Abstract
Background
The recombinant zoster vaccine had over 90% efficacy in preventing herpes zoster in clinical trials. However, its effectiveness outside of a clinical trial setting has not been investigated. This study aimed to assess the effectiveness of the recombinant zoster vaccine in general practice.
Methods
A de-identified administrative claims database, the OptumLabs Data Warehouse, was used to conduct this retrospective cohort study to assess the effectiveness of the recombinant zoster vaccine against herpes zoster in nonimmunocompromised, vaccine age–eligible individuals enrolled in the database for ≥365 days.
Results
A total of 4 769 819 adults were included in this study, with 173 745 (3.6%) adults receiving 2 valid doses of the recombinant zoster vaccine. The incidence rate of herpes zoster was 258.8 (95% confidence interval [CI], 230.0–289.4) cases per 100 000 person-years in vaccinated persons compared with 893.1 (95% CI, 886.2–900.0) in unvaccinated persons. Recombinant zoster vaccine effectiveness was 85.5% (95% CI, 83.5–87.3%) overall, with an effectiveness of 86.8% (95% CI, 84.6–88.7%) in individuals 50 to 79 years old compared with 80.3% (95% CI, 75.1–84.3%) in individuals aged 80 and older. In patients with a history of live zoster vaccine within 5 years of study inclusion, vaccine effectiveness was 84.8% (95% CI, 75.3–90.7%).
Conclusions
Recombinant zoster vaccine effectiveness against herpes zoster was high in a real-world setting. Given the low vaccine coverage and high effectiveness, a major public health effort is needed to identify and address barriers to vaccination and increase immunization rates.
Keywords: herpes zoster, recombinant zoster vaccine, infectious disease, epidemiology, vaccine effectiveness, shingrix vaccine, real world evidence
This study provides the first evidence of effectiveness of the recombinant zoster vaccine in preventing herpes zoster in a general practice setting. Overall, vaccine effectiveness was 85.5% (95% confidence interval, 83.5–87.3%).
(See the Major Article by Izurieta et al on pages 941–8 and the Editorial Commentary by Harpaz on pages 957–60.)
From 1994 to 2018, the incidence of herpes zoster (HZ; shingles) increased annually by 3.1%, from 286.0 to 579.6 cases per 100 000 person-years [1]. Although HZ incidence rates have begun stabilizing among adults ages 60 and above, rates have continued to increase in ages 20 through 59 from 2007 through 2018 [1–4]. Caused by reactivation of the varicella zoster virus from a posterior dorsal root ganglion, HZ typically presents as a painful, dermatomal rash [5, 6]. The risk of HZ increases with age and development of this condition can lead to long-term sequelae such as postherpetic neuralgia and blindness as well as an increased risk of stroke and heart attack [7–9]. Pain associated with HZ can significantly reduce quality of life and is associated with the loss of over 60 000 quality-adjusted life-years and $2.4 billion in direct medical costs and productivity losses annually in the United States [10]. Given this large disease burden, preventative measures are important to reduce the risk of zoster.
There are 2 vaccines that have been developed to protect against HZ. Zoster vaccine live (ZVL; Zostavax; Merck, Sharp & Dohme) is a live attenuated vaccine that was licensed by the Food and Drug Administration in 2006 for adults aged 60 and older and expanded to ages 50 and older in 2011 [11, 12]. Both clinical trials and real-world studies showed that the vaccine only offered approximately 50% protection against HZ [13–15]. Recombinant zoster vaccine (RZV; Shingrix; GlaxoSmithKline) is a 2-dose subunit vaccine that was licensed by the Food and Drug Administration in 2017 for adults aged 50 or older [16]. Approval for RZV was based on evidence from the Zoster Efficacy Study in Adults 50 Years of Age and Older (ZOE-50), which demonstrated a 97.2% reduction in HZ among adults aged 50 and older [17]. Due to the vaccine’s greater efficacy in comparison to ZVL, RZV is currently the preferred vaccine for HZ prevention in immunocompetent adults, and US production of ZVL was discontinued on 1 July 2020 [12, 18].
Although RZV has proven efficacy in clinical trials, there is a lack of information on RZV effectiveness in a real-world setting. It is crucial to assess RZV effectiveness due to significant differences between trial and clinical settings such as differences in the health of vaccinees and methods for HZ diagnosis. Vaccine coverage for HZ has historically been low and HZ incidence rates are continuing to increase in middle-aged adults, highlighting the need to address this public health issue [2, 3, 19, 20]. To further our understanding of the benefits of vaccination and provide guidance on vaccination efforts, this study assessed RZV effectiveness in a real-world setting.
METHODS
Setting
A retrospective cohort study was conducted using the de-identified healthcare claims database OptumLabs Data Warehouse (OLDW). OLDW is a real-world database that contains administrative claims and electronic health record data for over 200 million individuals who enrolled in commercial insurance, Medicare Advantage, or Medicare Part D. Comparisons between the US Census and the OLDW demonstrate that distributions in age, sex, race/ethnicity, and region are comparable between the 2. OLDW is nationally representative for commercial enrollees, with a higher proportion of enrollees in the South and Central regions.
Inclusion and Exclusion Criteria
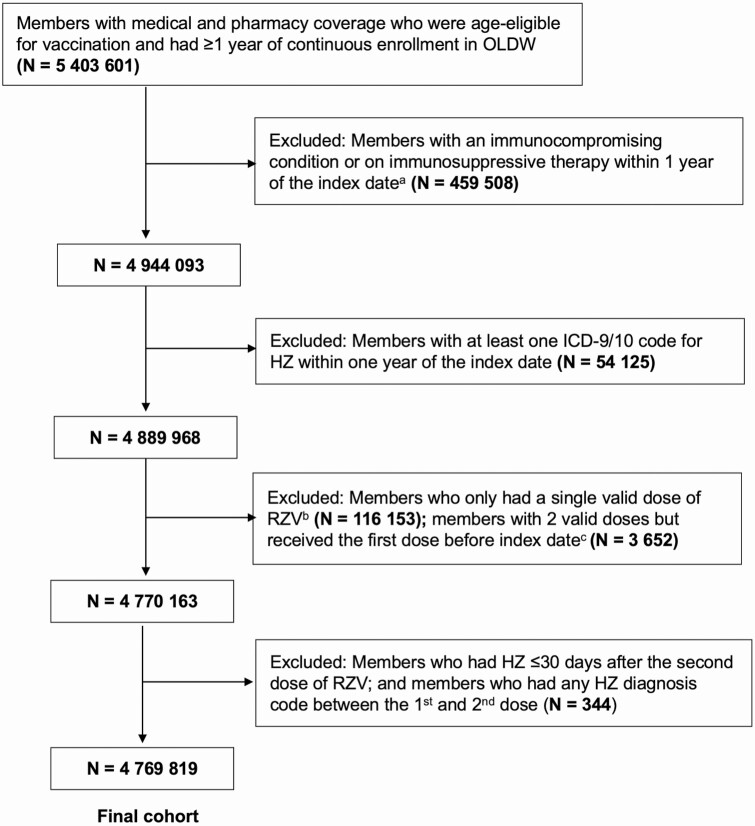
The date that the patient became eligible for inclusion in the study was considered the index date. A patient reached the index date once they met 2 criteria: (1) reached age 50 in 2018 or 2019, meeting age eligibility for RZV based on recommendations from the Advisory Committee on Immunization Practices (ACIP) [12], and (2) had at least 365 days of continuous enrollment in OLDW. A patient’s age was estimated by his/her year of birth, as the exact date of birth is unavailable to researchers to maintain patient confidentiality. From 1 January to 31 December 2018, patients who were aged 50 and above with at least 1 year of continuous enrollment were included in the cohort. From 1 January to 31 December 2019, only patients who turned 50 years old in 2019 and had 1 year of continuous enrollment were included. This was done to exclude individuals who may have had previous RZV vaccination prior to being included in the OLDW (139 individuals excluded who received a first dose of RZV prior to 1 January 2018, 0.08% of the total vaccinated cohort). Cohort selection details are shown in Figure 1.
Figure 1. .
Flow diagram of inclusion and exclusion criteria for study cohort. aIndex date was defined as the date at which an individual was eligible for study inclusion. bOf the 116 153 individuals who only had a single valid dose of RZV, 106 462 (91.7%) of them completed 1 dose during the study period, with 9691 (8.3%) of the individuals having an improperly spaced second dose. cTwo valid doses of RZV were defined as receiving the second dose between 30 and 210 days after the first dose. Abbreviations: HZ, herpes zoster; ICD, International Classification of Diseases; OLDW, OptumLabs Data Warehouse; RZV, recombinant zoster vaccine.
Individuals with a diagnosis of HZ, as determined by an International Classification of Diseases (ICD), 9th revision, or 10th revision, code (for HZ: ICD-9 053.xx; ICD-10 B02.xx; for PHN: ICD-9 053.1X; ICD-10 B02.2X) were included. An HZ diagnosis was identified in any clinical setting (inpatient hospital, long-term care, emergency department, outpatient hospital, office visits, and unclassified visits as defined by OLDW) and using any diagnostic position from physician claims and facility claims. Individuals who were classified as immunocompromised within 1 year of the index date were excluded. Immunocompromised status was defined as an ICD-9 or ICD-10 code for human immunodeficiency virus, AIDS, leukemia, or lymphoma (see Supplementary Box 1), or a prescription for immunosuppressive medications (see Supplementary Box 2) [21]. To provide adequate time for a protective immune response to develop after RZV, patients with HZ occurring between the first and second doses of RZV and up to 30 days after the second dose of RZV were excluded. Patients with only a single dose of RZV were also excluded.
Exposure and Outcome
Receipt of RZV was identified by a Current Procedural Terminology (CPT) code (90 750 for RZV) or in pharmacy claims by the brand name for RZV. A patient was deemed vaccinated after receipt of the second dose of RZV. Following the recommendations provided by the ACIP and to give the immune system adequate time to generate a response, a second dose was valid if it occurred 30 to 210 days after the first dose. Individuals who received a second dose of RZV less than 30 days or greater than 210 days after the first dose were excluded. The outcome of interest was the first diagnosis of HZ that occurred during the study follow-up.
Covariates and Follow-up Period
Time-fixed covariates that were identified as potential confounders included sex (female, male, unknown), race/ethnicity (Asian, Black, Hispanic, White, unknown), insurance type (commercial, Medicare Advantage), region (Midwest, Northeast, South, West, unknown), and vaccination with ZVL in the 1 year prior to index date. (See Supplementary Methods 1 for race/ethnicity and region information.)
Time-varying covariates included age, healthcare utilization (inpatient stay, long-term care, emergency department, outpatient hospital, and office visits), age-adjusted Charlson comorbidity index, and systemic antiviral use, and were updated for each 6-month period [22, 23]. Inpatient stay, long-term care, emergency department visit, and antiviral use were categorized as binary variables for each 6-month period due to the low number of events among this cohort. Outpatient hospital visits and office visits were treated as continuous variables for each 6-month period. Antiviral medications included valacyclovir (Valtrex; GlaxoSmithKline), acyclovir (Zovirax; GlaxoSmithKline), and famciclovir (Famvir; Novartis). Receipt of ZVL was identified by either a CPT code (90 736) or in pharmacy claims by the brand name for ZVL. Patients contributed to unvaccinated person-time until receipt of 2 valid doses of RZV, after which they contributed to vaccinated person-time. A 6-month period was split into 2 if vaccination occurred during the interval.
Patients were followed from the index date until one of the following events occurred: HZ diagnosis, development of immunocompromised status (see Supplementary Box 1), ZVL receipt, disenrollment from the insurance plan, or end of the study period on 31 December 2019.
Statistical Analysis
Incidence rates of HZ for each year postvaccination were computed as the number of HZ cases per 100 000 person-years, and the corresponding 95% confidence intervals (CIs) were estimated assuming occurrence of HZ followed a Poisson distribution. Cox proportional hazards regression models were used to estimate the HR associated with RZV. The Cox models were stratified by birth year and used calendar time as the time scale. We reported person-time in 6-month periods for the analysis. Inverse probability weighting (IPTW) was used to control for confounding. Time-updated IPTW was estimated using logistic regression, including fixed and time-updated covariates for both the treatment and censoring (see Supplementary Methods 2). The models were weighted by the product of the IPTW and inverse probability of censoring weight to estimate adjusted HRs. We assessed covariate balance improvement through inverse weighting by comparing standardized mean differences in the unweighted and weighted samples (Supplementary Table 1) [24]. The 95% CIs for the model were estimated using robust standard errors, which are conservative for inverse weighted estimators [25]. Vaccine effectiveness was estimated as follows: 1 − HR × 100%.
Subgroup analyses were performed by age group, sex, race/ethnicity, region, and prior history of ZVL. Each subgroup was chosen in order to assess for the potential effect that biological differences (eg, sex, age) and environmental factors (eg, region) may have on RZV effectiveness. We conducted a sensitivity analysis in which we required individuals who were age-eligible for RZV to have 5 years instead of 1 year of continuous enrollment prior to the index date, with assessment of ZVL during this time. This time period was chosen given previous evidence that ZVL demonstrates significant efficacy up to 5 years postvaccination [26]. Individuals with immunocompromised status or a history of HZ during the 5-year period were excluded in this sensitivity analysis. Finally, we determined the null E-value for the primary analysis to assess the potential effect of unmeasured confounders on the observed association.
All statistical analyses were conducted in R (version 3.6.3; The R Project for Statistical Computing, Vienna, Austria; http://www.r-project.org). Only de-identified data were available for analysis. This study received approval from the Institutional Review Board of the University of California, San Francisco, and was performed in accordance with the tenets of the Declaration of Helsinki.
RESULTS
A total of 4 769 819 individuals with 7 300 036 person-years were included in this study. The median age of all patients at the index date was 65 years old (interquartile range [IQR], 56–73 years). Table 1 presents the demographic characteristics at the index date. A total of 173 745 (3.6%) individuals received 2 valid doses of RZV. Median duration of follow-up was 7.0 months (IQR, 2.8–13.0 months) after vaccination. The median age at index date was 72 years old (IQR, 69–77 years) for vaccinated individuals and 64 years old (IQR, 56–73 years) for unvaccinated individuals. Females, White individuals, and individuals from the South were the most common demographic groups within the vaccinated and unvaccinated cohorts.
Table 1.
Characteristics of the Study Population at the Index Date by Vaccination Status
| Characteristic | Unvaccinated (n = 4 596 074) | Vaccinated (n = 173 745) | Overall (n = 4 769 819) |
|---|---|---|---|
| Age, median (IQR), years | 64 (56–73) | 72 (69–77) | 65 (56–73) |
| Sex | |||
| Male | 2 204 186 (48.0) | 72 397 (41.7) | 2 276 583 (47.7) |
| Female | 2 389 353 (52.0) | 100 849 (58.0) | 2 490 202 (52.2) |
| Unknown | 2535 (0.0) | 499 (0.3) | 3034 (0.0) |
| Race/ethnicity | |||
| Asian | 144 129 (3.1) | 6100 (3.5) | 150 299 (3.1) |
| Black | 513 228 (11.2) | 12 643 (7.3) | 525 871 (11.0) |
| Hispanic | 467 968 (10.2) | 8263 (4.8) | 476 231 (10.0) |
| White | 2 989 949 (65.1) | 128 967 (74.3) | 3 118 916 (65.4) |
| Unknowna | 480 800 (10.5) | 17 772 (10.2) | 498 572 (10.5) |
| Insurance type | |||
| Commercial | 2 443 126 (53.2) | 20 098 (11.6) | 2 463 224 (51.6) |
| Medicare Advantage | 2 152 948 (46.8) | 153 647 (88.4) | 2 306 595 (48.4) |
| Region | |||
| Midwest | 1 121 882 (24.4) | 56 774 (32.7) | 1 178 656 (24.7) |
| Northeast | 602 870 (13.1) | 18 698 (10.8) | 621 568 (13.0) |
| South | 2 155 141 (46.9) | 76 121 (43.8) | 2 231 262 (46.8) |
| West | 644 985 (14.0) | 22 127 (12.7) | 667 112 (14.0) |
| Unknownb | 71 196 (1.5) | 25 (0.0) | 71 221 (1.5) |
| Healthcare utilizationc | |||
| Inpatient stay, % with at least 1 visit | 402 062 (8. 7) | 15 902 (9.2) | 417 964 (8.8) |
| Long-term care, % with at least 1 visit | 94 481 (2.1) | 2426 (1.4) | 96 907 (2.0) |
| Emergency room visit, % with at least 1 visit | 959 170 (20.9) | 34 068 (19.6) | 993 238 (20.8) |
| Outpatient visits, median (IQR) | 1 (0–3) | 2 (1–5) | 1 (0–3) |
| Office visits, median (IQR) | 5 (2–11) | 9 (5–16) | 6 (2–11) |
| Charlson comorbidity index,d median (IQR) | 2 (1–4) | 3 (3–4) | 2 (1–4) |
Values are reported as n (%) unless otherwise indicated. The index date was defined as the date at which an individual was eligible for study inclusion.
Abbreviations: IQR, interquartile range; OLDW, OptumLabs Data Warehouse.
aThe unknown race/ethnicity category includes individuals with either unknown or missing race/ethnicity.
bThe unknown region category includes individuals in either unknown or other regions. In OLDW, the predefined region categories are Midwest, Northeast, South, West, Other, Unknown. The Other and Unknown categories were combined given the low prevalence of herpes zoster in each individual group.
cHealthcare utilization was assessed in the 1 year prior to the index date.
dCharlson comorbidity index was assessed in the 1 year prior to the index date.
There were 298 cases of HZ with a total follow-up time of 115 125 person-years in the vaccinated cohort. For the unvaccinated cohort, there were 64 169 HZ cases with a total follow-up of 7 184 911 person-years (Table 2). The incidence rate of HZ was 258.8 (95% CI, 230.6–289.4) cases per 100 000 person-years in vaccinated individuals compared with 893.1 (95% CI, 886.2–900.0) cases per 100 000 person-years in the unvaccinated individuals.
Table 2.
Incidence of Herpes Zoster per 100 000 Person-Years by Baseline Characteristics and Recombinant Zoster Vaccination Status From 2018 to 2019
| Unvaccinated | Vaccinated | Rate Ratioa (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| No. of Cases | No. of Person-Years | Incidence Rate (95% CI) | No. of Cases | No. of Person-Years | Incidence Rate (95% CI) | ||
| Overall | 64 169 | 7 184 911 | 893.1 (886.2, 900.0) | 298 | 115 125 | 258.8 (230.6, 289.4) | .29 (.26, .32) |
| Age group (years)b | |||||||
| 50–59 | 15 424 | 2 252 215 | 684.8 (674.1, 695.7) | <11 | 2019 | <544.8 (282.9, 933.0) | <.80 (.44, 1.44) |
| 60–69 | 17 326 | 2 040 881 | 848.9 (836.4, 861.7) | >34 | 22 934 | >148.3 (103.8, 203.8) | >.17 (.12, .24) |
| 70–79 | 19 920 | 1 926 358 | 1034.1 (1019.8, 1048.5) | 161 | 65 423 | 246.1 (210.0, 286.1) | .24 (.20, .28) |
| 80+ | 11 499 | 965 456 | 1191.0 (1169.4, 1212.9) | 92 | 24 750 | 371.7 (300.8, 452.9) | .31 (.25, .38) |
| Sex | |||||||
| Male | 24 142 | 3 422 927 | 705.3 (696.4, 714.2) | 92 | 48 288 | 190.5 (154.2, 232.1) | .27 (.22, .33) |
| Female | 39 977 | 3 757 202 | 1064.0 (1053.6, 1074.5) | 206 | 66 458 | 310.0 (269.5, 354.2) | .29 (.25, .33) |
| Unknown | 50 | 4782 | 1045.6 (781.9, 1362.8) | 0 | 379 | 0.0 | 0.0 |
| Race/ethnicity | |||||||
| Asian | 2018 | 226 738 | 890.0 (851.7, 929.4) | 19 | 3968 | 478.9 (294.5, 727.5) | .54 (.34, .85) |
| Black | 5873 | 801 219 | 733.0 (714.4, 751.9) | 22 | 8702 | 252.8 (161.2, 373.6) | .34 (.23, .52) |
| Hispanic | 6053 | 706 369 | 856.9 (835.5, 878.7) | 16 | 5432 | 294.6 (172.8, 463.3) | .34 (.21, .56) |
| White | 43 606 | 4 683 783 | 931.0 (922.3, 939.8) | 218 | 85 339 | 255.5 (223.0, 290.9) | .27 (.24, .31) |
| Unknownc | 6619 | 766 801 | 863.2 (842.6, 884.2) | 23 | 11 685 | 196.8 (127.0, 288.6) | .23 (.15, .34) |
| Insurance type | |||||||
| Commercial | 25 422 | 3 501 871 | 726.0 (717.1, 734.9) | 22 | 11 320 | 194.4 (124.0, 287.2) | .27 (.18, .41) |
| Medicare Advantage | 38 747 | 3 683 040 | 1052.0 (1041.6, 1062.5) | 276 | 103 805 | 265.9 (235.7, 298.5) | .25 (.22, .28) |
| Region | |||||||
| Midwest | 16 266 | 1 779 281 | 914.2 (900.2, 928.3) | 93 | 36 028 | 258.1 (209.2, 314.2) | .28 (.23, .35) |
| Northeast | 8704 | 959 821 | 906.8 (887.9, 926.0) | 30 | 12 647 | 237.2 (162.1, 332.5) | .26 (.18, .37) |
| South | 31 038 | 3 350 810 | 926.3 (916.0, 936.6) | 141 | 51 904 | 271.7 (229.2, 319.0) | .29 (.25, .35) |
| West | 8115 | 982 217 | 826.2 (808.3, 844.3) | 34 | 14 527 | 234.0 (163.9, 321,8) | .28 (.20, .40) |
| Unknownd | 46 | 112 782 | 40.8 (30.1, 53.7) | 0 | 19 | 0.0 | 0.0 |
Abbreviations: CI, confidence interval; OLDW, OptumLabs Data Warehouse.
aRate ratio was computed as incidence rate in vaccinated group/incidence rate in the unvaccinated group.
bFor cell counts <11, OptumLabs requires values to be reported as <11 rather than reporting actual values in order to protect patient privacy. In addition, in order to prevent back-calculation, the value in another cell in the same subgroup is lowered and reported with a greater than sign to keep the total case count the same. The incidence rate for vaccinated person-time for ages 50–59 is calculated for n = 11 to further prevent back-calculation and maintain individual privacy.
cThe unknown race/ethnicity category includes individuals with either unknown or missing race/ethnicity.
dThe unknown region category includes individuals in either unknown or other regions. In OLDW, the predefined region categories are Midwest, Northeast, South, West, Other, Unknown. The Other and Unknown categories were combined given the low prevalence of herpes zoster in each individual group.
Inverse weighting markedly improved covariate balance between vaccinated and unvaccinated groups (Supplementary Figure 1). In the unweighted sample, 12 (52%) out of the 23 covariates had standardized mean differences of greater than 0.1.
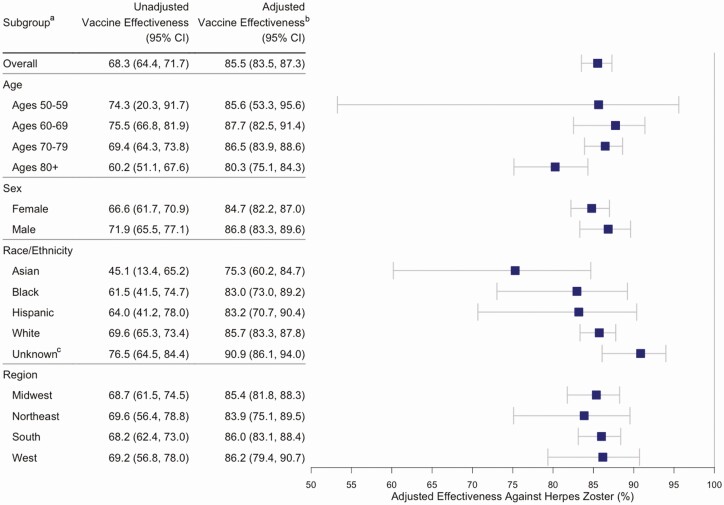
Overall adjusted vaccine effectiveness was 85.5% (95% CI, 83.5–87.3%), with an effectiveness of 86.8% (95% CI, 84.6–88.7%) in individuals aged 50 to 79 years old compared with 80.2% (95% CI, 75.1–84.3%) in individuals aged 80 and older. In the E-value analysis to assess the effect of unmeasured confounding on RZV effectiveness, the null E-value was 12.8. The estimates of vaccine effectiveness were similar by sex, race/ethnicity, and region subgroups. Although the point estimate was lower for Asians compared with other races, the CIs overlap (Figure 2).
Figure 2. .
Recombinant zoster vaccine effectiveness by age, sex, race/ethnicity, and region from 2018 to 2019. aValues are reported as percentages. bVaccine effectiveness was adjusted using inverse probability weighting for age, sex, race, region, prior zoster vaccine live, antiviral use, healthcare utilization, and Charlson comorbidity index. cThe unknown race/ethnicity category includes individuals with either unknown or missing race/ethnicity. Abbreviation: CI, confidence interval.
In the sensitivity analysis requiring 5 years of continuous enrollment prior to the index date, a total of 1 569 520 individuals with 2 039 740 person-years were included. The overall RZV effectiveness was 86.7% (95% CI, 83.3–89.4%). In individuals with a history of ZVL within 5 years prior to the index date, the incidence rate of HZ was 239.4 (95% CI, 142.8–371.8) cases per 100 000 person-years in individuals vaccinated with RZV and 754.5 (95% CI, 718.4–791.7) cases per 100 000 person-years in unvaccinated individuals. In individuals with no history of ZVL within 5 years prior to the index date, the incidence rate of HZ was 260.9 (95% CI, 205.9–325.0) cases per 100 000 person-years in individuals vaccinated with RZV and 908.1 (95% CI, 895.0–921.3) cases per 100 000 person-years in unvaccinated individuals. In the cohort with no history of ZVL, unadjusted vaccine effectiveness was 76.1% (95% CI, 70.0–81.0%) and adjusted effectiveness was 87.1% (95% CI, 83.4–89.9%). In adults with a history of ZVL within 5 years prior to the index date, unadjusted vaccine effectiveness was 71.2% (95% CI, 53.4–82.2%) and adjusted effectiveness was 84.8% (95% CI, 75.3–90.7%).
DISCUSSION
Adjusted RZV effectiveness against HZ was 85.5% overall. Recombinant zoster vaccine was effective across both sexes, all races/ethnicities, and all geographic regions. Sensitivity analyses showed comparable RZV effectiveness between individuals with and without a history of ZVL vaccination within 5 years of the index date.
The RZV was approved for adults aged 50 and over following the results of the ZOE-50 study. In this trial, RZV had an overall efficacy between 96.6% and 97.9% among all age groups 50 and above [17]. In a follow-up study in individuals aged 70 and above (ZOE-70), RZV efficacy was 89.8% [27]. Given that the median age of individuals in this study was 72 years old at the index date, the observed effectiveness of RZV was still high, at 85.5%. The live vaccine demonstrated an initial efficacy of 60.1% in clinical trials and approximately 50% in clinical practice settings [13, 15, 21, 28]. The analysis presented here shows that RZV effectiveness outside a clinical trial setting was in line with the efficacy from the ZOE-50 and ZOE-70 trials.
The primary trials found no significant difference in RZV efficacy with age, while the results in our study suggest a lower effectiveness among individuals aged 80 and older. The Shingles Prevention Study also showed that ZVL had significantly decreased efficacy in individuals aged 80 years and older, although follow-up real-world studies failed to find a decrease in ZVL effectiveness with age [13, 15, 21, 29, 30]. Immunosenescence could explain the decline in RZV effectiveness seen here, although protection against HZ was still 80% in this oldest age group [31, 32].
Vaccine effectiveness also remained high among individuals with a history of ZVL within 5 years of study inclusion. There is little information on RZV effectiveness within this subpopulation. Prior ZVL was an exclusion in the RZV clinical trials, although the US Centers for Disease Control and Prevention recommends that individuals with prior ZVL get vaccinated with RZV [12, 17]. The results shown here provide reassuring evidence for RZV vaccination among individuals with a history of ZVL.
Understanding the effectiveness of RZV in practice, outside of clinical trial settings, is crucial given the differences between clinical trial and general practice settings. The clinical trials had standardized protocols for vaccine storage, administration, and HZ diagnosis. In general practice, patients often have more health comorbidities. Patients may have a history of prior ZVL vaccination, which was an exclusion in the primary trial. Although there is some evidence that HZ incidence may be continuing to increase in middle-aged adults while stabilizing in those aged 50 and above, older individuals continue to be at higher risk for HZ [1–3, 33].
Vaccine coverage for RZV was 3.6%. The recent introduction of the vaccine and reported shortages could have contributed to the poor uptake [34]. It will be crucial to assess for potential barriers to RZV uptake moving forward. More research and public health efforts are needed to identify and address potential barriers to HZ vaccination in order to protect more individuals from this common condition.
Strengths and Limitations
This study utilizing a large commercial claims database provides novel information on the effectiveness of RZV in a real-world setting. Recombinant zoster vaccine is covered by commercial insurance, Medicare Part D, and Medicare Advantage, making exposure misclassification unlikely. Using IPTW helped minimize differences between the vaccinated and unvaccinated groups [35]. Having a sensitivity analysis requiring 5 years of continuous enrollment allowed for better ascertainment of prior ZVL history. Adjusting for a history of systemic antiviral use, which would have protected against HZ, also helped minimize bias.
Although the generalizability of this analysis may be limited since patients had commercial insurance, it is worth noting that nearly three-quarters of eligible adults were enrolled in Medicare Part D and approximately one-third were enrolled in Medicare Advantage in 2019 [36, 37]. Fewer data were available for enrollees aged 50 to 59 years old, decreasing the precision of estimates for this age group. Although we used IPTW to control confounding and selection bias, residual bias could have still been present due to unmeasured confounders. We estimated E-values to quantify the minimum strength of association on the risk ratio scale that an unmeasured confounder must have with both the treatment and outcome to shift the observed treatment–outcome association to the null. In previous studies, the relative risk of factors such as gender, race, and chronic health conditions on HZ was between 1 to 3. An E-value of 12.8 suggests residual bias from unmeasured confounding is unlikely to explain RZV effectiveness in this study [38–40]. Recombinant zoster vaccine efficacy waned over the 3 years of the trial. We were unable to assess waning due to the relatively short follow-up time of this study.
Conclusions
Recombinant zoster vaccine has demonstrated a higher effectiveness than ZVL in both clinical trial and general practice settings. The vaccine greatly reduced the risk of developing herpes zoster, regardless of age, sex, and prior vaccination with the live zoster vaccine. Given the morbidity caused by HZ, major public efforts will be needed to continue supporting vaccination and to identify and address barriers to RZV uptake.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Nina Veeravalli, Lead Research Analyst at OptumLabs, for providing logistical and administrative support related to accessing the OptumLabs Data Warehouse.
Financial support. This work was supported by the National Eye Institute and Office of Research on Women’s Health at the National Institutes of Health (grant number R01 EY028739; to N. R. A.) and the OptumLabs Data Warehouse (research credit to N. R. A.). The Department of Ophthalmology at the University of California, San Francisco, is supported by an unrestricted grant from the Research to Prevent Blindness Foundation; a core grant from the National Eye Institute at the National Institutes of Health (grant number EY06190); and the That Man May See Foundation.
Potential conflicts of interest. Y. S., E. K., C. L. K., B. F. A., T. C. P., and N. R. A. received nonfinancial support from OptumLabs (Cambridge, MA) outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes zoster and postherpetic neuralgia: changing incidence rates from 1994 to 2018 in the United States [manuscript published online ahead of print 23 August 2020]. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis 2019; 69:341–4. [DOI] [PubMed] [Google Scholar]
- 3.Wolfson LJ, Daniels VJ, Altland A, Black W, Huang W, Ou W. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: updated evidence from observational databases, 1991–2016. Clin Infect Dis 2020; 70:995–1002. [DOI] [PubMed] [Google Scholar]
- 4.Kong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence rate of herpes zoster ophthalmicus: a retrospective cohort study from 1994 through 2018. Ophthalmology 2020; 127:324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams WW, Lu P, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ 2017;66(n o. SS-11):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30. [PubMed] [Google Scholar]
- 8.Erskine N, Tran H, Levin L, et al. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One 2017; 12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis AR, Sheppard J. Herpes zoster ophthalmicus review and prevention. Eye Contact Lens Sci Clin Pract 2019; 45:286–91. [DOI] [PubMed] [Google Scholar]
- 10.Harvey M, Prosser LA, Rose AM, Ortega-Sanchez IR, Harpaz R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain 2020; 161:361–8. [DOI] [PubMed] [Google Scholar]
- 11.Baylor NW; US Food and Drug Administration (FDA) . Approval letter—Zostavax. 2006. Available at: https://www.fda.gov/vaccines-blood-biologics/vaccines/zostavax. Accessed 10 June 2020.
- 12.Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 14.Tseng HF, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Incidence of herpes zoster among children vaccinated with varicella vaccine in a prepaid health care plan in the United States, 2002–2008. Pediatr Infect Dis J 2009; 28:1069–72. [DOI] [PubMed] [Google Scholar]
- 15.Baxter R, Bartlett J, Fireman B, et al. Long-term effectiveness of the live zoster vaccine in preventing shingles: a cohort study. Am J Epidemiol 2018; 187:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration (FDA). BLA approval—zoster vaccine recombinant. 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581750.pdf. Accessed 10 June 2020.
- 17.Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 18.Merck & Co., Inc. Product discontinuation notice: ZOSTAVAX® (Zoster Vaccine Live). 2020. Available at: https://www.merckvaccines.com/wp-content/uploads/sites/8/2020/06/US-CIN-00033.pdf. Accessed 16 September 2020.
- 19.Aris E, Montourcy M, Esterberg E, Kurosky SK, Poston S, Hogea C. The adult vaccination landscape in the United States during the Affordable Care Act era: results from a large retrospective database analysis. Vaccine 2020; 38:2984–94. [DOI] [PubMed] [Google Scholar]
- 20.Williams WW, Lu P-J, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Surveill Summ. 2016; 65(no. SS-1): 1–36. [DOI] [PubMed] [Google Scholar]
- 21.Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–6. [DOI] [PubMed] [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–51. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016; 35:5642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 28.Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izurieta HS, Wernecke M, Kelman J, et al. Effectiveness and duration of protection provided by the live-attenuated herpes zoster vaccine in the Medicare population ages 65 years and older. Clin Infect Dis 2017; 64:785–93. [DOI] [PubMed] [Google Scholar]
- 30.Morrison VA, Johnson GR, Schmader KE, et al. ; Shingles Prevention Study Group . Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner A, Garner-Spitzer E, Jasinska J, et al. Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep 2018; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord JM. The effect of aging of the immune system on vaccination responses. Hum Vaccines Immunother 2013; 9:1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng HF, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis 2016; 213:1872–5. [DOI] [PubMed] [Google Scholar]
- 34.Nania R. Shingles vaccine shortage persists. 2019. Available at: https://www.aarp.org/health/conditions-treatments/info-2019/shingles-vaccine-in-short-supply.html. Accessed 11 September 2020.
- 35.Normand SLT, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001; 54:387–98. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser Family Foundation. Medicare advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/. Accessed 10 June 2020.
- 37.Kaiser Family Foundation. 10 Things to know about Medicare part D coverage and costs in 2019. Available at: https://www.kff.org/report-section/10-things-to-know-about-medicare-part-d-coverage-and-costs-in-2019-data-note/. Accessed 10 June 2020.
- 38.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019; 321:602–3. [DOI] [PubMed] [Google Scholar]
- 39.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology 2018; 29:e45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.