Abstract
Background
Current guidelines recommend community-wide mass azithromycin for trachoma, but a targeted treatment strategy could reduce the volume of antibiotics required.
Methods
In total, 48 Ethiopian communities were randomized to mass, targeted, or delayed azithromycin distributions. In the targeted arm, only children aged 6 months to 5 years with evidence of ocular chlamydia received azithromycin, distributed thrice over the following year. The primary outcome was ocular chlamydia at months 12 and 24, comparing the targeted and delayed arms (0–5 year-olds, superiority analysis) and the targeted and mass azithromycin arms (8–12 year-olds, noninferiority analysis, 10% noninferiority margin).
Results
At baseline, the mean prevalence of ocular chlamydia in the 3 arms ranged from 7% to 9% among 0–5 year-olds and from 3% to 9% among 8–12 year-olds. Averaged across months 12–24, the mean prevalence of ocular chlamydia among 0–5 year-olds was 16.7% (95% confidence interval [CI]: 9.0%–24.4%) in the targeted arm and 22.3% (95% CI: 11.1%–33.6%) in the delayed arm (P = .61). The final mean prevalence of ocular chlamydia among 8–12 year-olds was 13.5% (95% CI: 7.9%–19.1%) in the targeted arm and 5.5% (95% CI: 0.3%–10.7%) in the mass treatment arm (adjusted risk difference 8.5 percentage points [pp] higher in the targeted arm, 95% CI: 0.9 pp–16.1 pp higher).
Conclusions
Antibiotic treatments targeted to infected preschool children did not result in significantly less ocular chlamydia infections compared with untreated communities and did not meet noninferiority criteria relative to mass azithromycin distributions. Targeted approaches may require treatment of a broader segment of the population in areas with hyperendemic trachoma.
Keywords: trachoma, chlamydia, mass drug administration, antibacterial agents, Africa
Ethiopian communities were randomized to 1 of 3 arms: triannual azithromycin treatments targeted to preschool children infected with ocular chlamydia, community-wide mass azithromycin, or delayed treatment. Targeted treatments did not reduce ocular chlamydia infections among untreated community members.
The World Health Organization (WHO) includes annual community-wide antibiotic distributions in its trachoma elimination strategy in order to clear the ocular strains of chlamydia that cause the disease [1]. However, treatment is most important for preschool children, because this age group has more prevalent trachoma, higher infectious loads, and a longer duration of infections [2–6]. These children likely form a core group responsible for most transmission [7, 8]. Targeting antibiotic treatments to preschool children could remove this source of community transmission while having the ancillary benefit of limiting selection of antimicrobial resistance and reducing cost [8–13].
We conducted a cluster-randomized trial to assess the effectiveness of a targeted antibiotic strategy for trachoma. The study was set in Ethiopia, which continues to have persistently high trachoma despite years of repeated mass azithromycin distributions. Cluster-randomization was important because we were interested both in the direct and indirect effects of community-wide antibiotic distributions [14]. We hypothesized that antibiotic treatments targeted to infected preschool children would be superior to absence of treatment in terms of reducing ocular chlamydia and noninferior to community-wide antibiotics.
METHODS
Study Design
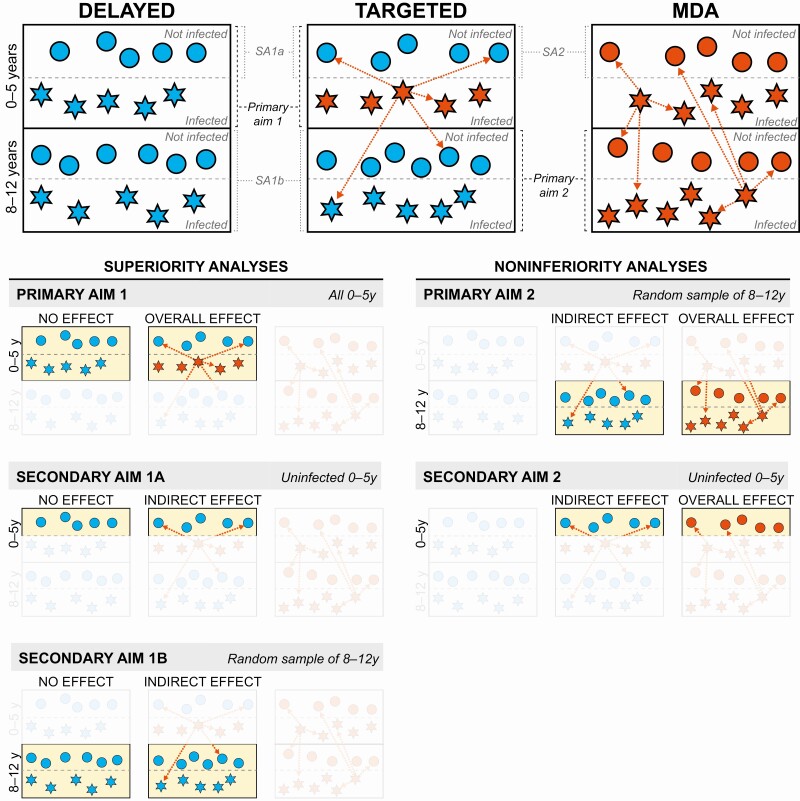
The Targeted Antibiotic Intervention for Trachoma in Under-Fives (TAITU) trial was a parallel-group, multiple-arm, cluster-randomized trial set in WagHemra Zone, Amhara Region, Ethiopia, whose field activities ranged from 9 November 2015 until 18 March 2018. Eligible clusters were randomized to 1 of 3 arms: (A) annual mass drug administration (MDA) of azithromycin to the entire community, (B) targeted triannual azithromycin treatments directed to 0–5 year-old children with ocular chlamydia, and (C) delayed treatment. The primary outcome was ocular chlamydia infection, assessed 12 and 24 months after baseline in 2 study populations: children aged 0–5 years of age (ie, those most likely to transmit ocular chlamydia) and children 8–12 years of age (ie, children who should never have received a targeted treatment earlier in the study but should still have a relatively high burden of ocular chlamydia and thus provide adequate statistical power). Mass antibiotic treatments convey a direct effect for the person receiving the drug and also an indirect effect for other community members by preventing transmission that could have occurred. The direct and indirect effects hypothesized in this trial are shown in Figure 1 [15]. Ethical approval was obtained from the University of California, San Francisco, Emory University, the Ethiopian Food and Drug Authority, and the Ethiopian Ministry of Innovation and Technology. Verbal consent was obtained from guardians given high illiteracy in the study area. The manual of procedures and statistical analysis plan are available online (https://osf.io/bu8qs/ and https://osf.io/zq3md/, respectively).
Figure 1.
Schematic showing potential direct and indirect effects in the 3 treatment arms. Each box represents a community in one of the three treatment arms, stratified by age group (0–5 y on top, 8–12 y on bottom) and infection status at baseline (infected on bottom, not infected on top). Circles represent uninfected children and stars represent infected children. Red symbols receive antibiotic treatment and blue symbols do not. Arrows represent potential indirect effects of treatment through reduced transmission. Children in the mass drug administration (MDA) communities experienced both the direct and indirect effects of antibiotics. In the targeted communities, the infected children experienced both direct and indirect effects of antibiotics, but the uninfected children could only benefit from indirect effects. The delayed-treatment communities received no antibiotics at all. Primary analysis 1 assessed whether the overall effect (ie, the direct and indirect effects at the community level) of targeted treatments was superior to no treatment; secondary analyses (SA) 1A and 1B assessed whether the indirect effects of targeted treatment were superior to no treatment. Primary analysis 2 and SA 2 assessed whether the indirect effects of targeted treatments were noninferior to the overall effect (ie, community-level direct and indirect effects) of mass azithromycin.
Eligibility
The unit of randomization was the “gott,” an Ethiopian demographic unit termed a village for this report. The study population consisted of 48 villages situated in 3 contiguous districts (ie, Sekota Zuria, Sekota Ketema, and Gazgibella) that had not been selected for a concurrent randomized trial in the same study area [16]. The largest population center of each district was excluded because trachoma is less common in urban areas, and villages further than a 3-hour walk from the nearest gravel road were not included due to the logistical complexities of reaching such communities [17]. All consenting community members were enrolled in the study and offered treatment according to the assigned treatment arm. Annual monitoring for trachoma was offered in each village to all children aged 0–5 years and to a random sample of 30 children aged 8–12 years. A separate random sample of 8–12 year-olds was drawn for each monitoring visit based on an annual census performed 2–4 weeks earlier, so any given child might or might not have been selected for multiple monitoring visits.
Setting
The study area had received mass azithromycin distributions annually from May 2009 to June 2015, with a supplemental treatment in October 2014. Trachoma impact surveys done in 2012 and 2014 found trachomatous inflammation, follicular (TF) in 59% of 1–9 year-old children, the highest in the region [18, 19].
Randomization
Once the baseline census and monitoring visits were complete, the trial biostatistician performed simple random sampling to allocate villages in a 1:1:1 ratio to annual mass azithromycin distributions, targeted azithromycin, or delayed treatment using the statistical program R (R for Statistical Computing, Vienna, Austria). The study coordinator assigned the allocated intervention. Allocation was concealed by performing the randomization after all communities had been enrolled. Study participants were not masked due to the nature of the intervention. Laboratory staff were masked to treatment allocation, implemented by using only a 5-digit random number to label specimens.
Monitoring
Monitoring visits were performed approximately 1 month following the annual census, at baseline, month 12, and month 24. All children aged 0–5 years and a random sample of 30 children 8–12 years of age were offered conjunctival swabbing of the everted right upper eyelid with a Dacron swab. Swabs were stored on ice in the field, then at −20ºC for up to 4 weeks, then transported on ice to Bahir Dar, Ethiopia, where they were stored in a −20ºC freezer until processed. Swabs from the same village and age group were pooled in groups of 5, then processed for the presence of Chlamydia trachomatis DNA with the Abbott RealTime assay on the m2000 platform. Swabs from positive pools of 0–5 year-olds were subsequently tested individually and the results used to determine which children to treat in the targeted treatment arm. Village-level prevalence measures in the older age group were determined using maximum likelihood estimation based on pooled results. Although chlamydial results were used for treatment decisions only in the targeted azithromycin arm, testing was done identically for all treatment arms in a masked fashion, with a processing time goal of <2 months.
Intervention
In villages randomized to annual distributions, all community members 6 months or older on the most recent census were offered a single dose of oral azithromycin (20 mg/kg for children using height-based approximation, 1 g for adults). Children under 6 months of age, pregnant women, and those allergic to macrolide antibiotics were offered 2 tubes of topical tetracycline, to be used twice daily for 6 weeks. The mass treatment coverage was ≥80% per village, as per WHO guidelines [1]. In villages randomized to targeted treatment, children aged 0–5 years at baseline who tested positive for chlamydia were offered 3 20 mg/kg doses of azithromycin approximately every 4 months over the first year of the trial (ie, triannual treatment), and children aged 0–5 years at the month 12 census who tested positive were offered the same treatment schedule over the second year of the trial. No antibiotics were distributed in the delayed treatment arm until the conclusion of the trial. Treatment visits in the annual and targeted treatment arms occurred over the same 2-week period after the ocular chlamydia testing was complete; this was approximately 3 months after the preceding monitoring visit.
Statistical Considerations
Two primary analyses were prespecified, performed in an intention-to-treat fashion without adjustment for postrandomization covariates. Statistical significance was determined by Monte Carlo permutation (10 000 permutations). Because the 2 main analyses compared outcomes from a different age group and in a different pair of arms, the trial’s Data and Safety Monitoring Committee afforded a 2-sided alpha of 0.05 for each analysis.
Targeted vs Delayed Treatment
The aim of the first primary analysis was to determine whether the overall effect of the targeted treatment arm (ie, both the direct and indirect effects of antibiotic treatments) was superior to no treatment in terms of reducing ocular chlamydia infection. Specifically, the vector of individual-level dichotomous chlamydia results in the 0–5 year-old age group from months 12 and 24 was modeled in a mixed effects logistic regression model, using baseline chlamydia results and treatment assignment as covariates and a random intercept for village. Two secondary analyses specifically assessed for an indirect effect of targeted antibiotics: (i) a Poisson regression modeling counts of incident cases of ocular chlamydia among those negative at baseline and thus untreated in the targeted arm, using time from the baseline monitoring visit as the offset, and (ii) a village-level analysis for the 8–12 year-old age group (Figure 1).
Targeted vs Mass Treatment
The aim of the second primary analysis was to determine whether the indirect effect for untreated community members in the targeted-treatment arm could approach the overall effect (ie, both direct and indirect effect) had they actually received antibiotic treatment. In a noninferiority analysis, the village-level prevalence of ocular chlamydia among individuals aged 8–12 years was compared between the targeted azithromycin arm and the mass azithromycin arm. Specifically, the final 24-month prevalence values for each village were modeled in a linear regression, with baseline prevalence values and treatment assignment as covariates. A noninferiority test was conducted using a prespecified noninferiority margin of 10%, based on a 2-sided 95% confidence interval (CI) for the difference in 8–12 year-old prevalence between groups. A secondary analysis was performed among children aged 0–5 years who were not infected at baseline using a Poisson regression model as specified above but comparing the targeted and mass treatment arms (Figure 1). Square root transformation of prevalence outcomes improved normality of model residuals but did not change conclusions; we report untransformed outcomes to ease interpretation.
Sample Size
Assuming 30 children per community, a loss to follow-up of 15%, a community-level intraclass correlation coefficient (ICC) of 0.01 for ocular chlamydia infection, a final chlamydia prevalence of 15% in the control arm, a correlation of 0.5 between individual-level baseline and final chlamydia status, and an alpha of 0.05, then 16 communities per arm would provide >80% power to detect an absolute effect size of 7.5% between the targeted and delayed arms. This same sample size would provide at least 80% power to test a noninferiority margin of a 10% difference between the study arms, assuming a standard deviation of 0.10, a correlation of 0.50 between the baseline and final prevalence, and an alpha of 0.05. Correlation parameters were based on our own previous published and unpublished studies in Ethiopia [20].
RESULTS
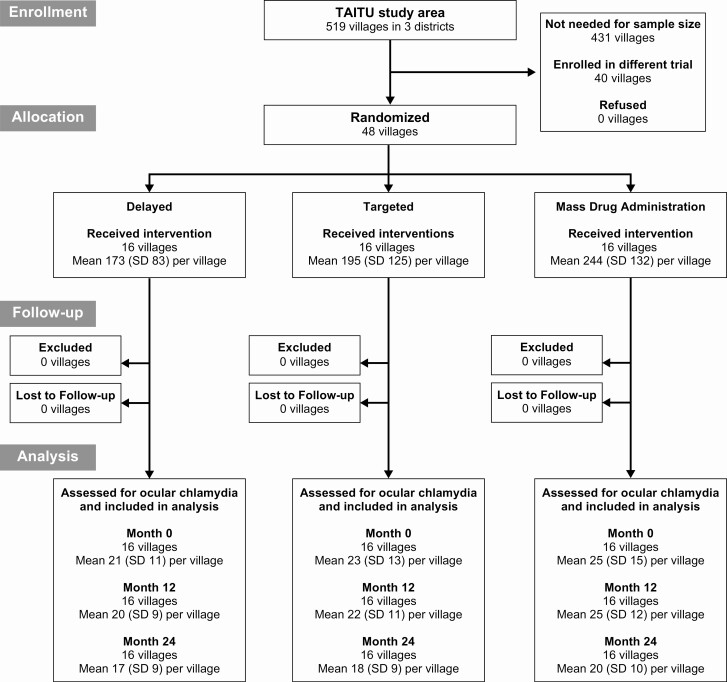
The trial flow is shown in Figure 2. Baseline demographic characteristics for the 3 treatment arms are shown in Table 1. The baseline prevalence of ocular chlamydia was similar in the 3 arms, with a mean prevalence ranging from 7.8% to 9.3% among 0–5 year-olds and from 3.0% to 8.6% among 8–12 year-olds (Supplementary Table 1). All communities received their allocated study intervention, with high antibiotic coverage to those eligible for treatment (Table 2). All communities in the MDA arm achieved at least 80% coverage during both years of the study. In the targeted treatment arm, all 37 (100%) children testing positive for ocular chlamydia at the baseline visit received all 3 azithromycin treatments, as did 114 of 118 (97%) testing positive at month 12, including 6 children who were positive at both time points. The 4 children eligible for targeted treatment who did not receive the full 3 doses moved out of the study area before all doses could be administered. No communities in the delayed treatment arm mistakenly received treatment. No communities were lost to follow-up.
Figure 2.
Trial flow. Abbreviation: SD, standard deviation.
Table 1.
Baseline Characteristics Per Community by Study Arm, as Assessed From a Population Census
| Mean (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Delayed (N = 16) | MDA (N = 16) | Targeted (N = 16) | |||
| Households, no. | 40 | (30–49) | 54 | (37–70) | 42 | (29–56) |
| Persons, no. | 173 | (128–217) | 244 | (174–315) | 195 | (128–262) |
| Age fraction, % | ||||||
| 0–5 years | 18% | (16–20%) | 20% | (19–21%) | 18% | (17–20%) |
| 6–7 years | 7% | (6–8%) | 6% | (6–7%) | 6% | (6–7%) |
| 8–12 years | 14% | (12–15%) | 15% | (14–15%) | 15% | (14–17%) |
| ≥13 years | 62% | (59–64%) | 59% | (58–61%) | 60% | (57–63%) |
| Sex fraction, % | 49% | (47–52%) | 50% | (49–51%) | 50% | (48–53%) |
| Elevation, m | 2140 | (2019–2260) | 2052 | (1939–2164) | 2117 | (2005–2228) |
| Distance from main town, km | 19.1 | (14.1–24.1) | 17.6 | (12.1–23.1) | 19.4 | (14.4–24.5) |
Abbreviations: CI, confidence interval; MDA, mass drug administration.
Table 2.
Treatment Coverage of Eligible Populations per Study Community, as Assessed from a Population Census
| Mean (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDA (N = 16) | Targeted (N = 16) | |||||||
| Characteristic | First Year | Second Year | First Year | Second Year | ||||
| All ages | ||||||||
| Total, no. | 244 | (174–315) | 252 | (166–338) | 195 | (128–262) | 228 | (155–300) |
| Eligible for treatment, no. | 244 | (174–315) | 252 | (166–338) | 2 | (1–4) | 10 | (4–15) |
| Received treatment, no.a | 216 | (156–275) | 216 | (145–286) | 2 | (1–4) | 9 | (4–14) |
| Coverage (eligible), % | 89% | (87–91%) | 86% | (84–89%) | 100% | (>99–100%) | 98% | (96–100%) |
| Coverage (total), % | 89% | (87–91%) | 86% | (84–89%) | 1% | (>0–2%) | 3% | (2–5%) |
| 0–5 years | ||||||||
| Total, no. | 49 | (33–66) | 49 | (28–70) | 38 | (22–53) | 40 | (27–52) |
| Eligible for treatment, no. | 49 | (33–66) | 49 | (28–70) | 2 | (1–4) | 10 | (4–15) |
| Received treatment, no.a | 45 | (30–61) | 44 | (26–62) | 2 | (1–4) | 9 | (4–14) |
| Coverage (eligible), % | 92% | (89–96%) | 91% | (87–95%) | 100% | (>99–100%) | 98% | (96–100%) |
| Coverage (total), % | 92% | (89–96%) | 91% | (87–95%) | 7% | (2–12%) | 17% | (7–27%) |
| 8–12 years | ||||||||
| Total, no. | 36 | (26–46) | 38 | (25–50) | 30 | (20–40) | 34 | (23–46) |
| Eligible for treatment, no. | 36 | (26–46) | 38 | (25–50) | 0 | (0–0) | 0 | (0–0) |
| Received treatment, no.a | 33 | (24–43) | 33 | (22–44) | 0 | (0–0) | 0 | (0–0) |
| Coverage (eligible), % | 93% | (90–96%) | 88% | (83–92%) | 0% | (0–0%) | 0% | (0–0%) |
| Coverage (total), % | 93% | (90–96%) | 88% | (83–92%) | 0% | (0–0%) | 0% | (0–0%) |
No one in the 16 delayed treatment villages was eligible for or received treatment during the study. The mean total population in the delayed treatment arm was 173 (95% CI: 128–217) for the first year and 186 (95% CI: 141–231) for the second year; the mean population of 0–5 year-olds was 31 (95% CI: 22–40) for the first year and 34 (95% CI: 25–43) for the second year; the mean population of 8–12 year-olds was 24 (95% CI: 17–31) for the first year and 25 (95% CI: 18–32) for the second year.
Abbreviations: CI, confidence interval; MDA, mass drug administration.
a Indicates a single dose of azithromycin for the MDA group and all 3 treatments for the targeted group.
Targeted vs Delayed Treatment
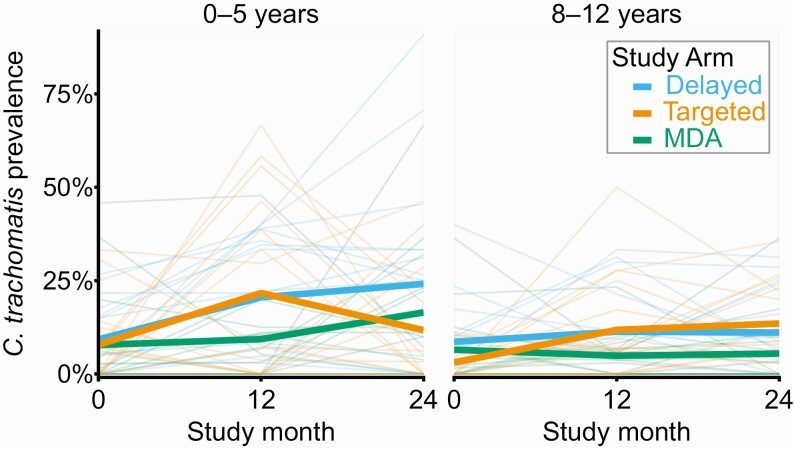
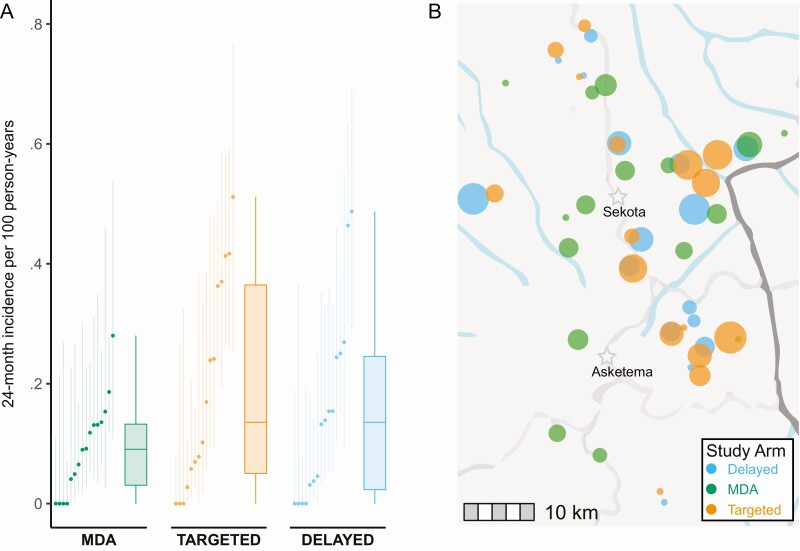
Village-level prevalence among children 0–5 years old increased in all arms over the two monitoring visits (Figure 3 and Supplementary Table 1). Averaged over the month 12 and 24 follow-up visits, the mean prevalence of ocular chlamydia among children aged 0–5 years was 22.3% (11.1%–33.6%) in the delayed treatment arm and 16.7% (9.0%–24.4%) in the targeted treatment arm. Compared with the delayed treatment arm, the targeted treatment arm had fewer ocular chlamydia infections detected among 0–5 year-olds over the two monitoring visits of the study—although the difference was not statistically significant: adjusted odds ratio (OR) 0.75 (95% CI: .25–2.26; P = .61; primary analysis 1). The prevalence of ocular chlamydia among 0–5 year-olds decreased markedly in the targeted arm during the second year of the study (Figure 3), but a sensitivity analysis of the month 24 village-level prevalence showed no significant difference between arms (mean prevalence 12.9 pp lower in targeted arm, 95% CI: 27.7 points lower to 2.4 points higher; P = .11; population-weighted analysis, adjusted for baseline prevalence). No evidence for an indirect effect of antibiotics was found in a secondary analysis assessing the incidence of new chlamydia infections among 0–5 year-olds over the 24-month study (mean incidence 19.1 [95% CI: 9.8 to 28.5] new infections per 100 person years in targeted villages versus 15.1 [95% CI: 6.7 to 23.5] per 100 person years in delayed villages; adjusted risk difference [RD] 2.9 percentage points [pp] (95% CI: −13.6 pp to 19.4 pp; P = .74; secondary analysis 1a; Figure 4). Prevalence figures for the 8–12 year-old age group are shown for each study village in Figure 3 and Supplementary Table 1; chlamydial infection among this older age group did not differ between the 2 arms at 24 months, providing no evidence of an indirect antibiotic effect (mean prevalence 2.5 pp higher in the targeted treatment arm, 95% CI: 5.8 pp lower to 10.8 pp higher; secondary analysis 1b; P = .53).
Figure 3.
Village-level prevalence of ocular chlamydia, stratified by age group. The left-hand panel shows the prevalence in a random sample of 0–5 year-olds and the right-hand panel in a random sample of 8–12 year-olds; a new random sample was selected at each monitoring visit. Heavy lines summarize group averages. Abbreviation: MDA, mass drug administration.
Figure 4.
Incidence of new ocular chlamydia infections over 24 months among children ages 0–5 years not infected at baseline. Panel A depicts for each treatment arm the incidence estimate and 95% confidence interval for each village on the left, and a box and whiskers plot summarizing all villages on the right. Panel B depicts the same data on a map of the study area, with each marker corresponding to a village, and markers sized according to the magnitude of the incidence estimate. The dark gray line represents the Zonal border; the light gray lines represent the major roads; the blue lines represent surface water; and the stars represent the 2 district administrative centers in the study area (base map: OpenStreetMap). Abbreviation: MDA, mass drug administration.
Targeted vs Mass Treatment
At month 24, the mean prevalence of ocular chlamydia among 8–12 year-olds was 5.5% (95% CI: .3%–10.7%) in the MDA arm and 13.5% (95% CI: 7.9%–19.1%) in the targeted azithromycin arm. The prevalence of ocular chlamydia at month 24 was on average 8.5 pp higher in the targeted azithromycin arm than the MDA arm after adjusting for baseline prevalence, with a 95% CI of 0.9 pp to 16.1 pp higher (ie, not meeting the prespecified criteria for noninferiority, which would have required the upper bound to be lower than 10%; primary analysis 2). We performed an analogous comparison of the 0–5 year-old age group by comparing new chlamydial infections among children with a negative chlamydia test at baseline (who were thus not eligible for treatment in the targeted arm); this analysis found a mean 24-month incidence of 19.1 new infections per 100 person-years in the targeted arm compared with 9.2 per 100 person-years in the MDA arm (adjusted RD 10.6 pp, 95% CI: −2.6 pp to 23.8 pp; P = .13; secondary analysis 2; Figure 4). Other pairwise village-level comparisons are shown in Supplementary Table 2.
DISCUSSION
This cluster-randomized trial showed no significant differences in ocular chlamydia between communities assigned to mass antibiotic distributions, targeted antibiotic treatments, or delayed treatment over a 2-year study period. Analyses designed to assess whether targeted antibiotic treatments had an indirect effect for untreated community members failed to show any statistically significant differences.
In the present study, the putative core group was identified from the population of preschool children based on the presence of C. trachomatis from a conjunctival swab, because hyper-transmitters would be expected to be more frequently infected with ocular chlamydia at any given time. Preschool children were purposefully chosen because chlamydia infections were likely to be most frequent and of highest load in this age group, thus offering the chance to catch many or even most of the infections while limiting the number of children requiring testing. In hindsight, the testing approach was likely too restrictive in this hyperendemic setting, because the prevalence of infection was not much lower among 8–12 year-olds, suggesting that some among this age stratum were also likely hyper-transmitters. It is possible that the effect of our targeting strategy would be different in areas with less trachoma. Future efforts at targeting antibiotic treatments in trachoma-hyperendemic settings might choose to widen the target population or alternatively to treat all household members of an infected child, because the entire household would be at increased risk of infection and hence transmission.
The results of the present study underscore the challenges facing antibiotic programs to eliminate trachoma from the most hyperendemic regions. The study area had received repeated rounds of mass azithromycin for the 7 years before the study, and yet the prevalence of ocular infection was still high at baseline. Infection tended to increase over the duration of the study in both monitored age groups. Even the arm continuing to receive annual mass azithromycin experienced an increase in infection from month 12 to month 24, indicating a force of infection stronger than has been observed in prior studies in Amhara [21, 22]. The lack of efficacy of continued mass azithromycin may not be generalizable outside this region of Ethiopia, which has some of the most hyperendemic trachoma in the world. Moreover, some of the surrounding communities enrolled in a separate trial did not receive antibiotics during the study period, so it is possible that infections originating outside the study communities eventually overwhelmed the specific study interventions, leading to an increase in transmission (ie, contamination). Nonetheless, this study provides more evidence that annual treatments alone are unlikely to eliminate trachoma in places with extremely hyperendemic disease [22]. New strategies are needed for these areas, perhaps incorporating additional antibiotic treatments or supplementing antibiotics with programs to improve water, sanitation, and hygiene.
This trial had several limitations. As mentioned above, cluster-randomized trials are at risk of contamination from nearby clusters receiving a different treatment, and in this case, it is possible infections were introduced by neighboring communities receiving less antibiotics. Moreover, the targeted treatment arm experienced a delay between swabbing and treatment, during which time infected children may have spread ocular chlamydia to other children. The relatively small number of clusters and small size of some clusters may have reduced statistical power, although sensitivity analyses taking village size into account did not change the conclusions. The targeted treatment strategy appeared to be more effective during the second year of the trial, perhaps because more infected children were identified and treated at the 12-month visit. It is unclear whether a targeted treatment strategy would be more effective in areas that had not previously received many years of mass azithromycin, or in areas with less prevalent trachoma.
In summary, a cluster-randomized trial conducted in the Amhara region of Ethiopia with hyperendemic trachoma failed to find evidence that antibiotic treatments targeted to infected pre-school children would provide a community-wide benefit. An intervention more intensive than the status quo of annual mass azithromycin distributions is likely to be necessary for controlling trachoma in areas with a high prevalence of disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank administrative staff members of the Carter Center Ethiopia (Sintayehu Gebresillasie, Mesfin Seifu, Mulat Tarekegn, Habtamu Workneh, and Mulat Zerihun), the field staff in WagHemra, Ethiopia (Birhanu Abebe, Hirut Afewereke, Daniel Alem, Dagmawi Ali, Girma Amare, Yinges Amsale, Fire Assefa, Lasta Atnafie, Zerf Ayele, Kalkidan Belay, Mesele Berie, Mekides Berihun, Yordanos Beyene, Biniam Birhanu, Amare Bitew, Desalegn Chanie, Habtamu Debash, Hirut Dessie, Yohhanes Dessie, Tayech Dessie, Desallegn Emagnu, Mehari Engedaw, Abebe Fentaw, Daniel G/egziabher, Abeba G/hiwot, Birhane G/kidan, Gebremedhin Geberezabeher, Mequant Geta, Asefash Getachew, Tegegne Getaneh, Genet Getawoy, Belaynesh Getu, Tsega Giroum, Awoke Habtie, Melaku Hiluf, Adna Kasaye, Lubaba Kassaye, Habtam Kindie, Demelash Kinfe, Aregawi Mammo, Dinke Mammo, Tru Mammo, Meseret Mengestie, Selam Mengestu, Mefikrseb Mesfin, Samuel Melku, Amare Misene, Emebet Moges, Habtu Moges, Yibeltal Mohammed, Abraham Negash, Marye Nigatie, Abdulatif Oumer, Kenedi Shegaw, Assefa Sissay, Bethelehem Tadesse, Debas Tadesse, Mekuanint Tametew, Mandefro Tebekaw, Ashenafy Teka, Samrawit Tsegaye, Yirgalem Tsegaye, Assefa Tsehayu, Solomon Wagnew, Getachew Wodaj, Mimi Wondmu, Marye Workie, Mesfin Wudu, Tigst Zerihun), the trial’s Data and Safety Monitoring Committee (William Barlow [chair], Leslie Hyman, Art Reingold, Serge Resnikoff, Larry Schwab, and Carrie Thiessen), and our National Institutes of Health Program Officer Don Everett.
Financial support. This work was supported by the National Eye Institute at the National Institutes of Health (grant number U10EY023939), That Man May See, the Peierls Foundation, the Alta California Eye Research Foundation, and Research to Prevent Blindness. Abbott Laboratories donated the m2000 RealTime molecular diagnostics system and consumables. The International Trachoma Initiative donated the azithromycin.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Solomon AW, World Health Organization , London School of Hygiene and Tropical Medicine, International Trachoma Initiative. Trachoma control: a guide for programme managers. Geneva: World Health Organization, 2006. [Google Scholar]
- 2.Bailey R, Duong T, Carpenter R, Whittle H, Mabey D. The duration of human ocular Chlamydia trachomatis infection is age dependent. Epidemiol Infect 1999; 123:479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet 2003; 362:198–204. [DOI] [PubMed] [Google Scholar]
- 4.Solomon AW, Holland MJ, Alexander ND, et al. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med 2004; 351:1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West ES, Munoz B, Mkocha H, et al. Mass treatment and the effect on the load of Chlamydia trachomatis infection in a trachoma-hyperendemic community. Invest Ophthalmol Vis Sci 2005; 46:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton MJ, Holland MJ, Makalo P, et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet 2005; 365:1321–8. [DOI] [PubMed] [Google Scholar]
- 7.Lietman T, Porco T, Dawson C, Blower S. Global elimination of trachoma: how frequently should we administer mass chemotherapy? Nat Med 1999; 5:572–6. [DOI] [PubMed] [Google Scholar]
- 8.House JI, Ayele B, Porco TC, et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet 2009; 373:1111–8. [DOI] [PubMed] [Google Scholar]
- 9.Frick KD, Lietman TM, Holm SO, Jha HC, Chaudhary JS, Bhatta RC. Cost-effectiveness of trachoma control measures: comparing targeted household treatment and mass treatment of children. Bull World Health Organ 2001; 79:201–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Schémann JF, Guinot C, Traore L, et al. Longitudinal evaluation of three azithromycin distribution strategies for treatment of trachoma in a sub-Saharan African country, Mali. Acta Trop 2007; 101:40–53. [DOI] [PubMed] [Google Scholar]
- 11.Laming AC, Currie BJ, DiFrancesco M, Taylor HR, Mathews JD. A targeted, single-dose azithromycin strategy for trachoma. Med J Aust 2000; 172:163–6. [DOI] [PubMed] [Google Scholar]
- 12.Holm SO, Jha HC, Bhatta RC, et al. Comparison of two azithromycin distribution strategies for controlling trachoma in Nepal. Bull World Health Organ 2001; 79:194–200. [PMC free article] [PubMed] [Google Scholar]
- 13.Atik B, Thanh TT, Luong VQ, Lagree S, Dean D. Impact of annual targeted treatment on infectious trachoma and susceptibility to reinfection. JAMA 2006; 296:1488–97. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin-Chung J, Abedin J, Berger D, et al. Spillover effects on health outcomes in low- and middle-income countries: a systematic review. Int J Epidemiol 2017; 46:1251–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin-Chung J, Arnold BF, Berger D, et al. Spillover effects in epidemiology: parameters, study designs and methodological considerations. Int J Epidemiol 2018; 47:332–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittberg DM, Aragie S, Tadesse W, et al. WASH Upgrades for Health in Amhara (WUHA): study protocol for a cluster-randomised trial in Ethiopia. BMJ Open 2021; 11:e039529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemayehu M, Koye DN, Tariku A, Yimam K. Prevalence of active trachoma and its associated factors among rural and urban children in Dera woreda, northwest Ethiopia: a comparative cross-sectional study. Biomed Res Int 2015; 2015:570898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart AEP, Zerihun M, Gessese D, et al. Progress to eliminate trachoma as a public health problem in Amhara national regional state, Ethiopia: results of 152 population-based surveys. Am J Trop Med Hyg 2019; 101:1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash SD, Chernet A, Moncada J, et al. Ocular Chlamydia trachomatis infection and infectious load among pre-school aged children within trachoma hyperendemic districts receiving the SAFE strategy, Amhara region, Ethiopia. PLoS Negl Trop Dis 2020; 14:e0008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoller NE, Gebre T, Ayele B, et al. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. Int Health 2011; 3:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebre T, Ayele B, Zerihun M, et al. Comparison of annual versus twice-yearly mass azithromycin treatment for hyperendemic trachoma in Ethiopia: a cluster-randomised trial. Lancet 2012; 379:143–51. [DOI] [PubMed] [Google Scholar]
- 22.Keenan JD, Tadesse Z, Gebresillasie S, et al. Mass azithromycin distribution for hyperendemic trachoma following a cluster-randomized trial: a continuation study of randomly reassigned subclusters (TANA II). PLoS Med 2018; 15:e1002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.