Abstract
Background
Daily co-trimoxazole is recommended for African adults living with human immunodeficiency virus (HIV) irrespective of antiretroviral treatment, immune status, or disease stage. Benefits of continued prophylaxis and whether co-trimoxazole can be stopped following immune reconstitution are unknown.
Methods
We conducted a randomized controlled trial at 2 sites in Malawi that enrolled adults with HIV with undetectable viral load and CD4 count of >250/mm3 and randomized them to continue daily co-trimoxazole, discontinue daily co-trimoxazole and begin weekly chloroquine, or discontinue daily co-trimoxazole. The primary endpoint was the preventive effect of co-trimoxazole prophylaxis against death or World Health Organization (WHO) HIV/AIDS stage 3–4 events, using Cox proportional hazards modeling, in an intention-to-treat population.
Results
1499 adults were enrolled. The preventive effect of co-trimoxazole on the primary endpoint was 22% (95% CI: −14%–47%; P = .20) versus no prophylaxis and 25% (−10%–48%; P = .14) versus chloroquine. When WHO HIV/AIDS stage 2 events were added to the primary endpoint, preventive effect increased to 31% (3–51%; P = .032) and 32% (4–51%; P = .026), respectively. Co-trimoxazole and chloroquine prophylaxis effectively prevented clinical malaria episodes (3.8 and 3.0, respectively, vs 28/100 person-years; P < .001).
Conclusions
Malawian adults with HIV who immune reconstituted on ART and continued co-trimoxazole prophylaxis experienced fewer deaths and WHO HIV/AIDS stage 3–4 events compared with prophylaxis discontinuation, although statistical significance was not achieved. Co-trimoxazole prevented a composite of death plus WHO HIV/AIDS stage 2–4 events. Given poor healthcare access and lack of routine viral load monitoring, co-trimoxazole prophylaxis should continue in adults on ART after immune reconstitution in sub-Saharan Africa.
Clinical Trials Registration. NCT01650558.
Keywords: HIV infection, trimethoprim-sulfamethoxazole, chloroquine, malaria, Africa
The role of co-trimoxazole prophylaxis among adults on antiretroviral therapy in Africa has not been characterized. In this clinical trial, continuing co-trimoxazole prevented mild to moderate infections and may save lives in areas of poor access to healthcare.
Before widespread antiretroviral therapy (ART) use in sub-Saharan Africa, studies demonstrated that daily trimethoprim-sulfamethoxazole (co-trimoxazole) prophylaxis reduced morbidity and mortality among adults living with human immunodeficiency virus (HIV) by preventing pneumonia, sepsis, malaria, and diarrhea [1–4]. Based on studies showing that co-trimoxazole prophylaxis for Pneumocystis jirovecii pneumonia and toxoplasmosis, common opportunistic infections, can be safely stopped at the CD4 cell count threshold of 200/mm3 in North America and Europe [5, 6], co-trimoxazole is prescribed in these settings until the CD4 cell count reaches 200/mm3. Several African studies have explored the possibility of discontinuing co-trimoxazole prophylaxis [3, 7–10], but none included mortality as a powered endpoint or identified a clinical or laboratory threshold at which co-trimoxazole prophylaxis ceases to impact survival and severe morbidity. Whether co-trimoxazole can safely be discontinued after successful ART initiation has not been directly tested in a randomized controlled study.
Routine co-trimoxazole prophylaxis has continued with expanded access to ART throughout sub-Saharan Africa, consistent with 2014 World Health Organization (WHO) guidelines that call for co-trimoxazole continuation among adults, adolescents, and children regardless of CD4 cell count or WHO clinical stage for settings in which malaria and systemic bacterial infection are highly prevalent [11]. In Malawi, co-trimoxazole prophylaxis is recommended to continue for life for all persons with HIV. We designed a clinical trial to evaluate the effects of co-trimoxazole prophylaxis on morbidity and mortality among Malawian adults living with HIV infection after good response to ART. We also aimed to distinguish benefits due to the antibacterial and antimalarial effects of co-trimoxazole in the southern region of Malawi where 26% of Plasmodium falciparum prevalence is detected in children [12]. Chloroquine-susceptible P. falciparum parasites now predominate in Malawi [13], and chloroquine offers highly effective malaria prophylaxis without providing prevention of bacterial infection.
METHODS
Study Design
We conducted an investigator-initiated, 2-center, randomized, open-label, controlled noninferiority trial at outpatient health facilities in Malawi, Central Africa, to compare standard-of-care prophylaxis with daily co-trimoxazole with discontinuation of standard-of-care co-trimoxazole prophylaxis and starting weekly chloroquine prophylaxis or discontinuation of standard-of-care co-trimoxazole prophylaxis, as published previously [14]. The trial protocol was approved by the University of Malawi College of Medicine Research Ethics Committee and the University of Maryland Institutional Review Board.
Participants
Adults with HIV who were on ART for at least 6 months and fulfilled all other inclusion and exclusion criteria were enrolled at Ndirande Health Centre, Blantyre, and Zomba Central Hospital, Zomba. Undetectable HIV viral load (<400 copies/mL) and CD4 count of 250/mm3 or greater were required at screening, and those with severe acute illness or who required chronic treatment or secondary prophylaxis for toxoplasmosis or tuberculosis were excluded. A complete list of the inclusion and exclusion criteria is provided in the Supplementary Appendix. All participants provided written informed consent. The first-line ART in Malawi was stavudine/lamivudine/nevirapine and changed to tenofovir/lamivudine/efavirenz in 2013.
Randomization and Masking
Participants were randomly assigned in a 1:1:1 ratio to continue standard-of-care daily co-trimoxazole prophylaxis (160 mg trimethoprim/800 mg sulfamethoxazole) [15], discontinue co-trimoxazole and start weekly chloroquine prophylaxis (300–310 mg chloroquine base), or discontinue co-trimoxazole prophylaxis. An internet-based data-entry system with hard-copy backup was used to randomize participants, who began their prophylaxis treatment assignment at enrollment. Treatment arms were not masked.
Procedures
All participants were seen at follow-up visits every 4–12 weeks (every 4 weeks for the first 24 weeks to ensure compliance, then every 12 weeks thereafter) and when ill. Participants underwent CD4 cell count and HIV viral load testing every 24 weeks. Clinical monitoring for adverse events took place at all visits to determine seriousness, relatedness, and expectedness. Antiretroviral therapy and illness management followed routine clinical practice per Malawi Ministry of Health guidelines. When participants experienced a WHO HIV/AIDS stage 3 or 4 event, their study treatment assignment was discontinued and they restarted routine co-trimoxazole prophylaxis for the duration of the study. Participants who became pregnant were placed on routine co-trimoxazole prophylaxis during pregnancy and resumed their study treatment assignment after delivery.
Diagnostic Criteria
Participants were considered to have confirmed bacterial infection if they had a positive bacterial culture from blood or other normally sterile site and a suspected bacterial infection if they had a clinical diagnosis consistent with a bacterial etiology based on investigator assessment. Diagnostic criteria for HIV/AIDS stage 3 or 4 events were based on the WHO clinical staging document of 2006 and AIDS Clinical Trials Group (ACTG) diagnostic criteria (see Supplementary Appendix). Participants with an illness consistent with malaria were tested for malaria by microscopy and diagnosed with clinical malaria if the blood smear demonstrated P. falciparum infection.
Outcomes
The primary outcome was occurrence of a severe event, defined as death or a WHO HIV/AIDS stage 3 or 4 event [16]. The secondary outcomes were prevalence of detectable HIV viral load; occurrence of CD4 cell count less than 200 cells/mm3; occurrence of WHO HIV/AIDS stage 2, 3, or 4 event or death; occurrence of bacterial infections; occurrence of P. falciparum clinical malaria defined as infection identified on a thick blood smear with malaria symptoms; occurrence of grade 3–5 adverse events; and the rate of treatment discontinuation. A primary endpoint review committee, blinded to study treatment assignment, used prespecified criteria to adjudicate clinical outcomes as previously described [14] (see Supplementary Appendix for case definitions for WHO HIV/AIDS stage 2–4 events). Participant adherence to ART and to study treatment, if appropriate, was assessed at each scheduled follow-up visit using pill counts and administration of a standardized adherence questionnaire. Participant adverse events were captured during scheduled and unscheduled study visits or by guardian report and supplemented with available clinic or hospital records. Events were assessed for relationship to study treatment, if appropriate, seriousness, severity, expectedness, and time to resolution/stabilization.
Statistical Analysis
The protocol-defined measure of preventive effect for the primary endpoint (time to first of death or WHO HIV/AIDS stage 3–4 event) was 1 – hazard ratio (HR) (co-trimoxazole/experimental). Hence, when the preventive effect is positive, it reflects benefit for co-trimoxazole prophylaxis relative to the experimental arm. This noninferiority study design tested the null hypothesis that the preventive effect of co-trimoxazole is 0.35 or greater. Each experimental group would be declared noninferior to co-trimoxazole if the upper bound of the 95% confidence interval (CI) of the preventive effect was less than .35. No multiplicity adjustment was planned to account for the performance of 2 primary comparisons (co-trimoxazole vs each experimental arm). The initial sample size of 900 participants was calculated to have 80% power to reject the null hypothesis. Because the interim event rate was lower than expected, the steering committee decided to expand the study to include an additional 600 participants and extend follow-up for an additional 2 years.
Time-to-event endpoints were summarized using the Kaplan-Meier method. Hazard ratios and 95% CIs were estimated by a Cox proportional hazards model with a single covariate for treatment group, and 2-sided log-rank P values for superiority were calculated. All analyses were conducted using the intention-to-treat population, and participants were censored at their last visit. Secondary endpoint analyses considered total number of events using Poisson regression modeling. Detectable HIV viral load, CD4 cell count less than 200 cells/mm3, suspected bacterial infections, P. falciparum clinical malaria, and grade 3–5 adverse events were compared by analyzing the incidence of events in chloroquine and no prophylaxis versus co-trimoxazole using exact Poisson regression. All P values were 2-sided tests of superiority. Data were analyzed using SAS version 9.4 (SAS Institute). The standing National Institutes of Health (NIH) Division of AIDS Complications and Coinfections Data Safety and Monitoring Board oversaw the study and reviewed safety data at least annually. This trial is registered with ClinicalTrials.gov as NCT01650558.
RESULTS
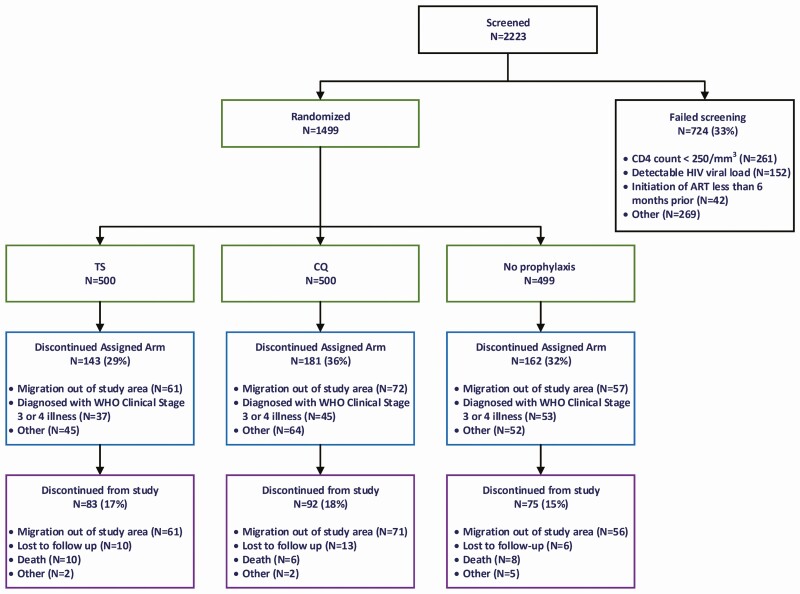
From 14 December 2012 to 26 August 2016, 1499 participants were recruited. A total of 4958 person-years of observation (pyo) were accrued, and 1249 (83%) enrolled participants completed the study (Figure 1). The most common reasons for screening failure were CD4 cell count less than 250/mm3 and detectable HIV viral load.
Figure 1.
Enrollment and randomization of participants. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; WHO, World Health Organization.
Participant demographics are summarized in Table 1 and reveal similar characteristics among study groups. Previous WHO HIV/AIDS stage 2, 3, and 4 data were obtained from ART clinic records and participant reporting. After study enrollment, 486 of 1499 participants permanently discontinued their treatment arm but continued to be maintained in the study cohort, including 135 due to WHO HIV/AIDS stage 3 or 4 event, 17 due to toxicity, and 4 due to participant preference. One hundred ninety-four women became pregnant during the study and were temporarily switched to routine co-trimoxazole until after delivery (Supplementary Table 1).
Table 1.
Baseline Demographic and Clinical Data
| Co-trimoxazole | Chloroquine | No Prophylaxis | Total | |
|---|---|---|---|---|
| Age (years) | ||||
| n | 500 | 500 | 499 | 1499 |
| Mean (SD) | 39.1 (9.7) | 38.7 (9.6) | 39.4 (10.1) | 39.1 (9.8) |
| Male, n/N (%) | 125/500 (25) | 126/500 (25) | 113/499 (`23) | 364/1499 (24) |
| CD4 count (cells/mm3) | ||||
| n | 498 | 500 | 498 | 1496 |
| Mean (SD) | 568.9 (237.2) | 550.8 (226.0) | 585.2 (245.3) | 568.3 (236.5) |
| Hemoglobin (g/dL) | ||||
| n | 498 | 500 | 499 | 1497 |
| Mean (SD) | 13.2 (1.7) | 13.2 (1.6) | 13.2 (1.6) | 13.2 (1.6) |
| Absolute neutrophil count (×103/µL) | ||||
| n | 481 | 481 | 480 | 1442 |
| Mean (SD) | 2.1 (0.9) | 2.1 (0.9) | 2.1 (0.9) | 2.1 (0.9) |
| White blood cell count (×103/µL) | ||||
| n | 498 | 500 | 499 | 1497 |
| Mean (SD) | 4.6 (1.4) | 4.6 (1.3) | 4.8 (1.4) | 4.7 (1.4) |
| Platelets (×103/µL) | ||||
| n | 498 | 500 | 499 | 1497 |
| Mean (SD) | 256.3 (81.1) | 263.9 (85.7) | 266.8 (81.7) | 262.3 (82.9) |
| Weight (kg) | ||||
| n | 500 | 500 | 499 | 1499 |
| Mean (SD) | 56.9 (9.6) | 56.6 (9.2) | 57.5 (11.3) | 57.0 (10.1) |
| WHO performance status, n/N (%) | ||||
| 1 (asymptomatic, normal activity) | 497/500 (99) | 498/500 (>99) | 498/499 (>99) | 1493/1499 (>99) |
| 2 (symptomatic, normal activity) | 2 (<1) | 1 (<1) | 1 (<1) | 4 (<1) |
| Not obtained | 1 (<1) | 1 (<1) | 0 | 2 (<1) |
| Previous WHO stage 2+ event, n/N (%) | 271/499 (54) | 277/500 (55) | 294/499 (59) | 842/1498 (56) |
| Previous WHO stage 3+ event, n/N (%) | 210/499 (42) | 203/500 (41) | 202/499 (40) | 615/1498 (41) |
| Previous WHO stage 4 event, n/N (%) | 47/499 (9) | 36/500 (7) | 34/499 (7) | 117/1498 (8) |
Column header counts and denominators are the number of randomized participants. Table only includes participants with nonmissing data for each measure. Abbreviation: WHO, World Health Organization.
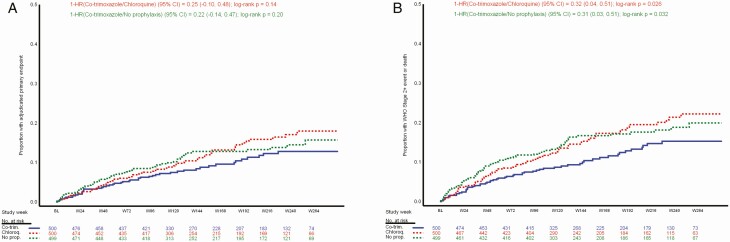
A total of 179 WHO HIV/AIDS stage 3 or 4 endpoint events from 157 individual participants were detected during follow-up and confirmed by the adjudication committee. When 13 reported deaths that were not adjudicated to be due to WHO HIV/AIDS stage 3 or 4 events were included, a total 170 participants experienced 192 primary endpoint events (Tables 2 and 3). Most participants with a primary endpoint event (88%) experienced only 1 event. A Kaplan-Meier plot for time to first primary endpoint event is presented in Figure 2A and shows no statistically significant difference between groups (co-trimoxazole vs chloroquine, P = .14; co-trimoxazole vs no prophylaxis P = .20); the preventive effect (1 − HR) and 95% CIs estimated by Cox proportional hazards did not achieve statistical significance to declare either noninferiority to co-trimoxazole or superiority of co-trimoxazole (co-trimoxazole vs no prophylaxis: .22; −.14–.47; P = .20; co-trimoxazole vs chloroquine: .25; −.10–.48; P = .14). The primary endpoint incidence rates in the co-trimoxazole, chloroquine, and no-prophylaxis arms were 3.3, 4.2, and 4.2 per 100 pyo, respectively (Table 3). Although the observed event rate in the co-trimoxazole arm was lower than in the chloroquine and no-prophylaxis arms, neither comparison detected a statistically significant difference. Twenty-four deaths were reported: 10 in the co-trimoxazole group, 6 in the chloroquine group, and 8 in the no-prophylaxis group. Eleven deaths were caused by WHO HIV/AIDS stage 3 or 4 events. Causes of death are listed by study treatment arm (Supplementary Table 2).
Table 2.
Participants Who Experienced WHO Stage 2, 3, or 4 Events or Death by Treatment Arm
| Participants With Any Event | Co-trimoxazole (n = 500), n (%) | Chloroquine (n = 500), n (%) | No Prophylaxis (n = 499), n (%) | Total (N = 1499), n (%) |
|---|---|---|---|---|
| Adjudicated WHO stage 3+ event or deatha | 48 (10) | 62 (12) | 60 (12) | 170 (11) |
| WHO stage 2 event, adjudicated WHO stage 3+ event, or deathb | 57 (11) | 80 (16) | 79 (16) | 216 (14) |
Column header counts and denominators are the number of randomized participants. Participants are included in each row if they had any event of that type. Abbreviation: WHO, World Health Organization.
aIncludes deaths not otherwise classified as a WHO stage 3+ event.
bInvestigator-reported WHO stage 2 events are included because WHO stage 2 events are not adjudicated. Deaths are included if not otherwise classified as a WHO stage 2 event per investigator or adjudicated WHO stage 3+ event.
Table 3.
Incidence of Primary and Secondary Endpoint Events by Treatment Arm
| Co-trimoxazole vs Chloroquine | Co-trimoxazole vs No prophylaxis | |||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | Co-trimoxazole (n = 500, Pt-years = 1654), n (ratea) | Chloroquine (n = 500, Pt-years = 1646), n (ratea) | No Prophylaxis (n = 499, Pt-years = 1658), n (ratea) | Total (N = 1499, Pt-years = 4958), n (ratea) | Rate Ratio (95% CI) | P | Rate Ratio (95% CI) | P |
| Adjudicated WHO stage 3+ event or deathb | 54 (3.3) | 69 (4.2) | 69 (4.2) | 192 (3.9) | .78 (.55, 1.11) | .17 | .79 (.55, 1.12) | .18 |
| WHO stage 2 event, adjudicated WHO stage 3+ event, or deathc | 66 (4.0) | 94 (5.7) | 97 (5.8) | 257 (5.2) | .70 (.51, .96) | .026 | .68 (.50, .93) | .017 |
| Malariad, e | 63 (3.8) | 49 (3.0) | 465 (28.0) | 577 (11.6) | 1.28 (.49, 3.33) | .61 | .14 (.07, .27) | <.001 |
| Bacterial infectiond, f | 437 (26.4) | 591 (35.9) | 586 (35.3) | 1614 (32.6) | .74 (.63, .87) | <.001 | .75 (.64, .88) | <.001 |
| Grade 3+ adverse eventd | 209 (12.6) | 244 (14.8) | 239 (14.4) | 692 (14.0) | .85 (.66, 1.10) | .21 | .88 (.68, 1.12) | .31 |
Column header counts are the number of randomized participants. Abbreviations: CI, confidence interval; Pt-years, patient-years; WHO, World Health Organization.
aIncidence rate per 100 participant-years of observation through the last visit in the database.
bIncludes deaths not otherwise classified as a WHO Stage 3 + event.
cInvestigator-reported WHO Stage 2 events are included because WHO Stage 2 events are not adjudicated. Deaths are included if not otherwise classified as a WHO Stage 2 event per investigator or adjudicated WHO Stage 3 + event.
dRate ratio CIs and P values were adjusted for overdispersion using the Pearson’s chi-square scale. Rate ratios present the rate in co-trimoxazole participants relative to participants in the experimental group.
eAdverse event report of malaria or if no adverse event report, laboratory-confirmed malaria.
fLaboratory-confirmed bacterial infection or unconfirmed, suspected bacterial infection.
Figure 2.
A, Time to first adjudicated primary endpoint event. B, Time to first WHO stage 2+ event or death. Abbreviations: BL, baseline; Chloroq, chloroquine; CI, confidence interval; Co-trim, co-trimoxazole; HR, hazard ratio; No prop, no prophylaxis; WHO, World Health Organization.
When WHO HIV/AIDS stage 2 events were added to the primary endpoint of deaths and WHO HIV/AIDS stage 3 and 4 events, 216 participants experienced 257 events (Tables 2 and 3). The event rates of death or WHO HIV/AIDS stage 2, 3, or 4 illness in the co-trimoxazole, chloroquine, and no-prophylaxis arms were 4.0, 5.7, and 5.8 per 100 pyo, respectively. A Kaplan-Meier plot for time to death or to first WHO HIV/AIDS stage 2, 3, or 4 event is presented in Figure 2B and shows that time to first WHO HIV/AIDS stage 2+ event or death was longer in the co-trimoxazole arm versus the chloroquine (P = .026) and no-prophylaxis (P = .032) arms; the preventive effect and 95% CIs estimated by Cox proportional hazards achieved statistical significance to declare superiority of co-trimoxazole (co-trimoxazole vs no prophylaxis: .31; .03–.51; P = .032; co-trimoxazole vs chloroquine: .32; .04–.51; P = .026).
Participants on co-trimoxazole prophylaxis experienced a lower rate (26.4 per 100 pyo) of suspected or confirmed bacterial infections compared with those on chloroquine (35.9 per 100 pyo, P < .001) or no prophylaxis (35.3 per 100 pyo, P < .001) (Table 3). When comparing suspected bacterial infections by system organ class/preferred term, participants in the chloroquine and no-prophylaxis groups had increased rates of furuncle, abscess, pelvic inflammatory disease, cellulitis, paronychia, pyelonephritis, and dental caries relative to the co-trimoxazole prophylaxis group. In addition to these diagnoses, persons in the chloroquine group had increased rates for the following conditions relative to the co-trimoxazole group: urinary tract infection, wound infection, wound sepsis, and subcutaneous abscess. Persons in the no-prophylaxis group had increased risk for the following conditions relative to the co-trimoxazole group: bacterial conjunctivitis, limb abscess, acute tonsillitis, and acne (Supplementary Table 3, Supplementary Appendix).
Participants on co-trimoxazole prophylaxis experienced fewer P. falciparum clinical malaria episodes (3.8 per 100 pyo) than those on no prophylaxis (28.0 per 100 pyo, P < .001) and equivalent malaria episodes compared with chloroquine prophylaxis (3.0 per 100 pyo, P = .61) (Table 3).
The prevalence of detectable HIV viral load at post-baseline time points was 5% (24/491) in the co-trimoxazole arm, 7% (36/488) in the chloroquine arm, and 7% (33/489) in the no-prophylaxis arm. Likewise, the prevalence of CD4 cell count less than 200 cells/mm3 at all post-baseline time points was 5% (24/491) in the co-trimoxazole arm, 5% (23/488) in the chloroquine arm, and 6% (27/489) in the no-prophylaxis arm. Results were not statistically different by study arm.
Grade 3–5 adverse events occurred at a slightly lower rate in the co-trimoxazole group (12.6 per 100 pyo) compared with the chloroquine and no-prophylaxis groups (14.8 and 14.4 per 100 pyo, respectively), but this result was not statistically significant (P = .21 and .31, respectively) (Table 3 and Supplementary Table 4). Participants in the co-trimoxazole arm experienced increased grade 3+ neutropenia at a rate of 2.5 per 100 pyo, compared with 1.6 and 1.1 in the chloroquine and no-prophylaxis groups, respectively (Supplementary Table 4).
Discussion
This study is the first clinical trial in adults living with HIV in sub-Saharan Africa randomized to continue or discontinue co-trimoxazole prophylaxis with the primary objective to measure subjects with mortality and HIV-associated morbidity in the ART era. Discontinuing co-trimoxazole prophylaxis did result in increased death and WHO HIV/AIDS stage 3 and 4 events when compared with chloroquine or no prophylaxis, but this finding was not statistically significant. When the prespecified secondary endpoint of combining deaths or WHO HIV/AIDS stage 2, 3, and 4 events was compared among study arms, participants receiving co-trimoxazole prophylaxis experienced about one-third fewer events than participants on chloroquine or no prophylaxis. This modest but significant preventive effect was attributable to fewer bacterial infections in the co-trimoxazole group compared with the chloroquine and no-prophylaxis arms. Co-trimoxazole also provided effective protection against P. falciparum malaria, similar to prophylaxis with chloroquine, a drug known to be highly effective in Malawi [13, 17]. Differences in loss of viral suppression or decreases in CD4 cell count were not detected in the 3 groups.
The lack of statistically significant protection of co-trimoxazole prophylaxis against the primary endpoint likely resulted from a lower-than-expected event rate. The actual rate of 3.9 per 100 pyo was much less than the expected rate of 6.8 per 100 pyo, an estimate based on initial data over the first 1.5 years of follow-up in the current study. With our sample size and observed primary endpoint rate, the trial would have 80% or higher power to detect a 42% or higher protective effect of co-trimoxazole. Our results are similar to previous observational studies in adults and a clinical trial in children that demonstrated that continued co-trimoxazole therapy does not impact mortality but does lead to decreased overall risks of infections, hospitalizations, malaria, and diarrhea [7–9, 18].
Co-trimoxazole prophylaxis causes hematologic toxicity, including neutropenia [19, 20]. Adults living in sub-Saharan Africa with HIV on ART may be at increased risk for such hematologic toxicities [21–23], and we did find higher rates of neutropenia in the co-trimoxazole group. Despite this, participants on co-trimoxazole prophylaxis experienced fewer bacterial infections than participants on chloroquine or no prophylaxis, suggesting that neutropenia did not increase the risk of infections.
Although prophylaxis to prevent bacterial infections and malaria is appealing, its use, even in a targeted population, should be weighed against disadvantages, including cost, toxicity, pill burden, supply-chain demands, and antimicrobial resistance (AMR). The WHO has long advocated for interventions to combat AMR, including promoting judicious use of antimicrobials in the community [24]. The WHO 2014 global surveillance report on AMR documented the magnitude of co-trimoxazole resistance to common community-acquired infections, including urinary tract infections and pneumonia [25]. This reduced antimicrobial efficacy is particularly concerning for areas where few alternative antibiotics are available to treat infections. Co-trimoxazole prophylaxis use increases AMR among enteric organisms [26, 27], as well as to pneumococci that develop cross-resistance to penicillin [28], but these concerns have to be balanced against preventive effects against death and disease.
While our findings, based on a large dataset generated from extensive follow-up, are robust, generalizability may be limited by the strict eligibility criteria, geographically specific disease risk, and the intensive and high-quality clinical and laboratory follow-up the participants received. In this context of extensive access to clinical care, infections were detected and treated at early stages before they could progress to the severe and even life-threatening conditions that are common outcomes in low-resource settings. Extrapolating our findings to populations without similar access to ART, HIV viral load and CD4 monitoring and high-quality clinical care, and with a different disease burden should be done with caution [29–31]. An additional study limitation is the open-label design that was mitigated by blinding adjudication committee members to treatment arm, although endpoint detection bias by study clinicians could have influenced results and WHO HIV/AIDS stage 2 events were not adjudicated due to the high frequency of events and lack of objective evidence for these diagnoses. Our diagnoses for all WHO HIV/AIDS endpoint did follow standardized diagnostic criteria and were routinely reviewed by the supervising physicians.
In conclusion, after immune reconstitution, co-trimoxazole prophylaxis continued to provide modest benefit in preventing bacterial infections and malaria and was safe and well tolerated by adults on successful ART in Malawi. Other important considerations that may impact decisions about co-trimoxazole prophylaxis policy include the impact on AMR, ART adherence, cost, and procurement. Continuing co-trimoxazole prophylaxis may be most beneficial in areas with high malaria prevalence and in circumstances where regular HIV viral load and CD4 monitoring, as well as diagnostics and treatments for acute infections, are not readily available. We suggest that co-trimoxazole be continued in these settings, in accordance with the current WHO recommendations. However, when routine HIV management and clinical care in sub-Saharan Africa expands to include routine viral load and CD4 monitoring and diagnostic testing and treatment for bacterial infections, it may be possible to safely discontinue co-trimoxazole prophylaxis in healthy individuals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Authors’ contributions. M. B. L., R. G. M., N. N., O. M. N., T. H. D., M. K., F. A. M., L. T. G., W. N., E. M., T. E. T., J. M., C. V. P., J. J. v. O., and M. K. L. conceived the study, developed the protocol, developed the standardized operating procedures, conducted study procedures and ethical submissions, and reviewed the manuscript. M. D. and A. T. developed the statistical analysis plan and conducted analyses and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments. We thank the members of the study team for their many and invaluable contributions, the members of the National Institutes of Health (NIH) Division of AIDS Coinfections and Complications Data and Safety Monitoring Board for their oversight, the members of the endpoint review committee (Johnstone Kumwenda, MBChB, FRCP, University of Malawi College of Medicine, Blantyre, Malawi; Henry C. Mwandumba, MBChB, PhD, Malawi-Liverpool-Wellcome Trust, Blantyre, Malawi; Moffat J. Nyirenda, PhD, Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda; Camilla Rothe, MD, DTM&H, Ludwig-Maximilians-University of Munich, Munich, Germany), and NIH Division of AIDS staff (Sarah Read, MD; Beverly Alston-Smith, MD; Ellen DeCarlo, BSN; Chris Lambros, PhD). We are also grateful to the participants and the communities of Ndirande and Zomba for their dedication and commitment to this trial.
Deidentified individual data that underlie the results reported in this article will be made available to researchers who provide a methodologically sound proposal beginning 9 months and ending 36 months after article publication. Proposals should be directed to mlaufer@som.umaryland.edu.
Disclaimer. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (grant number U01AI089342) with additional support from grant number K24AI114996 to M. K. L.
Potential conflicts of interest. A. T. performed analysis for Data and Safety Monitoring Board reports through Statistics Collaborative, Inc. T. E. T. reports membership on the Novartis Institute for Tropical Diseases Strategic Advisory Board and the Novartis Institute for Tropical Diseases Malaria Advisory Council. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Anglaret X, Chêne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 1999; 353:1463–8. [DOI] [PubMed] [Google Scholar]
- 2.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d’Ivoire: a randomised controlled trial. Lancet 1999; 353:1469–75. [DOI] [PubMed] [Google Scholar]
- 3.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004; 364:1428–34. [DOI] [PubMed] [Google Scholar]
- 4.Chintu C, Bhat GJ, Walker AS, et al. ; CHAP Trial Team . Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 2004; 364:1865–71. [DOI] [PubMed] [Google Scholar]
- 5.Mussini C, Pezzotti P, Antinori A, et al. ; Changes in Opportunistic Prophylaxis (CIOP) Study Group . Discontinuation of secondary prophylaxis for Pneumocystis carinii pneumonia in human immunodeficiency virus-infected patients: a randomized trial by the CIOP Study Group. Clin Infect Dis 2003; 36:645–51. [DOI] [PubMed] [Google Scholar]
- 6.Lopez Bernaldo de Quiros JC, Miro JM, Pena JM, et al. A randomized trial of the discontinuation of primary and secondary prophylaxis against Pneumocystis carinii pneumonia after highly active antiretroviral therapy in patients with HIV infection. Grupo de Estudio del SIDA 04/98. N Engl J Med 2001; 344:159–67. [DOI] [PubMed] [Google Scholar]
- 7.Walker AS, Ford D, Gilks CF, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet 2010; 375:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anywaine Z, Levin J, Kasirye R, et al. ; COSTOP Research Team . Discontinuing cotrimoxazole preventive therapy in HIV-infected adults who are stable on antiretroviral treatment in Uganda (COSTOP): a randomised placebo controlled trial. PLoS One 2018; 13:e0206907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JD, Moore D, Degerman R, et al. HIV-infected Ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/μL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis 2012; 54:1204–11. [DOI] [PubMed] [Google Scholar]
- 10.Polyak CS, Yuhas K, Singa B, et al. Cotrimoxazole prophylaxis discontinuation among antiretroviral-treated HIV-1-infected adults in Kenya: a randomized non-inferiority trial. PLoS Med 2016; 13:e1001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2014. [PubMed] [Google Scholar]
- 12.National Malaria Control Programme, Ministry of Health, Government of Malawi. Malawi malaria indicator survey 2017. Malawi malaria indicator survey 2017. Lilongwe, Malawi: Ministry of Health, Government of Malawi, : 2018. [Google Scholar]
- 13.Laufer MK, Thesing PC, Eddington ND, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med 2006; 355:1959–66. [DOI] [PubMed] [Google Scholar]
- 14.Laurens MB, Mungwira RG, Nyirenda OM, et al. TSCQ study: a randomized, controlled, open-label trial of daily trimethoprim-sulfamethoxazole or weekly chloroquine among adults on antiretroviral therapy in Malawi: study protocol for a randomized controlled trial. Trials 2016; 17:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malawi Ministry of Health. Clinical management of HIV in children and adults: Malawi integrated guidelines. Lilongwe, Malawi: Malawi Ministry of Health. 2011. [Google Scholar]
- 16.World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance: African region. Geneva, Switzerland: World Health Organization, 2005. [Google Scholar]
- 17.Frosch AE, Laufer MK, Mathanga DP, et al. Return of widespread chloroquine-sensitive Plasmodium falciparum to Malawi. J Infect Dis 2014; 210:1110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med 2014; 370:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imrie KR, Prince HM, Couture F, Brandwein JM, Keating A. Effect of antimicrobial prophylaxis on hematopoietic recovery following autologous bone marrow transplantation: ciprofloxacin versus co-trimoxazole. Bone Marrow Transplant 1995; 15:267–70. [PubMed] [Google Scholar]
- 20.Woods WG, Daigle AE, Hutchinson RJ, Robison LL. Myelosuppression associated with co-trimoxazole as a prophylactic antibiotic in the maintenance phase of childhood acute lymphocytic leukemia. J Pediatr 1984; 105:639–44. [DOI] [PubMed] [Google Scholar]
- 21.Ssali F, Stöhr W, Munderi P, et al. ; DART Trial Team . Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antivir Ther 2006; 11:741–9. [DOI] [PubMed] [Google Scholar]
- 22.Moh R, Danel C, Sorho S, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Côte d’Ivoire. Antivir Ther 2005; 10:615–24. [DOI] [PubMed] [Google Scholar]
- 23.Toure S, Gabillard D, Inwoley A, Seyler C, Gourvellec G, Anglaret X. Incidence of neutropenia in HIV-infected African adults receiving co-trimoxazole prophylaxis: a 6-year cohort study in Abidjan, Côte d’Ivoire. Trans R Soc Trop Med Hyg 2006; 100:785–90. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. The evolving threat of antimicrobial resistance – options for action. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 25.World Health Organization. Antimicrobial resistance: global report on surveillance 2014. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 26.Luvsansharav UO, Wakhungu J, Grass J, et al. Exploration of risk factors for ceftriaxone resistance in invasive non-typhoidal Salmonella infections in western Kenya. PLoS One 2020; 15:e0229581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powis KM, Souda S, Lockman S, et al. Cotrimoxazole prophylaxis was associated with enteric commensal bacterial resistance among HIV-exposed infants in a randomized controlled trial, Botswana. J Int AIDS Soc 2017; 20:e25021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seid M, Beyene G, Alemu Y, et al. Does cotrimoxazole prophylaxis in HIV patients increase the drug resistance of pneumococci? A comparative cross-sectional study in southern Ethiopia. PLoS One 2020; 15:e0243054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One 2011; 6:e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Kop ML, Thabane L, Awiti PO, et al. Advanced HIV disease at presentation to care in Nairobi, Kenya: late diagnosis or delayed linkage to care? A cross-sectional study. BMC Infect Dis 2016; 16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndlovu Z, Burton R, Stewart R, et al. Framework for the implementation of advanced HIV disease diagnostics in sub-Saharan Africa: programmatic perspectives. Lancet HIV 2020; 7:e514–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.