Abstract
Growth factors signaling through the phosphoinositide 3-kinase/Akt pathway promote cell survival. The mechanism by which the serine/threonine kinase Akt prevents cell death remains unclear. We have previously shown that Akt inhibits the activity of DEVD-targeted caspases without changing the steady-state levels of Bcl-2 and Bcl-xL. Here we show that Akt inhibits apoptosis and the processing of procaspases to their active forms by delaying mitochondrial changes in a caspase-independent manner. Akt activation is sufficient to inhibit the release of cytochrome c from mitochondria and the alterations in the inner mitochondrial membrane potential. However, Akt cannot inhibit apoptosis induced by microinjection of cytochrome c. We also demonstrated that Akt inhibits apoptosis and cytochrome c release induced by several proapoptotic Bcl-2 family members. Taken together, our results show that Akt promotes cell survival by intervening in the apoptosis cascade before cytochrome c release and caspase activation via a mechanism that is distinct from Bad phosphorylation.
Apoptosis is an essential process for the development and tissue homeostasis of most multicellular organisms. Deregulation of apoptosis has been implicated in the pathogenesis of many disease states, including neurodegenerative disorders and cancer (37). One mechanism by which apoptosis is regulated is through the availability of mediators of survival signals, such as certain growth factors and cytokines and cell-matrix contact (32, 33).
The activation of growth factor receptors leads to multiple intracellular effects, including alterations in gene expression, protein synthesis, mitogenesis, cytoskeletal rearrangements, differentiation, and cell survival. The pathway leading to the activation of phosphoinositide 3-kinase (PI 3-kinase) and its downstream effector the serine/threonine kinase Akt (also called protein kinase B [PKB]) by growth factor receptors has emerged as the major mechanism by which growth factors promote cell survival (reviewed in reference 28). Upon activation, PI 3-kinase phosphorylates membrane phosphoinositides at the D-3 position. These phospholipids act as second messengers that mediate the diverse cellular functions of PI 3-kinase (13). One function of these second messengers is the activation of Akt. The amino terminus of Akt contains a pleckstrin homology domain that is thought to directly bind the phospholipid products of PI 3-kinase. This binding recruits Akt to the membrane and induces a conformational change that allows the phosphorylation of Akt by the phosphoinositide-dependent kinases I (PDKI) and PDKII at residues Thr308 and Ser473, respectively. Phosphorylation of Akt results in full activation of Akt kinase activity and the subsequent regulation of multiple cellular processes, including the transmission of growth factor-dependent survival signal (reviewed in references 7 and 28). Recent genetic studies in Drosophila melanogaster have corroborated these results and have suggested an important in vivo role for Akt in the regulation of apoptosis (3, 36).
Although Akt promotes cell survival in a variety of cell types and blocks apoptosis induced by diverse apoptotic stimuli, the step at which Akt abrogates the apoptotic cascade is not known. Because Akt is blocking apoptosis both in mammalian cells and in Drosophila, it is likely that the mechanism by which Akt intervenes in the apoptotic cascade is evolutionarily conserved.
The apoptotic cascade in mammalian cells is a multistep process (reviewed in reference 15). In most cases, the apoptotic cascade is initiated by loss of integrity of the outer mitochondrial membrane accompanied by release of cytochrome c. Following mitochondrial release, cytochrome c is thought to bind and induce a conformational change in the apoptotic protease activating factor (Apaf-I) that results in the cleavage and activation of caspase-9 and a subsequent activation of a caspase cascade that executes the cell death (reviewed in reference 38). However, the mechanism of cytochrome c translocation from mitochondria into the cytosol and the regulation of this translocation remained unclear.
The critical regulators of the apoptosis cascade are the Bcl-2 family members. These family members include antiapoptotic proteins such as Bcl-2 and Bcl-xL and proapoptotic members such as Bad, Bid, and Bik (the BH3 subfamily) and Bax, and Bak (the Bax subfamily) (reviewed in references 1 and 23). Many members of this family, such as Bcl-2 and Bcl-xL, are predominantly localized to the outer membrane of mitochondria, while others interact with mitochondria indirectly. For example, the proapoptotic protein Bad does not contain a mitochondrial targeting sequence but rather localizes to mitochondria, in a phosphorylation-dependent manner, by heterodimerization with other Bcl-2 family members (42, 44, 47). The mechanism by which the Bcl-2 family members regulate cell death remains unclear but may involve regulation of mitochondrial integrity (23). Bcl-2 family members may function as ion channels that regulate the osmotic balance of mitochondria and the release of apoptogenic molecules from mitochondria such as cytochrome c. Alternatively, Bcl-2/Bcl-xL may function by binding and sequestering Apaf-I from interacting with caspase-9, thus preventing Apaf-I–caspase-9 complex (apoptosome) formation and subsequent caspase activation (reviewed in reference 15).
Elucidation of the mechanism by which Akt intervenes in the apoptotic cascade is important not only for determining growth factor-mediated cell survival but also for understanding the genesis of a wide range of human cancers. This is in light of the observation that the PTEN phosphatase tumor suppressor implicated in a wide range of human cancers is a negative regulator of Akt (16, 27, 35, 43).
It was suggested that Akt inhibits apoptosis, at least in part, by phosphorylating and inactivating the proapoptotic Bcl-2 family member, Bad (8, 31). Here we provide evidence for a more general mechanism by which Akt inhibits apoptosis that is not dependent on Bad phosphorylation. We show that Akt promotes cell survival by maintaining mitochondrial integrity and by inhibiting the release of cytochrome c and alterations in mitochondrial membrane potential induced by multiple apoptotic stimuli, in a caspase-independent manner.
MATERIALS AND METHODS
Retroviral vectors.
The puromycin resistance gene of pBabePuro was replaced with the enhanced green fluorescence protein (eGFP) gene to construct pBabe(eGFP) (10). pSSFV Bad and pSSFV Bad112/136 (gift from Stanley Korsmeyer) were digested with EcoRI, and Bad coding sequences were inserted into the EcoRI site of pBabe(eGFP). pSSFV murine Bax (gift from Craig Thompson, University of Chicago) was digested with EcoRI, and Bax was inserted into the EcoRI site of pBabe(eGFP). pCDNA3 HA-Bak and HA-Bik (provided by G. Chinnandurai, St. Louis University, St. Louis, Mo.) were digested with HindIII and EcoRI and inserted into the BamHI and EcoRI sites of pBabe(eGFP).
Cell culture and retrovirus production.
Cell culture and quantitation of apoptosis by 4′,6-diamidino-2-phenylindole (DAPI) staining were performed as previously described (20). For production of high-titer retroviruses expressing proapoptotic proteins, Phoenix ecotropic packaging cells were plated at approximately 50 to 75% confluency in 15-cm-diameter tissue culture plates and transfected by a modified calcium phosphate method. After 2 days, viral supernatant was collected and passed through a 0.22-mm-pore-size filter. Virus was added to cells in the presence of Polybrene (8 μg/ml). Infected cells were visualized under an inverted fluorescence microscope. Infected populations exhibiting between 85 and 95% green cells were used as polyclonal cell line for further experimentation.
Antibodies.
Bad was detected with a hamster monoclonal anti-Bad antibody (product no. 13361S, PharMingen, La Jolla, Calif.) used at a concentration of 1 μg/ml. Cytochrome c and cytochrome c oxidase (COX) subunit IV were detected with anti-cytochrome c clone 7H8.2C12 (1 μg/ml; PharMingen) and anti-COX clone 20E8-C12 (1 μg/ml; Molecular Probes), respectively. Biotinylated proteins were detected with horseradish peroxidase-conjugated strepavidin (Zymed, San Francisco, Calif.) used at a concentration of 1 μg/ml.
Cytochrome c immunostaining.
Rat1a cells were plated at a density of 125,000 cells per 3-cm-diameter dish on glass coverslips, fixed in phosphate-buffered saline (PBS) containing 4% formaldehyde and 0.2% saponin, and then stained with Hoechst 33258 (1 μg/ml) for 20 min. The fixed cells were incubated in blocking buffer (PBS containing 10% fetal calf serum [FCS] and 0.2% Triton X-100) for 30 min and then for additional 30 min in PBS containing 0.2% saponin, 2% bovine serum albumin (BSA), and 1 μg of anti-cytochrome c antibody (clone 6H2.B4; PharMingen) per ml. Cells were then washed three times in blocking buffer and incubated for 30 min in PBS containing 2% BSA, 0.2% saponin, and 1 μg of tetramethylrhodamine isothiocyanate-conjugated anti-mouse antibody (Jackson ImmunoResearch, West Grove, Pa.) per ml. Cells were then rinsed three times in blocking buffer, and coverslips were mounted onto slides.
Microinjection.
Cells were microinjected on a Leica DMIRB inverted microscope using an Eppendorf (Madison, Wis.) micromanipulator (5171) and Transjector (5246) microinjection system. Injection needles (femtotips) were purchased from Eppendorf (product no. 5242 942.008). Rat1a cells were plated on glass coverslips at a density of 125,000 cells per 3-cm-diameter dish. Cells were injected (pressure, 100 hPa; time, 0.3 s) with a buffer containing 50 mM HEPES (pH 7.5), 50 mM NaCl, 0.2% Texas red (Mr, 10,000; lysine fixable; (Molecular Probes), and either BSA (1 mg/ml; FisherBiotech BP1600-100) or cytochrome c (1 mg/ml; Sigma C7752). Following microinjection, coverslips were fixed for 3 min with PBS containing 4% formaldehyde and the stained with Hoechst (1 μg/ml).
Subcellular fractionation.
Cells were scraped into 1 ml of Mito buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 10 μM phenylmethylsulfonyl fluoride, 1× complete protease inhibitors [product no. 1697498, Boehringer, Mannheim, Germany]). Cells were then mechanically lysed at 4°C with 40 strokes of a cell homogenizer (outer diameter, 0.1575 in.; inner diameter, 0.1560 in.). The cell lysate was then spun at 1,000 × g for 10 min, and the supernatant was further spun at 13,000 × g for an additional 20 min. This pellet was resuspended in 100 μl of Laemmli buffer and termed the mitochondrial pellet. The supernatant was incubated on ice with 20% trichloroacetic acid to precipitate cytochrome c. After 30 min, the supernatant was spun at 13,000 × g for 20 min; the pellet was then rinsed in 1 ml of diethyl ether on ice and respun at 13,000 × g for 10 min. The diethyl ether was removed, and pellet was allowed to dry on ice. The pellet was then resuspended in 70 μl of Laemmli buffer and termed the supernatant.
DiOC6 labeling and FACS analysis.
Cells were loaded with 50 nM 3′,3′-dihexyloxacarbo-cyanine iodide (DiOC6) at 37°C for 10 min. Floating cells were collected, and attached cells were trypsinized and resuspended in PBS. Cells were spun at 3,000 × g, and fractions were pooled and rinsed in PBS. Cells were resuspended in 1 ml of ice-cold PBS and subjected to fluorescence-activated cell sorting (FACS) analysis.
RESULTS
Akt inhibits UV light-mediated cell death and caspase activation in Rat1a cells.
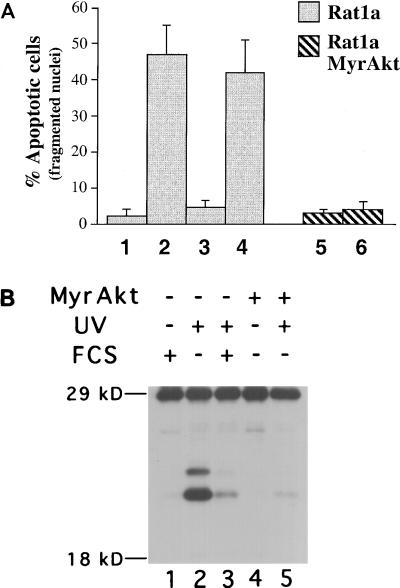
To further understand the mechanism by which Akt mediates cell survival, we examined the ability of Akt to inhibit UV light-induced cell death. Rat1a cells were placed in Dulbecco modified Eagle medium (DMEM) containing no serum and then irradiated with UV light (50 Jo/m2). The percentage of apoptotic cells was assessed 5 h following UV irradiation by staining with DAPI as previously described (20). Fragmented and condensed nuclei were scored as apoptotic. We observed a marked acceleration of apoptosis following UV irradiation of Rat1a cells (Fig. 1A; P < 0.005). When 10% FCS was added to the cells, immediately after irradiation, there was a significant attenuation of this cell death (P < 0.005). Preincubation of Rat1a cells with the PI 3-kinase inhibitor wortmannin was sufficient to block the survival signal generated by FCS (Fig. 1A). These results implicate PI 3-kinase in transmitting the FCS-generated survival signal and are in agreement with previous results demonstrating that PI 3-kinase can transmit an antiapoptotic signal (19–21, 46).
FIG. 1.
Akt inhibits UV-mediated nuclei fragmentation, caspase activation, and apoptosis in Rat1a cells. (A) Percentage of apoptotic cells scored by DAPI staining of Rat1a (columns 1 to 4) and Rat1a/MyrAkt (columns 5 and 6) cells. Cells were either mock treated or treated with 200 nM wortmannin for 30 min (columns 4 and 6). Cells were then irradiated with UVC light (260 nm) at a dose of 50 Joules/m2 (lanes 2 to 6). Medium containing either 0% FCS (columns 1, 2, and 5 to 7) or 10% FCS (columns 3 and 4) was then added. After an additional 4.5 h, cells were DAPI stained and scored for nuclear condensation. Averages (± standard error) of at least 300 cells from three independent experiments are shown. (B) Akt inhibits the activation of at least two caspases during UV-mediated apoptosis. Rat1a (lanes 1 to 3) and Rat1a/MyrAkt (lanes 4 and 5) cells were deprived of serum for 2 h and irradiated with 50 J of UVC light. The medium was then changed to DMEM with 0% FCS (lanes 2, 4, and 5) or 10% FCS (lanes 1 and 3). After an additional 5 h, extracts were made and labeled with the biotin-conjugated YVAD-AMK (Calbiochem, La Jolla, Calif.). Extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel, and the Western blot was incubated with horseradish peroxidase-conjugated streptavidin (1 μg/ml).
To study the effect of Akt signaling on UV-induced cell death, we used a polyclonal Rat1a cell line that stably expressing an activated form of Akt consisting of the Src myristoylation signal fused to the c-Akt coding sequence (MyrAkt) (20). MyrAkt exhibits constitutive kinase activity independently of PI 3-kinase activity and growth factor stimulation (2, 14, 20). Rat1a cells expressing MyrAkt (Rat1a/MyrAkt cells) were significantly protected from UV-induced apoptosis even in the presence of wortmannin (Fig. 1A; P < 0.001). Thus, Akt activation is sufficient to protect Rat1a cells from UV-induced cell death, consistent with previous results (24).
We have previously established that Akt prevents the induction of caspase activity that cleaves poly(ADP-ribosyl) polymerase and a fluorescently conjugated DEVD substrate (20). Procaspases are converted from inactive zymogens to their active forms by proteolytic cleavage and assembly into tetrameric complexes (reviewed in reference 29). To determine whether Akt was blocking the activity or the processing and activation of the procaspases, we used an in vitro system for detecting and identifying cleaved and activated caspases. This system uses a nonhydrolyzable caspase recognition peptide tagged with both a fluoroacyloxymethylketone moiety (which covalently binds the target proteins) and a biotin tag which enables their detection by immunoblot analysis (11, 12). Extracts from Rat1a and Rat1a/MyrAkt cells were labeled and subjected to immunoblot analysis. After irradiation, we observed the appearance of two species of labeled proteins that migrated with mobility similar to that of activated caspases, at approximately 21 and 23 kDa, as previously described (11) (Fig. 1B, lane 2). FCS and MyrAkt were both sufficient to prevent the appearance of these bands (lanes 3 and 5). Using a fluorescently conjugated caspase substrate as previously described (20), we observed induction of caspase activity that is correlated with the appearance of the biotinylated bands (data not shown). These results suggest that Akt not only inhibits caspase activity but also blocks the processing of caspases from inactive zymogens to their active forms.
Akt inhibits the release of cytochrome c from mitochondria following UV irradiation.
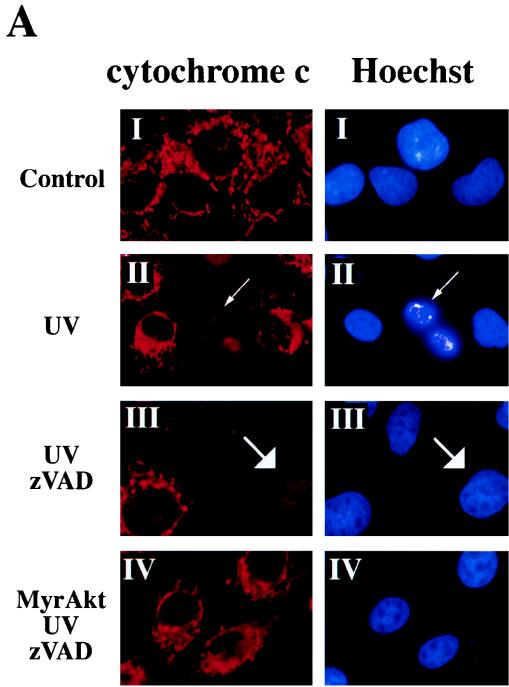
The major mechanism of processing and activation of procaspases in mammalian cells is mediated by the Ced-4 homologue Apaf-I. Apaf-I is activated by binding to cytochrome c which is released from mitochondria in the early stage of apoptosis (reviewed in reference 15). We therefore examined whether Akt is capable of regulating the release of cytochrome c from mitochondria. Immunofluorescence staining of Rat1a cells with an anti-cytochrome c antibody gave a punctate staining pattern characteristic of mitochondrial localization (Fig. 2A, panel I). The mitochondrial dyes DiOC6 and MitoTracker-CMXRos gave indistinguishable patterns of fluorescence (see below), suggesting that the cytochrome c antibody did indeed recognize cytochrome c within mitochondria. Treatment of Rat1a cells with UV light induced cytoplasmic shrinkage, nuclear fragmentation, chromatin condensation, and loss of mitochondrial cytochrome c staining (Fig. 2A, panel II). Quantitation of the percentage of cells with diffuse cytochrome c fluorescence showed a marked increase in cytochrome c release following UV irradiation (Fig. 2B; P < 0.001).
FIG. 2.
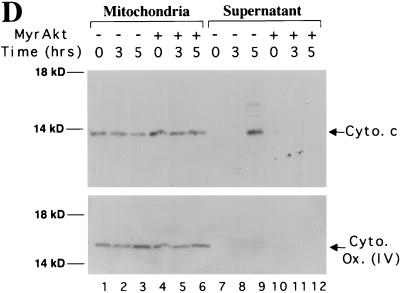
Akt prevents the release of cytochrome c from mitochondria in a caspase-independent manner. (A) Cytochrome c immunostaining and Hoechst staining of Rat1a and Rat1a/MyrAkt cells. Fixation and staining of the cells was done 3.5 h after treatment as described in Materials and Methods. (I) Rat1a cells were deprived of serum for 20 min. (II) Rat1a cells were deprived of serum for 20 min and then irradiated with UV light. Arrows indicates diffused cytochrome c staining and nucleus with condensed chromatin in a cell undergoing apoptosis. (III) Rat1a cells were pretreated with 100 mM zVAD for 20 min, deprived of serum, and UV irradiated. Arrows indicate diffused cytochrome c staining and the corresponding nucleus of a cell protected from apoptosis. (IV) Rat1a/MyrAkt cells were treated as for panel III. (B) Quantitation of cells with diffused cytochrome (cyto) c staining. Averages (± standard error) of at least 100 cells from three independent experiments are shown. (C) Preincubation of Ra1a cells with zVAD is sufficient to prevent apoptosis as measured by DAPI staining. Cells were deprived of serum for 20 min and then either mock treated or treated with 100 μM zVAD for 20 min followed by UV irradiation (50 J/m2), as indicated. After an additional 5 h, cells were DAPI stained and scored for nuclear condensation. Averages (± standard errors) of at least 300 cells from three independent experiments are shown. (D) Rat1a (lanes 1 to 3 and 7 to 9) and Rat1a/MyrAkt (lanes 4 to 6 and 10 to 12) cells were deprived of serum for 20 min and then irradiated with 50 J of UV light as described for panel A. Cells were mechanically lysed and separated into mitochondrial and supernatant fractions 0, 3, and 5 h following irradiation as described in Materials and Methods. Fractions were subjected to SDS-PAGE (15% gel) followed by immunoblot analysis. Cytochrome c (Cyto. c) and COX IV [Cyto. Ox. subunit (IV)] are indicated by arrows. Positions of molecular weight markers are indicated on the left.
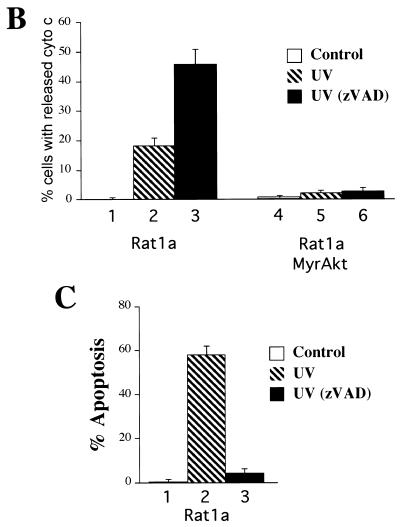
To determine whether the observed cytochrome c release is indeed the trigger of cell death or a consequence of caspase activation, we used the irreversible broad-range caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl-ketone (zVAD). Preincubation of Rat1a cells with zVAD was sufficient to prevent the fragmentation and condensation of nuclei following UV irradiation (Fig. 2C; P < 0.001). The release of cytochrome c from mitochondria, however, was not inhibited by zVAD (Fig. 2A, panel III; Fig. 2B). These results are consistent with previous results showing that translocation of cytochrome c from mitochondria is independent of caspase activity (4, 26, 40). A greater percentage of UV-irradiated Rat1a cells exhibited a loss of punctate cytochrome c immunostaining when pretreated with zVAD (Fig. 2B). This was not unexpected because zVAD prevented Rat1a cells from lifting off the coverslip following UV exposure (data not shown). In the absence of zVAD, many irradiated Rat1a cells had lifted off the coverslip and consequently were not scored. UV induced accumulation of cells with diffuse cytochrome c staining, and this was prevented by MyrAkt in the presence and in the absence of zVAD (Fig. 2A, panel IV; Fig. 2B, P < 0.002 and 0.001 respectively). These results suggest that Akt is sufficient to prevent the loss of punctate cytochrome c immunostaining immediately following UV irradiation.
To corroborate the immunofluorescence data, Rat1a and Rat1a/MyrAkt cells were mechanically lysed and the subcellular localization of cytochrome c was investigated following exposure to UV irradiation. Cytochrome c normally resides within mitochondria between the inner and outer membranes. In proliferating Rat1a cells, we observed cytochrome c in the mitochondrial fraction and not in the cytoplasmic fraction (Fig. 2D). The mitochondrial marker COX IV is also localized in the mitochondrial fraction and is absent from the cytoplasmic fraction. Following UV irradiation, cytochrome c translocated from the mitochondrial into the cytoplasmic fraction whereas COX IV remained in the mitochondrial fraction. In Rat1a/MyrAkt cells, cytochrome c did not translocate into the cytoplasmic fraction following UV irradiation (Fig. 2D). These results corroborate our immunofluorescence data and demonstrate that Akt is sufficient to maintain cytochrome c within mitochondria following UV irradiation of Rat1a cells.
Akt prevents the release of cytochrome c induced by multiple apoptotic stimuli.
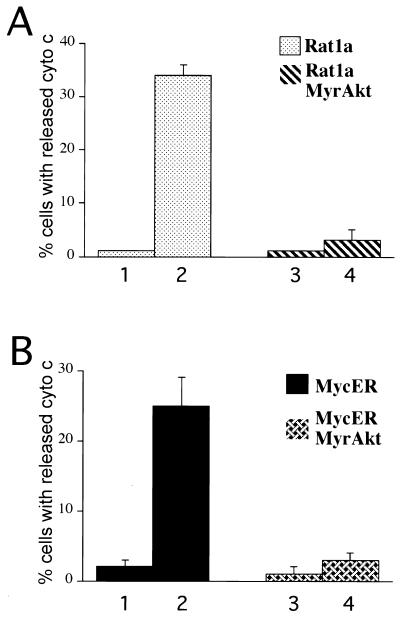
We extended these results by testing the ability of Akt to inhibit cytochrome c release from two additional inducers of apoptosis, the topoisomerase inhibitor etoposide and overexpression of Myc. Treatment of Rat1a cells with etoposide resulted in a release of cytochrome c, as measured by loss of punctate cytochrome c immunofluorescence (Fig. 3A; P < 0.001). Activated Akt was sufficient to prevent the etoposide-mediated release of cytochrome c (Fig. 3A; P < 0.001). To study Myc-mediated apoptosis we used a conditionally active Myc allele (MycER) that permits conditional activation of Myc activity upon addition of the β-estradiol analog 4-hydroxytamoxifen (4-HT). Rat1a cells expressing MycER (Rat1a/MycER cells) and cells expressing MycER and MyrAkt (Rat1a/MycER/MyrAkt cells) were deprived of serum in the presence of 4-HT. Activation of Myc induced a large increase in the percentage of cells exhibiting diffuse cytochrome c immunostaining (Fig. 3B; P < 0.001). Activated Akt was sufficient to prevent this translocation of cytochrome c (Fig. 3B; P < 0.001). These data demonstrate that in Rat1a cells, cytochrome c is released in response to a wide range of apoptotic stimuli and that activation of Akt is sufficient to prevent this release.
FIG. 3.
Akt prevents the release of cytochrome c induced by multiple apoptotic stimuli. (A) Akt inhibits etoposide-mediated cytochrome c release. Rat1a (columns 1 and 2) and Rat1a/MyrAkt cells (columns 3 and 4) were either mock treated (columns 1 and 3) or treated with 10 μM etoposide (columns 2 and 4) for 3 h in the presence of 100 μM zVAD. Cells were then fixed and immunostained for cytochrome (cyto) c as described in Fig. 2. Averages (± standard errors) of at least 100 cells from four independent experiments are shown. (B) Akt inhibits Myc-mediated cytochrome c release. The percentage of cells demonstrating diffuse cytochrome c immunostaining was quantitated. Rat1a/MycER (columns 1 and 2) and Rat1a/MycER/MyrAkt cells (columns 3 and 4) were incubated overnight in DMEM with 2.5% FCS with (columns 2 and 4) or without (columns 1 and 3) 1 μM 4-HT. The next day, cells were placed in DMEM with 0.5% FCS and 100 μM zVAD for 3 h. Cells were then fixed and immunostained for cytochrome c as described for Fig. 2. Averages (± standard errors) of at least 100 cells from four independent experiments are shown.
Akt prevents alterations in mitochondrial dye uptake following UV exposure.
Alterations in the mitochondrial inner membrane potential (Ψm) have been implicated in the execution of apoptosis (reviewed in references 15 and 23). Pharmacological studies have suggested that the loss of inner membrane potential (ΔΨm) is due to alterations in permeability transition (PT) pores; multiprotein complexes located between the inner and outer membranes. Artificial induction of PT pores opening can trigger caspase activation, and pharmacological inhibition of PT can prevent nuclear DNA condensation and apoptosis. In many cases, however, ΔΨm collapse is preceded by cytochrome c release and caspase activation (4, 22, 40, 45). A recent study has presented evidence supporting a causative role for a hyperpolarization and swelling of mitochondria, and the subsequent cytochrome c release, in the induction of apoptosis (40).
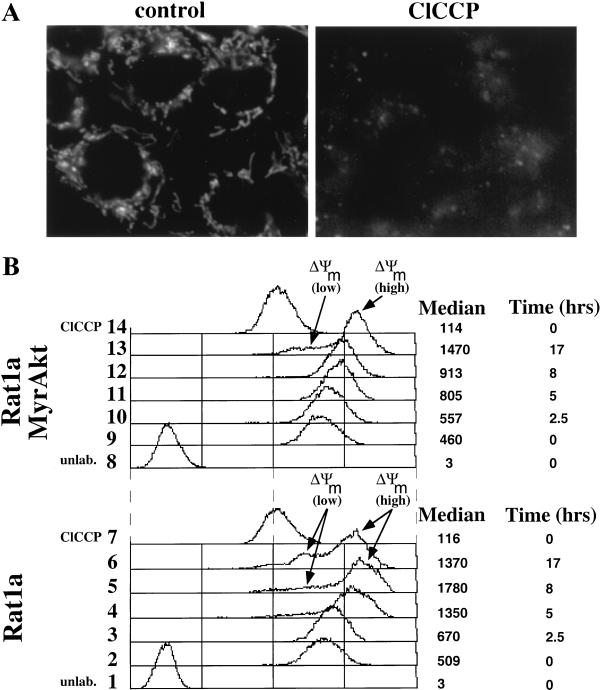
To determine if alterations in mitochondrial potential occur in our system, we measured the uptake of the mitochondrial specific dyes DiOC6 and MitoTracker-CMXRos in Rat1a cells undergoing apoptosis. Proliferating Rat1a cells loaded with DiOC6 (Fig. 4A) or MitoTracker-CMXRos (data not shown) exhibited a punctate fluorescence characteristic of a mitochondrial labeling pattern indistinguishable from the cytochrome c immunofluorescence pattern (Fig. 2A). Pretreatment of Rat1a cells with the protonophore carbonyl cyanide m-chlorophenylhydrazone (ClCCP), which dissipates the mitochondrial inner membrane proton gradient, substantially inhibited dye uptake, suggesting that dye uptake requires an intact membrane potential (Fig. 4A). Dye uptake was quantitated by flow cytometry (Fig. 4B). Proliferating Rat1a and Rat1a/MyrAkt cells took up similar amounts of DiOC6. Five hours after UV irradiation, we observed the appearance of a population of cells that had lost membrane potential (ΔΨm low). Activated Akt delayed the appearance of ΔΨm low cells until 17 h following irradiation (Fig. 4B). Five hours after irradiation, >45% of Rat1a cells exhibited fragmented and condensed nuclei (Fig. 1A), while only less than 10% of the cells had lost membrane potential. These results strongly suggest that in our system, ΔΨm low is a late event that occurs as a consequence of apoptosis. Interestingly, we observe an initial threefold increase in dye incorporation following irradiation (ΔΨm high), which is also significantly attenuated by MyrAkt (Fig. 4B). Thus, Akt activation, like Bcl-xL overexpression (40), is sufficient to delay increases in mitochondrial dye uptake and hyperpolarization. We obtained virtually identical results when these experiments were repeated using the mitochondrial dye MitoTracker-CMXRos (data not shown). The initial increase in dye uptake may reflect swelling of the mitochondria which precedes cytochrome c release. Thus, Akt inhibits cytochrome c release by delaying the early changes in the mitochondria immediately after the exposure to the apoptotic stimulus.
FIG. 4.
Akt is sufficient to prevent alterations in mitochondrial dye uptake. (A) DiOC6 labeling of mitochondria requires an intact inner membrane potential. (B) FACS analysis of UV-irradiated Rat1a and Rat1a/MyrAkt cells incubated with DiOC6. Rat1a and Rat1a/MyrAkt cells were subjected to UV irradiation as described for Fig. 1, either mock treated or treated with 100 mM ClCCP for 5 min, and then loaded with DiOC6 as described in Materials and Methods. Proliferating Rat1a cells loaded with DiOC6 were defined as having a fluorescence of 500 arbitrary units.
We found that ectopic expression of Bcl-2 delay mitochondrial changes for longer time than Akt (data not shown). This is likely because the MyrAkt kinase activity declines with time upon growth factor withdrawal due to the limited pool of the D-3 phosphoinositides that are required for full activation of Akt by PDKI and PDKII. As we have previously shown, the ability of Akt to promote cell survival is dependent on its kinase activity (20).
Akt cannot inhibit apoptosis induced by microinjection of cytochrome c.
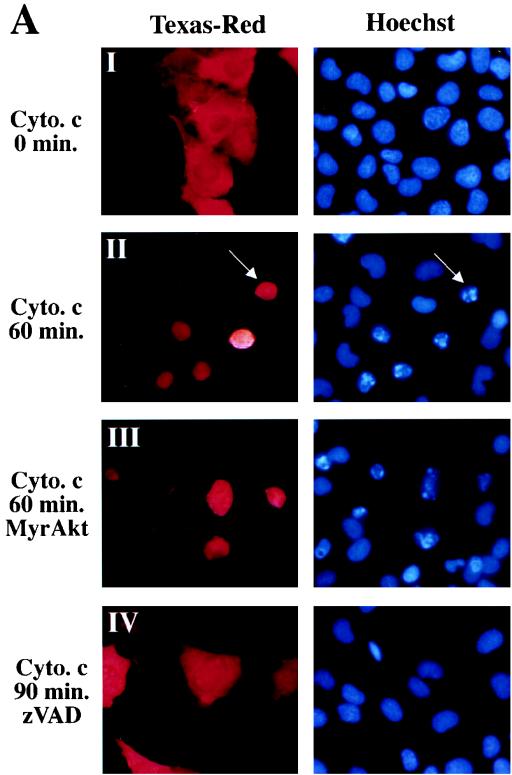
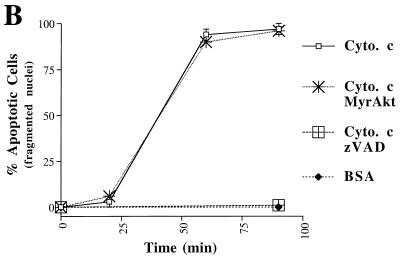
Microinjection of holo-cytochrome c can induce apoptosis in some cell lines (9, 25). We tested whether microinjection of holo-cytochrome c was able to induce cell death in Rat1a cells and whether Akt could prevent cytochrome c-induced cell death. Rat1a cells were injected with 0.1 to 10 mg of horse heart holo-cytochrome c per ml together with the dye Texas red. We observed a dose-dependent induction of apoptosis, as judged by fragmentation of nuclei beginning with 0.3 mg of cytochrome c per ml (data not shown). Because 1 mg/ml was the lowest concentration of cytochrome c that reproducibly induced apoptosis, we used this concentration for further experiments. Injection of 1 mg of cytochrome c per ml induced Rat1a cells to undergo apoptosis within 60 min of microinjection (Fig. 5; P < 0.001). Injection of 1 mg of BSA per ml did not induce any significant fragmentation of nuclei (Fig. 5B). Preincubation with zVAD was sufficient to prevent nuclear fragmentation induced by cytochrome c microinjection (Fig. 5; P < 0.001). Cells expressing MyrAkt were not protected from cytochrome c-induced cell death (Fig. 5). Taken together, these results demonstrate that although Akt can prevent cytochrome c release, it is unable to inhibit apoptosis after cytochrome c has accumulated in the cytoplasm. Therefore, the inhibition of cytochrome c release from mitochondria is a major mechanism by which Akt prevents cell death.
FIG. 5.
Akt cannot block apoptosis induced by microinjection of cytochrome c. (A) Hoechst staining and Texas red fluorescence of microinjected cells. Rat1a (panels I, II, and IV) and Rat1a/MyrAkt (panel III) cells were mock treated or treated with 100 mM zVAD (panel IV) for 20 min and then microinjected with 0.2% Texas red and 1 mg of cytochrome (cyto.) c per ml. After the time indicated on the left, cells were fixed. (B) Time course of apoptosis in Rat1a and Rat1a/MyrAkt cells microinjected with cytochrome c. Cells exhibiting fragmented and condensed nuclei were scored as apoptotic. Rat1a and Rat1a/MyrAkt cells were injected with 1 mg of cytochrome c per ml and scored for nuclear condensation 0, 20, 60, and 90 min after microinjection. Alternatively, Rat1a cells were either mock treated or treated with 100 μM zVAD, then injected with BSA (1 mg/ml) or cytochrome c (1 mg/ml), fixed, and scored after 90 min. Averages (± standard errors) of at least 50 cells from three independent experiments are shown.
Akt inhibits apoptosis induced by overexpression of the proapoptotic Bcl-2 family members.
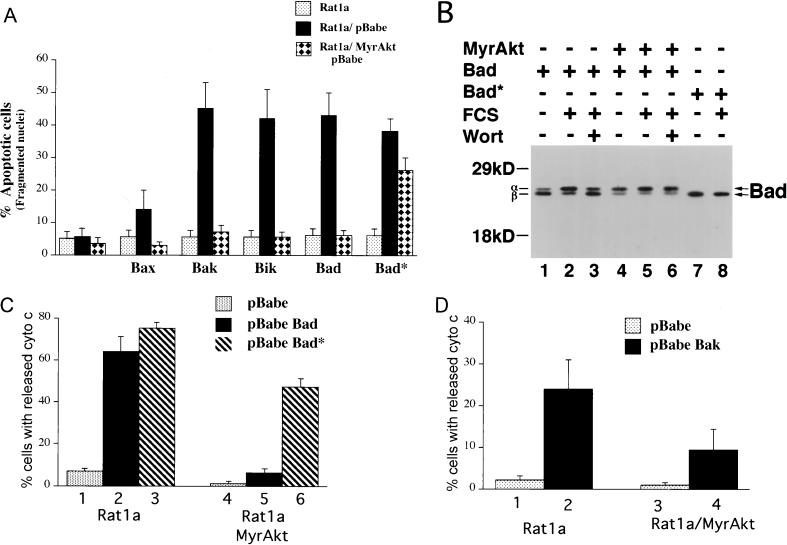
We have presented evidence that Akt prevents the disruption of mitochondrial integrity following apoptotic stimuli. As Bcl-2 family members also control mitochondrial integrity (reviewed in reference 1), it is possible that Akt functions by regulating this family of proteins. We have shown previously that Akt does not change the steady-state expression levels of the Bcl-2 and Bcl-xL proteins (20). We therefore examined the ability of Akt to inhibit apoptosis mediated by the proapoptotic members of the Bcl-2 family. Overexpression of Bax, Bak, Bik, and Bad induced a significant increase in apoptosis in Rat1a cells upon serum deprivation that was significantly attenuated by activated Akt (Fig. 6A).
FIG. 6.
Akt inhibits apoptosis and cytochrome c release induced by overexpression of the proapoptotic Bcl-2 family members. (A) Akt inhibits apoptosis accelerated by overexpression of Bax, Bak, Bik, and Bad. Rat 1a and Rat1a/MyrAkt cells were infected with eGFP retroviruses expressing Bax, Bak, Bik, Bad, and the Bad* (see Materials and Methods). Apoptosis was scored by DAPI staining 16 h following serum deprivation, a time point at which Rat 1a cells do not exhibit significant apoptosis. Cells were seeded at a density of 50,000 cells/3-cm-diameter dish. The next day cells were placed in DMEM containing 0% FCS for 16 h. Averages (± standard errors) of at least 300 cells from three independent experiments. (B) Akt induces a mobility shift of ectopically expressed Bad in Rat1a cells. Rat1a (lanes 1 to 3, 7, and 8) and Rat1a/MyrAkt (lanes 4 to 6) cells were infected with retrovirus expressing Bad or Bad*. Forty-eight hours following infection, cells were deprived of serum for 2 h. Cells were either mock treated (lanes 1, 2, 4, 5, 7, and 8) or treated with 200 nM wortmannin (Wort; lanes 3 and 6) for 30 min. Cells were then either mock treated (lanes 1, 4, and 7) or stimulated with 20% FCS for 30 min (lanes 2, 3, 5, 6, and 8). Cells were lysed, and extracts were subjected to SDS-PAGE (12% gel). Bad was detected with a hamster monoclonal anti-Bad antibody (PharMingen clone 13361S), and the different phosphorylation states are indicated with arrows. Positions of molecular weight standards are indicated on the left. (C) Akt prevents cytochrome c release accelerated by overexpression of Bad. Rat1a (columns 1 to 3) and Rat1a/MyrAkt (columns 4 to 6) cells demonstrating diffuse cytochrome c immunostaining were quantitated. Cells were treated as described for Fig. 2. Two hours (a time point at which there is no significant cytochrome c release in Rat 1a cells) after UV irradiation cells were fixed and immunostained for cytochrome c localization. Averages (± standard errors) of at least 100 cells from three independent experiments are shown. (D) Akt prevents cytochrome c release accelerated by overexpression of Bak. Cells were treated and scored for cytochrome c release as described for panel C.
Bad is phosphorylated in hemapoietic cells treated with interleukin-3 at two sites, serine 112 (S112) and serine 136 (S136) (41, 47). Akt has recently been shown to phosphorylate and inactivate Bad in primary neurons and in vitro (8, 31). Indeed, in Rat1a cells, activated Akt was unable to efficiently inhibit apoptosis mediated by a mutant of Bad (Bad*) in which the two serine residues were converted to alanine (Fig. 6A). Immunoblot analysis of Rat1a cells expressing Bad and Bad* demonstrated that activated Akt was sufficient to induce a mobility shift of Bad indicative of phosphorylation. In proliferating Rat1a cells, Bad existed as a doublet (data not shown). The slower- and faster-migrating bands were termed α and β, respectively. In Rat1a cells deprived of FCS, the majority of Bad existed as the faster-migrating β species (Fig. 6B, lane 1). Treatment of the cells with FCS resulted in a shift in mobility of Bad to the slower-migrating α species (lane 2). Pretreatment of the cells with wortmannin prevented this serum-induced mobility shift (lane 3). In Rat1a/MyrAkt cells, Bad migrated predominantly as the α species, irrespective of the presence or absence of serum or wortmannin (lanes 5 to 7). Bad* migrated exclusively as the β species irrespective of the presence or absence of serum and/or wortmannin (lanes 7a and 8). The presence of MyrAkt was unable to induce a mobility shift in Bad*-expressing cells (data not shown). Thus, in Rat1a cells, Akt is sufficient to induce a mobility shift of ectopically expressed Bad that is dependent on S112 and S136.
We next investigated whether Bad-induced apoptosis involves cytochrome c release and whether Akt is capable of preventing this release. Cytochrome c release was measured in UV-treated Rat1a and Rat1a/MyrAkt cells expressing Bad or Bad*. Under these conditions, ectopic expression of Bad and Bad* increased the percentage of cells with diffuse cytochrome c immunostaining (Fig. 6C; P < 0.002 <0.001). Akt was able to prevent release of cytochrome c induced by Bad and to a lesser extent by Bad* (Fig. 6C; P < 0.001). We then determined whether the mechanism by which Akt inhibits apoptosis mediated by other proapoptotic members of the Bcl-2 family is also through the inhibition of cytochrome c release. Because Bak is a potent accelerator of apoptosis in Rat1a cells, we examined the ability of Akt to inhibit the cytochrome c release induced by overexpression of Bak, as a representative of the Bax subfamily. Overexpression of Bak also elicited an increase in cytochrome c release that was significantly inhibited by Akt (Fig. 6D). These results show that Akt inhibits apoptosis and cytochrome c release induced by both the BH3 subfamily and the Bax subfamily of the proapoptotic Bcl-2 family. Furthermore, these results suggest that Bad is not the only target for Akt upstream of cytochrome c release (see discussion below).
DISCUSSION
In mammalian cells, the serine/threonine kinase Akt/PKB is widely recognized as the major mediator of growth factor-promoted cell survival, but the step at which it abrogates the apoptosis cascade remained unknown. A critical early step is initiated by loss of integrity of the outer mitochondrial membrane accompanied by release of cytochrome c. Cytochrome c then binds to and activates the Ced-4 homologue Apaf-I, which in turn activates caspase-9, resulting in the execution of a caspase cascade culminating in caspase-3 and caspase-7. In the present study, we demonstrated that Akt can prevent the activation and the processing of the caspases by maintaining mitochondrial integrity and by preventing the critical early step of cytochrome c release. The data for dye uptake (Fig. 4) suggest that Akt can block also the swelling of the mitochondria which preceded cytochrome c release, as was shown for Bcl-xL (40). While mitochondrial step of apoptosis is at least in part controlled by Bcl-2 family of proteins, we failed to detected changes in the levels of such proteins upon introduction of constitutively active Akt (20). One possible candidate that may mediate the regulation of mitochondrial integrity by Akt is the proapoptotic Bcl-2 family member Bad. Bad has been shown to be directly phosphorylated and inactivated by Akt. Our results are consistent with Bad being a downstream target of Akt-mediated mitochondrial integrity in cells overexpressing Bad. Although this phenomenon is important for Akt-mediated survival of cells overexpressing Bad, we have presented evidence for the existence of a more general, Bad-independent mechanism of Akt action. First, in this study we have demonstrated that Akt can overturn effects of proapoptotic Bcl-2 family members (Bik, Bak, and Bax) that lack Akt phosphorylation sites. Second, Akt showed small yet reproducible and statistically significant protection from the effects of an overexpressed Bad mutant that is insensitive to Akt phosphorylation (Fig. 6A). Moreover, Akt can completely protect from a certain threshold level of unphosphorylated Bad, because there is always a significant fraction of unphosphorylated Bad when wild-type Bad is overexpressed (Fig. 6B, lanes 4 to 6). Importantly, these phenomena were observed in Rat1a cells that lack any detectable Bad protein (19a). Third, Akt promotes survival of cells of various tissues origins and in response to diverse stimuli. Bad-deficient mice, however, lack major apoptotic defects (22a), and their primary fibroblasts are as sensitive to serum withdrawal as the wild-type cells (19a). Finally, while this report was in review, it was shown that Akt is unlikely to be the major kinase that phosphorylates Bad in vivo (18).
An alternative route of Akt action was suggested by the finding that Akt can directly phosphorylate and inactivate caspase-9 (6). The latter is expected to have an effect analogous to that of the caspase inhibitor zVAD. In contrast, our data indicate that Akt (but not zVAD) blocks release of mitochondrial cytochrome c in a caspase-independent manner (Fig. 2), while zVAD (but not Akt) attenuates cell death upon cytochrome c microinjection (Fig. 5). Noteworthy, caspase-9 deficiency interferes with apoptosis without preventing cytochrome c release (17).
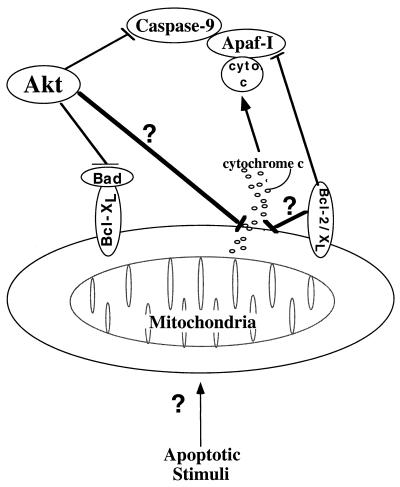
Taken together, our findings demonstrate that Akt intervenes in the apoptotic pathway independently of caspases by inhibiting mitochondrial changes prior to cytochrome c release. In addition, to this mechanism that counteracts the effects of diverse apoptotic stimuli and proapoptotic proteins, there are more specific mechanisms such as phosphorylation of Bad and caspase-9 (Fig. 7). Although our results do not support Akt-mediated cell survival through inhibition of caspase-9, we cannot completely rule out the possibility that in addition to preventing cytochrome c release, Akt phosphorylates and inactivates caspase-9 and other caspases. This mechanism together with the phosphorylation of Bad could be alternative or safeguard mechanisms for Akt-mediated cell survival.
FIG. 7.
Schematic illustration showing Akt antiapoptotic activities. Akt promotes cell survival by intervening in the apoptosis cascade upstream of cytochrome c (cyto c) release. Akt may maintain the integrity of the mitochondria by a general unknown mechanism or by a specific mechanism of Bad phosphorylation. By analogy to Bcl-2 and Bcl-xL, which inhibit apoptosis post-cytochrome c release through binding of Apaf-I, Akt can also inhibit apoptosis by phosphorylation and inactivation of caspase-9.
The exact mechanism by which Akt maintains mitochondrial integrity is not clear. It is possible that Akt is antagonizing the initial damage to the mitochondria induced by the apoptotic stimuli. Because the nature and the identity of this early event are not known, it is unclear how Akt regulates the integrity of the mitochondria. However, it is likely that this mechanism is evolutionarily conserved because Akt exhibits antiapoptotic effects in both mammalian and Drosophila cells (3, 36).
The mechanism by which Akt maintains the integrity of mitochondria is likely to be similar to the effect of growth factors on mitochondria. It has been recently shown that growth factor deprivation decreases the accessibility of mitochondrial matrix to ADP, which in turn elicits hyperpolarization of the inner mitochondrial membrane and matrix swelling. Bcl-xL prevents cell death upon growth factor withdrawal by bypassing the inaccessibility of mitochondria to ADP, thereby inhibiting hyperpolarization and swelling of mitochondria (39). Because activated Akt can prevent the initial swelling and hyperpolarization of mitochondria, it is likely that it inhibits cell death by maintaining accessibility of mitochondria to ADP.
While this report was in review, another possible mechanism by which Akt promotes cell survival was suggested: Akt prevents the expression of Fas ligand upon growth factor withdrawal (5). This implies that in serum-deprived cells, the apoptotic signal is relayed to mitochondria via a caspase-8-dependent pathway which is sensitive to zVAD (26). Our observations, however, do not support this prediction because zVAD fails to prevent cytochrome c release, although it is capable of inhibiting the onset of subsequent apoptotic changes.
Both the apoptotic cascade and Akt are conserved in Caenrhabditis elegans and in Drosophila. Thus, it is expected that Akt will also have a role in regulating apoptosis in nematodes and flies. Indeed, Akt was shown in C. elegans to be a downstream effector of insulin-like growth factor receptor and PI 3-kinase (30). Thus far, there is no evidence that Akt plays a role in regulating apoptosis in C. elegans. In this regard, it is worth noting that although the fundamental apoptotic machinery is conserved between C. elegans and mammals, no role in apoptosis has been attributed to cytochrome c in C. elegans. Because Akt inhibits apoptosis by preventing cytochrome c release, it may not have a role in the regulation of apoptosis in C. elegans. Interestingly, in mammalian cells, Bcl-2 can intervene in two steps of apoptosis: it can prevent cytochrome c release and can also physically interact with Apaf-I to inhibit its function similar to the interaction between Ced-9 and Ced-4 in C. elegans (reference 1 and Fig. 7). This may explain the ability of Bcl-2, at least in some cases, to inhibit apoptosis after cytochrome c release (34). It was shown that Akt is required in Drosophila for blocking apoptosis in the embryos but has only modest effect on apoptosis in the Drosophila eye (3, 36). It remains to be seen whether Drosophila has also adopted cytochrome c as an apoptosis cofactor.
ACKNOWLEDGMENTS
We thank Maura O’Leary for technical assistance, R. Dekoter and H. Singh for pBabe(eGFP), E. Eves and M. Rosner for use of the microinjection apparatus, S. Korsmeyer for communicating results before publication, and C. Palfrey for critical reading of the manuscript.
This work was supported by grants CA71874 and AG16927 from the National Institutes of Health to N.H.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Transduction of interleukin-2 antiapoptotic and proliferative signal via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E, Newmeyer D D, Green D R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:318–321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 7.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 9.Duckett C S, Li F, Wang Y, Tomaselli K J, Thompson C J, Armstrong R C. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1998;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eves E M, Xiong W, Bellacosa A, Kennedy S G, Tsichlis P N, Rosner M R, Hay N. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol. 1998;18:2143–2152. doi: 10.1128/mcb.18.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y. Multiple species of CPP32 and MCh2 are the major active caspases present in apoptotic cells. EMBO J. 1997;16:2271–2281. doi: 10.1093/emboj/16.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fearnhead H O, McCurrach M E, O’Neill J, Zhang K, Lowe S W, Lazebnik Y A. Oncogene-dependent apoptosis in extracts from drug-resistant cells. Genes Dev. 1997;11:1266–1276. doi: 10.1101/gad.11.10.1266. [DOI] [PubMed] [Google Scholar]
- 13.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 14.Gingras A C, Kennedy S G, O’Leary M A, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green D, Reed J. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 16.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 17.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman S A, Lowe S W, Penninger J M, Mak T W. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 18.Harada H, Becknell B, Wilm M, Mann M, Jun-shen Huang L, Taylor S S, Scott J D, Korsmeyer S J. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 19.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 19a.Kennedy, S. G., and N. Hay. Unpublished data.
- 20.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 21.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 22a.Korsmeyer, S. Personal communication.
- 23.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 24.Kulik G, Klippel A, Weber M J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Srinivasan A, Wang Y, Armstrong R C, Tomaselli K J, Fritz L C. Cell-specific induction of apoptosis by microinjection of cytochrome c. J Biol Chem. 1997;272:30299–30305. doi: 10.1074/jbc.272.48.30299. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Zhu H, Xu C, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Simpson L, Takahashi M, Miliaresis C, Myers M P, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998;58:5667–5672. [PubMed] [Google Scholar]
- 28.Marte B M, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 30.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3 induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 32.Raff M C. Social controls of cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 33.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 34.Rosse T. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 35.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 36.Staveley B E, Ruel L, Jin J, Stambolic V, Mastronardi F G, Heitzler P, Woodgett J R, Manoukian A S. Genetic analysis of protein kinase B (AKT) in drosophila. Curr Biol. 1998;8:599–602. doi: 10.1016/s0960-9822(98)70231-3. [DOI] [PubMed] [Google Scholar]
- 37.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 38.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:312–316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 39.Vander Heiden M G, Chandel N S, Schumacker P T, Thompson C B. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- 40.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 41.Wang H-G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Yin X-M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]