Abstract
Nicotine use remains highly prevalent with tobacco and e-cigarette products consumed worldwide. However, increasing evidence of transgenerational epigenetic inheritance suggests that nicotine use may alter behavior and neurobiology in subsequent generations. We tested the effects of chronic paternal nicotine exposure in C57BL6/J mice on fear conditioning in F1 and F2 offspring, as well as conditioned fear extinction and spontaneous recovery, nicotine self-administration, hippocampal cholinergic functioning, RNA expression, and DNA methylation in F1 offspring. Paternal nicotine exposure was associated with enhanced contextual and cued fear conditioning and spontaneous recovery of extinguished fear memories. Further, nicotine reinforcement was reduced in nicotine-sired mice, as assessed in a self-administration paradigm. These behavioral phenotypes were coupled with altered response to nicotine, upregulated hippocampal nicotinic acetylcholine receptor binding, reduced evoked hippocampal cholinergic currents, and altered methylation and expression of hippocampal genes related to neural development and plasticity. Gene expression analysis suggests multigenerational effects on broader gene networks potentially involved in neuroplasticity and mental disorders. The changes in fear conditioning similarly suggest phenotypes analogous to anxiety disorders similar to post-traumatic stress.
Keywords: cholinergic, hippocampus, learning, multigenerational, nicotine, transgenerational
1 |. INTRODUCTION
Accumulating evidence suggests that the impact of exposure to drugs of abuse extends beyond the individual to affect physiological and behavioral phenotypes in unexposed offspring.1–3 Characterization of nicotine’s effects across generations is critical considering the prevalence of tobacco product use4 and the dramatic rise of e-cigarette use.5 Through its effects on brain cholinergic systems, nicotine exposure produces marked alterations in brain function that may underlie nicotine addiction and contribute to increased risk for psychiatric disorders, including depression6 and anxiety.7 Epigenetic modifications downstream of cholinergic activation may allow for persistent effects on cellular and circuit function.8,9 Until recently, it was believed that these epigenetic modifications were erased upon establishment of the germline and thus sequestered from subsequent generations. However, epigenetic modifications, including DNA methylation, histone posttranslational modifications, and noncoding RNAs, acquired in one generation can be inherited in the next generation.10,11 These epigenetic modifications may mediate multigenerational and transgenerational effects of parental nicotine exposure on offspring behavior and neurobiology.
Rodent studies from multiple, independent laboratories have begun to identify multigenerational and transgenerational consequences of parental nicotine exposure. This work has thus far identified effects of parental nicotine exposure on depressive- and anxiety-like phenotypes,1 cognitive flexibility,2 attention deficit hyperactivity disorder (ADHD)–like behaviors,3 and gene expression.1,2 The multigenerational and transgenerational consequences of nicotine exposure may affect endophenotypes involved in nicotine addiction and mental health. For example, we have shown that nicotine exposure modulates contextual fear conditioning, a model of hippocampus-dependent fear learning that is related to vulnerability to mental health disorders such as post-traumatic stress disorder (PTSD) and addiction.12–14 Nicotine’s effects on contextual fear learning are modulated by the hippocampus.15,16 We have found that acute nicotine exposure enhances hippocampus-dependent fear learning,15,17 impairs extinction of contextual fear,18,19 and augments spontaneous recovery of contextual fear following extinction.18 However, the multigenerational and transgenerational effects of paternal nicotine exposure on these phenotypes have not been studied. Furthermore, no previous studies have characterized nicotine’s multigenerational effects on cholinergic function. Multigenerational inheritance refers to phenotypes arising in the generation immediately following exposed individuals, whereas transgenerational inheritance consists of germ-line-mediated inheritance of epigenetic information between generations in the absence of direct environmental influences that lead to phenotypic variation.11 Here, we examined both multigenerational and transgenerational effects of paternal nicotine exposure on contextual and cued fear learning in the F1 and F2 generation as well as on nicotine self-administration, hippocampal nicotinic acetylcholine receptor (nAChR) binding, hippocampal cholinergic functioning, hippocampal gene expression, and hippocampal DNA methylation in the F1 generation. We hypothesize that paternal nicotine exposure will impact fear conditioning, hippocampal gene expression, and function in offspring and grand-offspring.
2 |. METHODS AND MATERIALS
2.1 |. Subjects
Subjects were male and female C57BL/6J mice (8–20 weeks of age, Jackson Laboratory, Bar Harbor, ME). With the exception of housing for harem breeding, all animals were group-housed with a 12-hourr light/dark cycle and ad libitum access to food and water. During self-administration, subjects were food-restricted to 85% to 90% of their free-feeding body weight and water was provided ad libitum. All behavioral testing occurred between 9:00 AM and 6:00 PM. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by Penn State University, Temple University, or University of California Irvine IACUC committees.
2.2 |. Paternal nicotine exposure
Males (8 weeks) received 0.9% sterile saline or nicotine hydrogen tartrate salt (12.6 mg/kg/day, free base weight—Fisher Scientific, Waltham, MA or MP Biomedical, Santa Ana, CA) dissolved in 0.9% sterile saline, delivered subcutaneously via osmotic mini-pumps (Alzet, Model 1004, Durect, Cupertino, CA) for 28 days. This dose produces plasma nicotine and cotinine levels comparable with those seen in moderate human smokers.20,21
2.3 |. Generation of F1 and F2 mice
The half-life of nicotine in mice is approximately 6 minutes.22 It has been shown previously that the impacts of nicotine withdrawal dissipate by 4 days post-nicotine removal.23–25 Therefore, a 4-day delay between nicotine treatment and breeding was implemented to ensure systemic elimination of nicotine prior to breeding. Male mice were placed into cages with two naïve C57BL/6 J females (8–20 weeks of age) for 2 weeks to generate F1 offspring. F2 mice were generated by mating naïve male F1 mice with naïve females.
2.4 |. Fear conditioning
Fear conditioning and extinction procedures have been described in detail previously.19 Briefly, mice were trained and tested in noise-attenuating chambers (18.8 × 20 × 18.3 cm, 65 dB background noise; MED Associates, St. Albans, VT). F1 and F2 mice were fear conditioned with two conditioned stimulus (CS, 30 s, 85-dB white noise)–unconditioned stimulus (US, 2 s, 0.57-mA foot shocks) pairings separated by 120 seconds. To examine the acute effects of nicotine on fear conditioning in F1 and F2 mice, offspring received acute nicotine (0.09 mg/kg, NIC freebase weight, i.p.; nicotine hydrogen tartrate salt, Fisher Scientific) or saline (SAL) 2 to 4 minutes prior to training and testing sessions. Twenty-four hours after training, the mice were returned to the training context for 5 minutes to assess contextual freezing. After contextual testing, the mice were placed in distinct chambers to assess cued fear learning. Experimenters blinded to conditions assessed freezing, defined as the absence of voluntary movement aside from respiration, via an unbiased time sampling method.19 To examine potential ceiling effects during cued testing, a separate cohort of F1 mice received identical training with only one CS-US pairing, and contextual fear extinction and spontaneous recovery were also examined. Fear extinction occurred over five consecutive sessions beginning the day after contextual and cued fear testing. Following the final extinction session, the mice were left undisturbed in their home cages for 7 days and then retested in the training context for spontaneous recovery.
To determine if any observed differences in fear conditioning were due to differences in shock sensitivity, anxiety, or more broad learning deficits, male and female NIC- and SAL-Sired animals were additionally tested in open field, shock sensitivity, elevated plus maze (EPM), and novel object recognition paradigms (see Supporting Information for full methods and results).
2.5 |. Food and intravenous nicotine self-administration
A separate cohort of adult SAL-Sired and NIC-Sired F1 mice were used for food and nicotine self-administration studies. Beginning at 6 weeks of age, male F1 mice were weighed, mildly food-restricted to 85% to 90% of their free-feeding body weight, and then trained to press a lever in an operant chamber (Med Associates) for food chow pellets (20 mg; TestDiet, Richmond, IN) under a fixed-ratio 5, time out 20 seconds (FR5TO20 sec) schedule of reinforcement (see Supporting Information for full methods). Once stable responding was achieved (>25 pellets per session across three subsequent sessions), subjects were jugular vein catheterized under isoflurane (1%–3%)/oxygen vapor anesthesia, as previously described.26 Mice were allowed greater than or equal to 72 hours to recover from surgery before access to respond again for food reward. The re-establishment of food responding ensures that the mice have sufficiently recovered post-intravenous surgery and exhibit normal operant responding following a delay in access to the operant chambers. The mice were then permitted to acquire intravenous (IV) nicotine self-administration during 1-hour daily sessions, 6 to 7 days per week (nicotine hydrogen tartrate salt dissolved in 0.9% sterile saline, 0.03 mg/kg/infusion, free base weight; MP Biomedical, Santa Ana, CA). IV nicotine was delivered by a Razel syringe pump (Med Associates). Each session had two retractable levers (one active, one inactive). Completion of the response criteria on the active lever resulted in the delivery of an IV nicotine infusion (0.03 ml infusion volume; FR5TO20 sec schedule). Responses on the inactive lever were recorded but had no scheduled consequences. Following eight acquisition sessions at 0.03 mg/kg/infusion, the infusion dose switched to 0.1 mg/kg/infusion for six sessions. For each dose, mean intake of the last three sessions was used for statistical analyses. Catheters were flushed daily with physiological sterile saline (0.9% w/v) containing heparin (100 USP units/ml). Catheter patency was verified with Brevital (methohexital sodium, Eli Lilly, Indianapolis, IN) following the nicotine self-administration phase. To assess relapse-related behavior, the mice were tested for incubation of craving after the session immediately following the last 0.1 mg/kg/infusion dose of IV nicotine self-administration; in this procedure, the mice are permitted to respond on the active lever but receive no infusions of nicotine. On the first baseline incubation session (day 1), mice were placed in operant chambers under the FR5TO20 sec schedule with contingent cue light activation. Thereafter, the mice were housed in home cages for 20 days. On day 21 of abstinence, the mice were examined for incubation of craving, with the active lever cue light being delivered under the FR5TO20 sec schedule. Studies were conducted by experimenters blinded to group conditions, and behavioral responses were automatically recorded by MedAssociates software.
2.6 |. Nicotinic acetylcholine receptor binding
A radioligand binding assay16 was performed using hippocampi from 8-week old NIC-Sired (5 M and 10F) and SAL-Sired (9 M and 6F) F1 mice. Samples were homogenized using lysis buffer (5 mM Tris +5 mM EDTA +5 mM EGTA), centrifuged at 100 000 g for 30 minutes at 4°C, resuspended in lysis buffer, and centrifuged again. Pellets were resuspended in Tris/10% sucrose buffer and incubated with [3H] Epibatidine ([3H]EB) (~2 nM based on27,28) (specific activity 54.1 Ci/mmol, PerkinElmer, Boston, MA) for 1 hour at room temperature. [3H]EB was chosen for nAChR binding, as previous results showed that hippocampal heteromeric α4β2 nAChRs mediate the effects of nicotine on fear conditioning.17 Nonspecific binding was assessed in the presence of 300 μM nicotine (nicotine hydrogen tartrate salt dissolved in Tris Buffer, free base concentration). [3H]EB-bound nAChRs were filtered (24-well cell harvester, Brandel Co, Gaithersburg, MD), and a liquid scintillation counter (Tri-Carb 2810 TR, Perkin Elmer, Boston, MA) measured filter radioactivity. Specific binding, expressed as fmol/mg tissue, was calculated as the difference between total and nonspecific binding.16
2.7 |. In vivo amperometric cholinergic recordings
A separate cohort of naïve 10- to 20-week-old NIC-Sired and SAL-Sired F1 mice were used to assess alterations in hippocampal cholinergic transmission using amperometry. Ceramic-based microelectrodes (Center for Microelectrode Technology, Lexington, KY), with 4 (15 × 333 μm) platinum recording sites arranged in pairs (upper and lower), were coated with the choline oxidase (EC Number 1.1.3.17; Sigma-Aldrich, St. Louis, MO), as reported previously.29 Electrodes were electropolymerized with meta-phenylenediamine (m-PD; Sigma-Aldrich, St. Louis, MO) to enhance selectivity for detecting choline currents. Microelectrodes with a sensitivity of greater than or equal to 3pA/μM and limit of detection less than or equal to 400 nM for choline were used to provide a sensitive index of Acetylcholine (ACh) release.30 Animals were anesthetized with urethane (1.2–1.5 g/kg, i.p.), and enzyme-coated microelectrodes were stereotaxically lowered into dorsal (A/P −1.7 mm, M/L ± 1.5 mm, D/V −2.3 mm) or ventral (A/P −3.1 mm, M/L ± 3.0 mm, D/V −4.3 mm) hippocampus. Ventral and dorsal hippocampus were evaluated separately, as they differentially contribute to contextual fear conditioning: the ventral hippocampus (vHPC) has a more prominent role in fear association and expression, while the dorsal hippocampus (dHPC) is critical for contextual memory.31 Ag/AgCl reference electrodes were implanted into contralateral rostral cortex.
Amperometric recordings were conducted at 2 Hz by applying a fixed potential of +0.7 V, and data were digitized (FAST-16 potentiostat, Quanteon, Nicholasville, KY). Background currents were stabilized for 60 minutes, then drugs were applied into the hippocampus using a glass capillary (tip diameter:15 μm) attached to the electrode. Depolarization-evoked ACh release was measured by applying either brief pulses of potassium (KCl 70 mM; 100 nL) or NIC (1 mM freebase, nicotine tartrate; 100 nL) at 2 to 10 psi every 2 minutes. Recordings were counterbalanced for hippocampal region (dorsal or ventral) and drug (potassium or NIC). Choline signal amplitudes were measured by change in current on enzyme-coated channel from baseline current and converted into μM equivalents of choline based on in vitro calibration. Self-referencing was adopted to eliminate artifacts by subtracting currents from sentinel channels.29 Microelectrode placement was verified by Nissl staining of coronal hippocampal sections (Figure S1). Averages of two responses per drug manipulation per animal were used for statistical analysis.
2.8 |. Statistical analysis
Statistical comparisons were performed using SPSS (IBM, Armonk, NY) or GraphPad Prism (La Jolla, CA, USA). Outliers were determined by values 2 standard deviations above the mean. If an outlier was detected, the information is included in the results section. The criterion for significance was set at α = .05. Statistical analysis was initially performed including sex as a factor for all experiments testing both male and female offspring. Analyses were collapsed across sex when three-way or two-way interactions with sex were not detected (P > .05). Data were analyzed by t test, 1-way, or 2-way ANOVA, as appropriate. Significant main or interaction effects were followed by LSD post hoc comparisons. Repeated measures ANOVAs were followed by Bonferroni post hoc comparison with correction for multiple comparisons. If unequal variances were detected, Welch’s t test for unequal variances was utilized and degrees of freedom were rounded down.
2.9 |. RNA/DNA isolation
Adult F1 mice (8 weeks old; n = 3 M and 3 F per group) were euthanized via cervical dislocation. Hippocampi were rapidly dissected into ventral and dorsal portions (in a 1:1 ratio), pooled from left and right sides, and flash frozen on dry ice. DNA and RNA were co-isolated and purified using an AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA). RNA and DNA concentration and quality were evaluated using NanoDrop2000 (NanoDrop, Wilmington, DE) and Agilent Bioanalyzer (Agilent, Santa Clara, CA). For RNA extractions, minimum RNA Integrity Number (RIN) was 8.5.
2.10 |. Transcriptome analysis via RNA sequencing
Libraries were prepared by the Huck Institutes of the Life Sciences Genomics Core Facility (Penn State University) for 150 bp single-end reads using the Illumina TruSeq Stranded mRNA Library Prep Kit (Illumina, San Diego, CA) and sequenced on the Illumina HiSeq 2500 in rapid run mode (three consecutive runs with approximately 10 million reads per sample). FASTQ files were quality checked via FASTQC and possessed mean per read Phred quality scores greater than 30 (ie, less than 0.1% sequencing error). FASTQ files were aligned to a mouse reference genome (mm10; UCSC Genome Browser) using TopHat (v2.1.0)32 on Galaxy Project33 (http://galaxyproject.org/). Cufflinks and Cuffmerge (v2.2.1.0)34 were used to assemble transcripts from mapped reads and merge transcript files for final transcriptome assembly. False discovery rate (FDR) adjusted P values were computed for differential gene expression from NIC-Sired and SAL-Sired samples using Cuffdiff (v2.2.1.3),34 with a standard FDR cut-off of 0.05.35 Transcriptome datasets were deposited to Gene Expression Omnibus.
2.11 |. Enrichment analysis
Differentially expressed genes were analyzed using ingenuity pathway analysis (IPA, run December 2018; www.qiagen.com/ingenuity; Qiagen, Redwood City, CA, USA)36 in order to reveal potential enrichment of associative biological networks. Run parameters specified a maximum of 35 molecules per gene network and restricted analysis to mammalian CNS tissue or cell lines. Statistical significance for enrichment was determined using a right-tailed Fisher’s exact test corrected for multiple testing. The Enrichr database was also used to compute enrichment scores for ranked terms derived from a subset of the 133 available gene set libraries (http://amp.pharm.mssm.edu/Enrichr/).37
2.12 |. Targeted bisulfite sequencing
DNA co-isolated with RNA (see Section 2.9) was used for DNA methylation analysis. RNA-seq identified 952 and 162 differentially expressed genes in vHPC and dHPC, respectively. The 1010 unique genes from these combined lists were selected for enrichment in bisulfite-seq using a custom SeqCap Epi Enrichment System (Roche, Pleasonton, CA, USA; Table S1).38 Targeted bisulfite sequencing was performed at the Penn State Huck Institutes of the Life Sciences Genomics Core Facility. Libraries were constructed using the KAPA Hyper Prep kit (Kapa Biosystems, Wilmington, MA). Sodium bisulfite-converted libraries were PCR amplified and enriched for selected genomic regions using a custom capture probe set (SeqCap Epi Choice Probes; Roche, Pleasonton, CA, USA). Captured DNA was sequenced on the Illumina HiSeq 2500 using 100 nt paired-end reads. FASTQ files were quality checked via FASTQC. Illumina adapter sequences were removed and low-quality bases were trimmed using Trimmomatic.39 Low-quality base trimming was performed with a sliding window approach, trimming when the average quality within a four base pair window fell below a threshold of 20, with a minimum read length of 35. After trimming, FASTQ files possessed mean per read Phred quality scores greater than 30 (ie, less than 0.1% sequencing error). Trimmed reads were mapped to the mouse reference genome (mm10) using Bowtie240 implemented in Bismark.41 Methyl_extract within Bismark was used to extract CpG methylation information and to create methylation reports. MethylKit42 was used for analysis of differentially methylated regions (DMRs). Methylation status was summarized over nonoverlapping windows of 500 base pairs and differential methylation analysis was performed, with a standard FDR of 0.05.35 Datasets have been deposited to Gene Expression Omnibus.
3 |. RESULTS
3.1 |. Paternal nicotine enhances contextual fear conditioning and reverses acute nicotine enhancement of contextual fear conditioning in F1 and F2 generation mice
NIC-Sired and SAL-Sired F1 male and female mice were fear conditioned following acute SAL or NIC administration (0.09 mg/kg i.p., Figure 1A). Complete analysis of baseline, pre-CS, and CS freezing is included in Supporting Information. A 3-way ANOVA of contextual freezing with sire treatment, acute drug treatment, and sex as factors revealed a significant sire × acute drug treatment interaction (F(1,36) = 32.75, P < .001). Because there was no significant interaction between sex and sire or acute drug treatment, a 2-way ANOVA collapsed across sex was performed and revealed a significant sire treatment × acute drug treatment interaction (F(1,40) = 20.96, P < .001). Post hoc comparisons indicated that saline-treated NIC-Sired F1 mice exhibited augmented contextual fear conditioning compared with saline-treated SAL-Sired F1 mice (t20 = 2.73, P < .05). In line with previous findings,43 acute NIC at 0.09 mg/kg produced enhanced contextual fear conditioning in SAL-Sired mice (t22 = 2.99, P < .01). However, acute NIC at 0.09 mg/kg impaired contextual fear conditioning in NIC-sired mice (t18 = 3.36, P < .01). Overall, context freezing levels in NIC-Sired NIC mice were comparable with those observed in SAL-Sired SAL mice at both 0.09 mg/kg (P > .05).
FIGURE 1.

Paternal nicotine enhances contextual fear conditioning and attenuates acute nicotine enhancement of fear conditioning. A, Contextual freezing was significantly higher in NIC-Sired + SAL compared with SAL-Sired + SAL controls. Acute nicotine at 0.09 mg/kg enhanced contextual fear conditioning in SAL-Sired but significantly reduced contextual fear conditioning in NIC-Sired animals (n = 10–12 per group). B, Contextual freezing was significantly higher in NIC-grandsired + SAL compared with SAL-grandsired + SAL controls (n = 9–11 per group). Error bars indicate standard error of the mean (SEM), *P < .05
Additionally, male and female NIC- and SAL-Sired animals were tested in shock sensitivity (Figure S2), elevated plus maze (EPM, Figure S3), open field, and novel object recognition paradigms (Figure S4, see Supporting Information for full methods and results). With the exception of NIC-Sired females in EPM (who showed increased anxiety-like behaviors) and NIC-Sired animals in shock sensitivity (who showed decreased vocal reactivity to shock, which would not confound the enhanced fear learning multigenerational phenotype), no differences were detected between NIC- and SAL-Sired mice.
To determine if impaired contextual fear conditioning continued into the next generation (F2), NIC-grandsired and SAL-grandsired male and female F2 mice were bred from naïve F1 male mice. Complete analysis of baseline, pre-CS, and CS freezing is included in Supporting Information. A 3-way ANOVA of contextual freezing was performed with grandsire treatment, acute drug treatment, and sex as independent factors (Figure 1B). Because there was no significant interaction between sex and sire or acute drug treatment, a 2-way ANOVA collapsed across sex was performed. A significant main effect of sire (F(1,37) = 9.88, P < .01) was found, and post hoc comparisons indicated that NIC-grandsired mice exhibited augmented contextual fear conditioning compared with SAL-grandsired mice (t39 = 3.04, P < 0.01). In addition, SAL-grandsired mice administered acute NIC had enhanced contextual fear conditioning (t19 = 2.41, P = 0.026) but acute NIC did not enhance contextual fear conditioning in NIC-grandsired mice.
3.2 |. Paternal nicotine enhances cued fear conditioning in F1 generation mice
To examine whether a ceiling effect precluded detection of group differences for cued fear conditioning (see Supporting Information), a separate group of F1 mice was trained with one CS-US pairing. In this cohort, enhancement of contextual fear conditioning (t7 = 3.21, P < .05) as well as cued fear conditioning was found in NIC-Sired mice (t6 = 2.41, P < .05; Figure 2A).
FIGURE 2.
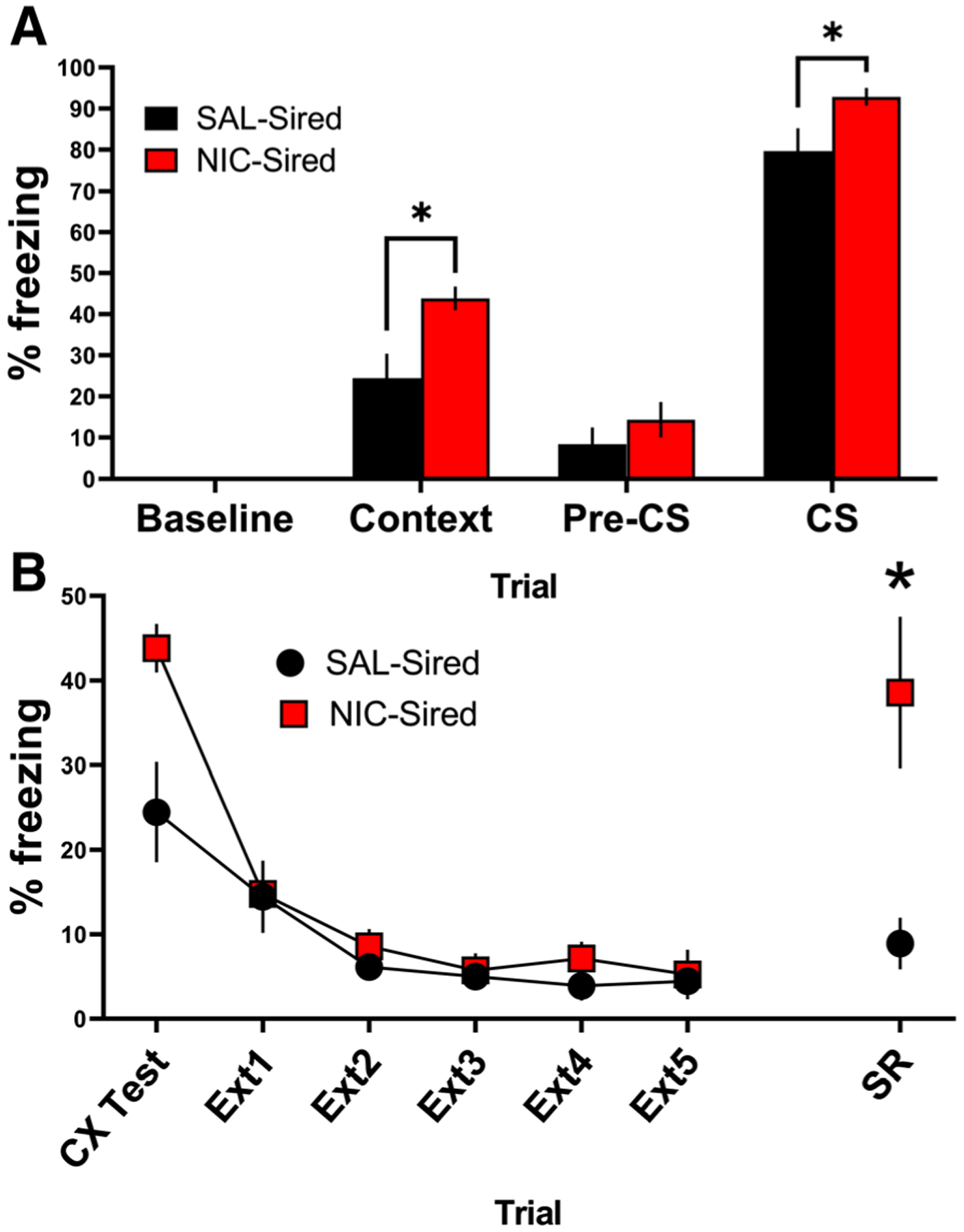
Paternal nicotine enhances cued fear conditioning and spontaneous recovery of fear memory. To examine potential ceiling effects during cued testing, a separate cohort of F1 mice received identical training with only one CS-US pairing. A, Both contextual and cued fear conditioning were augmented in NIC-Sired mice compared with SAL-Sired mice trained with 1 CS-US pairing (n = 8–10 per group). B, Paternal nicotine exposure did not affect extinction of contextual fear but enhanced spontaneous recovery of fear memory 7 days following the final extinction session (n = 8–10 per group). Error bars indicate standard error of the mean (SEM), *P < .05
3.3 |. Paternal nicotine enhances spontaneous recovery of contextual fear memory in F1 generation mice
The cohort of F1 mice that received one CS-US pairing were subsequently tested for extinction and spontaneous recovery of contextual fear memory. F1 NIC-Sired mice showed normal fear extinction but displayed enhanced spontaneous recovery of contextual fear memory relative to SAL-Sired mice (t7 = 3.38, P < 0.05; Figure 2B).
3.4 |. Paternal nicotine decreases nicotine self-administration
Prior to training for nicotine self-administration, the subjects were analyzed for their ability to learn an operant task to obtain food reward and no differences were observed (Supporting information, Figure S5). To test potential effects of sire nicotine exposure on nicotine reinforcement in F1 offspring, acquisition of IV nicotine self-administration (0.03 mg/kg/infusion) was assessed in a 2-way mixed design ANOVA, which identified a main effect of session (F(7,119) = 13.60, P < .001) and a session × sire treatment interaction (F(7,119) = 5.00, P < .001). However, post hoc tests did not reveal any statistically significant differences between the groups on each of the eight acquisition sessions (Figure 3A). The number of active and inactive lever presses were then analyzed to determine if the groups maintained an across-session preference for the active lever during acquisition (Figure 3B), which identified a main effect of session (F(7,238) = 24.18, P < .001) and a session × sire treatment interaction (F(21,238) = 11.40, P < .001). Post hoc analysis revealed that the groups differed on the first day of nicotine self-administration. The NIC-Sired group exhibited greater active lever pressing compared with the SAL-Sired group. This effect may either represent a greater level of drug-seeking behavior on the first day of exposure, perseverance of responding for food reward, and/or decreased cognitive flexibility in transitioning responding from food to drug. However, this difference did not persist across further sessions. SAL-Sired mice exhibited a consistent statistically significant preference for the active lever over their inactive lever (post hoc P < .01), but NIC-Sired mice did not exhibit this maintained preference for sessions 3 to 8.
FIGURE 3.
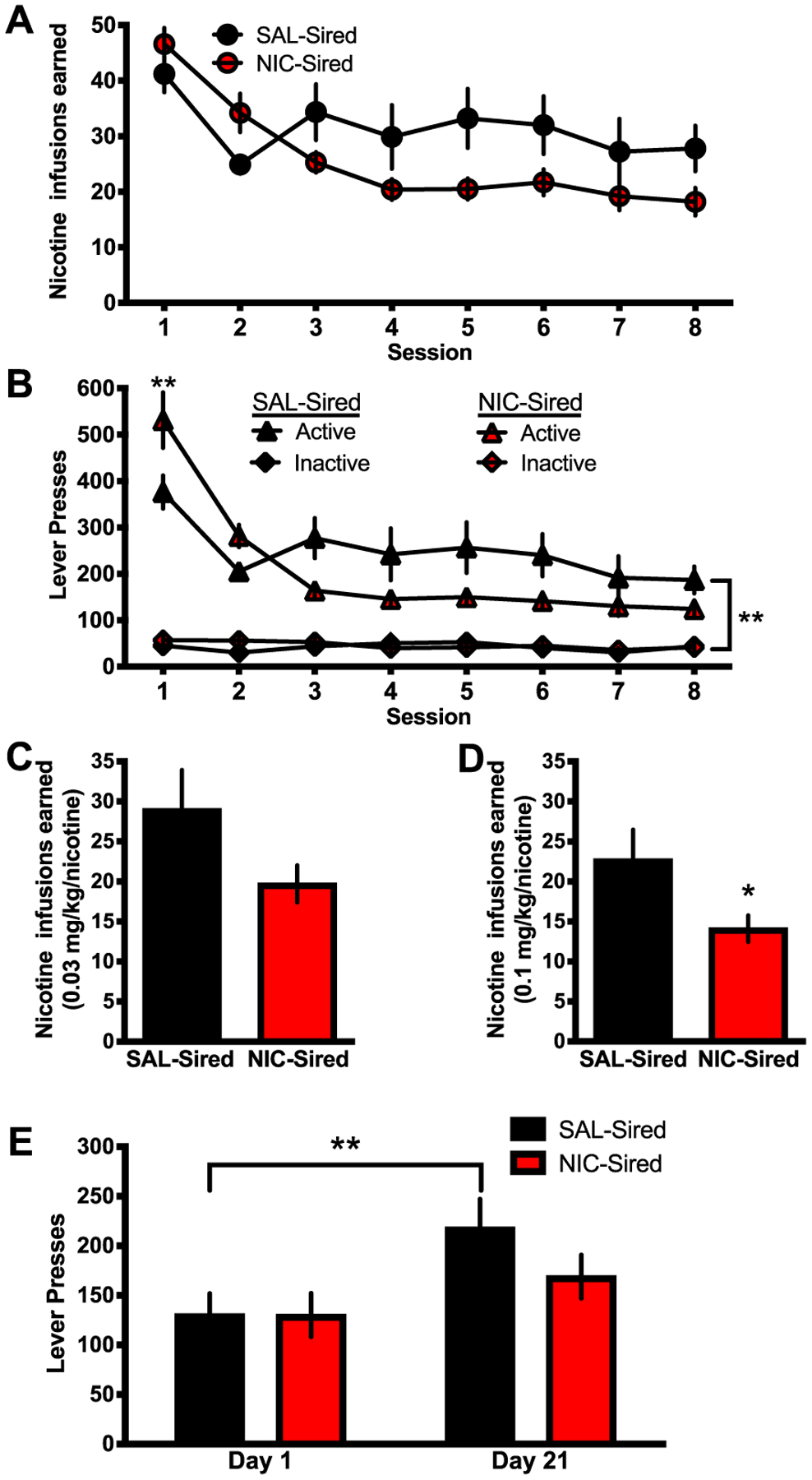
Paternal nicotine reduces nicotine self-administration. A, NIC- and SAL-Sired male mice (n = 9–10 per group) did not differ in the total number of infusions earned for each session during the acquisition period on the 0.03 mg/kg/infusion dose. B, During acquisition, the number of active and inactive lever presses significantly differed on the first session, with NIC-Sired mice nicotine exhibiting a greater number of active lever presses compared with SAL-Sired mice. However, across subsequent sessions, NIC-Sired mice decreased responding, resulting in no significant differences between their active and inactive number of lever presses across sessions 3 to 8. In contrast, SAL-Sired animals exhibited a consistent statistically significant preference for the active lever over their inactive lever. C, Mean number of nicotine infusions across the three last acquisition sessions did not significantly differ between NIC- and SAL-Sired mice. D, At a moderate dose of 0.1 mg/kg/infusion, NIC-Sired mice self-administered a significantly lower number of nicotine infusion. E, Incubation of craving assessment revealed a significant increase in responding on the previously active lever after 21 days of abstinence only for SAL-Sired mice. Error bars indicate standard error of the mean (SEM), *P < .05
To further examine potential group differences while controlling for variability during the initial phase of acquisition, the mean number of nicotine infusions were examined for the last three sessions, a time at which the subjects displayed more consistent responding for nicotine (Figure 3C). The groups did not significantly differ in the mean number of nicotine infusions (P > .05). Thereafter, the mice were transitioned to a 0.1 mg/kg/infusion dose of nicotine, previously shown to be preferred in adult C57BL6/J mice.44 At this dose, NIC-Sired mice self-administered a lower number of infusions (t17 = 2.20, P < .05; Figure 3D). For incubation of craving behavior, which is considered a measure of increased drug seeking during abstinence, a 2-way mixed design ANOVA with session and sire treatment identified a main effect of session (F(1,17) = 16.90, P < .001). While SAL-Sired animals exhibited an incubation effect with greater responding on day 21 of abstinence as compared with day 1, NIC-Sired mice did not display an increase in nicotine seeking behavior (P < .01).
3.5 |. Paternal nicotine exposure alters hippocampal cholinergic binding and function
High-affinity hippocampal heteromeric nAChR binding was upregulated in NIC-Sired F1 mice (t28 = 2.14, P < .05; SAL-Sired = 1.21 ± 0.043, NIC-Sired = 1.34 ± 0.044). One subject (NIC-Sired) was removed because binding values were two standard deviations above the mean.
Amperometric recordings of potassium- and nicotine-evoked ACh currents were assessed in F1 dHPC and vHPC. Due to uneven sample sizes per sex, sex was not included as a preliminary factor in these analyses. KCl depolarization-evoked cholinergic signals did not differ between SAL- and NIC-Sired mice in dHPC (P > .05; Figure 4A); however, local nicotine application resulted in a significant reduction in cholinergic signal amplitudes in NIC-Sired mice (t8 = 2.33, P < .05; Figure 4C). In vHPC, ACh release was decreased in NIC-Sired mice following application of KCl (t8 = 2.60, P < .05; Figure 4B) or nicotine (t8 = 2.98, P < .05; Figure 4D).
FIGURE 4.
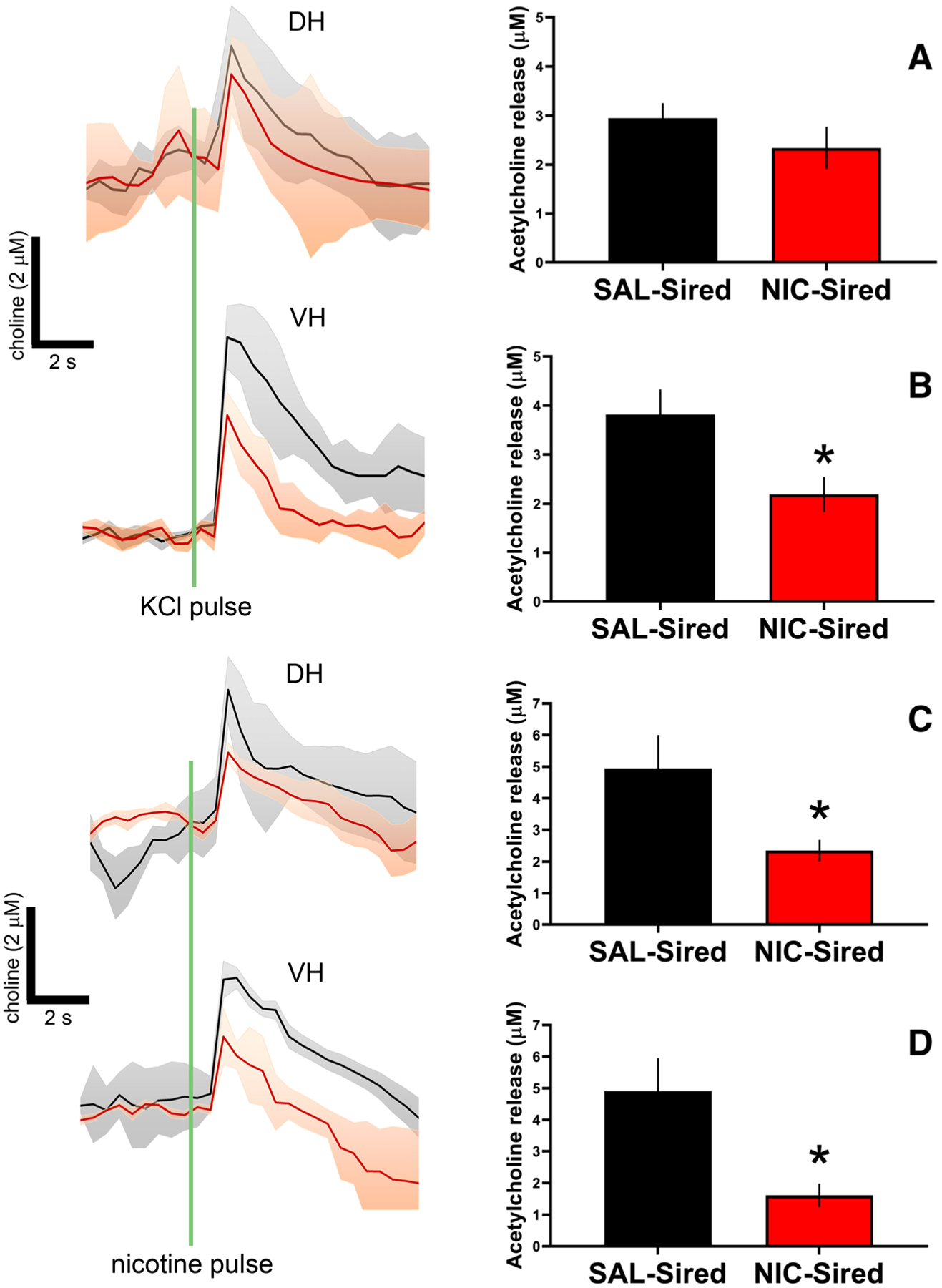
Paternal nicotine reduces cholinergic signaling in hippocampus. A, dHPC population choline signals evoked by KCl-induced terminal depolarization. No significant differences were detected between NIC- and SAL-Sired animals (n = 5 per group). B, vHPC population choline signals evoked by KCl-induced terminal depolarization were reduced in NIC-Sired mice. C, Nicotine-evoked population dHPC choline signals were reduced in NIC-Sired mice. D, Nicotine-evoked population vHPC choline signals were reduced in NIC-Sired mice. No effects of sex on cholinergic signaling were observed. Error bars indicate standard error of the mean (SEM), *P < .05
3.6 |. Paternal nicotine exposure differentially alters dorsal and ventral hippocampal gene expression
F1 hippocampal transcriptome analysis via RNA-sequencing revealed 952 differentially expressed genes in vHPC (FDR = 0.05; Table S2). Of these genes, 612 were downregulated and 340 were upregulated in NIC-Sired mice. In dHPC, only 162 genes were differentially expressed in NIC-Sired mice compared with SAL-Sired mice (FDR = 0.05). Of these 162 genes, 86 were downregulated and 76 were upregulated. One hundred three genes with altered gene expression overlapped between vHPC and dHPC.
3.7 |. Paternal nicotine exposure alters transcriptional pathways involved in nervous system development
In vHPC, IPA analysis identified the top network “Neurological Disease, Organismal Injury and Abnormalities, Cell Death and Survival” (score = 41, Table S3) and the second top network “Nervous System Development and Function, Tissue Morphology, Neurological Disease” (score = 23). The top five Molecular and Cellular Functions categories were: “Cell morphology” (88 molecules), “Cellular Assembly and Organization” (79 molecules), “Cellular Development” (96 molecules), “Cellular Function and Maintenance” (79 molecules), and “Cellular Growth and Proliferation” (87 molecules). The top Physiological System Development and Function was “Nervous System Development and Function” (175 molecules), and some of the top Diseases and Disorders Functions include “Neurological Disease” (second, 191 molecules) and “Psychological Disorders” (fourth, 90 molecules) (Table S4).
Complementary to the IPA results, enrichment analysis using Enrichr provided further evidence for alterations in cellular growth and development in vHPC, with top GO biological terms including “RNA splicing,” “response to unfolded protein,” and “regulation of cell growth” and “protein stabilization” (Table S5). Correspondingly, “spliceosomal complex” was identified as a top GO cellular term. KEGG pathway analysis via Enrichr additionally pointed to spliceosome functioning and MAPK signalling as potentially affected pathways.
Despite a considerably shorter list of differentially expressed genes in dHPC compared with vHPC, similar dHPC-enriched pathways and terms were identified (Tables S3 and S4). IPA analysis identified the top network “Behavior, Neurological Disease, Organismal Injury and Abnormalities” (score = 24) and the second top network “Neurological Disease, Organismal Injury and Abnormalities, and Psychological Disorders (score = 20).
The top five Molecular and Cellular Functions categories in dHPC were “Cellular Development” (29 molecules), “Cellular Growth and Proliferation” (29 molecules), “Cell Morphology” (27 molecules), “Cellular Assembly and Organization” (23 molecules), and “Cellular Function and Maintenance” (25 molecules). “Nervous System Development and Function” was again identified as a top enriched term under the Physiological System Development and Function classification (second, 44 molecules). Top enriched terms under the Diseases and Disorders Function classification included “Neurological Disease” (1st, 51 molecules), and “Psychological Disorders” (fifth, 31 molecules). Enrichment analysis using Enrichr identified multiple different GO biological terms for dHPC compared with vHPC, including “regulation of neuron death” and “brain development” (Table S5), which is complementary to the IPA molecular and cellular function “Cell Death and Survival.”
To further explore the functional role of differentially expressed transcripts overlapping between the dHPC and vHPC, differentially expressed genes common to both regions (103 total) were evaluated. Overlapping differentially expressed transcripts between the two regions were all downregulated or upregulated in the same direction, suggesting common alterations in transcriptional pathways across brain regions in NIC-sired mice. The top five Molecular and Cellular Functions categories identified by IPA were “Cell Death and Survival” (17 molecules), “Cellular Movement” (10 molecules), “Cell-to-Cell Signaling and Interaction” (17 molecules), “Cellular Growth and Proliferation” (18 molecules), and “Cell Morphology” (17 molecules) (Table S4).
Differentially expressed genes unique to dHPC and vHPC were subsequently analyzed separately in IPA in order to test for divergent neurobiological adaptions between the two regions (Table S6). No enriched canonical pathways overlapped between the unique vHPC unique dHPC analyses. Top enriched canonical pathways unique to vHPC (44 total) included “Calcium signaling” and “Glucocorticoid receptor signaling,” while top dHPC canonical pathways (eight total) included “Thyroid Hormone Metabolism” and “Retinoic acid Mediated Apoptosis Signaling.” Enriched diseases and functions unique to vHPC (295 total) included “Formation of [Hippocampus] Ammon’s Horn” and “Quantity of Cellular Protrusions” while uniquely dHPC enriched diseases and functions terms (111 total) included “Inflammation of white matter” and “Demyelination.”
3.8 |. Paternal nicotine exposure alters hippocampal DNA methylation
A targeted DNA methylation analysis was performed to determine if altered DNA methylation in corresponding regulatory regions accounted for the differential gene expression in NIC-Sired F1 offspring. Targets included the 1114 differentially expressed genes identified in either dHPC or vHPC. In vHPC, 11 differentially methylated regions (DMRs) were detected, with eight showing increased methylation and three showing decreased methylation (Table 1). Of the 11 DMRs, 10 were located in regions associated with a gene that exhibited altered expression in vHPC. In the dHPC, 30 DMRs were detected, with 15 showing increased methylation and 15 showing decreased methylation. Of the 30 DMRs, 29 were located in regions associated with a gene that exhibited altered expression in dHPC.
TABLE 1.
Paternal nicotine alters hippocampal DNA methylation
| Region | Gene | Chr | Mb (start) | FC (methylation) | q value | FC (RNAseq) | q value (RNAseq) |
|---|---|---|---|---|---|---|---|
| vHPC | Fez1 | 8 | 69.155 | −12.171 | 0.030 | −1.183 | 0.028 |
| vHPC | Flrt2 | 12 | 95.693 | −6.366 | 0.048 | 1.271 | 0.002 |
| vHPC | Nostrin | 2 | 69.149 | −4.504 | 0.048 | −1.444 | 0.037 |
| vHPC | Pnpla2 | 7 | 141.45 | 4.869 | 0.000 | −1.355 | 0.006 |
| vHPC | Bag3 | 7 | 128.52 | 6.304 | 0.046 | −1.275 | 0.038 |
| vHPC | Ksr1 | 11 | 79.081 | 7.618 | 0.048 | −1.258 | 0.002 |
| vHPC | Tnni2 | 7 | 142.44 | 8.137 | 0.019 | −13.092 | 0.002 |
| vHPC | Tshz2 | 2 | 169.84 | 9.376 | 0.022 | −1.301 | 0.002 |
| vHPC | Rrbp1 | 2 | 143.98 | 9.493 | 0.030 | −1.373 | 0.002 |
| vHPC | Fkbp5 | 17 | 28.506 | 9.879 | 0.046 | −1.345 | 0.002 |
| vHPC | Thsd4 | 9 | 60.372 | 16.559 | 0.046 | N/A | n.s. |
| dHPC | Gm45906 | 7 | 81.463 | −12.829 | 0.006 | N/A | n.s. |
| dHPC | Slc16a7 | 10 | 125.34 | −12.688 | 0.000 | N/A | n.s. |
| dHPC | Hspb1 | 5 | 135.89 | −11.900 | 0.006 | −1.805 | 0.007959 |
| dHPC | Gap43 | 16 | 42.254 | −10.569 | 0.029 | −1.328 | 0.007959 |
| dHPC | Cacna2d1 | 5 | 16.116 | −9.572 | 0.029 | N/A | n.s. |
| dHPC | Col16a1 | 4 | 130.09 | −9.480 | 0.029 | N/A | n.s. |
| dHPC | Slc4a5 | 5 | 89.123 | −9.433 | 0.029 | 1.713 | 0.0384228 |
| dHPC | Pcp4 | 16 | 96.504 | −9.387 | 0.033 | N/A | n.s. |
| dHPC | Nos1 | 5 | 117.79 | −8.080 | 0.021 | −1.306 | 0.007959 |
| dHPC | Slit1 | 11 | 35.407 | −7.964 | 0.043 | −1.287 | 0.007959 |
| dHPC | Rusc2 | 4 | 43.419 | −6.961 | 0.029 | N/A | n.s. |
| dHPC | Evc2 | 5 | 37.417 | −6.266 | 0.029 | N/A | n.s. |
| dHPC | Adra1b | 11 | 43.872 | −5.503 | 0.021 | 1.732 | 0.007959 |
| dHPC | Rbms1 | 2 | 60.794 | −4.204 | 0.044 | N/A | n.s. |
| dHPC | Gnb4 | 3 | 32.588 | −4.024 | 0.033 | N/A | n.s. |
| dHPC | Tril | 6 | 53.818 | 1.248 | 0.017 | N/A | n.s. |
| dHPC | Msi2 | 11 | 88.574 | 4.730 | 0.033 | N/A | n.s. |
| dHPC | Pcnt | 10 | 76.397 | 5.178 | 0.029 | N/A | n.s. |
| dHPC | Sipa1l2 | 8 | 125.54 | 7.669 | 0.033 | N/A | n.s. |
| dHPC | Tssc1 | 12 | 28.83 | 7.904 | 0.009 | N/A | n.s. |
| dHPC | Ncor2 | 5 | 125.13 | 10.035 | 0.006 | N/A | n.s. |
| dHPC | Slc7a11 | 3 | 50.3 | 10.959 | 0.006 | N/A | n.s. |
| dHPC | Lgr4 | 2 | 109.97 | 11.717 | 0.006 | N/A | n.s. |
| dHPC | Ksr1 | 11 | 89.02 | 12.221 | 0.006 | −1.242 | 0.0459173 |
| dHPC | Nmb | 6 | 49.056 | 12.571 | 0.000 | N/A | n.s. |
| dHPC | Akap8l | 17 | 32.351 | 14.520 | 0.006 | N/A | n.s. |
| dHPC | Tshz2 | 2 | 169.91 | 17.806 | 0.017 | N/A | n.s. |
| dHPC | Lgr4 | 2 | 109.92 | 18.785 | 0.005 | N/A | n.s. |
| dHPC | Sipa1l3 | 7 | 29.444 | 20.474 | 0.005 | N/A | n.s. |
Abbreviations: Chr, chromosome; dHPC, dorsal hippocampus; FC, fold-change; Mb, megabase; N/A, not applicable (ie, not on differential gene expression list); n.s., not significant; vHPC, ventral hippocampus.
4 |. DISCUSSION
Increased understanding of epigenetic processes in conjunction with recent data, including the present findings, have challenged traditional understanding of inheritance. Factors beyond genotype alone may determine phenotypes in subsequent generations, and exposures within a generation may not be sequestered from progeny. The present study suggests that the detrimental health effects of nicotine exposure may transcend individual exposure and affect subsequent generations. We identified multigenerational and transgenerational effects of preconception paternal nicotine exposure in C57BL/6J mice on Pavlovian fear conditioning, resulting in stronger fear memories in F1 and F2 progeny. Paternal nicotine exposure also resulted in decreased nicotine self-administration and attenuated relapse-related behaviors, suggesting a greater aversive response to nicotine. In support of these behavioral differences, multigenerational alterations in hippocampal cholinergic function and epigenetic processes were observed. Together, these results point to changes in nervous system function in the offspring of nicotine-exposed mice resulting in altered behavioral phenotypes.
F1 and F2 offspring of male mice exposed to nicotine exhibited enhanced contextual and cued fear conditioning. Despite no differences in contextual fear extinction between NIC- and SAL-Sired F1 mice, NIC-Sired mice showed enhanced spontaneous recovery of contextual fear memories. Importantly, no differences in shock sensitivity between NIC- and SAL-Sired mice that could account for increased fear conditioning were found. Enhanced fear conditioning may suggest generalized enhancement of learning processes as opposed to modulation of processes more specific to fear learning. However, no changes in novel object recognition, operant food training, or open field locomotion were observed in NIC-Sired mice, although a sex-specific effect of increased EPM open arm time in NIC-Sired female mice was identified. While this does not rule out potential modifications to other learning systems or cognitive processes, these findings together suggest that fear learning may be more sensitive to the multigenerational and transgenerational effects of paternal nicotine exposure. Moreover, these findings suggest altered cholinergic function in NIC-Sired animals. Nicotine modulates contextual fear conditioning. Whereas acute nicotine enhances contextual fear conditioning,15,45 withdrawal from chronic nicotine disrupts contextual fear conditioning.16,21 In the present study, acute nicotine enhanced contextual fear conditioning in F1 and F2 mice from saline-treated mice. In contrast, acute nicotine-disrupted contextual fear conditioning in NIC-Sired mice and had no effect in NIC-grandsired mice, which may point to altered cholinergic functioning in the hippocampus.
The effects of paternal nicotine exposure on subsequent nicotine self-administration in the F1 generation also points to disrupted cholinergic function. During acquisition of IV nicotine self-administration at the lower dose, the groups did not differ in the number of nicotine infusions, although an increase in the number of active lever presses was found in the NIC-Sired group. This suggests that the NIC-Sired mice may have exhibited a perseverance of responding for food reward and/or decreased cognitive flexibility in transitioning responding from food to drug. However, it is also worthwhile to note that the groups did not differ on day 1 of incubation of craving, which represents an extinction session (eg, no nicotine infusions during session), and thus, this effect appears to have been present when reinforcers are switched but not in the absence of a reinforcer during an extinction session. The NIC-Sired mice also exhibited decreased nicotine self-administration at the moderate dose, which aligns with recent work identifying decreases in alcohol, cocaine, and opioid administration associated with parental alcohol, cocaine, and morphine exposure (eg Vassoler et al,46 as reviewed in Goldberg and Gould47). The observed reduction in nicotine self-administration may be attributed to either decreased sensitivity to the rewarding effects of nicotine and/or increased sensitivity to the aversive effects of nicotine. Indeed, the groups differed at the moderate nicotine dose but not the lower nicotine dose, which supports the notion of an increased aversive response with the higher dose. Interestingly, we also found a lack of incubation of craving on day 21 in NIC-Sired mice following self-administration at the moderate dose, suggesting that decreased nicotine-seeking behaviors could be related to an aversion-associated memory for nicotine. Although various neural substrates may underlie these effects on nicotine intake and relapse-related responding, a recent study found that decreased DNA methyltransferase in the hippocampal CA1 region reduced morphine self-administration.48 This finding, along with the known function of cholinergic hippocampal function in learning and memory processes, further supports the notion of disrupted nicotine-mediated processing in the hippocampus of NIC-Sired mice.
Along these lines, NIC-Sired mice exhibited increased hippocampal high-affinity nAChR binding. We also found reductions in potassium-evoked ACh release in vHPC as well as in nicotine-evoked ACh release in both dHPC and vHPC of NIC-Sired animals. Changes in depolarization-evoked ACh release reflect altered cholinergic function downstream of receptor binding, while changes in nicotine-evoked ACh release reflect altered nAChR function. These data are in line with previous findings of upregulated high-affinity nAChR binding following decreased nAChR function.49 Because both potassium-evoked and nicotine-evoked ACh release were altered in the vHPC of NIC-Sired mice, vHPC may be more sensitive to the multigenerational effects of paternal nicotine exposure.
DHPC is known to modulate contextual fear conditioning.31,50 Inhibition of vHPC disrupts both cued and contextual fear conditioning51,52 and vHPC cholinergic lesions impair cued fear conditioning.53 We have also shown that direct nicotine infusion into dHPC enhances contextual fear conditioning while infusion into vHPC disrupts contextual fear conditioning.15 vHPC may also modulate spontaneous recovery of contextual fear memories, as inactivation of vHPC-prelimbic circuitry decreases spontaneous recovery of contextual fear memories.54 While other brain regions involved in fear conditioning, such as the amygdala,55 may also be affected by paternal nicotine exposure, these findings along with the present data suggest that changes in vHPC function may be responsible for altered fear conditioning in NIC-Sired mice.
We hypothesized that the multigenerational effects of paternal nicotine exposure may be related to changes in transcriptional effectors acting upstream of these neural systems. Genome-wide transcriptional sequencing in vHPC and dHPC of F1 generation mice identified 1114 differentially expressed genes between NIC- and SAL-Sired mice. This difference was greater in vHPC (952) versus dHPC (162), in line with the greater change in vHPC relative to dHPC cholinergic function and alterations to both contextual and cued fear conditioning. Subsequent pathway analysis suggested broad alterations to transcriptional pathways associated with glucocorticoid signaling and neural development/plasticity in both hippocampal regions. In order to identify potential adaptations specific to vHPC, pathway analysis was performed using only transcripts specific to either hippocampal subregion. No enriched IPA canonical pathways overlapped between the vHPC and dHPC, which are functionally distinct subregions of the hippocampus.31 When genes that overlapped between dHPC and vHPC were removed, top enriched canonical pathways unique to vHPC included “Glucocorticoid Receptor Signaling,” suggesting a unique, additional alteration to glucocorticoid functioning in this region compared with dHPC.
With the aim of identifying upstream epigenetic regulators that may act on gene expression, we performed targeted DNA methylation sequencing using the compiled list of dHPC and vHPC differentially expressed genes identified from RNA-sequencing. Surprisingly, we found only 11 DMRs in vHPC and 30 DMRs in dHPC between NIC- and SAL-Sired animals. Although this is unexpected given the much higher number of differentially expressed transcripts in vHPC, DNA methylation is only one of several regulatory factors that can impact gene expression and DNA methylation does not consistently translate into altered gene expression.56
Of the 11 vHPC DMRs, seven exhibited methylation patterns consistent with the direction of differential transcription (decreased transcription with increased DNA methylation and increased transcription with reduced methylation). Differentially methylated genes in the vHPC included Fkbp5, Ksr1, and Pnpla2. Interestingly, Fkbp5 and Ksr1 transcription was disrupted in one behavioral mouse model of PTSD,57 where mice were exposed to an electric footshock and then presented situational reminders. Fkbp5 encodes a glucocorticoid receptor chaperone whose functioning has been associated with a maladaptive prolonged stress response in individuals with PTSD and other anxiety disorders.58 Specifically, human studies show that Fkbp5 methylation and transcription correlate with severity of PTSD symptoms, such that increased methylation and decreased transcription predict more severe PTSD symptomology.59,60 Fkbp5 expression modulates HPA-axis functioning, which is thought to mediate its involvement in PTSD.59,61 Our finding of enhanced spontaneous recovery of fear memory in conjunction with dysregulation of transcriptional pathways associated with glucocorticoid signaling in NIC-Sired animals may point to increased vulnerability to PTSD-like phenotypes.
In dHPC, DMR patterns were largely inconsistent with the direction of differential transcript expression found by RNA-sequencing, which suggests that changes in vHPC DNA methylation produced by paternal nicotine exposure are more consequential in terms of impacting gene expression than those in dHPC. This is in line with our identification of a greater number of differentially expressed transcripts and more exaggerated changes in cholinergic transmission in NIC-Sired vHPC compared with dHPC. Our targeted sequencing approach may have limited the ability to detect potential transcriptional regulation by distally methylated sequences. Future investigations including analysis of genome-wide DNA methylation, histone modifications, and small RNA expression will provide a more complete interpretation of these findings.
A potential limitation of our nicotine exposure design is the focus on paternal nicotine exposure to investigate the multigenerational and transgenerational impact of nicotine exposure. Although other studies finding multi/transgenerational phenotypes following paternal drug exposure, including cocaine46 and morphine,62 found no differences in maternal care, it is possible that paternal nicotine exposure may impact maternal care. Future studies investigating the impact on maternal care are warranted. As our current focus was on paternal exposure, future work should also compare impacts of paternal vs maternal exposure.
Overall, the present findings provide a novel understanding for the multigenerational and transgenerational effects of nicotine exposure, which are supported by a growing literature characterizing multigenerational and transgenerational effects of drug exposure (as reviewed in Goldberg and Gould47). This study was the first to test contextual fear conditioning in F1 and F2 offspring of nicotine-exposed males and identified enhanced fear memory formation and spontaneous recovery of fear memories. This study was also the first to identify altered nicotine self-administration and incubation of craving in F1 nicotine-exposed offspring. Differential methylation in genes associated with PTSD and HPA-axis dysregulation as well as concurrent disruptions in stress-related transcriptional pathways were found in NIC-Sired mice. Paternal nicotine was also associated with decreased hippocampal cholinergic function and increased hippocampal nAChR binding. Interestingly, PTSD patients that did not smoke show significantly higher mesiotemporal cortical high-affinity nAChR binding63 and PTSD is associated with greater fear conditioning and spontaneous recovery of extinguished fear memories.64 Together, our findings suggest that nicotine exposure may have a multigenerational impact of increasing offspring susceptibility to PTSD-like symptomology. This finding along with other recent findings showing multigenerational effects of nicotine exposure on cognitive flexibility2 suggest that the negative health outcomes of nicotine exposure cast a wider net than previously thought.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Institute on Drug Abuse (T.G., DA017949 and 1U01DA041632; V.P., DA037421; and C.D.F., DA039658), Jean Phillips Shibley Endowment (T.J.G.), and Penn State University (T.J.G.). The Penn State Huck Institutes of the Life Sciences Genomics Core facility completed all RNA-sequencing and bisulfite-sequencing, with an acknowledgement to Dr. Craig Paul for his assistance.
Funding information
National Institute on Drug Abuse, Grant/Award Numbers: DA017949, DA037421, DA039658, DA041632 and 1U01DA041632; Pennsylvania State University; Penn State University; Jean Phillips Shibley Endowment
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Dai J, Wang Z, Xu W, et al. Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b. Sci Rep. 2017;7(1):7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy DM, Morgan TJ Jr, Lowe SE, et al. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol. 2018;16(10):e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. Transgenerational transmission of hyperactivity in a mouse model of ADHD. J Neurosci. 2014;34(8):2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings KM, Proctor RN. The changing public image of smoking in the United States: 1964–2014. Cancer Epidemiol Biomarkers Prev. 2014;23(1):32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang LL, Kowitt SD, Sutfin EL, Patel T, Ranney LM, Goldstein AO. Electronic cigarette use among high school students and its association with cigarette use and smoking cessation, North Carolina Youth Tobacco Surveys, 2011 and 2013. Prev Chronic Dis. 2016;13:E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holliday ED, Nucero P, Kutlu MG, et al. Long-term effects of chronic nicotine on emotional and cognitive behaviors and hippocampus cell morphology in mice: comparisons of adult and adolescent nicotine exposure. Eur J Neurosci. 2016;44:2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284:2348–2351. [DOI] [PubMed] [Google Scholar]
- 8.Jung Y, Hsieh LS, Lee AM, et al. An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior. Nat Neurosci. 2016;19(7):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gitik M, Holliday ED, Leung M, et al. Choline ameliorates adult learning deficits and reverses epigenetic modification of chromatin remodeling factors related to adolescent nicotine exposure. Neurobiol Learn Mem. 2018;155:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird A Perceptions of epigenetics. Nature. 2007;447:396–398. [DOI] [PubMed] [Google Scholar]
- 11.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JA, Gould TJ. Associative learning, the hippocampus, and nicotine addiction. Curr Drug Abuse Rev. 2008;1:9–19. [DOI] [PubMed] [Google Scholar]
- 13.Kutlu MG, Parikh V, Gould TJ. Nicotine addiction and psychiatric disorders. Int Rev Neurobiol. 2015;124:171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh V, Kutlu MG, Gould TJ. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: current trends and perspectives. Schizophr Res. 2016;171:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology (Berl). 2013;225(1): 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl). 2006;184:345–352. [DOI] [PubMed] [Google Scholar]
- 18.Kutlu MG, Zeid D, Tumolo JM, Gould TJ. Pre-adolescent and adolescent mice are less sensitive to the effects of acute nicotine on extinction and spontaneous recovery. Brain Res Bull. 2018;138:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutlu MG, Gould TJ. Acute nicotine delays extinction of contextual fear in mice. Behav Brain Res. 2014;263:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. In: Nicotine psychopharmacology. Berlin, Heidelberg: Springer; 2009:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- 23.Gould TJ, Portugal GS, Andre JM, et al. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutlu MG, Oliver C, Huang P, Liu-Chen LY, Gould TJ. Impairment of contextual fear extinction by chronic nicotine and withdrawal from chronic nicotine is associated with hippocampal nAChR upregulation. Neuropharmacology. 2016;109:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. [DOI] [PubMed] [Google Scholar]
- 26.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomazzo E, MacArthur L, Yasuda RP, Wolfe BB, Kellar KJ. Quantitative analysis of the heteromeric neuronal nicotinic receptors in the rat hippocampus. J Neurochem. 2010;115:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30: 3518–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh V, Sarter M. Forebrain cholinergic systems and cognition: new insights based on rapid detection of choline spikes using enzyme-based biosensors. In: Microelectrode Biosensors. Totowa, NJ: Humana Press; 2013:257–277. [Google Scholar]
- 31.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afgan E, Baker D, van den Beek M, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44:W3–w10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5): 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001; 125(1–2):279–284. [DOI] [PubMed] [Google Scholar]
- 36.Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4): 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendt J, Rosenbaum H, Richmond TA, Jeddeloh JA, Burgess DL. Targeted bisulfite sequencing using the SeqCap epi enrichment system. Methods Mol Biol. 2018;1708:383–405. [DOI] [PubMed] [Google Scholar]
- 39.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27(11):1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akalin A, Kormaksson M, Li S, et al. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012;13(10):R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117(6):1276–1282. [DOI] [PubMed] [Google Scholar]
- 44.Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61(4):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102(1–2):31–39. [DOI] [PubMed] [Google Scholar]
- 46.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldberg LR, Gould TJ. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur J Neurosci. 2018;50(3):2453–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang JJ, Jiang FZ, Zheng W, et al. DNMT3a in the hippocampal CA1 is crucial in the acquisition of morphine self-administration in rats. Addict Biol. 2019;▪:▪-▪. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220(4593):214–216. [DOI] [PubMed] [Google Scholar]
- 50.Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111(1):104–113. [DOI] [PubMed] [Google Scholar]
- 51.Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126(1–2):159–174. [DOI] [PubMed] [Google Scholar]
- 52.Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118(1):97–110. [DOI] [PubMed] [Google Scholar]
- 53.Staib JM, Della Valle R, Knox DK. Disruption of medial septum and diagonal bands of Broca cholinergic projections to the ventral hippocampus disrupt auditory fear memory. Neurobiol Learn Mem. 2018;152:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasquez JH, Leong KC, Gagliardi CM, Harland B, Apicella AJ, Muzzio IA. Pathway specific activation of ventral hippocampal cells projecting to the prelimbic cortex diminishes fear renewal. Neurobiol Learn Mem. 2019;161:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka M, Li H, Zhang X, et al. Region- and time-dependent gene regulation in the amygdala and anterior cingulate cortex of a PTSD-like mouse model. Mol Brain. 2019;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. [DOI] [PubMed] [Google Scholar]
- 59.Sarapas C, Cai G, Bierer LM, et al. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis Markers. 2011;30(2–3):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yehuda R, Daskalakis NP, Desarnaud F, et al. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front Psych. 2013;4:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yehuda R, Cai G, Golier JA, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. [DOI] [PubMed] [Google Scholar]
- 62.Li CQ, Luo YW, Bi FF, et al. Development of anxiety-like behavior via hippocampal IGF-2 signaling in the offspring of parental morphine exposure: effect of enriched environment. Neuropsychopharmacology. 2014;39(12):2777–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czermak C, Staley JK, Kasserman S, et al. beta2 Nicotinic acetylcholine receptor availability in post-traumatic stress disorder. Int J Neuropsychopharmacol. 2008;11(3):419–424. [DOI] [PubMed] [Google Scholar]
- 64.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


