Supplemental Digital Content is Available in the Text.
Updating a core outcome set for pediatric chronic pain interventions using an accepted framework and methodology will advance care for children with chronic pain.
Keywords: Pediatric chronic pain, Core outcome set, Interventions, Clinical trials, Clinical registries
Abstract
Appropriate outcome measures and high-quality intervention trials are critical to advancing care for children with chronic pain. Our aim was to update a core outcome set for pediatric chronic pain interventions. The first phase involved collecting providers', patients', and parents' perspectives about treatment of pediatric chronic pain to understand clinically meaningful outcomes to be routinely measured. The second phase was to reach consensus of mandatory and optional outcome domains following the OMERACT framework. A modified Delphi study with 2 rounds was conducted including 3 stakeholder groups: children with chronic pain (n = 93), their parents (n = 90), and health care providers who treat youth with chronic pain (n = 52). Quantitative and qualitative data from round 1 of the Delphi study were summarized to identify important outcomes, which were condensed to a list of 10 outcome domains. Round 2 surveys were analyzed to determine the importance of the 10 domains and their relative ranking in each stakeholder group. A virtual consensus conference was held with the steering committee to reach consensus on a set of recommended outcome domains for pediatric chronic pain clinical trials. It was determined, by unanimous vote, that pain severity, pain interference with daily living, overall well-being, and adverse events, including death, would be considered mandatory domains to be assessed in all trials of any type of intervention. Emotional functioning, physical functioning, and sleep were important but optional domains. Last, the research agenda identifies several important emerging areas, including biomarkers. Future work includes selecting appropriate validated measures to assess each outcome domain.
1. Introduction
Despite the high prevalence, cost, and impact of child and adolescent chronic pain, the evidence base for chronic pain interventions is limited and considered a priority to address.3,10 Appropriate outcome measures and high-quality intervention trials are critical to advancing care. In 2006, a pediatric working group of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT), conducted a 2-round Delphi poll and consensus meeting to identify core outcome domains for clinical trials of pain interventions in children.12 Eight domains were recommended for chronic pain trials: pain intensity, physical functioning, symptoms/adverse events, global satisfaction with treatment, emotional functioning, role functioning, sleep, and economic factors.
PedIMMPACT recommendations have been used to guide outcomes measurement in clinical registries and trials.2,15 However, there are concerns about the uptake of these recommendations over the past 12 years. Connolly et al.6 conducted a systematic review of reporting practices in 107 randomized controlled trials (RCTs) of pediatric chronic pain interventions. Nearly all trials included pain intensity as an outcome domain, but fewer than 35% included outcomes in any other recommended domain, suggesting insufficient use of this core outcome set (COS).
Although the reasons behind its limited uptake need to be further investigated, several concerns have been raised about the relevance of the PedIMMPACT recommendations and the process used to derive them. First, PedIMMPACT used a consensus process with a relatively small group of professionals/researchers in pain medicine and did not incorporate the perspectives of patients with chronic pain or their parents. Second, the group combined the consideration of acute and chronic pain outcomes in one consensus meeting, which may have artificially inflated similarities in the COSs for acute and chronic pain. Third, there is a lack of specificity in the recommendations to guide its use and no prioritization of domains (all are considered core outcomes).
Progress has been made in the field of rheumatology through the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT), an independent international initiative that includes health professionals and patient research partners focused on outcome measures and measurement methodology. One of their major contributions is the development of a systematic framework to help guide the work of choosing relevant outcome domains for clinical populations. Recently, integrating the perspectives of providers, patients, and families, the OMERACT framework was used to update a COS in juvenile idiopathic arthritis.13 Using the OMERACT framework to guide an update of pediatric chronic pain outcome domains is an advance that may improve relevance and provide a common language for measurement methodology.
Our aim is to update a COS for pediatric chronic pain interventions. The first phase of our study involved collecting a spectrum of providers', patients', and parents' perspectives about treatment of pediatric chronic pain to understand clinically meaningful outcomes that should be routinely measured. The second phase was to reach consensus of mandatory and optional outcome domains following the OMERACT framework. A future phase will include recommendations for validated measures to assess each outcome domain.
2. Methods
2.1. Study overview
We used the OMERACT recommended methods to choose outcome domains using provider, patient, and parent input followed by an expert consensus meeting. The steering committee was comprised of cochairs (T.M.P. and G.A.W.) who designed the project and an international panel of 5 other pediatric pain researchers. The group had a diversity of expertise in assessment, clinical trials, clinical practice, critical analysis, and outcomes (K.A.B., G.C., C.E., S.K.-Z., and A.L.S.). Three members of the steering committee had previously served on the 2006 PedIMMPACT consensus group (T.M.P., G.A.W., and C.E.). The steering committee met several times to develop the Delphi surveys and then to integrate the proposed domains into the OMERACT framework. The findings from the Delphi poll were distributed to the committee, and a virtual 1-day meeting was held to select final outcome domains.
2.2. Participants
A convenience sample of child and parent participants were recruited from a cohort who had previously participated in a multicenter clinical trial of a pain self-management app.14 This cohort was selected because they had a range of chronic pain conditions, represented multiple clinics across the United States, and had previous experience with a clinical trial. Children had chronic pain (defined as pain present for at least 3 months) with or without a concurrent chronic health condition and had been evaluated or treated in 1 of 8 pain or gastroenterology clinics across the United States.
To select a range of providers caring for children with chronic pain, we recruited a convenience sample of providers from 3 sources: (1) Seattle Children's Hospital (including pain medicine, pediatrics, adolescent medicine, gastroenterology, rehab medicine, hematology/oncology, etc.), (2) the 5 children's hospitals that were involved in the clinical trial,14 and (3) from an announcement on the American Society of Pediatric Hematology/Oncology listserv. Eligibility criteria for the providers included physicians, nurse practitioners, psychologists, and rehabilitation therapists caring for children and adolescents with chronic pain as part of their clinical practice. This study was conducted under 2 separate protocols that were either approved or considered exempt by the Institutional Review Board at Seattle Children's Hospital.
2.3. Modified Delphi study
A modified Delphi study with 2 rounds was conducted to understand the most important domains to use in clinical trials or longitudinal clinical registries as reported by children and adolescents with chronic pain, their parents, and health care providers who treat youth with chronic pain. Surveys were administered by a REDCap9 interface hosted by the University of Washington. Providers, children, and their parents were invited to participate in the study through an email invitation, explaining the purpose of the study, providing instructions, and a REDCap link to access and complete the study survey. Consent language was included in the instructions, and completion of the survey was considered implicit informed consent. All completers from round 1 were invited to participate in round 2. Children and parents were sent a $20 (US) gift card on questionnaire completion in each round (up to $40 total). Providers were entered into a lottery to win a $100 gift card for each round of the survey.
Round 1: parent and child surveys were designed following the process outlined by Sinha19 for COSs for pediatric chronic conditions. Round 1 surveys for children and parents included open-ended questions about their perspectives on how well their treatments for chronic pain have been working and how to identify when a pain treatment should be changed because of lack of improvement. They were also asked about any worries related to treatments they are receiving for chronic pain. Round 1 provider surveys were also open-ended and asked the participant to list up to 10 beneficial outcomes that they find clinically most important in treating children and adolescents with chronic pain. Providers were also asked about harmful effects or outcomes by listing up to 10 harmful outcomes that they consider important in recommending changing a child's treatment for chronic pain. These same questions were asked for outcomes for longitudinal registries.
Demographic information (eg, age, sex, and pain condition) on children was available from our previous trial. Providers supplied their professional background information and years in practice on the surveys. Demographic information was not obtained for parents.
Round 1 open-ended responses were analyzed qualitatively to summarize responses from providers, children/teens, and parents. Data were sorted and analyzed using NVivo software.17 We conducted thematic analysis with an inductive approach. After initial familiarization with the data, one researcher (U.R.P.) completed coding of the data and in collaboration with the first author (T.P.), generated themes. The 2 coders reached consensus regarding each disagreement (27% of responses required discussion), and most stemmed from ambiguous patient/parent responses. Inconsistencies were addressed after reviewing all themes, which were also defined and named as outcome domains, and further refined for use in Round 2 surveys. Definitions for each domain were developed considering themes across stakeholder groups.
Round 2: surveys were sent only to children, parents, and providers who had completed Round 1. All stakeholder groups completed the same survey structure using the list of 10 domains identified in Round 1. Round 2 surveys included a description of a clinical trial and of an outcome domain in lay language modeled after Sinha et al.19 Participants rated how important they perceived each of the 10 domains to be for assessing outcomes of pediatric chronic pain interventions using a scale of 0 (not important) to 10 (most important). Then, they selected the 5 domains most important to them and ranked them by level of importance from first to fifth. A write-in option was available to propose any other domains (not included) that they believed were important to be measured in a clinical trial for pediatric chronic pain.
Round 2 data were summarized using item level means and medians using STATA v.14 software. Rankings were determined by assigning weights to each rank, calculating a “priority ranking score” for each domain, and comparing scores to establish a rank of the domain.
3. Results
3.1. Modified Delphi study
In round 1, 136 child–parent dyads and 97 providers were invited to participate in the study. Of those invited, we received completed surveys from 235 stakeholders, of which 93 were children/adolescents, 90 were parents, and 52 were providers. Round 2 surveys were sent to the 235 individuals who completed round 1. Completion rates were high with a total of 215 stakeholders completing round 2 surveys (91% response rate) of which 86 were children/adolescents, 85 were parents, and 44 were providers. Figure 1 shows the participant flow through the study.
Figure 1.
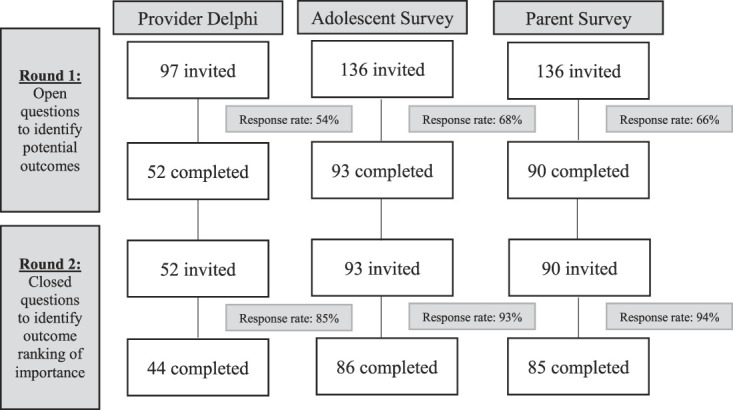
Study flowchart showing participants in each round.
Table 1 shows demographic and professional background information for children and providers. Child participants ranged in age from 11 to 19 years (mean = 15.8, SD = 2.2). Children had various pain conditions including abdominal pain (28%), musculoskeletal pain (16%), head pain (12%), and neuropathic pain (11%); 15.1% had a concurrent chronic health condition (eg, sickle cell disease, chronic pancreatitis). Half of the providers surveyed were physicians, about a quarter were psychologists, 15% were nurses, and 6% were physical therapists. Most common specialty areas were pain medicine, hematology and oncology, neurology, and gastroenterology. The experience level (in terms of years of experience) was variable, ranging from less than 5 years to more than 15 years of experience. There were no differences in demographic (age and sex) or clinical variables (type of pain condition) between children who completed round 1 and round 2 surveys or in those who chose to participate in the survey vs nonresponders. There were no differences between the providers in specialty or career stage who participated in each round.
Table 1.
Delphi poll and survey respondent characteristics.
| Respondent characteristics | Phase 1, n = 235 | Phase 2, n = 215 |
|---|---|---|
| Groups | ||
| Children | 93 | 86 |
| Parents | 90 | 85 |
| Providers | 52 | 44 |
| Patient demographics | ||
| Age (mean, SD) | 15.0 (2.1) | 15.0 (2.1) |
| Sex (n, %) | ||
| Female | 78 (84) | 71 (83) |
| Male | 15 (16) | 15 (17) |
| Type of pain condition (n, %) | ||
| Abdominal pain | 29 (31) | 27 (31) |
| Musculoskeletal pain | 15 (16) | 15 (17) |
| Orofacial and head pain | 11 (12) | 11 (13) |
| Neuropathic pain | 10 (11) | 9 (10) |
| Spine pain | 11 (12) | 9 (10) |
| Unspecified chronic pain | 9 (10) | 8 (9) |
| Chest pain | 2 (2) | 2 (2) |
| Missing/unknown | 6 (6) | 5 (6) |
| Provider characteristics | ||
| Professional background (n, %) | ||
| Physician | 27 (52) | 22 (50) |
| Psychologist | 13 (25) | 12 (27) |
| Registered nurse/nurse practitioner | 8 (15) | 7 (16) |
| Physical therapist | 3 (6) | 3 (7) |
| Physician assistant | 1 (2) | 0 (0) |
| Practice specialty (n, %) | ||
| Pain medicine | 21 (40) | 17 (39) |
| Hematology and oncology | 10 (19) | 10 (23) |
| Neurology | 4 (8) | 4 (9) |
| Gastroenterology | 4 (8) | 2 (5) |
| Adolescent medicine | 2 (4) | 2 (5) |
| Rehabilitation medicine | 2 (4) | 2 (5) |
| Gynecology | 2 (4) | 0 |
| Others (eg, orthopedics, orthopedic surgery, and sports medicine) | 7 (13) | 7 (16) |
| Years of experience (y; n, %) | ||
| 0-5 | 16 (31) | 15 (34) |
| 6-10 | 16 (31) | 12 (27) |
| 11-15 | 9 (17) | 11 (25) |
| ≥16 | 11 (21) | 6 (14) |
Quantitative and qualitative data from round 1 were summarized to identify outcomes of importance. Providers listed a mean of 7.2 beneficial outcomes and 4.8 harmful outcomes. Because provider responses were almost identical in the separate queries for outcome domains for clinical trials and for longitudinal registries, these data were combined.
Qualitative coding was conducted in several phases. First, within each stakeholder group, themes were identified from the round 1 surveys and coded into outcome domains. Frequency counts of outcome domains were computed for each stakeholder group to identify most commonly endorsed outcomes. Table 2 shows the 17 domains that were identified across stakeholder groups. Second, across stakeholder groups, further classification was performed to condense the 17 domains to a list of 10 outcome domains to use in round 2 surveys based on frequency of endorsement. Some further refinement was performed by the steering committee to reduce overlapping domains and increase fit with the OMERACT framework. Table 3 shows how the domains fit the OMERACT 2.0 framework core areas (death, life impact, and pathophysiological manifestations).5 Considering themes across stakeholder groups, definitions for each outcome domain were developed.
Table 2.
Initial 17 domains identified from phase 1 stakeholder input.
| Domains identified | Web survey of teens | Web survey of parents | Delphi poll of providers |
|---|---|---|---|
| 1. Impact on diet | ✓ | ✓ | ✓ |
| 2. Reduced physical activity | ✓ | ✓ | |
| 3. Missed school | ✓ | ✓ | ✓ |
| 4. Reduced social activities | ✓ | ✓ | |
| 5. Fatigue | ✓ | ✓ | ✓ |
| 6. Reduced mobility | ✓ | ✓ | |
| 7. Sleep problems | ✓ | ✓ | ✓ |
| 8. Limitations in independence and long-term potential | ✓ | ✓ | |
| 9. Reductions in quality of life | ✓ | ✓ | |
| 10. Using pain self-management skills | ✓ | ✓ | ✓ |
| 11. Impact on emotional or psychological functioning | ✓ | ✓ | ✓ |
| 12. Fear of pain | ✓ | ✓ | |
| 13. Pain severity | ✓ | ✓ | ✓ |
| 14. Side effects of treatments | ✓ | ✓ | ✓ |
| 15. Occurrence of nonpain symptoms | ✓ | ✓ | |
| 16. Use of opioids | ✓ | ||
| 17. Uncertainty about long-term outcomes | ✓ | ✓ |
Table 3.
Shortlist of 10 domains identified for round 2 surveys.
| Domains organized by OMERACT 2.0 core areas |
|---|
| Adverse events* |
| Side effects |
| Life impact |
| Pain interference |
| Quality of life |
| Physical functioning |
| Treatment satisfaction |
| Pain self-management skills |
| Opioid medication use |
| Pathophysiological manifestations |
| Pain severity |
| Emotional functioning |
| Sleep |
Death is the core area used in OMERACT 2.0, but the committee chose to use adverse events as an outcome, recognizing the rarity of death as an outcome of pediatric chronic pain interventions.
Round 2 surveys were analyzed to determine the importance of each domain and its relative ranking in each stakeholder group. Table 4 shows the mean and median importance ratings for each of the 10 domains presented in the round 2 surveys by stakeholder. All 3 stakeholder groups gave the highest importance ratings to quality of life. Although they were ranked in a slightly different order for each group, there was consistency across the 3 groups on their ranking of the 5 most important domains: quality of life, pain severity, emotional functioning, physical functioning, and pain interference. However, there was a divergence of opinion on some domains, most notably the importance of opioid medication use was perceived differently by children and parents vs providers, with providers deeming it to be more important.
Table 4.
Importance ratings and final rankings of the 10 domains based on Round 2 stakeholder input.
| Domain | Teens (n = 86) | Parents (n = 85) | Providers (n = 44) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Importance rating (mean, SD) | Importance rating (median, IQR) | Priority ranking score | Final rank | Importance rating (mean, SD) | Importance rating (median, IQR) | Priority ranking score | Final rank | Importance rating (mean, SD) | Importance rating (median, IQR) | Priority ranking score | Final rank | |
| Quality of life | 8.2 (2.6) | 9 (7-10) | 256 | 1 | 9.0 (1.6) | 10 (9-10) | 283 | 1 | 9.2 (1.2) | 10 (9-10) | 132 | 2 |
| Pain severity | 8.1 (2.3) | 9 (7-10) | 241 | 2 | 9.0 (1.6) | 10 (8-10) | 268 | 2 | 7.9 (2.3) | 9 (6-10) | 57 | 4 |
| Emotional functioning | 7.9 (2.5) | 9 (7-10) | 227 | 3 | 8.9 (1.6) | 10 (8-10) | 200 | 3 | 8.8 (1.2) | 9 (8-10) | 53 | 5 |
| Physical activity | 7.4 (2.4) | 8 (6-10) | 128 | 4 | 8.4 (2.1) | 9 (7-10) | 90 | 5 | 9.0 (1.2) | 9 (9-10) | 75 | 3 |
| Pain interference | 8.0 (2.4) | 9 (7-10) | 115 | 5 | 8.9 (1.6) | 10 (8-10) | 117 | 4 | 9.6 (0.7) | 10 (9-10) | 171 | 1 |
| Sleep | 7.7 (2.4) | 8 (7-10) | 105 | 6 | 8.7 (1.6) | 9 (8-10) | 65 | 6 | 8.3 (1.7) | 9 (7-10) | 36 | 8 |
| Pain self-manag skills | 7.5 (2.3) | 8 (6-9) | 74 | 7 | 8.6 (1.9) | 9 (8-10) | 117 | 4 | 8.3 (1.6) | 8 (7-10) | 41 | 7 |
| Side effects | 7.2 (2.7) | 8 (6-9) | 65 | 8 | 8.1 (2.1) | 8 (7-10) | 38 | 8 | 7.7 (1.9) | 8 (7-9) | 17 | 9 |
| Treatment satisfaction | 8.0 (2.2) | 8 (7-10) | 54 | 9 | 8.4 (1.9) | 9 (8-10) | 58 | 7 | 8.7 (1.2) | 9 (8-10) | 43 | 6 |
| Opioid medication use | 4.4 (3.7) | 4.5 (0-8) | 24 | 10 | 6.3 (3.6) | 7 (3-10) | 36 | 9 | 7.7 (2.3) | 8 (7-10) | 14 | 10 |
IQR, interquartile range.
3.2. Steering committee consensus meeting
In April 2020, once the Delphi study was completed, a virtual consensus conference was held with the steering committee with the goal to reach consensus on a set of recommended outcome domains for pediatric chronic pain clinical trials and longitudinal registries. The meeting agenda included presentations and discussions about the goals of the project, earlier work performed by PedIMMPACT and other COSs, how to apply the OMERACT framework, and methodology to consider for future measure selection.
The committee had extensive discussion related to quality of life as an outcome domain, recognizing overlap with several other domains of physical or psychosocial health that are typically incorporated into quality of life assessment. The committee judged that including quality of life, physical functioning, and emotional functioning as separate domains were redundant. To improve specificity and reduce overlap, the committee decided to separate out the primary domains that comprise quality of life. A patient's overall (global) well-being represents a unique aspect of quality of life not captured by the other important domains identified by stakeholders. Thus, the committee decided to modify the domain quality of life to “overall well-being.”
The committee also reviewed the treatment satisfaction domain, which was operationalized in the survey as the patient's global perception of improvement in response to treatment. There were several issues with the domain that were considered. First, in the initial round surveys, all 3 stakeholder groups mentioned descriptors about improvements over time in the child's independence, activities, etc. but did not specifically describe this as “response to treatment,” although this label was used in the Delphi poll. Across the stakeholder groups, the importance ratings for treatment satisfaction/response to treatment were lower compared with other domains. Thus, the committee did not vote to include this domain in the final COS. However, the committee discussed that the assessment of overall well-being could potentially capture overall perception of improvement in addition to other aspects of well-being.
There was also extensive discussion on patient and practitioner perspectives on opioid use. Use of opioids for chronic pain is a source of intense public and political debate in the United States and Canada because of the widespread misuse of prescription and nonprescription drugs, although this is not the case internationally.11 Moreover, there is overall limited use of opioids for pediatric chronic pain, making the domain not relevant for all trials. Many clinical trials of treatments for chronic pain (especially in pediatrics) also explicitly exclude individuals using opioid medications. The consensus, therefore, was that opioid use should be considered as a delivery of care outcome concerned with attitudes or appropriateness of this specific treatment, and although opioid use may be relevant for some trials (eg, opioid sparing outcomes for a pharmacological intervention), it should not be a core outcome domain.
Following the OMERACT methodology and their 3 layer onion framework,5 the committee prioritized the domains obtained in the Delphi study as: (1) core set of domains mandatory for all trials, (2) important domains with optional inclusion, and (3) research agenda (eg, domains that are exploratory and need further research). The onion concept was recently updated to include within the inner core set domains that are mandatory for specific circumstances (eg, pain diagnoses), but the committee did not identify any domains that fit this purpose.
During the meeting, it was determined, by unanimous vote, that pain severity, pain interference with daily living, overall well-being, and adverse events, including death, would be considered mandatory domains to be assessed in all trials of any type of intervention. Emotional functioning, physical functioning, and sleep were important but optional domains. Last, the research agenda identifies several important emerging areas, including biomarkers. Slight adjustments to labels for outcome domains and definitions were made after the meeting. Final domains are shown in Figure 2, and final definitions are shown in Table 5.
Figure 2.
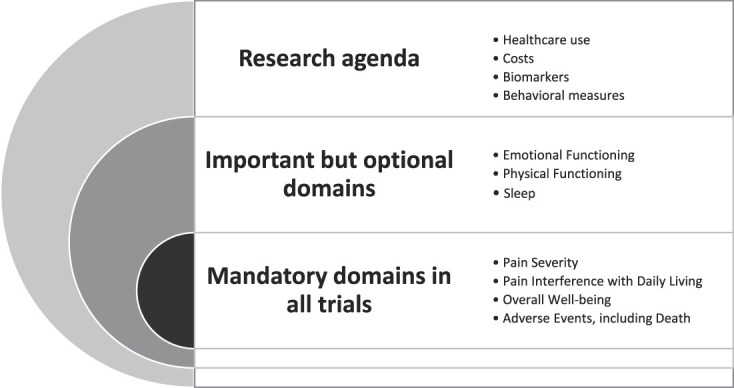
Final domains from steering committee consensus using OMERACT2.0 domain framework.
Table 5.
Definitions of final outcome domains.
| 1. Pain interference with daily living—how much pain interferes with engagement in social, physical, and recreational activities. |
| 2. Pain severity—perception of the severity of pain including how intense pain is and how frequently it occurs. |
| 3. Overall well-being—perception of overall (global) well-being (eg, satisfaction with health and life) |
| 4. Sleep—quantity and quality of sleep (eg, problems with falling sleep). |
| 5. Physical functioning—ability to perform physical activities |
| 6. Emotional functioning—psychological and emotional well-being (such as experiencing anxiety or depression). |
| 7. Adverse events—an unwanted symptom or reaction from a treatment (eg, stomach upset, vomiting, fatigue, etc.). |
4. Discussion
The study objectives were to update a COS for pediatric chronic pain interventions using an accepted framework and methodology. Our goal was to advance the earlier efforts conducted by the initial PedIMMPACT group12 to incorporate scientific innovations made in outcome measurement, include patients' perspectives, and use a more rigorous methodology. In particular, the initial PedIMMPACT group was limited in its representativeness, including only a small number of professionals in pediatric pain. Moreover, the participants in the Delphi poll and the consensus panel were the same, and the group combined a focus on acute and chronic pain interventions, potentially conflating similarities in the domains identified. Our updated methodology for the COS for pediatric chronic pain interventions had 3 key improvements: (1) precision is increased by focusing only on domains for chronic pain interventions, (2) key stakeholder input from children with chronic pain, parents, and a range of healthcare providers was obtained, and (3) a steering committee applied the OMERACT methodology to have a conceptual framework in which to organize the domains for chronic pain interventions.
In our first phase of work presented in this report, we conducted a 2-round Delphi poll with children with chronic pain, their parents, and healthcare providers. Then, our steering committee reached consensus on a COS layered within the OMERACT framework that includes 4 mandatory domains to be assessed in all clinical trials of pediatric chronic pain interventions: pain severity, pain interference with daily living, overall well-being, and adverse events. In addition, 3 optional domains important for inclusion in some trials of chronic pain interventions were identified: emotional functioning, physical functioning, and sleep. The onion concept was recently updated to include within the inner core set domains that are mandatory for specific circumstances (eg, for specific diagnoses). The consensus was not to use this category but rather recommend that all trials in pediatric chronic pain include the mandatory domains to make it possible to compare outcomes across trials and ultimately develop an evidence base that allows patients and providers to choose a treatment based on known beneficial effects and potential risks.
By contrast, the initial PedIMMPACT recommendations were for 8 domains for chronic pain trials: pain intensity, physical functioning, symptoms and adverse events, global satisfaction with treatment, emotional functioning, role functioning, sleep, and economic factors. There are some areas of similarity in domains of both pain and functional outcomes. However, a major difference in the new COS is the prioritization of domains that may allow for better matching recommendations to use. One of the problems with the PedIMMPACT guidance is that 8 domains were recommended for all trials, which was burdensome, and also challenging to find valid assessments in each domain.
Findings from our Delphi poll and surveys indicated many commonalities in perceptions of important outcomes across stakeholder groups. The top 5 highest rated outcomes were almost identical between stakeholders. However, there were also some differences. For example, children and parents did not think that taking opioid medications for chronic pain was a relevant outcome, whereas providers did. Children and parents' descriptions of treatment benefits were incorporated into the definitions of each domain. The relevancy of our new COS for pediatric chronic pain to research and clinical care is greatly enhanced by including the patient/family voice.8,20
Related, the committee discussed the issue of opioid use as a potential outcome domain. We eventually concluded that the use of opioids in North America is a current issue topmost on many providers' minds because of the ongoing societal problem of opioid misuse and addiction16,21, but this is not reflective of perspectives in other regions. Indeed, there is an inequality in consumption of opioid analgesics because of differences in access and resources that has grown over time, with estimates in 2015 that almost 6.5 billion people lived in countries where opioid analgesic consumption was low, very low, or extremely low.18 Although opioids are rarely prescribed for chronic pain in pediatrics, they may have been given undue focus by providers in our Delphi study. We acknowledge that outcomes relating to delivery of care such as patient preference, acceptability of interventions, and appropriateness of interventions can be conceptualized within the core area of life impact. However, given the numerous issues outlined and lack of support from all stakeholders for including opioid use as a domain, we excluded it from consideration in our recommendations.
Pain intensity has been the primary endpoint in clinical trials of pediatric chronic pain interventions.6 Instead, we recommend that all RCTs for any intervention in pediatric chronic pain should include pain interference with daily living, overall well-being, and adverse events, in addition to pain severity. This recommendation is also consistent with movement in the US Food and Drug Administration to broaden endpoint guidance for analgesic trials to include measures of function.1 Moreover, our recommendation extends conclusions from multiple review articles regarding outcomes in clinical trials in pediatric chronic pain.7 For example, Birnie et al.4 construct an evidence and gap map from systematic reviews for treatments in pediatric chronic pain showing the predominance of studies measuring pain intensity but few studies providing any evidence for nonpain outcomes. Because our new COS is applicable across all types of treatments (ie, psychological, analgesic, and integrative), as investigators incorporate the COS into their clinical trials, it will begin to address gaps in knowledge of intervention effects for pediatric chronic pain.
There are other movements in evidence-based medicine that this COS could also align with including individualizing treatments for patients and shared decision-making with providers. In particular, consistent use of this COS across chronic pain interventions will produce evidence on benefits and risks of each treatment along a set of common outcome domains. Dissemination of this evidence may then arm patients with knowledge to make informed decisions with their providers based on assessment of risk/benefit profiles and their own individual preferences. Patients may be able to individualize evaluation of treatment benefits (ie, tying benefit of treatment to what actually matters to each individual patient) by choosing treatments that have known benefit in domains that are of the most individual importance to them (eg, choosing a treatment that helps improve sleep over a treatment that helps improve mood).
We also highlight that the COS is considered appropriate for both clinical trial and longitudinal clinical outcomes. Our survey with providers suggested that there was no differentiation of outcomes for the purpose of a clinical trial vs a longitudinal registry. Thus, similar to what has already been performed with PedIMMPACT recommendations, we encourage those using longitudinal clinical outcomes in clinical databases and registries to follow the same guidance.
Our findings should be interpreted in light of several limitations. First, we recruited convenience samples for the surveys, which were limited to North American participants and will likely not reflect viewpoints from other regions. In addition, provider participants did not equally represent the full range of specialty areas who care for children with chronic pain and did not include primary care providers (because of our limitations reaching other professional societies). Similarly, the child and adolescent patient group did not represent the full range of chronic pain conditions. We believe it is a strength that this group had previous experience with a clinical trial but recognize that other patients may have different perspectives. Moreover, our steering committee was comprised of psychologists with methodological expertise in trials and outcome measures. Inclusion of patients, administrators, policymakers, and other professionals in the steering committee might have led to different conclusions and interpretation and prioritization of outcomes.
The patient sample was limited to older children and adolescents (ages 11 and above) and did not include the viewpoint of younger children. It is possible that certain outcomes may be more important to younger children rather than adolescents. Although the OMERACT framework was generally identified as relevant, there were domains generated by stakeholders that were not easily classified into the core areas. For example, the domain “self-management skills” does not readily fit the OMERACT 2.0 core areas and instead represents an intermediate or mechanistic outcome of interest to many psychological interventions. In addition, the core area “death” is not typically described as an outcome of pediatric chronic pain interventions, so the steering committee focused instead on the area adverse events which may include serious adverse events including death as well as side effects.
Future iterations of the COS are expected and encouraged to advance its utility. Future work may specifically address some of the sampling limitations in our study (eg, broader range of providers and international representation) or may focus on refining the domains (eg, evaluating applicability across different diagnoses or interventions). Our committee's next step includes identifying relevant validated outcome measures and reaching consensus on recommendations for measures for each of the identified domains.
In conclusion, this report describes an updated COS for pediatric chronic pain that advances recommendations for measuring outcomes in all trials for any intervention in pediatric chronic pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplemental video content
Video content associated with this article can be found online at http://links.lww.com/PAIN/B314.
Acknowledgements
The authors recognize the generous support of the Mayday Fund in providing grant support for the project (PIs: G.A. Walco, T.M. Palermo). The authors thank the children, parents, and providers who participated in the study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Gary A. Walco, Email: gary.walco@seattlechildrens.org.
Unmesha Roy Paladhi, Email: uroy@uw.edu.
Kathryn A. Birnie, Email: kathryn.birnie@ucalgary.ca.
Geert Crombez, Email: Geert.Crombez@UGent.be.
Rocio de la Vega, Email: rociovegapsicologa@gmail.com.
Christopher Eccleston, Email: hssce@bath.ac.uk.
Susmita Kashikar-Zuck, Email: susmita.kashikar-zuck@cchmc.org.
Amanda L. Stone, Email: amanda.l.stone@vumc.org.
References
- [1].Berde CB, Walco GA, Krane EJ, Anand KJ, Aranda JV, Craig KD, Dampier CD, Finkel JC, Grabois M, Johnston C, Lantos J, Lebel A, Maxwell LG, McGrath P, Oberlander TF, Schanberg LE, Stevens B, Taddio A, von Baeyer CL, Yaster M, Zempsky WT. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics 2012;129:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bhandari RP, Feinstein AB, Huestis SE, Krane EJ, Dunn AL, Cohen LL, Kao MC, Darnall BD, Mackey SC. Pediatric-Collaborative Health Outcomes Information Registry (Peds-CHOIR): a learning health system to guide pediatric pain research and treatment. PAIN 2016;157:2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Birnie KA, Dib K, Ouellette C, Dib MA, Nelson K, Pahtayken D, Baerg K, Chorney J, Forgeron P, Lamontagne C, Noel M, Poulin P, Stinson J. Partnering for Pain: a Priority Setting Partnership to identify patient-oriented research priorities for pediatric chronic pain in Canada. CMAJ Open 2019;7:E654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Birnie KA, Ouellette C, Amaral TD, Stinson JN. Mapping the evidence and gaps of interventions for pediatric chronic pain to inform policy, research, and practice: a systematic review and quality assessment of systematic reviews. Can J Pain 2020;4:129–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d'Agostino MA, Conaghan PG, Bingham CO, III, Brooks P, Landewe R, March L, Simon LS, Singh JA, Strand V, Tugwell P. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014;67:745–53. [DOI] [PubMed] [Google Scholar]
- [6].Connolly MR, Chaudari JY, Yang X, Ward N, Kitt RA, Herrmann RS, Krane EJ, LeBel AA, Smith SM, Walco GA, Weisman SJ, Turk DC, Dworkin RH, Gewandter JS. Design and reporting characteristics of clinical trials of select chronic and recurrent pediatric pain conditions: an analgesic, anesthetic, and addiction clinical trial translations, innovations, opportunities, and networks systematic review. J Pain 2019;20:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eccleston C, Fisher E, Howard RF, Slater R, Forgeron P, Palermo TM, Birnie KA, Anderson BJ, Chambers CT, Crombez G, Ljungman G, Jordan I, Jordan Z, Roberts C, Schechter N, Sieberg CB, Tibboel D, Walker SM, Wilkinson D, Wood C. Delivering transformative action in paediatric pain: a lancet child & adolescent health commission. Lancet Child Adolesc Health 2021;5:47–87. [DOI] [PubMed] [Google Scholar]
- [8].Gwara M, Smith S, Woods C, Sheeren E, Woods H. International children's advisory network: a multifaceted approach to patient engagement in pediatric clinical research. Clin Ther 2017;39:1933–8. [DOI] [PubMed] [Google Scholar]
- [9].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metdata-drive methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liossi C, Anderson AK, Howard RF, NC-CCi Pain, Palliative C. Development of research priorities in paediatric pain and palliative care. Br J Pain 2017;11:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martins SS, Sampson L, Cerda M, Galea S. Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. Am J Public Health 2015;105:e29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain 2008;9:771–83. [DOI] [PubMed] [Google Scholar]
- [13].Morgan EM, Munro JE, Horonjeff J, Horgan B, Shea B, Feldman BM, Clairman H, Bingham CO, III, Thornhill S, Strand V, Alongi A, Magni-Manzoni S, van Rossum MAJ, Vesely R, Vojinovic J, Brunner HI, Harris JG, Horton DB, Lovell DJ, Mannion M, Rahimi H, Ravelli A, Ringold S, Ruperto N, Schrandt MS, Shenoi S, Shiff NJ, Toupin-April K, Tzaribachev N, Weiss P, Consolaro A. Establishing an updated core domain set for studies in juvenile idiopathic arthritis: a report from the OMERACT 2018 JIA workshop. J Rheumatol 2019;46:1006–13. [DOI] [PubMed] [Google Scholar]
- [14].Palermo TM, de la Vega R, Murray C, Law E, Zhou C. A digital health psychological intervention (WebMAP Mobile) for children and adolescents with chronic pain: results of a hybrid effectiveness-implementation stepped-wedge cluster randomized trial. PAIN 2020;161:2763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T, Tai G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. PAIN 2016;157:174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pielech M, Lunde CE, Becker SJ, Vowles KE, Sieberg CB. Comorbid chronic pain and opioid misuse in youth: knowns, unknowns, and implications for behavioral treatment. Am Psychol 2020;75:811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].QSR. NVivo Qualitative Data Analysis Software for Windows, v.10. Victoria: QSR International Pty Ltd., 2012. [Google Scholar]
- [18].Scholten W, Christensen AE, Olesen AE, Drewes AM. Analyzing and benchmarking global consumption statistics for opioid analgesics 2015: inequality continues to increase. J Pain Palliat Care Pharmacother 2020;34:1–12. [DOI] [PubMed] [Google Scholar]
- [19].Sinha IP, Gallagher R, Williamson PR, Smyth RL. Development of a core outcome set for clinical trials in childhood asthma: a survey of clinicians, parents, and young people. Trials 2012;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tsang VWL, West L, Woods C, Koh CJ, McCune S, Mullin T, Smith SR, Gaillard S, Claverol J, Nafria B, Preston J, Dicks P, Thompson C. Role of patients and parents in pediatric drug development. Ther Innov Regul Sci 2019;53:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yaster M, McNaull PP, Davis PJ. The opioid epidemic in pediatrics: a 2020 update. Curr Opin Anaesthesiol 2020;33:327–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video content associated with this article can be found online at http://links.lww.com/PAIN/B314.


