Abstract
Background:
Wearable sensors allow for direct measurement of upper limb (UL) performance in daily life.
Objective:
To map the trajectory of UL performance and its relationships to other factors post stroke.
Methods:
Participants (n = 67) with 1st stroke and UL paresis were assessed at 2, 4, 6, 8, 12, 16, 20, and 24 weeks after stroke. Assessments captured UL impairment (Fugl-Meyer), capacity for activity (Action Research Arm Test), and performance of activity in daily life (accelerometer variables of use ratio and hours of paretic limb activity), along with other potential modifying factors. We modeled individual trajectories of change for each measurement level and the moderating effects on UL performance trajectories.
Results:
Individual trajectories were best fit with a 3-parameter logistic model, capturing the rapid growth early after stroke within the longer data collection period. Plateaus (90% of asymptote) in impairment (bootstrap mean±SE: 32±4 days post stroke) preceded those in capacity (41±4 days). Plateau in performance, as measured by the use ratio (24±5 days), tended to precede plateaus in impairment and capacity. Plateau in performance, as measured by hours of paretic activity (41±6 days), occurred at a similar time to that of capacity and slightly lagged impairment. Modifiers of performance trajectories were capacity, concordance, UL rehabilitation, depressive symptomatology, and cognition.
Conclusions:
UL performance in daily life approached plateau 3 to 6 weeks post stroke. Individuals with stroke started to achieve a stable pattern of UL use in daily life early, often before neurological impairments and functional capacity started to stabilize.
Keywords: Upper extremity, stroke, rehabilitation, outcome assessment, recovery
INTRODUCTION
Upper limb paresis and its resulting deficits are a major problem post stroke. Decades of research on recovery trajectories post stroke indicate that larger, rapid changes occur in the first few weeks, with smaller, slower changes occurring later.1–6 Changes in impairment generally precede changes in functional capacity by around one week, such that as movement control returns, individuals might regain the ability to execute functional tasks.1, 6 The World Health Organization separates the activity domain (i.e. the ability to execute functional tasks) into the capacity for activity vs. performance of activity in daily life.7 Capacity (or functional capacity) is what someone can do, assessed by standardized tests in structured, clinical or laboratory settings. Performance is what someone actually does in the unstructured, free-living environment (e.g. activities of daily living plus movements like arm swing during gait, gesturing, stretching, scratching). Note that this use of performance is different and distinct from using performance to describe the quality of a movement or the successful completion of an action of a standardized test. Advancements in wearable movement sensors now allow for direct measure of upper limb performance in daily life.8 The most common wearable sensors used are accelerometers, from which numerous clinically-relevant variables about upper limb activity can be computed.9–11 As with other indices of activity in daily life (e.g. steps/day as a measure of physical activity), wearable sensors provide a partial but not complete12 read out of upper limb performance in daily life. Emerging data suggest that upper limb capacity measures are not a good proxy for upper limb performance in daily life,13, 14 and open up new questions about trajectories of upper limb performance post stroke.
A few studies have explored upper limb performance early after stroke13, 15–17 or how it might change over time.18–20 Building on these, the purpose of the current study was to map the trajectory of upper limb performance and its relationships to impairment, capacity, and other factors over the course of stroke recovery. Biweekly and then monthly assessments were used to capture and then model precise time-courses of change out to nearly six months post stroke. Our hypothesis, based on work cited above,1 was that upper limb impairments improve first, followed by improvements in the capacity for activity and then improvements in upper limb performance in daily life. We further hypothesized that higher levels of performance would be driven by higher levels of capacity in the first month, the dominant hand being affected by stroke (i.e. concordance), and younger age at stroke onset, as these factors are typically linked with better outcomes.19, 21–23 Given that a key purpose for referring to and participating in upper limb rehabilitation services is to improve performance in daily life,24 knowledge gained from this study informs the content and timing of rehabilitation services. Later stabilization (plateaus) in performance would mean there is more time post stroke for motor rehabilitation interventions to be effective, while earlier stabilization would suggest that typical impairment and capacity-focused motor interventions might need to be paired with intentional health behavioral interventions25–27 to be effective in the early weeks after stroke.
METHODS
This study was a longitudinal, prospective cohort of persons with first time stroke. Participants were recruited from the stroke service of a large, urban United States hospital. First ever stroke survivors with residual upper limb paresis were enrolled within two weeks of their stroke. Inclusion criteria were: 1) within two weeks of a first-ever ischemic or hemorrhagic stroke, confirmed with neuroimaging; 2) presence of unilateral UL motor deficits within the first 24–48 hours post-stroke, as indicated by a National Institutes of Health Stroke Scale (NIHSS)28 Arm Item score of 1–4 or documented manual muscle test grade of <5 anywhere on the paretic UL; 3) able to follow a 2-step command, as measured by a NIHSS Command Item score of zero; and 4) anticipated return to independent or community living, as indicated by the acute stroke team. This last inclusion criteria removed potential participants from the sample who were to be discharged to and expected to stay in long-term care or hospice facilities, since these individuals are not typically referred to rehabilitation services with goals for improvement. Participants were excluded from the study if any of the following criteria were met: 1) history of previous stroke, neurological condition, or psychiatric diagnoses other than depression or anxiety; 2) presence of other comorbid conditions that may limit recovery (e.g. end-stage renal disease, stage IV cancer); 3) lived more than 90 minutes from study location; and 4) currently pregnant by self-report. The Human Research Protection Office at Washington University in St. Louis, MO approved this study and all participants provided written informed consent. Enrollment in this cohort was stopped early due to the COVID-19 global pandemic in March 2020. Beyond the safety of participants, it was possible that the significant disruption of daily life could influence performance data.
Participants completed eight assessment sessions over the first six months post-stroke. The assessment battery was administered by trained personnel at 2, 4, 6, 8, 12, 16, 20, and 24 weeks, with each assessment session lasting 30–60 minutes. These time points were chosen to capture anticipated rapid changes within the first 8 weeks and then slower changes out to 24 weeks. Data were collected within ± 3 days for the assessments at weeks 2 – 8 and ± 1 week for those at weeks 12 – 24. We have previously reported on the performance trajectories of an early portion of this cohort out to 12 weeks.18 Participants received medical and rehabilitation services in accordance with their overall plan of care; we recorded but did not control for the amount or type of rehabilitation services delivered to participants. Assessments were administered in inpatient hospital wards, other healthcare facilities, the research lab, and/or participants’ homes, depending on individual participant location and travel abilities.
Study Measures
Performance of upper limb activity in daily life was quantified via bilateral, wrist-worn, tri-axial accelerometers (Actigraph Link, Pensacola, FL). Accelerometers worn on the wrists provide quantification of upper limb movement. While the accelerometer data do not specify which activities are performed during the wearing time, variables computed from the data have been shown to be a valid and reliable measures of upper limb activity during the wearing time (i.e performance in daily life) in both healthy adults22, 29 and adults with stroke.9, 30–32 Briefly, participants wore the accelerometers for 24 hours at each assessment time point. The accelerometers were donned at the end of the visit (after completion of clinical measures) and worn for the following day. Once the accelerometers were returned, data were uploaded, visually inspected, and processed using Actilife 6 software (Actigraph Corp., Penacola, FL) and custom-written software, as per published protocols.8 Data were sampled at 30 Hz, band-pass filtered between frequencies of 0.25 and 2.5 Hz, and down sampled into 1-second epochs for each axis. Activity counts (unit of acceleration recorded for this device and software, 1 count = 0.001664 gravitational units [m/s2]) were combined across the three axes to create a single vector magnitude (√x2+y2+z2) for each second of data. The threshold for considering if the upper limb was active was when the vector magnitude was ≥ 2 activity counts in each one second epoch.31, 33 Seconds of activity were summed over the wearing period to arrive at activity duration variables. The use ratio (hours of paretic limb activity/hours of non-paretic limb activity) was chosen as the primary performance measure, based on its strong psychometric properties. 29, 31, 34, 35 The use ratio has a narrow distribution (mean ± SD of 0.95 ± 0.06) across the lifespan in neurologically-intact individuals.12, 29, 36 Because it is a ratio variable, it controls for the amount of activity recorded in the accelerometers that comes from arm swing during gait.30 Hours of paretic upper limb activity was used as a secondary measure of upper limb performance. These two variables quantify the symmetry and duration of performance of upper limb activity in daily life, but not the magnitude or variability. Other accelerometer variables quantifying these additional dimensions of movement were calculated in this cohort, but are not reported here, as they are strongly correlated to these simple duration measures and had similar trajectories over time early after stroke.18
Capacity for upper limb activity was quantified by the Action Research Arm Test (ARAT)37, 38 and impairment was quantified with the upper extremity portion of the Fugl-Meyer motor assessment (UEFM),39 both well-established, criterion-rated scales.40 Sample demographics included age, sex, race, ethnicity, handedness, and rehabilitation service utilization. Other measures taken at 2, 12 and 24 weeks were: the Center for Epidemiologic Studies Depression (CES-D) scale41 to quantify depressive symptomatology, the Montreal Cognitive Assessment (MoCA) to quantify common vascular cognitive impairments,42 and the Unstructured Mesulam to quantifying hemispatial neglect.43
Statistical Analyses
Analyses were done in R version 4.0.2, employing nonlinear longitudinal multilevel modeling44 with the nlme package.45 Longitudinal multilevel analyses (measures nested within people) is the preferred method for these data given it does not require the same number of assessments across participants, can account for missing data, and can minimize noise in the clinical measures.44, 46 First, individual participant trajectories for the longitudinal impairment, capacity, and performance data were modeled. Various models were initially assessed (e.g. polynomial, logistic, asymptotic regression) to determine the best approach; the logistic model was conceptually and empirically superior to the alternatives. Trajectories were best fit with a 3-parameter logistic model (see Equation), capturing the rapid growth earlier after stroke within the longer data collection period, eventuating in an asymptote. The three parameters were: ϕ1, the upper asymptote, ϕ2, the time value corresponding to the inflection point, and ϕ3 the “growth rate” or compression of the growth curve. The upper asymptote, ϕ1, is the upper value at which the curve fit eventually flattens, i.e. where subsequent points in time did not change the value. The inflection point, ϕ2, is the time post stroke when the curve transitions from an increase in the rate of rise to a decrease in the rate of rise. The last parameter, ϕ3, can be considered a scaling factor related to how quickly or slowly the curve reaches asymptote. As ϕ3 increases, the logistic curve approaches the horizontal asymptote more slowly. While the values of ϕ1 have clear and easy to understand biological relevance, the values of ϕ2 and ϕ3 are less biologically relevant.
| Equation: |
Models were fit with fixed effects (the parameters) and as many random effects as could be estimated (see Results). If models with random effects for more than one parameter did not converge to solutions, they were discarded in favor of the fixed effect model for that parameter. All models had random asymptotes (ϕ1) and some models had random inflection points (ϕ2); no models could be estimated with the remaining parameter, ϕ3, as random. Final models were chosen by selecting the model for each measure with the lowest Akaike (AIC) Information Criteria. The AIC estimates the relative amount of information lost by the model, so the lower AIC (less information lost) the better the fit of the model to the data.
Second, hypotheses about timing of trajectories were evaluated by determining when each measure approached its plateau, operationally defined as the time when the measure achieved 90% of its upper asymptote, ϕ1.47 The values of interest here are not direct parameters in the model, but instead derived from the model effects. Since the theoretical sampling distributions of these values are unknown, case resampling bootstrapping (iterations = 2000) was used to estimate values and 95% confidence intervals. Bias-corrected and accelerated confidence intervals48, 49 were chosen over other confidence interval estimates because, theoretically, the distributions could be asymmetrical and the amount of asymmetry could vary. For each bootstrap sample, fixed effects were estimated, and from these, new estimates for the plateau time were calculated as well as pairwise differences among the plateau times. Across the 2000 bootstrap samples, distributions of plateau times and pairwise differences were obtained, and from these, the confidence intervals were calculated. Statistical significance in the pairwise comparisons occurred when the 95% CI of the differences did not include zero. This is equivalent to a p value of 0.05. Confidence intervals that overlapped with zero but where ≥ 90% of the interval was above zero were labeled statistical trends.
And third, hypotheses about moderators of performance trajectories were evaluated for their influence on model parameters. The moderator analyses can be conceptualized as looking for interactions between the moderator and the model parameters. Models attempted to include moderators of each logistic parameter (ϕ1, ϕ2, ϕ3) and were trimmed when models could not be estimated by inclusion of a particular moderator (due to lack of variability, estimation problems). Consequently, moderators of ϕ1 were always tested; occasionally moderators of ϕ2 could be included. Moderators of ϕ3 could not be included in any model due to insufficient variability. The most complex models that could be fit are reported here. Plateau analyses were conducted for the moderator models using bootstrapping methods and address whether plateau differences for levels of a moderator are significantly different. Seven variables were evaluated as moderators, one at a time, for their effect on use ratio and paretic hours trajectories. Potential moderators evaluated included: capacity (ARAT score at four weeks), concordance (when the dominant limb is the paretic limb), age, upper limb rehabilitation utilization, and CES-D, MoCA, and Mesulam scores. Impairment was not tested as a moderator of performance in its own right because it is highly correlated to capacity and would be expected to mediate its influence via capacity. The ARAT score entered was from the 4 week assessment since that was expected to occur before, but potentially close in time, to the plateau values. Upper limb rehabilitation service utilization was entered as the number of measurement periods that a participant reported receiving services. The CES-D, MoCA, and Mesulam scores were from 2 weeks post stroke, since that was the only time point from which these values were available before anticipated plateaus (other available values were at 12 and 24 weeks).
RESULTS
Seventy-three individuals were recruited with 67 included in the analysis. Figure 1 shows the flow diagram for this observational cohort. The percentages of the 67 participants providing data at each time point were: 100% at 2 wks, 88% at 4 wks, 82% at 6 wks, 79% at 8 wks, 73% at 12 wks, 67% at 16 wks, 57% at 20 wks, and 61% at 24 wks. Participant demographics are provided in Table 1. A majority of the sample received rehabilitation services for the upper limb out to at least 8 weeks post stroke. The three-parameter logistic model was the best fit with the data across all measurement levels. Figure 2A shows a generic, 3-parametric logistic curve to illustrate the model and parameters. The lowest AIC values were generated by models with random effects for ϕ1 (the upper asymptote) and ϕ2 (the time of the inflection point) for the impairment and capacity data, but with random effects only for ϕ1 for the performance data. Model parameters are provided in the top part of Table 2. Figure 2B shows an individual example of model fit for each measurement, with arrows marking the time to plateau (90% of the asymptote). Figures 2C–2F show the predicted data for impairment (2C), capacity (2D) and performance measures (2E, 2F) for all subjects. As can be seen in Figure 2C–2F, the sample spanned a broad range of severity in impairment (UEFM), capacity (ARAT), and performance (use ratio and paretic hours). With respect to absolute scores on the scales, no participants had the maximum UEFM (66) or ARAT (57) scores at the two week time point (ave. time post stroke = 14 ± 2 days). Six participants had UEFM scores between 61 and 65 and seven participants had ARAT scores between 51 and 56. On the low end of the scales at the two week time point, 20 participants had UEFM scores between 0 and 10, while 30 participants had ARAT scores between 0 and 10.
Figure 1.
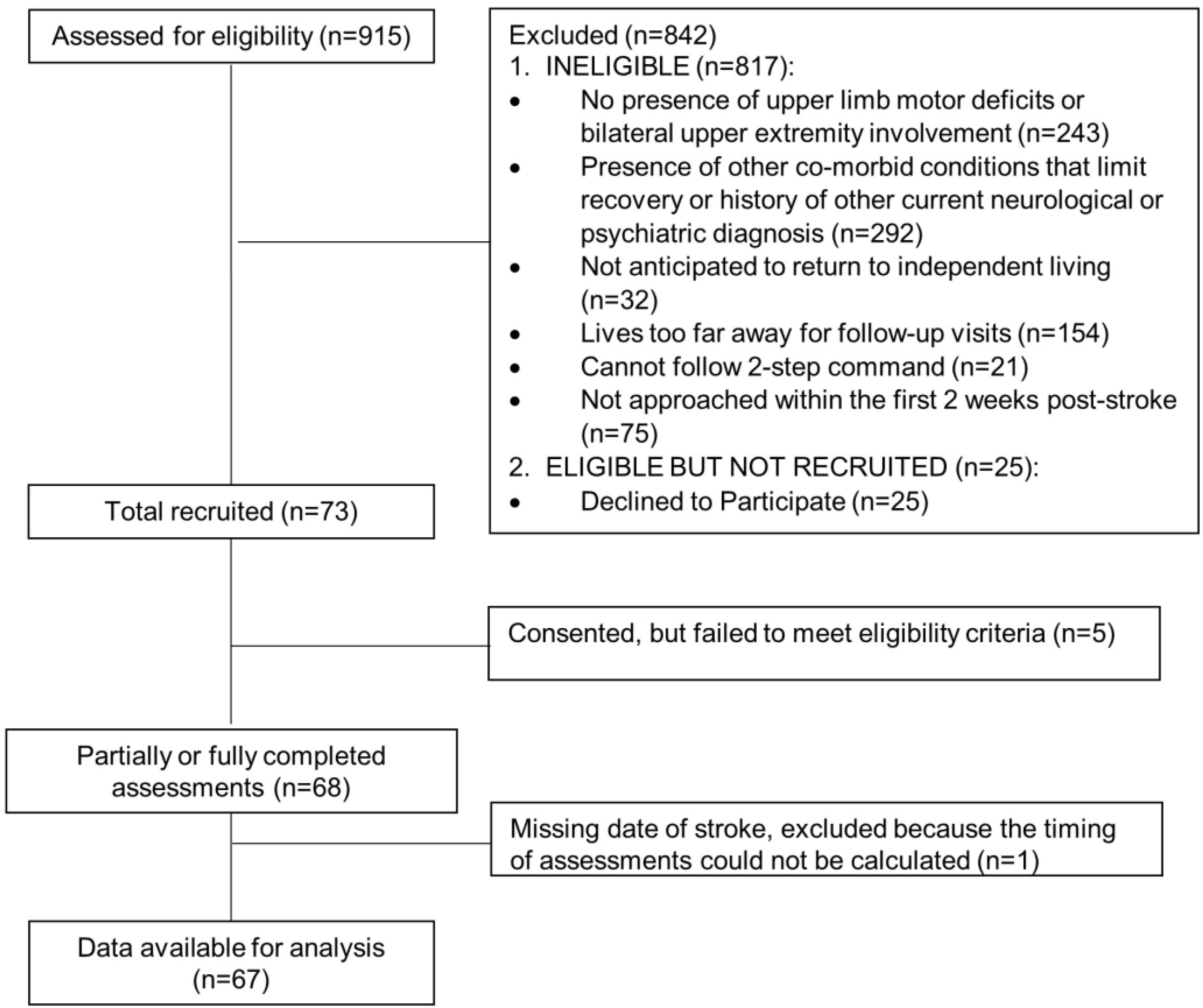
STROBE chart of participant enrollment.
Table 1.
Participant demographics. Values are mean ± SD, percentage, or median (1st quartile, 3rd quartile).
| Total sample (n = 67) | |
|---|---|
|
| |
| Age, yrs | 68 ± 10 |
|
| |
| Sex | 45% Female 55% Male |
|
| |
| Race | 58% Caucasian |
| 40% Black or African American | |
| 2% Asian or Pacific Islander | |
|
| |
| Ethnicity | 100% Non-Hispanic |
|
| |
| Days from stroke to consent | 7.0 ± 3.2 |
|
| |
| Stroke type | 87% Ischemic |
| 13% Hemorrhagic | |
|
| |
| Stroke location | 61% Cortical |
| 36% Subcortical | |
| 2% Cortical & subcortical | |
| 1% Posterior circulation | |
|
| |
| Affected side | 64% Left |
| 35% Right | |
|
| |
| Concordance* | 39% |
|
| |
| Portion receiving UL rehabilitation services | |
| Week 2 | 87% |
| Week 4 | 69% |
| Week 6 | 63% |
| Week 8 | 61% |
| Week 12 | 46% |
| Week 16 | 33% |
| Week 20 | 24% |
| Week 24 | 22% |
|
| |
| UEFM at 2 wks | 35 (9, 55) |
|
| |
| ARAT at 2 wks | 14 (0, 40) |
|
| |
| ARAT at 4 wks | 26 (3, 48) |
|
| |
| CES-D at 2 wks | 15 (6, 21) |
|
| |
| MoCA at 2 wks | 18 (14, 22.5) |
|
| |
| Unstructured Mesulam at 2 wks (Left – right errors) | 0 (0, 2.5) |
ARAT: Action Research Arm test, range 0–57; CES-D: Center for Epidemiological Studies – Depresssion Scale, range 0–60; MoCA: Montreal Cognitive Assessment, range 0–30; UEFM: Upper Extremity Fugl-Meyer assessment, range 0–66.
Dominant limb = paretic limb
Figure 2.
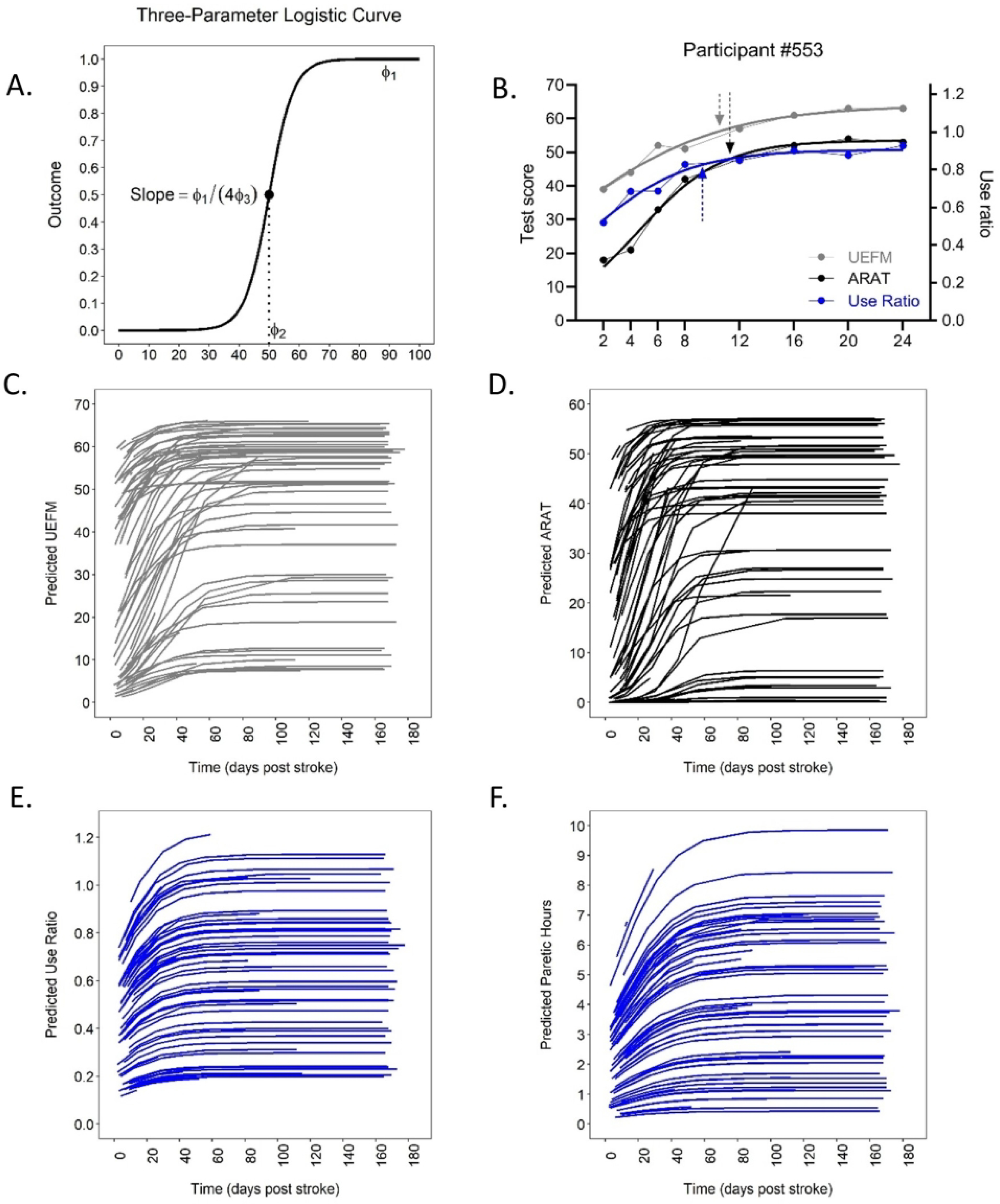
A: Generic 3-parameter logistic curve to illustrate the model and parameters. Most participant data fit the upper half of the model, as shown in the individual example in B. B: Individual examples showing data and fit with model, gray = UEFM, black = ARAT, and blue = use ratio. The UEFM and ARAT data correspond to the left y-axis and the use ratio corresponds to the right y-axis. Arrows represent time to plateau (90% of asymptote). Predicted data from each participant for impairment (C), capacity (D), and performance (E&F).
Table 2.
Model parameters, including modifiers of performance trajectories. Values are model estimates ± SE.
| Model estimates without modifiers | |||
|---|---|---|---|
| ϕ1 | ϕ2 | ϕ3 | |
| Impairment: UEFM Df = 336 |
45.97 ± 2.47*** | 3.90 ± 2.85 | 13.02 ± 0.77*** |
| Capacity: ARAT Df = 337 |
35.64 ± 2.62*** | 19.79 ± 2.86*** | 9.28 ± 0.47*** |
| Performance: Use ratio Df = 326 |
0.65 ± 0.04*** | −5.60 ± 4.93† | 13.51 ± 3.17*** |
| Performance: Paretic hours Df = 326 |
4.27 ± 0.34*** | 4.90 ± 2.97 | 16.53 ± 3.02*** |
|
Contributions of modifiers to Use Ratio model Values are interactions of the modifier and parameter indicating the amount by which the logistic parameter changes with a one unit change in the moderator. | |||
| ARAT | 0.01 ± 0.0009*** | −0.55 ± 0.13*** | -- |
| Concordance | 0.17 ± 0.07* | 10.8 ± 3.91** | -- |
| Age | −0.005 ± 0.004 | -- | -- |
| Upper limb rehabilitation | −0.01 ± 0.02 | 3.53 ± 1.14** | -- |
| CES-D | −0.008 ± 0.004* | 0.09 ± 0.20 | -- |
| MoCA | 0.008 ± 0.005 | −0.7 ± 0.27 | -- |
| Unstructured Mesulam | −0.001 ± 0.007 | −0.21 ± 0.36 | -- |
|
Contributions of modifiers to Paretic Hours model Values are interactions of the modifier and parameter indicating the amount by which the logistic parameter changes with a one unit change in the moderator. | |||
| ARAT | 0.08 ± 0.01*** | −0.43 ± 0.11*** | -- |
| Concordance | 1.65 ± 0.66* | 6.57 ± 4.13 | -- |
| Age | −0.05 ± 0.03 | -- | -- |
| Upper limb rehabilitation | −0.24 ± 0.04 | 2.59 ±0.92** | -- |
| CES-D | −0.09 ± 0.03** | −0.24 ± 0.26 | -- |
| MoCA | 0.09 ± 0.05 | −0.66 ± 0.29* | -- |
| Unstructured Mesulam | −0.04 ± 0.07 | −0.68 ± 0.62 | -- |
ϕ1: upper asymptote in units of the scale; i.e. the average value into which the curve flattens, and does not increase further over time.
ϕ2: inflection point in days; i.e. the average time when the curve starts to slow its growth. Note that some individual participant curves fit best only to the upper part of the curve, such that the inflection point occurs on or before the time of stroke.
ϕ3: “growth rate” best viewed as a scaling factor for how quickly the asymptote, or flattening of the curve is achieved.
ARAT: Action Research Arm Test; CES-D: Center for Epidemiologic Studies – Depression Scale; Df: degrees of freedom; MoCA: Montreal Cognitive Assessment; UEFM: Upper extremity Fugl-Meyer Assessment.
--: models that tried to estimate these interaction parameters did not converge.
p ≤ 0.05
p < 0.01
p < 0.001 Note that the statistical significance of each parameter reflects whether or not the value is different from zero, not the fit of the model.
The negative value for ϕ2 indicates that the best model places the inflection point of the curve prior to the stroke.
Changes in the performance trajectories slowed or stopped surprisingly early after stroke, with the use ratio reaching plateau around 3 weeks (bootstrap mean ± SE: 24 ± 5 days) and hours of paretic activity reaching plateau around 6 weeks (41 ± 6 days). Figure 3 shows the timing of plateaus for each of the measures, obtained from the bootstrapping procedures. As hypothesized, plateaus in impairment as measured by the UEFM significantly preceded those of capacity as measured by the ARAT (bootstrap paired mean difference ± SE, 95% CI: 8 ± 4, 1 – 16 days). Plateau in performance as measured by the use ratio trended toward preceding plateaus in impairment (8 ± 5, −3 – 17 days), while significantly preceding plateaus in capacity (16 ± 5, 7 – 26 days). Plateau in performance as measured by hours of paretic activity was timed similarly to the plateau in capacity (1 ± 7, −11 – 16 days). Plateau in hours of paretic activity appeared to lag plateaus in impairment, but this did not achieve significance (9 ± 7, −4 – 24 days). Comparison of the two performance measures indicated that the use ratio plateaued significantly prior to the paretic hours of use (17 ± 7, 2 – 31 days). Since final models for each measure did not have the same fixed vs. random parameters, we repeated the plateau analyses requiring the same fixed (Φ2 and Φ3) and random (Φ1) parameters for each measure. The repeated analyses generated the same statistical conclusions as the one with the best-fit models.
Figure 3.
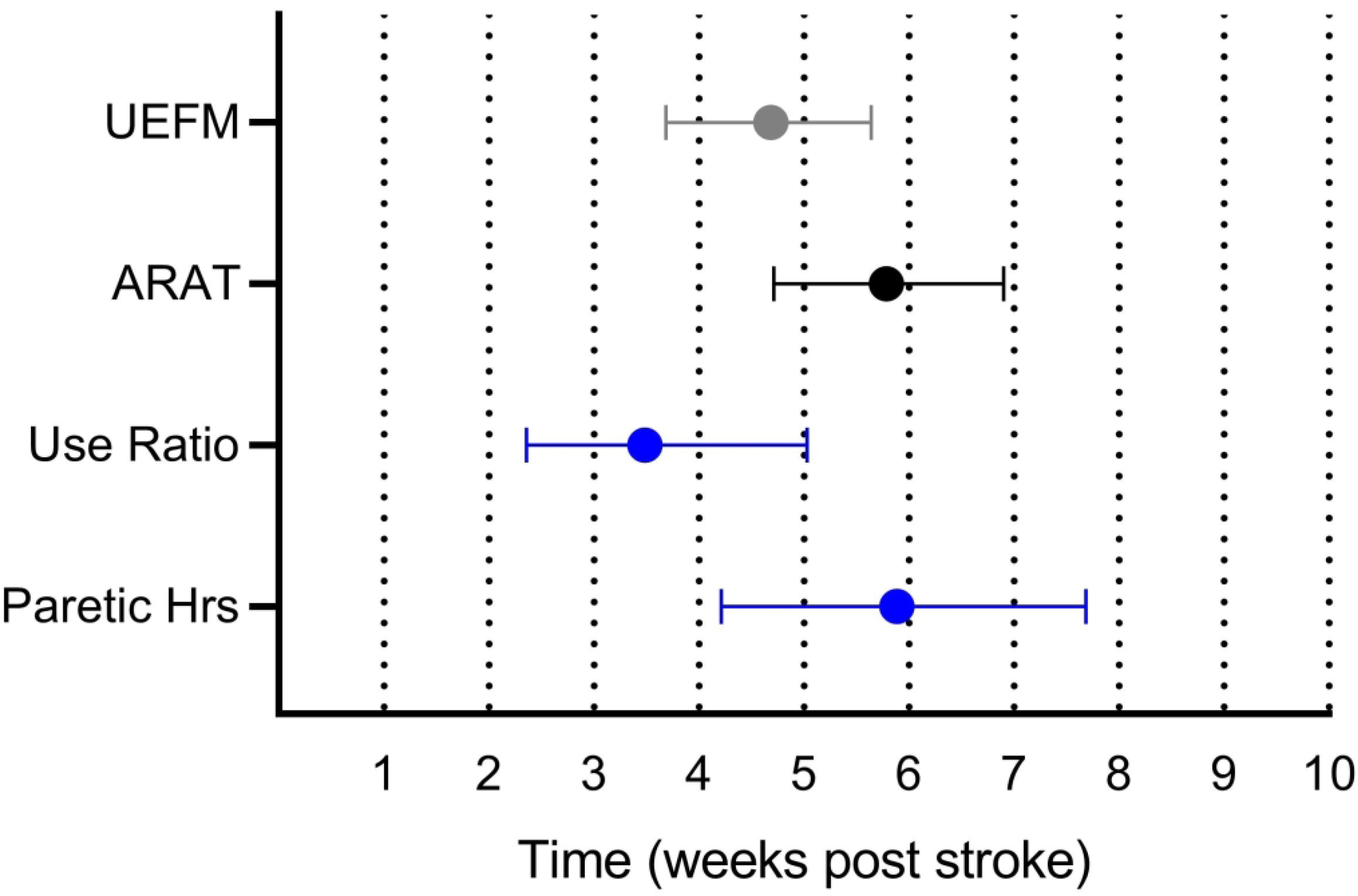
Timing of plateaus across measures obtained from bootstrapping procedures. Values are means and 95% confidence intervals.
Five of the seven potential moderators influenced performance trajectories. Estimated contributions for each moderator to the use ratio and paretic hours model parameters are provided in the bottom of Table 2. These are the numerical estimates of the interaction between the moderator and the parameter. For example, for every 1 point increase in ARAT at 4 weeks, there is an associated increase of 0.01 for the eventual asymptote in the use ratio and an associated increase of 0.08 hrs (~5 min) for the eventual asymptote in paretic hours. Likewise, when the dominant limb is the paretic limb (concordance), the eventual asymptote is increased by 0.17 for the use ratio and 1.65 hrs for paretic hours. Figure 4 is a graphical illustration of some of the significant moderator effects. Here, predicted performance trajectories of higher (+1 SD) vs. lower (−1 SD) levels of each moderator are graphed to better communicate the effect of the moderator on the performance trajectories and assist the reader in interpreting the numerical values in the bottom of Table 2. As hypothesized, better capacity was associated with quicker (sooner inflection point, ϕ2) and better performance recovery (higher asymptote, ϕ1, Figure 4 A&B). Eventual performance achieved was better but took longer for those with a concordant dominant and paretic limb (Figure 4 C&D). More depressive symptoms were associated with lower eventual upper limb performance but did not affect rate (Figure 4 E&F). Less upper limb rehabilitation was associated with quicker recovery. Finally, more early cognitive deficits led to later recovery of performance measured by paretic hours, but did not reach significance for the use ratio. Other factors evaluated did not moderate upper limb performance trajectories.
Figure 4.
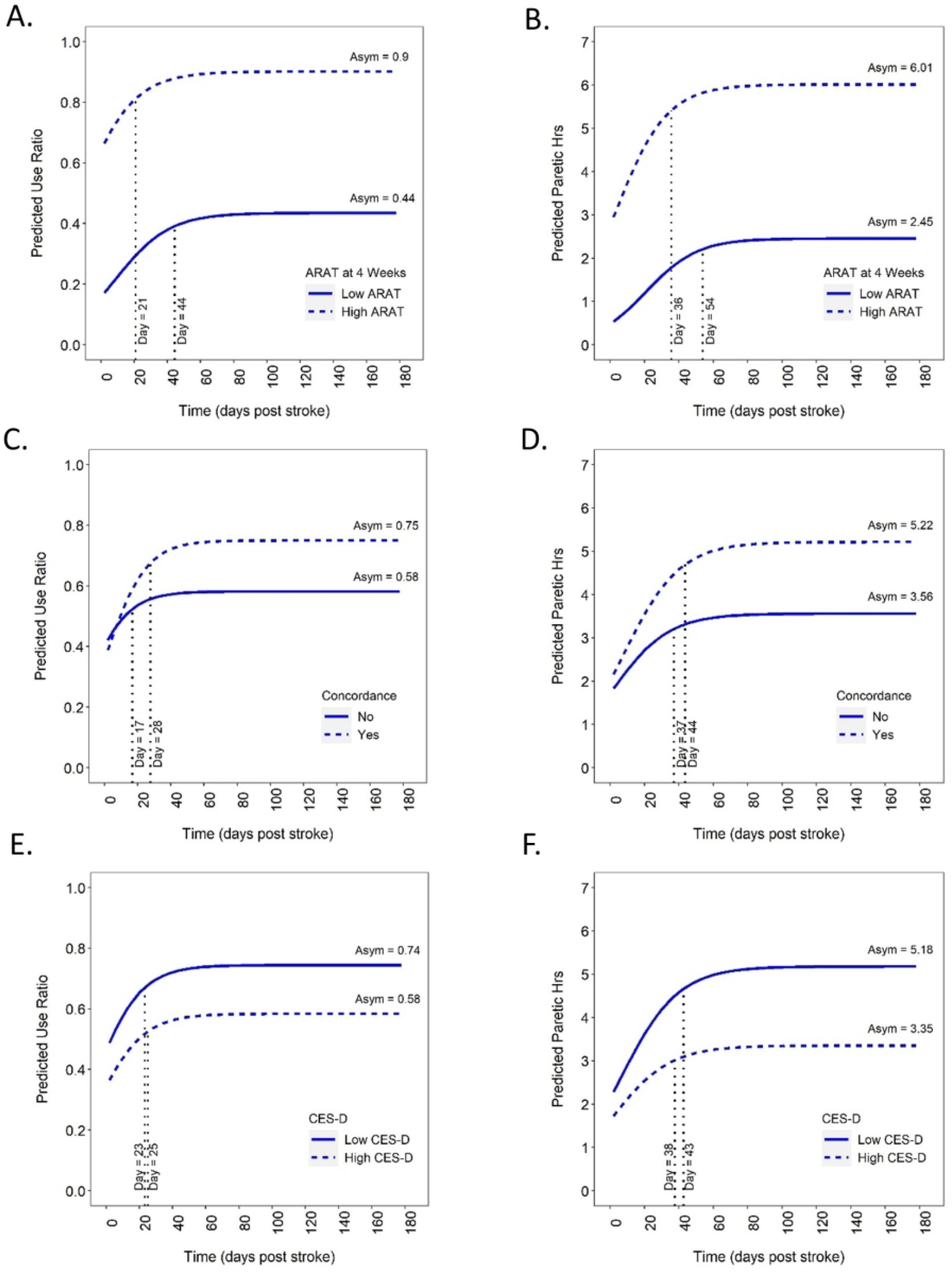
Illustration of predicted higher (+ 1 SD) vs. lower (−1 SD) moderator scores on performance trajectories. Data are modeled estimates at the moderator value, not cohorts of patients. A&B: ARAT score at four weeks; C&D: concordance; E&F: CES-D score at 2 weeks.
DISCUSSION
Upper limb performance in daily life, as measured by accelerometry, started to stabilize between three and six weeks post stroke, on average. Plateaus in performance did not lag plateaus in impairment and capacity, but instead slightly preceded or occurred at the same time. Factors that were associated with better eventual performance in daily life were better capacity, concordant dominant and affected limb, less upper limb rehabilitation services, and no or minimal depressive symptomatology. Factors that were associated with faster rates of change were better capacity, discordant dominant and affected limb, less rehabilitation, and no or minimal cognitive deficits.
A major finding of this study is that individuals approach a stable pattern of upper limb performance in daily life surprisingly early after stroke. The upper limb performance trajectories mapped here are largely consistent with previous reports and build on them by extending the measurement points and including more participants,18 filling in intermediate time points,13, 19, 20 and modeling individual trajectories to pinpoint individual time of plateau instead of assessing time point differences.20 Our data indicate that upper limb performance in home and community life becomes fixed relatively early, even in the presence of ongoing upper limb rehabilitation services. Over 60% of the sample was still receiving services at eight weeks post stroke, as shown in Table 1. One upper limb performance variable, the use ratio, plateaued sooner than the other, paretic hours of activity. While neither variable is a complete measure of upper limb performance,12 validity of the use ratio is better established34 and is not influenced by walking activity.33 Trajectories of paretic hours in this study could potentially have been influenced by small increases in walking (~45 minute/day increase from 3–12 weeks, from Figure 3C of Regterschot et al.) during this same time period.20
A second finding of this study is that upper limb performance changes do not lag, and in the case of the use ratio, may actually precede changes in the impairment and capacity measures. Thus, motor habits in daily life may form before neurological and functional recovery stabilize. This was opposite our hypothesis. One explanation is that initial experiences after a disruptive event strongly reinforce future behaviors (either positively or negatively) for many months.50, 51 Specific to stroke, attempting and not succeeding in using the affected limb for a particular activity leads to learned non-use.15, 52, 53 Another potential explanation is the idea that individuals consider themselves in a holding pattern “waiting to get better”.54 Anecdotally, this idea is often voiced by research participants and patients in routine care, that they are “waiting for their arm/leg to improve” and then they plan to increase activity in daily life after discharge from services. Within the data set, we did not see any evidence that capacity needs to exceed a certain threshold in order for performance to change, as suggested from modeling of self-reported performance data.55 A clever paper modeling data from EXCITE trial participants has suggested that a person has to improve above a threshold on a capacity test (Wolf Motor Function Test) in order for improved capacity to translate into improved performance in daily life (self-report of performance on the Motor Activity Log).55 While we did not directly test the threshold hypothesis, the earlier stabilization of the use ratio compared to stabilization of capacity, and the linear moderating effect of capacity on the performance trajectories do not support this idea. There may also be differences in self-reported vs. sensor-based trajectories of upper limb performance, since measurements of one vs. the other can be inconsistent.56
Five factors modified upper limb performance trajectories after stroke. Consistent with previous reports of moderate, positive correlations between the two levels of measurement (for review see35), better upper limb capacity was associated with faster rates of improvement and better eventual upper limb performance. When the dominant limb was the affected limb, eventual overall performance was better but it took longer to reach plateau. This finding, along with the early plateaus in performance suggest that perhaps individuals are willing to persist longer in regaining daily use of the dominant vs. non-dominant limb, especially in a world built for right-handers. The negative moderating effect of rehabilitation services likely reflects that service utilization here is a proxy for severity of motor problems. People with mild motor problems recover quickly and no longer need services, while those with more severe problems continue to engage in rehabilitation. There was only a small amount of variance in reported service delivery before and during the time of plateau (i.e. high percentages in Table 1 for weeks 2–6). It is also worth noting that upper limb performance is not assessed in routine clinical care34 (our study did not provide data back to the clinicians or patients), and clinical interventions are typically focused on impairment and/or capacity but not performance, despite recent recommendations.27 Our findings that higher levels of depressive symptomology and cognitive deficits reduce and slow recovery of upper limb performance, respectively, reinforce the importance of assessing these domains and targeting them for treatment.57 Looking forward, these data open up the testable hypothesis that treatment to improve depression and/or cognition might also improve upper limb performance in daily life.
Three key limitations need to be considered in interpreting these data. First, the sample size was small and recruited from one large, academic, mid-western, United States hospital. Data from additional participants and additional sites are needed to assess the generalizability of our findings. Second, the measures of upper limb performance (like those of impairment and capacity) are far from perfect.12 The measures capture global upper limb movement quantity, and do not fully capture movement quality11 nor distal movements that might occur independently of wrist and other upper limb movements.58 We have reported here on measures of movement duration and symmetry of movement duration, but have found strong correlations and similar time course of changes between these measures and measures of magnitude and variability. Given the challenges at all levels of measurement, these results can be considered an initial picture about the relative timing of the impairment, capacity and performance of the upper limb. And third, it is a challenge to interpret the clinical relevance of some of the statically significant modifiers found here. The two most relevant modifying effects may be capacity and concordance, since they had a reasonable impact on the performance variables. Other modifying variables, such as depressive symptomatology may not matter unless someone is severely affected on the CES-D.
Conclusions
Upper limb performance in daily life as measured by wearable motion sensors approached plateau within three to six weeks post stroke, at the same time or just before plateaus in impairment and capacity. Our results need to be validated in a larger sample, preferably across countries and healthcare systems in order to evaluate how trajectories are influenced by neurobiology, human behaviors, and access to and content of rehabilitation services. These data suggest that now is the time to add direct measures of upper limb performance into clinical practice and potentially change or augment intervention strategies to include a focus on behavior in daily life.27
Acknowledgments:
We thank Christine Gordon for her assistance with data collection and the many participants who came in repeatedly for their assessments.
Sources of funding:
NIH R01HD068290, T32HD007434, TL1TR002344
Footnotes
Disclosures: None
REFERENCES
- 1.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. May1995;76(5):406–12. [DOI] [PubMed] [Google Scholar]
- 2.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. March32000;39(5):835–41. [DOI] [PubMed] [Google Scholar]
- 3.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22(3–5):281–99. [PubMed] [Google Scholar]
- 4.Ramsey LE, Siegel JS, Lang CE, Strube M, Shulman GL, Corbetta M. Behavioural clusters and predictors of performance during recovery from stroke. Nat Hum Behav. 2017;1 doi: 10.1038/s41562-016-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Vliet R, Selles RW, Andrinopoulou ER, et al. Predicting Upper Limb Motor Impairment Recovery after Stroke: A Mixture Model. Ann Neurol. March2020;87(3):383–393. doi: 10.1002/ana.25679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortes JC, Goldsmith J, Harran MD, et al. A Short and Distinct Time Window for Recovery of Arm Motor Control Early After Stroke Revealed With a Global Measure of Trajectory Kinematics. Neurorehabil Neural Repair. June2017;31(6):552–560. doi: 10.1177/1545968317697034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Towards a common language for Functioning, Disability, and Health: ICF. 2002.
- 8.Lang CE, Waddell KJ, Klaesner JW, Bland MD. A Method for Quantifying Upper Limb Performance in Daily Life Using Accelerometers. J Vis Exp. April212017;(122)doi: 10.3791/55673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbin MA, Bailey RR, Lang CE. Validity of body-worn sensor acceleration metrics to index upper extremity function in hemiparetic stroke. J Neurol Phys Ther. April2015;39(2):111–8. doi: 10.1097/NPT.0000000000000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbin MA, Waddell KJ, Lang CE. Acceleration Metrics Are Responsive to Change in Upper Extremity Function of Stroke Survivors. Arch Phys Med Rehabil. December92014;doi:S0003–9993(14)01282–9 [pii] 10.1016/j.apmr.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth J, Klaesner JW, Lang CE. Relationships between accelerometry and general compensatory movements of the upper limb after stroke. J Neuroeng Rehabil. October202020;17(1):138. doi: 10.1186/s12984-020-00773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BA, Lang CE. Sensor Measures of Symmetry Quantify Upper Limb Movement in the Natural Environment Across the Lifespan. Arch Phys Med Rehabil. June2019;100(6):1176–1183. doi: 10.1016/j.apmr.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rand D, Eng JJ. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabil Neural Repair. January2012;26(1):76–84. doi:1545968311408918 [pii] 10.1177/1545968311408918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddell KJ, Strube MJ, Bailey RR, et al. Does Task-Specific Training Improve Upper Limb Performance in Daily Life Poststroke? Neurorehabil Neural Repair. March2017;31(3):290–300. doi: 10.1177/1545968316680493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barth J, Geed S, Mitchell A, Lum PS, Edwards DF, Dromerick AW. Characterizing upper extremity motor behavior in the first week after stroke. PLoS One. 2020;15(8):e0221668. doi: 10.1371/journal.pone.0221668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebruers N, Truijen S, Engelborghs S, Nagels G, Brouns R, De Deyn PP. Actigraphic measurement of motor deficits in acute ischemic stroke. Cerebrovasc Dis. 2008;26(5):533–40. doi: 10.1159/000160210 [DOI] [PubMed] [Google Scholar]
- 17.Iacovelli C, Caliandro P, Rabuffetti M, et al. Actigraphic measurement of the upper limbs movements in acute stroke patients. J Neuroeng Rehabil. December42019;16(1):153. doi: 10.1186/s12984-019-0603-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waddell KJ, Strube MJ, Tabak RG, Haire-Joshu D, Lang CE. Upper Limb Performance in Daily Life Improves Over the First 12 Weeks Poststroke. Neurorehabil Neural Repair. October2019;33(10):836–847. doi: 10.1177/1545968319868716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rand D, Eng JJ. Predicting daily use of the affected upper extremity 1 year after stroke. J Stroke Cerebrovasc Dis. February2015;24(2):274–83. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.039 [DOI] [PubMed] [Google Scholar]
- 20.Regterschot GRH, Bussmann JBJ, Fanchamps MHJ, Meskers CGM, Ribbers GM, Selles RW. Objectively measured arm use in daily life improves during the first six months poststroke: a longitudinal observational cohort. Journal of Neuroengineering and Rehabilitation. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. September2006;20(3):380–9. doi: 10.1177/1545968305284528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey RR, Klaesner JW, Lang CE Quantifying real-world upper limb activity in nondisabled adults and adults with chronic stroke. Neurorehabilitation and neural repair. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konig IR, Ziegler A, Bluhmki E, et al. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke. June2008;39(6):1821–6. [DOI] [PubMed] [Google Scholar]
- 24.Waddell KJ, Birkenmeier RL, Bland MD, Lang CE. An exploratory analysis of the self-reported goals of individuals with chronic upper-extremity paresis following stroke. Disabil Rehabil. May2016;38(9):853–7. doi: 10.3109/09638288.2015.1062926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glanz K, Rimer BK, Viswanath K. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; 2015. [Google Scholar]
- 26.Glasgow RE, Goldstein MG, Ockene JK, Pronk NP. Translating what we have learned into practice. Principles and hypotheses for interventions addressing multiple behaviors in primary care. Am J Prev Med. August2004;27(2 Suppl):88–101. doi: 10.1016/j.amepre.2004.04.019 [DOI] [PubMed] [Google Scholar]
- 27.Dobkin BH. Behavioral self-management strategies for practice and exercise should be included in neurologic rehabilitation trials and care. Curr Opin Neurol. December2016;29(6):693–699. doi: 10.1097/WCO.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brott T, Adams HP Jr., Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. July1989;20(7):864–70. [DOI] [PubMed] [Google Scholar]
- 29.Bailey RR, Lang CE. Upper-limb activity in adults: referent values using accelerometry. J Rehabil Res Dev. 2013;50(9):1213–22. doi: 10.1682/JRRD.2012.12.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uswatte G, Giuliani C, Winstein C, Zeringue A, Hobbs L, Wolf SL. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: evidence from the extremity constraint-induced therapy evaluation trial. Arch Phys Med Rehabil. October2006;87(10):1340–5. doi:S0003–9993(06)00529–6 [pii] 10.1016/j.apmr.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 31.Uswatte G, Miltner WH, Foo B, Varma M, Moran S, Taub E. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke. March2000;31(3):662–7. [DOI] [PubMed] [Google Scholar]
- 32.Bailey RR, Klaesner JW, Lang CE. An accelerometry-based methodology for assessment of real-world bilateral upper extremity activity. PLoS One. 2014;9(7):e103135. doi: 10.1371/journal.pone.0103135 PONE-D-14–14991 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB. Ambulatory monitoring of arm movement using accelerometry: an objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch Phys Med Rehabil. July2005;86(7):1498–501. [DOI] [PubMed] [Google Scholar]
- 34.Lang CE, Barth J, Holleran CL, Konrad JD, Bland MD. Implementation of Wearable Sensing Technology for Movement: Pushing Forward into the Routine Physical Rehabilitation Care Field. Sensors (Basel). October102020;20(20)doi: 10.3390/s20205744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayward KS, Eng JJ, Boyd LA, Lakhani B, Bernhardt J, Lang CE. Exploring the role of accelerometersin the measurement of real world upper limb use after stroke. Brain Impairment. 2016;17(1):16–33. [Google Scholar]
- 36.Hoyt CR, Van AN, Ortega M, et al. Detection of Pediatric Upper Extremity Motor Activity and Deficits With Accelerometry. JAMA Netw Open. April52019;2(4):e192970. doi: 10.1001/jamanetworkopen.2019.2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. Jan-Feb 2008;22(1):78–90. doi:1545968307305353 [pii] 10.1177/1545968307305353 [DOI] [PubMed] [Google Scholar]
- 38.Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther. Apr-Jun 2013;26(2):104–15. doi: 10.1016/j.jht.2012.06.005 S0894–1130(12)00074–9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 40.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized Measurement of Sensorimotor Recovery in Stroke Trials: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair. September2017;31(9):784–792. doi: 10.1177/1545968317732662 [DOI] [PubMed] [Google Scholar]
- 41.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 42.Koski L Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36(1):6–18. doi: 10.1159/000352051 [DOI] [PubMed] [Google Scholar]
- 43.Rengachary J, d’Avossa G, Sapir A, Shulman GL, Corbetta M. Is the posner reaction time test more accurate than clinical tests in detecting left neglect in acute and chronic stroke? Arch Phys Med Rehabil. December2009;90(12):2081–8. doi:S0003–9993(09)00679–0 [pii] 10.1016/j.apmr.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long JD. Longitudinal data analyses for the behavioral sciences using R. Sage Publications; 2012. [Google Scholar]
- 45.nlme: Linear and Nonlinear Mixed Effects Models. 2020. https://CRAN.R-project.org/package=nlme
- 46.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; 2002. [Google Scholar]
- 47.Feldman LS, Cao J, Andalib A, Fraser S, Fried GM. A method to characterize the learning curve for performance of a fundamental laparoscopic simulator task: defining “learning plateau” and “learning rate”. Surgery. August2009;146(2):381–6. doi: 10.1016/j.surg.2009.02.021 [DOI] [PubMed] [Google Scholar]
- 48.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. May152000;19(9):1141–64. doi:::aid-sim479>3.0.co;2-f [DOI] [PubMed] [Google Scholar]
- 49.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. August1996;11(3):189–212. [Google Scholar]
- 50.Ouellette JA, Wood W. Habit and intention in everyday life: the multiple processes by which past behavior predicts future behavior. Psychol Bull. 1998;124(1):54–57. [Google Scholar]
- 51.Wood W, Tam L, Witt MG. Changing circumstances, disrupting habits. J Pers Soc Psychol. June2005;88(6):918–933. doi: 10.1037/0022-3514.88.6.918 [DOI] [PubMed] [Google Scholar]
- 52.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. April1993;74(4):347–54. [PubMed] [Google Scholar]
- 53.Taub E The behavior-analytic origins of constraint-induced movement therapy: an example of behavioral neurorehabilitation. Behav Anal. Fall 2012;35(2):155–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chin LF, Hayward KS, Brauer SG. Factors Influencing Paretic Upper Limb Use During First 4 Weeks After Stroke: A Cross-Sectional Accelerometry Study. Am J Phys Med Rehabil. February12021;100(2):153–160. doi: 10.1097/PHM.0000000000001539 [DOI] [PubMed] [Google Scholar]
- 55.Schweighofer N, Han CE, Wolf SL, Arbib MA, Winstein CJ. A functional threshold for long-term use of hand and arm function can be determined: predictions from a computational model and supporting data from the Extremity Constraint-Induced Therapy Evaluation (EXCITE) Trial. Phys Ther. December2009;89(12):1327–36. doi:ptj.20080402 [pii] 10.2522/ptj.20080402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waddell KJ, Lang CE. Comparison of Self-Report Versus Sensor-Based Methods for Measuring the Amount of Upper Limb Activity Outside the Clinic. Arch Phys Med Rehabil. September2018;99(9):1913–1916. doi: 10.1016/j.apmr.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winstein CJ, Stein J, Arena R, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. May42016;doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 58.Rowe JB, Friedman N, Chan V, Cramer SC, Bachman M, Reinkensmeyer DJ. The variable relationship between arm and hand use: a rationale for using finger magnetometry to complement wrist accelerometry when measuring daily use of the upper extremity. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4087–90. doi: 10.1109/EMBC.2014.6944522 [DOI] [PubMed] [Google Scholar]


