Abstract
Background
CYD-TDV, a live, attenuated, tetravalent dengue vaccine, has been approved for the prevention of symptomatic dengue in previously dengue exposed individuals. This post hoc analysis assessed hospitalized and severe virologically confirmed dengue (VCD) over the complete 6-year follow-up of 3 CYD-TDV efficacy studies (CYD14, CYD15, and CYD23/CYD57).
Methods
The main outcomes were hazard ratios (HRs) for hospitalized or severe VCD by baseline dengue serostatus, focusing on those who were seropositive, and by age at immunization (<9 years/≥9 years). Baseline dengue serostatus was measured or inferred using several methods. Hospitalized VCD cases were characterized in terms of clinical signs and symptoms and wild-type viremia level. Antibody persistence was assessed up to 5 years after the last injection.
Results
In those aged ≥9 years and baseline seropositive, CYD-TDV protected against hospitalized and severe VCD over 6 years compared to placebo (HR [95% confidence interval] multiple imputation from month 0 method, .19 [.12–.30] and .15 [.06–.39]; other methods were consistent). Vaccine protection was observed over the different study periods, being highest during the first 2 years. Evidence for a decreased risk of hospitalized and severe VCD was also observed in seropositive participants aged 6–8 years. Clinical signs and symptoms, and quantified dengue viremia from participants with hospitalized VCD were comparable between groups.
Conclusions
CYD-TDV demonstrated robust protection against hospitalized and severe VCD over the entire 6-year follow-up in participants who were seropositive and ≥9 years old. Protection was also observed in seropositive 6–8 year-olds.
Clinical Trials Registration: NCT00842530, NCT01983553, NCT01373281, NCT01374516.
Keywords: dengue, CYD-TDV, serostatus, VCD
The final completed 6-year follow-up of 3 CYD-TDV randomized trials in Asia and Latin America confirms the influence of age and prior dengue infection on vaccine effect, indicating protection against hospitalized and severe dengue in seropositives ≥6 years old.
CYD-TDV (Dengvaxia®, Sanofi Pasteur) is a recombinant, live, attenuated, tetravalent dengue vaccine. Vaccine efficacy against symptomatic virologically confirmed dengue (VCD) was assessed during the first 25 months of 2 Phase III studies (CYD14 and CYD15), and a Phase IIb study (CYD23) [1–3]. All 3 efficacy studies had long-term follow-up safety phases to monitor the incidence of VCD hospitalization for an additional 4 years (totaling 6 years of follow-up), in compliance with World Health Organization (WHO) recommendations [4]; interim results have been published [5–9].
The CYD-TDV efficacy trials assessed baseline dengue serostatus in a subset of participants by measuring antibodies with a 50% plaque reduction neutralization test (PRNT50) [1–3]. Accounting for approximately 7.5%–20% of participants, these immunogenicity subsets did not allow for precise estimates of the risk of hospitalized or severe VCD (independent data monitoring committee [IDMC] definition) by baseline dengue serostatus. To overcome this limitation, blood samples collected from all participants after the third vaccination were used to retrospectively determine baseline serostatus using a dengue anti-nonstructural protein-1 (NS1) IgG enzyme-linked immunosorbent assay (ELISA), which detected anti-NS1 antibodies induced by wild-type dengue [10, 11]. A post hoc case-cohort study, assessing vaccine performance by baseline serostatus on interim data from these studies, showed protection of CYD-TDV against severe and hospitalized VCD in those who were dengue seropositive using 3 methods [9]. However, an increased risk was observed for vaccinated persons who had not been previously exposed to dengue. Based on this, the vaccine was recommended by WHO for people with evidence of prior dengue infection [12].
This post hoc analysis assessed hospitalized and severe VCD cases over the entire 6-year follow-up of the 3 trials (CYD14, CYD15, and CYD23/57). We describe the results by baseline serostatus, focusing on those classified as seropositive, and by age at first vaccination (≥9 years and <9 years). We present additional methods to assess baseline dengue serostatus to increase specificity, especially among individuals <9 years old where there is an increased risk of false dengue seropositivity. We also describe signs and symptoms, viremia, and neutralizing antibody persistence over the entire long-term follow up period.
METHODS
Study Design
The study designs of the 2 Phase III trials (CYD14 [NCT01373281] and CYD15 [NCT01374516]) and the Phase IIb trial (CYD23/CYD57 [NCT00842530/NCT01983553]) have been reported elsewhere [1–3, 5]. The study designs were almost identical, with participants randomized (2:1) to receive CYD-TDV or placebo at months 0, 6, and 12. CYD14 was conducted in 5 Asia-Pacific countries in participants aged 2–14 years, CYD15 was conducted in 5 Latin American countries in participants aged 9–16 years, and CYD23 was conducted in 1 province in Thailand in participants aged 4–11 years with the follow-up phase conducted in participants enrolled in the subsequent CYD57. The first 2 years were active surveillance of symptomatic VCD (years 1–2; active phase), and the following 4 years involved surveillance for hospitalized and severe VCD (years 3–6; long-term safety follow-up phase); active surveillance was reactivated during years 5–6 (surveillance expansion period, SEP).
Outcomes
The main parameters of this post hoc analysis were hazard ratios (HRs) or relative risks (RRs) for hospitalized VCD and severe VCD due to any serotype, by baseline serostatus, and by age at first dose (≥9 years or <9 years). Other parameters assessed among seropositive participants were: HRs for hospitalized VCD during each time period (years 1–2, year 3, year 4 and years 5–6), for each serotype (serotypes 1–4), and in additional age groups (2–5 years and 6–8 years); attributable risks for hospitalized and severe VCD; clinical signs and symptoms in hospitalized VCD cases; and dengue strain viremia levels from hospitalized VCD acute samples.
Methods for the confirmation of dengue, determination of dengue severity and the clinical signs and symptoms collected for all hospitalized dengue cases in the 3 studies have been described previously [1–3, 5]. Dengue viremia was assessed by reverse transcription polymerase chain reaction (RT-PCR), including a virus standard enabling expression as a concentration of log10 plaque-forming units (PFU)/mL.
The persistence of dengue antibodies was determined yearly by the PRNT50 assay in seropositive participants from the CYD14 and CYD15 immunogenicity subsets.
Additional endpoints included: annual incidence rate and RRs for hospitalized and severe VCD in the randomized populations from each study, regardless of serostatus, and the RR for hospitalized VCD in baseline seropositive or seronegative participants from the immunogenicity subsets.
Statistical Analyses
Safety and efficacy analyses were descriptive. They were performed on the Full Analysis Set for Efficacy (FASE) from the source studies, or subsets by covariates. The FASE comprised all participants who received ≥1 injection by the group to which they were randomized.
In NS1 analyses, month 0 (M0) PRNT50 serostatus (threshold of 10 [1/dil] to categorize immunogenicity) was measured or imputed in those with missing baseline values through multiple imputation (MI) at M0 or using super learner for targeted minimum loss-based estimation (TMLE) approaches, as previously described [10]. Serostatus was also determined in complementary analyses by dengue anti-NS1 immunoglobulin G (IgG) ELISA assay readout at M13, with seropositivity defined as ≥9 EU/mL (threshold 9 [NS1 M13 (Th9)]) [10]. Sensitivity analyses included: seropositivity by M13 anti-NS1 ELISA defined as ≥50 EU/mL (strict seropositive), and measured or imputed PRNT90 (threshold of 10 [1/dil] to categorize immunogenicity) at M0 by MI, to increase specificity, especially in individuals <9 years where there is an increased risk of false dengue seropositivity with PRNT50 due to a lower pre-test probability of prior dengue infection.
For NS1 analyses by baseline dengue status, several statistical methods were used, as described previously [10]. Briefly, a weighted Cox regression model was used for the MI and NS1 methods with HRs and corresponding 95% confidence intervals (CI) presented for individual studies and for the pooled analysis. TMLE was used to estimate cumulative incidences of dengue, from which RR was calculated for individual studies and for the pooled analysis. For MI and NS1 methods, attributable risks were calculated as between-group differences in estimated cumulative incidence over 6 years. The MI and TMLE approaches included cases from M0 onward; the analyses by M13 anti-NS1 ELISA levels included cases from M13 onward.
Incidence data for results regardless of serostatus and in the immunogenicity subsets were presented as total numbers of cases, annual incidence, RR (calculated as the ratio of annual incidence rates in the CYD-TDV vs placebo groups), and 2-sided 95% CIs.
For the clinical signs and symptoms of dengue infection in the NS1 M13 (Th9) positive population, proportions were calculated for categorical variables, and median and ranges were calculated for numeric variables. Viremia in acute blood samples was analyzed in terms of proportion with quantified viremia and mean concentrations, standard deviation (SD) and range.
The statistical analyses were carried out with SAS® software version 9.4 (SAS Institute, Cary, North Carolina, USA) and R statistical software version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Participant flow through the 3 studies is summarized in Supplementary Figures 1–3. The number of participants completing year 6 were: 3036 (94.8%) in CYD57, 9874 (96.1%) in CYD14, and 16 319 (78.2%) in CYD15.
Risk of Hospitalized and Severe VCD
Risk in Seropositive Participants
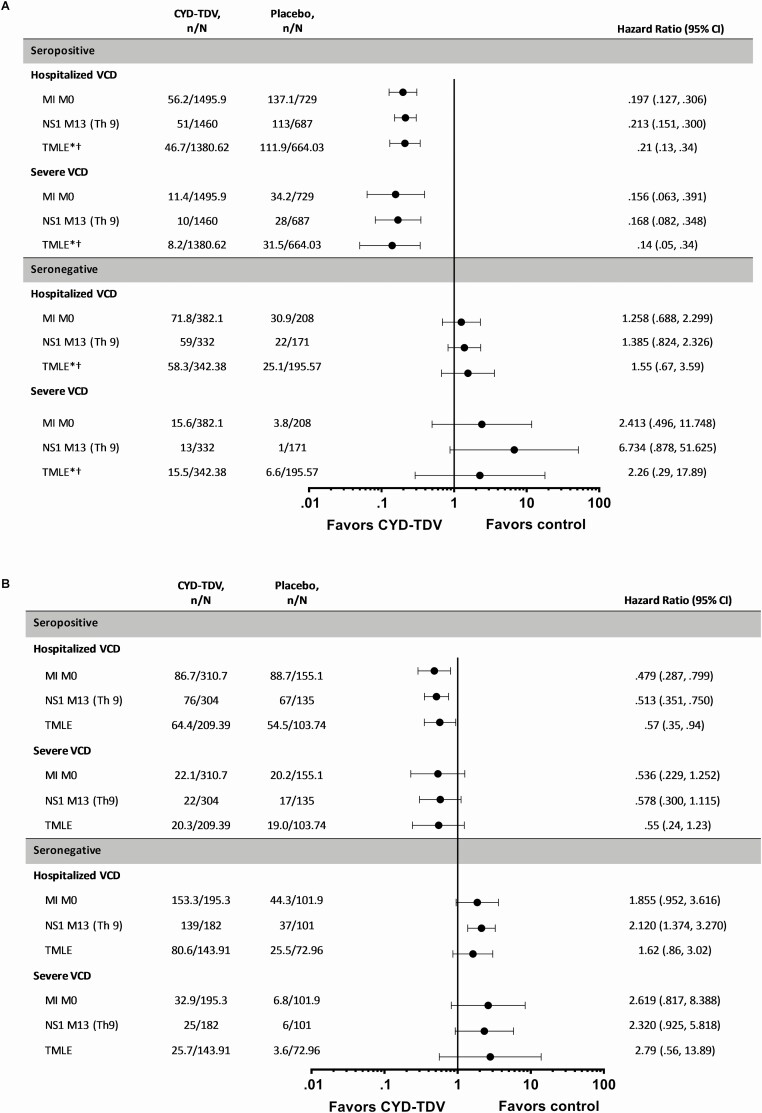
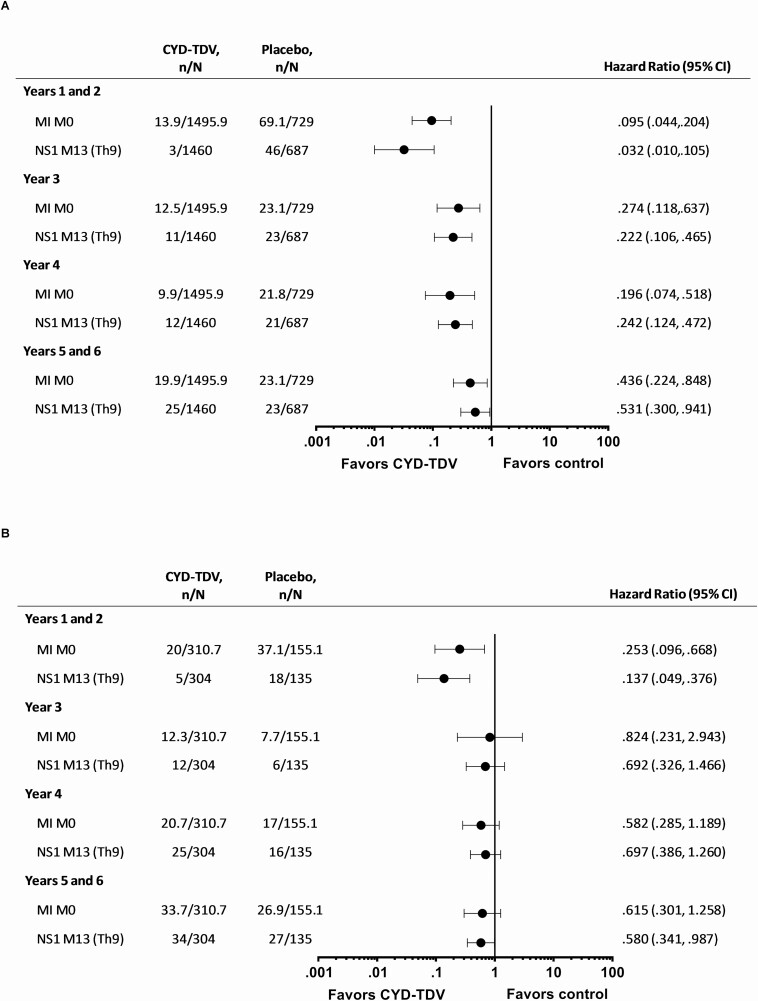
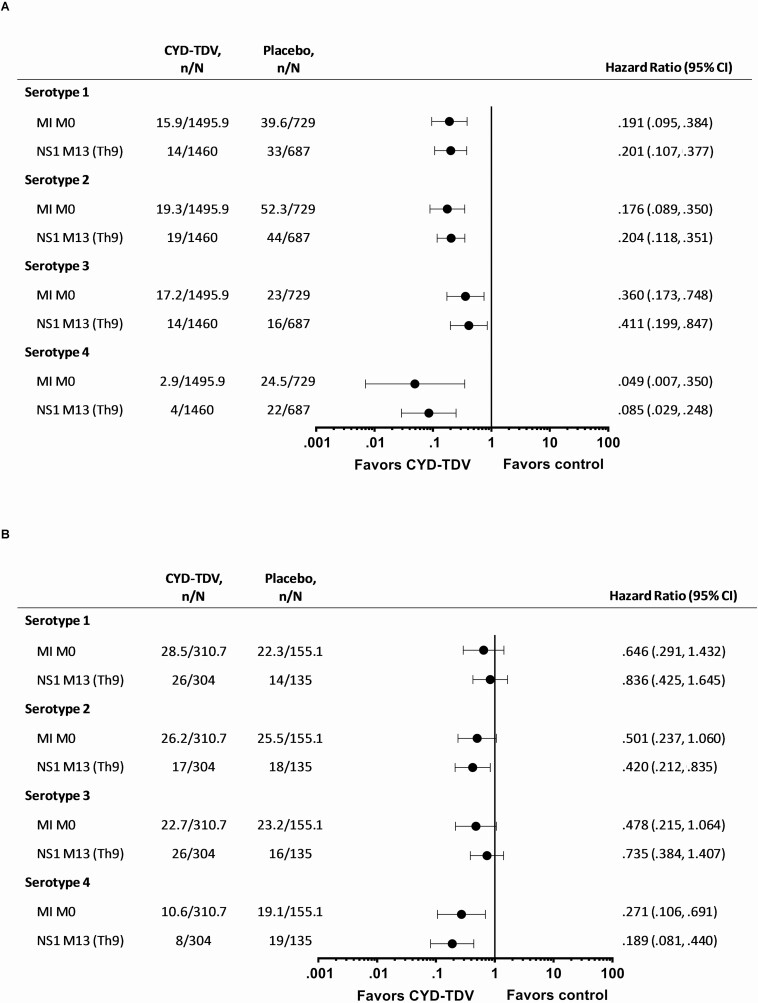
For each method, in those aged ≥9 years and seropositive at baseline, the risk for hospitalized VCD over the 6-year follow-up was consistently lower with CYD-TDV compared to placebo (Figure 1A; Supplementary Table 1). The dengue hospitalization risk reduction was maintained across all study periods over the 6-year follow-up, with the lowest HRs for years 1–2 (Figure 2A; Supplementary Table 2). The risk for hospitalized VCD was reduced across all dengue serotypes, being lowest for serotype 4 (Figure 3A; Supplementary Table 3). The risk for severe VCD was also lower with CYD-TDV compared to placebo in those seropositive by each of the methods (Figure 1A; Supplementary Table 4). The attributable risk of dengue hospitalization per 1000 vaccinees over the 6-year follow-up ranged from –15.7 to –13.2 for hospitalized VCD and from –4.1 to –3.4 for severe VCD (Table 1).
Figure 1.
Risk of hospitalized VCD and severe VCD in the pooled studies (CYD14, CYD15, and CYD23/57) over the 6-year period in those seropositive or seronegative by MI M0, TMLE*, and NS1 M13 (Th9) in participants aged (A) ≥9 years and (B) <9 years. Study group classified as treated (participants included in the CYD-TDV group if they received at least one injection of CYD-TDV vaccine). n represents the number of subjects fulfilling the item listed; N represents the total number of subjects selected in subcohort. NS1 M13 (Th9), dengue anti-nonstructural protein-1 (NS1) IgG ELISA at M13 with seropositivity defined as ≥9 EU/mL (threshold 9). For all MI approaches n and N are average numbers from 10 iterations of multiple imputations. For NS1 M13 (Th9) participants with VCD cases before M13 were excluded from the analyses. *TMLE data presented as relative risk (95% CI), N divided by 10; pooled TMLE data for CYD14 and CYD15. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; MI, multiple imputation; M0, baseline; TMLE, targeted minimum loss-based estimation; VCD, virologically confirmed dengue.
Figure 2.
Risk of hospitalized VCD for each time period for the pooled studies (CYD14, CYD15, CYD23/57) over the 6-year period in participants seropositive by MI M0, NS1 M13 (Th9) and aged (A) ≥9 years, (B) <9 years. Study group classified as treated (participants included in the CYD-TDV group if they received at least 1 injection of CYD-TDV vaccine). n represents the number of subjects fulfilling the item listed; N represents the total number of subjects selected in subcohort. For all MI approaches n and N are average numbers from 10 iterations of multiple imputations. For NS1 M13 (Th9) participants with VCD cases before M13 were excluded from the analyses. Years 1 and 2 correspond to the active phase of the studies, and years 5 and 6 correspond to the surveillance expansion period. Abbreviations: CI, confidence interval; VCD, virologically confirmed dengue.
Figure 3.
Risk of hospitalized VCD for each serotype in the pooled studies (CYD14, CYD15, CYD23/57) over the 6-year period in participants seropositive by MI M0, NS1 M13 (Th9) and aged (A) ≥9 years and (B) <9 years. Study group classified as treated (participants included in the CYD-TDV group if they received at least 1 injection of CYD-TDV vaccine). n represents the number of subjects fulfilling the item listed; N represents the total number of subjects selected in subcohort. NS1 M13 (Th9), dengue anti-non-structural protein-1 (NS1) IgG ELISA at M13 with seropositivity defined as ≥9 EU/mL (threshold 9). For all MI approaches n and N are average numbers from 10 iterations of multiple imputations. For NS1 M13 (Th9) participants with VCD cases before M13 were excluded from the analyses. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; MI, multiple imputation; M0, baseline; VCD, virologically confirmed dengue.
Table 1.
Attributable Risk and Cumulative Incidence Estimates for the Pooled Studies (CYD14, CYD15, and CYD23/57) Over the 6-year Period in Participants Aged ≥9 and <9 Years old, According to Baseline Serostatus by MI M0, NS1 M13 (Th9) and TMLE
| Estimated incidence, per 1000 (95% CI) | ||||
|---|---|---|---|---|
| Age | Endpoint | CYD-TDV | Placebo | Attributable Risk (CYD-TDV–placebo) (per 1000) (95% CI) |
| Seropositive | ||||
| ≥9 years | Hospitalized VCD | |||
| MI M0 | 4.21 (2.42, 6.00) | 19.93 (15.92, 23.93) | –15.71 (–20.05, –11.37) | |
| NS1 M13 (Th9) | 3.71 (2.66, 4.75) | 16.95 (13.61, 20.29) | –13.25 (–16.74, –9.75) | |
| TMLE | 3.61 (2.04, 5.18) | 17.32 (13.62, 21.03) | –13.7 (–17.7, –10) | |
| Severe VCD | ||||
| MI M0 | 0.85 (0.15, 1.54) | 4.95 (3.15, 6.75) | –4.10 (–6.02, –2.18) | |
| NS1 M13 (Th9) | 0.76 (0.29, 1.22) | 4.19 (2.61, 5.76) | –3.43 (–5.08, –1.78) | |
| TMLE | 0.63 (0.11, 1.16) | 4.66 (2.78, 6.54) | –4.0 (–6.0, –2) | |
| <9 years | Hospitalized VCD | |||
| MI M0 | 28.91 (18.33, 39.49) | 58.24 (38.27, 78.21) | –29.33 (–51.28, –7.38) | |
| NS1 M13 (Th9) | 24.95 (18.79, 31.08) | 49.48 (35.34, 63.40) | –24.52 (–39.84, –9.21) | |
| TMLE | 29.42 (18.76, 40.08) | 51.47 (34.33, 68.60) | –22.0 (–42.2, –2) | |
| Severe VCD | ||||
| MI M0 | 7.28 (2.77, 11.79) | 13.31 (6.41, 20.21) | –6.03 (–14.16, 2.10) | |
| NS1 M13 (Th9) | 7.43 (4.25, 10.59) | 12.69 (6.33, 19.00) | –5.26 (–12.34, 1.82) | |
| TMLE | 9.55 (4.36, 14.74) | 17.42 (6.91, 27.93) | –7.9 (–19.6, 4) | |
| Seronegative | ||||
| ≥9 years | Hospitalized VCD | |||
| MI M0 | 21.14 (13.58, 28.71) | 16.35 (7.42, 25.28) | 4.79 (–6.56, 16.14) | |
| NS1 M13 (Th9) | 19.74 (14.12, 25.32) | 13.98 (7.80, 20.11) | 5.76 (–2.56, 14.07) | |
| TMLE | 17.52 (10.42, 24.62) | 11.32 (2.99, 19.65) | 6.2 (–4.7, 17) | |
| Severe VCD | ||||
| MI M0 | 4.49 (1.59, 7.39) | 1.98 (–.96, 4.92) | 2.51 (–1.52, 6.54) | |
| NS1 M13 (Th9) | 4.47 (1.90, 7.04) | .67 (.00, 1.97) | 3.80 (.92, 6.69) | |
| TMLE | 4.24 (1.47, 7.00) | 1.87 (–1.80, 5.54) | 2.4 (–2.2, 7) | |
| <9 years | Hospitalized VCD | |||
| MI M0 | 79.78 (58.34, 101.23) | 44.12 (19.28, 68.96) | 35.67 (4.03, 67.30) | |
| NS1 M13 (Th9) | 77.37 (60.93, 93.52) | 37.37 (23.47, 51.07) | 40.00 (18.65, 61.35) | |
| TMLE | 57.29 (-4.21,74.58) | 35.45 (15.97, 54.93) | 21.8 (-4.2, 48) | |
| Severe VCD | ||||
| MI M0 | 17.19 (8.74, 25.63) | 6.81 (–.28, 13.91) | 10.37 (–0.39, 21.14) | |
| NS1 M13 (Th9) | 13.82 (8.11, 19.49) | 6.22 (1.09, 11.32) | 7.60 (–0.05, 15.25) | |
| TMLE | 17.63 (8.52, 26.75) | 6.32 (–3.28, 15.92) | 11.3 (-1.9, 25) | |
All incidence and attributable risks are presented cumulatively over the study period up to month 72. Subjects with VCD cases before M13 were excluded from the NS1 M13 (Th9) analyses. Data pooled from the CYD14, CYD15, and CYD23/57 studies, except TMLE where data are pooled from the CYD14 and CYD15 studies.
Abbreviations: CI, confidence interval; MI, multiple imputation; M0, baseline; NS1 M13 (Th9), dengue anti-nonstructural protein-1 (NS1) IgG ELISA at M13 with seropositivity defined as ≥9 EU/mL (threshold 9); TMLE, targeted minimum loss-based estimation; VCD, virologically confirmed dengue.
In those who were <9 years and seropositive, there was a lower risk for hospitalized VCD with CYD-TDV compared to placebo for each method (Figure 1B; Supplementary Table 5). The HR for hospitalized VCD among CYD-TDV versus placebo recipients was below 1 for all time periods, but the upper limit of CI was below 1 only for years 1–2 (Figure 2B; Supplementary Table 6). The risk of hospitalized VCD was less than 1 for all 4 serotypes, however the upper limits of the CI crossed 1 for all but serotype 4 (Figure 3B; Supplementary Table 7). The results for severe VCD in this age group showed HR/RR below 1; however, the 95% CI crossed 1 (Figure 1B; Supplementary Table 8). The estimated attributable risk of dengue hospitalization over the 6-year follow-up ranged from –29.3 to –22.0 per 1000 vaccinees, and from –7.9 to –5.3 per 1000 vaccinees for severe VCD (Table 1).
In those aged 6–8 years and seropositive, the risk of hospitalized VCD was lower with CYD-TDV compared to placebo for each method; however, the upper bounds of the 95% CI crossed 1 in CYD23/57 (Table 2; Supplementary Table 9). The risk for hospitalized VCD among CYD-TDV versus placebo recipients was consistently below 1 for all time periods, with the upper bound of the HR CI <1 for years 1–2 and years 5–6 (Table 2). In those aged 2–5 years the risk of hospitalized VCD was also lower, but the upper bounds of the 95% CI crossed 1, except for the PRNT90 method (Table 2); favorable HRs were observed mostly during the first 2 years (Table 2). The risk for severe VCD was reduced in the 6–8 years age group, with the upper bounds of the 95% CI sparing the null value with all methods except for MI M0 (Supplementary Table 10). In the 2–5 years age group the RR for severe VCD was below 1 for all methods except for strict seropositive (Supplementary Table 10).
Table 2.
Risk of Hospitalization for VCD in Participants Aged 2–5 Years or 6–8 Years at Enrollment and Classified as Dengue Seropositive by MI M0 and NS1 M13 (Th9) in the Pooled Sudies (CYD14 and CYD23/57) Over the 6-Year Period, and for Each Time Period
| Number of Participants With Cases | Risk of Dengue Hospitalization | ||||
|---|---|---|---|---|---|
| CYD-TDV, n (N) | Placebo, n (N) | Hazard Ratio | 95% CI | P-value | |
| Participants 2–5 years | |||||
| Entire 6-year period | |||||
| MI M0 | 40.7 (146.5) | 28.1 (70.6) | .692 | (.362, 1.326) | .265 |
| NS1 M13 (Thr 9) | 40 (149) | 23 (60) | .712 | (.396, 1.279) | .256 |
| Years 1 and 2 | |||||
| MI M0 | 9.4 (146.5) | 10.8 (70.6) | .403 | (.114, 1.429) | .156 |
| NS1 M13 (Thr 9) | 3 (149) | 6 (60) | .231 | (.055, 0.974) | .046 |
| Year 3 | |||||
| MI M0 | 5.6 (146.5) | 1.7 (70.6) | 1.696 | (.181, 15.931) | .642 |
| NS1 M13 (Thr 9) | 5 (149) | 2 (60) | .700 | (.238, 2.061) | .517 |
| Year 4 | |||||
| MI M0 | 7.5 (146.5) | 6 (70.6) | .478 | (.168, 1.359) | .166 |
| NS1 M13 (Thr 9) | 11 (149) | 6 (60) | .676 | (.278, 1.641) | .387 |
| Years 5 and 6 | |||||
| MI M0 | 18.2 (146.5) | 9.6 (70.6) | 1.051 | (.370, 2.985) | .925 |
| NS1 M13 (Thr 9) | 21 (149) | 9 (60) | 1.051 | (.464, 2.381) | .905 |
| Participants 6–8 years | |||||
| Entire 6-year period | |||||
| MI M0 | 46 (164.2) | 60.6 (84.5) | .381 | (.208, .696) | .002 |
| NS1 M13 (Thr 9) | 36 (155) | 44 (75) | .404 | (.243, .670) | <.001 |
| Years 1 and 2 | |||||
| MI M0 | 10.6 (164.2) | 26.3 (84.5) | .192 | (.068, .546) | .003 |
| NS1 M13 (Thr 9) | 2 (155) | 12 (75) | .088 | (.019, .400) | .002 |
| Year 3 | |||||
| MI M0 | 6.7 (164.2) | 6 (84.5) | .596 | (.137, 2.583) | .482 |
| NS1 M13 (Thr 9) | 7 (155) | 4 (75) | .712 | (.256, 1.981) | .516 |
| Year 4 | |||||
| MI M0 | 13.2 (164.2) | 11 (84.5) | .664 | (.238, 1.847) | .428 |
| NS1 M13 (Thr 9) | 14 (155) | 10 (75) | .724 | (.332, 1.582) | .418 |
| Years 5 and 6 | |||||
| MI M0 | 15.5 (164.2) | 17.3 (84.5) | .419 | (.182, .965) | .041 |
| NS1 M13 (Thr 9) | 13 (155) | 18 (75) | .349 | (.166, .731) | .005 |
Study group classified as treated (participants included in the CYD-TDV group if they received at least one injection of CYD-TDV vaccine). n represents the number of subjects fulfilling the item listed; N represents the total number of subjects selected in subcohort. For all MI approaches n and N are average numbers from 10 iterations of multiple imputations.
For NS1 M13 (Th9) and strict seropositive, participants with VCD cases before M13 were excluded from the analyses.
Abbreviations: CI, confidence interval; VCD, virologically confirmed dengue.
Risk in Seronegative Participants
In those who were ≥9 years old and seronegative at baseline, there was an increased risk of hospitalized VCD for CYD-TDV versus placebo; however, the 95% CI crossed 1 (Figure 1A); there was a similar pattern for severe VCD. The estimated attributable risk over the 6-year period ranged from 4.8 to 6.2 per 1000 vaccinees for hospitalized VCD and from 2.4 to 3.8 per 1000 vaccinees for severe VCD (Table 1).
In participants who were <9 years and seronegative, there was an increased risk of hospitalized and severe VCD in the CYD-TDV group (Figure 1B). The estimated attributable risk over the follow-up ranged from 21.8 to 40.0 per 1000 vaccinees for hospitalized VCD and 7.6 to 11.3 per 1000 vaccinees for severe VCD (Table 1).
Risk Irrespective of Serostatus
Regardless of baseline serostatus, the risk for hospitalized VCD over the 6 years of follow-up in individuals ≥9 years was lower for CYD-TDV versus placebo recipients (Supplementary Table 11). In children <9 years, the RR for hospitalized VCD over the 6-year period was below 1, with the 95% CI upper limit greater than 1, although the RR was slightly above 1 for years 3–6 (Supplementary Table 11). Results for severe VCD were consistent (Supplementary Table 12).
Risk in the Seropositive Participants of the Immunogenicity Subsets
Among participants from the immunogenicity subsets and who were seropositive by PRNT50, the risk of hospitalized VCD was lower with CYD-TDV versus placebo; in the pooled analyses the upper bounds of the CI were below 1 for participants ≥9 years and just above 1 for participants <9 years (Supplementary Table 13).
Clinical Signs and Symptoms
In those classified as seropositive by NS1 M13 (Th9) there were no cases of severe VCD that were WHO Grade IV, and 4 cases that were Grade III; 3 in the CYD-TDV group (all in individuals <9 years) and 1 in the placebo group (in an individual ≥9 years). All severe cases recovered.
There were no differences in the clinical signs and symptoms between the vaccine and placebo groups in either age group (Supplementary Table 14). There were no deaths related to the vaccine in any group.
Dengue Viremia
In participants seropositive by NS1 M13 (Th9), the mean quantified non serotype-specific dengue viremia from participants with hospitalized VCD was comparable between treatment groups in those ≥9 and <9 years (Supplementary Table 15).
Dengue Antibody Persistence
The persistence of dengue antibodies in seropositive participants over the 6-year study period is presented in Supplementary Figures 4 and 5; in both age groups, following a peak in antibodies with the third dose of vaccine at year 1, GMTs decreased in years 2 and 3, remaining level until year 6; higher titers were observed in those ≥9 years compared to those <9 years. In the placebo groups antibody titers increased in years 4 and 5, especially in CYD15.
DISCUSSION
This study provides an extended analysis of the risk of hospitalized and severe VCD cases over the entire 6-year safety follow-up of the CYD-TDV phase III and IIb studies, CYD14, CYD15, and CYD23/57, focusing on those inferred to be seropositive. This study expands upon previous analyses of the phase IIb and III CYD-TDV studies as it considers the entire 6-year follow-up period for each study and utilizes stricter definitions for seropositives among the range of methods used, allowing a clearer assessment of protection in children <9 years. Furthermore, the descriptions of clinical signs and symptoms, viremia data, and long-term immune responses provide a more comprehensive picture of the effects of CYD-TDV over the 6 years after vaccination.
This study demonstrated protection against hospitalized and severe VCD with CYD-TDV was seen across all 6 years of study in individuals aged ≥9 years and dengue seropositive at baseline; the highest levels of protection were observed during years 1–2. These results are consistent with the decline in neutralizing dengue antibody titers during years 2 and 3 in participants from CYD14 and CYD15; titers then levelled off and persisted up to year 6. Previous analyses have shown an association between post-CYD-TDV dose 3 titers and the probability of dengue disease, where the higher the titer, the lower the probability of the disease [13]. This was also observed for hospitalized VCD cases during the follow-up of CYD14 and CYD15 [14]. In addition to the decline in antibody levels in the vaccine groups, a concurrent increase was observed in antibody levels in the placebo groups. This increase is likely due to flavivirus exposure over time, whereby an increasing proportion of the placebo group gains natural immunity to dengue, therefore reducing their risk for further dengue infection and potentially impacting the observed protection against hospitalized VCD.
In the current indicated age population (≥9 years), over 6 years, the decreased attributable risk translated into approximately 13–16 hospitalized dengue cases prevented per 1000 seropositive vaccinees, and approximately 3–4 severe dengue cases prevented per 1000 vaccinees. There was a high level of protection against hospitalized dengue to each of the four serotypes in seropositive participants aged ≥9 years; ~80% risk reduction for serotypes 1 and 2, ~60% risk reduction for serotype 3, and ~95% risk reduction for serotype 4.
In those <9 years old at randomization who were determined to be seropositive, the rates of hospitalized and severe VCD were approximately 45–50% lower in the CYD-TDV group than in the placebo group, although this estimated protection was significant for only years 1–2. Additional analyses were conducted for those 2–5 years or 6–8 years at randomization; in those aged 6–8 years and seropositive, vaccine protection was observed against hospitalized and severe VCD cases, suggesting that this age group may also benefit from CYD-TDV. This was observed for each time period with more conclusive results during years 1–2 and 5–6, but also supportive point estimates for years 3 and 4. Estimates were less precise for years 3 and 4 as these were computed over 1 year. The apparent discrepancy between year 3/4 and the other periods may also be in part related to the differences in surveillance methods used between the study phases. The results observed over the last 2 years are particularly supportive of the duration of protection in this age group, consistent with the protection observed against symptomatic VCD during the SEP of these studies [15]. In seropositives aged 2–5 years, HR estimates for hospitalized and severe VCD cases were higher than in those 6–8 years.
Sensitivity analyses including seropositivity by M13 anti-NS1 ELISA ≥50 EU/mL (strict seropositive), and PRNT90 at M0 by MI, showed consistent results with other methods; confirming the protection against severe VCD in 6–8 years-olds.
The underlying mechanism of this observed effect modification by age (with lower protection in the younger age group) is unknown but could be related to age-related differences in the maturity of the immune system [16]. During the long-term follow-up, antibody levels in the 2–5 years age group showed a more pronounced decrease compared to older age groups (data not shown). Young children are also intrinsically at higher risk to develop clinically significant capillary damage during acute dengue illness compared with older individuals [17–19].
Potential study limitations due to the analytical methods used to determine serostatus and the assumptions made have been described previously [10]. A further limitation of this study is that there may be some variation between countries or regions on practices for hospitalization for dengue. However, results for hospitalized VCD cases were consistent with results for the more objective outcome of severe VCD.
In this study including the entire six-year follow-up of the 3 efficacy studies, CYD-TDV demonstrated robust protection against hospitalized and severe VCD in those who were seropositive and ≥9 years old. Protection was also observed in seropositive participants aged 6–8 years, who could also benefit from CYD-TDV vaccination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank the participants included in the study and their parents, the investigators of CYD14, CYD15, CYD23 and CYD57, coordinators and study teams. Editorial assistance with the preparation of the manuscript was provided by Nwoza Eshun and Nicola Truss PhD, inScience Communications, Springer Healthcare Ltd, UK. Funding for this assistance was provided by Sanofi Pasteur. The authors thank Aline Richetin-Guilluy, Nathalie Cochet, and Pierre Carteron (Sanofi Pasteur) for their assistance and Jean-Sébastien Persico for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur.
Financial support. This work was supported by Sanofi Pasteur.
Author contributions. All authors critically reviewed the manuscript, gave their final approvals, and are accountable for accuracy and integrity.
Potential conflicts of interest. R. F., T. L., L. S., D. L. C., D. C., A. P. P., C. F., B. Z., T. M., Y. W., C. D., M. B., C. V., O. H., S. S., G. D., and F. N. are employees of Sanofi Pasteur. M. B. reports stock ownership in Sanofi Pasteur. C. D. reports being a shareholder and full-time employee for Sanofi. B. Z. and C. F. report shares from Sanofi Pasteur. L. S. and C. V. report personal fees from Sanofi Pasteur. A. L. reports grants from Sanofi Pasteur. A. B. was an employee of Sanofi Pasteur when this study was conducted. H. R. develops clinical trials for various pharmaceutical companies. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Capeding MR, Tran NH, Hadinegoro SR, et al. ; CYD14 Study Group . Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358–65. [DOI] [PubMed] [Google Scholar]
- 2.Villar L, Dayan GH, Arredondo-García JL, et al. ; CYD15 Study Group . Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113–23. [DOI] [PubMed] [Google Scholar]
- 3.Sabchareon A, Wallace D, Sirivichayakul C, et al. . Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380:1559–67. [DOI] [PubMed] [Google Scholar]
- 4.Hombach J. Guidelines for clinical trials of dengue vaccine in endemic areas. J Clin Virol 2009; 46(Suppl 2):S7–9. [DOI] [PubMed] [Google Scholar]
- 5.Limkittikul K, Hattasingh W, Chansinghakul D, et al. . Long-term safety follow-up of children from a randomized, controlled phase IIb proof-of-concept efficacy study of the live, attenuated, tetravalent dengue vaccine (CYD-TDV) in Thailand. Asia Pac J Trop Med 2019; 12:396–403. [Google Scholar]
- 6.Arredondo-García JL, Hadinegoro SR, Reynales H, et al. ; CYD-TDV Dengue Vaccine Study Group . Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin Microbiol Infect 2018; 24:755–63. [DOI] [PubMed] [Google Scholar]
- 7.Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. ; CYD-TDV Dengue Vaccine Working Group . Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. [DOI] [PubMed] [Google Scholar]
- 8.Gailhardou S, Skipetrova A, Dayan GH, et al. . Safety overview of a recombinant live-attenuated tetravalent dengue vaccine: pooled analysis of data from 18 clinical trials. PLoS Negl Trop Dis 2016; 10:e0004821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigne C, Dupuy M, Richetin A, et al. . Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum Vaccin Immunother 2017; 13:2004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sridhar S, Luedtke A, Langevin E, et al. . Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379:327–40. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento EJM, Huleatt JW, Cordeiro MT, et al. . Development of antibody biomarkers of long term and recent dengue virus infections. J Virol Methods 2018; 257:62–8. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Dengue vaccine: WHO position paper. Wkly Epidemiol Rec 2018; 93:457–76. [Google Scholar]
- 13.Moodie Z, Juraska M, Huang Y, et al. . Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J Infect Dis 2018; 217:742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert PB, Huang Y, Juraska M, et al. . Bridging efficacy of a tetravalent dengue vaccine from children/adolescents to adults in highly endemic countries based on neutralizing antibody response. Am J Trop Med Hyg 2019; 101:164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan GH, Langevin E, Gilbert PB, et al. . Assessment of the long-term efficacy of a dengue vaccine against symptomatic, virologically-confirmed dengue disease by baseline dengue serostatus. Vaccine 2020; 38:3531–6. [DOI] [PubMed] [Google Scholar]
- 16.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015; 282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders KL, Nguyet NM, Chau NV, et al. . Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg 2011; 84:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trung DT, Wills B. Systemic vascular leakage associated with dengue infections: the clinical perspective. Curr Top Microbiol Immunol 2010; 338:57–66. [DOI] [PubMed] [Google Scholar]
- 19.Wills BA, Oragui EE, Dung NM, et al. . Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis 2004; 190:810–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.