Abstract
Background
Poor control of diabetes mellitus (DM) increases active tuberculosis (TB) risk. Understanding risk factors for latent TB infection (LTBI) in this population and intervention completion rates is crucial for policy making.
Methods
Under a collaborative multidisciplinary team consisting of public health professionals, endocrinologists, and pulmonologists, patients aged >45 years with poorly controlled DM (pDM), defined as having a glycated hemoglobin level of ≥9% within the preceding year, were enrolled by endocrinologists from 2 hospitals; these patients underwent LTBI screening by using QuantiFERON (QFT). Once-weekly isoniazid and rifapentine for 12 weeks (3HP) or daily isoniazid for 9 months (9H) was administered by pulmonologists. QFT-positivity predictors were evaluated using logistic regression. Completion rates and safety were also investigated.
Results
Among 980 patients with pDM (age: 64.2 ± 9.7 years), 261 (26.6%) were QFT-positive. Age, DM duration, chronic kidney disease stage ≥3, and dipeptidyl peptidase-4 inhibitor use, not using metformin, were associated with QFT-positivity. Preventive therapy (3HP: 138; 9H: 62) was administered in 200 (76.6%) QFT-positive patients. The completion rates of 3HP and 9H were 84.1% and 79.0%, respectively (P = .494). Nine (6.5%) and zero patients in the 3HP and 9H groups, respectively, developed systemic drug reactions (P = .059); 78.3% and 45.2% had ≥1 adverse drug reactions (P < .001); and post-treatment QFT conversion rates were 32% and 20%, respectively (P = .228).
Conclusions
LTBI prevalence exceeds 25% in elderly patients with pDM. Under care from a collaborative multidisciplinary team, the completion rate of preventive therapy, regardless of regimen could approach, or even exceed 80% in this population.
Keywords: diabetic mellitus, latent tuberculosis infection, preventive therapy, rifapentine, treatment outcome
The latent tuberculosis infection prevalence exceeds 25% in elderly patients with poorly controlled diabetes mellitus (DM), particularly those with a long DM duration. The completion rate of preventive therapy, regardless of the regimen, approaches 80% in this population under a collaborative framework.
Diabetes mellitus (DM) is a crucial risk factor for tuberculosis (TB) in the elderly population [1, 2] and is associated with poor TB outcomes [3]. However, the actual effect of DM on the risk of latent TB infection (LTBI) remains controversial. The LTBI prevalence in patients with DM was observed to be more than twice of that in nondiabetic people in population-based studies conducted in the United States (11.6% vs 4.6% [4]) and Taiwan (21.1% vs 9.7 [5]). A systematic review revealed that the odds ratio (OR) for LTBI in DM patients was 1.18 (95% confidence interval [CI]: 1.06–1.30), with low statistical heterogeneity across studies [6]. Nevertheless, the situation is even worse among patients with poorly controlled DM (pDM) whom may be at higher risk for TB infection and disease according to several studies [2, 5, 7–9]. A study reported that every 1% increase in glycated hemoglobin (HbA1c) level resulted in a 1.13-fold increase (95% CI: 1.04–1.22) in the prevalence of TB infection [8]. Given the increasing burden of DM in TB endemic areas, programmatic interventions targeting the coepidemic population for LTBI are essential to eradicate TB.
Because of the paucity of studies evaluating the safety and efficacy of LTBI treatment, TB preventive therapy (TPT) for patients with DM has not been strongly recommended by the World Health Organization (WHO) [10]. Instead, the WHO has emphasized to select target population based on local epidemiology and resources [10]. Despite having a protective effect of 85%–90%, the traditional 9-month daily isoniazid (9H) regimen is difficult to implement because of the unacceptably long treatment duration [11]. Compared with the 9H regimen, the 3-month weekly rifapentine plus isoniazid (3HP) regimen has a similar efficacy in TB prevention [12–16], a lower hepatotoxicity risk [12, 13, 17], a 10% higher completion rate [12–15] and to be more cost-effective [18]. However, the 3HP regimen flaws into a significantly higher risk of adverse events other than hepatotoxicity, particularly flu-like syndrome and systemic drug reactions (SDRs) [17], as well as potential drug interactions with antidiabetic drugs [19]. Studies have not yet evaluated the completion rate and safety profile of 3HP in patients with DM, preventing the widespread use of the 3HP regimen in this high-TB-risk population.
In this pilot project funded by the Taiwan Center for Disease Control (CDC), patients with pDM were enrolled by endocrinologists and treated by pulmonologists from a collaborative multidisciplinary team in 2 hospitals. We reported the completion rates of screening and preventive therapy for LTBI with a special emphasis on the 3HP regimen.
METHODS
Study Design and Population
This prospective study was conducted at a medical center in Taichung and a regional hospital in Kaohsiung between April 2018 and June 2020 in a collaborative setting involving public health professionals, endocrinologists, and pulmonologists. This study was approved by the institutional ethics committees of both hospitals (see Supplementary Material for details).
Because an HbA1c level of >9% was reported to increase infection risk in DM patients in the United States [20], and a study conducted in Taiwan [7] revealed that DM patients with an HbA1c level of >9% had a 3.55-fold higher risk of having smear-positive pulmonary TB compared with nondiabetic controls, 9% was used as the cutoff value for HbA1c within the recent 12 months to define pDM in this study. From endocrinology clinics, patients with pDM aged >45 years were enrolled. Patients were excluded if they were close contacts of patients with pulmonary TB, pregnant, seropositive for human immunodeficiency virus (HIV), had active TB at enrollment, or had a history of TB disease.
Programmatic Settings for LTBI Screening and Treatment
This is the first study to our knowledge to include endocrinologists in an LTBI intervention program in Taiwan. Before the recruitment of patients, public health professionals and pulmonologists provided necessary information and knowledge regarding LTBI intervention in high-risk population to endocrinologists. Case selection criteria and study protocols were then established by this multidisciplinary team. Regular study meetings were held monthly and as needed to review and discuss the process and related issues of the study.
Potential study participants (see study proposal in Supplementary Material) were initially interviewed by endocrinologists. Those who fulfilled case selection criteria and provided informed consent were screened for LTBI by using the QuantiFERON-TB (QFT) Gold In-Tube (Qiagen, Valencia, California, USA). Those who were QFT-positive were referred to pulmonologists’ clinic for further evaluation of their indications and suitability for TPT.
Either 9H or 3HP was offered for LTBI treatment in current study (see study proposal in Supplementary Material). Because all expenses of LTBI screening and treatment in this study were covered by the official budget of Taiwan CDC, one preventive regimen versus the other was recommended after considering patients’ convenience and safety. First, potential severe drug-drug interactions were screened. If no contraindication was noted, the 3HP regimen was preferred. For patients with concomitant liver diseases or those with abnormal baseline liver function test results, the 3HP regimen was preferred. After the pulmonologist in charge explained the advantages and disadvantages of both regimens in detail, the final choice of the regimen was made through shared decision making [21]. In addition to isoniazid and/or rifapentine, pulmonologists simultaneously prescribed acetaminophen for symptom relief if a patient developed adverse drug reactions (ADRs) such as fever or aches. Pulmonologists also informed endocrinologists to evaluate the blood sugar level of patients during preventive therapy.
Programmatic Settings for Monitoring ADRs
Regardless of the regimen, all participants joined the directly observed therapy (DOT) program [22]. ADRs were assessed through phone interview or on communication apps within 2 days after each 3HP dose or every 2 weeks during 9H treatment and when any ADR occurred by either the official case manager in the hospital or DOT supporters in the community. All of these individuals were trained and qualified by the Taiwan CDC [23]. Hemogram, liver, and kidney function tests were performed every 2 weeks in the first month, monthly in the following 2 months, and every 2 months thereafter during treatment and when patients developed SDRs (see study proposal in Supplementary Material). If participants agreed, the QFT test was repeated after TPT completion.
The severity of ADRs, hepatotoxicity [24], and SDRs [17] was defined in accordance with previous reports (see study proposal in Supplementary Material). Pulmonologists in charge evaluated the causal relationship between drugs and ADRs by calculating Naranjo scores [25] and subsequently provided appropriate management.
All participants were followed up until premature termination, active TB development, or 1 week after treatment completion.
Outcome Assessment
The aims of the current study were to evaluate the QFT-positive rate in patients with pDM and the TPT completion rate in each regimen. The QFT response was defined as the difference in the interferon-gamma level between TB antigen and nil tubes, with a level of ≥0.35 IU/mL indicating QFT-positivity in accordance with manufacturer’s instructions. Completion of the 3HP and 9H regimens was defined as completing 12 doses within 16 weeks and 270 doses within 12 months, respectively.
We investigated the predictors of QFT-positivity and analyzed the safety profile of each TPT regimen as well as the effect of each regimen on the completion rate. In addition, the QFT conversion rate after the completion of TPT was assessed.
Statistical Analysis
Patients’ demographic profiles, clinical characteristics, and laboratory data were obtained. Student t test and the Mann-Whitney U test were performed to analyze intergroup differences in continuous variables depending on the normality. Categorical variables were compared using either the χ 2 test or Fisher exact test, as appropriate. Multivariate logistic regression was used to calculate the adjusted OR (aOR), 95% CI, and P values for potential risk factors for QFT-positivity and permanent discontinuation of TPT. Participants were excluded from the treatment outcome analysis if TPT was not administered. Statistical significance was set at a 2-sided P value of < .05. All statistical analyses were performed using SPSS, version 20.0 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Study Population
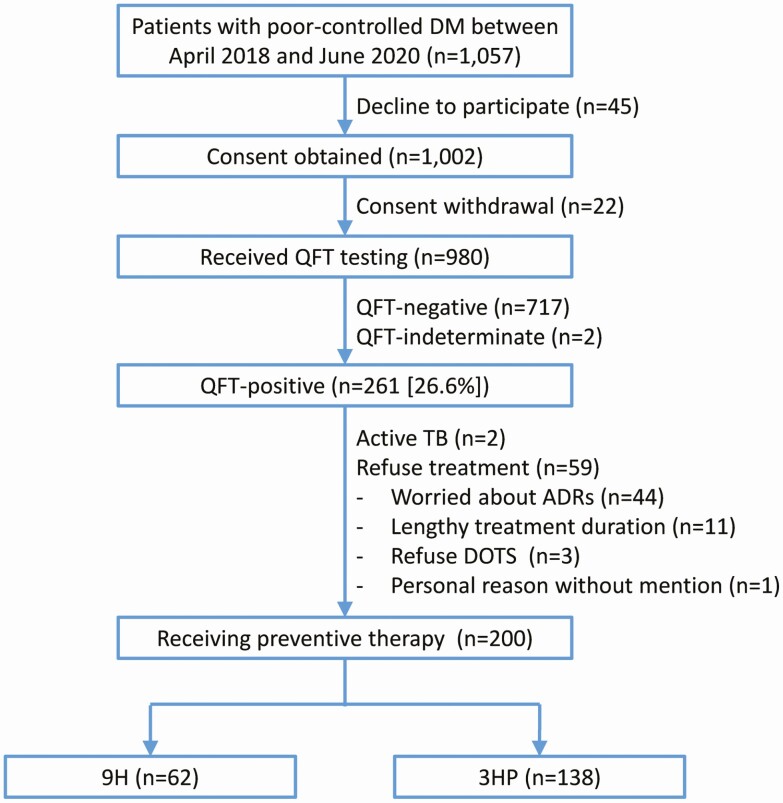
Between April 2018 and June 2020, a total of 1057 patients with pDM (age [mean ± standard deviation]: 64.3 ± 9.6 years) were eligible for recruitment (Figure 1). Among them, 980 (92.7%) received QFT testing (age: 64.2 ± 9.7 years), 261 (26.7%) were QFT-positive, and 2 (0.2%) had an indeterminate QFT result. Among 261 QFT-positive patients, 2 (0.8%) were diagnosed as having active TB, and 59 (22.6%) refused undergoing TPT (75% of them due to concern of ADRs). Of the remaining 200 (76.6%) patients with pDM, 62 (31.0%) and 138 (69.0%) subsequently underwent the 9H and 3HP regimens, respectively.
Figure 1.
Case selection process. Abbreviations: 3HP, 3-month weekly isoniazid plus rifapentine; 9H, 9-month daily isoniazid; ADR, adverse drug reaction; DM, diabetes mellitus; DOTS, directly observed treatment short course; QFT, QuantiFERON test; TB, tuberculosis.
Characteristics of Patients With QFT-Positivity or Negativity
Among 978 patients with pDM (age: 64.2 ± 9.6), 55.3% were men, and 10.5% had a body mass index (BMI) of ≥27 kg/m2, defined as indicating obesity by the Health Promotion Administration, Ministry of Health and Welfare of Taiwan (Table 1). Compared with QFT-negative patients, patients with a positive QFT result were more likely to have a BMI of ≥27 kg/m2, systemic comorbidities, and a longer DM duration and less likely to receive metformin and sodium–glucose cotransporter 2 (SGLT2) inhibitors. No significant difference was observed in the income status and educational level between both groups. Baseline laboratory results were similar between both groups, except that the QFT-positive group had a higher average platelet count (227 ± 66 vs 208 ± 107 K/µL, P = .001) and a lower aspartate transaminase level (24.3 ± 9.8 vs 26.5 ± 12.9 U/L, P = .010) (Supplementary Table 1).
Table 1.
Baseline Characteristics of Patients With Poorly Controlled Diabetes Mellitus (DM)
| Patients Receiving QFT testing | Patients Receiving TPT | |||||
|---|---|---|---|---|---|---|
| Total (n = 978) | QFT-negative (n = 717) | QFT-positive (n = 261) | TPT (n = 200) | 3HP (n = 138) | 9H (n = 62) | |
| Male sex | 541 (55.3%) | 396 (55.2%) | 145 (55.6%) | 112 (56.0%) | 77 (55.8%) | 35 (56.5%) |
| Age | 64.2 ± 9.6 | 63.3 ± 9.8 | 66.1 ± 8.6* | 65.6 ± 8.5 | 63.5 ± 7.8 | 70.3 ± 8.2# |
| BMI (kg/m2) | 26.2 ± 4.5 | 26.2 ± 4.7 | 26.3 ± 4.0 | 26.4 ± 3.7 | 26.5 ± 3.7 | 26.1 ± 3.7 |
| <18.5 | 18 (1.8%) | 15 (2.1%) | 3 (1.1%) | 1 (0.5%) | 1 (0.7%) | 0 |
| 18.5 ≤ BMI < 24 | 291 (29.8%) | 221 (30.8%) | 70 (26.8%) | 52 (26.0%) | 34 (24.6%) | 18 (29.0%) |
| 24 ≤ BMI < 27 | 566 (57.9%) | 418 (58.3%) | 147 (56.3%) | 113 (56.5%) | 80 (58.0%) | 33 (53.2%) |
| ≥27a | 103 (10.5%) | 63 (8.8%) | 40 (15.3%)* | 34 (17.0%) | 23 (16.7%) | 11 (17.7%) |
| Smoking status | ||||||
| Never smoker | 708 (72.4%) | 525 (73.2%) | 183 (70.1%) | 142 (71.0%) | 98 (71.0%) | 44 (71.0%) |
| Ex-smoker | 132 (13.5%) | 91 (12.7%) | 41 (15.7%) | 30 (15.0 %) | 18 (13.0%) | 12 (19.4%) |
| Current smoker | 138 (14.1%) | 101 (14.1%) | 37 (14.2%) | 28 (14.0%) | 22 (15.9%) | 6 (9.7%) |
| Low incomeb | 10 (1.0%) | 7 (1.0%) | 3 (1.1%) | 3 (1.5%) | 2 (1.4%) | 1 (1.6%) |
| Highest education level | ||||||
| Primary school or lower | 268 (27.4%) | 188 (26.2%) | 80 (30.7%) | 58 (29.0%) | 35 (25.4%) | 23 (37.1%) |
| Middle school | 217 (22.2%) | 152 (21.2%) | 65 (24.9%) | 54 (27.0%) | 34 (24.6%) | 20 (32.3%) |
| High school | 262 (26.8%) | 198 (27.6%) | 64 (24.5%) | 46 (23.0%) | 37 (26.8%) | 9 (14.5%) |
| College or higher | 231 (23.6%) | 179 (25.0%) | 52 (19.9%) | 42 (21.0%) | 32 (23.2%) | 10 (16.1%) |
| Comorbidities | ||||||
| Hyperlipidemia | 673 (68.8%) | 496 (69.2%) | 177 (67.8%) | 137 (68.5%) | 96 (69.6%) | 41 (66.1%) |
| Hypertension | 606 (62.0%) | 426 (59.4%) | 180 (69.0%)* | 144 (72.0%) | 99 (71.1%) | 45 (72.6%) |
| CKD stage ≥3 | 278 (28.4%) | 202 (28.2%) | 76 (29.1%) | 59 (29.5%) | 34 (24.6%) | 25 (40.3%)# |
| Coronary artery disease | 191 (19.5%) | 133 (18.5%) | 58 (22.2%) | 41 (20.5%) | 25 (18.1%) | 16 (25.8%) |
| Old CVA | 124 (12.7%) | 81 (11.3%) | 43 (16.5%)* | 32 (16.0%) | 19 (13.8%) | 13 (21.0%) |
| Cancer | 119 (12.2%)c | 92 (12.8%) | 27 (10.3%) | 22 (11.0%)d | 13 (9.4%) | 9 (14.5%) |
| Congestive heart failure | 59 (6.0%) | 35 (4.9%) | 24 (9.2%)* | 16 (8.0%) | 12 (8.7%) | 4 (6.5%) |
| COPD | 53 (5.4%) | 37 (5.2%) | 16 (6.1%) | 11 (5.5%) | 9 (6.5%) | 2 (3.2%) |
| Autoimmune disease | 51 (5.2%)c | 39 (5.4%) | 12 (4.6%) | 9 (4.5%)d | 7 (5.1%) | 2 (3.2%) |
| Asthma | 41 (4.2%) | 27 (3.8%) | 14 (5.4%) | 11 (5.5%) | 7 (5.1%) | 4 (6.5%) |
| Bronchiectasis | 18 (1.8%) | 15 (2.1%) | 3 (1.1%) | 2 (1.0%) | 2 (1.4%) | 0 |
| Hepatitis B | 29 (3.0%) | 20 (2.8%) | 9 (3.4%) | 8 (4.0%) | 4 (2.9%) | 4 (6.5%) |
| Hepatitis C | 15 (1.5%) | 11 (1.5%) | 4 (1.5%) | 3 (1.5%) | 2 (1.4%) | 1 (1.6%) |
| DM status | ||||||
| Duration (years) | 9.5 ± 6.8 | 9.0 ± 6.5 | 11.0 ± 7.5* | 11.4 ± 7.2 | 11.4 ± 7.6 | 11.2 ± 6.4 |
| Maximum HbA1c (%) | 10.9 ± 1.7 | 11.0 ± 1.7 | 10.9 ± 1.5 | 10.9 ± 1.4 | 10.9 ± 1.4 | 10.9 ± 1.5 |
| HbA1c (%) at enrollment | 9.5 ± 1.5 | 9.5 ± 1.5 | 9.4 ± 1.5 | 9.4 ± 1.4 | 9.2 ± 1.3 | 9.7 ± 1.4# |
| Anti-diabetic medication | ||||||
| Insulin | 530 (54.2%) | 383 (53.4%) | 147 (56.3%) | 115 (57.5%) | 79 (57.2%) | 36 (58.1%) |
| Metformin | 686 (70.1%) | 526 (73.4%) | 160 (61.3%)* | 122 (61.0%) | 89 (64.5%) | 33 (53.2%) |
| DDP-4 inhibitor | 484 (49.5%) | 349 (48.7%) | 145 (55.6%) | 112 (56.0%) | 74 (53.6%) | 38 (61.3%) |
| Sulfonylurea | 428 (43.8%) | 324 (45.2%) | 104 (39.8%) | 81 (40.5%) | 56 (40.6%) | 25 (40.3%) |
| Thiazolidinedione | 260 (26.6%) | 200 (27.9%) | 60 (23.0%) | 47 (23.5%) | 33 (23.9%) | 14 (22.6%) |
| SGLT2 inhibitor | 225 (23.0%) | 177 (24.7%) | 48 (18.4%)* | 37 (18.5%) | 36 (26.1%) | 1 (1.6%)# |
| Glinide | 76 (7.8%) | 49 (6.8%) | 27 (10.3%) | 19 (9.5%) | 11 (8.0%) | 8 (12.9%) |
| GLP-1 agonist | 56 (5.7%) | 42 (5.9%) | 14 (5.4%) | 13 (6.5%) | 12 (8.7%) | 1 (1.6%) |
| α-glucosidase inhibitor | 53 (5.4%) | 35 (4.9%) | 18 (6.9%) | 11 (5.5%) | 7 (5.1%) | 4 (6.5%) |
| Lipid lowering agent | ||||||
| Statin | 616 (63.0%) | 460 (64.2%) | 156 (59.8%) | 120 (60.0%) | 84 (60.9%) | 36 (58.1%) |
| Fibrate | 77 (7.9%) | 52 (7.3%) | 25 (9.6%) | 19 (9.5%) | 15 (10.9%) | 4 (6.5%) |
| QFT (IU/mL) | ||||||
| Nil | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.5* | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.3 ± 0.3 |
| Mitogen | 8.9 ± 1.9 | 8.9 ± 2.0 | 9.1 ± 1.7* | 9.1 ± 2.5 | 9.0 ± 2.8 | 9.5 ± 1.4 |
| TB antigen—Nil | 0.8 ± 1.7 | 0.18 ± 0.3 | 2.7 ± 2.6* | 2.7 ± 2.6 | 2.7 ± 2.6 | 2.6 ± 2.5 |
Data are either presented as the mean ± standard deviation or a number (%).
Abbreviations: 3HP, 3-month weekly isoniazid plus rifapentine; 9H, 9-month daily isoniazid; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DPP4, dipeptidyl peptidase 4; GLP-1, Glucagon-like peptide-1; HbA1c, glycated haemoglobin; SGLT2, sodium–glucose cotransporter 2; TB, tuberculosis; TPT, tuberculosis preventive therapy.
*P < .05 between QFT-positive and QFT-negative groups.
#P < .05 between 3HP and 9H groups.
aBMI ≥27 kg/m2 was recommended as the definition of obesity by the Health Promotion Administration, Ministry of Health and Welfare of Taiwan.
bThe definition of low income was personal income <471.6 USD/month.
c, dPlease see supplementary material for details.
Predictors of QFT-Positivity in Patients With pDM
Multivariate logistic regression analysis results revealed that age (aOR [95% CI] for per year increment: 1.02 [1.00–1.04], P = .026), DM duration (1.04 [1.02–1.07], P < .001), chronic kidney disease, stage ≥3 (1.80 [1.23–2.65], P = .003), metformin use (0.56 [0.39–0.80], P = .001), and dipeptidyl peptidase-4 inhibitor use (1.51 [1.08–2.13], P = .018) were independent predictors of QFT-positivity (Table 2).
Table 2.
Independent Factors Associated With QuantiFERON Positivity in Patients With Poorly Controlled Diabetes Mellitus (DM)
| Variables | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Age (per year increment) | 1.02 | 1.00–1.04 | .026 |
| Duration of DM (per year increment) | 1.04 | 1.02–1.07 | <.001 |
| Chronic kidney disease, stage ≥3 | 1.80 | 1.23–2.65 | .003 |
| Metformin use | 0.56 | .39–.80 | .001 |
| Use of dipeptidyl peptidase 4 inhibitor | 1.51 | 1.08–2.13 | .018 |
Abbreviations: CI, confidence interval; DM, diabetic mellitus; OR, odds ratio.
Variables in Table 1 except for laboratory data were entered into the multivariate regression model.
Characteristics and Outcomes of Patients With pDM Who Received TPT
The mean age of 200 patients with pDM who received TPT was 65.6 years, and the male to female ratio was 1.27 (Table 1). Baseline characteristics were similar between the 3HP (n = 138) and 9H (n = 62) groups, except that patients in the 9H group were older on average (70.3 ± 8.2 vs 63.5 ± 7.8 years, P < .001), had a higher prevalence of CKD stage ≥3 (40.3% vs 24.6%, P = .024), had a higher average HbA1c level (%) at enrolment (9.7 ± 1.4 vs 9.2 ± 1.3, P = .050), and were less likely to receive SGLT2 inhibitors (1.6% vs 26.1%, P < .001). Baseline laboratory results were similar between the 2 groups, except that the 9H group had a lower average hemoglobin level (12.9 ± 1.6 vs 13.9 ± 1.9 g/dL, P < .001) and a higher average creatinine level (1.3 ± 0.9 vs 1.0 ± 0.4 mg/dL, P = .018) (Supplementary Table 2).
Among those receiving TPT, the completion rates of the 3HP and 9H groups were 84.1% and 79.0% (P = .494), respectively (Table 3). ADRs were the cause of permanent TPT discontinuation in 20 (14.5%) patients receiving the 3HP regimen and 8 (12.9%) patients receiving the 9H regimen (P = .764).
Table 3.
Treatment Course and Outcome of Patients Undergoing Either the 3-Month Weekly Isoniazid Plus Rifapentine (3HP) or 9-Month Daily Isoniazid (9H) Regimen
| Total (n = 200) | 3HP (n = 138) | 9H (n = 62) | P-value | |
|---|---|---|---|---|
| Complete treatment | 165 (82.5%) | 116 (84.1%) | 49 (79.0%) | .494 |
| No adverse drug reactions | 59 (29.5%) | 30 (21.7%) | 29 (46.8%) | <.001 |
| Permanent discontinuation | 35 (17.5%) | 22 (15.9%) | 13 (21.0%) | .494 |
| Dose received | 5.0 ± 2.7 | 56.7 ± 40.8 | ||
| Cause of discontinuation | ||||
| Adverse drug reaction | 28 (14.0%) | 20 (14.5%) | 8 (12.9%) | .764 |
| Systemic drug reaction | 6 (3.0%) | 6 (4.3%) | 0 | .223 |
| Hypotension | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Flu-like syndrome | 5 (2.5%) | 5 (3.6%)a | 0 | .301 |
| Urticaria | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Hepatotoxicity | 4 (2.0%) | 2 (1.4%) | 2 (3.2%) | .776 |
| Other adverse drug reactions | 18 (9.0%) | 12 (8.7%) | 6 (9.7%) | .822 |
| Patient refusal | 5 (2.5%) | 2 (1.4%) | 3 (4.8%) | .352 |
| Other reasons | 2 (1.0%) | 0 | 2 (3.2%)b | .176 |
Data are presented as either the mean ± standard deviation or a number (%). The denominator of each calculation of percentage is the case number of each corresponding age group.
aOne had both flu-like syndrome and urticaria.
bOne died of myocardial infarction, and the other died of septic shock.
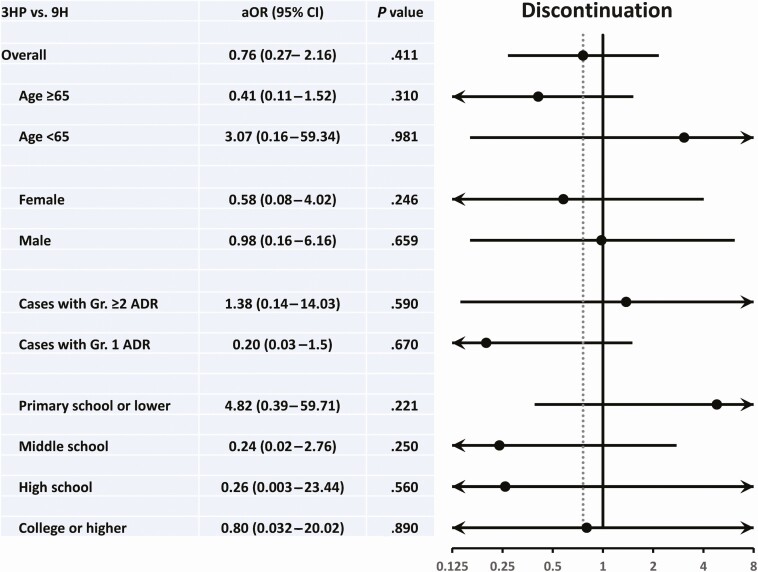
The results of multivariate logistic regression analysis including all variables, except for the QFT value listed in Table 1 revealed that the regimen (3HP vs 9H) was not a significant predictor of permanent TPT discontinuation among 200 patients with pDM who received TPT (0.76 [0.27–2.16], P = .609) and all subgroups (Figure 2 and Supplementary Table 3). Both low-income status and educational level were also not significant predictors.
Figure 2.
Forest plots showing the adjusted odds ratio (aOR) and 95% confidence interval (CI) of the impact of regimen on permanent discontinuation of tuberculosis preventive therapy in the overall study population and different subgroups. All variables listed in Table 1, except for QFT data, were considered in the statistical models.
Abbreviations: 3HP, 3-month weekly isoniazid plus rifapentine; 9H, 9-month daily isoniazid; ADR, adverse drug reaction; DM, diabetes mellitus; DPP4, dipeptidyl peptidase 4.
Safety Profile of 3HP and 9H
Among patients with pDM who received TPT, 78.3% and 53.2% of those in the 3HP and 9H groups experienced ≥1 ADR, respectively (P < .001). Detailed ADRs are presented in Table 4 and Supplementary Figure 1. SDRs occurred in 9 (6.5%) patients receiving 3HP, with flu-like syndrome occurring in 89% of them, resulting in permanent discontinuation of 3HPin 6 (67%). One patient experienced hypotension (blood pressure: 82/55 mmHg) during 3HP treatment. Grade-3 hepatotoxicity (definition in the study proposal) occurred only in 3HP group (0.7% vs 0%, P = .689). Two other patients in the 3HP group developed grade-3 toxicity (one had hypertension up to 201/179 mmHg, and the other one had severe dizziness requiring an emergency department visit).
Table 4.
Details of Adverse Drug Reactions (ADRs) in Patients Receiving 3-Month Weekly Isoniazid and Rifapentine (3HP) or 9-Month Daily Isoniazid (9H)
| Total (n = 200) | 3HP (n = 138) | 9H (n = 62) | P-value | |
|---|---|---|---|---|
| Any ADR | 141 (70.5%) | 108 (78.3%) | 33 (53.2%) | <.001 |
| Systemic drug reaction | 9 (4.5%) | 9 (6.5%) | 0 | .091 |
| Flu-like syndrome | 8 (4.0%) | 8 (5.8%) | 0 | .122 |
| Hypotension | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Urticaria | 2 (1.0%) | 2 (1.4%) | 0 | .854 |
| Hepatotoxicity | 8 (4.0%) | 4 (2.9%) | 4 (6.5%) | .426 |
| Grade 3 | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Grade 2 | 3 (1.5%) | 1 (0.7%) | 2 (3.2%) | .473 |
| Gastrointestinal ADRs | 93 (46.5%) | 78 (56.5%) | 15 (24.2%) | <.001 |
| Nausea | 53 (26.5%) | 42 (30.4%) | 11 (17.7%) | .060 |
| Gr. 2 | 26 (13.0%) | 23 (16.7%) | 3 (4.8%) | .021 |
| Epigastralgia | 29 (14.5%) | 25 (18.1%) | 4 (6.5%) | .030 |
| Gr. 2 | 18 (9.0%) | 16 (11.6%) | 2 (3.2%) | .056 |
| Anorexia | 29 (14.5%) | 20 (14.5%) | 9 (14.5%) | .997 |
| Gr. 2 | 5 (2.5%) | 3 (2.2%) | 2 (3.2%) | .961 |
| Diarrhea | 9 (4.5%) | 9 (6.5%) | 0 | .091 |
| Flu-like symptoms | 88 (44.0%) | 74 (53.6%) | 14 (22.6%) | <.001 |
| Dizziness | 59 (29.5%) | 51 (37.0%) | 8 (12.9%) | .001 |
| Gr. 3 | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Gr. 2 | 13 (6.5%) | 11 (8.0%) | 2 (3.2%) | .343 |
| Malaise | 44 (22.0%) | 40 (29.0%) | 4 (6.5%) | <.001 |
| Gr. 2 | 6 (3.0%) | 5 (3.6%) | 1 (1.6%) | .747 |
| Lethargy | 27 (13.5%) | 21 (15.2%) | 6 (9.7%) | .289 |
| Myalgia and arthralgia | 24 (12.0%) | 24 (17.4%) | 0 | <.001 |
| Gr. 2 | 13 (6.5%) | 13 (9.4%) | 0 | .029 |
| Headache | 22 (11.0%) | 21 (15.2%) | 1 (1.6%) | .004 |
| Gr. 2 | 9 (4.5%) | 9 (6.5%) | 0 | .091 |
| Fever | 20 (10.0%) | 20 (14.5%) | 0 | .002 |
| Gr. 2 | 14 (7.0%) | 14 (10.1%) | 0 | .021 |
| Febrile sensation and flush | 20 (10.0%) | 19 (13.8%) | 1 (1.6%) | .008 |
| Gr. 2 | 2 (1.0%) | 2 (1.4%) | 0 | .854 |
| Chills | 11 (5.5%) | 11 (8.0%) | 0 | .051 |
| Gr. 2 | 3 (1.5%) | 3 (2.2%) | 0 | .589 |
| URT symptoms | 10 (5.0%) | 9 (6.5%) | 1 (1.6%) | .262 |
| Gr. 2 | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Cutaneous ADRs | 33 (16.5%) | 18 (13.0%) | 15 (24.2%) | .049 |
| Rash | 15 (7.5%) | 9 (6.5%) | 6 (9.7%) | .622 |
| Gr. 2 | 10 (5.0%) | 4 (2.9%) | 6 (9.7%) | .092 |
| Itching | 25 (12.5%) | 13 (9.4%) | 12 (19.4%) | .049 |
| Gr. 2 | 8 (4.0%) | 3 (2.2%) | 5 (8.1%) | .115 |
| Vasculitis | 1 (0.5%) | 0 | 1 (1.6%) | .680 |
| Cardiovascular | 19 (9.5%) | 17 (12.3%) | 2 (3.2%) | .043 |
| Palpitation | 9 (4.5%) | 9 (6.5%) | 0 | .091 |
| Gr. 2 | 3 (1.5%) | 3 (2.2%) | 0 | .589 |
| Hypertension | 9 (4.5%) | 7 (5.1%) | 2 (3.2%) | .831 |
| Gr. 3 | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Gr. 2 | 2 (1.0%) | 2 (1.4%) | 0 | .854 |
| Numbness | 5 (2.5%) | 4 (2.9%) | 1 (1.6%) | .961 |
| Gr. 2 | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
| Fluctuated glucose control | 7 (3.5%) | 4 (2.9%) | 3 (4.8%) | .784 |
| Gr. 2 | 1 (0.5%) | 1 (0.7%) | 0 | .680 |
Data are presented as number (%). The denominator of each calculation of percentage is the number of cases in each corresponding group.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; Gr., grade; T-Bil, total bilirubin; ULN, upper limit of normal; URT, upper respiratory tract.
The most common ADRs were gastrointestinal symptoms (56.5%) and flu-like symptoms (53.6%) in the 3HP group and gastrointestinal symptoms (24.2%) and cutaneous reactions (24.2%) in the 9H group (Table 4). Fluctuating glucose control was noted in 4 (2.9%) patients in the 3HP group and 3 (4.8%) patients in the 9H group (P = .784); all instances except one were grade 1 in severity.
QFT Conversion Rate After TPT
After completing their treatment, 47 patients (34.1%) in the 3HP group and 35 (56.5%) in the 9H group received a follow-up QFT test. Both groups exhibited a significant reduction in the QFT response after TPT (both P < .001; paired t-test; Supplementary Figure 2). The QFT response (P = .720) and QFT conversion rate (32% vs 20%, P = .228) after TPT were insignificantly different between the 2 groups.
DISCUSSION
The results of the current pilot study demonstrated that through the collaboration of public health professionals, endocrinologists, and pulmonologists, programmatic LTBI intervention could lead to an LTBI screening rate of 92.7% and a completion rate of 82.5% among patients receiving TPT. Three major findings of this study were as follows: First, approximately one-quarter of patients with pDM had LTBI. The prevalence of LTBI was higher than that in TB close contacts (15%) [26] and patients receiving hemodialysis (19.3%) [27] in Taiwan; both populations are recommended by the WHO as targets for LTBI treatment [10]. The finding suggested that patients with pDM should be considered as the priority group for LTBI interventions from a public health perspective, particularly elderly people with a long DM duration and impaired renal function. Second, despite the higher rate of ADRs (mostly grade 1 and 2 in severity) under the 3HP regimen and the long duration of the 9H regimen, the completion rate was 80% for both regimens, implying that creating a collaborative multidisciplinary team and efficient public health program may be essential. In the 3HP cohort described in our recently published study [16], the 3HP completion rate in pDM patients without the inclusion of a collaborative multidisciplinary team was 77.3% (n = 44), approximately 7% lower than that in the current study (P = .303). Finally, metformin may be protective against TB infection.
Because of the limited resources of public health and medical systems, cost-effectiveness is always a major concern in national TB programs. The results of a simulation model demonstrated that LTBI screening using interferon-gamma release assay in the United States was cost-effective only when the prevalence of LTBI approached 25% [28]. A study conducted in South Korea revealed that the prevalence of LTBI exerted a strong effect on the incremental cost-effectiveness ratio [29]. Therefore, although the whole diabetic population should not be prioritized, programmatic screening and treatment for LTBI should be considered for elderly patients with pDM.
The results of this study suggested that the use of metformin seems protective against LTBI. Metformin is recommended as a first-line therapy for DM and might be beneficial in TB treatment. A systematic review including 12 observational studies reported that metformin significantly reduced the risk of TB-related mortality and shortened the time to sputum conversion [30]. In addition, metformin may reduce LTBI risk in DM patients [31, 32]. The protective effect of metformin might be due to its ability to enhance the function of phagolysosomes, modulate the innate host response to Mycobacterium tuberculosis, and reduce the chronic inflammation of the infected lung [33].
The optimal TPT regimen for patients with DM remains unclear. Among WHO-recommended regimens, 3HP is recognized for its shorter course duration and simplicity, leading to a higher completion rate than the 9H regimen, regardless of its high rate of ADRs [34]. In patients with DM, 3HP poses more safety concerns because rifapentine is a potent inducer of cytochrome P450, interfering with the metabolism of oral antidiabetic drugs [19]. In the current study, only 3.5% of patients with pDM (2.9% in 3HP group and 4.8% in 9H group) experienced mild fluctuation in glucose level. Although no significant difference in the discontinuation rate was observed between 3HP and 9H regimens in the whole study population and subgroups, the point estimate favors 9H in those who were aged <65 years and received education up to primary school or lower. Additional studies are necessary to confirm the findings.
More than three-quarters of the 3HP group developed ADRs, mainly grade 1 or 2 in severity. Nonetheless, the completion rates of the 3HP and 9H regimens were both high (84.1% and 79.0%, respectively) under care from the present collaborative multidisciplinary team. Several crucial and unique features may explain the success. The first is forming a multidisciplinary task force and conducting regular meetings to discuss the study progress. Second, the benefits of LTBI screening were emphasized by patients’ regular endocrinologists, with whom they can be expected to have had rapport, and participants were referred to pulmonologists qualified by the Taiwan CDC for LTBI treatment once an QFT-positive result obtained. Third, decisions regarding whether patients should receive TPT and the choice of the preventive regimen were determined through shared decision making, during which the advantages and disadvantages are well explained. Fourth, TPT medications were administered along with a symptom reliever in case of ADRs. Fifth, during TPT, endocrinologists monitored changes in the blood sugar level to consolidate patients’ safety and adherence. Finally, the entire treatment course was supervised by DOT supporters and case managers who were trained and qualified in promoting adherence and reporting and managing ADRs.
The current study has some limitations. First, the decreased M. tuberculosis-specific interferon-gamma response in patients [35] may have compromised the validity of LTBI diagnosis. Second, because therapeutic drug monitoring was not performed, we cannot speculate about the responsible drug or drug interactions causing ADRs. Third, because of the limited number of cases, we could not compare the risk of incident TB, which is the most critical outcome of TPT, between different regimens.
CONCLUSIONS
Because the prevalence of LTBI exceeds 25% in elderly patients with pDM, programmatic LTBI interventions integrating health professionals, endocrinologists, and pulmonologists can facilitate the successful implementation of LTBI policy to achieve high screening and completion rates, regardless of the regimen.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. The authors thank the Taiwan Center for Disease Control and the Departments of Health of Taichung City Government and Kaohsiung City Government for supporting this study. The authors also thank Dr Gwan-Han Shen, who supervised Laboratory No. 114 at Taichung Veterans General Hospital and passed away in 2014. We hold you dear in our memory.
Authors’ contribution. Manuscript writing and figure: H.-L. H., W.-C. H.
Study design: H.-L. H., W.-C. H., J.-Y. W., I.-T. L., I.-W. C.
Data collection: H.-L. H., W.-C. H., K. D. L., S.-S. L., M.-R. L., C.-S. C., M.-H. C.
Data analysis and interpretation: P.-L. L., C.-C. S., J.-Y. W., I.-T. L., I.-W. C.
Manuscript revision: J.-Y. W., I.-T. L.
Financial support. This study was supported by the Taiwan Centers for Disease Control, the Ministry of Science and Technology (grant numbers MOST 107-2314-B-037-106-MY3, MOST 109-2314-B-037-085-MY3), and the Kaohsiung Municipal Ta-Tung Hospital (grant numbers KMTTH-108-R007, KMTTH-109-R001). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lee PH, Fu H, Lai TC, Chiang CY, Chan CC, Lin HH. Glycemic control and the risk of tuberculosis: a cohort study. PLoS Med 2016; 13:e1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Q, Lu P, Martinez L, et al. Undiagnosed diabetes mellitus and tuberculosis infection: a population-based, observational study from eastern China. Diabetes Metab Res Rev 2020; 36:e3227. [DOI] [PubMed] [Google Scholar]
- 3.Restrepo BI. Diabetes and tuberculosis. Microbiol Spectr 2016; 4:10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron MM, Shaw KM, Bullard KM, Ali MK, Magee MJ. Diabetes is associated with increased prevalence of latent tuberculosis infection: findings from the National Health and Nutrition Examination Survey, 2011–2012. Diabetes Res Clin Pract 2018: 139:366–379. [DOI] [PubMed] [Google Scholar]
- 5.Lin CH, Kuo SC, Hsieh MC, et al. ; Changhua Research Alliance for Tuberculosis Elimination . Effect of diabetes mellitus on risk of latent TB infection in a high TB incidence area: a community-based study in Taiwan. BMJ Open 2019; 9:e029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MR, Huang YP, Kuo YT, et al. Diabetes mellitus and latent tuberculosis infection: a systemic review and meta-analysis. Clin Infect Dis 2016: 64:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang CY, Bai KJ, Lin HH, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One 2015; 10:e0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez L, Zhu L, Castellanos ME, et al. Glycemic control and the prevalence of tuberculosis infection: a population-based observational study. Clin Infect Dis 2017; 65:2060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merza MA, Savo AAS, Jaafer M. Risk of latent tuberculosis infection among diabetic patients in Azadi Teaching Hospital, Duhok province: a case control study. Asian J Med Bio Res 2018: 4:227–232. [Google Scholar]
- 10.World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management.2018. Available at: https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed 21 February 2021.
- 11.Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med 2017; 167:248–55. [DOI] [PubMed] [Google Scholar]
- 12.Sterling TR, Villarino ME, Borisov AS, et al. ; TB Trials Consortium PREVENT TB Study Team . Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 13.Sun HY, Huang YW, Huang WC, et al. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: a multicentre randomised controlled trial in Taiwan. Tuberculosis (Edinb) 2018; 111:121–6. [DOI] [PubMed] [Google Scholar]
- 14.Chen YM, Liao TL, Chen HH, Chen DY. Three months of once-weekly isoniazid plus rifapentine (3HP) in treating latent tuberculosis infection is feasible in patients with rheumatoid arthritis. Ann Rheum Dis 2018; 77:1688–9. [DOI] [PubMed] [Google Scholar]
- 15.Lin SY, Chiu YW, Lu PL, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection in hemodialysis patients: high rates of adverse events. J Microbiol Immunol Infect 2019: 52:158–162. [DOI] [PubMed] [Google Scholar]
- 16.Huang HL, Lee MR, Cheng MH, et al. Impact of age on outcome of rifapentine-based weekly therapy for latent tuberculosis infection. Clin Infect Dis 2020:ciaa1741. doi: 10.1093/cid/ciaa1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterling TR, Moro RN, Borisov AS, et al. ; Tuberculosis Trials Consortium . Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT tuberculosis study. Clin Infect Dis 2015; 61:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doan TN, Fox GJ, Meehan MT, et al. Cost-effectiveness of 3 months of weekly rifapentine and isoniazid compared with other standard treatment regimens for latent tuberculosis infection: a decision analysis study. J Antimicrob Chemother 2019; 74:218–27. [DOI] [PubMed] [Google Scholar]
- 19.Zheng C, Hu X, Zhao L, Hu M, Gao F. Clinical and pharmacological hallmarks of rifapentine’s use in diabetes patients with active and latent tuberculosis: do we know enough? Drug Des Devel Ther 2017; 11:2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol 2016; 4:148–58. [DOI] [PubMed] [Google Scholar]
- 21.Saheb Kashaf M, McGill ET, Berger ZD. Shared decision-making and outcomes in type 2 diabetes: a systematic review and meta-analysis. Patient Educ Couns 2017; 100:2159–71. [DOI] [PubMed] [Google Scholar]
- 22.Bloss E, Chan PC, Cheng NW, Wang KF, Yang SL, Cegielski P. Increasing directly observed therapy related to improved tuberculosis treatment outcomes in Taiwan. Int J Tuberc Lung Dis 2012; 16:462–7. [DOI] [PubMed] [Google Scholar]
- 23.Chan PC, Chen CH, Chang FY. External review of the National Tuberculosis Program and the development of strategy and targets post 2015 in Taiwan. J Formos Med Assoc 2014: 113:775–777. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services. National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1. 2017. Available at https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf
- 25.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45. [DOI] [PubMed] [Google Scholar]
- 26.Taiwan Centers for Disease Control. IT’S TIME! At-risk groups urged to get LTBI screening as Taiwan continues campaign to end TB.2019. Available at: https://www.cdc.gov.tw/En/Bulletin/Detail/cHsOe6RCP5mIwPAOJRDeDw?typeid=158. Accessed 21 February 2021.
- 27.Wu CH, Su HA, Chou CA, et al. An observational study on prevalence of latent tuberculosis infection and outcome of 3HP treatment in patients under hemodialysis in Taiwan. J Formos Med Assoc 2020. doi: 10.1016/j.jfma.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Tasillo A, Salomon JA, Trikalinos TA, Horsburgh CR Jr, Marks SM, Linas BP. Cost-effectiveness of testing and treatment for latent tuberculosis infection in residents born outside the United States with and without medical comorbidities in a simulation model. JAMA Intern Med 2017; 177:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohn H, Kim HY, Lee SH. Cost-effectiveness of contact screening strategies for tuberculosis among high-school adolescents in South Korea. Int J Tuberc Lung Dis 2018; 22:496–503. [DOI] [PubMed] [Google Scholar]
- 30.Yu X, Li L, Xia L, et al. Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: a systematic review. BMC Infect Dis 2019; 19:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leow MK, Dalan R, Chee CB, et al. Latent tuberculosis in patients with diabetes mellitus: prevalence, progression and public health implications. Exp Clin Endocrinol Diabetes 2014; 122:528–32. [DOI] [PubMed] [Google Scholar]
- 32.Magee MJ, Salindri AD, Kornfeld H, Singhal A. Reduced prevalence of latent tuberculosis infection in diabetes patients using metformin and statins. Eur Respir J 2019: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yew WW, Chan DP, Chang KC, Zhang Y. How does metformin act as a host-directed agent in tuberculosis associated with diabetes mellitus? J Thorac Dis 2020; 12:1124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njie GJ, Morris SB, Woodruff RY, Moro RN, Vernon AA, Borisov AS. Isoniazid-rifapentine for latent tuberculosis infection: a systematic review and meta-analysis. Am J Prev Med 2018; 55:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JC, Jarlsberg LG, Grinsdale JA, et al. Reduced sensitivity of the QuantiFERON(®) test in diabetic patients with smear-negative tuberculosis. Int J Tuberc Lung Dis 2015; 19:582–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.