Abstract
Background
Some contacts of patients with tuberculosis remain negative on tests for tuberculosis infection, despite prolonged exposure, suggesting they might be resistant to Mycobacterium tuberculosis infection. The objective of this multinational study was to estimate the proportion of household contacts resistant to M. tuberculosis (resisters).
Methods
We conducted a longitudinal study enrolling index patients enrolled in treatment for pulmonary multidrug- or rifampin-resistant tuberculosis and their household contacts. Contacts were tested for tuberculosis infection with a tuberculin skin test (TST) and interferon-gamma release assay (IGRA) at baseline and after 1 year. Exposure was quantified based on index patients’ infectiousness, index patient and household contact interaction, and age. We explored multiple definitions of resistance to tuberculosis infection by varying TST negativity cutoffs (0 vs <5 mm), classification of missing test results, and exposure level.
Results
In total, 1016 contacts were evaluated from 284 households; 572 contacts aged ≥5 years had TST and longitudinal IGRA results available. And 77 (13%) or 71 (12%) contacts were classified as resisters with a <5 mm or 0 mm TST threshold, respectively. Among 263 highly exposed contacts, 29 (11%) or 26 (10%) were classified as resisters using TST cutoffs of <5 mm and 0 mm, respectively. The prevalence of resisters did not differ substantially by sex, age, human immunodeficiency virus (HIV) coinfection, or comorbid conditions.
Conclusions
At least 10% of household contacts can be classified as resistant to tuberculosis infection, depending on the definition used, including those with high exposure. Further studies to understand genetic or immunologic mechanisms underlying the resister phenotype may inform novel strategies for therapeutics and vaccines.
Keywords: tuberculosis, infection, exposure, resisters
Some contacts of patients with tuberculosis are resistant against tuberculosis infection, despite prolonged exposure. We estimated that depending on definition used, 10–21% of household contacts can be classified as resistant to tuberculosis infection, including those with high exposure.
INTRODUCTION
Tuberculosis (TB) is a major public health problem and the leading cause of infectious disease-related mortality globally. In 2018, an estimated 10 million people fell ill with TB, and 1.2 million people with TB died worldwide [1]. An additional 23–32% of the world’s population is infected with Mycobacterium tuberculosis (Mtb) and can remain asymptomatic for decades (ie, TB infection) [2]. Although risk factors for infection with Mtb and progression to active TB disease have been identified, 10–50% of household contacts (HHCs) of TB patients remain negative on tuberculin skin test (TST) despite substantial exposure (eg, living in the same house or same room) to an infectious source case [3–5]. These individuals may be resistant to Mtb infection, commonly referred to as resisters or resister phenotype. Limited knowledge about factors that mediate resistance to Mtb infection after exposure to an infectious case has hampered development of prevention measures for TB, including a TB vaccine [6, 7].
Challenges in identifying individuals who are truly infected with Mtb, given the lack of a gold standard for determining TB infection, have limited rigorous research of resister phenotype to date. The 2 main methods used for the diagnosis of TB infection are TST and interferon-gamma release assays (IGRA) [5, 8, 9]. However, both methods measure the immune response to Mtb, rather than the infection itself. Some of the disadvantages of these methods are false negativity due to anergy, persistent positivity after clearance of infection, and false-positivity of TST due to nontuberculosis mycobacteria and Bacillus Calmette-Guérin (BCG) vaccination [10, 11]. In addition, discordance between TST and IGRA is common and ranges from 8% to 15% [12–14], making clinical interpretation challenging.
Similar to measuring TB infection, the objective measurement of TB exposure is also challenging. Although a validated TB exposure risk score has been developed for children [15], there is no standard methodology for assessing the extent of exposure across all ages and in diverse epidemiological settings, making it difficult to distinguish between resisters and those who are TST- or IGRA-negative due to insufficient exposure. Exposure to Mtb is affected both by clinical characteristics of the index patient (bacillary burden, or presence of cavities as clinical proxy) and the intensity and duration of exposure, such as proximity of contact and ventilation in the home [16, 17].
In preparation for an interventional trial, a longitudinal study was conducted to investigate the feasibility of identifying, recruiting, and characterizing adult index patients and their HHCs. Using data from this study, we aimed to: (1) estimate the proportion of household contacts of rifampicin-resistant (RR) and multidrug-resistant (MDR) TB patients who might be resistant to Mtb infection, using varying definitions of infection and exposure; and (2) evaluate factors associated with resistance to Mtb among HHCs.
METHODS
Study Design and Setting
A multicenter longitudinal study was conducted during 2015–2017 at 16 study sites in 8 high and middle TB burden countries worldwide: Botswana (1 site), Brazil (1), Haiti (1), India (2), Kenya (1), Peru (2), South Africa (7), and Thailand (1). These sites are now participating in a randomized clinical trial to test the safety and efficacy of a novel drug, delamanid, compared to standard of care isoniazid, for prevention of TB among HHCs (NCT03568383).
Study Population
The study population consisted of a convenience sample of adult index patients who initiated treatment for pulmonary RR/MDR-TB within 6 months preceding study enrollment, and their HHCs. A HHC was defined as a person who lived in the same dwelling unit or plot of land as the index patient and shared the same housekeeping arrangements. Further details about eligibility and enrollment of index patients and HHCs are provided in the methods section of the supplementary material and have been previously described elsewhere [18].
Data Collection
Data collection procedures in the parent study have been previously described and details are also provided in the supplementary material [18]. HHCs were tested for TB infection by TST and/or IGRA using QuantiFERON Gold or Gold-in-tube (Qiagen, Venlo, The Netherlands). TST was not performed at 2 study sites in Peru and 1 study site in South Africa due to reagent shortages. Quantitative results for IGRA were not recorded, and results <0.35 IU/mL were classified as negative. Among HHCs ≥5 years of age with a negative or missing result on IGRA at baseline, IGRA was repeated at 1 year of follow-up. Because the focus of the parent study was to inform the upcoming clinical trial, repeated testing was not performed among children <5 considering that this subpopulation is considered high risk regardless of their LTBI testing results. TST was not repeated in any age group. Previous human immunodeficiency virus (HIV) testing and TB history in HHCs was obtained through self-report during interviews. An HIV test was performed for those with unknown HIV status or last negative result documented more than 1 year prior to study entry. All HHCs not already on TB treatment were screened for active TB both at baseline and after 1-year follow-up with a symptom questionnaire, chest radiograph, and a collected sputum sample, as well as a gastric aspirate for children, if disease was suspected.
Definitions of Exposure and Resistance to Mtb
The primary outcome of interest was resistance to Mtb infection among adult and child HHCs ≥5 years of age. Inclusion criteria for the main analysis were the following: (1) age ≥5 years; (2) available TST result at baseline; and (3) complete IGRA result, defined as either positive IGRA result at baseline or valid results from 1-year follow-up testing (ie, positive or negative), if the baseline IGRA was negative or missing. Borderline/indeterminate IGRA results were counted as missing (n = 8 at baseline and n = 1 at the follow-up testing).
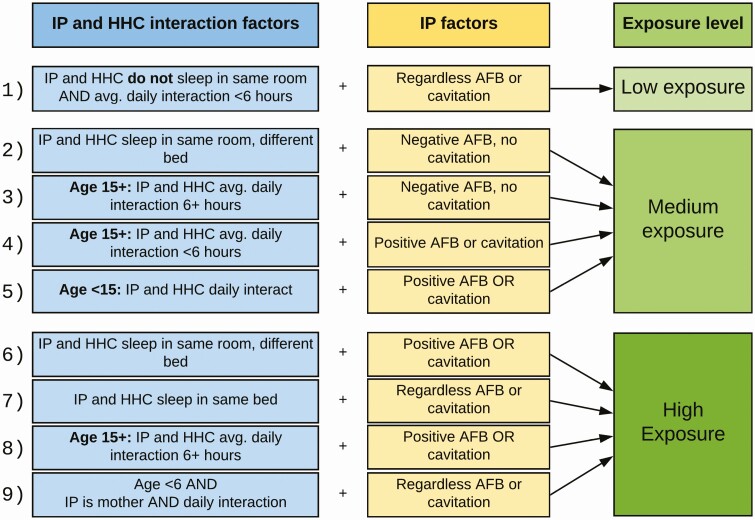
To assess the level of exposure among HHCs, a 3-level variable was created by adapting factors validated in published literature, which included infectiousness factors of index patient, index patient and HHC interaction factors, and age of HHC [15, 19]. Although all HHCs were considered exposed to an infectious TB case, we combined these factors to classify HHCs into low-, medium-, or high-exposure categories for the purposes of our analysis (Figure 1).
Figure 1.
Components of exposure score and classification scheme. Abbreviations: AFB, acid-fast bacilli; HHC, household contact; IP, index patient.
There is no universally accepted definition of resistance to Mtb infection or validated tools for assessing level of exposure in adults. As such, we used several classification criteria that varied the specificity of the definition of resister phenotype. Specifically, we explored different definitions of TB infection by varying the cutoff for baseline TST negativity (0 mm vs <5 mm). We also varied how we classified HHCs with incomplete TST or IGRA test results. Finally, we conducted a subset analysis to assess how the proportion of resister phenotype changed if we restricted to only those with high exposure. Our primary outcome definition was based on the most restrictive definition of resistance (ie, high specificity).
Data Analysis
Descriptive analysis was conducted on index patients, HHCs and household characteristics. The distribution of resisters across households was examined to identify any potential association between resisters and their household characteristics. Multivariable analysis using log-binomial regression was used to identify factors associated with resister phenotype. The models were fitted using generalized estimating equations (GEE) using a working exchangeable correlation structure to account for the clustering of contacts within households, and prevalence ratios with confidence intervals using robust standard errors were calculated. In order to evaluate whether any difference between groups was due to actual resistance to Mtb rather than lack of exposure, all models were adjusted for the 3-level exposure variable. Statistical analyses were carried out using SAS 9.4 and R package “digraph.”
Ethical Considerations
The parent study was approved by the local IRB or Ethics Committee at each site. Written informed consent was obtained from all participants and assent in children as per local guidelines. This secondary analysis of resister phenotype was also approved by the institutional review board (IRB) at Emory University.
RESULTS
Description of Study Population
A total of 284 index patients were enrolled. The highest proportion of index patients were enrolled in South Africa (121, 43%), followed by India (58, 20%) and Peru (54, 19%). There were 102 (36%) index patients who were HIV-positive, 135 (48%) with a positive acid-fast bacilli (AFB) smear result, and 138 (49%) with a positive Xpert result prior to study enrollment. Of 184 index patients with documented chest X-ray, 80 (43%) had cavitation.
A total of 1016 HHCs were included and evaluated (Supplementary Table 1). 913 (90%) of them were ≥5 years old, of which 63 (7%) were HIV-infected (including both newly diagnosed and known HIV infection), 89 (9%) had been previously treated for TB, and 212 (23%) reported current or past smoking. TST and IGRA discordance at baseline was observed in approximately 31% of HHCs, regardless of TST cutoff.
Proportion of Resister Phenotype Based on Varying Definitions of Infection and Exposure
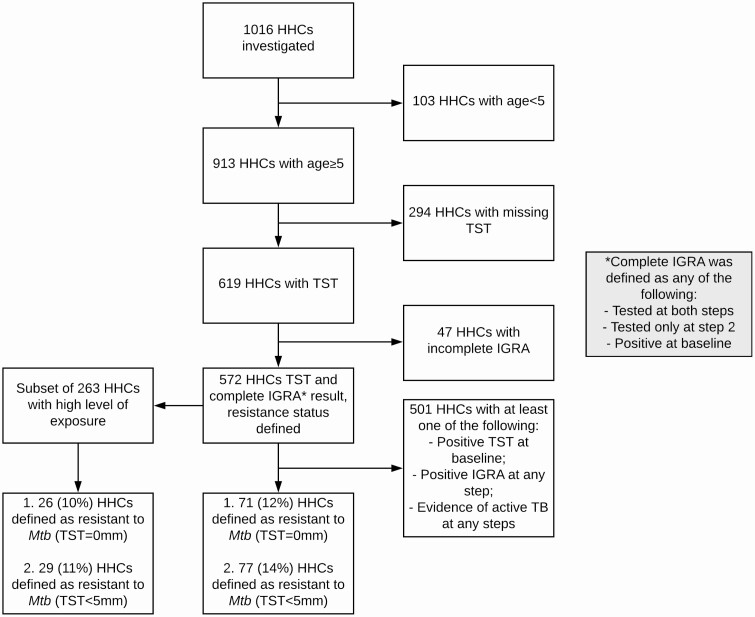
Using a restrictive (ie, high specificity) definition of resistance to Mtb as TST of <5 mm, IGRA <0.35 IU/mL after 1 year of follow-up, no evidence of active TB at baseline or 1-year follow-up, and all levels of exposure, we were able to determine resister status for 572 HHCs ≥5 years of age based on the availability of complete TST and IGRA test results (Figure 2, Table 1). Among these, 77 (13%) HHCs were classified as resisters (Table 1, Supplemental Table 2). Using the most restrictive TST cutoff of 0 mm, there were 71 (12%) HHCs classified as resisters (Supplemental Table 3). Of the 572 HHCs, 263 were highly exposed. Among these highly exposed HHCs, 29 (11%) were classified as resisters using a TST of <5 mm, and 26 (10%) with a TST of 0 mm (Figure 1, Table 1).
Figure 2.
Flow diagram of household contacts with complete testing and exposure data to determine resistance to Mtb infection. Abbreviations: HHC, household contact; IGRA, interferon-gamma release; Mtb, Mycobacterium tuberculosis; TB, tuberculosis; TST, tuberculin skin test.
Table 1.
Proportion of Household Contacts Classified as Resistant to Mtb Infection Using Varying Definitions for Infection and Exposure
| All HHCs | Highly Exposed HHCs | |||||
|---|---|---|---|---|---|---|
| Criteria for Defining HHC as a resistera | HHCs Included | n (%) Resistant, TST Cutoff <5 mm | n (%) Resistant, TST Cutoff 0 mm | HHCs Included | n (%) Resistant, TST Cutoff <5 mm | n (%) Resistant, TST Cutoff 0 mm |
| TST- and IGRA2-b | 572 | 77 (13.5) | 71 (12.4) | 263 | 29 (11.0) | 26 (10.0) |
| TST- and IGRA2-; TST- and IGRA1- and IGRA2 missing | 616 | 101 (16.4) | 93 (15.1) | 285 | 38 (13.3) | 35 (12.3) |
| All tests negative or any test negative while others missing | 898 | 188 (20.9) | 180 (20.0) | 415 | 63 (15.2) | 60 (14.5) |
Abbreviations: HHC, household contact; IGRA1, interferon gamma release assay at baseline; IGRA2, interferon gamma release assay after 1-year follow-up; Mtb, Mycobacterium tuberculosis; TB, tuberculosis; TST, tuberculin skin test at baseline.
a Age ≥5 was a required criterion for inclusion in the analysis population. No evidence of active TB was required criteria for classifying a HHC as a resister in all definitions.
b IGRA2 was only performed if IGRA1 was negative or missing.
Expanding our analysis population to include HHCs with baseline TST and IGRA data available but no follow-up IGRA testing, we were able to determine resister status for additional 44 HHCs, with total of 616 HHCs included (Table 1). Among them, 101 (16%) HHCs were classified as resisters using TST <5 mm and 93 (15%) using 0 mm. When exposure was added to the definition, 285 HHCs were highly exposed; among these, 38 (13%) were classified as resisters using a TST of <5 mm and 35 (12%) using 0 mm.
Finally, including HHCs with at least 1 negative TST or IGRA test at baseline or follow-up, resister status was available for additional 282 HHCs, with a total of 898 HHCs included (Table 1). Among these, 188 (21%) HHCs were resistant to Mtb infection using TST <5 mm and 180 (20%) using 0 mm. Among 415 HHCs in this group who were highly exposed, 63 (15%) were resisters using a TST of <5 mm and 60 (14%) using 0 mm.
Characteristics of Participants With Resistance to Mtb Infection
Table 2 shows the characteristics of HHCs with the resister phenotype, using the most restrictive TST (0 mm cutoff) and IGRA definitions, along with exposure level. The proportion of resisters differed by country and ranged from 0% in Brazil to 46% in Thailand. However, proportion in 2 of the largest enrolling countries (India and South Africa) was similar. The proportion of resisters did not differ substantially by sex, age, employment status, HIV coinfection, or comorbid diabetes or asthma. The only factor with considerable variation in proportion of resisters was based on the relationship to the index patient, where HHCs who were first-degree relatives (eg, parent, spouse, sibling) had a lower proportion of resisters than other cohabitants. Substance and alcohol use among HHCs were also associated with a lower proportion of resisters, although tobacco use was not. In multivariable analysis, we were unable to find any meaningful clinical or behavioral factor associated with the resister phenotype (Supplementary Table 4).
Table 2.
Characteristics of Household Contacts With Resistance to Mtb Infection (0 mm TST cutoff), for All Levels of Exposure and for Highly Exposed HHCs
| All Levels of Exposure | High Exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistant | Not Resistant | Resistant | Not Resistant | |||||||||
| Total | % (column) | n | % (row) | n | % (row) | Total | % (column) | n | % (row) | n | % (row) | |
| Total | 572 | 71 | 12% | 501 | 88% | 263 | 26 | 10% | 237 | 90% | ||
| Qualitative exposure variable | ||||||||||||
| Low exposure | 75 | 13% | 18 | 24% | 57 | 76% | ||||||
| Medium exposure | 234 | 41% | 27 | 12% | 207 | 88% | ||||||
| High exposure | 263 | 46% | 26 | 10% | 237 | 90% | 263 | 26 | 10% | 237 | 90% | |
| HHC demographics | ||||||||||||
| Countrya | ||||||||||||
| Botswana | 28 | 5% | 10 | 36% | 18 | 64% | 15 | 6% | 5 | 33% | 10 | 67% |
| Brazil | 9 | 2% | 0 | 0% | 9 | 100% | 7 | 3% | 0 | 0% | 7 | 100% |
| Haiti | 38 | 7% | 3 | 8% | 35 | 92% | 19 | 7% | 1 | 5% | 18 | 95% |
| India | 159 | 28% | 16 | 10% | 143 | 90% | 68 | 26% | 7 | 10% | 61 | 90% |
| Kenya | 6 | 1% | 0 | 0% | 6 | 100% | 5 | 2% | 0 | 0% | 5 | 100% |
| Peru | 0 | 0 | - | 0 | - | 0 | - | 0 | 0 | - | ||
| South Africa | 304 | 53% | 29 | 10% | 275 | 90% | 145 | 55% | 12 | 8% | 133 | 92% |
| Thailand | 28 | 5% | 13 | 46% | 15 | 54% | 4 | 2% | 1 | 25% | 3 | 75% |
| Sex | ||||||||||||
| Male | 233 | 41% | 35 | 15% | 198 | 85% | 97 | 37% | 8 | 8% | 89 | 92% |
| Female | 339 | 59% | 36 | 11% | 303 | 89% | 166 | 63% | 18 | 11% | 148 | 89% |
| Age, y | ||||||||||||
| <15 | 113 | 20% | 18 | 16% | 95 | 84% | 38 | 14% | 4 | 11% | 34 | 89% |
| 15+ | 459 | 80% | 53 | 12% | 406 | 88% | 225 | 86% | 22 | 10% | 203 | 90% |
| Employed | ||||||||||||
| Yes | 145 | 25% | 17 | 12% | 128 | 88% | 69 | 26% | 9 | 13% | 60 | 87% |
| No | 425 | 74% | 54 | 13% | 371 | 87% | 193 | 73% | 17 | 9% | 176 | 91% |
| Refused | 2 | 0% | 0 | 0% | 2 | 100% | 1 | 0% | 0 | 0% | 1 | 100% |
| Highest education | ||||||||||||
| None | 107 | 19% | 15 | 14% | 92 | 86% | 49 | 19% | 7 | 14% | 42 | 86% |
| Primary | 207 | 36% | 23 | 11% | 184 | 89% | 93 | 35% | 7 | 8% | 86 | 92% |
| Secondary | 214 | 37% | 29 | 14% | 185 | 86% | 99 | 38% | 11 | 11% | 88 | 89% |
| College/University or higher | 44 | 8% | 4 | 9% | 40 | 91% | 22 | 8% | 1 | 5% | 21 | 95% |
| Relationship of index patient to HHC | ||||||||||||
| Spouse | 42 | 7% | 5 | 12% | 37 | 88% | 35 | 13% | 5 | 14% | 30 | 86% |
| Cohabitant | 34 | 6% | 7 | 21% | 27 | 79% | 15 | 6% | 4 | 27% | 11 | 73% |
| Child | 78 | 14% | 8 | 10% | 70 | 90% | 34 | 13% | 3 | 9% | 31 | 91% |
| Mother | 66 | 12% | 6 | 9% | 60 | 91% | 36 | 14% | 2 | 6% | 34 | 94% |
| Father | 40 | 7% | 2 | 5% | 38 | 95% | 16 | 6% | 1 | 6% | 15 | 94% |
| Sibling | 104 | 18% | 8 | 8% | 96 | 92% | 45 | 17% | 4 | 9% | 41 | 91% |
| Missing/other | 208 | 36% | 35 | 17% | 173 | 83% | 82 | 31% | 7 | 9% | 75 | 91% |
| Comorbidities | ||||||||||||
| HIV | ||||||||||||
| Positive | 45 | 8% | 5 | 11% | 40 | 89% | 26 | 10% | 3 | 12% | 23 | 88% |
| Negative | 444 | 78% | 50 | 11% | 394 | 89% | 208 | 79% | 18 | 9% | 190 | 91% |
| Missing | 83 | 15% | 16 | 19% | 67 | 81% | 29 | 11% | 5 | 17% | 24 | 83% |
| Diabetes | 19 | 3% | 2 | 11% | 17 | 89% | 9 | 3% | 2 | 22% | 7 | 78% |
| COPD | 7 | 1% | 0 | 0% | 7 | 100% | 3 | 1% | 0 | 0% | 3 | 100% |
| Asthma | 18 | 3% | 2 | 11% | 16 | 89% | 7 | 3% | 0 | 0% | 7 | 100% |
| HHC behavioral factors | ||||||||||||
| Ever smoke tobacco | 139 | 24% | 13 | 9% | 126 | 91% | 76 | 29% | 6 | 8% | 70 | 92% |
| Currently smoke tobacco | 116 | 20% | 12 | 10% | 104 | 90% | 64 | 24% | 5 | 8% | 59 | 92% |
| HHC use substancesb | 44 | 8% | 1 | 2% | 43 | 98% | 21 | 8% | 0 | 0% | 21 | 100% |
| HHC drank alcohol | 38 | 7% | 3 | 8% | 35 | 92% | 22 | 8% | 0 | 0% | 22 | 100% |
| Index patient characteristicsc | ||||||||||||
| Index patient’s cavitation | ||||||||||||
| Yes | 199 | 35% | 27 | 14% | 172 | 86% | 118 | 45% | 15 | 13% | 103 | 87% |
| No | 190 | 33% | 20 | 11% | 170 | 89% | 74 | 28% | 6 | 8% | 68 | 92% |
| Unknown | 183 | 32% | 24 | 13% | 159 | 87% | 71 | 27% | 5 | 7% | 66 | 93% |
| Index patient’s AFB resultd | ||||||||||||
| Negative | 229 | 40% | 27 | 12% | 202 | 88% | 58 | 22% | 5 | 9% | 53 | 91% |
| Scanty positive | 56 | 10% | 6 | 11% | 50 | 89% | 38 | 14% | 5 | 13% | 33 | 87% |
| (1+) | 52 | 9% | 4 | 8% | 48 | 92% | 33 | 13% | 1 | 3% | 32 | 97% |
| (2+) | 70 | 12% | 10 | 14% | 60 | 86% | 42 | 16% | 3 | 7% | 39 | 93% |
| (3+) | 120 | 21% | 16 | 13% | 104 | 87% | 83 | 32% | 8 | 10% | 75 | 90% |
| Unknown | 45 | 8% | 8 | 18% | 37 | 82% | 9 | 3% | 4 | 44% | 5 | 56% |
Abbreviations: AFB, acid-fast bacilli; COPD, chronic obstructive pulmonary disease; HHC, household contact; HIV, human immunodeficiency virus; Mtb, Mycobacterium tuberculosis; TST, tuberculin skin test.
a TST was not performed in Peru due to reagent shortage. All countries followed the same protocol, and there was no other substantial difference across countries in terms of study procedures.
b In the past 12 months, has the household contact used any other substances (eg, marijuana, cocaine, etc.).
c Based on testing before or at study enrollment, whichever result was highest.
d Numbers included in this section are of HHCs who have an index patient with given characteristic, not the number of index patients itself.
Distribution of Participants With Resistance to Mtb Infection Across Households
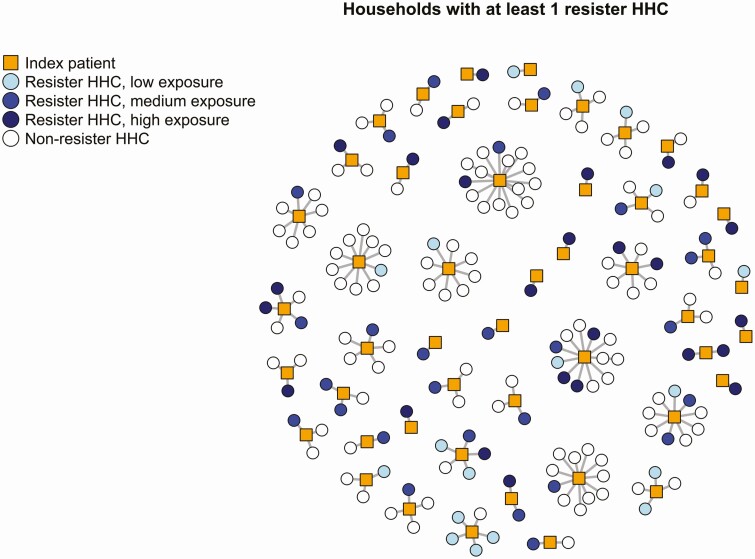
The 71 HHCs among all exposure levels who were classified as resisters to Mtb infection based on the definition with 0 mm TST, and all exposure levels were distributed across 49 (17%) households in 5 countries (Figure 3). Visual examination of household clusters suggests uneven distribution of that resister phenotype across households, with several households with large clusters of resister HHCs (Figure 3). The median number of HHCs in these households was 3 (interquartile range [IQR] 2–5). Among the 38 households with >1 HHCs and ≥1 resister HHC, the percentage of HHCs resistant to Mtb infection varied from 8% to 100% (median 33%, IQR 25%–50%).
Figure 3.
Clustering of persons with resistancea to Mtb infection in the subset of households with at least 1 resistor household contact (HHC; n = 49). Abbreviations: HHC, household contact; Mtb, Mycobacterium tuberculosis. aUsing the definition with 71 resisters (all levels of exposure, tuberculin skin test [TST] 0 mm, negative follow-up interferon-gamma release [IGRA]).
DISCUSSION
In this multinational study, we examined the proportion of individuals resistant to Mtb infection among HHCs who were exposed to active RR/MDR-TB index patients diagnosed in the preceding 6 months. Overall, we found that depending on the definition used, 10–21% of HHCs were resistant to Mtb infection, despite living in the same household as an infectious index patient. Even after applying stringent criteria to define resistance to Mtb with high specificity (0 mm induration on TST at baseline and negative IGRA at 1 year), at least 10% of HHCs were resistant to Mtb infection. To our knowledge, this is the first multinational study using consistent methods across countries to characterize a resister phenotype, supporting the idea that this phenotype exists in various geographic contexts.
The concept of resistance to Mtb infection has long existed; however, few rigorous studies characterizing the proportion and predictors of resister phenotype have been carried out to date. There are several notable challenges in studying resistance to Mtb infection. These include: the inherent limitations of TST and IGRA to detect the presence of Mtb infection; the lack of standardized methodology in measuring exposure to infectious index patients; a still rudimentary understanding of infectiousness of index patients; and no clear definition of resistance to Mtb, which incorporates these measures of infection, exposure, and infectiousness. In this study, we attempted to address these challenges using multiple strategies to improve the specificity of the definition. We used a combination of TST and IGRA—at baseline and after 1 year of follow-up, as well as using differing cutoffs of TST results—to examine the influence of varying definitions on the proportion of HHCs found to be resisters. Similarly, we created an exposure variable incorporating differing durations and proximity that contacts have spent with index patients, as well as clinical factors of the index patients’ TB disease that have been previously associated with infectiousness. Finally, we utilized several definitions of resistance to Mtb to identify how each of these components impacts the proportion of resister HHCs identified.
Using our most stringent definition, which required a TST of 0 mm, a negative IGRA result after 1 year of follow-up, and a high level of exposure, 10% of HHCs were classified as resistant. As each of these components were varied stepwise in definitions that increased sensitivity, the proportion of HHCs classified as resisters increased. The most sensitive definition (HHCs with at least 1 negative TST or IGRA test at baseline or follow-up) classified 21% of HHCs as being resisters. Based on these data, future researchers can vary the definition of resistance to Mtb to suit their purposes. For example, very strict definition with high specificity could be used in studies that involve assessment of immune or genetic markers and might have limited resources for laboratory testing due to high cost. Other studies that aim to identify individuals potentially resistant to Mtb could use more encompassing definition with higher sensitivity for initial screening purposes. Regardless of the definition that is used to identify resisters, however, it is clear that a considerable group of HHCs remain uninfected with Mtb, lending considerable strength to the long-standing notion that resistance to Mtb infection exists.
We did not find any meaningful behavioral or clinical factors associated with resister phenotype, despite our large sample size. We hypothesize that resistance to Mtb infection has an underlying biologic mechanism, mediated through innate immune responses, genetic or epigenetic factors. If that hypothesis is true, we would not expect that behavioral or clinical factors would be associated with resistance to Mtb, because the underlying biologic mechanisms would not be expected to vary by these factors. This hypothesis is further supported by recent studies that found enhanced innate immune response and distinct adaptive immune response profile among resister HHCs. [20, 21] However, more definitive acceptance of this hypothesis is hindered by the lack of tools to accurately assess the extent of exposure.
The findings of this large multinational study contribute to an evidence base from published single-site studies. A recent study in India found that the proportion of HHCs who were persistently LTBI-negative was 8.4% overall and 6.5% among HHCs with high exposure. [22]. The slightly higher estimates in our analysis could be explained by the fact that only IGRA testing was repeated after 1 year of follow-up in our study, although both IGRA and TST were repeated in the study from India, and lack of infection was defined as negative TST and negative IGRA at both baseline and up to 12 months following exposure to index patient. Due to common discrepancy between TST and IGRA, if TST were also repeated in our study, some of the HHCs currently classified as resisters in our definitions could have positive TST, and the total proportion of resisters would decrease. Similar to our analysis, the study from India did not identify any epidemiologic factors associated with resister phenotype. Two other studies from Uganda describe HHCs who remain persistently TST negative after exposure to index patient and report similar findings: Ma et al (2014) report that 11.7% of HHCs were persistently TST negative after 2 years of follow-up, and Stein et al (2018) report 10.7% of HHCs persistently TST-negative after at least 1 year of follow-up [19, 23]; IGRA testing was not done in either of these 2 studies. The participants from the latter study were later retraced, and 82.7% of those who were originally persistently TST-negative were concordantly negative on TST and IGRA after an average of 9.5 years, suggesting that resistance to Mtb is a stable phenomenon [24].
The exact mechanism of resistance against Mtb infection remains unknown. Simmons et al (2018) discuss several potential explanations for persistently negative TB infection tests [25]. One hypothesis suggests that HHCs have negative TST or IGRA test results only because they have not had substantial exposure to index patient. Our analysis, as well as studies from India and Uganda, suggest that resistance to Mtb infection is observed even when we restrict the study population to highly exposed HHCs. Another explanation suggests that the resister phenotype is actually observed due to false-negative results in tests of TB infection. A recent study in Uganda found that HHCs classified as resisters had developed non-interferon-gamma immune response to Mtb, which could explain negative results on traditional TB infection testing methods [21]. Whether this means that these contacts are actually infected and at higher risk of active TB or whether this different type of immune response provides protection to progression to TB has yet to be determined and will require larger studies that will synthesize epidemiologic, genetic and immunologic data.
Our study had several limitations. First, the study population was not a random sample of all eligible individuals in the target population, which might have introduced selection bias. Second, TST was not performed on almost one-third of the initial study population due in part to a shortage of reagent, which limited our ability to determine their TB infection status and reduced our final sample size. Third, repeated testing after 1 year of follow-up was only performed using IGRA without TST and was not performed at all among children <5 years. For this reason, we had to exclude a large proportion of participants from our main analysis, including children with age <5 years. Fourth, we did not have quantitative results of IGRA testing available. IGRA is interpreted as positive if the result is above the cutoff of 0.35 IU/mL. However, borderline results around this cutoff have been shown to fluctuate substantially. We were not able to explore how our findings would change using different cutoffs for IGRA [26–28].
Despite these limitations, our study contributes to the emerging literature that supports the resister phenotype as part of the spectrum of TB infection to disease. This is the first report to our knowledge that describes the proportion of resister phenotype using multiple definitions and explores the extent to which different classification schemes might affect the proportion of resisters. Immunogenomic studies of resister phenotype are important in order to improve our understanding of genetic or immunologic mechanisms underlying resistance to Mtb infection, which will in turn help in developing novel strategies for vaccines, diagnostics, and therapies for TB infection and disease.
Supplementary Material
Notes
Acknowledgments. The authors thank the study participants and their families. They also thank the site investigators, study teams, and protocol team of A5300/I2003.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health, under award numbers UM1AI068634, UM1AI068636 UM1AI106701, UM1A1068616, UM1AI068632, UM1AI068616, UM1AI106716, and by NICHD contract number HHSN275201800001I and R01AI139406 (PI Gandhi). This work was supported in part by the NIH Fogarty International Center Global Infectious Diseases grant number D43TW007124 to D. B.; K24AI114444, Emory TB Research Unit grant number U19AI111211 and Emory Center for AIDS Research grant P30AI051519 to N. G.; grant numbers UM1AI069465, R01HD081929, and R01AI39406 to A. G., and the National South African Research Foundation through a SARCHi Chair to A. H. C. U. reports that ACTG provided institutional grant support and was sponsor of the original feasibility study. G. C. reports that the Aurum Institute received a grant from ACTG/DAIDS to implement the PHOENIx Feasibility study.
Potential conflicts of interest. S. S. reports grants from ViiV Healthcare. M. H. reports research grants from NIH, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization, 2019. [Google Scholar]
- 2.Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: a re-estimation using mathematical modelling. PLoS Med 2016; 13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitaker JA, Mirtskhulava V, Kipiani M, et al. Prevalence and incidence of latent tuberculosis infection in Georgian healthcare workers. PloS One 2013; 8: e58202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health 1968; 16:26–35. [DOI] [PubMed] [Google Scholar]
- 5.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8:359–68. [DOI] [PubMed] [Google Scholar]
- 6.Ottenhoff TH. The knowns and unknowns of the immunopathogenesis of tuberculosis. Int J Tuberc Lung Dis 2012; 16:1424–32. [DOI] [PubMed] [Google Scholar]
- 7.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol 2012; 12:581–91. [DOI] [PubMed] [Google Scholar]
- 8.Fox GJ, Nhung NV, Sy DN, et al. Contact investigation in households of patients with tuberculosis in Hanoi, Vietnam: a prospective cohort study. PLoS One 2012; 7:e49880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma SK, Mohanan S, Sharma A. Relevance of latent TB infection in areas of high TB prevalence. Chest 2012; 142:761–73. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso JD, Mody RM, Olsen CH, Harrison LH, Santosham M, Aronson NE. The long-term effect of Bacille Calmette-Guérin vaccination on tuberculin skin testing: a 55-year follow-up study. Chest 2017; 152:282–94. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Report No: 1057-5987 (Print) 1057-5987 (Linking). 2000. [PubMed] [Google Scholar]
- 12.Dorman SE, Belknap R, Graviss EA, et al. ; Tuberculosis Epidemiologic Studies Consortium . Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 2014; 189:77–87. [DOI] [PubMed] [Google Scholar]
- 13.Arend SM, Thijsen SF, Leyten EM, et al. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med 2007; 175:618–27. [DOI] [PubMed] [Google Scholar]
- 14.Jones-López EC, White LF, Kirenga B, et al. Cough aerosol cultures of Mycobacterium tuberculosis: insights on TST / IGRA discordance and transmission dynamics. PLoS One 2015; 10:e0138358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandalakas AM, Kirchner HL, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis 2012; 16:1033–9. [DOI] [PubMed] [Google Scholar]
- 16.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med 2007; 4:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braden CR. Infectiousness of a university student with laryngeal and cavitary tuberculosis. Investigative team. Clin Infect Dis 1995; 21:565–70. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Swindells S, Kim S, et al. Feasibility of identifying household contacts of rifampin- and multidrug-resistant tuberculosis cases at high risk of progression to tuberculosis disease. Clin Infect Dis 2020; 70:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma N, Zalwango S, Malone LL, et al. ; Tuberculosis Research Unit (TBRU) . Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis 2014; 14:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verrall AJ, Schneider M, Alisjahbana B, et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J Infect Dis 2020; 221:1342–50. [DOI] [PubMed] [Google Scholar]
- 21.Lu LL, Smith MT, Yu KKQ, et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med 2019; 25:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mave V, Chandrasekaran P, Chavan A, et al. ; CTRIUMPH RePORT India Study Team . Infection free “resisters” among household contacts of adult pulmonary tuberculosis. PLoS One 2019; 14:e0218034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein CM, Zalwango S, Malone LL, et al. Resistance and susceptibility to Mycobacterium tuberculosis infection and disease in tuberculosis households in Kampala, Uganda. Am J Epidemiol 2018; 187:1477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein CM, Nsereko M, Malone LL, et al. Long-term stability of resistance to latent Mycobacterium tuberculosis infection in highly exposed tuberculosis household contacts in Kampala, Uganda. Clin Infect Dis 2019; 68:1705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons JD, Stein CM, Seshadri C, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol 2018; 18:575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzorka JW, Bossink AWJ, Franken WPJ, et al. Borderline QuantiFERON results and the distinction between specific responses and test variability. Tuberculosis (Edinb) 2018; 111:102–8. [DOI] [PubMed] [Google Scholar]
- 27.Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med 2015; 191:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nienhaus A, Ringshausen FC, Costa JT, Schablon A, Tripodi D. IFN-γ release assay versus tuberculin skin test for monitoring TB infection in healthcare workers. Expert Rev Anti Infect Ther 2013; 11:37–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.