Biological hydroxylation, O-demethylation, and aryl side-chain oxidation were integrated in a cell for lignin valorization.
Abstract
Converting lignin components into a single product is a promising way to upgrade lignin. Here, an efficient biocatalyst was developed to selectively produce gallate from lignin components by integrating three main reactions: hydroxylation, O-demethylation, and aryl side-chain oxidation. A rationally designed hydroxylase system was first introduced into a gallate biodegradation pathway–blocked Rhodococcus opacus mutant so that gallate accumulated from protocatechuate and compounds in its upper pathways. Native and heterologous O-demethylation systems were then used, leading to multiple lignin-derived methoxy aromatics being converted to gallate. Furthermore, an aryl side-chain oxidase was engaged to broaden the substrate spectrum. Consequently, the developed biocatalyst showed that gallate yields as high as 0.407 and 0.630 g of gallate per gram of lignin when alkaline-pretreated lignin and base-depolymerized ammonia fiber explosion lignin were applied as substrates, respectively. These results suggested that this rationally developed biocatalyst enabled the lignin valorization process to be simple and efficient.
INTRODUCTION
Lignin, the most abundant renewable aromatic biopolymer in nature, makes up 10 to 35% of lignocellulosic biomass (1). It is estimated that there is approximately 300 billion tons of lignin source in the biosphere, and this number still increases annually, providing an enormous carbon pool (2). With the development of lignocellulose biorefineries, the cellulose and hemicellulose components of lignocellulosic biomass can be efficiently converted to bioethanol and other bioproducts. Nevertheless, most lignin components are designed to be burned for energy supply in the current biorefinery concept (3). Searching for a green and sustainable way to upgrade lignin is critical for the most complete utilization of lignocellulosic resources.
Structurally, lignin is mainly composed of multiple phenylpropane derivatives. On the basis of the number of methoxy groups, these phenylpropane derivatives can be mainly divided into three units, namely, p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), which contain zero, one, and two methoxy groups in the aromatic ring. These units are linked with the chemical bonds of β-O-4, α-O-4, β-5, β-β, etc. (2, 4). The diverse methoxy and other side-chain groups on the aromatic rings, as well as the multiple connected chemical bonds, endow lignin with a vast number of functional groups and heterogeneous properties. Plentiful functional groups in lignin—e.g., methoxy, phenolic and aliphatic hydroxyl, benzyl alcohol, noncyclic benzyl ether, and carbonyl groups—not only influence its reactivity but also lead to the different composition of monolignols in plant species (2). With the increasing focus on lignin valorization, multiple depolymerization methods have been developed to depolymerize lignin to multiple low–molecular weight fragments, which can be further converted to valuable products by chemical or biological approaches (5–9).
Nevertheless, the heterogeneity of lignin commonly leads to multiple depolymerization products, which requires complicated processes for product separation and purification. For instance, there are generally p-coumarate, ferulate, p-hydroxybenzoate, vanillate, as well as some other low- and high-molecular lignin residues in alkaline-pretreated lignin liquors (10). It is desirable to convert multiple lignin components into single valuable products (11). Many notable studies have been performed for this purpose. For instance, lignin can be selectively transformed into terephthalic acid with combined oxidation, demethoxylation, and carbonylation of mixed lignin-derived monomers (12); into guaiacol with decarbonization and demethoxylation of lignin-derived alkyl-syringol and alkylguaiacol (13); and into phenol by removing the methoxy functionalities and alkyl side groups of lignin-derived aromatics (14, 15). However, these conversions commonly contain several sequential reaction steps; thus, it requires more than one reactor to complete the conversion, leading to a time-consuming process. Furthermore, the diversity of lignin components often contributes to undesired side reactions if the conditions are not well controlled.
Recently, the biological valorization of lignin has attracted increasing attention because some microorganisms can funnel multiple lignin components into a signal product by their elaborate metabolic pathways. As commonly recognized, lignin depolymerization in nature is mainly initiated with fungi (soft rot, white rot, and brown rot fungi, etc.), which can excrete powerful extracellular ligninolytic enzymes—including laccases, peroxidases, and some accessory enzymes—for the depolymerization of high–molecular weight lignin (2, 6, 16, 17). Thereafter, some bacteria assimilate the generated low–molecular weight lignin components for carbon and energy supply, during which the lignin components are converted into carbon dioxide and some bioproducts (18, 19). For instance, H-type monolignol p-coumarate can be converted to p-hydroxybenzoate with Fcs [feruloyl–coenzyme A (CoA) synthetase], Ech (enoyl-CoA hydratase/aldolase), and Vdh (aldehyde dehydrogenase), and the resultant p-hydroxybenzoate can be further hydroxylated to protocatechuate by PobA (p-hydroxybenzoate hydroxylase); G-type monolignol vanillate can be O-demethylated to protocatechuate by VanAB in Pseudomonas strains (20). Compared to chemical catalysts, these biological enzymes not only have great potential in the selective conversion of lignin components but also can be integrated in a chassis cell, making biocatalysis a potentially efficient method for lignin upgrading.
Here, Rhodococcus opacus PD630, a well-known strain that can use multiple lignin-derived aromatic compounds for cell growth (21), was selected as a chassis to selectively convert as many lignin components as possible into a single aromatic compound with integrated biocatalysis. Using gallate (3,4,5-trihydroxybenzoic acid)—a value-added compound that has been widely exploited in the food, pharmaceutical, and cosmetic industries (22, 23)—as a target product, we integrated three main biocatalytic reactions of hydroxylation, O-demethylation, and aryl side-chain oxidation in R. opacus PD630 by introducing exogenous biocatalytic pathways and enhancing endogenous biocatalytic systems (Fig. 1). In particular, the constructed R. opacus PD630 biocatalyst can efficiently valorize alkaline-pretreated lignin and base-depolymerized ammonia fiber explosion (AFEX) lignin components to gallate with a signal bioreactor compared with traditional chemical approaches that require several reactors. This simple and efficient biocatalytic process can provide a reference for producing other aromatic products from lignin components, as well as producing target products from mixed substrates.
Fig. 1. Catalyzing G-/H-/S-type lignin components into gallate with multiple-step reactions and the integrated one-pot biocatalytic route reported in this study.
RESULTS
Identification and blockage of the gallate degradation pathway in R. opacus PD630
In previous studies, lignin and lignin-derived aromatics were converted to microbial lipids (24), cis, cis-muconate (25–28), polyhydroxyalkanoate (19), pyridine–dicarboxylic acids (29, 30), vanillin (31), vanillylamine (32), itaconate (33), pyruvate, lactate (34), succinate (35), alkane, wax ester (36), etc. by native or modified microbial metabolic pathways. Considering that lignin is the most abundant renewable aromatic polymer in the biosphere, retaining its native aromatic structure and converting it to aromatic compounds is promising, avoiding the energy consumed in the ring opening of aromatic nuclei. To realize this hypothesis, we selected gallate as a target product considering that it can be formulated by modification of multiple lignin-derived aromatics. Moreover, gallate is also a valuable platform chemical for pharmaceutical, food, and chemical industries. To accumulate gallate in R. opacus PD630, there must be no gallate biodegradation pathways in the microorganism. Thus, wild R. opacus PD630 was first tested for its gallate degradation capability. As shown in fig. S1, when cultivated with 2 to 15 mM gallate as the sole carbon source, R. opacus PD630 survived well without an obvious lag phase. Moreover, the gallate in the culture broth was rapidly degraded. According to previous studies, gallate biodegradation pathways have been reported in Sphingomonas sp. SYK-6 (37), Pseudomonas putida KT2440 (38), and Novosphingobium aromaticivorans DSM12444 (39), in which gallate is converted to 4-oxalomesaconate by gallate 3,4-dioxygenzase (DesB) or protocatechuate 4,5-dioxygenase (LigAB) with 3,4-cleavage or 4,5-cleavage for further metabolism. However, no information about gallate biodegradation in R. opacus PD630 has been reported in previous studies.
To unravel gallate biodegradation in R. opacus PD630, we first performed protein sequence alignments of the total protein of R. opacus PD630 with DesB, as well as with LigAB. The results indicated that there was no protein similar to DesB (WP_014074660.1) and LigAB (A: WP_014075577.1; B: WP_014075576.1) in R. opacus PD630. Thus, instead of 3,4- or 4,5-cleavage mode, R. opacus PD630 may cleave gallate in other ways. In our previous study, a putative catechol 2,3-dioxygenase (AHK27425.1) in R. opacus PD630 was demonstrated to be responsible for the cleavage of methylcatechol (27). Moreover, it was reported that R. opacus PD630 can degrade protocatechuate with a protocatechuate 3,4-dioxygenase (β chain: AHK32653.1; α chain: AHK32654.1) (21, 40–43). Considering the similar structures of methylcatechol, protocatechuate, and gallate, it was assumed that the putative catechol 2,3-dioxygenase and protocatechuate 3,4-dioxygenase might be attributed to gallate degradation in R. opacus PD630. To verify this assumption, the putative catechol 2,3-dioxygenase and protocatechuate 3,4-dioxygenase genes were deleted from R. opacus PD630, generating R. opacus PD630-△c and R. opacus PD630-△p. Then, R. opacus PD630, R. opacus PD630-△c, and R. opacus PD630-△p were cultivated with 1 mM gallate. As presented in fig. S2, gallate was completely consumed by R. opacus PD630 and R. opacus PD630-△p within 6 hours (fig. S2, A and B); in contrast, only a slight gallate reduction was observed in the culture broth of R. opacus PD630-△c (fig. S2C), which might have been caused by autoxidation of gallate. The results suggested that the putative catechol 2,3-dioxygenase (AHK27425.1) was likely responsible for gallate degradation in R. opacus PD630. To further investigate the autoxidation of gallate, 1 mM gallate was added into the sterile M9 medium, and the obtained solution was maintained at 200 rpm, 30°C. The results showed that gallate autoxidation degree in the group without R. opacus PD630-△c (fig. S2E) was much higher than that with R. opacus PD630-△c (fig. S2C). The cause for the different gallate autoxidation profiles is likely that, with R. opacus PD630-△c, most oxygen was consumed by incubated cells, which resulted in a low oxygen level in the culture broth and hence a low gallate autoxidation. Thus, keeping the dissolved oxygen in the culture broth at a low level will be beneficial for preventing gallate autoxidation.
The alignments of the putative catechol 2,3-dioxygenase (AHK27425.1) with the reported DesB and LigAB, two dioxygenases responsible for the 3,4-cleavage or 4,5-cleavage of gallate, were performed. The results indicated that the similarities between the putative catechol 2,3-dioxygenase (AHK27425.1) and DesB/LigAB were relatively low (fig. S3, A and B). To further demonstrate the function of the putative catechol 2,3-dioxygenase, we heterologously expressed it in Escherichia coli BL21 (DE3), and the obtained recombinant E. coli BL21-C23D was cultivated with gallate. As shown in Fig. 2A and fig. S4, the signal peak of gallate disappeared quickly when E. coli BL21-C23D was cultivated with gallate, whereas when the parental E. coli BL21-pET28a was cultivated with gallate, no significant decrease in gallate concentration was observed. Further alignments of the putative catechol 2,3-dioxygenase (AHK27425.1) with some verified extradiol dioxygenases were conducted (fig. S5). The results suggested that the highly conserved amino acid residues responsible for substrate ring open in AHK27425.1 were consistent with those in verified extradiol dioxygenases, e.g., the Fe2+-binding sites His158, His221, and Glu272, which were defined as vital residues of extradiol dioxygenases for binding of iron (44). Besides, a phylogenetic tree of AHK27425.1 and verified extradiol dioxygenases indicated that the putative catechol 2,3-dioxygenase (AHK27425.1) from R. opacus PD630 belonged to family I.2 and was close to the I.2.D and I.2.E subclasses (fig. S6) (44). These results suggested that the putative catechol 2,3-dioxygenase (AHK27425.1) belongs to the extradiol dioxygenases species. All of the above information indicated that the putative catechol 2,3-dioxygenase was the key enzyme for gallate degradation in R. opacus PD630.
Fig. 2. Identification of the key functional genes involved in gallate degradation in R. opacus PD630.
(A) E. coli BL21-pET28a and E. coli BL21-C23D were cultivated with 10 mM gallate. High-performance liquid chromatography (HPLC) analysis showed that E. coli BL21-C23D efficiently degraded gallate. (B) The putative reaction involved in gallate degradation in R. opacus PD630 (red).
It has demonstrated that protocatechuate can be transformed to 5-carboxy-2-hydroxymuconate-6-semialdehyde (5CHMS) in Paenibacillus sp. strain JJ-1b with protocatechuate 2,3-dioxygenase (45). In consideration of the similar structures of protocatechuate and gallate, the most likely product of gallate with 2,3-cleaveage mode is 5-carboxy-2,3-dihydroxymuconate-6-semialdehyde (Fig. 2B), and relevant gallate degradation mechanisms in R. opacus PD630 need further study. With the deletion of the putative catechol 2,3-dioxygenase gene, the obtained R. opacus PD630 mutant lost the gallate degradation capability and was further applied for gallate production from lignin components.
Converting lignin-derived aromatics to gallate by a rational biological hydroxylation system
Rational hydroxylation of protocatechuate for gallate production
According to previous studies, protocatechuate is one of the most important metabolic nodes during lignin biodegradation, and many lignin-derived compounds—e.g., vanillin, vanillate, ferulate, and p-coumarate—can be funneled to protocatechuate for further metabolism (2, 19, 27). Thus, if protocatechuate can be converted to gallate, then many G- and H-type lignin components can also be converted to gallate. Compared with the structures of protocatechuate and gallate, it was found that if the protocatechuate is exclusively hydroxylated at the C-5 position, then it would be converted to gallate. In an earlier study, p-hydroxybenzoate was hydroxylated into protocatechuate by PobA, a flavin-dependent monooxygenase that can use one oxygen atom from O2 to hydroxylate p-hydroxybenzoate, in P. aeruginosa (46). Thereafter, two key amino residues, Tyr201 and Tyr385, in PobA were demonstrated to be vital to p-hydroxybenzoate hydroxylation in P. aeruginosa (47). With the mutation PobA* (Tyr385Phe), protocatechuate could be successfully hydroxylated into gallate, whereas the turnover rate remained low. To enhance the catalytic activity of PobA* (Tyr385Phe), Chen and colleagues (48) further introduced a previously unidentified mutation site, Thr294Ala, to PobA* (Tyr385Phe). As a result, the catalytic ability of the double mutant PobA** (Tyr385Phe/Thr294Ala) increased fourfold compared with that of the single Tyr385Phe mutant toward protocatechuate. Here, the endogenous PobA of R. opacus PD630 was aligned with verified PobAs from Pseudomonas aeruginosa and Corynebacterium glutamicum. As shown in fig. S3C, the endogenous PobA of R. opacus PD630 showed a high similarity to those of P. aeruginosa and C. glutamicum. In particular, the endogenous PobA of R. opacus PD630 has the reported threonine and tyrosine residues that are responsible for the catalytic activity and substrate specificity of PobA at positions 295 and 386, respectively. Then, site-directed mutagenesis was performed for endogenous PobA to achieve the goal of gallate production from protocatechuate, generating PobA* (Tyr386Phe) and PobA* (Tyr386Phe/Thr295Ala).
As shown in fig. S7, pobA*, pobA**, and wild pobA were expressed in the E. coli BL21 (DE3) system, and then the in vitro protocatechuate hydroxylation activities of these three enzymes were tested. Figure 3A indicates that the signal peak of gallate appeared when PobA* and PobA**, rather than PobA, were applied as catalysts. In detail, 0.40 and 0.494 mM gallate were produced from 1 mM protocatechuate within 8 min when PobA* and PobA** were applied as catalysts. Further kinetic parameter analysis of PobAs suggested that PobA** exhibited a high affinity toward protocatechuate compared with PobA* (fig. S8 and table S1). These results suggested that the mutation of Tyr386Phe endowed PobA with the protocatechuate hydroxylation ability and that the double mutations of Tyr386Phe/Thr295Ala further enhanced the obtained protocatechuate hydroxylation ability.
Fig. 3. Rational hydroxylation of protocatechuate to gallate in R. opacus PD630-△c△p.
(A) HPLC analysis of three reaction systems: The gallate (**) signal peak appeared and the protocatechuate (*) peak decreased when PobA* and PobA** were applied, rather than PobA. (B) The conversion curve of protocatechuate to gallate with R. opacus PD630-GA0 as a biocatalyst: No gallate generation was detected and the protocatechuate concentration remained nearly constant. (C) The conversion curve of protocatechuate to gallate with R. opacus PD630-GA1 as a biocatalyst: Protocatechuate was efficiently converted to gallate. The data shown in (B) and (C) are presented as the means ± SD of three biological replicates.
To further realize in vivo gallate production from protocatechuate and compounds in its upper pathways, both the native protocatechuate and gallate biodegradation pathways were blocked in R. opacus PD630, generating the mutant strain R. opacus PD630-△c△p (fig. S9). Then, the pobA** and pobA (as a control) genes were introduced into R. opacus PD630-△c△p with the help of the replicable plasmid pK18mob-PB264. As shown in Fig. 3B, with 1 mM protocatechuate as the substrate, no gallate generation was observed in the culture broth of R. opacus PD630-GA0, and the protocatechuate concentration remained nearly unchanged because of blockade of the corresponding pathways. In contrast, when cultivated with R. opacus PD630-GA1, 1 mM protocatechuate was almost completely consumed within 24 hours, along with the production of 0.87 mM gallate and a gallate yield of 91.59%.
Catalyzing p-hydroxybenzoate and p-coumarate to gallate with the designed hydroxylation module
To further verify the availability of the designed hydroxylation module in R. opacus PD630, we tested two typical lignin-derived aromatics, p-hydroxybenzoate and p-coumarate, for gallate production. According to our previous study, both p-hydroxybenzoate and p-coumarate can be degraded with protocatechuate as an intermediate in wild R. opacus PD630 (27). Thus, it was expected that these two compounds could be further converted to gallate with the designed hydroxylation module. As presented in Fig. 4A, with 1 mM p-hydroxybenzoate as the substrate, 0.94 mM gallate was obtained at 36 hours, which indicated that the designed hydroxylation module was efficient for gallate production from p-hydroxybenzoate. In particular, 0.14 mM protocatechuate accumulated during the p-hydroxybenzoate conversion process, suggesting that the conversion speed from p-hydroxybenzoate to protocatechuate was higher than that of protocatechuate to gallate in this process. Thus, further enhancement of the protocatechuate to gallate module is essential for efficient gallate production from lignin by the R. opacus PD630 mutant in the future. As a linkage of hemicellulose and lignin, p-coumarate is one of the main available aromatic compounds in lignocellulosic hydrolysates, especially in the hydrolysate of grass species (10, 49). As shown in Fig. 4B, when 1 mM p-coumarate was applied as substrate, the highest gallate concentration reached 0.81 mM at 12 hours with a yield of 81%. Here, the accumulation of protocatechuate in p-coumarate conversion process was less than that in the p-hydroxybenzoate conversion process, which was mainly attributed to the longer pathway to protocatechuate from p-coumarate compared with that from p-hydroxybenzoate (27). In summary, with the introduction of the designed hydroxylation module, R. opacus PD630-GA1 can efficiently convert both protocatechuate and aromatics with protocatechuate as a metabolic intermediate for gallate production.
Fig. 4. Production of gallate from H-type lignin components with the designed hydroxylation module.
(A) p-Hydroxybenzoate and (B) p-coumarate were applied as substrates, respectively. The data are presented as the means ± SD of three biological replicates.
Converting lignin-derived aromatics to gallate by two biological O-demethylation systems
Gallate production from G-type lignin components by the native Rieske nonheme iron monooxygenase–based O-demethylation system
Methoxy groups are among the abundant groups in lignin components. As reported, methoxy groups account for appropriately 11.0 weight % of the kraft lignin (11, 50). The disposal of methoxy aromatics is a huge challenge for lignin valorization. With chemical routes, demethylation of lignin-derived aromatics frequently occurs by cleavage of C─O bonds, which is done at high temperature and often in continuous flow reactors to avert hydrogenation of aromatic rings (7). Recently, the biological O-demethylation reaction has attracted increasing attention for lignin valorization (51–53). For instance, the O-demethylation reaction of vanillate to protocatechuate in Pseudomonas strains can be realized by VanAB, a heterodimeric enzyme consisting of the terminal oxygenase VanA and the reductase VanB. During the O-demethylation process, both NADH (reduced form of nicotinamide adenine dinucleotide) and NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) act as electron donors via a Rieske nonheme iron monooxygenase mechanism. Meanwhile, the by-product formaldehyde is generated, which can be further metabolized for amino acid synthesis and energy supply (20, 54). VanAB was also found in Rhodococcus jostii RHA1, a well-known member of Rhodococcus species, and the vanA gene deletion strain lost the capability of growing on vanillate (55). Recent multiomic elucidation of aromatic catabolism suggested the existence of VanAB in R. opacus PD630, and the by-product formaldehyde along with O-demethylation was mainly degraded by means of a mycothiol-dependent pathway to CO2 or involved in cell metabolism by the ribulose monophosphate pathway (42).
On the basis of our previous study, R. opacus PD630 can use vanillate and ferulate, both of which contain one methoxy group, as the sole carbon source for cell growth (27). With the genome information, similar enzymes (AHK27805.1 and AHK34193.1) for VanAB were mined in R. opacus PD630. To verify the effectiveness of this Rieske nonheme iron monooxygenase system, we tested the methoxy aromatics, vanillate and ferulate, for gallate production by R. opacus PD630-GA1. As shown in Fig. 5A, 1 mM vanillate was exhausted within 10 hours, and the highest gallate concentration of 0.95 mM was obtained at 32 hours with a yield of 95% from vanillate. According to our previous study, ferulate can be converted to vanillate via a CoA-dependent β-oxidation pathway in R. opacus PD630 with feruloyl-CoA, 4-hydroxy-3-methoxyphenyl-β-hydroxypropionyl-CoA, 4-hydroxy-3-methoxybenzoyl acetyl-CoA, and vanillyl-CoA as intermediates (27). As shown in Fig. 5B, ferulate was depleted in the first 4 hours, and the highest gallate concentration reached 0.95 mM at 36 hours with a yield of 95%. From these results, it was demonstrated that the native Rieske nonheme iron monooxygenase–based O-demethylation system in R. opacus PD630 is active, and relevant R. opacus PD630 mutants can selectively convert the G-type lignin compounds to gallate efficiently.
Fig. 5. Gallate production from G-type lignin components by native Rieske nonheme iron monooxygenase based O-demethylation system in R. opacus PD630.
(A) Vanillate and (B) ferulate were applied as substrates, respectively. The data are presented as the means ± SD of three biological replicates.
Gallate production from S-type lignin compounds by introduction of the tetrahydrofolate-dependent O-demethylation system
In addition to G-type lignin components, S-type lignin compounds also have methoxy groups. Moreover, the number of methoxy groups in S-type lignin compounds is two compared to one methoxy group in G-type lignin compounds. There has been no report on the metabolic pathways for S-type lignin compounds in R. opacus PD630. To verify the effectiveness of the native O-demethylation system on S-type lignin compounds, we first used an S-type lignin monolignol syringate as a substrate for gallate production. As shown in Fig. 6A, within 48 hours, only 0.002 mM gallate was generated by R. opacus PD630-GA1 with an initial OD600 (optical density at 600 nm) value of 0.8, along with 0.76 mM residual syringate in the culture broth, indicating that the native O-demethylation ability of R. opacus PD630 on syringate was quite weak. The weak O-demethylation on syringate in native R. opacus PD630 might occur with Rieske nonheme iron monooxygenase VanAB (table S2) (56). To enhance the utilization of syringate, we introduced two O-demethylase genes of desA and ligM from Sphingobium sp. SYK-6 to R. opacus PD630-△p△c, generating R. opacus PD630-GA2. As shown in Fig. 6B, 0.088 mM gallate was generated by R. opacus PD630-GA2 within 48 hours, and the syringate was decreased to 0.74 mM at 48 hours, suggesting that this exogenous O-demethylation system had little effect on syringate conversion in R. opacus PD630. As described in previous studies, tetrahydrofolate (H4folate) acts as an assistant in C1 metabolism during the O-demethylation process of syringate in Sphingobium sp. SYK-6 (18). In vitro enzyme catalysis also verified that LigM could catalyze 3-O-methylgallate to gallate only when H4folate existed in the reaction system (57). Seemingly, the mechanisms of O-demethylation in Sphingobium sp. SYK-6 are different from those in Pseudomonas: H4folate was essential for O-demethylation of lignin-derived aromatics in Sphingobium sp. SYK-6 (57, 58), and the generated methyl groups were involved in methionine and serine synthesis for cell growth and metabolism (59). Considering the low yield of gallate from syringate with the introduced O-demethylation system, it was speculated that the H4folate in R. opacus PD630-GA2 might be insufficient for the introduced O-demethylation process. Thus, 0.5 mM H4folate was added to R. opacus PD630-GA2 culture broth. As a result, the highest gallate concentration reached 0.146 mM and the syringate concentration was decreased to 0.60 mM (Fig. 6B), which verified that H4folate is indeed crucial for the O-demethylation of syringate.
Fig. 6. Gallate production from the S-type lignin compound syringate by introducing a heterogeneous H4folate-dependent O-demethylation system and H4folate regeneration system, as well as increasing the cell inoculation volume.
(A) Syringate (1 mM) was cultivated with R. opacus PD630-GA1, and only 0.002 mM gallate was produced. (B) Syringate (1 mM) was cultivated with R. opacus PD630-GA2: condition 1, no extra H4folate; condition 2, additional 0.5 mM H4folate. (C) Syringate (1 mM) was cultivated with R. opacus PD630-GA3 with the initial OD600 value of 0.8. (D) Syringate (1 mM) was cultivated with R. opacus PD630-GA3 with the initial OD600 value of 5.0. The data are presented as the means ± SD of three biological replicates.
To reduce the cost of additional H4folate, an in vivo H4folate regeneration system is preferred. As reported, during the O-demethylation of syringate and vanillate in Sphingomonas sp. SYK-6, H4folate is transformed to CH3-H4folate, and then CH3-H4folate is regenerated to H4folate with a series of reactions, among which two enzymes, MetF and LigH, are vital to H4folate recycling (57–59). Moreover, if the H4folate regeneration process is blocked, then the accumulation of CH3-H4folate will decrease the O-demethylation efficiency (60). Thus, constructing an in vivo H4folate recycling system is supposed to be beneficial to the O-demethylation reaction; the plasmid pK18mob-PB264-GA2 bearing metF and ligH from Sphingomonas sp. SYK-6 along with desA and ligM was subsequently introduced to R. opacus PD630-△p△c, generating R. opacus PD630-GA3. As shown in Fig. 6C, when R. opacus PD630-GA3 was applied as a biocatalyst, 0.35 mM gallate was produced from a 1 mM syringate, an improvement of 3.98-fold compared with that using R. opacus PD630-GA2 as a biocatalyst without extra H4folate. The proven function of metF and ligH genes in R. opacus PD630-GA3 boosted the gallate yield from syringate, whereas the productivity and yield were still unsatisfactory. To address this issue, we increased the initial cell inoculation volume to an OD600 value of 5.0. As shown in Fig. 6D, the syringate was depleted in 24 hours, and the gallate concentration reached 1.00 mM at 36 hours with a yield of 100%. With the combined strategy of introducing a heterogeneous O-demethylation system and H4folate regeneration system, as well as increasing the cell inoculation volume, the S-type lignin compound syringate was efficiently converted to gallate.
In addition to tailoring methoxy groups in lignin-derived compounds to valuable aromatics, the utilization of methoxy groups in lignin has also attracted increasing attention in recent years. For instance, Mei and colleagues (11) converted the methoxy groups in lignin to acetic acid, in which carbon monoxide, catalyst, and high pressure were involved. With the increasing focus on the utilization of C1 compounds, many bacterial enzymes involving in methyl group utilization have been investigated, because these groups are also important C1 resources. In addition to the Rieske nonheme iron monooxygenase–based and H4folate-dependent O-demethylase systems mentioned in this study, a promiscuous P450 aryl-O-demethylase, consisting of a cytochrome P450 protein from the family CYP255A2 (GcoA, WP_020419855.1) and a three-domain reductase (GcoB, WP_020419854.1), was first characterized from Amycolatopsis sp. ATCC39116 in 2018. Substrate spectrum tests suggested that this promiscuous P450 aryl-O-demethylase system can efficiently function on a wide variety of lignin-derived aromatics, including guaiacol, 3-methoxycatechol, 2-methylanisole, anisole, guaethol, vanillin, and syringol (51). Not long after the first P450 demethylase system found in Amycolatopsis sp., similar systems were also found in R. rhodochrous MK007070, R. rhodochrous EP4, and R. jostii RHA1 (53, 61). In R. rhodochrous EP4, two P450 demethylases, AgcA (belonging to the CYP255A1 family) and GcoA (belonging to the CYP255A2 family), which shared 65 and 76% amino acid sequence identities with GcoA in Amycolatopsis sp. ATCC39116 were verified. Similarly, both AgcA and GcoA were also confirmed in R. jostii RHA1. Further studies indicated that AgcAs could catalyze the O-demethylation of 4-alkylguaiacols but had a low specificity for guaiacol (53). The coexistence of these two kinds of P450 demethylases potentially endow these R. rhodochrous species with powerful assimilation ability for abundant methylated aromatics. Rational engineering on these P450 demethylases could allow the recombinant proteins converting extensive lignin-derived aromatic compounds, which is beneficial to funnel-methylated aromatics to target compounds for lignin valorization (52, 62). We thus searched for the potential P450 demethylases in R. opacus PD630 based on the genome information, and the results indicated that WP_005251375.1 in R. opacus PD630 exhibited the highest similarity to both reported GcoA and AgcA (fig. S10). Tests for alkylguaiacol assimilation capabilities of R. opacus PD630 and functional characterization of the protein WP_005251375.1 will further the understanding of the O-demethylation system in R. opacus PD630.
Aryl side-chain oxidation of lignin-derived aromatics for gallate production
In addition to the abovementioned hydroxylation and O-demethylation reactions, aryl side-chain oxidation is also widely applied in lignin valorization. As mentioned in previous studies, multiple valuable aromatic compounds have been obtained from lignin components by different oxidation approaches. For instance, some benzoquinones can be obtained from lignin compounds by C1-Cα oxidation; some phenolics can be achieved by the side-chain oxidation of lignin components (7). Here, to widen the substrate spectrum for gallate production, a side-chain oxidation reaction was used to convert a typical monolignol syringaldehyde to syringate and, further, to gallate. First, the gallate production capability of R. opacus PD630-GA3 was tested with 1.0 mM syringaldehyde as the substrate. As a result, 0.51 mM syringyl alcohol was generated without any gallate detected in 24 hours (fig. S11). Considering the high gallate production efficiency from syringate by R. opacus PD630-GA3, the absent gallate production capability from syringaldehyde suggested that there were no distinctive enzymes for oxidizing syringaldehyde to syringate. Instead, the production of 0.51 mM syringyl alcohol indicated that syringaldehyde was reduced to the alcohol form. A similar reduction in aromatic aldehydes also appeared in our previous studies that when cultivated with R. opacus PD630 mutants, most vanillin was converted to vanillyl alcohol, instead of vanillate (27). To improve the yield of gallate from lignin components, these aromatic aldehydes should also be effectively integrated into the gallate production funnel.
According to previous studies, the aldehyde dehydrogenase DesV, which can oxidize syringaldehyde to its acid form with nicotinamide adenine dinucleotide as an electron acceptor, has been characterized from Sphingobium sp. SYK-6 (63). To verify the effectiveness of DesV in R. opacus PD630, the corresponding gene was introduced into R. opacus PD630-△p△c, generating R. opacus PD630-DesV. After cultivation with 1 mM syringaldehyde, a 0.99 mM syringate was generated by R. opacus PD630-DesV (fig. S11), indicating that DesV was efficient for syringaldehyde conversion in R. opacus PD630. Thereafter, all confirmed functional genes involved in the abovementioned hydroxylation, O-demethylation, and aryl side-chain oxidation reactions—including pobA**, desA, ligM, metF, ligH, and desV—were integrated into plasmid pK18mob-PB264-GA3 (fig. S12) and introduced into R. opacus PD630-△p△c, generating R. opacus PD630-GA4. As shown in Fig. 7A, with an initial OD600 value of 5.0, R. opacus PD630-GA4 completely catalyzed 1 mM syringaldehyde into syringate in the first 4 hours, which was further converted to gallate with a concentration as high as 1.00 mM, indicating the effectiveness of the introduced aryl side-chain oxidation system.
Fig. 7. Gallate production from syringaldehyde and coniferyl alcohol with heterologous and native aryl side-chain oxidation systems, respectively.
(A) Syringaldehyde (1 mM) was cultivated with R. opacus PD630-GA4. (B) Gallate production with 1 mM coniferyl alcohol as substrate and R. opacus PD630-GA4 as a biocatalyst. The data are presented as the means ± SD of three biological replicates.
In addition to aromatic aldehydes, some aromatic alcohols can also be oxidized to corresponding products. Using coniferyl alcohol, one of the main components in softwood lignin (64), as an example, it was reported that coniferyl alcohol can be catalyzed to coniferyl aldehyde and further to ferulate (65). According to our previous studies, there are native coniferyl alcohol metabolic pathways in R. opacus PD630, with ferulate and vanillate as important intermediates. From the above information, vanillate can be converted to gallate with native O-demethylation and an introduced hydroxylation system. Thus, in theory, coniferyl alcohol can also be converted to gallate with vanillate as an intermediate. As presented in Fig. 7B, when 1 mM coniferyl alcohol and R. opacus PD630-GA4 were applied as the substrate and biocatalyst, respectively, the intermediates of ferulate and vanillate were detected at concentrations of 0.35 mM at 2 hours and 0.31 mM at 4 hours, respectively. The highest gallate concentration reached 0.93 mM at 32 hours with a yield of 93% from coniferyl alcohol, suggesting that coniferyl alcohol was transformed into gallate with continuous reactions of aryl side-chain oxidation, O-demethylation, and hydroxylation reactions.
On the basis of previous studies, various methods can be used for breaking the side chain of lignin and depolymerizing lignin into low–molecular weight fragments, which contain multiple aryl side chains (7). In theory, with appropriate oxidation methods, the obtained low–molecular weight lignin fragments can be directly converted to specific aromatic alcohols, aldehydes, and acids (7). In the current work, the aryl side-chain oxidation system was the primary reaction for producing gallate from syringaldehyde, as well as coniferyl alcohol, by R. opacus PD630-GA4. In addition, the O-demethylation, β-oxidation, and hydroxylation reactions also participated in these upgrading processes.
Gallate production from mixed lignin-derived aromatics
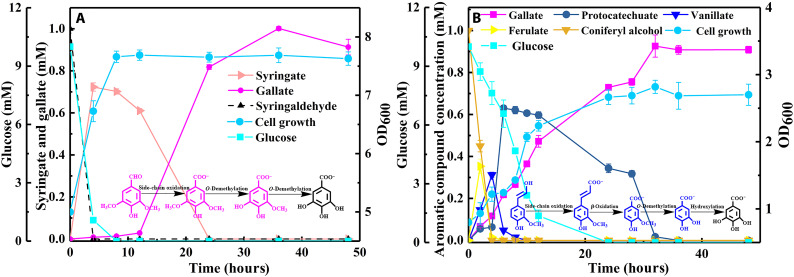
To further demonstrate our design in which gallate can be produced from multiple lignin-derived compounds with integrated hydroxylation, O-demethylation, and aryl side-chain oxidation systems, we applied mixed G/H/S-type aromatics—including 1 mM p-coumarate, 1 mM ferulate, 1 mM vanillate, 1 mM p-hydroxybenzoate, 1 mM syringaldehyde, and 1 mM sinapate—as substrates for gallate production. As shown in Fig. 8A, p-coumarate, ferulate, vanillate, and p-hydroxybenzoate were depleted within 4 hours, while protocatechuate reached the highest concentration of 3.44 mM. Syringaldehyde was completely transformed into syringate in the first 2 hours, and syringate was subsequently O-demethylated to gallate. It was hypothesized that sinapate could be converted to syringaldehyde by FerAB (FerA: feruloyl-CoA synthetase; FerB: feruloyl-CoA hydratase/lyase) with β-oxidation and further oxidized to syringate by LigV in N. aromaticivorans (39). Here, the results showed that the consumed sinapate was low, and 0.56 mM sinapate remained in the culture broth at 48 hours, which indicated that the corresponding enzymes might be less active in R. opacus PD630. To further investigate sinapate metabolism in R. opacus PD630, 1 mM sinapate was used to cultivate R. opacus PD630 and R. opacus PD630-GA4 with initial OD600 values of 0.8 and 5.0, respectively (fig. S13). Sinapate was barely consumed in R. opacus PD630 within 120 hours of cultivation. Nevertheless, sinapate was decreased to 0.677 mM along with the accumulation of 0.01 mM gallate in R. opacus PD630-GA4 culture broth, which was consistent with the low sinapate consumption in mixed aromatics.
Fig. 8. Gallate production from mixed lignin-derived aromatics, alkaline-pretreated lignin, and base-depolymerized AFEX lignin using R. opacus PD630-GA4 as a biocatalyst.
(A) Mixed aromatics of 1 mM p-coumarate, 1 mM ferulate, 1 mM vanillate, 1 mM p-hydroxybenzoate, 1 mM syringaldehyde, and 1 mM sinapate as substrate. (B) Gallate production from 2.5% alkaline-pretreated lignin (v/v, lignin solution/fermentation medium). (C) Gallate production from 5.0% alkaline-pretreated lignin (v/v, lignin solution/fermentation medium). (D) Gallate production from 10.0% alkaline-pretreated lignin (v/v, lignin solution/fermentation medium). (E) Gallate production from base-depolymerized AFEX lignin (0.25 g/liter). (F) Gallate production from base-depolymerized AFEX lignin (0.5 g/liter). The data are presented as the means ± SD of three biological replicates.
As mentioned above, the hydroxylation of protocatechuate is a vital scenario for gallate production from lignin components, whereas the conversion rate of protocatechuate to gallate was relatively low after 12 hours when mixed aromatic compounds were applied as substrates. Gallate production from glucose has been carried out in some engineered E. coli strains, and protocatechuate also accumulates in most cases (48). Here, to explore gallate productivity from protocatechuate in detail, a gradient concentration of protocatechuate was tested with an initial OD600 of 0.8 or 5.0 for R. opacus PD630-GA4. As shown in fig. S14A, when the initial protocatechuate concentration was 2 mM and the initial OD was 0.8, protocatechuate was completely consumed, and the final gallate concentration reached 1.86 mM with a yield of 93.00%. However, when the protocatechuate concentration was increased to 3 and 4 mM, the gallate yield decreased significantly to 88.33 and 84.50%, respectively (fig. S14, B and C). When the initial OD600 value was increased to 5.0, 6.75 mM gallate was produced from 10 mM protocatechuate with a gallate yield of 67.50% (fig. S14D). To unravel the underlying mechanisms for the decreased gallate yield at a high protocatechuate concentration, gene expression levels of some vital genes in R. opacus PD630-GA4 were determined at 8 and 24 hours. The results showed an appropriately 40% decrease in expression of pobA** and desA at 24 hours compared with those at 8 hours, although kanamycin (50 μg/ml) was added in the culture broth to maintain the stable existence of pK18mob-PB264-GA3 plasmid (fig. S15). It is important to note that 10 mM glucose, the main supplier for carbon source, adenosine triphosphate (ATP), cofactors, etc., was consumed up within 8 to 20 hours in most cases of this study. The glucose depletion in the late fermentation stage might have contributed to the decreased gene expression levels, and maintaining a reasonable glucose level in culture broth could potentially be beneficial to a high gallate yield. Future studies—including enhancing the hydroxylation efficiency for protocatechuate of PobA** with rational engineering, applying a fed-batch fermentation to maintain the glucose concentration in culture broth, as well as enhancing the strain stability by integrating functional genes into genome—could be performed to improve the gallate production with high concentrations of substrate.
Gallate production from alkaline-pretreated lignin and base-depolymerized AFEX lignin
As a proof of concept, R. opacus PD630-GA4 with integrated hydroxylation, O-demethylation, and aryl side-chain oxidation systems was inoculated into different concentrations of alkaline-pretreated lignin prepared from corn stover for gallate production. To reduce the inhibition of cell growth by high concentrations of lignin components, we diluted alkaline-pretreated lignin to 2.5% (v/v) and 5% (v/v). As shown in Fig. 8 (B and C), when 2.5% (v/v) and 5% (v/v) alkaline-pretreated lignin streams were applied, p-coumarate and ferulate, the two main components in the lignin stream, were rapidly depleted in the first 4 hours. Meanwhile, the protocatechuate reached 0.38 and 1.16 mM and was subsequently completely converted to gallate. The highest gallate concentration reached 1.40 mM (0.239 g/liter) at 8 hours for the 2.5% (v/v) alkaline-pretreated lignin stream and 2.48 mM (0.422 g/liter) at 32 hours for the 5% (v/v) alkaline-pretreated lignin stream. Significantly, with a 2.5% (v/v) alkaline-pretreated lignin stream as the substrate, the highest yield of 0.407 g of gallate per gram of lignin was achieved (table S3), suggesting great potential for lignin upgrading. However, when the alkaline-pretreated lignin stream was increased to 10% (v/v), protocatechuate accumulated at a concentration of 1.43 mM at 48 hours, and the gallate concentration only reached 1.67 mM (0.284 g/liter) (Fig. 8D). This result was consistent with the previously mentioned phenomenon that the gallate yields decreased with high protocatechuate concentrations (fig. S14D). To quantify the gallate production process from lignin, we determined lignin concentration by the protocol from the National Renewable Energy Laboratory (NREL; lignin contents are shown in table S4) (66). As predicted, p-coumarate and ferulate were two major components of the prepared lignin solution (table S5) (10). On the basis of the results of this study, both p-coumarate and ferulate are suitable substrates for gallate production with theoretical molar conversion rates of 100% by the constructed biocatalytic system. Nevertheless, gallate was not only produced from p-coumarate and ferulate in the lignin stream, but in fact, gallate yields were 150 and 134.31% based on the contents of these two substrates in 2.5% (v/v) and 5% (v/v) alkaline-pretreated lignin streams, respectively, suggesting that other lignin-derived aromatics also contributed to gallate production (table S6). To further analyze the lignin structural changes during the biocatalysis process reported here, we conducted two-dimensional heteronuclear single-quantum coherence–nuclear magnetic resonance (2D-HSQC-NMR). The chemical shift region was assigned on the basis of previous reports (16, 67). Related signals showed that polymeric lignin remained after biocatalysis, including G-lignin units, H-lignin units, S-lignin units, methoxy groups [δC/δH 54 to 57/3.1 to 4.5 parts per million (ppm)], and β-O-4 structures [Aα (δC/δH 70 to 73/4.8 to 5.3)] (fig. S16, B to E). The amount of residual β-O-4 structures was consistent with previous studies that R. opacus PD630 has low lignin depolymerization capability (24, 27). Further, gel permeation chromatography (GPC) analysis showed that lignin components with low molecular weight were consumed when cultivated with R. opacus PD630-GA4 (fig. S17A). The combination of 2D-HSQC-NMR and GPC results suggested that R. opacus PD630-GA4 converted vast low–molecular weight lignin components, instead of high–molecular weight ones, to gallate, and appropriate lignin-depolymerization methods are beneficial to this biological lignin valorization process.
Furthermore, to verify the effectiveness of the constructed biocatalytic system, AFEX lignin, prepared from the AFEX pretreatment process, was also applied for gallate production. As shown in fig. S18, when 0.25, 0.5, and 1.0 g/liter of AFEX lignin were used as substrates for gallate production, the highest gallate concentrations of 0.012, 0.021, and 0.036 mM were obtained, respectively. The yields of gallate from AFEX lignin were relatively low compared with those from the abovementioned alkaline-pretreated lignin, and thus, base-catalyzed depolymerization was applied for AFEX lignin to enhance its biological availability. As shown in table S7, most AFEX lignin was dissolved in alkali liquor, whereas about 79.38% AFEX lignin was precipitated after pH adjustment to neutral, and there was soluble base-depolymerized lignin (1.47 g/liter) after pH adjustment. With soluble base-depolymerized AFEX lignin as a substrate, the gallate titers reached 0.888 mM at 8 hours from 0.25 g/liter of lignin, 1.853 mM at 48 hours from 0.5 g/liter of lignin, and 2.469 mM at 48 hours from 1.0 g/liter of lignin (Fig. 8, E and F, and fig. S19). Similar to alkaline-pretreated lignin, p-coumarate, ferulate, p-hydroxybenzoate, vanillate, and syringate were detected in base-depolymerized AFEX lignin solution. Gallate yields were approximately 159 and 167% based on the determined p-coumarate, ferulate, p-hydroxybenzoate, vanillate, and syringate in base-depolymerized AFEX lignin solution (0.25 and 0.5 g/liter), suggesting that other lignin components also contributed to gallate production (table S8). On the basis of the 2D-HSQC-NMR analysis for the AFEX lignin samples before and after the base-catalyzed depolymerization process, there was an obvious decrease in β-O-4 alkyl aryl ether signals after the base-catalyzed depolymerization process (fig. S16G), which confirmed the depolymerization of lignin in the alkaline solution. The GPC analysis also suggested that most generated low–molecular weight lignin components were consumed by R. opacus PD630-GA4 for gallate production (fig. S17B). The above results indicated that appropriate lignin depolymerization methods are beneficial to enhance the biological availability of lignin resources, and base-catalyzed lignin depolymerization method is just such an approach. Overall, gallate was produced from lignin and lignin-derived aromatics by integrated biocatalytic reactions, including hydroxylation, O-demethylation, and oxidation. With these catalytic reactions integrated in a chassis cell, a simple and efficient biocatalytic process for lignin valorization was realized instead of the traditional chemical approaches that require several reactors to realize these three reactions (12–15, 68).
DISCUSSION
Gallic acid is a phenolic acid widely exploited for its antioxidant, antimicrobial, anti-inflammatory, and anticancer activities (22). Currently, gallic acid is mainly obtained by hydrolysis of tannins with acids, bases, or microbial tannase (48). Nevertheless, no matter which hydrolysis method is applied, tannins are the most used substrate. The planting area and harvest time of relevant plants contribute to the fluctuation of tannin price and, further, gallic acid. Considering that lignin is the most abundant renewable aromatic resource on Earth, it is of great potential to prepare gallic acid from lignin. In this study, an efficient biocatalytic process was designed for converting lignin, the most abundant renewable aromatic in the atmosphere, to gallate with R. opacus PD630 mutants as biocatalysts. In detail, three main reactions of hydroxylation, O-demethylation, and aryl side-chain oxidation were integrated in a gallate degradation pathway–blocked R. opacus PD630 cell, and this developed biocatalyst can unify multiple G-lignin, H-lignin, and S-lignin–derived aromatics to gallate (Fig. 9). Ultimately, when lignin extracted from corn stover by an alkaline method was applied as a substrate for gallate production, a gallate yield as high as 0.407 g/g of lignin was obtained. In another case, a gallate yield of 0.630 g/g of lignin was obtained when base-depolymerized AFEX lignin solution was applied as the substrate. The constructed R. opacus PD630-GA4 biocatalyst provided a sustainable and convenient approach for producing gallate from lignin resources.
Fig. 9. Integrated biocatalytic reactions involved in gallate production from lignin by R. opacus PD630-GA4.
Lignin was depolymerized to aromatic monomers and further catalyzed to gallate with the combined hydroxylation, O-demethylation, and oxidation systems. C23D, putative catechol 2,3-dioxygenase; P34D, protocatechuate 3,4-dioxygenase; PobA**, p-hydroxybenzoate hydroxylase (Y386F/T295A); PobA, p-hydroxybenzoate hydroxylase; Fcs, feruloyl-CoA synthetase; Ech, enoyl-CoA hydratase/aldolase; Vdh, aldehyde dehydrogenase; CalA, coniferyl alcohol dehydrogenase; CalB, coniferyl aldehyde dehydrogenase; VanA, vanillate demethylase; VanB, vanillate O-demethylase oxidoreductase; DesA, syringate O-demethylase; LigM, vanillate/3-O-methyl gallate O-demethylase; LigH, 10-formyltetrahydrofolate synthetase; MetF, 5,10-methylenetetrahydrofolate reductase; DesV, benzaldehyde-derivatives dehydrogenase; GlyA, glycine hydroxymethyltransferase; FolD, methenyltetrahydrofolate cyclohydrolase; Ru5P, ribulose 5-phosphate; Hu6P, d-arabino-3-hexulose-6-phosphate; RuMP, ribulose monophosphate pathway; PPP, pentose phosphate pathway; 3-MGA, 3-O-methylgallate; AMP, adenosine monophosphate.
Numerous studies on lignin conversion have been conducted with R. opacus PD630 as a chassis cell, and some metabolic pathways for lignin-derived aromatics have been illustrated in R. opacus PD630 (21, 27, 40–43, 69). However, the endogenous genome of R. opacus PD630 is still a valuable resource to be mined, especially for genes related to the biological degradation/conversion of aromatic and heterogeneous compounds, because this strain was originally isolated from soils of a gas-working plant. In this study, 2,3-cleavage or 5,6-cleavage of gallate was predicated in R. opacus PD630, instead of the reported 3,4-cleavage or 4,5-cleavage in Sphingomonas sp. (37), P. putida (38), and N. aromaticivorans (39). This verified gallate cleavage enzyme also exhibited extradiol dioxygenase activity on 4-methyl-catechol in our previous studies (27). With the understanding of the cleavage mode of gallate, the complete pathway for gallate biodegradation in R. opacus PD630 can be unraveled in future studies.
With the gallate degradation pathway–blocked R. opacus PD630 as a chassis, a series of recombinant strains were constructed, and then multiple aromatic monomers, as well as two types of lignin, were applied as substrates to produce gallate with constructed R. opacus PD630 stains as biocatalysts. As a result, most tested aromatic monomers led to high gallate yields. In contrast, low gallate yields resulted from raw AFEX lignin samples. Nevertheless, alkali lignin and base-depolymerized AFEX lignin both led to high gallate yields. On the basis of previous studies and the process analysis in this study, lignin can be depolymerized to multiple aromatic monomers in alkaline conditions, which can be easily assimilated by R. opacus PD630. However, the inherent lignin-depolymerization ability of native R. opacus PD630 was weak, and, thus, many lignin oligomers and polymeric lignin remained unused. In nature, some fungi and bacteria can depolymerize lignin to low–molecular weight components by secreting laccase, peroxidase, dye-decolorizing peroxidase, etc. Consolidated simultaneous lignin depolymerization and product generation bioprocesses have been proven effective in some previous studies (16, 70). In this study, we also tried to construct a consolidated lignin biological conversion process for gallate production by adding laccase into the culture broth. However, there was no obvious increase in gallate production. Instead, decreased gallate productions were observed in some cases. Further studies demonstrated that laccase not only depolymerized lignin but also functioned on gallate. Therefore, it is not a practical approach to produce aromatic compounds sensitive to oxygen from lignin by adding ligninolytic enzymes because these enzymes generally can also function on aromatic products. To produce these compounds from lignin resources, enhancing lignin depolymerization with chemical approaches for microbial utilization is straightforward and effective (28).
In this study, the gallate yields were relatively high when low concentrations of substrates (both aromatic monomers and alkaline-pretreated lignin) were applied. In contrast, when high concentrations of substrates were applied, obvious decreases in gallate yields were observed. The decreased gallate yields might be due to a comprehensive result of several factors: (i) As the main supplier for carbon source, ATP, cofactors, etc., the initially added glucose was consumed up within 8 to 20 hours in most cases, resulting in a weak cell activity and hence a low substrate conversion efficiency. Maintaining a reasonable glucose level in culture broth will be beneficial to a high gallate yield. (ii) The mutant enzyme PobA** developed in this work exhibited high activity for protocatechuate at low concentrations. When the concentration of protocatechuate increased, the conversion of protocatechuate to gallate was inhibited, which was consistent with the accumulation of protocatechuate in some cases. Protein engineering on improving the activity of PobA** is essential for producing gallate from high concentrations of protocatechuate and other lignin-derived aromatics. (iii) Target genes were inserted into a free replicable plasmid for expression in this study, and a dose of kanamycin was added in the culture broth to maintain strain stability. A genome-integrated gene expression cassette may be helpful for both stable expression of target genes and reducing the expense on kanamycin, especially in industrial applications at a large scale. (iv) The autoxidation of gallate was observed with a high gallate titer. Controlling the dissolved oxygen at a low level may prevent autoxidation and lead to a higher gallate titer. Similarly, the pyrogallol titer was enhanced by adding a suitable oxygen-scavenging agent, ascorbic acid, to alleviate the autoxidation of produced pyrogallol (71). (v) The toxicity of the produced gallate toward host cells mainly limited the production of gallate. Converting toxic compounds to their nontoxic analogs is an alternative way to solve these issues (72). In some plants, gallate is converted to the β-glucogallin under gallate 1-O-galloyltransferase, which can provide a feasible approach for microbial gallate detoxification (73). Moreover, adaptive evolution is also a common strategy to improve microbial tolerance to multifarious aromatic compounds (72). The adaptive evolution for the constructed R. opacus PD630 will be beneficial for both improving its tolerance to the produced gallate and aromatic substrates.
This work also provides valuable information for the valorization of other resources. For instance, the O-demethylation of methoxy aromatics also plays a vital role in C1 compound utilization (59); the β-oxidation of long aliphatic chain–substituted aromatics is an alternative method for acetyl-CoA generation. Converting lignin into biobased polymers or polymer building blocks has recently drawn enormous interest, during which the functionalization of phenol moieties was the main strategy for polymer production (7, 64). Both of the mentioned biological hydroxylation and O-demethylation systems in this study can be useful for efficiently introducing phenolic hydroxyl groups into lignin-derived phenolics. In addition, this study can also provide a reference for the production of other aromatics from lignin resources, as well as for the utilization of other resources with mixed components.
MATERIALS AND METHODS
Media, strains, primers, plasmids, and chemicals
Luria-Bertani (LB) medium consisting of yeast extract (5 g/liter), NaCl (10 g/liter), and tryptone (10 g/liter) was used for routine inoculation of E. coli, R. opacus PD630, and their derivatives. M9 medium (pH 7.0) consisting of Na2HPO4·12H2O (13.56 g/liter), KH2PO4 (6 g/liter), NaCl (1 g/liter), NH4Cl (2 g/liter), MgSO4·7H2O (0.492 g/liter), CaCl2 (0.111 g/liter), and 10 ml of Hoagland trace element solution (Sigma-Aldrich, H2395) with 10 mM glucose and different doses of lignin components was used for gallate production. When needed, kanamycin was added to the medium to a final concentration of 50 μg/ml. The strains and primers used in this study are listed in tables S9 and S10.
Plasmid construction and DNA manipulation
The replication plasmid pK18mob-PB264, constructed by combining pK18mob and R. opacus replicon PB264, was applied as a skeleton plasmid for gene expression in R. opacus strains. The ligations of the target gene expression cassette and plasmid were performed with the ClonExpress II One Step Cloning Kit (Vazyme Biotech Co. Ltd., Nanjing, China). The nucleotide fragments of metF and ligH were amplified from Sphingobium sp. SYK-6 using primers in table S10. The nucleotide fragments of desV, desA, and ligM were optimized on the basis of the codon bias of R. opacus species (table S11) and synthesized by Genscript Biotech (Nanjing, China).
Protein expression and purification
Target nucleotide fragments from the R. opacus PD630 genome were amplified with the relevant primers in table S10 and then inserted into pET-28a. The verified recombinant vector was introduced to E. coli BL21 (DE3) for protein expression. In detail, the recombinant E. coli strain was cultivated in 100 ml of LB medium at 37°C until its OD600 value reached approximately 0.6, and then isopropyl β-d-1-thiogalactopyranoside was added to the broth at a final concentration of 0.2 mM to induce the expression of target genes. The induction of protein expression was performed at 30°C and 250 rpm for 24 hours, and then the cells were harvested with centrifugation at 8000g for 30 min. After washing with 50 mM tris-HCl (pH 8.0), the obtained cells were lysed with an ultrasonic breaker, and the crude extract was centrifuged at 10,000g for 30 min. The supernatant was loaded onto Ni–nitrilotriacetic acid resin (GE Healthcare), and the target protein was eluted with 5 ml of 50 mM tris-HCl buffer (pH 8.0) containing 200 mM imidazole, followed by analysis with SDS–polyacrylamide gel electrophoresis. The concentration of the obtained protein was determined by the Bradford method.
In vitro hydroxylation of protocatechuate with purified enzymes
The in vitro protocatechuate hydroxylation system—which contained 100 mM tris-HCl (pH 8.0), 10 μM flavin adenine dinucleotide, 1 mM NADPH, 50 nM purified protein, and 1 mM protocatechuate, with three purified PobAs—was constructed as described by Chen et al. (48). The reaction was initiated with protocatechuate addition, and then the mixture was incubated at 30°C for 8 min. Thereafter, the reaction was terminated with 100 mM HCl, and the terminated reaction mixture was subsequently measured with high-performance liquid chromatography (HPLC) to determine the consumption of protocatechuate and generation of gallate.
Cultivation of relevant microorganisms
For microorganism cultivation, a positive colony was inoculated into 5 ml of LB liquid medium until its OD600 reached approximately 1.0. Then, the culture broth was transferred into 50 ml of LB liquid medium and cultivated at 250 rpm and 30°C. After 24 hours of cultivation, cells were harvested by centrifugation at 4000 rpm and washed twice with physiological saline [0.9% NaCl (w/w)]. Then, the washed cells were inoculated into M9 medium containing 10 mM glucose and relevant lignin components with an initial OD600 value of 0.8 or 5.0. The inoculated broth was cultivated at 250 rpm and 30°C. Each experiment was performed in triplicate. Cell growth was determined with a spectrophotometer, and the concentrations of lignin-derived aromatics and gallate were determined by HPLC.
Preparation of alkaline-pretreated lignin from corn stover
Lignin extraction from corn stover was performed as described by Rodriguez et al. (10) with some modifications: 100 g of corn stover was added into a 2-liter reactor containing 2% NaOH solution at a solid loading of 10% (w/v) and then the reaction system was maintained at 120°C for 40 min. After the reaction, the solid and liquid fractions were separated by filtration with gauze, and the pH of the obtained slurry was adjusted to 7.0 with 5 M H2SO4. Then, the neutralized solution was centrifuged at 10,000 rpm for 40 min and filtered through filter paper, and soluble lignin was obtained. The prepared lignin solution was filtered with a 0.22-μm filter and then stored at −20°C until use, dubbed alkaline-pretreated lignin. When needed, the prepared alkaline-pretreated lignin was added to the culture broth for lignin conversion by constructed R. opacus PD630 biocatalysts.
Preparation of AFEX lignin and base-depolymerized AFEX lignin
AFEX lignin was prepared from corn stover with a classical AFEX pretreatment. The pretreated biomass was repeatedly and extensively hydrolyzed with cellulase, hemicellulase, and protease to remove cellulose, hemicellulose, and protein components. Then, the solid residues were washed twice with deionized water and freeze-dried for further studies. The solid residues were designated as AFEX lignin. For preparation of base-depolymerized AFEX lignin, base-catalyzed lignin depolymerization was conducted using the abovementioned methods in the “Preparation of alkaline-pretreated lignin from corn stover” section.
Determination of lignin-derived aromatics, gallate, and glucose
The concentrations of lignin-derived aromatics and gallate were analyzed by an HPLC system (Shimadzu, LC-20A). Before analysis, all samples were centrifuged at 13,000 rpm for 20 min. The supernatant was separated with a reversed-phase Poroshell EC-C18 column (4 μm, 4.6 mm by 150 mm; Agilent Technologies). A two-phase mobile phase system was used: Solvent A was water with 0.1% formic acid, and solvent B was acetonitrile with 0.1% formic acid. The gradient of the mobile phase was set as follows: 8 to 26% solvent B for 5 min, 26 to 8% solvent B for 3 min, and 8% solvent B for an additional 2 min. The flow rate of the mobile phase was 1.5 ml/min, and the column temperature was maintained at 60°C. Target compounds were quantified on the basis of the peak areas at an ultraviolet (UV) absorbance of 280 nm. The HPLC system equipped with a Bio-Rad Aminex HPX-87H column and a diode array detector was used for glucose analysis. The mobile phase of the HPLC system was 0.005 M sulfuric acid with a flow rate of 0.6 ml/min.
Lignin quantification
Lignin quantification was performed according to the protocol “Determination of Structural Carbohydrates and Lignin in Biomass” (66) from NREL. The lignin content in this work included insoluble and soluble lignin, which were determined by oven and UV detection as described in the protocol, respectively. Alkaline-pretreated lignin and base-depolymerized AFEX lignin were freeze-dried first for lignin quantitation, and the detailed procedures are described as follows. Briefly, 0.3 g of lignin sample (dry weight, ODWsample) was added into a tared pressure tube with 3 ml of 72% sulfuric acid and mixed thoroughly for 1 min. The tube was maintained in a 30°C water bath for 60 min, and 84 ml of deionized water was subsequently added. Then, the tube was placed in an autoclave at 121°C for 60 min until the hydrolysates were cooled to room temperature.
For acid-insoluble lignin (AIL), the hydrolysate was filtered with a previously weighed filtering crucible (Weightcrucible), and the filtrate was captured in a filtering flask (Volumefiltrate). The remaining solids in filtering crucibles were washed with deionized water and dried at 105°C to constant weight. The weight of the crucible and dry residue (Weightcrucible plus AIR) was recorded when the sample was cooled to room temperature. The crucibles and residue were placed in a muffle furnace at 575°C for 6 hours, and the weight (Weightcrucible plus ash) was recorded when the sample was cooled to room temperature. The AIL was calculated using the following formula
For acid-soluble lignin (ASL), the filtrate was diluted to its absorbance in the range of 0.7 to 1.0 at 340 nm with a UV-visible spectrophotometer, and the dilution and absorbance (UVabs) were recorded. The amount of ASL was calculated using the following formula
where, ε = the absorptivity of the biomass at a specific wavelength (30 liter/g cm for corn stover lignin); Volumefiltrate = the volume of the filtrate, 86.73 ml; and Pathlength = the pathlength of UV-visible cell in centimeters.
Two-dimensional heteronuclear single-quantum coherence–nuclear magnetic resonance
The 2D 1H-13C HSQC-NMR procedures were carried out as Li et al. described (74). Briefly, a 100-mg freeze-dried lignin sample was dissolved in 1 ml of dimethyl sulfoxide–d6 and centrifuged at 12,000 rpm for 2 min, and the supernatant was used for detection. The standard Bruker pulse sequence “hsqcetgpsi.2” was used. The spectral widths of 1H and 13C were 13.02 and 220.00 ppm, respectively. HSQC-NMR data and plots were processed with MestReNova. As for preparation of lignin samples, the liquors before and after biocatalysis were centrifuged at 13,000 rpm for 40 min, and the resulted supernatants were filtrated through 0.22-μm filters. The filtrate pH was then adjusted to 1.0 with 2 M H2SO4, which led to relevant lignin components precipitated from filtrates. The obtained precipitate was washed twice with 20 mM H2SO4 to remove salts and then free-dried for further analysis (75).
GPC analysis
The methodology for GPC used in this study was referenced from Rodriguez et al. (10). In detail, a 20-mg prepared lignin sample was acetylated with an acetic anhydride (0.5 ml) and pyridine (0.5 ml) mixture at 40°C for 24 hours. Then, the reaction was terminated with the addition of 0.2 ml of methanol. The solvent was evaporated in a nitrogen gas atmosphere, and the sample was dried at 40°C overnight. Following dissolution in tetrahydrofuran (THF), the sample was filtered with 0.45-μm polytetrafluoroethylene filters for GPC analysis. Agilent HPLC equipped with three GPC columns (Polymer Laboratories; 7.5-mm inner diameter by 300-mm length) was used for GPC analysis, and THF was used as the eluent with a flow rate of 1 ml/min. Polystyrene was used to build a standard curve at an absorbance of 260 nm with a diode array detector. The samples used for GPC analysis were prepared with the same acid precipitation method as for 2D-HSQC-NMR sample preparation.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number 21808111) and the National Natural Science Foundation of Jiangsu Province (grant numbers BK20170829 and BK20170037). Author contributions: C.C., H.Z., S.C., and Z.X. performed the experiments, analyzed the data, and participated in experiment design. C.C. and Z.X. wrote the draft manuscript. Z.X. and M.J. conceived and designed the experiments, supervised the work, analyzed the data, and confirmed the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S19
Tables S1 to S11
REFERENCES AND NOTES
- 1.Chio C., Sain M., Qin W., Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sust. Energ. Rev. 107, 232–249 (2019). [Google Scholar]
- 2.Becker J., Wittmann C., A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 37, 107360 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ragauskas A. J., Beckham G. T., Biddy M. J., Chandra R., Chen F., Davis M. F., Davison B. H., Dixon R. A., Gilna P., Keller M., Langan P., Naskar A. K., Saddler J. N., Tschaplinski T. J., Tuskan G. A., Wyman C. E., Lignin valorization: Improving lignin processing in the biorefinery. Science 344, 1246843 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Boerjan W., Ralph J., Baucher M., Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Rahimi A., Ulbrich A., Coon J. J., Stahl S. S., Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Bugg T. D. H., Williamson J. J., Rashid G. M. M., Bacterial enzymes for lignin depolymerisation: New biocatalysts for generation of renewable chemicals from biomass. Curr. Opin. Chem. Biol. 55, 26–33 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Sun Z., Fridrich B., de Santi A., Elangovan S., Barta K., Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 118, 614–678 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakzeski J., Bruijnincx P. C., Jongerius A. L., Weckhuysen B. M., The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Shuai L., Amiri M. T., Questell-Santiago Y. M., Heroguel F., Li Y., Kim H., Meilan R., Chapple C., Ralph J., Luterbacher J. S., Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez A., Salvachúa D., Katahira R., Black B. A., Cleveland N. S., Reed M., Smith H., Baidoo E. E. K., Keasling J. D., Simmons B. A., Beckham G. T., Gladden J. M., Base-catalyzed depolymerization of solid lignin-rich streams enables microbial conversion. ACS Sustain. Chem. Eng. 5, 8171–8180 (2017). [Google Scholar]
- 11.Mei Q., Liu H., Shen X., Meng Q., Liu H., Xiang J., Han B., Selective utilization of the methoxy group in lignin to produce acetic acid. Angew. Chem. Int. Ed. Eng. 56, 14868–14872 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Song S., Zhang J., Gozaydin G., Yan N., Production of terephthalic acid from corn stover lignin. Angew. Chem. Int. Ed. Eng. 58, 4934–4937 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Shen X., Meng Q., Mei Q., Liu H., Yan J., Song J., Tan D., Chen B., Zhang Z., Yang G., Han B., Selective catalytic transformation of lignin with guaiacol as the only liquid product. Chem. Sci. 11, 1347–1352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X., Ludenhoff J. M., Dirks M., Ouyang X., Boot M. D., Hensen E. J. M., Selective production of biobased phenol from lignocellulose-derived alkylmethoxyphenols. ACS Catal. 8, 11184–11190 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan J., Meng Q., Shen X., Chen B., Sun Y., Xiang J., Liu H., Han B., Selective valorization of lignin to phenol by direct transformation of Csp2-Csp3 and C-O bonds. Sci. Adv. 6, eabd1951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvachúa D., Katahira R., Cleveland N. S., Khanna P., Resch M. G., Black B. A., Purvine S. O., Zink E. M., Prieto A., Martínez M. J., Martínez A. T., Simmons B. A., Gladden J. M., Beckham G. T., Lignin depolymerization by fungal secretomes and a microbial sink. Green Chem. 18, 6046–6062 (2016). [Google Scholar]
- 17.Del Cerro C., Erickson E., Dong T., Wong A. R., Eder E. K., Purvine S. O., Mitchell H. D., Weitz K. K., Markillie L. M., Burnet M. C., Hoyt D. W., Chu R. K., Cheng J. F., Ramirez K. J., Katahira R., Xiong W., Himmel M. E., Subramanian V., Linger J. G., Salvachua D., Intracellular pathways for lignin catabolism in white-rot fungi. Proc. Natl. Acad. Sci. U.S.A. 118, e2017381118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamimura N., Takahashi K., Mori K., Araki T., Fujita M., Higuchi Y., Masai E., Bacterial catabolism of lignin-derived aromatics: New findings in a recent decade: Update on bacterial lignin catabolism. Environ. Microbiol. Rep. 9, 679–705 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Linger J. G., Vardon D. R., Guarnieri M. T., Karp E. M., Hunsinger G. B., Franden M. A., Johnson C. W., Chupka G., Strathmann T. J., Pienkos P. T., Beckham G. T., Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. U.S.A. 111, 12013–12018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez J. I., Minambres B., Garcia J. L., Diaz E., Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ. Microbiol. 4, 824–841 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Xie S., Sun S., Lin F., Li M., Pu Y., Cheng Y., Xu B., Liu Z., da Costa Sousa L., Dale B. E., Ragauskas A. J., Dai S. Y., Yuan J. S., Mechanism-guided design of highly efficient protein secretion and lipid conversion for biomanufacturing and biorefining. Adv. Sci. 6, 1801980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes F. H. A., Salgado H. R. N., Gallic acid: Review of the methods of determination and quantification. Crit. Rev. Anal. Chem. 46, 257–265 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Badhani B., Sharma N., Kakkar R., Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 5, 27540–27557 (2015). [Google Scholar]
- 24.Kosa M., Ragauskas A. J., Lignin to lipid bioconversion by oleaginous Rhodococci. Green Chem. 15, 2070 (2013). [Google Scholar]
- 25.Vardon D. R., Franden M. A., Johnson C. W., Karp E. M., Guarnieri M. T., Linger J. G., Salm M. J., Strathmann T. J., Beckham G. T., Adipic acid production from lignin. Energy Environ. Sci. 8, 617–628 (2015). [Google Scholar]
- 26.Kohlstedt M., Starck S., Barton N., Stolzenberger J., Selzer M., Mehlmann K., Schneider R., Pleissner D., Rinkel J., Dickschat J. S., Venus J., van Duuren J. B. J. H., Wittmann C., From lignin to nylon: Cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab. Eng. 47, 279–293 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Cai C., Xu Z., Xu M., Cai M., Jin M., Development of a Rhodococcus opacus cell factory for valorizing lignin to muconate. ACS Sustain. Chem. Eng. 8, 2016–2031 (2020). [Google Scholar]
- 28.Barton N., Horbal L., Starck S., Kohlstedt M., Luzhetskyy A., Wittmann C., Enabling the valorization of guaiacol-based lignin: Integrated chemical and biochemical production of cis,cis-muconic acid using metabolically engineered Amycolatopsis sp ATCC 39116. Metab. Eng. 45, 200–210 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Spence E. M., Calvo-Bado L., Mines P., Bugg T. D. H., Metabolic engineering of Rhodococcus jostii RHA1 for production of pyridine-dicarboxylic acids from lignin. Microb. Cell Factories 20, 15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mycroft Z., Gomis M., Mines P., Law P., Bugg T. D. H., Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways. Green Chem. 17, 4974–4979 (2015). [Google Scholar]
- 31.Sainsbury P. D., Hardiman E. M., Ahmad M., Otani H., Seghezzi N., Eltis L. D., Bugg T. D. H., Breaking down lignin to high-value chemicals: The conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem. Biol. 8, 2151–2156 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Manfrao-Netto J. H. C., Lund F., Muratovska N., Larsson E. M., Parachin N. S., Carlquist M., Metabolic engineering of Pseudomonas putida for production of vanillylamine from lignin-derived substrates. Microb. Biotechnol., doi:10.1111/1751-7915.13764 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmore J. R., Dexter G. N., Salvachúa D., Martinez-Baird J., Hatmaker E. A., Huenemann J. D., Klingeman D. M., Peabody G. L., Peterson D. J., Singer C., Beckham G. T., Guss A. M., Production of itaconic acid from alkali pretreated lignin by dynamic two stage bioconversion. Nat. Commun. 12, 2261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson C. W., Beckham G. T., Aromatic catabolic pathway selection for optimal production of pyruvate and lactate from lignin. Metab. Eng. 28, 240–247 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Hong C. Y., Ryu S. H., Jeong H., Lee S. S., Kim M., Choi I. G., Phanerochaete chrysosporium multienzyme catabolic system for in vivo modification of synthetic lignin to succinic acid. ACS Chem. Biol. 12, 1749–1759 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Salmela M., Lehtinen T., Efimova E., Santala S., Santala V., Alkane and wax ester production from lignin-related aromatic compounds. Biotechnol. Bioeng. 116, 1934–1945 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Kasai D., Masai E., Miyauchi K., Katayama Y., Fukuda M., Characterization of the gallate dioxygenase gene: Three distinct ring cleavage dioxygenases are involved in syringate degradation by Sphingomonas paucimobilis SYK-6. J. Bacteriol. 187, 5067–5074 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nogales J., Canales A., Jimenez-Barbero J., Garcia J. L., Diaz E., Molecular characterization of the gallate dioxygenase from Pseudomonas putida KT2440. The prototype of a new subgroup of extradiol dioxygenases. J. Biol. Chem. 280, 35382–35390 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Cecil J. H., Garcia D. C., Giannone R. J., Michener J. K., Rapid, parallel identification of catabolism pathways of lignin-derived aromatic compounds in Novosphingobium aromaticivorans. Appl. Environ. Microbiol. 84, e01185-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoneda A., Henson W. R., Goldner N. K., Park K. J., Forsberg K. J., Kim S. J., Pesesky M. W., Foston M., Dantas G., Moon T. S., Comparative transcriptomics elucidates adaptive phenol tolerance and utilization in lipid-accumulating Rhodococcus opacus PD630. Nucleic Acids Res. 44, 2240–2254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeLorenzo D. M., Henson W. R., Moon T. S., Development of chemical and metabolite sensors for Rhodococcus opacus PD630. ACS Synth. Biol. 6, 1973–1978 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Henson W. R., Campbell T., DeLorenzo D. M., Gao Y., Berla B., Kim S. J., Foston M., Moon T. S., Dantas G., Multi-omic elucidation of aromatic catabolism in adaptively evolved Rhodococcus opacus. Metab. Eng. 49, 69–83 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Roell G. W., Carr R. R., Campbell T., Shang Z., Henson W. R., Czajka J. J., Martin H. G., Zhang F., Foston M., Dantas G., Moon T. S., Tang Y. J., A concerted systems biology analysis of phenol metabolism in Rhodococcus opacus PD630. Metab. Eng. 55, 120–130 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Eltis L. D., Bolin J. T., Evolutionary relationships among extradiol dioxygenases. J. Bacteriol. 178, 5930–5937 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasai D., Fujinami T., Abe T., Mase K., Katayama Y., Fukuda M., Masai E., Uncovering the protocatechuate 2,3-cleavage pathway genes. J. Bacteriol. 191, 6758–6768 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Entsch B., van Berkel W. J., Structure and mechanism of para-hydroxybenzoate hydroxylase. FASEB J. 9, 476–483 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Eschrich K., van der Bolt F. J., de Kok A., van Berkel W. J., Role of Tyr201 and Tyr385 in substrate activation by p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Eur. J. Biochem. 216, 137–146 (1993). [DOI] [PubMed] [Google Scholar]
- 48.Chen Z., Shen X., Wang J., Wang J., Yuan Q., Yan Y., Rational engineering of p-hydroxybenzoate hydroxylase to enable efficient gallic acid synthesis via a novel artificial biosynthetic pathway. Biotechnol. Bioeng. 114, 2571–2580 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Janusz G., Pawlik A., Sulej J., Swiderska-Burek U., Jarosz-Wilkolazka A., Paszczynski A., Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 41, 941–962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.C. L. Chen, Determination of methoxyl groups, in Methods in Lignin Chemistry, S. Y. Lin, C. W. Dence, Eds. (Springer Series in Wood Science, Springer Berlin Heidelberg, 1992), pp. 465–472. [Google Scholar]
- 51.Mallinson S. J. B., Machovina M. M., Silveira R. L., Garcia-Borras M., Gallup N., Johnson C. W., Allen M. D., Skaf M. S., Crowley M. F., Neidle E. L., Houk K. N., Beckham G. T., DuBois J. L., McGeehan J. E., A promiscuous cytochrome P450 aromatic O-demethylase for lignin bioconversion. Nat. Commun. 9, 2487 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machovina M. M., Mallinson S. J. B., Knott B. C., Meyers A. W., Garcia-Borras M., Bu L., Gado J. E., Oliver A., Schmidt G. P., Hinchen D. J., Crowley M. F., Johnson C. W., Neidle E. L., Payne C. M., Houk K. N., Beckham G. T., McGeehan J. E., DuBois J. L., Enabling microbial syringol conversion through structure-guided protein engineering. Proc. Natl. Acad. Sci. U.S.A. 116, 13970–13976 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fetherolf M. M., Levy-Booth D. J., Navas L. E., Liu J., Grigg J. C., Wilson A., Katahira R., Beckham G. T., Mohn W. W., Eltis L. D., Characterization of alkylguaiacol-degrading cytochromes P450 for the biocatalytic valorization of lignin. Proc. Natl. Acad. Sci. U.S.A. 117, 25771–25778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hibi M., Sonoki T., Mori H., Functional coupling between vanillate-O-demethylase and formaldehyde detoxification pathway. FEMS Microbiol. Lett. 253, 237–242 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Chen H. P., Chow M., Liu C. C., Lau A., Liu J., Eltis L. D., Vanillin catabolism in Rhodococcus jostii RHA1. Appl. Environ. Microbiol. 78, 586–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notonier S., Werner A. Z., Kuatsjah E., Dumalo L., Abraham P. E., Hatmaker E. A., Hoyt C. B., Amore A., Ramirez K. J., Woodworth S. P., Klingeman D. M., Giannone R. J., Guss A. M., Hettich R. L., Eltis L. D., Johnson C. W., Beckham G. T., Metabolism of syringyl lignin-derived compounds in Pseudomonas putida enables convergent production of 2-pyrone-4,6-dicarboxylic acid. Metab. Eng. 65, 111–122 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Abe T., Masai E., Miyauchi K., Katayama Y., Fukuda M., A tetrahydrofolate-dependent O-demethylase, LigM, is crucial for catabolism of vanillate and syringate in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 187, 2030–2037 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masai E., Sasaki M., Minakawa Y., Abe T., Sonoki T., Miyauchi K., Katayama Y., Fukuda M., A novel tetrahydrofolate-dependent O-demethylase gene is essential for growth of Sphingomonas paucimobilis SYK-6 with syringate. J. Bacteriol. 186, 2757–2765 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varman A. M., He L., Follenfant R., Wu W., Wemmer S., Wrobel S. A., Tang Y. J., Singh S., Decoding how a soil bacterium extracts building blocks and metabolic energy from ligninolysis provides road map for lignin valorization. Proc. Natl. Acad. Sci. U.S.A. 113, E5802–E5811 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao L., Yu L. L., Zhang J. J., Xie X. T., Tao Q., Yan X., Hong Q., Qiu J. G., He J., Ding D. R., A tetrahydrofolate-dependent methyltransferase catalyzing the demethylation of dicamba in Sphingomonas sp. strain Ndbn-20. Appl. Environ. Microbiol. 82, 5621–5630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Hidalgo J., Ravi K., Kure L. L., Liden G., Gorwa-Grauslund M., Identification of the two-component guaiacol demethylase system from Rhodococcus rhodochrous and expression in Pseudomonas putida EM42 for guaiacol assimilation. AMB Express 9, 34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellis E. S., Hinchen D. J., Bleem A., Bu L., Mallinson S. J. B., Allen M. D., Streit B. R., Machovina M. M., Doolin Q. V., Michener W. E., Johnson C. W., Knott B. C., Beckham G. T., McGeehan J. E., DuBois J. L., Engineering a cytochrome P450 for demethylation of lignin-derived aromatic aldehydes. JACS Au 1, 252–261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamimura N., Goto T., Takahashi K., Kasai D., Otsuka Y., Nakamura M., Katayama Y., Fukuda M., Masai E., A bacterial aromatic aldehyde dehydrogenase critical for the efficient catabolism of syringaldehyde. Sci. Rep. 7, 44422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duval A., Lawoko M., A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 85, 78–96 (2014). [Google Scholar]
- 65.Lubbers R. J. M., Dilokpimol A., Visser J., Makela M. R., Hilden K. S., de Vries R. P., A comparison between the homocyclic aromatic metabolic pathways from plant-derived compounds by bacteria and fungi. Biotechnol. Adv. 37, 107396 (2019). [DOI] [PubMed] [Google Scholar]
- 66.A. Sluiter, B.H.R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton, Determination of Structural Carbohydrates and Lignin in Biomass (Tech. Rep. NREL/TP-510-42618, National Renewable Energy Laboratory, 2012).
- 67.Mansfield S. D., Kim H., Lu F., Ralph J., Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 7, 1579–1589 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Ouyang X., Huang X., Boot M. D., Hensen E. J. M., Efficient conversion of pine wood lignin to phenol. ChemSusChem 13, 1705–1709 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holder J. W., Ulrich J. C., DeBono A. C., Godfrey P. A., Desjardins C. A., Zucker J., Zeng Q., Leach A. L., Ghiviriga I., Dancel C., Abeel T., Gevers D., Kodira C. D., Desany B., Affourtit J. P., Birren B. W., Sinskey A. J., Comparative and functional genomics of Rhodococcus opacus PD630 for biofuels development. PLOS Genet. 7, e1002219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salvachúa D., Karp E. M., Nimlos C. T., Vardon D. R., Beckham G. T., Towards lignin consolidated bioprocessing: Simultaneous lignin depolymerization and product generation by bacteria. Green Chem. 17, 4951–4967 (2015). [Google Scholar]
- 71.Wang J., Shen X., Yuan Q., Yan Y., Microbial synthesis of pyrogallol using genetically engineered Escherichia coli. Metab. Eng. 45, 134–141 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Li M., Liu C., Yang J., Nian R., Xian M., Li F., Zhang H., Common problems associated with the microbial productions of aromatic compounds and corresponding metabolic engineering strategies. Biotechnol. Adv. 41, 107548 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Muir R. M., Ibanez A. M., Uratsu S. L., Ingham E. S., Leslie C. A., McGranahan G. H., Batra N., Goyal S., Joseph J., Jemmis E. D., Dandekar A. M., Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia). Plant Mol. Biol. 75, 555–565 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., He Y., Zhang L., Xu Z., Ben H., Gaffrey M. J., Yang Y., Yang S., Yuan J. S., Qian W. J., Yang B., Discovery of potential pathways for biological conversion of poplar wood into lipids by co-fermentation of Rhodococci strains. Biotechnol. Biofuels 12, 60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z. H., Olson M. L., Shinde S., Wang X., Hao N., Yoo C. G., Bhagia S., Dunlap J. R., Pu Y., Kao K. C., Ragauskas A. J., Jin M., Yuan J. S., Synergistic maximization of the carbohydrate output and lignin processability by combinatorial pretreatment. Green Chem. 19, 4939–4955 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S19
Tables S1 to S11