregoing literature. High mean diffusivity, axial diffusivity, and radial diffusivity in our ADHD+PAE participants may therefore be signals of abnormal neurodevelopment in FASD, including fewer axons, thinner axons, and dysmyelination.
Within-Group Correlations Between Neurobehavioral and Neuroimaging Metrics
The third major finding involved relations between Trail Making performance and diffusion tensor imaging metrics, between Tower Test performance and magnetic resonance spectroscopy metabolite levels and between magnetic resonance spectroscopy and diffusion tensor imaging metrics (Tables 4 and 5). Unlike our pilot (O’Neill et al. 2019), group-mean differences in fractional anisotropy were not observed between ADHD+PAE and ADHD-PAE; possibly these are restricted to the anterior corona radiata. Within ADHD+PAE, however, Trails Number-Letter Switching (Trails B) performance was better for higher fractional anisotropy and worse for higher radial diffusivity. The Trail Making Test largely interrogates cognitive speed and fluidity (Salthouse 2011) or, alternatively, motor performance, selective attention, working memory, and cognitive flexibility (Koch et al. 2013). Thereby, Number-Letter Switching is the more challenging of the two principal test conditions. We are unaware of prior diffusion tensor imaging studies of the Trail Making Test specifically in ADHD or FASD. In healthy controls (Koch et al. 2013; Kucukboyaci et al. 2012; Ohtani et al. 2017), however, and in multiple disorders (Genova et al. 2013; Konrad et al. 2012; Kourtidou et al. 2013; Liu et al. 2017; Matsui et al. 2014; Pérez-Iglesias et al. 2010) superior performance on various Trails metrics accompanied higher fractional anisotropy or, in a few cases, lower values of the more seldom analyzed indices mean diffusivity or radial diffusivity (Kourtidou et al. 2013; Matsui et al. 2014), but there are few studies in children. Within pediatric ADHD+PAE, present results associate lower fractional anisotropy and higher radial diffusivity with inferior cognitive flexibility, although we did not see this in our pediatric ADHD-PAE sample. In our TD child sample, the cognitively less challenging Trail Making Test Visual Scanning score increased with fractional anisotropy, decreased with mean diffusivity and radial diffusivity, and decreased with glutamate and creatine.
Within ADHD+PAE, the Tower Test Total Achievement Score (higher for better performance) correlated negatively with glutamate (Fig. 4), creatine, and choline and Mean First Move Time (lower for better performance) correlated positively with N-acetylaspartate (Fig. 4) and glutamate. The Tower Test measures the cognitive function of “planning” (Phillips et al. 2001; Shallice et al. 1982). It provides an index of executive functions such as establishing and maintaining instructional set, rule learning, spatial planning, and response inhibition (Homack et al. 2005). These functions may underlie key abnormal behaviors associated with PAE such as difficulty following rules, problem solving (Green et al. 2009; Kodituwakku et al. 1995; Mattson et al. 1999), self-monitoring behavior, and controlling impulses (Mattson and Riley 1998). We are aware of no previous studies that examined the relation between magnetic resonance spectroscopy and Tower Test metrics. Higher levels of the magnetic resonance spectroscopy neurometabolites in white matter may represent a preponderance of axons and, thus indirectly, undermyelination of nervous tissue in support of the above-listed symptoms. For both Trails and the Tower Test, scores did not differ significantly between ADHD+PAE and ADHD-PAE, but relationships of these scores with neuroimaging metrics did. In this indirect sense, the two tests may potentially discriminate between the ADHD etiologies (Mattson et al. 2010, 2013; Nguyen et al. 2014).
Glutamate, in addition to differing between ADHD+PAE and ADHD-PAE, correlated with certain executive function measures in ADHD+PAE or in TD. In FASD, magnetic resonance spectroscopy has previously identified glutamate effects in parietal white matter in neonates (Howells et al. 2016) or in a cerebellar voxel containing mostly white matter in children (du Plessis et al. 2014). Although glutamate levels did not differ between ADHD+PAE and TD, glutamate may yet play a role in white matter pathology in PAE. Howells et al. (2016) pointed out that glutamate triggers myelination by activating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors on immature oligodendrocytes (Karadottir and Attwell 2006). AMPA and NMDA receptor expression arises following neuregulin and brain-derived neurotrophic factor (BDNF) release (Lundgaard et al. 2013) which may be attenuated in FASD (Balaszczuk et al. 2013). Undermyelination may contribute to ADHD symptoms in these children, a mechanism that may not apply in familial ADHD-PAE. The preclinical literature, while rarely measuring gross-tissue metabolite levels as in human in vivo magnetic resonance spectroscopy, does demonstrate long-term abnormalities of ionotropic and metabotropic glutamate receptors following PAE (Costa et al. 2000; Dettmer et al. 2003; Galindo et al. 2004; Valenzuela et al. 2010). Even moderate PAE alters NMDA receptor subunit composition, negatively affecting long term potentiation (Brady et al. 2013); and AMPA receptors, involved in fast glutamatergic neurotransmission critical for memory formation (Hayashi et al. 2007; Kawabe et al. 2007). The effects are dependent upon the amount and timing of the dose, and consequently result in heterogeneous findings throughout the brain (Costa et al. 2000; Valenzuela et al. 2010). Given that NMDA and AMPA receptors have altered sensitivity to glutamate in PAE, even normal glutamate levels may impact neurotransmission negatively. This may explain why stimulant medications, which increase glutamate availability (White et al. 2018) are not as efficacious in ADHD+PAE as in ADHD-PAE; while glutamate-receptor modulators such as aniracetam (Black 2005; Ito et al. 1990) which slows receptor deactivation (Jin et al. 2005; Suppiramaniam et al. 2001) improved learning and memory in juvenile animals with PAE (Vaglenova et al. 2007; Vaglenova and Petkov 2001). Continued clinical and preclinical investigation of glutamate in PAE may reveal additional therapeutic promise.
Choline findings were less prominent in this study than in some prior FASD magnetic resonance spectroscopy studies (Astley et al. 1995, 2009; Cortese et al. 2006; du Plessis et al. 2014; Fagerlund et al. 2006; Gonçalves et al. 2009). Specifically, the low choline found in anterior corona radiata in our pilot (O’Neill et al. 2019) was not identified in supraventricular white matter more broadly. Choline, however, was a factor in correlations with neurobehavioral or diffusion tensor imaging metrics. The potential of choline therapies for PAE remains unclear and thereby choline warrants continued investigation with magnetic resonance spectroscopy.
N-acetylaspartate and creatine were involved in relatively few statistically significant correlations. Effects of FASD on N-acetylaspartate have been detected in some prior investigations (Cortese et al. 2006; du Plessis et al. 2014; Fagerlund et al. 2006). Cortese et al.’s observation of elevated N-acetylaspartate to creatine ratio in FASD was in left caudate, a nucleus not sampled in the present investigation. du Plessis et al. (2014) measured below-normal N-acetylaspartate in FASD in deep cerebellar nuclei and surrounding white matter, again a region not sampled here. Alongside frontal and parietal cortices, the thalamus, and the cerebellar dentate, Fagerlund et al. (2006) did find below-normal N-acetylaspartate to choline ratio and N-acetylaspartate to creatine ratio in frontal white matter in FASD. They attributed their findings to elevated creatine and choline rather than to diminished N-acetylaspartate in FASD. Our study, benefitting from more participants and more advanced magnetic resonance spectroscopy technology, failed to find group-mean differences in N-acetylaspartate, creatine, or choline, although there were correlations with executive function involving each of these metabolites. We identified no prior study citing effects of FASD on creatine, although several studies used creatine as a denominator in forming ratios. Given the creatine-related effects reported in Fagerlund et al. (2006) and here, caution is advised in interpreting such findings.
Interestingly, the correlations between diffusion tensor imaging and magnetic resonance spectroscopy metrics differed across groups (Table 5, Fig. 5). fractional anisotropy, for example, increased with glutamate (and choline) in ADHD-PAE but decreased with glutamate (and creatine) in TD; mean diffusivity correlated positively with choline in ADHD+PAE, but negatively in TD. Perhaps this reflects variant composition of white matter across the groups, e.g., in terms of preponderance of axons vs. myelin vs. fluid space.
Limitations
Limitations of this study include a modest sample size. The total number of subjects reported our study is 70 (23 ADHD+PAE, 19 ADHD-PAE, 28 TD). This is larger than many of the previous studies that are discussed above. However, a few studies that used only magnetic resonance spectroscopy (Astley et al. 2009) or only diffusion tensor imaging (Treit et al. 2017; Lebel et al. 2008) were larger. Ours is thereby the largest study that used both methods. Our sample size is too small to make conclusions regarding the impact of gender, subject age and treatments. Our ADHD+PAE group was 1.0–1.5 years younger than the other two groups. As stated above, ADHD+PAE has earlier onset, which shifts the recruitment pool to younger ages. Although all metrics were adjusted for age, ideally all groups would be age-matched. Use of psychotropic medication was more frequent in the ADHD+PAE than in the ADHD-PAE group (Table 1). Behavioral symptoms are often quite florid in ADHD+PAE, leading to drug treatment, even though such treatment is less efficacious. By far, the most commonly prescribed medications in our ADHD+PAE sample were stimulants and adrenergic agents such as guanfacine and atomoxetine. Participants were asked not to take stimulant medications on the days of scan or assessment. Still, a better comparison would be with an equally medicated ADHD-PAE group. Our analysis was restricted to supraventricular white matter. It would be valuable to explore further white matter regions as well as cortex and subcortical nuclei using similar methods. The magnetic resonance spectroscopic imaging technology used in this study sampled a substantial amount of gray matter adjacent to the interhemispheric fissure. An evaluation of magnetic resonance spectroscopy findings in gray matter for the three subject groups is currently underway.
Conclusions
In summary, pediatric ADHD associated with prenatal alcohol exposure may be distinguished from familial ADHD by greater hyperactivity and higher glutamate and mean diffusivity, axial diffusivity, and radial diffusivity in supraventricular white matter. Within ADHD+PAE, scores on the Trail Making Test correlate with fractional anisotropy and radial diffusivity while performance on the Tower Test correlate with certain magnetic resonance spectroscopy neurometabolite levels. The relationships between diffusion tensor imaging and magnetic resonance spectroscopy metrics also differ between ADHD+PAE, ADHD-PAE, and TD. These results suggest distinct white matter pathology in ADHD+PAE versus ADHD-PAE with impact on executive functions. It may be feasible to leverage the present neurobehavioral and neuroimaging effects to implement objective algorithms for differential diagnosis of these two etiologies of ADHD leading to more specific and effective treatments. Furthermore the combination of advanced neuroimaging and neurobehaviorial assessment is expected to contribute to a better understanding of the neuropathology associated with ADHD subtypes in general.
Funding
This research was supported by grant NIAAA R01 AA025066 (PIs: M. J. O’Connor and J. G. Levitt) from the National Institutes of Health.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Adisetiyo V, Tabesh A, Di Martino A, Falangola MF, Castellanos FX, Jensen JH, Helpern JA (2014) Attention-deficit/hyperactivity disorder without comorbidity is associated with distinct atypical patterns of cerebral microstructural development. Hum Brain Mapp 35:2148–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger JR, Stanovich J, Lai J, Armstrong C, Feusner JD, Levitt J, O’Neill J (2016) Performance validation of a new software package for analysis of 1H-MRS. ISMRM Workshop on MR Spectroscopy: From Current Best Practice to Latest Frontiers. Lake Constance, Germany, 14-17 August 2016 [Google Scholar]
- American Psychiatric Association; (2013) Diagnostic and statistical manual of mental disorders, 5th edn. Arlington, VA [Google Scholar]
- Aoki Y, Cortese S, Castellanos FX (2018) Research review: Diffusion tensor imaging studies of attention-deficity/hyperactivity disorder: meta-analyses and reflections on head motion. J Child Psychol Psychiatry 59(3):193–202 [DOI] [PubMed] [Google Scholar]
- Astley SJ, Weinberger E, Shaw DW, Richards TL, Clarren SK (1995) Magnetic resonance imaging and spectroscopy in fetal ethanol exposed Macaca nemestrina. Neurotoxicol Teratol 17(5):523–530 [DOI] [PubMed] [Google Scholar]
- Astley SJ, Richards T, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowik T, Kraegel P, Maravilla K (2009) Magnetic resonance spectroscopy outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Magn Reson Imaging 27:760–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung WY, Mar S, Benzinger TL (2013) Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med 5:427–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaszczuk V, Bender C, Pereno G, Beltramino CA (2013) Binge alcohol-induced alterations in BDNF and GDNF expression in central extended amygdala and pyriform cortex on infant rats. Int J Devel Neurosci 31:287–296 [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D, (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–455 [DOI] [PubMed] [Google Scholar]
- Black MD (2005) Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits A review of preclinical data. Psychopharmacology 179:154–163 [DOI] [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK (2013) Moderate Prenatal Alcohol Exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci 33:1062–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK (2007) Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57:688–695 [DOI] [PubMed] [Google Scholar]
- Carlsson ML (2000) On the role of cortical glutamate in obsessive-compulsive disorder and attention deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr Scand 102:401–413 [DOI] [PubMed] [Google Scholar]
- Carlsson ML (2001) On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive-compulsive disorder and attention deficit hyperactivity disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 25:5–26 [DOI] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. Fetal alcohol syndrome: guidelines for referral and diagnosis National Center on Birth Defects and Developmental Disabilities Department of Health and Human Services. 2004.
- Chen L, Huang X, Lei D, He N, Hu X, Chen Y, Li Y, Zhou J, Guo L, Kemp GJ, Gong QY (2015) Microstructural abnormalities of the brain white matter in attention-deficit/hyperactivity disorder. J Psychiatry Neurosci 40:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff R, Combs-Orme T, Risley-Curtiss C, Heisler A (1994) Assessing the health status of children entering foster care. Pediatrics 93:594–601 [PubMed] [Google Scholar]
- Coles CD (2001) Fetal alcohol exposure and attention: moving beyond ADHD. Alcohol Res Health 25:199–203 [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith, Platzman KA, Falek A (1991) Effects of prenatal alcohol exposure and at school age. I. Physical and cognitive development. Neurotoxicol Teratol 13:357–367 [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE (1997) A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 21:150–161 [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD, CIFASD (2015) Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Maternal Child Health J 19:2605–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L (2014) A macroscopic view of microstructure: using diffusion-weighted images to infer damage, repair, and plasticity of white matter. Neurosci 276:14–28 [DOI] [PubMed] [Google Scholar]
- Conners CK, Pitkanen J, Rzepa SR (2011) Conners 3rd Edition (Conners3; Conners 2008). In Kreutzer JS, DeLuca J, & Caplan B (Eds.). Encyclopedia of clinical neuropsychology. NY: Springer. [Google Scholar]
- Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Black V, Hannigan JH (2006) Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: preliminary findings in the caudate nucleus. Neurotoxicol Teratol 28:597–606 [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF (2000) A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res 24:706–715 [PubMed] [Google Scholar]
- Courvoise H, Hooper SR, Fine C, Kwock L, Castillo M (2004) Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: preliminary findings. J Neuropsychiatry Clin Neurosci 16:63–69 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001) Manual for the Delis-Kaplan Executive Function System. Psychological Corporation, San Antonio, TX [Google Scholar]
- Dettmer TS, Barnes A, Iqbal U, Bailey CD, Reynolds JN, Brien JF, Valenzuela CF (2003) Chronic prenatal ethanol exposure alters inoptropic gluatamate receptor subunit protein levels in the adult guinea pig cerebral cortex. Alcohol Clin Exp Res 27:677–681 [DOI] [PubMed] [Google Scholar]
- Doig J, McLennan JD, Gibbard WB (2008) Medication effects on symptoms of attention-deficit/hyperactivity disorder in children with fetal alcohol spectrum disorder. J Child Adolesc Psychopharmacol 18:365–371 [DOI] [PubMed] [Google Scholar]
- Donald KA, Roos A, Fouche JP, Koen N, Howells FM, Woods RP, Zar HJ, Narr KL, Stein DJ (2015) A study of the effects of prenatal alcohol exposure on white matter microstructural integrity at birth. Acta Neuropsychiatrica 27:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S, Hansen DB, Vella J, Bond P, Harper G, Zammit C, Valentino M, Fern R (2018) Vesicular glutamate release from central axons contributes to myelin damage. Nat Commun 9:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis L, Jacobson JL, Jacobson SW, Hess AT, van der Kouwe A, Avison MJ, Molteno CD, Stanton ME, Stanley JA, Peterson BS, Meintjes EM (2014) An in vivo (1)H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 38:1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D, Perlov E, Maier S, Feige B, Nickel K, Goll P, Bubl E, Lange T, Glauche V, Graf E, Ebert D, Sobanski E, Philipsen A, Tebartz van Elst L (2015) Normal neurochemistry in the prefrontal and cerebellar brain of adults with Attention-Deficit Hyperactivity Disorder. Front Behav Neurosci 9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht V, Scherer A, Rassek M, Witsack HJ, Modder U (2002) Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiol 222:410–418 [DOI] [PubMed] [Google Scholar]
- Fagerlund A, Heikkinen S, Autti-Rämö I, Korkman M, Timonen M, Kuusi T, Riley EP, Lundbom N (2006) Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 30:2097–2104 [DOI] [PubMed] [Google Scholar]
- Frankel F, Paley B, Marquardt R, O’Connor M (2006) Stimulants, neuroleptics, and children’s friendship training for children with fetal alcohol spectrum disorders. Child Adolesc Psychopharmacol 16:777–789 [DOI] [PubMed] [Google Scholar]
- Friedrich P, Fraenz C, Schlüter C, Oclenburg S, Mädler B, Güntürkün O, Genç E (2020) The relationship between axon density, myelination, and fractional anisotropy in the human corpus callosum. Cereb Cortex 30:2042–2056 [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN (2007) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119:733–741 [DOI] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP (2009) Characterization of white matter microstructure in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 33:514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo R, Frausto S, Wolff C, Caldwell KK, Perrone-Bizzozero NI, Savage DD (2004) Prenatal ethanol exposure reduces mGluR5 receptor number and function in the dentate gyrus of adult off-spring. Alcohol Clin Exp Res 28:1587–1597 [DOI] [PubMed] [Google Scholar]
- Genova HM, DeLuca J, Chiaravalloti N, Wylie G (2013) The relationship between executive functioning, processing speed and white matter integrity in multiple sclerosis. J Clin Exp Neuropsychol 35:631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass L, Ware AL, Crocker N, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN (2013) Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Neuropsychological deficits associated with heavy prenatal alcohol exposure are not exacerbated by ADHD. Neuropsychology 27:713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass L, Ware AL, Mattson SN (2014) Neurobehavioral, neurologic, and neuroimaging characteristics of fetal alcohol spectrum disorders. Handb Clin Neurol 125:435–462 [DOI] [PubMed] [Google Scholar]
- Gonçalves R, Vasconcelos MM, Faleiros LO, Cruz LC Jr, Domingues RC, Brito AR, Werner J Jr, Herdy GV (2009) Proton magnetic resonance spectroscopy in children with fetal alcohol spectrum disorders. Arq Neuro-Psiquiatria 67:254–261 [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN (2009) Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry 50:688–697 [DOI] [PubMed] [Google Scholar]
- Green CR, Lebel C, Rasmussen C, Beaulieu C, Reynolds JN (2013) Diffusion tensor imaging correlates of saccadic reaction time in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 37:1499–1507 [DOI] [PubMed] [Google Scholar]
- Hai T, Duffy H, Lemay JF, Swansburg R, Climie EA, MacMaster FP (2017) Neurochemical correlates of executive function in children with Attnetion-Deficit Hyperactivity Disorder. J Can Acad Child Adolesc Psychiatry 29:15–25 [PMC free article] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O’Neill J, Alger JR, Luders E, Philips OR, Caplan R, Toga AW, McCracken J, Narr KL (2008) Reduced white matter integrity in attention-deficit hyperactivity disorder. NeuroReport 19:1705–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Boehm N, Grucker D, Ghandour MS (2006) Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 83:392–402 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Yoshihara T, Ichitani Y (2007) Involvement of hippocampal metabotropic glutamate receptors in radial maze performance. NeuroReport 18:719–723 [DOI] [PubMed] [Google Scholar]
- Homack S, Lee D, Riccio CA (2005) Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol 27:599–609 [DOI] [PubMed] [Google Scholar]
- Howells FM, Donald KA, Rood A, Woods RP, Zar HZ, Narr KL, Stein DJ (2016) Reduced glutamate in white matter of male neonates exposed to alcohol in utero: a 1H-magnetic resonance spectroscopy study. Metab Brain Dis 31:1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley MA, Marais A, May PA (2016) Updated clinical guidelines for diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138:2015–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Tanabe S, Kohda A, Sugiyama H (1990) Allosteric potentiation of quisqualate receptors by a nootropic drug airacetam. J Physiol 424:533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janve VA, Zu Z, Yao SY, Li K, Zhang FL, Wilson KJ, Ou X, Does MD, Subramaniam S, Gochberg DF (2013) The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis lesions. Neuroimage 74:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM (2005) Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci 25:9027–9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, Kulikovsky Y, Wertelecki W, Pedersen TL, Chambers CD, CIFASD, (2015) The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol 49:647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for affective disorders and schizphrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988 [DOI] [PubMed] [Google Scholar]
- Káradóttir R, Attwell D (2006) Combining patch-clamping of cells in brain slices with immunocytochemical labeling to define cell type and developmental stage. Nature Protocols 1:1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe K, Iwasaki T, Ichitani Y (2007) Repeated treatment with N-methyl-d-aspartate antagonists in neonatal, but not adult, rats causes long-term deficits of radial-arm maze learning. Brain Res 1169:77–86 [DOI] [PubMed] [Google Scholar]
- Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD (2010) The plausibility of maternal nutritional status being a contribut-ing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. BioFactors 36:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CE, Thompson DK, Chen J, Leemans A, Adamson CL, Inder TE, Cheong JL, Doyle LW, Anderson PJ (2016) Axon density and axon orientation dispersion in children born preterm. Hum Brain Mapp 9:3080–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DR, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC (2012) Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neuobiiol Aging 33:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Wagner G, Schachtzabel C, Schultz CC, Güllmar D, Reichenbach JR, Sauer H, Schlösser RGM, (2013) Age-dependent visuomotor performance and white matter structure: a DTI study. Brain Struct and Func 218:1075–1084 [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD (1995) Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res 19:1558–1564 [DOI] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Lorscheider M, Bernow N, Thümmel M, Chai C, Pfeifer P, Stoeter P, Scheurich A, Fehr C (2012) Broad Disruption of Brain White Matter Microstructure and Relationship with Neuropsychological Performance in Male Patients with Severe Alcohol Dependence. Alcohol Alcoholism 47:118–126 [DOI] [PubMed] [Google Scholar]
- Kourtidou P, McCauley SR, Bigler ED, Traipe E, Wu TC, Chu ZD, Hunter JV, Li X, Levin HS, Wilde EA (2013) Centrum semiovale and corpus callosum integrity in relation to information processing speed in patients with severe traumatic brain injury. J Head Trauma Rehab 28:433–441 [DOI] [PubMed] [Google Scholar]
- Kucukboyaci NE, Girard HM, Hagler DJ Jr, Kuperman J, Tecoma ES, Iragui VJ, Halgren E, McDonald CR (2012) Role of fronto-temporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. J Int Neuropsychol Soc 18:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D (2007) Vesicular glutamate release from axons in white matter. Nat Neurosci 10:311–320 [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, Moffitt TE (2004) Co-occurrence of ADHD and low IQ has genetic origins. Am J Med Gen Part B (Neuropsychiatric Gen) 124B:41–47 [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, Asherson P (2014) The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. J Abnorm Child Psychol 42:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova. (2017) Global prevalence of fetal alcohol spectrum disorder among children and youth: a systematic review and meta-analysis. JAMA Pediatr 171:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O’Neill J, Alger J, Narr KL (2013) White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J Am Acad Child Adolesc Psychiatry 52:431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C (2008) Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 32:1732–1740 [DOI] [PubMed] [Google Scholar]
- Lei D, Ma J, Du X, Shen G, Jin X, Gong Q (2014) Microstructural abnormalities in the combined and inattentive subtypes of attention deficit hyperactivity disorder: a diffusion tensor imaging study. Scientific Rep 4:6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang P, Yin L, Shu N, Zhao T, Xing Y, Li F, Zhao Z, Li K, Han Y (2017) White Matter Abnormalities in Two Different Subtypes of Amnestic Mild Cognitive Impairment. PLOS ONE 12 (1):e0170185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HOB, Franklin RJM, ffrench-Constant C, Attwell D, Káradóttir RT (2013) Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLoS Biology 11 (12):e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Weibman D, Halperin JM, li X, (2019) A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front Hum Neurosci 13:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, Hu X (2005) Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res 29:1214–1222 [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Carrey N, Sparkes S, Kusumakar V (2003) Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol Psychiat 53:184–187 [DOI] [PubMed] [Google Scholar]
- Mädler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL (2008) Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging 26:874–888 [DOI] [PubMed] [Google Scholar]
- Maltezos S, Horder J, Coghlan S, Skirrow C, O’Gorman R, Lavender TJ, Mendez MA, Mehta M, Daly E, Xenitidis K, Paliokosta E, Spain D, Pitts M, Aherson P, Lythgoe DJ, Barker GJ, Murphy DG (2014) Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study. Transl Psychiatry 4:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt CB (2009) Non-linear least squares fitting in IDL with MPFIT. arXiv 0902:2850 [Google Scholar]
- Matsui JT, Vaidya JG, Johnson HJ, Magnotta VA, Long JD, Mills JA, Lowe MJ, Sakaie KE, Rao SM, Smith MM, Paulsen JS (2014) Diffusion weighted imaging of prefrontal cortex in prodromal Huntington’s Disease. Hum Brain Mapp 35:1562–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP (1998) A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res 22:279–294 [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EO (1999) Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 23:1808–1815 [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund Å, Autti–Rämö I, Jones KL, May PA, the CIFASD (2010) Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 34:1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT (2011) Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev 21:81–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP, CIFASD (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KE, Jones KL, Hoyme HE (2018) Prevalence of fetal alcohol spectrum disorders in 4 US communities. J Am Med Assoc 319:474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Biederman J, Wozniak J, Mick E, Aleardi M, Wardrop M, Renshaw PF (2006) Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: a proton magnetic resonance spectroscopy study. Am J Psychiatry 163:316–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Hong DS, Shepard S, Moore T (2017) Linking ADHD to the neural circuitry of attention. Trends Cog Sci 21:474–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaijen J, Lythgoe DJ, Zwiers MP, Hartman CA, Hoekstra PJ, Buitelaar JK, Aarts E (2018) Anterior cingulate cortex glutamate and its association with striatal functioning during cognitive control. Eur Neuropsychophrmacol 28:381–391 [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT (2011) Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adol Psychiatry 50:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J, Miller J, Mukherjee P, Huppi PS (2002) Diffusion tensor imaging of normal and injured developing human brain: a technical review. NMR Biomed 15:543–552 [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Glass L, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD, (2014) The clinical utility and specificity of parent report of executive function among children with prenatal alcohol exposure. J Int Neuropsychol Soc 20:704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD (2016) Randomized, double-blind, placebo controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. American J Clin Nutrition 104:1683–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ (2014) Mental health outcomes associated with prenatal alcohol exposure: genetic and environmental factors. Curr Dev Disord Rep. 10.1007/s40474-014-0021-7 [DOI] [Google Scholar]
- Oesterheld JR, Kofoed L, Tervo R, Fogas B, Wilson A, Fiechtner H (1998) Effectiveness of methylphenidate in Native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: a controlled pilot study. J Child Adolesc Psychopharmacol 8:39–48 [DOI] [PubMed] [Google Scholar]
- Ohtani T, Nestor PG, Bouix S, Newell D, Melonakos ED, McCarley RW, Shenton ME, Kubicki M (2017) Exploring the neural substrates of attentional control and human intelligence: Diffusion tensor imaging of prefrontal white matter tractography in healthy cognition. Neurosci 341:52–60 [DOI] [PubMed] [Google Scholar]
- O’Malley KD, Nanson J (2002) Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Can J Psychiatr 47:349–354 [DOI] [PubMed] [Google Scholar]
- O’Neill J, Levitt JG, Alger JR (2013) Magnetic resonance spectroscopy studies of attention deficit hyperactivity disorder. In: Blüml S, Panigrahy A (eds) MR spectroscopy of pediatric brain disorders. Springer, NY, pp 229–276 [Google Scholar]
- O’Neill J, O’Connor M, Yee V, Ly R, Narr K, Alger J, Levitt J (2019) Differential neuroimaging indices in prefrontal white matter in prenatal alcohol-associated ADHD versus idiopathic ADHD. BDR 111:797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Dammers J, Kan CC, Vasquez AA, Schene AH, Buitelaar J, Franke B (2015) Deviant white matter structure in adults with attention-deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog Neuro-Psychopharmacol Biol Psychiatry 63:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peadon E, Rhys-Jones B, Bower C, Elliott EJ (2009) Systematic review of interventions for children with Fetal Alcohol Spectrum Disorders. BMC Pediatr 9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Iglesias R, Tordesillas-Gutiérrez D, McGuire PK, Barker GJ, Roiz-Santiañez R, Mata I, de Lucas ME, Rodríguez-Sánchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B (2010) White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry 167:451–458 [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Hesslinger B, Buechert M, Ahrendts J, Feige B, Bubl E, Hennig J, Ebert D, Tebartz van Elst L (2007) Reduced cingulate glutamate/glutamine-to-creatine ratios in adult patients with attention deficit/hyperactivity disorder – a magnet resonance spectroscopy study. J Psychiatr Res 41:934–941 [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Matthies S, Drieling T, Maier S, Bubl E, Buechert M, Henning J, Ebert D, Tebartz van Elst L (2009) Spectroscopic findings in attention-deficit/hyperactivity disorder: review and meta-analysis. World J Biol Psychiatry 10:355–365 [DOI] [PubMed] [Google Scholar]
- Phillips LH, Wynn VE, McPherson S, Gilhooly KJ (2001) Mental planning and the Tower of London task. Quar J Exp Psychol, Section A 54:579–597 [DOI] [PubMed] [Google Scholar]
- Quattlebaum J, O’Connor MJ (2013) Higher-functioning children with prenatal alcohol exposure: Is there a specific neurocognitive profile? Child Neuropsychol 18:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C (2014) A Guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res 39:1–36 [DOI] [PubMed] [Google Scholar]
- Raldiris TL, Bowers TG, Towsey C (2018) Comparisons of intelligence and behavior in children with fetal alcohol spectrum disorder and ADHD. J Attn Disord 22:959–970 [DOI] [PubMed] [Google Scholar]
- Rasmussen C (2005) Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 29:1359–1367 [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP (1999) Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res 23:1070–1076 [PubMed] [Google Scholar]
- Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, Sagvolden T (2006) Response variability in Attention-Deficit/Hyperactivity Disorder: a neuronal and glial energetics hypothesis. Behav Brain Func 2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD (2008) Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res 1237:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T (2011) What cognitive abilities are involved in trailmaking performance? Intelligence 39:222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CAM, Boulby PA, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH (2007) Diffusion tensor imaging of post mortem multiple sclerosis. Brain NeuroImage 35:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CAM, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH (2008) Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med 59:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD (2016) Adolescent choline supplementation attenuates working memory deficits in rats exposed to alcohol during the third trimester equivalent. Alcohol Clin Exp Res 40:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T (1982) Specific impairments of planning. Phil Trans Roy Soc London. Series B, Biol Sciences 298:199–209 [DOI] [PubMed] [Google Scholar]
- Sherbaf FG, Aarabi MH, Yazdi MH, Haghshomar M (2019) White matter microstructure in fetal alcohol spectrum disorders: a systematic review of diffusion tensor imaging studies. Hum Brain Mapp 40:1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J, Nanson J, Snyder R, Block G (1997) A study of stimulant medication in children with FAS. In: Streissguth A, Kanter J (eds) Overcoming and Preventing Secondary Disabilities in Fetal Alcohol Syndrome and Fetal Alcohol Effects. University of Washington Press, Seattle, WA, pp 64–77 [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA, (1998) Automated spectral analysis III: Application to in vivo proton MR Spectroscopy and spectroscopic imaging. Magn Reson Med 40:822–831 [DOI] [PubMed] [Google Scholar]
- Soliva JC, Morena A, Fauguet J, Bielsa A, Carmona S, Gispert JD, Rovira M, Bulbena A, Vilarroya O (2010) Cerebellar neurometabolite abnormalities in pediatric attention/deficit hyperactivity disorder: a proton MR spectroscopic study. Neurosci Lett 470:60–64 [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436 [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722 [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140 [DOI] [PubMed] [Google Scholar]
- Srinavasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D (2005) Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 128:1016–1025 [DOI] [PubMed] [Google Scholar]
- Streissguth AP, O’Malley K (2000) Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semi Clin Neuropsychiatry 5:177–190 [DOI] [PubMed] [Google Scholar]
- Suppiramaniam V, Bahr BA, Sinnarajah S, Owens K, Rogers G, Yilma S et al. (2001) Member of the Ampakine class of memory enhancers prolongs the single channel open time of reconstituted AMPA receptors. Synapse 40:154–158 [DOI] [PubMed] [Google Scholar]
- Svatkova A, Nestrasil I, Rudser K, Goldenring Fine J, Bledsoe J, Semrud-Clikeman M (2016) Unique white matter microstructural patterns in ADHD presentations-a diffusion tensor imaging study. Hum Brain Mapp 37:3323–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafazoli S, O’Neill J, Bejjani A, Ly R, Salamon N, McCracken JT, Alger JR, Levitt JG (2013) 1H MRSI of middle frontal gyrus in pediatric ADHD. J Psychiatr Res 47:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Barnea-Goraly N, Reiss AL (2012) Diffusion tensor imaging reveals white matter abnormalities in attention-deficit/hyperactivity disorder. Psychiatry Res 202:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, Meintjes EM (2015) A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum Brain Mapp 36:170–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Tran TD (2012) Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus 22:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP (2000) Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol 22:703–711 [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM (2004) Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol 26:35–45 [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD (2007) Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci 121:120–130 [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD (2009) Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol 31:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, Botteron KN (2001) Is attention-deficit/hyperactivity disorder an energy deficiency syndrome? Biol Psychiatry 50:151–158 [DOI] [PubMed] [Google Scholar]
- Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, Alexander L, Gill M, Birmaher B, Sylvester R, Rice D, Deep A, Kaufman J (2020) Development of three Web-based computerized versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary validity data. J Am Acad Child Adol Psychiatry 59:309–325 [DOI] [PubMed] [Google Scholar]
- Treit S, Chen Z, Zhou D, Baugh L, Rasmussen C, Andrew G, Pei J, Beaulieu C (2017) Sexual dimorphism of volume reduction but not cognitive deficit in fetal alcohol spectrum disorders: a combined diffusion tensor imaging, cortical thickness and brain volume study. Neuroimage Clin 15:284–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Petkov VV (2001) Can nootropic drugs be effective against the impact of ethanol teratogenicity on cognitive performance? Eur Neuropsychopharm 11:33–40 [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Pandiella N, Wijayawardhane N, Vaithianathan T, Birru S, Breese C, Suppiramaniam V, Randal C (2007) Aniracetam reversed learning and memory deficits following prenatal ethanol exposure by modulating functions of synaptic AMPA receptors. Neuropsychopharmacology 33:1071–1083 [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Puglia MP, Zucca S (2010) Focus on neurotransmitter systems. Alcohol Res Health: J National Institute on Alcohol Abuse and Alcoholism 34:106–120 [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, Hoekstra PJ, Franke B, Buitelaar JK, Oosterlaan J (2014) Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry 53:790–9.e3 [DOI] [PubMed] [Google Scholar]
- Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, Cross AH, Song SK (2015) Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain 138:1223–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011) Wechsler Abbreviated Scale of Intelligence, 2nd edn (WASI-II). NCS Pearson, San Antonio [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M (2009) About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260 [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO (2006) Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 30:1799–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, (2011) What Does Diffusion Tensor Imaging Reveal About the Brain and Cognition in Fetal Alcohol Spectrum Disorders?. Neuropsychology Rev 21:133–147 [DOI] [PubMed] [Google Scholar]
- White TL, Monnig MA, Walsh EG, Nitenson AZ, Harris AD, Cohen RA, Porges EC, Woods AJ, Lamb DG, Boyd CA, Fekir S (2018) Psychostimulant drug effects on glutamate, Glx, and creatine in the anterior cingulate cortex and subjective response in healthy humans. Neuropsychopharmacol 43:1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Fink BA, Fuglestad AJ, Eckerle JK, Boys CJ, Sandness KE, Radke JP, Miller NC, Lindgren C, Brearley AM, Zeisel SH (2020) Georgieff MK Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodevelop Disord 12:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K, Govindaraju V, Soher BJ, Maudsley AA (1998a) Automated spectral analysis I: Formation of a priori information by spectral simulation. Magn Reson Med 40:812–815 [DOI] [PubMed] [Google Scholar]
- Young K, Soher BJ, Maudsley AA, (1998b) Automated spectral analysis II: application of wavelet shrinkage for characterization of non-parameterized signals. Magn Reson Med 40:816–821 [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE (2007) Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 10:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]