OVERVIEW
Depression results in more years lived with disability than any other disease, and ranks fourth in terms of disability-adjusted life years.1–3 Projections are that, by 2020, depression will be second only to heart disease in its contribution to the global burden of disease (measured by disability-adjusted life years).4 As the population ages, successive cohorts of older adults will experience depressive disorders.4 Late-life depression (LLD) carries additional risk for suicide, medical comorbidity, disability, and family caregiving burden.5–7 Although treatment is effective in reducing symptoms, it is less successful in achieving and maintaining remission and in averting years lived with disability. Although response and remission rates to pharmacotherapy and electroconvulsive therapy (ECT) are comparable with those in midlife depression, relapse rates are higher,8 underscoring the challenge not only to achieve but also to maintain wellness.
This article reviews the evidence base for LLD treatment options and provides a more in-depth analysis of treatment options for difficult-to-treat LLD variants (eg, psychotic depression, vascular depression). Treatment algorithms are also reviewed based on predictors of response and novel treatment options that represent promising leads.
Standard Treatment
Pharmacotherapy
Approximately two-thirds of patients presenting with severe forms of depression respond to antidepressant treatment. However, older, frail people are particularly vulnerable to antidepressant side effects, especially cardiovascular and anticholinergic side effects, and this can compromise compliance and effectiveness of treatment.9
Acute Treatment
A recent meta-analysis of acute pharmacological trials revealed a paucity of placebo-controlled trials in older depressed populations.10 Using a 50% reduction in the Hamilton Rating Scale for Depression (HRSD) as the primary outcome measure, the meta-analysis reported an overall number needed to treat (NNT) of 8 (95% confidence interval [CI] 5,11) when all antidepressant classes were collapsed together.10 The analysis of each class was similar: for tricyclic antidepressants (TCAs) the NNT was 5 (95% CI 3,9) and for selective serotonin reuptake inhibitors (SSRIs) the NNT was 8 (95% CI 5,11). Because the confidence intervals between TCAs and SSRIs overlap substantially, these data do not support that one drug class is more effective than another.10 A limitation of the studies examined in this meta-analysis is that they were efficacy trials, excluding subjects with comorbid psychiatric illnesses, medical comorbidity and poor treatment response history, thus limiting generalizability.10 Moreover, the largest trials conducted so far showed a large placebo response rate and a significant number of subjects who do not respond or who have residual depressive symptoms.10
In a large (N = 728) trial, Nelson and colleagues11 used the HRSD to determine the symptoms that showed the greatest improvement during treatment: depressed mood (effect size [ES] 0.93), decreased interest and activity (ES 0.86), psychic anxiety (ES 0.65), guilt (ES 0.63), suicidal ideation/behavior (ES 0.6). Consequently, the investigators compared the results with those obtained using 5 other scales (Montgomery Asberg Depression Rating Scale, the Keller Brief Depression Rating Scale, Yale Depression Inventory, Quick Inventory of Depressive Symptoms, Inventory of Depressive Symptoms) and reported that there is considerable agreement among the scales with regard to symptoms sensitive to change during treatment of LLD.11
LLD is also more varied in its clinical presentations than its midlife equivalent. Thus, instruments currently used to define depression might not capture the entire spectrum or phenotype of depressive disorders in the elderly. Moreover, instruments such as HRSD or Montgomery Asberg Depression Rating Scale are difficult to use on a regular base in the real-life environment of the currently overcrowded outpatient clinics. Self-report measures, such as PHQ9, provide a more practical, easy-to-use tool for measure-based care, and fit in well with the strategies of depression care management.10
An effectiveness trial of older depressed outpatients reported a post hoc analysis for participants treated with citalopram in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) analyzing the correlation between age of onset of the first MD episode and clinical outcome.12 Remission rates (defined by a 16-item Quick Inventory of Depressive Symptomatology-Self-rated) were not statistically different between earlier onset (age of onset <55 years; 30.8%) and late onset (31.9%).12
A 2006 Cochrane Review on the use of antidepressants in the elderly examined the efficacy of antidepressant classes, compared the withdrawal rates associated with each class, and described the side effect profile of antidepressants for treating depression in patients age 55 years and older.9 The review did not find any differences in efficacy between classes of antidepressants, although it reported that TCAs are associated with a higher withdrawal rate because of side effect experiences (Table 1).9 The small number of studies restricted the validity of subgroup analysis on different populations (outpatient/inpatients/community volunteers/nursing home residents).9 Because few trials used standardized instruments to report side effects, the Cochrane Review used an analysis of withdrawal rates and described the ratios of the number of side effects experienced by patients treated with each antidepressant class. Thus, TSA recipients experienced more gastrointestinal side effects (4.6 side effects experienced by 10 TCA recipients compared with 2.9/10 SSRI recipients) and more neuropsychiatric side effects (4.1 side effects experienced by 10 TCA recipients compared with 2.3/10 SSRI recipients). However, nausea and vomiting were experienced by a greater percentage of SSRI recipients.9 A STAR*D report on melancholiform depression in midlife reported that the presence of melancholic features was associated with significantly reduced remission rates to SSRI (8.4% compared with 24.1% in nonmelancholiform depression).13
Table 1.
Comparing antidepressants for acute treatment of LLD: duration of treatment
| Primary Outcome Efficacy (Change in HDRS) | Secondary Outcome (Withdrawal Rates) | |||
|---|---|---|---|---|
| No. of Trials | RR | No. of Trials | Withdrawal Rates | |
| 16 | 26 | |||
| TCAs vs SSRIs | 9 trials | No difference (RR 1.07, CI 0.94–1.22) | 14 | SSRI<TCAs (RR 1.36, CI 1.09–1.70) |
| TCAs vs MAOIs | 2 studies | RR 1.16, CI 0.74–1.83 | 3 | ND (RR 0.91, CI 0.64–1.29) |
| TCAs vs atypicalsa | 4 trials | RR 0.84, CI 0.51–1.38 | 8 | ND (RR 0.96, CI 0.75–1.24) |
| SSRIs vs MAOIs | 1 trial | RR 0.81, CI 0.55–1.20 | 1 | ND (RRs not given) |
| MAOIs vs atypical | No trial | No trial | ||
| SSRIs vs atypicals | No trial | No trial | ||
Abbreviations: CI, confidence interval; RR, risk ratio; HDRS, Hamilton Depression Rating Scale; MAOI, monoamine oxidase inhibitors.
Atypical antidepressants: tianeptine, mirtazepine, reboxetine, buspirone, milnaciprin, bupropion.
Data from Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev 2006;1:CD003491.
Overall, these studies underscore the similar incomplete response to antidepressant treatment across the life cycle and highlight the challenge to develop novel, more efficacious treatment strategies, especially for patients who do not respond fully to first-line treatments. The goal of acute, or short-term, treatment is full remission of symptoms. The goal of longer-term treatment is prevention of recurrence. Getting well is important but not enough. It is staying well that counts.
Maintenance Treatment
There is limited consensus about the length of long-term maintenance pharmacotherapy after a first episode of depression, most experts recommending 6 to 12 months of pharmacotherapy after a first episode of depression in old age.14 Recurrence rates in LLD range from 50% to 90% in a period of 2 to 3 years.15 Thus, the goal of the treatment is not only acute recovery but also prevention of recurrence.16 There are few controlled studies on the efficacy of maintenance antidepressant medication. Maintenance nortriptyline (plasma steady-state level 80–120 ng/mL), monthly interpersonal therapy (IPT), and the combination of the 2 were superior to placebo in preventing recurrence for 3 years among patients with LLD with a history of multiple episodes.17 Citalopram (dose 20–40 mg/d)18 but not sertraline (50–100 mg/d)19 have differed from placebo in 2 randomized trials following subjects for 48 and 100 weeks respectively. The most recent study to date to test the efficacy of an SSRI in maintenance treatment of LLD tested the efficacy of 2-year maintenance treatment with paroxetine and monthly interpersonal therapy.16 Major depression recurred in 35% of the patients receiving paroxetine and psychotherapy, 37% of those receiving paroxetine and clinical management sessions (30-minute visits with no specific therapy, questions about symptoms and possible side effects), 68% of those receiving placebo and psychotherapy, and 58% of those receiving placebo and clinical management sessions.16 The relative risk of recurrence among patients receiving placebo was 2.4 times that among those receiving paroxetine (dose 10–40 mg/d).16 Moreover, patients treated with paroxetine for 2 years were less likely to have recurrent depression, whereas maintenance psychotherapy did not prevent recurrences.16 Patients in their first lifetime episodes also benefited from maintenance treatment of 2 years, thus not supporting the conventional wisdom and practice of limiting continuation treatment to 6 to 12 months following remission from acute treatment.16
Another important and clinically relevant aspect of maintenance treatments is that the NNT is around 4, in contrast with an NNT of 7 to 8 in acute treatment. In comparison, 4 large trials of statins found that the number of patients needed to be treated with statins for 5 years to prevent another myocardial infarction was 21,16 indicating a larger clinical effect size for maintenance antidepressant pharmacotherapy.
Psychotherapy
Given the propensity to multiple side effects noticed in the elderly, psychotherapy may represent a safer alternative. Most guidelines advocate the additional benefit of supporting antidepressant medication with psychosocial interventions.6,17,20 An expert-consensus guideline from 2001 considered cognitive behavioral therapy (CBT), problem-solving therapy (PST), IPT, and supportive therapy as first-line psychosocial interventions, whereas psychodynamic therapy received a more controversial rating (26% of the experts rated this as first line and 36% as third line).14 Overall, the expert consensus recommended psychotherapy as an adjunctive treatment to medication, except for mild depression or dysthymia, for which psychotherapy alone was considered an alternate initial treatment strategy.14 The more commonly prescribed psycho-therapies are developed from cognitive therapy, which focuses on dysfunctional beliefs; they include CBT, PST, and behavioral activation. Numerous descriptive studies have examined the technical issues in adapting these therapies to aging populations: emphasizing behavioral techniques, repeating information, a slower pace, and using different sensory modalities.21 Thus, given the executive dysfunctions described in LLD,22 several experts advocated for the use of PST23,24 that uses behavioral activation and explicitly trains patients to select and solve daily problems as a way of increasing self-efficacy and overcoming the feelings of helplessness at the core of depression.
However, there is little evidence based on randomized controlled trials that specifically examines the efficacy of various types of psychotherapy in older people.
A Cochrane Review from 2007 identified 9 trials of CBT and psychodynamic therapy, 7 of these providing comparison data between CBT and controls.20 CBT was more effective than waiting list controls, whereas there was no difference in treatment effect between CBT and psychodynamic therapy. However, the superiority CBT to waiting list was maintained only when assessed via the HRSD; it disappeared when using the Geriatric Depression Scale (GDS).20 All the trials analyzed had small sample sizes, the inclusion criteria allowed for both major depression and dysthymia, included both clinical populations as well as community volunteers,25 with duration varying from 4 to 24 weeks.20 The investigators concluded that, although CBT-derived therapies seem to be superior to waiting list control, the small size of the meta-analysis, the high dropout rates, and the heterogeneity of the study populations and the interventions limited the ability to generalize these findings to clinical populations.20
One more recent randomized, controlled trial reported that in 4 months, CBT was more effective than treatment as usual or talking control (supportive therapy) for late-life depressed subjects (total N = 204).26 Another randomized, controlled trial showed that, in a period of 12 weeks, PST was superior to supportive therapy in older adults with major depression and executive dysfunction.27 Integrating the results of 89 controlled studies of LLD acute treatment, a recent meta-analysis28 reported that both pharmacotherapy and psychotherapy render comparable, moderate-to-large effect sizes (Cohen d 0.62–0.69 for pharmacotherapy studies and 0.83–1.09 for psychotherapy studies).
ECT
Older depressed patients are often frailer and particularly prone to the side effects of antidepressants. ECT has been established as particularly effective in LLD.29 Although it is still controversial, ECT seems to be a safe treatment even in elderly with comorbid cardiovascular illness, dementia, or Parkinson disease.30
From the various randomized controlled trials of ECT for elderly people (>60 years old), only 4 trials were eligible for inclusion in the Cochrane meta-analysis, 1 comparing the efficacy of real ECT versus simulated ECT, 2 comparing the efficacy of unilateral versus bilateral ECT, and the other comparing the efficacy of weekly ECT with three times weekly ECT. However, the various methodological problems did not allow the investigators to perform a quantitative comparative analysis of these studies.31 The investigators concluded that neither the efficacy of unilateral compared with bilateral ECT, nor of the 3-week ECT compared with weekly ECT, has been convincingly proved.31 Moreover, studies that establish the long-term effects of ECT or those comparing the safety and efficacy of ECT with antidepressants in subpopulations such as elderly depressed with dementia or vascular disease are still needed.31
Post-ECT maintenance treatment with pharmacotherapy are discussed later.
Difficult-to-treat LLD
In general, the pharmacological treatment of LLD is only partially successful, with about 50% of patients improving with antidepressant monotherapy to the point of full response or remission. Many factors predict a difficult-to-treat depression, including clinical profile (comorbid anxiety, psychotic symptoms, poor sleep, low self-esteem), high medical burden, coexisting cognitive impairment.32 Partial response poses the risk of chronic relapsing depression, nonadherence to other treatments for coexisting medical disorders, family caregiver burden, and suicide.
Treatment-resistant Depression
Treatment-resistant depression reportedly affects up to one-third of older depressed patients.33 Before labeling an episode of depression as treatment resistant, it is important to ensure that the diagnosis is correct and that the patient has received an adequate dose of treatment, for an appropriate length of time, to assess the presence of comorbid physical and psychiatric conditions.34 Pharmacological options of treatment-resistant depression can be grouped in 2 categories: switching or combining. In the first case, treatment is switched within or between classes of antidepressants and thus avoids polypharmacy and potential increased side effects and medication costs.35 Combination strategies have the advantage of building on achieved improvements and are recommended when partial response has already been obtained. The most frequently used augmenting agents are lithium, atypical antipsychotics, and thyroid hormones. A sequential treatment protocol compared augmentation with lithium, switching to monoamine oxidase inhibitors (MAOI) or to ECT in elderly with partial acute response to either venlafaxine or nortriptyline.36 Augmentation with lithium was the best treatment option in this group for both efficacy and tolerability.36 So far, the combination of antidepressants and atypical antipsychotics (aripiprazole and olanzapine) are the only approved augmenting agents for treatment-resistant depression. A recent pilot study in older adults using aripiprazole augmentation reported that 50% of the 24 incomplete responders to prior sequential treatment with SSRI and serotonin-norepinephrine reuptake inhibitor pharmacotherapy remitted in 12 weeks with the addition of aripiprazole (mean daily dose 10 mg) and remission was sustained during 6 months of continuation treatment.37
Several experimental, less well studied alternatives use central nervous system stimulants such as methylphenidate, modafinil, ω-3 fatty acids, lamotrigine, topiramate, herbal supplements, or β-blockers.38–40
Although there is no equivalent in the elderly of the STAR*D trial in midlife adults, Dew and colleagues41 reported a cumulative response rate of more than 80% to successive augmentation strategies, a rate similar to that reported in the STAR*D trials.
Therefore, if patients stay the course in depression care management with evidence-based pharmacotherapy, most eventually reach full response or remission. Eliciting treatment adherence is an important part of depression care management and usually involves working with family care givers in building a therapeutic alliance.42
Anxious Depression
Comorbid anxiety is common in late-life depression, having a prevalence of up to 65% in clinical samples.43,44 Several studies reported that greater severity of anxiety is associated with increased risk of withdrawal from treatment, decreased response to acute antidepressant treatment, and a longer time to both response and remission.45 In a controlled, randomized trial, we reported that high pretreatment levels of anxiety symptoms increased not only the risk of nonresponse in acute treatment but also the risk of recurrence of depression in the first 2 years after response to antidepressant treatment.45 Also, persistent severe symptoms of anxiety after 6 weeks of treatment were associated with longer time and lower rates of remission of LLD.46 Among anxiety symptoms, worry more than panic predicted longer time to response and earlier recurrence in subjects with LLD treated with paroxetine (Fig. 1).47
Fig. 1.
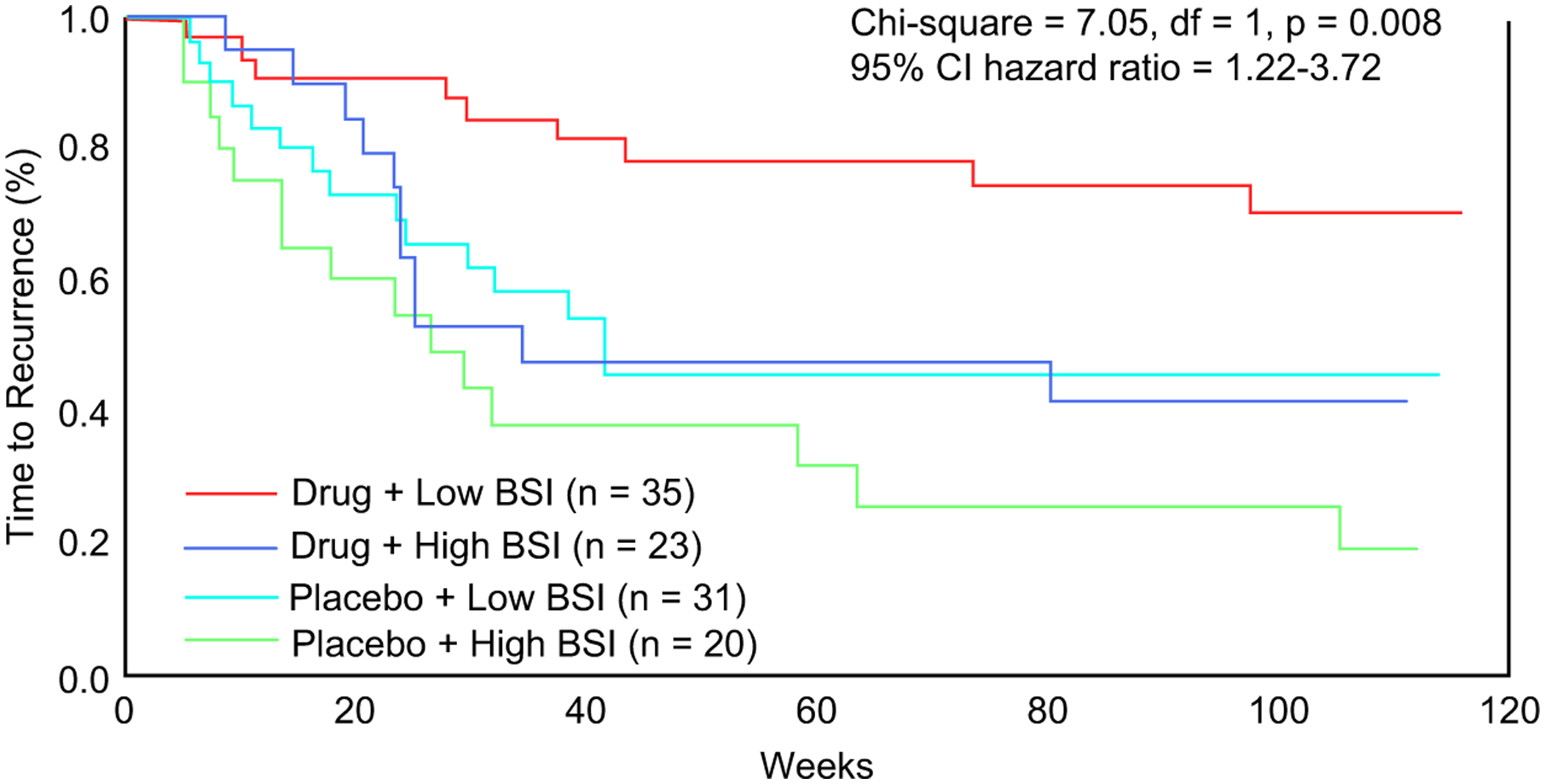
Comorbid anxiety symptoms and time to recurrence of late-life depression. (From Andreescu C, Lenze EJ, Dew MA, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry 2007;190:347; with permission.)
Psychotic Depression
High rates of major depressive disorder (MDD) with psychotic features (as high as 45%) have been reported in elderly inpatients with depression.48,49 Psychotic depression is associated with poorer short-term outcome, longer time to recovery, greater disability, and greater mortality than MDD without psychosis.50 There have been only 2 randomized controlled pharmacotherapy trials of psychotic depression in older people. The first examined the efficacy of an antidepressant alone (nortriptyline, target plasma level 100 ng/mL) versus a combination of antidepressant and antipsychotic (notriptyline plus placebo/perphenazine, mean does 18.9 mg/d).51 The categorical response was mediocre with both the antidepressant alone and with the combination (44% and 50% respectively), the investigators hypothesizing that the low response rate might have been caused by the heterogeneity of pathogenesis of psychotic depression in older patients, some of whom might have had incipient dementia.51 The higher frailty of older patients often leads to the use of ECT early in the course of the treatment.52 The second controlled trial examined for 12 weeks the efficacy of olanzapine (dose 5–20 mg/d) plus placebo or a combination of olanzapine and sertraline (50–150 mg/d) in patients with psychotic depression, and reported the results in the subgroup (more than 60 years old).53 The combination of olanzapine plus sertraline was associated with a greater remission rate than olanzapine monotherapy (41.9% vs 23.9%, χ2 = 9.53, P = .02).53
Although practice guidelines recommend the use of an antidepressant and an antipsychotic for the treatment of psychotic depression, an analysis regarding the use of pharmacotherapy in psychotic depression revealed that, with usual care, only 5% of subjects received an adequate dose of an antidepressant and a high dose of an antipsychotic.54 The intensity of pharmacotherapy in the combination trials was significantly associated only with the duration of current depressive episode. Most subjects (84%) received no antipsychotic or, at best, subtherapeutic doses of antipsychotics, and only about half of them (48%) received therapeutic doses of antidepressants.54 The high proportions of patients who did not receive antipsychotics or received low doses of antipsychotics may be to the result of a lack of recognition of psychotic features.54
ECT has been reported to show a response rate of 87% in a mixed sample of psychotic and nonpsychotic depressed subjects55 but there is a rapid increase in depressive symptoms after ECT.55 However, pharmacotherapy may be more practical in community settings. Post-ECT maintenance treatment seems to be more effective when Li is combined with an antidepressant than when the antidepressant (nortriptyline) is used alone (39% relapse rate for the combination versus 60% relapse rate for antidepressant monotherapy).55
Vascular Depression
The vascular depression hypothesis was formulated in 1997 and postulates that cere-brovascular disease can predispose, precipitate, or perpetuate a depressive syndrome in older adults.55 Depressed older adults with subcortical ischemic lesions often have a distinct clinical presentation with motor retardation, apathy, disability, increased risk of dementia, and a low familial load of depression.55 Most,56–58 but not all,59 studies documented poorer response to antidepressants for patients with depression and subcortical vascular lesions. SSRIs have so far been of limited efficacy in depressed patients with subcortical vascular lesions. Some experts recommended the use of dopamine-acting agents (especially in depressed subjects with frontostriatal impairment) or psychotropics with cathecholaminergic activity that might promote recovery following ischemic events.60 Two studies examined the advantages of using adjuvant calcium channel blockers, concluding that the augmentation of fluoxetine treatment with nimodipine leads to better treatment results and lower rates of recurrence at 8 months.61,62
Depression in the Context of Cognitive Impairment
Cognitive impairment in LLD is a core feature of the illness, contributing to disability and impaired quality of life. In a recent randomized controlled trial, the investigators tested the efficacy of added donepezil to antidepressant treatment in improving cognitive performance and reducing recurrences of depression in 2 years of maintenance treatment.63 The overall response rate to open escitalopram (followed by duloxetine and duloxetine plus aripiprazole as needed) was about 65%. During double-blind, placebo-controlled maintenance treatment for 2 years (with adjunctive donepezil or placebo), patients randomly assigned to donepezil had small improvement in cognitive function but substantially greater rates of recurrent major depressive episodes, compared with placebo. In the subgroup of patients with mild cognitive impairment (MCI) at the start of maintenance treatment (n = 57), 3 of 30 patients on donepezil (10%) converted to dementia within 2 years, versus 9 of 27 (33%) on placebo (Fisher exact P = .05). The investigators concluded that augmentation of maintenance pharmacotherapy with cholinesterase inhibitors in older adults with depression depends on a careful weighing of benefits and risks, especially in those with MCI. There seems to be no benefit in patients without MCI.63
Major depression affects about 25% of patients with Alzheimer disease (AD) and it is a major cause of disability, being associated with increased impairment in the quality of life and activities in daily life (ADLs), greater caregiver burden, increased physical aggression, and increased risk of suicide.64 Various studies have investigated the treatment response in depression comorbid with cognitive impairment but most included subjects with major depression but also with depressive symptoms, or subjects with various grades of cognitive impairment. Few studies focused on patients with MDD and AD. Moclobemid, citalopram, and clomipramine were found to be superior to placebo in the short-term treatment of depression in AD.65–67 A 12-week randomized controlled trial showed that sertraline (mean dose 95 mg/d) was superior to placebo (effect size 0.85) in treating MDD in patients with AD.64 Sertraline-treated patients also had a trend toward less ADL decline, although there was no benefit to cognition as assessed by the Mini Mental State Examination at 12 weeks.64 However, a follow-up report examining the week 24 outcome of patients who participated in the trial found no between-groups differences in depression response or remission rates or secondary outcomes (such as ADL decline), concluding that sertraline may not be beneficial for long-term treatment of depression in AD.68 The association between damage to the locus coeruleus and depression in AD suggests a better efficacy of noradrenergic than serotoninergic antidepressants. We only found 1 study comparing the efficacy of citalopram and mianserin in elderly depressed subjects with dementia.69 On balance, the evidence supporting the efficacy of antidepressant pharmacotherapy for depression in AD is mixed and inconclusive.
Bipolar Depression
Manic and bipolar depressed patients represent 5% to 15% of patients presenting for acute treatment at geriatric psychiatry services.70 There are no systematic studies of the treatment of bipolar depression in the elderly,70 and clinicians usually rely on data from mixed-age studies, case reports, or uncontrolled trials.71 Various strategies have been proposed, including combinations of paroxetine and lithium72 and the preferred use of SSRIs and bupropion rather than tricyclics.70 Optimal mood-stabilizer dosing for lithium in bipolar elderly patients has not been assessed, and its tolerability in the elderly is a particular concern (increased cognitive impairment, neurological side effects, delirium, sick sinus syndrome, hypothyroidism, polyuria, edema).73 Experts recommend lower doses than for mixed-age patients (0.5–0.8 mEq/L), but lithium toxicity has been reported in the elderly even at moderate concentrations (0.5–0.8 mEq/L).70 Other recommendations include combination of lithium and an SSRI, the use of lamotrigine or other anticonvulsants, or the addition of an atypical antipsychotic.70 ECT should also be considered in patients with bipolar depression or rapid cycling symptoms refractory to pharmacotherapy, in suicidal patients, or those with inadequate food and fluid intake.70
A retrospective analysis of the efficacy of lithium (mean dose 750 mg/d) and lamotrigine (mean dose 240 mg/d) in the maintenance treatment geriatric bipolar disorder reported that lamotrigine but not lithium maintenance therapy significantly delayed time to intervention for a depressive episode.71
At this time there are no studies exploring the impact of cognitive impairment or of comorbid medical conditions on acute/long-term treatment of bipolar depression in the elderly.
Predictors of Treatment Response: Use of Treatment Decision-making Trees
Successful antidepressant treatment is one of the most effective ways to reduce disability, prevent morbidity, and improve quality of life in older depressed patients. However, LLD is often resistant to treatment and may exhibit a slower resolution of symptoms than midlife depression.74 The identification of predictors of treatment response would allow the clinicians to modify treatment options earlier in the course of the treatment.
Several studies explored the biological, clinical, and psychosocial predictors of treatment response in LLD (Table 2).
Table 2.
Predictors of treatment response in LLD
| Predictors of Treatment Response | Role | Study | |
|---|---|---|---|
| Biologic predictors | Serotonin transporter | S allele increases treatment resistance | Lotrich et al,110 2008 |
| REM sleep latency | Decreased REM sleep latency correlates with poor response | Reynolds et al,102 1991 | |
| Glucose cerebral metabolism | Reduced glucose metabolism in ACC and mPFC correlated with better response | Smith et al,103 1999 | |
| Increased metabolism in ACC | Predicts response to rTMS in vascular depression | Narushima et al,81 2010 | |
| Clinical predictors | Medical burden | Greater medical burden predicted slower recovery | Dew et al,41 2007 |
| Early symptom improvement | Predicted faster response | Mulsant et al,104 2006 | |
| Age of onset of first episode | Younger age at onset predicted poorer response | Dew et al,78 1997 | |
| Sleep disturbances | Baseline sleep disturbance predicted poorer response | Reynolds et al,102 1991 Dew et al,78 1997 | |
| Baseline HDRS scores | Higher score correlated with slower response | Gildengers et al,105 2005 | |
| Baseline anxiety | Increased baseline anxiety correlated with slower response | Andreescu et al,45 2007 | |
| Suicidal ideation | Baseline suicidal ideation correlated with longer time to response | Szanto et al,106 2003 | |
| Response to previous antidepressant treatment | Poor previous antidepressant response correlated with decrease rate of response | Tew et al,107 2006 | |
| Psychosocial predictors | Social support | Poor social support and poor family support correlated with poor response | Dew et al,78,108 1997 Martire et al,108 2007 |
| Social inequalities | Low income correlated with poorer response | Cohen et al,109 2006 | |
| Self-esteem | Higher self-esteem correlated with faster response | Gildengers et al,105 2005 | |
Abbreviations: ACC, anterior cingulate cortex; HDRS, Hamilton Depression Rating Scale; mPFC, medial prefrontal cortex; REM, rapid eye movement.
However, it may be difficult for clinicians to integrate the various predictors reported in the literature into a practical treatment strategy. In an analysis that pooled data from the acute treatment phase of 3 National Institute of Mental Health–funded treatment studies, we attempted to integrate and develop a hierarchy of clinical predictors of treatment response.75 Using signal detection theory,76 we built 2 different models by modulating the sensitivity threshold for each predictor of treatment response to obtain hierarchies of risk correlates with different patients’ characteristics.
Thus, for patients requiring an aggressive treatment approach (eg, patients with a high risk of suicide or severely disabled by their symptoms), the most significant predictor of treatment response was early symptom improvement (40% drop in Hamilton scores by 4 weeks), followed by lower levels of baseline anxiety and later age of onset of first episode of depression.75 No other clinical predictors, including adequacy of previous treatment (as measured by the antidepressant treatment history form [ATHF] score),77 race, recurrence, or baseline sleep disturbance reached significance levels (Fig. 2).
Fig. 2.
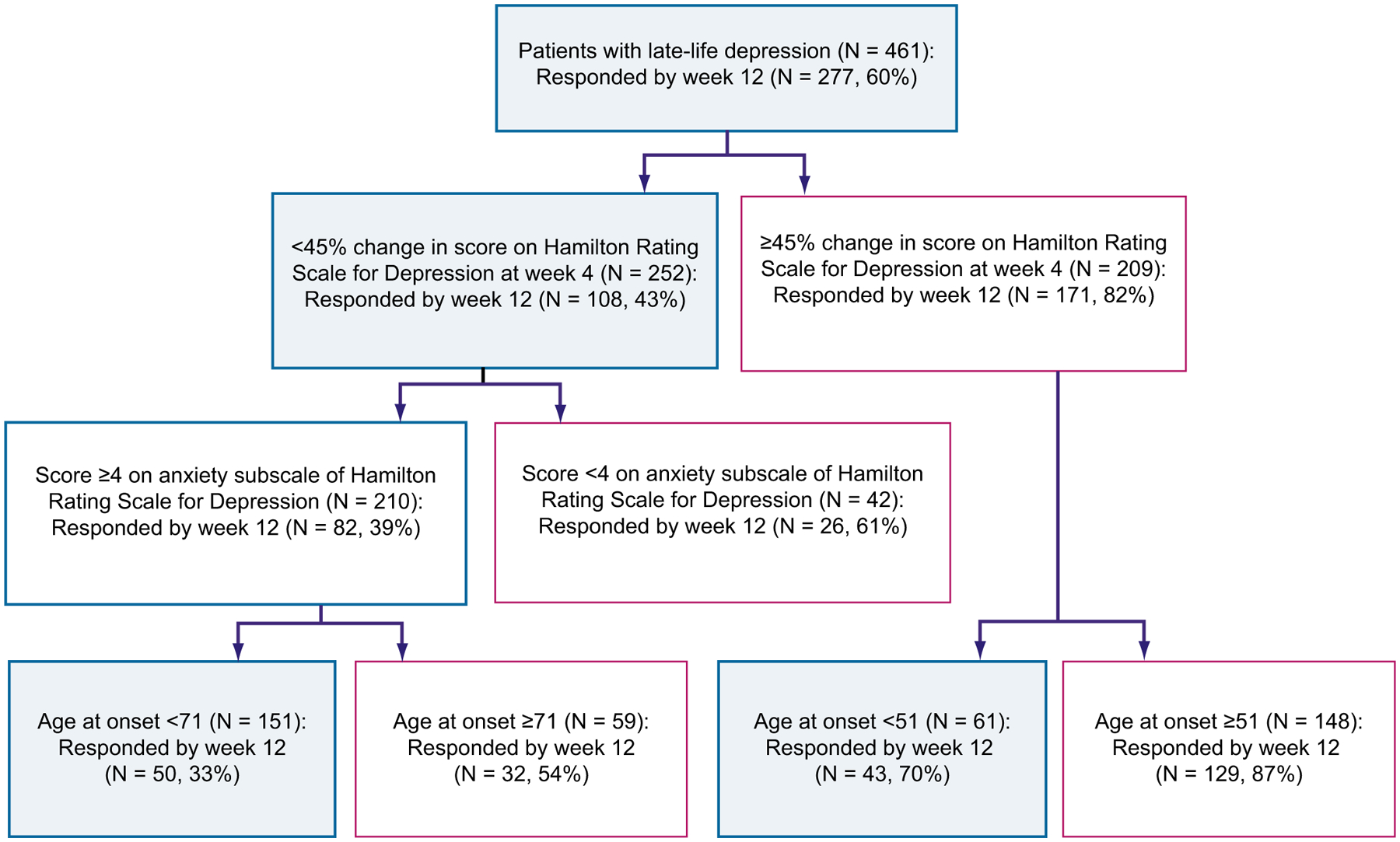
Hierarchy of predictors of treatment response with an aggressive treatment approach. Main outcome, full response status at week 12; proportion of responders at 12 weeks, 60%. A change in HRSD score at week 4 of less than 45% from baseline predicts a less-than-half (43%) chance of responding at week 12. For the patients in this group, higher baseline anxiety predicts a 39% chance of responding at week 12. For patients with a higher baseline anxiety, a younger age of onset predicts a 32% chance of responding at week 12. (From Andreescu C, Mulsant BH, Houck PR, et al. Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry 2008;165:859; with permission.)
For patients requiring a more conservative treatment approach (eg, patients with a history of multiple, unsuccessful, underdosed trials), the most significant predictor of treatment response was again early symptom improvement, followed by baseline anxiety and adequacy of previous antidepressant trials (Fig. 3).
Fig. 3.
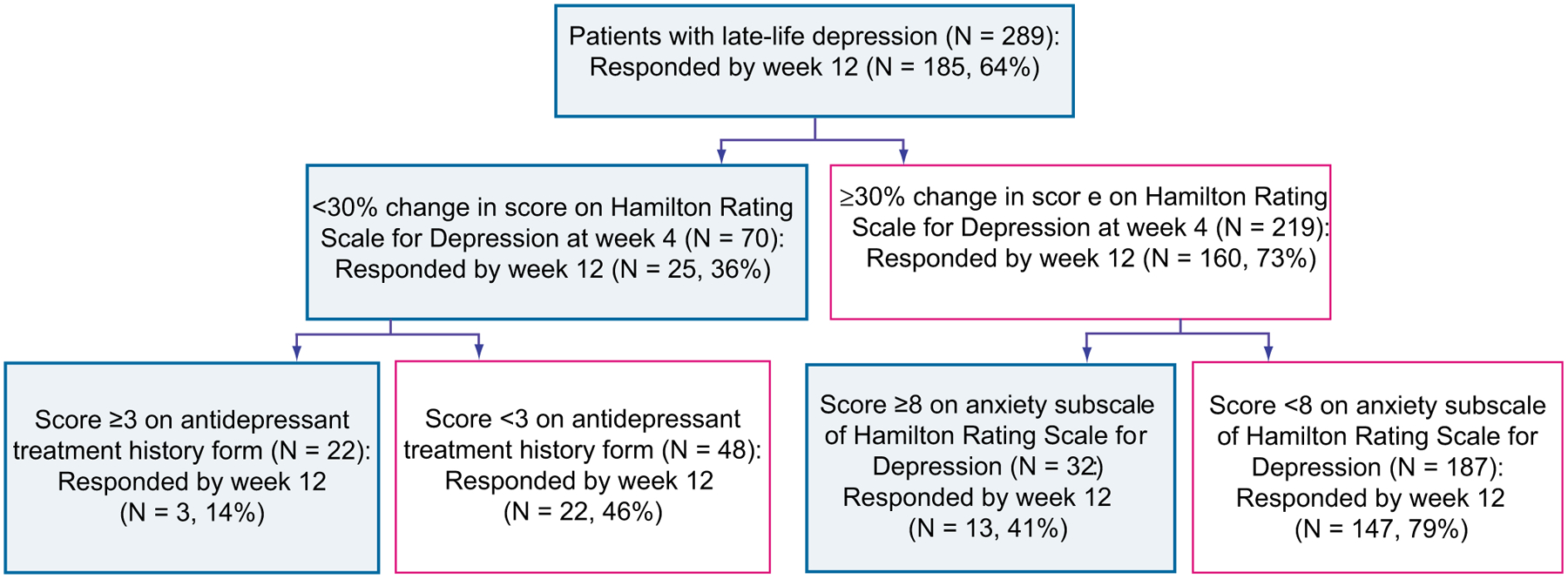
Hierarchy of predictors of treatment response with a conservative treatment approach. Main outcome, full response status at week 12; proportion of responders at 12 weeks, 64%. For the ATHF, a score greater than or equal to 3 indicates probably adequate antidepressant treatment history (trial of more than 4 weeks of an antidepressant at an adequate dose); ATHF les than 3 indicates inadequate antidepressant treatment history (trial of less than 4 weeks, or of more than 4 weeks but with an inadequate dose). High anxiety, at least moderate anxiety symptoms; low anxiety, mild or no anxiety symptoms. Change in HRSD at week 4 of less than 30% from baseline predicts a 35% chance of responding at week 12. For those subjects with a change in HRSD at week 4 of less than 30%, a history of at least 1 adequate antidepressant trial predicts a 13% chance of responding at week 12. For those subjects with a change in HRSD at week 4 higher than 30%, the next predictor is baseline anxiety. A higher baseline anxiety score predicts a lower chance of responding at 12 weeks (40%), whereas a lower baseline anxiety score predicts a 79% chance of responding at week 12. (From Andreescu C, Mulsant BH, Houck PR, et al. Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry 2008;165:860; with permission.)
These reports confirmed earlier work on the trajectory of acute response that emphasized that early symptom resolution predicts more stable long-term treatment response.78,79 Higher levels of acute or chronic stressors, poorer social support, younger age at onset, melancholiform features, older current age, and higher current anxiety also predicted a poorer response profile.78 The importance of early symptom resolution was further emphasized by the 2006 Maintenance Treatment Trial16 that noted that patients who needed adjunctive medication in acute treatment to get well also had a more brittle long-term response.
CHALLENGES AND FUTURE DIRECTIONS
Novel Treatment Options
Advances in LLD treatment include novel treatments, personalized treatment (according to depression type, individual characteristics), and strategies to improve access to and delivery of care.2
Novel treatments include transcranial magnetic stimulation (TMS), deep brain stimulation, vagus nerve stimulation (VNS), and magnetic seizure therapy.
TMS has been approved since 2008 as treatment of depression resistant to pharmacotherapy. High-frequency pulse (>1 Hz) repetitive TMS (rTMS) is usually applied to the left dorsolateral prefrontal cortex.80 A recent randomized, placebo-controlled trial indicated that rTMS may be beneficial for vascular depression (response 39%, remission 27% vs sham 7% and 4% respectively). Subgenual cingulate θ activity predicts treatment response in rTMS in vascular depression.81
Deep brain stimulation delivers a continuous train of repetitive, brief small voltage pulses mainly to the subgenual anterior cingulate cortex (ACC), an area that has been associated with treatment-resistant depression.82 More recent case reports delivered the voltage pulses to either deep brain structures such as nucleus accum-bens and ventral striatum.83
VNS was approved for treatment-resistant depression in 2005. The procedure stimulates the left cervical vagus nerve through low-frequency, chronic, intermittent-pulsed electric signals, stimulates areas involved in mood regulation (locus coeruleus, nucleus raphe magnus), and seems to modulate hippocampal neurogenesis.84 To our knowledge, there are no trials of VNS in LLD.
Several small trials examined the safety and efficacy of magnetic seizure therapy in depression. Its antidepressant effect seems to be less robust than that of ECT.2
Informed/personalized treatment uses neuroimaging techniques such as blood oxygenation level–dependent (BOLD) functional magnetic resonance imaging (fMRI) or diffusion tensor imaging (DTI).
In the last decade, there has been a rapid increase in the availability of magnetic resonance imaging (MRI), and it is likely that, in the near future, MRI accessibility will continue to increase, along with a decrease in scanning costs. If this trend continues, then using MRI to optimize the choice of medications for an individual with depression is possible. The identification of magnetic resonance (MR) markers of treatment response would allow for faster and more efficient trial rather than waiting 3 to 6 weeks to determine whether a new intervention is effective. Several MR markers of treatment response have been identified: lower activation of the rostral ACC at baseline, increased burden of white matter hyperintensities in the frontal regions, and lower fractional anisotropy in frontolimbic areas were associated with poor treatment response in either midlife85,86 or late-life studies of depression.87,88
Pharmacogenetics involves the use of molecular genetic information to assist in the prediction of drug efficacy and drug-induced adverse events. In a heterogeneous disorder such as LLD, pharmacogenetic data could be paramount for the development of individualized treatment approaches.89 Although the neuroimaging prediction of antidepressant response is not yet refined/cheap enough for clinical applications, genotyping assays are easy to do and their costs have rapidly decreased. Various candidate genes in the serotoninergic system (most notably the serotonin transporter polymorphism) have been associated with treatment response.90 The serotonin transporter gene (SLC6A4) also influences treatment response variability in LLD, mainly in the initial stages of treatment, through a gene-concentration interaction for SSRIs.91 In addition, elderly subjects carrying the S allele may be at increased risk of adverse drug reactions and may require a higher initial SSRI plasma concentration to obtain a response.91,92 Another recent candidate gene (OPRM1, the μ-opioid receptor) has been associated with citalopram response in the STAR*D sample.93 However, a recent study of 72 candidate genes that also used a genome-wide associate study assessing more than 500,000 SNPs reported modest results.94 None of the candidate genes provided evidence for association with response to antidepressants.94
Sequential treatment:
Pharmacotherapy followed by psychotherapy. A recent meta-analysis examined the efficacy of the sequential integration of psychotherapy and pharmacotherapy in reducing the risk of relapse and recurrence in MDD.95 The pooled risk ratio (RR = 0.79) suggested a relative advantage in preventing relapse and recurrence for the sequential administration of psychotherapy after successful response to acute-phase pharmacotherapy compared with control conditions.95
ECT followed by pharmacotherapy. Relapse rates after ECT remain high, with virtually all remitted patients relapsing within 6 months of stopping ECT.55 Most investigators have advocated the use of antidepressants or a combination of antidepressant and mood stabilizer (Li) after completion of ECT.55 Some experts recommended using antidepressants during ECT to prevent early relapses.96
rTMS followed by pharmacotherapy. In a recent study, subjects received citalopram (20 mg/d) after either rTMS or sham treatment, with mixed results (of the 12 subjects who responded to rTMS, 9 maintained response and 4 had a relapse of depression).97
Various other lines of investigations are being developed. For example, homocysteine has been correlated with increased risk of depression (most likely through the link between the folate/methylation cycles and depression). Lowering homocysteine levels would reduce the incidence and severity of depressive symptoms; a meta-analysis found that older adults with high homocysteine plasma levels have increased risk of depression (odds ratio 1.7).98
Health Services Perspectives
The greatest limitation in treatment of LLD concerns treatment access and delivery. In primary care settings, the diagnosis of depression is frequently missed and treatment is often inadequate.2 Studies such as Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT)5,99 and Improving Mood Promoting Access to Collaborative Care Treatment (IMPACT)100 have shown that collaborative care in primary care settings has better outcomes than usual care, and that downstream consequences of inadequately treated depression can be prevented. More importantly, a long-term, developmental perspective on depression across life span is needed.3 Regarding prevention as protection (prolonging lifespan and healthspan), participation in studies such as PROSPECT5 have been linked to lower rates of mortality from cancer at 4-year to 5-year follow-up.3
Many real-world challenges hinder implementing depression treatment recommendations, such as adequate funds, adequate management of various programs, overcoming barriers in training staff in intervention techniques, ensuring fidelity to established protocols, adequate support to evaluate outcomes, and ensuring accessibility.101 Partnership among researchers, health care providers, and policy makers is necessary to implement successful treatment protocols for depression in late life.101
Acknowledgments
Supported in part by P30 MH71944, K23 MH 086686, R01 MH 083660, the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry, and the UPMC Endowment in Geriatric Psychiatry.
Financial disclosure:
Carmen Andreescu has no conflict of interest to report. Charles F. Reynolds III has received pharmaceutical supplies for his NIH-sponsored research from GlaxoSmithKline, Pfizer Inc, Eli Lilly and Co, Bristol Meyers Squibb, Wyeth Pharmaceuticals, and Forest Pharmaceuticals.
REFERENCES
- 1.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–8. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos GS, Kelly RE Jr. Research advances in geriatric depression. World Psychiatry 2009;8:140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds CF. The cutting edge: prevention of depressive disorders. Depress Anxiety 2009;26:1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman DP, Perry GS. Depression as a major component of public health for older adults. Prev Chronic Dis 2008;5:A22. [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91. [DOI] [PubMed] [Google Scholar]
- 6.Charney DS, Reynolds CF, Lewis L, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry 2003;60:664–72. [DOI] [PubMed] [Google Scholar]
- 7.Stevens JA, Hasbrouck LM, Durant TM, et al. Surveillance for injuries and violence among older adults. MMWR CDC Surveill Summ 1999;48:27–50. [PubMed] [Google Scholar]
- 8.Mitchell AJ, Subramaniam H. Prognosis of depression in old age compared to middle age: a systematic review of comparative studies. Am J Psychiatry 2005;162:1588–601. [DOI] [PubMed] [Google Scholar]
- 9.Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev 2006;1:CD003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor WD, Doraiswamy PM. A systematic review of antidepressant placebo-controlled trials for geriatric depression: limitations of current data and directions for the future. Neuropsychopharmacology 2004;29:2285–99. [DOI] [PubMed] [Google Scholar]
- 11.Nelson JC, Clary CM, Leon AC, et al. Symptoms of late-life depression: frequency and change during treatment. Am J Geriatr Psychiatry 2005;13:520–6. [DOI] [PubMed] [Google Scholar]
- 12.Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry 2008;16:58–64. [DOI] [PubMed] [Google Scholar]
- 13.McGrath PJ, Khan AY, Trivedi MH, et al. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: a STAR*D report. J Clin Psychiatry 2008;69:1847–55. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Katz IR, Reynolds CF, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract 2001;7:361–76. [DOI] [PubMed] [Google Scholar]
- 15.Zis AP, Grof P, Webster M. Predictors of relapse in recurrent affective disorders. Psychopharmacol Bull 1980;16:47–9. [PubMed] [Google Scholar]
- 16.Reynolds CF, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med 2006;354:1130–8. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds CF, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. J Am Med Assoc 1999;281: 39–45. [DOI] [PubMed] [Google Scholar]
- 18.Klysner R, Bent-Hansen J, Hansen HL, et al. Efficacy of citalopram in the prevention of recurrent depression in elderly patients: placebo-controlled study of maintenance therapy. Br J Psychiatry 2002;19:29–35. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KC, Mottram PG, Ashworth L, et al. Older community residents with depression: long-term treatment with sertraline. Randomised, double-blind, placebo-controlled study. Br J Psychiatry 2003;182:492–7. [DOI] [PubMed] [Google Scholar]
- 20.Wilson KC, Mottram PG, Vassilas CA. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;1:CD004853. [DOI] [PubMed] [Google Scholar]
- 21.Grant RW, Casey DA. Adapting cognitive behavioral therapy for the frail elderly. Int Psychogeriatr 1995;7:561–71. [DOI] [PubMed] [Google Scholar]
- 22.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry 2004;61: 587–95. [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos GS, Raue P, Arean PA. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry 2003;11:46–52. [PubMed] [Google Scholar]
- 24.Alexopoulos GS, Raue PJ, Kanellopoulos D, et al. Problem-solving therapy for the depression-executive dysfunction syndrome of late life. Int J Geriatr Psychiatry 2008;23:782–8. [DOI] [PubMed] [Google Scholar]
- 25.Arean PA, Perri MG, Nezu AM, et al. Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. J Consult Clin Psychol 1993;61:1003–10. [DOI] [PubMed] [Google Scholar]
- 26.Serfaty MA, Haworth D, Blanchard M, et al. Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Arch Gen Psychiatry 2009;66:1332–40. [DOI] [PubMed] [Google Scholar]
- 27.Arean PA, Raue P, Mackin RS, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry 2010;167:1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006;163:1493–501. [DOI] [PubMed] [Google Scholar]
- 29.Flint AJ, Rifat SL. The treatment of psychotic depression in later life: a comparison of pharmacotherapy and ECT. Int J Geriatr Psychiatry 1998;13:23–8. [PubMed] [Google Scholar]
- 30.Rice EH, Sombrotto LB, Markowitz JC, et al. Cardiovascular morbidity in high-risk patients during ECT. Am J Psychiatry 1994;151:1637–41. [DOI] [PubMed] [Google Scholar]
- 31.van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev 2003;2:CD003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Driscoll HC, Karp JF, Dew MA, et al. Getting better, getting well: understanding and managing partial and non-response to pharmacological treatment of nonpsychotic major depression in old age. Drugs Aging 2007;24:801–14. [DOI] [PubMed] [Google Scholar]
- 33.Mulsant BH, Pollock B. Treatment-resistant depression in late-life. J Geriatr Psychiatry Neurol 1998;11:186–93. [DOI] [PubMed] [Google Scholar]
- 34.Flint AJ. Treatment-resistant depression in late life. CNS Spectr 2002;7:733–8. [DOI] [PubMed] [Google Scholar]
- 35.Shelton RC, Osuntokun O, Heinloth AN, et al. Therapeutic options for treatment-resistant depression. CNS Drugs 2010;24:131–61. [DOI] [PubMed] [Google Scholar]
- 36.Kok RM, Nolen WA, Heeren TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274–81. [DOI] [PubMed] [Google Scholar]
- 37.Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry 2009;70:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavretsky H, Park S, Siddarth P, et al. Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind placebo-controlled pilot trial. Am J Geriatr Psychiatry 2006;14:181–5. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt do Prado-Lima PA, Bacaltchuck J. Topiramate in treatment-resistant depression and binge-eating disorder. Bipolar Disord 2002;4:271–3. [DOI] [PubMed] [Google Scholar]
- 40.Thomas SP, Nandhra HS, Jayaraman A. Systematic review of lamotrigine augmentation of treatment resistant unipolar depression (TRD). J Ment Health 2010;19:168–75. [DOI] [PubMed] [Google Scholar]
- 41.Dew MA, Whyte EM, Lenze EJ, et al. Recovery from major depression in older adults receiving augmentation of antidepressant pharmacotherapy. Am J Psychiatry 2007;164:892–9. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds CF, Dew MA, Martire LM, et al. Treating depression to remission in older adults: a controlled evaluation of combined interpersonal psychotherapy versus escitalopram with depression care management. Int J Geriatr Psychiatry 2010;25:1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenze EJ. Comorbidity of depression and anxiety in the elderly. Curr Psychiatry Rep 2003;5:62–7. [DOI] [PubMed] [Google Scholar]
- 44.Beekman AT, de Beurs E, van Balkom AJ, et al. Anxiety and depression in later life: co-occurrence and communality of risk factors. Am J Psychiatry 2000;157: 89–95. [DOI] [PubMed] [Google Scholar]
- 45.Andreescu C, Lenze EJ, Dew MA, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry 2007;190:344–9. [DOI] [PubMed] [Google Scholar]
- 46.Greenlee A, Karp JF, Dew MA, et al. Anxiety impairs depression remission in partial responders during extended treatment in late-life. Depress Anxiety 2010;27:451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreescu C, Lenze E, Mulsant B, et al. High worry severity is associated with poorer acute and maintenance efficacy of antidepressants in late-life depression. Depress Anxiety 2009;26:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coryell W, Leon A, Winokur G, et al. Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry 1996;153:483–9. [DOI] [PubMed] [Google Scholar]
- 49.Maj M, Pirozzi R, Magliano L, et al. Phenomenology and prognostic significance of delusions in major depressive disorder: a 10-year prospective follow-up study. J Clin Psychiatry 2007;68:1411–7. [DOI] [PubMed] [Google Scholar]
- 50.Vythilingam M, Chen J, Bremner JD, et al. Psychotic depression and mortality. Am J Psychiatry 2003;160:574–6. [DOI] [PubMed] [Google Scholar]
- 51.Mulsant BH, Pollock BG, Nebes R, et al. A twelve-week, double-blind, randomized comparison of nortriptyline and paroxetine in older depressed inpatients and outpatients. Am J Geriatr Psychiatry 2001;9:406–14. [PubMed] [Google Scholar]
- 52.Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatr Ann 2006;36:31–8. [Google Scholar]
- 53.Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry 2009;66:838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreescu C, Mulsant BH, Peasley-Miklus C, et al. Persisting low use of antipsychotic in the treatment of major depression with psychotic features. J Clin Psychiatry 2007;68:194–200. [DOI] [PubMed] [Google Scholar]
- 55.Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy. J Am Med Assoc 2001;285:1299–307. [DOI] [PubMed] [Google Scholar]
- 56.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry 2010;67:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry 1995;37:151–60. [DOI] [PubMed] [Google Scholar]
- 58.Simpson S, Baldwin RC, Jackson A, et al. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med 1998;28:1015–26. [DOI] [PubMed] [Google Scholar]
- 59.Salloway S, Boyle PA, Correia S, et al. The relationship of MRI subcortical hyperintensities to treatment response in a trial of sertraline in geriatric depressed outpatients. Am J Geriatr Psychiatry 2002;10:107–11. [PubMed] [Google Scholar]
- 60.Ramasubbu R, Goodyear BG. Methylphenidate modulates activity within cognitive neural networks of patients with post-stroke major depression: a placebo-controlled fMRI study. Neuropsychiatr Dis Treat 2008;4:1251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taragano FE, Allegri R, Vicario A, et al. A double blind, randomized clinical trial assessing the efficacy and safety of augmenting standard antidepressant therapy with nimodipine in the treatment of ‘vascular depression’. Int J Geriatr Psychiatry 2001;16:254–60. [DOI] [PubMed] [Google Scholar]
- 62.Taragano FE, Bagnatti P, Allegri RF. A double-blind, randomized clinical trial to assess the augmentation with nimodipine of antidepressant therapy in the treatment of “vascular depression”. Int Psychogeriatr 2005;17:487–98. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds CF, Butters MA, Lopez O, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry 2011;68(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry 2003;60:737–46. [DOI] [PubMed] [Google Scholar]
- 65.Roth M, Mountjoy CQ, Amrein R. Moclobemide in elderly patients with cognitive decline and depression: an international double-blind, placebo-controlled trial. Br J Psychiatry 1996;168:149–57. [DOI] [PubMed] [Google Scholar]
- 66.Nyth AL, Gottfries CG, Lyby K, et al. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatr Scand 1992;86:138–45. [DOI] [PubMed] [Google Scholar]
- 67.Petracca G, Teson A, Chemerinski E, et al. A double-blind placebo-controlled study of clomipramine in depressed patients with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1996;8:270–5. [DOI] [PubMed] [Google Scholar]
- 68.Weintraub D, Rosenberg PB, Drye LT, et al. Sertraline for the treatment of depression in Alzheimer disease: week-24 outcomes. Am J Geriatr Psychiatry 2010;18:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karlsson I, Godderis J, Augusto De Mendonca Lima C, et al. A randomised, double-blind comparison of the efficacy and safety of citalopram compared to mianserin in elderly, depressed patients with or without mild to moderate dementia. Int J Geriatr Psychiatry 2000;15:295–305. [DOI] [PubMed] [Google Scholar]
- 70.Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry 2004;12:342–57. [DOI] [PubMed] [Google Scholar]
- 71.Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry 2005;13:305–11. [DOI] [PubMed] [Google Scholar]
- 72.Nemeroff CB. Advancing the treatment of mood and anxiety disorders: the first 10 years’ experience with paroxetine. Psychopharmacol Bull 2003;37(Suppl 1): 6–7. [PubMed] [Google Scholar]
- 73.Smith RE, Helms PM. Adverse effects of lithium therapy in the acutely ill elderly patient. J Clin Psychiatry 1982;43:94–9. [PubMed] [Google Scholar]
- 74.Whyte EM, Dew MA, Gildengers A, et al. Time course of response to antidepressants in late-life major depression: therapeutic implications. Drugs Aging 2004; 21:531–54. [DOI] [PubMed] [Google Scholar]
- 75.Andreescu C, Mulsant BH, Houck PR, et al. Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry 2008;165:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiernan M, Kraemer HC, Winkleby MA, et al. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods 2001;6:35–48. [DOI] [PubMed] [Google Scholar]
- 77.Oquendo MA, Baca-Garcia E, Kartachov A, et al. A computer algorithm for calculating the adequacy of antidepressant treatment in unipolar and bipolar depression. J Clin Psychiatry 2003;64:825–33. [DOI] [PubMed] [Google Scholar]
- 78.Dew MA, Reynolds CF, Houck PR, et al. Temporal profiles of the course of depression during treatment: predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry 1997;54:1016–24. [DOI] [PubMed] [Google Scholar]
- 79.Dew MA, Reynolds CF, Mulsant B, et al. Initial recovery patterns may predict which maintenance therapies for depression will keep older adults well. J Affect Disord 2001;65:155–66. [DOI] [PubMed] [Google Scholar]
- 80.Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry 2008;65: 268–76. [DOI] [PubMed] [Google Scholar]
- 81.Narushima K, McCormick LM, Yamada T, et al. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. J Neuropsychiatry Clin Neurosci 2010;22:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45:651–60. [DOI] [PubMed] [Google Scholar]
- 83.Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 2009;65:267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 2006;31:1345–55. [DOI] [PubMed] [Google Scholar]
- 85.MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J Psychiatry Neurosci 2009;34:343–9. [PMC free article] [PubMed] [Google Scholar]
- 86.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000;48:830–43. [DOI] [PubMed] [Google Scholar]
- 87.Gunning-Dixon FM, Walton M, Cheng J, et al. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord 2010;126(3): 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taylor WD, Kuchibhatla M, Payne ME, et al. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One 2008;3:e3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malhotra AK. The pharmacogenetics of depression: enter the GWAS. Am J Psychiatry 2010;167:493–5. [DOI] [PubMed] [Google Scholar]
- 90.Serretti A, Kato M, RonchiDe D, et al. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 2007;12: 247–57. [DOI] [PubMed] [Google Scholar]
- 91.Gerretsen P, Pollock BG. Pharmacogenetics and the serotonin transporter in late-life depression. Expert Opin Drug Metab Toxicol 2008;4:1465–78. [DOI] [PubMed] [Google Scholar]
- 92.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol 1986;95:15–20. [DOI] [PubMed] [Google Scholar]
- 93.Garriock HA, Tanowitz M, Kraft JB, et al. Association of mu-opioid receptor variants and response to citalopram treatment in major depressive disorder. Am J Psychiatry 2010;167:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uher R, Perroud N, Ng MY, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry 2010;167:555–64. [DOI] [PubMed] [Google Scholar]
- 95.Guidi J, Fava GA, Fava M, et al. Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychol Med 2011;41(2):321–31. [DOI] [PubMed] [Google Scholar]
- 96.Yildiz A, Mantar A, Simsek S, et al. Combination of pharmacotherapy with electroconvulsive therapy in prevention of depressive relapse: a pilot controlled trial. J ECT 2010;26:104–10. [DOI] [PubMed] [Google Scholar]
- 97.Robinson RG, Tenev V, Jorge RE. Citalopram for continuation therapy after repetitive transcranial magnetic stimulation in vascular depression. Am J Geriatr Psychiatry 2009;17:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almeida OP, McCaul K, Hankey GJ, et al. Homocysteine and depression in later life. Arch Gen Psychiatry 2008;65:1286–94. [DOI] [PubMed] [Google Scholar]
- 99.Alexopoulos GS, Katz IR, Bruce ML, et al. Remission in depressed geriatric primary care patients: a report from the PROSPECT study. Am J Psychiatry 2005;162:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. J Am Med Assoc 2002;288:2836–45. [DOI] [PubMed] [Google Scholar]
- 101.Snowden M, Steinman L, Frederick J. Treating depression in older adults: challenges to implementing the recommendations of an expert panel. Prev Chronic Dis 2008;5:A26. [PMC free article] [PubMed] [Google Scholar]
- 102.Reynolds CF, Hoch CC, Buysse DJ, et al. Sleep in late-life recurrent depression: changes during early continuation therapy with nortriptyline. Neuropsychopharmacology 1991;5:85–96. [PubMed] [Google Scholar]
- 103.Smith GS, Reynolds CF, Pollock B, et al. Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry 1999;156:683–9. [DOI] [PubMed] [Google Scholar]
- 104.Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol 2006;26:113–20. [DOI] [PubMed] [Google Scholar]
- 105.Gildengers AG, Houck PR, Mulsant BH, et al. Trajectories of treatment response in late-life depression: psychosocial and clinical correlates. J Clin Psychopharmacol 2005;25:S8–13. [DOI] [PubMed] [Google Scholar]
- 106.Szanto K, Mulsant BH, Houck P, et al. Occurrence and course of suicidality during short-term treatment of late-life depression. Arch Gen Psychiatry 2003; 60:610–7. [DOI] [PubMed] [Google Scholar]
- 107.Tew JD, Mulsant BH, Houck PR, et al. Impact of prior inadequate treatment exposure on response to antidepressant treatment in late life. Am J Geriatr Psychiatry 2006;14:957–65. [DOI] [PubMed] [Google Scholar]
- 108.Martire LM, Schulz R, Reynolds CF, et al. Impact of close family members on older adults’ early response to depression treatment. Psychol Aging 2008;23: 447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohen A, Houck PR, Szanto K, et al. Social inequalities in response to antidepressant treatment in older adults. Arch Gen Psychiatry 2006;63:50–6. [DOI] [PubMed] [Google Scholar]
- 110.Lotrich FE, Pollock BG, Kirshner M, et al. Serotonin transporter genotype interacts with paroxetine plasma levels to influence depression treatment response in geriatric patients. J Psychiatry Neurosci 2008;33(2):123–30. [PMC free article] [PubMed] [Google Scholar]


