Families participating in ACP with their AYA with cancer reported significantly greater positive caregiver appraisals and found the experience overwhelmingly worthwhile.
Abstract
BACKGROUND AND OBJECTIVES:
Little is known about how families respond to pediatric advance care planning. Physicians are concerned that initiating pediatric advance care planning conversations with families is too distressing for families. We examined the effect of family centered pediatric advance care planning intervention for teens with cancer (FACE-TC) advance care planning on families’ appraisals of their caregiving, distress, and strain.
METHODS:
In a randomized clinical trial with adolescents with cancer and their families conducted from July 2016 to April 2019 in 4 tertiary pediatric hospitals, adolescents and family dyads were randomly assigned at a 2:1 intervention/control ratio to either the 3 weekly sessions of FACE-TC (Advance Care Planning Survey; Next Steps: Respecting Choices; Five Wishes) or treatment-as-usual. Only the family member was included in this study. Generalized estimating equations assessed the intervention effect measured by Family Appraisal of Caregiving Questionnaire.
RESULTS:
Families’ (n = 126) mean age was 46 years; 83% were female, and 82% were white. FACE-TC families significantly increased positive caregiving appraisals at 3-months postintervention, compared with those in the control group (β = .35; 95% confidence interval [CI] 0.19 to 0.36; P = .03). No significant differences were found between groups for strain (β = −.14; 95% CI = −0.42 to 0.15; P = .35) or distress (β = −.01; CI = −0.35 to 0.32; P = .93).
CONCLUSIONS:
Families benefited from participation in FACE-TC, which resulted in positive appraisals of their caregiving for their child with cancer, while not significantly burdening them with distress or strain. Clinicians can be assured of the tolerability of this family-supported model.
What’s Known on This Subject:
Qualitative studies and a small pilot study reveal families are interested in pediatric advance care planning. However, clinicians hesitate to initiate such conversations, fearing distressing vulnerable families.
What This Study Adds:
Families randomly assigned to the FACE-TC pediatric advance care planning intervention evidenced a significantly greater positive appraisal of caregiving and overwhelmingly found the experience worthwhile, without undue distress or strain, compared with those in the control group.
Little is known about how well families respond to participating in pediatric advance care planning (pACP) for adolescents and young adults (AYAs) with cancer. Advance care planning (ACP) facilitates early conversations between persons living with a serious illness and their families about future medical treatment and care, including end of life, through exploration and understanding of the patients’ values, preferences, and goals for care and treatment.1–3 For AYAs, pACP aims to (1) give AYAs a voice in medical treatment decisions when they experience severe illness complications; (2) prepare families to support future health care decisions aligned with the AYAs’ goals of care; and (3) ensure communication and medical record documentation of these goals and treatment preferences for clinicians.
In reports from the Institute of Medicine, the authors indicated families were naturally reluctant to explore death and dying with their seriously ill child.4,5 Nevertheless, in a classic study, most bereaved parents who engaged in end-of-life conversations with their dying child reported no regret.6 Qualitative studies revealed a key element of pACP from the bereaved parents’ perspective was engagement in discussions with facilitators to reach a decision, taking into account parental readiness.7 Although parents reported it was difficult to engage in pACP, they considered it important, advocating for a sensitive, individualized, and gradual approach to sustain hope and quality of life.8 Consensus exists about developing integrative-care models for pediatric oncology patients incorporating cancer-directed, symptom-directed, and supportive care throughout the illness, as most consistent with parents’ preferences.9 One such model, family-centered (FACE) pACP, was developed to facilitate goals-of-care conversations and completion of advance directives between AYAs and their families. In a pilot trial, researchers tested FACE with AYAs living with cancer,10 as well as a full trial with AYAs living with HIV,11 and demonstrated that conversations between families and their AYAs, although sad, were worthwhile and, for families’ of AYAs with HIV, decreased anxiety compared with controls.12,13 Yet, fear of distressing already burdened families continues to be a barrier to clinicians initiating pACP conversations.14–17
We conducted a planned interim analysis of a secondary outcome from the first scientifically rigorous trial to directly measure families’ appraisal of their caregiving as a potential benefit-associated pACP. “Family” was operationally defined as the legal guardian(s) of the minor adolescent (ages 14–17 years) or chosen surrogate decision-maker(s) of the young adult (ages 18–21 years) with cancer. We hypothesized that at 3 months’ postintervention families randomly assigned to family centered pediatric advance care planning for teens with cancer (FACE-TC) intervention, compared with those assigned to the treatment-as-usual control group, would report (1) significantly greater levels of positive caregiver appraisal but (2) nonsignificant levels of caregiver distress and caregiver strain. Secondarily, we examined families’ satisfaction and emotional reactions to participation.
Methods
Study Design and Participants
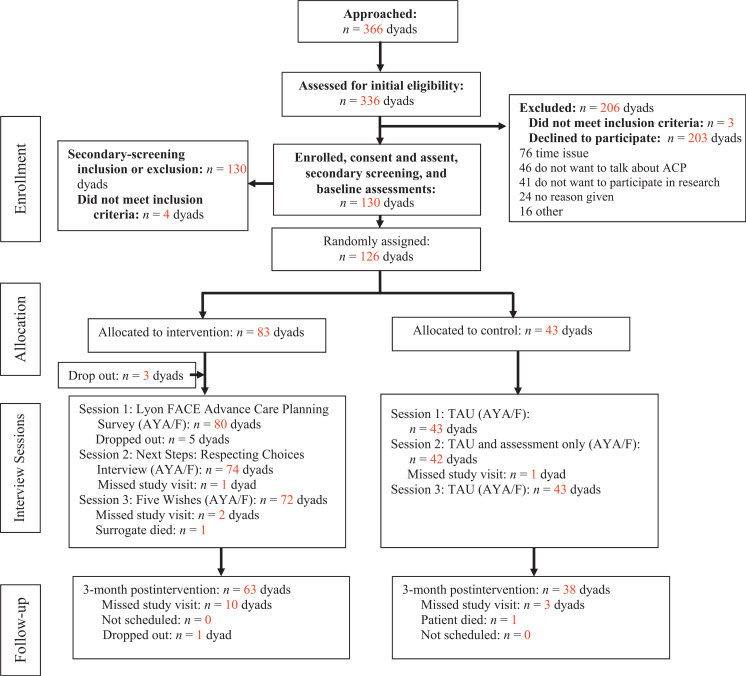
We conducted a 2-armed, single-blinded, intent-to-treat, randomized controlled clinical trial. Participants enrolled at the level of the AYA and family dyad. In this interim analysis, we examined only the family half of the dyads. AYA eligibility criteria included having ever been diagnosed with cancer, being aged ≥14 to <21 years, knowing cancer diagnosis, and being English-speaking. Family eligibility criteria included being aged ≥18.0 years, having provider determined not developmentally delayed, being English-speaking, and knowing patient’s diagnosis. Secondary screening for exclusion criteria occurred after enrollment for homicidality, suicidality, and psychosis to ensure competency to engage in shared decision-making. Dyads were recruited from tertiary pediatric hospitals: Akron Children’s Hospital, Children’s National Hospital, St. Jude Children’s Research Hospital, and University of Minnesota Masonic Children’s Hospital. Enrollment occurred between July 16, 2016, and April 30, 2019. Researchers completed 3 days of training on the protocol. Standardized procedures were enacted for validation of, implementation of, and fidelity to the protocol. Respecting Choices facilitators (nurses) achieved certification on the basis of program competency-based criteria, which included 6.5 hours of online training, with professional continuing education credits and two days of classroom skills-based education, followed by a practice video-based demonstration of competency. Monthly supervision and mentoring were provided on the basis of a review of video recordings of sessions and a competency-criteria checklist. There are minimal training costs associated with sessions 1 and 3, which were straightforward and required minimal training.
Institutional review boards at each site approved the protocol. Participants provided written informed consent and assent and were compensated. An external safety-monitoring committee monitored the trial annually. Protocol details are published elsewhere.18 We followed the Consolidated Standards of Reporting Trials guidelines. See the Consolidated Standards of Reporting Trials checklist.
Procedure
After consulting with the AYA’s oncologist, trained research assistants approached potentially eligible dyads face-to-face during hospital outpatient visits and inpatient admissions. AYA and family dyads were contacted until the targeted enrollment goal of 130 dyads was accomplished. This trial comprised 8 visits over 2 years. The first visit included enrollment, secondary screening, and, if eligible, completion of baseline questionnaires. Dyads were then randomly assigned at an allocation ratio of 2:1 (intervention/control) to either the FACE-TC intervention group or treatment-as-usual control group, by using computer-generated randomization triggered by baseline-assessment completion. Block randomization to condition by study site controlled for idiosyncrasies of site-specific effects.
Intervention and Treatment-as-Usual Control Conditions
FACE-TC
Three 60-minute sessions were conducted with a certified facilitator at weekly intervals. See Supplemental Table 5 for session goals and processes. FACE-TC provided an opportunity for AYAs and families to engage in a process of pACP that psychologically required the dyad to acknowledge that life is finite and think about quality of life near the end of life. The 3-session FACE-TC intervention was developed and adapted through a community-based participatory research approach.10,19 In session 1, the Lyon Advance Care Planning Survey was administered separately to AYAs and families to prepare the dyad for session 2 conversations. These survey results have been published.20 Session 2 was the Respecting Choices Next Steps pACP-facilitated conversation, a theoretically grounded and empirically tested goals-of-care conversation21 that results in documentation of preferences in a statement-of-treatment-preference form. Session 3 was the completion of the Five Wishes advance directive.22 Sessions 2 and 3 were attended by the dyad, and the conversations were facilitated.
After intervention completion, a Health Insurance Portability and Accountability Act–protected e-mail was sent by the facilitator to the treating oncologist summarizing the conversations and including a copy of the statement of treatment preferences and Five Wishes. The facilitator also sent a copy of the documents to medical records.
Treatment-as-Usual Comparison Condition
To minimize the burden to ill AYAs, we chose a treatment-as-usual comparison condition. Both groups were provided with an ACP handbook.23
Data Source and Measures
Self-report questionnaires were administered face-to-face to families at baseline and again at 3-months postintervention to determine the intervention effect on caregiving appraisals. Some 3-month assessments were completed by telephone. A satisfaction questionnaire was administered after session 2 (study visit 3) for intervention families or at study visit 3 for control families. Measures were administered by trained, blinded research assessors who were not the intervention facilitators. Assessors read the questions aloud in a private room and entered the responses immediately into the database through a laptop.
The demographic data form was administered at baseline to obtain family-reported sociodemographic information, including age, sex, race and ethnicity, education, employment status, and household income.
The Family Appraisal of Caregiving Questionnaire for Palliative Care (FACQ-PC)24 reveals changes over time in caregivers’ impacts from caregiving. There are 25 items, with 4 theoretically driven subscales: strain, distress, positive appraisals, and family well-being. Scores are from 5 (strongly agree) to 1 (strongly disagree). It has good internal consistency reliability and construct validity.24
The satisfaction questionnaire was developed and tested for the FACE protocol.19 Participants were asked “How did you feel about this session?” There are 13 items on a 5-point Likert scale, ranging from “strongly disagree” to “strongly agree.” The 2 subscales are as follows: subscale A (satisfaction [useful, helpful, load off my mind, satisfied, something I needed to do, courageous, and/or worthwhile]) and subscale B (emotional reaction [scared or afraid, too much to handle, harmful, angry, sad, and/or hurtful]). Higher scores on subscale A mean greater satisfaction. Higher scores on scale B mean stronger emotional reactions. Cronbach’s α was 0.84 for the satisfaction subscale score with this study population and 0.80 for the emotional reaction subscale score, indicating good reliability.
Adverse events were operationally defined by using a response pattern to the items on the satisfaction questionnaire following the institutional review board and safety monitoring committee guidance. See Supplemental Table 6 for operational definitions.
Covariates were family age, family sex, family race, household income, and AYA on active treatment.
Statistical Analysis
Sample Size Calculations
The method from Diggle et al25 was used to estimate the sample size needed for the generalized estimating equations (GEEs) model with two time points used in this study. Assuming a within-subject correlation of 0.20, the estimated sample size for testing a moderate effect size of 0.35 is n = 92. The sample size for this study is n = 102 at 3 months postintervention.
Statistical Procedures
Descriptive statistics, logistic regression, and GEEs were used. Before parametric testing, scale reliabilities were assessed by using Cronbach’s α, and composite subscale scores were used for data analyses. Analysis was by original assigned groups by using the intent-to-treat design. See Fig 1. Data were entered into Research Electronic Data Capture software version 8.10.18 (Vanderbilt University, Nashville, TN).
FIGURE 1.
Flow of participants through the trial. AYA/F, adolescent or young adult/family; TAU, treatment as usual.
For the caregiver appraisal outcome, the sample size was n = 126 families at baseline and n = 102 families at 3-months postintervention. GEE PROC GENMOD procedure SAS 9.4 (SAS Institute, Inc, Cary, NC) was used to test the effect of the intervention on the 4 family caregiver appraisal subscales at 3-months postintervention.
For the satisfaction questionnaire, data for n = 116 families were available. A two-sided Pearson’s χ2 test or 2-sided Fisher’s exact test was used for comparing the intervention and control families on the 13 items. A t test was used to compare the two intervention groups for the subscale scores. A linear regression model was used for testing the intervention effect on the subscale scores. Statistical significance was set to α = .05.
Results
Participant Characteristics
Among families (n = 126), the mean age was 46 (SD: 8.3) years, ranging in age from 19 to 67 years; 83% were female, 82% were white, 75% were mothers, 15% were fathers, and 8% had nonbiological patient-chosen surrogate decision-makers (Table 1). More than one-half had household incomes <200% of the Federal Poverty Level; 58% had less than a college education. AYAs (n = 126) had a mean age of 17 years and were 57% female and 79% white.
TABLE 1.
Social-Demographics for Families and Clinical Data by Two Groups (n = 126)
| Variable | FACE-TC (n = 83) | Treatment-as-Usual Control (n = 43) |
|---|---|---|
| Age | ||
| Mean (SD) | 45.6 (8.2) | 46.5 (8.4) |
| Range | 19–67 | 20–63 |
| Sex, n (%) | ||
| Female | 67 (80.7) | 37 (86.0) |
| Male | 16 (19.3) | 6 (14.0) |
| Race, n (%) | ||
| American Indian or Alaska Native | 0 (0.0) | 1 (2.3) |
| Asian | 3 (3.6) | 0 (0.0) |
| Black or African American | 10 (12.0) | 4 (9.3) |
| White | 68 (81.9) | 35 (81.4) |
| >1 race | 2 (2.4) | 3 (7.0) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 4 (4.8) | 0 (0.0) |
| Not Hispanic or Latino | 79 (95.2) | 42 (97.7) |
| Declined | 0 (0.0) | 1 (2.3) |
| Education, n (%) | ||
| No high school diploma or GED equivalency or high school or GED equivalency | 18 (21.7) | 7 (16.3) |
| Some college but no Bachelor’s degree | 31 (37.3) | 17 (39.5) |
| Bachelor’s degree, Master’s degree, doctorate, and/or professional degree | 34 (41.0) | 19 (44.2) |
| Income, n (%) | ||
| Equal to or below the federal poverty line | 21 (25.3) | 12 (27.9) |
| 101% to 200% of federal poverty line | 23 (27.7) | 14 (32.6) |
| 201% to 300% of federal poverty line | 14 (16.9) | 5 (11.6) |
| >300% of federal poverty line | 23 (27.7) | 10 (23.3) |
| Declined | 2 (2.4) | 2 (4.7) |
| Relationship after regrouping, n (%) | ||
| Biological | 77 (92.8) | 39 (90.7) |
| Nonbiological | 6 (7.2) | 4 (9.3) |
| On active treatment of AYA, n (%) | ||
| Yes | 20 (24.1) | 7 (16.3) |
| No | 63 (75.9) | 36 (83.7) |
GED, General Educational Development.
Initial eligibility criteria were met by 336 dyads. See Fig 1. Of these, 203 declined, and 3 were ineligible (39% participation rate). Of those who declined, the major reason given was lack of time (37% [76 of 203 dyads]), followed by at least 1 member of the dyad not wanting to talk about pACP (23% [46 of 203 dyads] and 20% (41 of 203 dyads) not wanting to participate in research. The predetermined sample size of 130 enrolled dyads was achieved.18 Of those dyads who began the intervention, 90% attended all 3 FACE-TC sessions.
Family Caregiver Appraisal
Cronbach’s α for the 4 family caregiver appraisals subscales were ≥0.70, and, therefore, they are reliable to use as composite scores: caregiver strain (0.90), positive caregiver appraisals (0.81), caregiver distress (0.70), and family well-being (0.83). The means, SDs, and effect sizes for each of the subscales are shown in Table 2.
TABLE 2.
Descriptive Statistics for the FACQ-PC Subscales at Baseline and 3-Month Postintervention Follow-Up
| Outcome | Baseline (n = 126) | 3 mo Postintervention Follow-up (n = 102a) | The Effect Size of Intervention At 3 mo | ||
|---|---|---|---|---|---|
| FACE-TC (n = 83), mean (SD) | Treatment-as-Usual Control (n = 43), mean (SD) | FACE-TC (n = 65), mean (SD) | Treatment-as-Usual Control (n = 37), mean (SD) | ||
| Caregiver strainb (higher strain = more strain) | 2.17 (0.75) | 2.34 (0.89) | 2.15 (0.88) | 2.39 (0.81) | 0.28 |
| Positive caregiving appraisalc (higher score = more positive) | 4.51 (0.46) | 4.61 (0.40) | 4.55 (0.45) | 4.47 (0.54) | 0.17 |
| Caregiver distressd (higher score = more distress) | 2.19 (0.78) | 2.44 (0.80) | 2.23 (0.90) | 2.49 (1.01) | 0.28 |
| Family well-beinge (higher score = better well-being) | 4.13 (0.67) | 4.25 (0.63) | 4.12 (0.70) | 4.09 (0.67) | 0.03 |
One control surrogate missed the visit.
Strain subscale items are as follows: as a caregiver, I feel… (tired and run down; my own health has suffered; I am losing control over my life; I don’t have enough time for myself; isolated and alone in caring for (patient’s name); have had to give up my social life to care for (patient); I have not been able to do my job or study as well as I would like; caring for (patient) creates financial difficulties.
Positive caregiving-appraisal items are as follows: caring for (patient) is satisfying; it is a privilege to care for (patient); caring for (patient) has made me feel closer to him or her; I am able to comfort (patient) when he or she needs it; I feel confident I can handle most problems in caring for (patient); I feel useful in my relationship with (patient); I am committed to caring for (patient).
Caregiver distress subscale items are as follows: I feel guilty about not being able to do more for (patient); I worry that I won’t be able to do enough to care for (patient); I feel anxious about caring for (patient); I feel depressed about caring for (patient); I feel sad about how life could have been for (patient).
Family well-being subscale items are as follows: our family works together to solve problems; our family is able to talk about our feelings with each other; I feel our family is closer because of caring for (patient); because of caring for (patient) our family is better able to cope with change; our family disagrees a lot caring for (patient); our family avoids discussing their fears and concerns about caring for (patient).
GEE tests of the intervention effect on the 4 family caregiver appraisal subscales at 3 months postintervention are shown in Table 3. The intervention had a significant effect on positive caregiving appraisals: positive caregiving appraisals were significantly greater in the intervention group compared with the control group (β for the interaction between time and intervention: β = .19; 95% confidence interval [CI]: 0.19 to 0.36; P = .03). There was no significant intervention effect compared with the control group for caregiver strain (β = −.14; 95% CI: −0.42 to 0.15; P = .35), caregiver distress (β = −.01; 95% CI: −0.35 to 0.32; P = .93), or family well-being (β = .16; 95% CI: −0.07 to 0.38; P = .17). Caregiver strain was positively associated with their child being on active treatment (β = .47; 95% CI: 0.16 to 0.78; P = .003). White families had less distress than families of color (β = −.47; 95% CI: −0.9 to 0.04; P = .03). Family members’ age, sex, and household income were not significantly associated with caregiver appraisals.
TABLE 3.
Selected GEE Models for the FACQ-PC Subscales (n = 222)
| Parameter | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Caregiver Stain | Positive Caregiving Appraisal | Caregiver Distress | Family Well-being | |||||
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Visit | ||||||||
| Baseline | — | — | — | — | — | — | — | — |
| 3 mo follow-up | 0.09 (−0.14 to 0.32) | .44 | −0.15 (−0.29 to −0.01) | .03 | 0.03 (−0.25 to 0.32) | .81 | −0.16 (−0.33 to 0.01) | .07 |
| Intervention group | ||||||||
| Control | — | — | — | — | — | — | — | — |
| FACE-TC | −0.24 (−0.54 to 0.06) | .11 | −0.07 (−0.22 to 0.08) | .37 | −0.26 (−0.56 to 0.04) | .08 | −0.13 (−0.38 to 0.11) | .29 |
| 3 mo FACE-TC | −0.14 (−0.42 to 0.15) | .35 | 0.19 (0.02 to 0.36) | .03 | −0.01 (−0.35 to 0.32) | .93 | 0.16 (−0.07 to 0.38) | .17 |
| Surrogate age | −0.01 (−0.03 to 0.01) | .21 | 0.01 (0 to 0.02) | .17 | 0 (−0.01 to 0.02) | .93 | 0 (−0.01 to 0.02) | .67 |
| Surrogate sex | ||||||||
| Male | — | — | — | — | — | — | — | — |
| Female | 0.12 (−0.15 to 0.39) | .38 | 0.07 (−0.11 to 0.26) | .44 | −0.05 (−0.4 to 0.31) | .79 | −0.08 (−0.34 to 0.19) | .56 |
| Surrogate race | ||||||||
| Multiracial | — | — | — | — | — | — | — | — |
| White | −0.17 (−0.57 to 0.24) | .42 | −0.07 (−0.25 to 0.1) | .42 | −0.47 (−0.9 to −0.04) | .03 | −0.12 (−0.43 to 0.2) | .46 |
| Poverty | ||||||||
| No | — | — | — | — | — | — | — | — |
| Yes | 0.03 (−0.28 to 0.33) | .86 | 0.15 (0 to 0.29) | .04 | 0.04 (−0.27 to 0.35) | .80 | 0.05 (−0.2 to 0.3) | .69 |
| On active treatment | ||||||||
| No | — | — | — | — | — | — | — | — |
| Yes | 0.47 (0.16 to 0.78) | .003 | −0.14 (−0.32 to 0.04) | .13 | 0.08 (−0.23 to 0.39) | .62 | 0.1 (−0.13 to 0.34) | .39 |
A total of 4 who declined to report household income at baseline and 2 who declined to report household income at 3 mo postintervention were excluded. —, the reference group for each parameter.
Satisfaction and Emotional Reaction
As shown in Table 4, FACE-TC families, compared with control families, were significantly more likely to report the experience to be worthwhile (73 of 75 [97%] vs 36 of 42 [86%]; P = .03), useful (42 of 74 [97%] vs 35 of 42 [83%]; P = .01), and something I needed to do (63 of 74 [85%] vs 23 of 42 [55%]; P < .001). Emotional reactions were not significantly different. FACE-TC families had a higher mean total score on positive satisfaction than those in the control group (mean: 28.1 [SD: 3.3] vs 24.9 [SD: 4.4]; P < .001). There was no significant difference on mean total scores on emotional reactions (mean: 11.4 [SD: 4.0] vs 12.4 [SD: 4.1]; P = .23). No adverse or serious adverse events were reported.
TABLE 4.
Satisfaction Questionnaire Items Dichotomized (Agree or Strongly Agree) by FACE pACP Versus Treatment-as-Usual Controls at Session 2 (n = 116)
| Satisfaction Questionnaire Items | Families (Agree or Strongly Agree) | Difference, % (95% CI) | P a | |
|---|---|---|---|---|
| FACE-TC pACP (n = 74), n (%) | Treatment-as-Usual (n = 42), n (%) | |||
| 1. It was useful. | 72 (97.3) | 35 (83.3) | 14.0 (2.0 to 25.8) | .01 |
| 2. It was helpful. | 71 (95.9) | 33 (78.6) | 17.4 (4.2 to 30.6) | .008 |
| 3. I felt scared or afraid. | 15 (20.3) | 11 (26.2) | −6.9 (−22.1 to 10.2) | .46 |
| 4. It felt like a load off my mind. | 29 (39.2) | 7 (16.7) | 22.5 (6.7 to 38.4) | .01 |
| 5. It was too much to handle. | 7 (9.5) | 2 (4.8) | 4.7 (−4.6 to 14.0) | .48 |
| 6. I felt satisfied. | 56 (76.7)b | 25 (59.5) | 17.2 (−0.5 to 34.9) | .05 |
| 7. It was harmful. | 1 (1.4) | 0 (0.0) | 1.4 (−1.3 to 4.0) | .99 |
| 8. I felt angry. | 1 (1.4) | 1 (2.4)b | −1.1 (−6.5 to 4.3) | .99 |
| 9. It was something I needed to do. | 63 (85.1) | 23 (54.8) | 30.4 (13.3 to 47.5) | <.001 |
| 10. I felt sad. | 31 (41.9) | 18 (42.9) | −1.0 (−19.7 to 17.8) | .92 |
| 11. I felt courageous. | 31 (41.9) | 11 (26.2) | 15.7 (−1.7 to 33.1) | .09 |
| 12. It felt hurtful. | 3 (4.1) | 3 (7.1) | −3.1 (−12.1 to 5.9) | .67 |
| 13. It was worthwhile. | 72 (97.3) | 36 (85.7) | 11.6 (0.4 to 22.8) | .03 |
Pearson’s χ2 test and Fisher’s exact test were used.
One missing data.
Controlling for age, sex, race, household income, and AYA on active treatment, linear regression models indicated (Supplemental Table 7; n = 111) the intervention had a significant effect on families’ positive satisfaction with participation, compared with the control group (β = 3.19; 95% CI: 1.74 to 4.63; P < .001), whereas there was no intervention effect on emotional reactions (β = −.80; 95% CI: −2.41 to 0.82; P = .33).
The survey ended with “Is there anything else you want to tell us about how you felt during this session (Respecting Choices interview)?” See Supplemental Tables 8 and 9 for all verbal responses from families.
Discussion
In the largest and most scientifically rigorous intervention trial to date of AYA pACP in cancer, families’ positive caregiving appraisals were significantly greater for FACE-TC families, compared with those in the control group. Although the effect size was small, the results from the satisfaction questionnaire confirm a positive outcome for FACE-TC families who, compared with those in the control group, overwhelmingly reported the experience as worthwhile, useful, and helpful, although strong feelings were elicited. Families with household incomes below the Federal Poverty Level reported more positive caregiving appraisals, regardless of study arm. The reason for the significance of the positive caregiving appraisals regardless of study arm for low-income families is unknown and unexamined in the research literature.
Consistent with our hypotheses, the FACE intervention did not significantly increase distress, compared with those in the controls group, and the effect size was small. However, persons of color were significantly more likely to experience caregiver distress. The finding may be associated with health disparities or other inequities and is consistent with evidence that racial and/or ethnic minority children and adolescents have a higher risk of death from treatment amenable cancer.26
Trial findings are also consistent with research from Feudtner et al27 on what it means to be a “good parent” to a seriously ill child, which includes “making informed medical decisions” and “advocating for my child with medical staff.”28 These are two key roles of families engaged in FACE-TC. Families learned about their child’s goals of care to facilitate future medical decisions, and facilitators asked families if they could honor their child’s treatment preferences in their child’s presence (ie, to act as their advocate with medical staff, if the need should arise).
Human subjects’ protections were effective because there were no adverse events reported. Results are consistent with our AYA FACE-HIV trial in which a similar balance was identified between the strong emotions elicited by talking about the possibility of bad outcomes for their child and value of learning their child’s representation of illness, hopes, fears, worries, and treatment preferences.11
This 2-arm, parallel, assessor-blinded, intent-to-treat, randomized clinical trial made it possible to draw conclusions about cause and effect. Standardized pACP conversation guidelines, facilitator certification, and fidelity monitoring insured consistency in the intervention and increased replicability. Valid and reliable measures increased reproducibility and ease of comparison with other research. The 4 study sites increased validity and the likelihood of influencing clinical practice. Generalizability was also increased by the geographically and economically diverse sample. Racial diversity enabled identification of racial differences, with families of color experiencing more caregiver distress. We achieved the enrollment goal of 130 AYA and family dyads. All AYAs receiving oncology care were included, reflecting recommendations that ACP occur at all stages for anyone with a serious illness.1 This trial was theoretically grounded in transactional stress and coping theory,29 positing that under conditions of chronic and severe stress, problem solving facilitates positive reappraisals.30
Generalizability is limited by the participation rate. Participants enrolled at rates similar to adult dyadic end-of-life studies, in which only 30% to 47% of eligible patients participate.31–35 The participation rate of 39% was below the study benchmark of 50%, which was achieved in our single-site trials.4,5 Alternative pACP models may be more appropriate for AYAs who cannot identify a surrogate decision-maker36 or who prefer to have the conversation with their clinician and family, rather than a facilitator.37 Social-desirability bias could have occurred with face-to-face administration of study questionnaires. However, this approach enabled monitoring of emotional reactions and controlled for issues of literacy, impaired vision, item comprehension, and questionnaire completeness. Providers were not interviewed. A future survey is planned of clinicians whose patients participated in this trial, by using a survey instrument developed in the pilot.38
Trial findings have potential practice implications. First, clinicians’ concerns that initiating pACP for children with life-limiting conditions and their families is distressing for parents14–17 should be mitigated by the finding that high-quality pACP conversations did not significantly increase family distress or strain. Although conversations were emotional, families found them overwhelmingly helpful, useful, and worthwhile. Similarly, honest and skillful conversations by oncologists with parents of children with cancer increased parental peace of mind and sense of purpose.39 Second, the FACE-TC model can assure intensivists that the first conversation about goals of care is not occurring in the ICU. Third, a pACP approach may prevent “decisional discord”40 about end-of-life treatment preferences. The National Cancer Institute has identified FACE pACP as an evidence-based intervention,41 ready to move into practice. Implementation science research could assist in identifying key factors required for successful implementation, including time and costs, which vary depending on the level of organizational leadership and investment in systems design (eg, improved electronic health records or planning documents), communication skills training for health care providers, consumer engagement and education, and ongoing quality improvement activities.
Conclusions
The FACE-TC trial revealed that pACP had, as one of its benefits, positive caregiver appraisals without increased distress, strain, or emotional burden, compared with those in the control group. Trial results provided evidence that the FACE-TC pACP model is safe, worthwhile, and caring. This evidence base met practice guidelines42,43 for an intervention that could be extended to other AYAs living with serious illnesses and their families.
Acknowledgments
We acknowledge the participating AYAs and their families and the following study staff who assisted with data collection: Jessica Thompkins, Elaine Churney, Kristine Allmendinger-Goertz, Jody Chrastek, Rachel Jenkins, Karuna Ramcharran, Alaina Martinez, Jessica Livingston, Sue Flesch, Robin Wilcox, Jennifer Zabrowski, and Melanie Gattas.
Glossary
- ACP
advance care planning
- AYA
adolescent and young adult
- CI
confidence interval
- FACE
family-centered
- FACE-TC
family centered pediatric advance care planning for teens with cancer
- FACQ-PC
Family Appraisal of Caregiving Questionnaire for Palliative Care
- GEE
generalized estimating equation
- pACP
pediatric advance care planning
Footnotes
Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available on publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to mlyon@childrensnational.org.
Dr Lyon conceptualized and designed the study, obtained funding, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Thompkins coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content; Drs Needle, Baker, and Friebert collected data from their sites and reviewed and revised the manuscript; Ms Briggs conceptualized the study and reviewed and revised the manuscript; Drs Wang and Ms Cheng had full access to all the data in this study, analyzed, and interpreted the data, take responsibility for the integrity of the data, conducted the data analysis, and contributed to interpretation of the findings; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02693665).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the United States National Institute of Nursing Research National Institutes of Health award R01NR014052-06. This research has been facilitated by the services and resources provided by the National Institutes of Health National Center for Advancing Translational Sciences to Children’s National, UL1TR0000075 and UL1RR031988. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Lyon is receiving grant support from the American Cancer Society outside the submitted work to translate and adapt family centered pediatric advance care planning for teens with cancer for Spanish speaking adolescents and their families. Ms Briggs is a codeveloper of Respecting Choices. She currenly consults for Respecting Choices a Division of the Coalition to Transform Advance Care-Innovations, Inc. Ms Briggs does not receive royalties for these activities; the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-048660.
References
- 1. Rietjens JAC, Sudore RL, Connolly M, et al. ; European Association for Palliative Care . Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–e551 [DOI] [PubMed] [Google Scholar]
- 2. Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000–1025 [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine (US) Committee on Palliative and End-of-Life Care for Children and Their Families. In: Field MJ, Behrman RE, eds.. When Children Die: Improving Palliative and End-of-Life Care for Children and Their Families. Washington (DC): National Academies Press; 2003 [PubMed] [Google Scholar]
- 5. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press; 2015 [PubMed] [Google Scholar]
- 6. Kreicbergs U, Valdimarsdóttir U, Onelöv E, Henter J-I, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351(12):1175–1186 [DOI] [PubMed] [Google Scholar]
- 7. Hein K, Knochel K, Zaimovic V, et al. Identifying key elements for paediatric advance care planning with parents, healthcare providers and stakeholders: a qualitative study. Palliat Med. 2020;34(3):300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lotz JD, Daxer M, Jox RJ, Borasio GD, Führer M. “Hope for the best, prepare for the worst”: a qualitative interview study on parents’ needs and fears in pediatric advance care planning. Palliat Med. 2017;31(8):764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bluebond-Langner M, Belasco JB, Goldman A, Belasco C. Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. J Clin Oncol. 2007;25(17):2414–2419 [DOI] [PubMed] [Google Scholar]
- 10. Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. Family-centered advance care planning for teens with cancer. JAMA Pediatr. 2013;167(5):460–467 [DOI] [PubMed] [Google Scholar]
- 11. Dallas RH, Kimmel A, Wilkins ML, et al. ; Adolescent Palliative Care Consortium . Acceptability of family-centered advanced care planning for adolescents with HIV. Pediatrics. 2016;138(6):e20161854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin C, Cheng I, Garvie P, D’Angelo L, Wang J, Lyon ME; Pediatric Palliative Care Consortium . The effect of family-centered (FACE®) pediatric advanced care planning intervention on family anxiety: a randomized controlled clinical trial for adolescents with HIV and their families. J Family Nursing. 2020;26(4):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin C, Cheng YI, Wang J, et al. ; for the Adolescent Palliative Care Consortium . Improving family health outcomes through pediatric advance care planning (pACP) for adolescents living with HIV/AIDS. In: 2019 Annual Pediatric Academic Societies (PAS) Meeting; April 24–May 1, 2019; Baltimore, MD [Google Scholar]
- 14. Marsac ML, Kindler C, Weiss D, Ragsdale L. Let’s talk about it: supporting family communication during end-of-life care of pediatric patients. J Palliat Med. 2018;21(6):862–878 [DOI] [PubMed] [Google Scholar]
- 15. Durall A, Zurakowski D, Wolfe J. Barriers to conducting advance care discussions for children with life-threatening conditions. Pediatrics. 2012;129(4). Available at: www.pediatrics.org/cgi/content/full/129/4/e975 [DOI] [PubMed] [Google Scholar]
- 16. Sanderson A, Hall AM, Wolfe J. Advance care discussions: pediatric clinician preparedness and practices. J Pain Symptom Manage. 2016;51(3):520–528 [DOI] [PubMed] [Google Scholar]
- 17. Lotz JD, Jox RJ, Borasio GD, Führer M. Pediatric advance care planning from the perspective of health care professionals: a qualitative interview study. Palliat Med. 2015;29(3):212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curtin KB, Watson AE, Wang J, Okonkwo OC, Lyon ME. Pediatric advance care planning (pACP) for teens with cancer and their families: design of a dyadic, longitudinal RCCT. Contemp Clin Trials. 2017;62:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyon ME, Garvie PA, Briggs L, He J, McCarter R, D’Angelo LJ. Development, feasibility, and acceptability of the Family/Adolescent-Centered (FACE) Advance Care Planning intervention for adolescents with HIV. J Palliat Med. 2009;12(4):363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friebert S, Grossoehme DH, Baker JN, et al. Congruence gaps between adolescents with cancer and their families regarding values, goals, and beliefs about end-of-life care. JAMA Netw Open. 2020;3(5):e205424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammes BJ, Briggs L. Respecting Choices: Palliative Care Facilitator Manual-Revised. LaCrosse, WI: Gundersen Lutheran Medical Foundation; 2007 [Google Scholar]
- 22. Towey J. The five wishes. Available at: https://fivewishes.org/. Accessed June 20, 2020
- 23. Wikinson D, Gillam L, Hynson J, Sullivan J, Yafis V. Caring Decisions: a Handbook for Parents Facing End-of-Life Decisions for Their Child. Parkville, Australia: The Royal Children’s Hospital; 2013. Available at: https://www.rch.org.au/uploadedFiles/Main/Content/caringdecisions/130890%20Caring%20Decisions%20book_v1.pdf. Accessed August 7, 2020 [Google Scholar]
- 24. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a Family Appraisal of Caregiving Questionnaire for Palliative Care. Psychooncology. 2006;15(7):613–622 [DOI] [PubMed] [Google Scholar]
- 25. Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 2018 [Google Scholar]
- 26. Delavar A, Barnes JM, Wang X, Johnson KJ. Associations between race/ethnicity and US childhood and adolescent cancer survival by treatment amenability. JAMA Pediatr. 2020;174(5):428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feudtner C, Walter JK, Faerber JA, et al. Good-parent beliefs of parents of seriously ill children. JAMA Pediatr. 2015;169(1):39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill DL, Faerber JA, Li Y, et al. Changes over time in good-parent beliefs among parents of children with serious illness: a two-year cohort study. J Pain Symptom Manage. 2019;58(2):190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med. 1997;45(8):1207–1221 [DOI] [PubMed] [Google Scholar]
- 30. Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60(5):317–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mackin ML, Herr K, Bergen-Jackson K, Fine P, Forcucci C, Sanders S. Research participation by older adults at end of life: barriers and solutions. Res Gerontol Nurs. 2009;2(3):162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirchhoff KT, Kehl KA. Recruiting participants in end-of-life research. Am J Hosp Palliat Care. 2008;24(6):515–521 [DOI] [PubMed] [Google Scholar]
- 33. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hudson P, Aranda S, McMurray N. Randomized controlled trials in palliative care: overcoming the obstacles. Int J Palliat Nurs. 2001;7(9):427–434 [DOI] [PubMed] [Google Scholar]
- 35. Lyon ME, Garvie PA, D’Angelo LJ, et al. ; Adolescent Palliative Care Consortium . Advance care planning and HIV symptoms in adolescence. Pediatrics. 2018;142(5):e20173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zadeh S, Pao M, Wiener L. Opening end-of-life discussions: how to introduce Voicing My CHOiCESTM, an advance care planning guide for adolescents and young adults. Palliat Support Care. 2015;13(3):591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fahner J, Rietjens J, van der Heide A, Milota M, van Delden J, Kars M. Evaluation showed that stakeholders valued the support provided by the Implementing Pediatric Advance Care Planning Toolkit. Acta Paediatr. 2020;110(1):237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacobs S, Perez J, Cheng YI, Sill A, Wang J, Lyon ME. Adolescent end of life preferences and congruence with their parents’ wishes: results of a survey of teens with cancer. Pediatr Blood Cancer. 2015;62(4):710–714 [DOI] [PubMed] [Google Scholar]
- 39. Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Peace of mind and sense of purpose as core existential issues among parents of children with cancer. Arch Pediatr Adolesc Med. 2009;163(6):519–524 [DOI] [PubMed] [Google Scholar]
- 40. Sisk BA, DuBois J, Kodish E, Wolfe J, Feudtner C. Navigating decisional discord: the pediatrician’s role when child and parents disagree. Pediatrics. 2017;139(6):e20170234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. National Cancer Institute. Family-centered advance care planning for teens with cancer (FACE-TC). Available at: https://ebccp.cancercontrol.cancer.gov/programDetails.do?programId=17054015. Accessed July 25, 2020
- 42. National Consensus Project for Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 2nd ed. Available at: www.nationalconsensusproject.org/guideline.pdf. Accessed August 28, 2020
- 43. Hinds PS, Oakes L, Furman W, et al. End-of-life decision making by adolescents, parents, and healthcare providers in pediatric oncology: research to evidence-based practice guidelines. Cancer Nurs. 2001;24(2):122–134, NaN–136 [DOI] [PubMed] [Google Scholar]