The medium-range order in GeO2 thin-film glasses is higher with elevated deposition temperature.
Abstract
Glasses are nonequilibrium solids with properties highly dependent on their method of preparation. In vapor-deposited molecular glasses, structural organization could be readily tuned with deposition rate and substrate temperature. Here, we show that the atomic arrangement of strong network-forming GeO2 glass is modified at medium range (<2 nm) through vapor deposition at elevated temperatures. Raman spectral signatures distinctively show that the population of six-membered GeO4 rings increases at elevated substrate temperatures. Deposition near the glass transition temperature is more efficient than postgrowth annealing in modifying atomic structure at medium range. The enhanced medium-range organization correlates with reduction of the room temperature internal friction. Identifying the microscopic origin of room temperature internal friction in amorphous oxides is paramount to design the next-generation interference coatings for mirrors of the end test masses of gravitational wave interferometers, in which the room temperature internal friction is a main source of noise limiting their sensitivity.
INTRODUCTION
Glasses are nonequilibrium, noncrystalline materials that retain in their structure at short- and medium-range (<2 nm) information about the history of preparation (1, 2). For melt-quenched glasses, a slow cooling rate toward the glass transition temperature (Tg) allows for an adequate configurational sampling that drives the system to lower energy states in the potential energy landscape (PEL). Physical vapor deposition is an efficient means of rapid cooling to produce glassy materials. Altering the deposition conditions, such as substrate temperature (Tsub) and deposition rate, makes it possible to manipulate the atomic ordering that, in turn, shapes the properties of the vapor-deposited glasses (3–6). For example, in vapor-deposited thin films of itraconazole, a glass-forming smectic liquid crystal, the orientation of the molecule’s long axis tends to align with the surface normal when the deposition rate is around 0.2 Å/s, whereas nearly isotropic orientation is preferred when the deposition rate is three orders of magnitude higher (7). In a similar fashion, Tsub plays a role in affecting the molecular packing in thin-film organic glasses. Depositing N,N′-bis(3-methylphenyl)-N,N′-diphenylbenzidine at around 0.8 Tg produces glasses with a strong horizontal orientation, while at 0.95 Tg, weak vertical orientation is observed (3, 8). In silico vapor deposition of glasses predicts that when depositing at the Kauzmann temperature, where the configurational entropy vanishes, it would be possible to achieve a uniform structural configuration characteristic of ultrastable glassy materials (9). It remains to be answered experimentally whether the atomic arrangement of strong network-forming glasses, such as amorphous SiO2 (a-SiO2) and GeO2 (a-GeO2), could be modified by altering the Tsub because the restructuring of strong covalent bonds is involved.
A question that follows is whether elevated temperature deposition of a-GeO2 would result in atomic rearrangements that alter the distribution of two-level systems (TLSs) in glassy materials. TLSs in the PEL model are used to describe the acoustic and thermal properties of amorphous solids at low temperatures (10). They are represented by asymmetric double-well potentials in some configuration coordinate. Transitions between the wells, which are thermally activated at temperatures above ~5 K, and quantum tunneling dominated at lower temperatures, are associated with the rearrangement of a small group of atoms at temperatures well below Tg (11). Recent insights into the properties of vapor-deposited glasses show that TLSs could possibly be drastically reduced at selected deposition conditions (2). In indomethacin thin-film glasses grown at 0.85 Tg, remarkable suppression of TLSs was found because of the particular molecular arrangement influenced by the deposition conditions (12). For network-forming glasses such as a-Si, the density of TLSs has been shown to be reduced by at least one order of magnitude when Tsub increased from 473 to 673 K (13, 14). This behavior has been ascribed to a more ordered amorphous network achieved with elevated temperature deposition. Motivated by these results, the gravitational wave community has explored the possibility to lower the density of TLSs through elevated temperature deposition of a-Ta2O5 as a way to reduce room temperature internal friction in the coatings of the end test mass mirrors (15, 16). Internal friction in amorphous materials is generally framed in terms of energy coupling from an elastic field into TLSs. The coupling causes excitations visualized as transitions between the two wells in a TLS. The relaxation of these excitations through various mechanisms characterizes the system’s internal friction (17, 18). Room temperature internal friction, in accord with the fluctuation-dissipation theorem (19), leads to thermally driven fluctuations in the amorphous coatings that limit the sensitivity of gravitational wave detectors (20–22). Recent work in a-Si thin films showed that room temperature internal friction reduced from around 2 × 10−4 to 0.5 × 10−4 when Tsub increased from 293 to 673 K (~ 0.80 Tg) (23, 24). Nevertheless, a correlation between structural organization and room temperature internal friction for strong network-forming glasses deposited at elevated Tsub is still lacking.
Here, we describe the modifications in the atomic configuration at medium range of a-GeO2 thin films vapor-deposited at elevated Tsubs. The lower Tg of GeO2, around 788 K (25), in comparison with Tg around 1475 K for SiO2 (26), makes the deposition at Tsub near Tg accessible. The signatures of structural ordering, obtained from Raman spectroscopy, distinctively show that the arrangement of GeO4 tetrahedra into six-membered rings increases when depositing at elevated Tsubs. It is also demonstrated that the deposition near Tg is more efficient than postgrowth annealing in modifying the atomic structure at medium range. These structural modifications correlate with the room temperature internal friction of a-GeO2 thin films, which decreases by as much as 44% when the film is deposited at 0.83 Tg. In combination, the results demonstrate a strong correlation between medium-range order and room temperature internal friction as predicted by theory.
RESULTS
The physical vapor deposition of amorphous oxides is characterized by hit-and-stick processes in which the atomic relaxation and formation of a more stable structure are constrained when Tsub is low. Elevated Tsub and postdeposition annealing alter the organizational state of the deposited glasses by enabling the system to explore nearby lower minima in the energy landscape through atomic rearrangements. For a-GeO2 that has strong directional covalent bonding, the most dominant structural order is at the medium range, which can be defined in terms of the connection of the GeO4 tetrahedra (27–29). The (Ge-O-Ge) connection chain has a ring shape with a size that is determined by the number of Ge atoms within the closed path. Rings of various sizes ranging from 3 to 10 with a maximum distribution of around six-membered rings are predicted by models of a-GeO2 (30). This structural information can be obtained from x-ray and neutron diffraction in combination with modeling (30, 31) or alternatively from Raman spectroscopy that is sensitive to local vibrations at the medium range in glasses.
The Raman spectra of a-GeO2 thin films, shown in Fig. 1, are characterized by strong peaks at around 430 and 510 cm−1. The former corresponds to the symmetric stretching of bridging oxygen in six-membered rings (A6) (32). The latter corresponds to the oxygen-breathing mode associated with three-membered rings (A3) (33, 34). Two Raman bands at 560 and 595 cm−1 assigned to the transverse optical and longitudinal optical asymmetric stretching of bridging oxygen, respectively (fig. S1), overlap with A3. This overlap introduces substantial uncertainty in the fitting of the A3 peak at 510 cm−1. The integrated A6 area was normalized to the total integrated area of the Raman spectrum between 200 and 700 cm−1 to evaluate the change in the population of six-membered rings. Figure 1 qualitatively contrasts the difference in the ring distribution of a-GeO2 samples deposited at Tsub = room temperature and Tsub = 0.83 Tg, which indicates that high-temperature deposition favors a larger fraction of six-membered rings, i.e., a more ordered structure, as described below.
Fig. 1. Raman scattering spectra of a-GeO2 thin films deposited at different temperatures.

Tsub = room temperature (r.t.; green) and Tsub = 0.83 Tg (purple). The A6 peak at around 430 cm−1 corresponds to the symmetric stretching of bridging oxygen in six-membered rings. The A3 peak at around 510 cm−1 corresponds to the breathing motion of bridging oxygen in three-membered rings. The peak at 337 cm−1 is assigned to the Ge “deformation” motion within the network (33, 34).
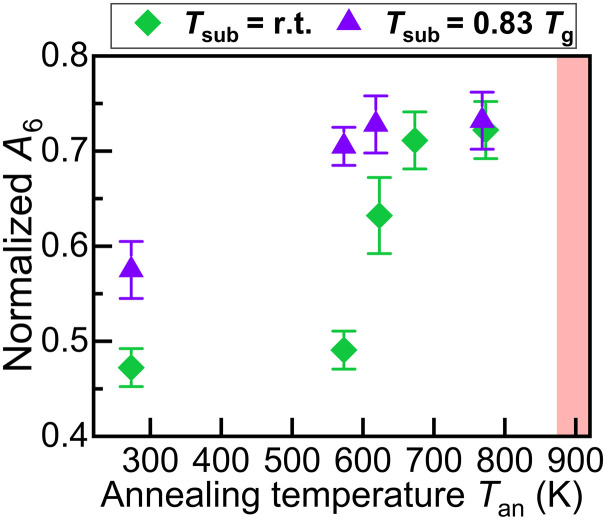
We focus first on the evolution of the ring distribution in a-GeO2 deposited at Tsub = room temperature as the system approaches lower energy states with postdeposition annealing, visualized as changes in normalized A6 in Fig. 2. A6 does not significantly vary after the first annealing step at an annealing temperature (Tan) = 573 K. A6 shows a pronounced increase of 29% to 0.63 ± 0.04 after annealing at Tan = 623 K. Annealing to higher temperature continues to relax the atomic structure, leading to an increase of A6 to 0.72 ± 0.03. Overall, the trend indicates that the population of large six-membered rings increases. The breakup of small (three- or four-membered) rings, qualitatively identified by a reduction in the A3 peak intensity, could be attributed to heavily strained intertetrahedral bridging bonds (35, 36). The increase in the population of six-membered rings indicates that an increased medium-range order is achieved in a-GeO2 thin films upon annealing.
Fig. 2. Structural relaxation in a-GeO2 thin films identified by changes in normalized A6 versus Tan.
Samples deposited at Tsub = room temperature and Tsub = 0.83 Tg are represented by green rhomboids and purple triangles, respectively. The temperature on each annealing step was held for 10 hours. The red shaded region in the figure indicates the start of crystallization at around 873 K for all samples.
Deposition at elevated Tsub brings more notable changes to the ring distribution in a-GeO2, as shown in Fig. 2. A6 is 0.58 ± 0.03 for the sample deposited at Tsub = 0.83 Tg and 0.47 ± 0.02 for the sample deposited at Tsub = room temperature. The larger fraction of six-membered rings confirms the formation of a more ordered atomic structure at elevated Tsub. Moreover, the results show that the Tan required to achieve a high medium-range order before crystallization, defined by A6 ~ 0.71, is lower at Tan = 573 K, when a-GeO2 is deposited at Tsub = 0.83 Tg, in comparison to Tan = 673 K for the a-GeO2 deposited at Tsub = room temperature. Further annealing of the sample deposited at Tsub = 0.83 Tg to Tan = 773 K resulted in increased normalized A6 ~ 0.72. All samples remained amorphous after annealing at Tan = 773 K for 10 hours (figs. S2 and S3). During room temperature deposition, incident sputtered particles rapidly lose their energy to the substrate before exploring the entire configuration space (37), whereas elevated Tsub promotes a larger sampling that leads to a higher degree of organization. It is through elevated Tsub that vapor-deposited glasses are able to achieve a more ordered structural organization (9, 38, 39).
The structural rearrangements at medium range play a role in lowering the potential energy of the glass system and are strongly dependent on the Tsub, as recently shown in molecular dynamic simulations of vapor-deposited a-SiO2 films (39). In comparison to a-SiO2 films deposited at Tsub well below Tg, the films with lower potential energy prepared at the optimal Tsub near Tg have a higher fraction and a narrower distribution of rings centered at six-membered rings, suggesting a greater structural uniformity.
The changes in the medium-range order of a-GeO2 and their impact in modifying thermally activated TLSs are assessed from the room temperature internal friction Q−1 of thin films deposited at different Tsub and postdeposition annealed. Q−1 will refer to internal friction at room temperature unless otherwise noted. Previous modeling and experimental results of amorphous oxides, such as a-Ta2O5, have suggested that an increased medium-range order correlates with the reduction in Q−1 (40, 41) at room temperature. The evolution of Q−1 for a-GeO2 deposited at Tsub = room temperature with annealing is shown in Fig. 3A. The as-prepared a-GeO2 thin film has a Q−1 of (2.98 ± 0.27) × 10−4, which reduces to (2.56 ± 0.52) × 10−4 after the first annealing step at Tan = 573 K. A significant decrease by 49% is obtained after the Tan = 623 K annealing step. Notably, the Tan after which Q−1 undergoes a sharp decrease, coincides with the turning point in the increase of normalized A6 in Fig. 2. Beyond Tan = 673 K, Q−1 plateaus at a value of (1.00 ± 0.13) × 10−4.
Fig. 3. Room temperature internal friction Q−1 of a-GeO2 thin films deposited at different temperatures.
(A) Tsub = room temperature (green), (B) Tsub = 0.60 Tg (orange), and (C) Tsub = 0.83 Tg (purple). The Tan to reach the lowest room temperature internal friction is Tan = 673 K, Tan = 623 K, and Tan = 573 K for samples deposited at Tsub = room temperature (green), Tsub = 0.60 Tg (orange), and Tsub = 0.83 Tg (purple), respectively.
Comparison of Q−1 for as-prepared a-GeO2 deposited at Tsub = room temperature, Tsub = 0.60 Tg, and Tsub = 0.83 Tg shows a steady decrease with an increase in Tsub. The a-GeO2 sample deposited at Tsub = 0.83 Tg has the lowest Q−1 = (1.66 ± 0.14) × 10−4, which is 44% less than Q−1 of the sample deposited at room temperature. Figure 3 shows a reduction in Q−1 with Tan for all samples, although the rate at which Q−1 reaches its minimum value is different for each one. The a-GeO2 thin film prepared at Tsub = 0.60 Tg reaches Q−1 = 1.00 × 10−4 after annealing at Tan = 623 K, yet the one prepared at Tsub = 0.83 Tg achieves this Q−1 after annealing at Tan = 573 K. Annealing beyond Tan = 623 K does not decrease Q−1 below 1.00 × 10−4 for the high-temperature deposited samples. It is also worth noting that the a-GeO2 thin film deposited at Tsub = 0.60 Tg (473 K) without thermal treatment has Q−1 = (2.39 ± 0.15) × 10−4, which is comparable to Q−1 = (2.56 ± 0.52) × 10−4 for the sample deposited at room temperature and annealed at Tan = 573 K for 10 hours. Considering that the same level Q−1 is obtained at a lower Tan during a significantly shorter deposition time of around 1 hour compared to annealing for 10 hours, it indicates a higher efficiency of elevated temperature deposition over annealing for reducing Q−1 of a-GeO2 thin films.
The inverse relationship of Q−1 with normalized A6 in Fig. 4 demonstrates a strong and direct correlation between lowering Q−1 and increasing medium-range order characterized by a larger fraction of six-membered rings in a-GeO2. Such observation echoes what has been found for vapor-deposited a-SiO2 (42), where a steady decrease in the population of three-membered rings is linked to the reduction in Q−1 during extended annealing. Similar behavior has also been observed in (43), in which analyses of grazing-incidence pair distribution functions of ZrO2-doped Ta2O5 reveal a systematic change in the medium-range order with Tan. Modeling shows that the atomic rearrangements that occur at medium range involve a decrease in the population of edge- and face-sharing polyhedra and an increase in corner-sharing ones, which agree with an expansion in the polyhedral ring connection. These modifications correlate with a steady decrease in Q−1 at room temperature. Further evidence directly linking the structural changes with TLSs for ZrO2-doped Ta2O5 is obtained from (44). In this work, the energy landscape of ZrO2-doped Ta2O5 was explored by searching for TLSs using molecular dynamic simulations. It is found that the TLSs can be sorted into two types depending on whether the cation-oxygen bond within the polyhedron breaks, namely, cage-breaking and non–cage-breaking transitions. The simulations show that a significant number of TLSs from cage-breaking events are responsible for room temperature Q−1 in the amorphous oxide. Elimination of these transitions by expanding the polyhedral connections, i.e., increasing the ring size, would thus result in lower Q−1. In combination, these theories provide a consistent interpretation of the correlation between Q−1 and medium-range order observed in a-GeO2 thin films.
Fig. 4. Room temperature internal friction Q−1 as a function of normalized A6 for a-GeO2 thin films deposited at different Tsub and subsequently annealed.
Samples deposited at Tsub = room temperature and Tsub = 0.83 Tg are represented by green and purple marks, respectively.
DISCUSSION
The results conclusively show that the atomic organization of strong network-forming a-GeO2 can be modified with elevated temperature deposition, similar to the behavior observed in organic glasses (3–8). The changes in the atomic arrangement are characterized by an increase in the fraction of six-membered rings. This structural reorganization corresponds to an increase in medium-range order, which is more notable when a-GeO2 is deposited at Tsub = 0.83 Tg compared to films deposited at Tsub = room temperature and subsequently annealed.
Furthermore, the results establish a strong correlation between medium-range order and room temperature internal friction in a-GeO2. The more ordered atomic arrangement leads to a reduction in room temperature internal friction of as much as 44% when the film is deposited at Tsub = 0.83 Tg compared to Tsub = room temperature. Under optimum deposition and annealing conditions, the room temperature internal friction of a-GeO2 reaches a value of Q−1 = 1.00 × 10−4, which is among the lowest in amorphous oxides and comparable to vapor-deposited a-SiO2 (42). These findings are relevant for identifying suitable amorphous oxide candidates for mirror coatings of the end test masses of gravitational wave interferometers, in which the coating’s room temperature internal friction is the main source of noise limiting their sensitivity.
MATERIALS AND METHODS
a-GeO2 thin films were deposited with a 4Wave Laboratory Alloy and Nanolayer System physical vapor deposition system (45, 46). A gridless ion source was used to generate low-energy Ar ions that were accelerated toward a high-purity Ge target negatively biased at 800 V. This created a sputter plume that deposited onto the substrate. Oxidation was achieved by flowing 6 standard cubic centimeters per minute of oxygen near the substrate surface. For high-temperature deposition, a heating lamp was positioned under the rotating substrate stage. The stage temperature was increased to the set point 10 min before deposition for equilibration purposes and maintained through the process. The rate for room temperature and high-temperature deposition was kept at 1.1 and 1.2 Å/s, respectively. The use of approximately the same deposition rate ruled out the influence of this parameter in atomic reorganization. Thermal annealing of each sample after deposition was carried out under ambient conditions by ramping up the temperature by 100 K/hour to Tan, holding at Tan for 10 hours, and ramping down at 100 K/hour to room temperature.
Raman scattering was performed with a HORIBA LabRAM HR Evolution Spectrometer. A frequency-doubled Nd:YAG laser of 532-nm wavelength and 10-mW average power was used for excitation. The laser beam was focused onto the sample’s surface using a ×100 objective. Three spectra of 60-s acquisition time were collected for each sample and averaged to improve the signal to noise. Deconvolution of the peaks in the Raman spectrum was carried out by fitting the peaks with Gaussian line shapes. The Raman spectrum contains a peak at 337 cm−1 that corresponds to the Ge motion within the network; A6 that corresponds to six-membered rings at 430 cm−1 (table S1); and the broad feature composed of the dominant A3 peak at 510 cm−1 associated with three-membered rings and peaks at 560 and 595 cm−1 associated with the Ge-O-Ge bending modes. In the fitting, the full width at half maximum of A3 was constrained to 75 cm−1 (47). The overlap of A3 with the peaks at 560 and 595 cm−1 produces a high uncertainty in the calculation of the A3 area. It is for this reason that we use the area of A6 normalized to the area of the spectrum from 200 to 700 cm−1 to represent changes in the population of six-membered rings.
Room temperature internal friction (Q−1) of the a-GeO2 thin films was measured with a ring down system (48, 49). The ~500-nm-thick a-GeO2 samples for this purpose were deposited on high-quality fused silica disk of 75-mm diameter and 1-mm thickness. A gentle nodal suspension method was used to support the sample inside a vacuum chamber held at a pressure below 10−6 Torr. After exciting the resonant mode, the oscillation amplitude at each frequency fi was tracked to obtain the decay time τi. The room temperature internal friction Qi−1 for each mode was then computed through the following relation
At each frequency, eight measurements were performed to obtain an averaged Qi−1. A mean value of Q−1 for each sample was then obtained by averaging the Qi−1 over a frequency range of 1 to 20 kHz.
Rutherford backscattering spectrometry (RBS) analyses were performed using He+ ions at 2.035 MeV with a 1.7-MV Tandetron accelerator. Spectra were acquired with samples on Si substrates tilted at 7° to minimize channeling. The scattered ions were collected at an angle of 10° from the beam. The experimental spectra were simulated using the SIMNRA software (50) to extract the composition and areal atomic density of the deposited layers. All samples were stoichiometric after annealing at 773 K (table S2).
Acknowledgments
The Raman scattering was performed at the Raman Microspectroscopy Laboratory in the Department of Geological Science at the University of Colorado-Boulder. Funding: This study was supported by the National Science Foundation (NSF) LIGO program through grant nos. 1710957 (L.Y. and C.S.M.) and 1708010 (M.F. and C.S.M.), NSF awards PHY-1707866 and PHY-1708175 (A.M., R.B., and M.M.F.), GBMF grant no. 6793 (A.M., K.P., R.B., and M.M.F.), FRQNT through the Regroupement québécois sur les matériaux de pointe (RQMP), and Natural Sciences and Engineering Research Council of Canada (M.C. and F.S.). Author contributions: L.Y., M.F., and C.S.M. conceived the experiment. L.Y. and M.F. performed the thin-film deposition and material spectroscopy analysis. G.V., A.A., and G.B. performed the room temperature internal friction measurements. A.M., R.B., K.P., and M.M.F. contributed to the XRD measurements. M.C. and F.S. contributed to the RBS measurements. All authors contributed to the preparation of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Section S1
Figs. S1 to S3
Tables S1 and S2
REFERENCES AND NOTES
- 1.Debenedetti P. G., Stillinger F. H., Supercooled liquids and the glass transition. Nature 410, 259–267 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Ediger M. D., Perspective: Highly stable vapor-deposited glasses. J. Chem. Phys. 147, 210901 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Dalal S. S., Walters D. M., Lyubimov I., de Pablo J. J., Ediger M. D., Tunable molecular orientation and elevated thermal stability of vapor-deposited organic semiconductors. Proc. Natl. Acad. Sci. U.S.A. 112, 4227–4232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalal S. S., Ediger M. D., Molecular orientation in stable glasses of indomethacin. J. Phys. Chem. Lett. 3, 1229–1233 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Bagchi K., Ediger M. D., Controlling structure and properties of vapor-deposited glasses of organic semiconductors: Recent advances and challenges. J. Phys. Chem. Lett. 11, 6935–6945 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Swallen S. F., Kearns K. L., Mapes M. K., Kim Y. S., McMahon R. J., Ediger M. D., Wu T., Yu L., Satija S., Organic glasses with exceptional thermodynamic and kinetic stability. Science 315, 353–356 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Bishop C., Gujral A., Toney M. F., Yu L., Ediger M. D., Vapor-deposited glass structure determined by deposition rate-substrate temperature superposition principle. J. Phys. Chem. Lett. 10, 3536–3542 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Gujral A., O’Hara K. A., Toney M. F., Chabinyc M. L., Ediger M. D., Structural characterization of vapor-deposited glasses of an organic hole transport material with X-ray scattering. Chem. Mater. 27, 3341–3348 (2015). [Google Scholar]
- 9.Singh S., Ediger M. D., de Pablo J. J., Ultrastable glasses from in silico vapour deposition. Nat. Mater. 12, 139–144 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Phillips W. A., Tunneling states in amorphous solids. J. Low Temp. Phys. 7, 351–360 (1972). [Google Scholar]
- 11.Rau S., Enss C., Hunklinger S., Neu P., Würger A., Acoustic properties of oxide glasses at low temperatures. Phys. Rev. B 52, 7179–7194 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Castañeda T., Rodríguez-Tinoco C., Rodríguez-Viejo J., Ramos M. A., Suppression of tunneling two-level systems in ultrastable glasses of indomethacin. Proc. Natl. Acad. Sci. U.S.A. 111, 11275–11280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Queen D. R., Liu X., Karel J., Metcalf T. H., Hellman F., Excess specific heat in evaporated amorphous silicon. Phys. Rev. Lett. 110, 135901 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Queen D. R., Liu X., Karel J., Jacks H. C., Metcalf T. H., Hellman F., Two-level systems in evaporated amorphous silicon. J. Non Cryst. Solids 426, 19–24 (2015). [Google Scholar]
- 15.Vajente G., Birney R., Ananyeva A., Angelova S., Asselin R., Baloukas B., Bassiri R., Billingsley G., Fejer M. M., Gibson D., Godbout L. J., Gustafson E., Heptonstall A., Hough J., MacFoy S., Markosyan A., Martin I. W., Martinu L., Murray P. G., Penn S., Roorda S., Rowan S., Schiettekatte F., Shink R., Torrie C., Vine D., Reid S., Adhikari R. X., Effect of elevated substrate temperature deposition on the mechanical losses in tantala thin film coatings. Class. Quantum Gravity 35, 075001 (2018). [Google Scholar]
- 16.LIGO Scientific Collaboration, Instrument Science White Paper 2020 (2020); https://dcc.ligo.org/LIGO-T2000407/public.
- 17.Gilroy K. S., Phillips W. A., An asymmetric double-well potential model for structural relaxation processes in amorphous materials. Philos. Mag. B 43, 735–746 (1981). [Google Scholar]
- 18.Hamdan R., Trinastic J. P., Cheng H. P., Molecular dynamics study of the mechanical loss in amorphous pure and doped silica. J. Chem. Phys. 141, 054501 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Levin Y., Internal thermal noise in the LIGO test masses: A direct approach. Phys. Rev. D 57, 659–663 (1998). [Google Scholar]
- 20.Miller J., Barsotti L., Vitale S., Fritschel P., Evans M., Sigg D., Prospects for doubling the range of Advanced LIGO. Phys. Rev. D 91, 062005 (2015). [Google Scholar]
- 21.Vajente G., Gustafson E. K., Reitze D. H., Precision interferometry for gravitational wave detection: Current status and future trends. Adv. Atom. Mol. Opt. Phys. 68, 75–148 (2019). [Google Scholar]
- 22.Granata M., Amato A., Cagnoli G., Coulon M., Degallaix J., Forest D., Mereni L., Michel C., Pinard L., Sassolas B., Teillon J., Progress in the measurement and reduction of thermal noise in optical coatings for gravitational-wave detectors. Appl. Optics 59, A229–A235 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Birney R., Steinlechner J., Tornasi Z., MacFoy S., Vine D., Bell A. S., Gibson D., Hough J., Rowan S., Sortais P., Sproules S., Tait S., Martin I. W., Reid S., Amorphous silicon with extremely low absorption: Beating thermal noise in gravitational astronomy. Phys. Rev. Lett. 121, 191101 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Miranda C. R., Antonelli A., Transitions between disordered phases in supercooled liquid silicon. J. Chem. Phys. 120, 11672–11677 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Brüning R., Crowell T., A method to determine the kinetics of a supercooled liquid by temperature scanning measurements applied to (Li, Na)acetate and GeO2. J. Non Cryst. Solids 248, 183–193 (1999). [Google Scholar]
- 26.Brüning R., On the glass transition in vitreous silica by differential thermal analysis measurements. J. Non Cryst. Solids 330, 13–22 (2003). [Google Scholar]
- 27.Elliott S. R., Medium-range structural order in covalent amorphous solids. Nature 354, 445–452 (1991). [Google Scholar]
- 28.Micoulaut M., Phillips J. C., Rings and rigidity transitions in network glasses. Phys. Rev. B 67, 104204 (2003). [Google Scholar]
- 29.Geissberger A. E., Galeener F. L., Raman studies of vitreous SiO2 versus fictive temperature. Phys. Rev. B 28, 3266–3271 (1983). [Google Scholar]
- 30.Kohara S., Suzuya K., Intermediate-range order in vitreous SiO2 and GeO2. J. Phys. Condens. Matter 17, S77–S86 (2005). [Google Scholar]
- 31.Shi R., Tanaka H., Distinct signature of local tetrahedral ordering in the scattering function of covalent liquids and glasses. Sci. Adv. 5, eaav3194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durben D. J., Wolf G. H., Raman spectroscopic study of the pressure-induced coordination change in GeO2 glass. Phys. Rev. B 43, 2355–2363 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Micoulaut M., Cormier L., Henderson G. S., The structure of amorphous, crystalline and liquid GeO2. J. Phys. Condens. Matter 18, R753–R784 (2006). [Google Scholar]
- 34.Henderson G. S., Neuville D. R., Cochain B., Cormier L., The structure of GeO2–SiO2 glasses and melts: A Raman spectroscopy study. J. Non Cryst. Solids 355, 468–474 (2009). [Google Scholar]
- 35.Hosono H., Ikuta Y., Kinoshita T., Kajihara K., Hirano M., Physical disorder and optical properties in the vacuum ultraviolet region of Amorphous SiO2. Phys. Rev. Lett. 87, 175501 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Song W., Li X., Wang B., Anoop Krishnan N. M., Goyal S., Smedskjaer M. M., Mauro J. C., Hoover C. G., Bauchy M., Atomic picture of structural relaxation in silicate glasses. Appl. Phys. Lett. 114, 233703 (2019). [Google Scholar]
- 37.Kaiser N., Review of the fundamentals of thin-film growth. Appl. Optics 41, 3053–3060 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Berthier L., Charbonneau P., Flenner E., Zamponi F., Origin of ultrastability in vapor-deposited glasses. Phys. Rev. Lett. 119, 188002 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Du T., Anoop Krishnan N. M., Smedskjaer M. M., Bauchy M., On the equivalence of vapor-deposited and melt-quenched glasses. J. Chem. Phys. 152, 164504 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Hart M. J., Bassiri R., Borisenko K. B., Véron M., Rauch E. F., Martin I. W., Rowan S., Fejer M. M., MacLaren I., Medium range structural order in amorphous tantala spatially resolved with changes to atomic structure by thermal annealing. J. Non Cryst. Solids 438, 10–17 (2016). [Google Scholar]
- 41.Amato A., Terreni S., Granata M., Michel C., Sassolas B., Pinard L., Canepa M., Cagnoli G., Observation of a correlation between internal friction and Urbach energy in amorphous oxides thin films. Sci. Rep. 10, 1670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granata M., Coillet E., Martinez V., Dolique V., Amato A., Canepa M., Margueritat J., Martinet C., Mermet A., Michel C., Pinard L., Sassolas B., Cagnoli G., Correlated evolution of structure and mechanical loss of a sputtered silica film. Phys. Rev. Mater. 2, 053607 (2018). [Google Scholar]
- 43.Prasai K., Jiang J., Mishkin A., Shyam B., Angelova S., Birney R., Drabold D. A., Fazio M., Gustafson E. K., Harry G., Hoback S., Hough J., Lévesque C., MacLaren I., Markosyan A., Martin I. W., Menoni C. S., Murray P. G., Penn S., Reid S., Robie R., Rowan S., Schiettekatte F., Shink R., Turner A., Vajente G., Cheng H.-P., Fejer M. M., Mehta A., Bassiri R., High precision detection of change in intermediate range order of amorphous zirconia-doped tantala thin films due to annealing. Phys. Rev. Lett. 123, 045501 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Jiang J., Mishkin A., Prasai K., Zhang R., Yazback M., Bassiri R., Fejer M. M., Cheng H.-P., Analysis of two-level systems and mechanical loss in amorphous ZrO2-doped Ta2O5 by non-cage-breaking and cage-breaking transitions. J. Chem. Phys. 154, 174502 (2021). [DOI] [PubMed] [Google Scholar]
- 45.West K. G., Lu J., Yu J., Kirkwood D., Chen W., Pei Y., Claassen J., Wolf S., Growth and characterization of vanadium dioxide thin films prepared by reactive-biased target ion beam deposition. J. Vac. Sci. Technol. A 26, 133–139 (2008). [Google Scholar]
- 46.Yang L., Fazio M., Vajente G., Ananyeva A., Billingsley G., Markosyan A., Bassiri R., Fejer M. M., Menoni C. S., Structural evolution that affects the room-temperature internal friction of binary oxide nanolaminates: Implications for ultrastable optical cavities. ACS Appl. Nano Mater. 3, 12308–12313 (2020). [Google Scholar]
- 47.Giacomazzi L., Umari P., Pasquarello A., Medium-range structural properties of vitreous germania obtained through first-principles analysis of vibrational spectra. Phys. Rev. Lett. 95, 075505 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Vajente G., Ananyeva A., Billingsley G., Gustafson E., Heptonstall A., Sanchez E., Torrie C., A high throughput instrument to measure mechanical losses in thin film coatings. Rev. Sci. Instrum. 88, 073901 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Cesarini E., Lorenzini M., Campagna E., Martelli F., Piergiovanni F., Vetrano F., Losurdo G., Cagnoli G., A “gentle” nodal suspension for measurements of the acoustic attenuation in materials. Rev. Sci. Instrum. 80, 053904 (2009). [DOI] [PubMed] [Google Scholar]
- 50.M. Mayer, SIMNRA User’s Guide (Report IPP 9/113, Max-Planck-Institut für Plasmaphysik, 1997).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Section S1
Figs. S1 to S3
Tables S1 and S2