Abstract
Background:
Myocarditis is a potentially fatal complication of ICI therapy. Data on the utility of CMR T1/T2 mapping in ICI myocarditis are limited.
Objectives:
To assess the value of cardiovascular magnetic resonance (CMR) T1/T2 mapping in patients with immune checkpoint inhibitor (ICI) associated myocarditis.
Methods:
In this retrospective study from an international registry of patients with ICI myocarditis, clinical and CMR findings (including T1/T2 maps) were collected. Abnormal T1/T2 were defined as 2 standard deviations (SD) above site (vendor/field-strength specific) reference values and a z-score was calculated for each patient. Major adverse cardiovascular events (MACE) were a composite of cardiovascular death, cardiogenic shock, cardiac arrest, and complete heart block.
Results:
Of 136 patients with ICI myocarditis with a CMR, 86 (63%) had T1 maps and 79 (58%) had T2 maps. Among the 86 patients (66.3±13.1 years), 36 (41.9%) had an LVEF <55%. Across all patients, mean z-scores for T1 and T2 values were 2.9±1.9 (p<0.001) and 2.2±2.1 (p<0.001) respectively. On Siemens 1.5T (n=67), native T1 (1079.0±55.5 vs. 1000.3±22.1ms, p<0.001) and T2 (56.2±4.9 vs. 49.8±2.2ms, p<0.001) values were elevated compared to reference values. Abnormal T1 and T2 values were seen in 78% and 43% of the patients, respectively. Applying the modified Lake Louse Criteria, 95% met the “non-ischemic myocardial injury” criteria and 53% met the “myocardial edema” criteria. Native T1 values had excellent discriminatory value for subsequent MACE with an AUC of 0.91 (95% CI 0.84–0.98). Native T1 values (HR 1.44, 95% CI 1.12, 1.84, for every 1 unit increase in z-score, p=0.004), but not T2 values were independently associated with subsequent MACE.
Conclusions:
The use of T1 mapping and application of the modified Lake Louise Criteria provides important diagnostic value, and T1 mapping provides prognostic value in patients with ICI myocarditis.
Keywords: cardiovascular magnetic resonance, immune checkpoint inhibitor, major adverse cardiovascular event, myocarditis, T1 mapping, T2 mapping, Lake Louise Criteria
Condensed Abstract
The diagnostic and prognostic value of T1 and T2 mapping in patients with ICI associated myocarditis is unknown. Using an international multicenter registry, we demonstrate that T1 (z-score 2.9±1.9) and T2 (z-score 2.2±2.1) values were elevated in patients with ICI myocarditis, as compared to site specific reference ranges. Abnormal T1/T2 values were seen in 78%/43%, respectively. Applying the modified Lake Louise Criteria, 95% met the non-ischemic myocardial injury criteria. Elevated T1 values, but not T2 values, were independently associated with MACE. Therefore, CMR native T1 mapping is a valuable clinical tool in the assessment of patients with ICI myocarditis.
INTRODUCTION
Immune checkpoint inhibitor (ICI) associated myocarditis is an uncommon immune-related adverse event (1). However, ICI myocarditis is associated with major adverse cardiac events (MACE) in up to 40% (2) with a case fatality rate of up to 25% (3). The diagnosis of myocarditis is usually based on clinical symptoms/signs, troponin elevation, cardiac imaging features and/or endomyocardial biopsy (EMB) (4); however, the latter is not commonly performed due to associated risks and the lack of widespread expertise. Among non-invasive methods, cardiovascular magnetic resonance (CMR) is the reference standard for both diagnosis and prognosis with non-ICI myocarditis (5–7). Recent work identified that components of the original Lake Louise Criteria for the diagnosis of non-ICI myocarditis were not universally present among patients with pathologically-confirmed ICI myocarditis. For example, among patients presenting with a preserved left ventricular ejection fraction (LVEF), late gadolinium enhancement (LGE) and abnormal T2-weighted imaging were observed in <50% of patients, and neither predicted MACE (2). Tissue characterization has evolved to include quantitative parametric mapping techniques. These techniques such as T1 and T2 mapping, have shown excellent diagnostic and prognostic value in patients with non-ICI myocarditis and are recommended in updated protocols (7–11). However, beyond case reports, there are limited data on the use of T1 and T2 mapping in patients with ICI myocarditis (12). In this study, the largest cohort of patients with ICI myocarditis, from multiple international centers, was leveraged to provide the first data on the application of T1 and T2 mapping to patients with ICI myocarditis.
METHODS
Patient cohort
This was a retrospective cohort study where consecutive patients with ICI myocarditis, diagnosed by a board-certified cardiologist using standard criteria (see below), at each site in an international multicenter registry, (2,13–15) and with available CMR with T1 or T2 mapping data were included. Follow-up started with first ICI administration. For each patient, the following was extracted from the medical records: demographics, cancer type, ICI treatment, prior cardiotoxic chemotherapy or radiation, cardiovascular risk factors, presentation, physical examination, initial troponin and natriuretic peptide (BNP) levels and peak values during hospitalization, electrocardiograms (ECG), echocardiographic data, CMR, EMB, and autopsy results. When available, echocardiographic global longitudinal strain (GLS) was measured as described (14). The study complied with the Declaration of Helsinki and was approved by each center’s institutional review committee, the requirement for written informed consent was waived.
Diagnosis of ICI myocarditis
ICI myocarditis was diagnosed in one of two ways: 1) presence of standard histopathological features (16); or 2) meeting the European Society of Cardiology (ESC) diagnostic criteria for clinically suspected myocarditis (6) (Supplemental Table 1). This standardized approach to the diagnosis of myocarditis has been used in multiple prior cohorts (17,18), including those with ICI myocarditis (2,13–15).
CMR protocol
The decision to undergo CMR at presentation with ICI myocarditis was at the discretion of the site practitioners. Applied CMR protocols complied with local institutional practices and were neither study-specified, nor aligned across sites; however, there were similarities in the key elements of the protocol. All CMR studies were either performed on a 1.5T or 3T Siemens (n=82) or 1.5T Philips (n=4) magnet including ECG gating, breath-holding, and local array coil signal reception (Supplement Table 2). Across all sites, exam protocols included cine balanced steady-state free precession (bSSFP) imaging for left ventricular functional/mass assessment (slice: 6–8 mm; gap: 0–2 mm) and T2-weighted imaging employing either T2 short tau inversion recovery (STIR) or spectral attenuated inversion recovery (SPAIR) techniques.
Pre-contrast T1 and T2 maps was performed in a single mid short axis slice (Supplement Table 2)(19). LGE images were performed 10–15 minutes after a gadolinium-based contrast agent (GBCA) (slice: 8 mm; gap: 0–2 mm); a subset of patients (n=19) also had T1 maps at 15 minutes post-contrast administration for quantification of extracellular volume fraction (ECV)(20,21).
For both, T1 and T2 maps, scanner/site specific motion correction was applied for map generation prior to further analysis. The CMR data, including T1 and T2 values, were interpreted at each site by experienced readers as part of clinical care. T1 and T2 values were measured using a single region of interest placed in the septal wall of the mid short axis slice; segments with LGE were excluded for T1 measurements. Site, CMR vendor, and field-strength specific normal T1/T2 reference values were obtained from each site (Supplemental Table 2). Abnormal T1/T2 values were defined as 2 standard deviations (SD) above the mean of the reference values as per the Society of Cardiovascular Magnetic Resonance (SCMR) recommendations (20). To enable combined analysis of multi-center/multi-vendor data, T1/T2 values were converted to a z-score (20) using the site-specific reference values derived as follows: (patient value – mean of reference range) / (SD of the reference range). As applied here, a z-score provides an assessment of how many SD each patient’s T1 or T2 value is above or below the mean for the normal range for each site, vendor, and CMR field-strength.
Adverse cardiovascular events
As prior (22–24), MACE were defined as a composite of cardiovascular death, cardiac arrest, cardiogenic shock, and complete heart block (CHB) requiring pacemaker. When multiple events occurred in a single patient, time to MACE was considered the time to first event. If cardiac arrest, cardiogenic shock, or CHB led to a death, this was considered a cardiac death. The end of follow-up was on July 19th, 2020.
Statistical Analysis
All data were first tested for normality using the Shapiro-Wilk test. Continuous variables were summarized as mean±SD or median (interquartile range) and compared between groups using Student’s t-tests or Wilcoxon rank sum tests. Categorical variables are presented as percentage and were compared between groups using the Fisher’s Exact test. A one sample T test was used to compare the z-scores to 0. Kaplan-Meier curves were generated for MACE and compared with the log-rank test. Univariable and multivariable Cox proportional hazards models (model 1: adjusting for age, sex; model 2: adjusting for age, sex, number of cardiovascular risk factors, and left ventricular ejection fraction [LVEF] by CMR) were performed to examine the association between T1 and T2 values and MACE. We performed a sensitivity analysis by including LGE in the multivariable model 2 when assessing the association between T1 and MACE. Proportional hazards assumption was tested using the Schoenfeld residuals method (25,26). The linearity assumption for continuous variables was tested by entering the square of the term into the model. Receiver-Operating-Characteristic (ROC) curves for MACE were generated for T1 and T2 related z-scores for all patients. A 2-sided P value <0.05 was considered significant. Analyses were performed with Stata15 (StataCorp, College Station, Texas).
RESULTS
Patient Characteristics
Amongst the 136 patients with a CMR in the ICI-registry, 86 with T1 maps were included, of whom 79 also had T2 maps (i.e. 79 patients had both T1 and T2 maps). Of the 86 patients, 38 were diagnosed using pathology (EMB (n=33), autopsy (n=5)) and 48 using the ESC diagnostic criteria (Supplemental Table 1) (6). Patient characteristics, cancer types, and cancer treatment are summarized in Table 1. The mean age was 66.3±13.1 years, 28 (32.6%) were female, 28 (32.6%) received combination ICI therapy. Obstructive coronary artery disease was excluded in 77/86 patients, either using coronary angiography (n=54), coronary computed tomography angiography (n=12) or stress tests with imaging (n=11). The clinical, imaging, and biomarker characteristics of patients who did (n=86) and did not have T1 mapping (N=50) in our CMR cohort were largely similar (Supplemental Tables 3 and 4).
Table 1:
Patient demographics, cancer and treatment details for all patients with ICI myocarditis and patients with abnormal versus normal T1 values.
| With T1 mapping‡ | Abnormal T1 values* | Normal T1 values | ||
|---|---|---|---|---|
| (N=86) | (N=67) | (N=19) | p† | |
| Age at start of ICI, years | 66.3±13.1 | 66.4±12.9 | 65.7±14.2 | 0.84 |
| Female | 28 (32.6) | 17 (25.4) | 11 (57.9) | 0.012 |
| CV risk factors | ||||
| • Hypertension | 47 (56.0) | 36 (54.6) | 11 (61.1) | 0.79 |
| • Diabetes mellitus | 15 (19.0) | 9 (15.0) | 0.18 | |
| • No CV risk factors | 23 (26.7) | 20 (30.0) | 3 (15.8) | 0.073 |
| Prior coronary artery disease | 11 (14.3) | 6 (10.2) | 5 (27.8) | 0.12 |
| Prior stroke | 3 (3.9) | 2 (3.3) | 1 (5.6) | 0.55 |
| Prior heart failure | 1 (1.3) | 1 (1.7) | 0 (0.0) | 1.00 |
| Chronic kidney disease | 4 (5.9) | 4 (7.7) | 0 (0.0) | 0.24 |
| Body mass index, kg/m2 | 27.8±6.3 | 27.2±6.3 | 29.4±6.3 | 0.20 |
| Primary cancer type | ||||
| Head and neck | 3 (3.5) | 1 (1.5) | 2 (10.5) | 0.12 |
| Breast | 4 (4.7) | 4 (6.0) | 0 (0.0) | 0.57 |
| Hodgkin’s lymphoma | 1 (1.2) | 1 (1.5) | 0 (0.0) | 1.00 |
| Melanoma | 37 (43.0) | 30 (44.8) | 7 (36.8) | 0.61 |
| Non-small cell lung cancer | 11 (12.8) | 8 (11.9) | 3 (15.8) | 0.70 |
| Pancreatic | 1 (1.2) | 1 (1.5) | 0 (0.0) | 1.00 |
| Renal cell carcinoma | 6 (7.0) | 6 (9.0) | 0 (0.0) | 0.33 |
| Glioblastoma | 1 (1.2) | 1 (1.5) | 0 (0.0) | 1.00 |
| Other | 22 (25.6) | 15 (22.4) | 7 (36.8) | 0.52 |
| Prior chemotherapy or radiation | ||||
| Radiation | 23 (26.7) | 15 (22.4) | 8 (42.1) | 0.14 |
| Anthracyclines | 8 (9.3) | 7 (10.5) | 1 (5.3) | 0.68 |
| ICI regimen | ||||
| Monotherapy | 58 (67.4) | 45 (67.2) | 13 (68.4) | 1.00 |
| • anti-PDl | 51 (59.3) | 38 (56.7) | 13 (68.4) | 0.43 |
| • anti-CTLA4 | 6 (7.0) | 6 (9.0) | 0 (0.0) | 0.33 |
| • anti-PDLl | 1 (12) | 1 (15) | 0 (0.0) | 1.00 |
| Dual therapy | 28 (32.6) | 22 (32.8) | 6 (31.6) | 0.49 |
Values are mean ± SD or n (%)
Percentages are represented as percentage of available data.
Abnormal T1 values were defined as values >2 standard deviation above the site, CMR vendor/field strength specific reference ranges.
Comparison between patients with normal versus abnormal T1 values was performed using the Student’s t-tests or Wilcoxon Rank Sum tests for continuous variables, as appropriate based on their normality and the Chi-squared test for categorical variables. Anti-CTLA4 = anti-cytotoxic T-lymphocyte-associated protein 4; anti-PD1 = anti-programmed cell death protein 1; anti-PDL1 = anti-programmed death-ligand 1; CMR = cardiovascular magnetic resonance; CV = cardiovascular; ICI = immune checkpoint inhibitors.
Diagnostic Tests
Physical exam, ECG, and biomarker findings are summarized in Table 2. Amongst the 86 included patients, 71(82.6%) were scanned with a 1.5T scanner (67 Siemens, 4 Philips) and 15 (17.4%) on 3T (all Siemens). Overall, 36 (41.9%) patients had a CMR LVEF of <55%. The mean CMR LVEF was reduced (51.3±13.8%), with abnormal LGE and T2 weighted imaging identified in 55.8% and 34.4% of these patients with available data, respectively. The average GLS values, measured with echocardiography, were reduced (median −14.3%, IQR −16.8%, −12.7%) in the subgroup with this data.
Table 2:
Clinical presentation of all patients with ICI myocarditis and patients with abnormal versus normal T1 values.
| With T1‡ mapping | Abnormal T1 values* | Normal T1 values | ||
|---|---|---|---|---|
| (N=86) | (N=67) | (N=19) | p† | |
| Time from starting ICI to admission for myocarditis, days | 57 (27, 110) | 59 (27, 116) | 37 (22, 82) | 0.27 |
| Myocarditis presentation | ||||
| Chest pain | 23 (26.7) | 17 (25.4) | 6 (31.6) | 0.57 |
| Shortness of breath | 52 (60.5) | 44 (65.7) | 8 (42.1) | 0.11 |
| Orthopnea | 16 (19.1) | 14 (21.2) | 2 (11.1) | 0.69 |
| Paroxysmal nocturnal dyspnea | 15 (17.7) | 15 (22.7) | 0 (0.0) | 0.036 |
| Fatigue | 29 (36.7) | 20 (32.8) | 9 (50.0) | 0.39 |
| Syncope | 6 (7.8) | 5 (8.3) | 1 (5.9) | 0.26 |
| Sudden cardiac death | 1 (1.3) | 1 (1.7) | 0 (0.0) | 0.23 |
| Palpitation | 19 (22.4) | 15 (22.7) | 4 (21.1) | 1.00 |
| Physical exam | ||||
| Jugular vein distention | 24 (28.2) | 21 (31.8) | 3 (15.8) | 0.14 |
| Crackles | 29 (34.5) | 26 (40.0) | 3 (15.8) | 0.11 |
| Lower extremity edema | 27 (32.1) | ^ 25 (38.5) | 2 (10.5) | 0.020 |
| SBP, mmHg | 125.9 ± 20.1 | 124.4 ± 20.4 | 130.9 ± 18.8 | 0.23 |
| DBP, mmHg | 74.2 ± 10.1 | 74.4 ± 9.1 | 73.7 ± 13.1 | 0.82 |
| Electrocardiogram at presentation | ||||
| Sinus rhythm | 70 (82.4) | 53 (80.3) | 17 (89.5) | 0.50 |
| ST-segment or T-wave changes | 43 (51.8) | 32 (48.5) | 11 (64.7) | 0.28 |
| Heart rate, beats/min | 82.7 ± 21.3 | 83.4 ± 22.5 | 78.5 ± 13.5 | 0.55 |
| Biomarkers | ||||
| Initial troponin T, ng/mL) | 1.3 (0.3, 28.4) | 1.3 (0.4, 15.6) | 21.0 (0.2, 67.4) | 0.63 |
| Peak troponin T, ng/mL | 2.0 (0.5, 96.7) | 2.0 (0.5, 54.4) | 16.2 (0.5, 114.6) | 0.73 |
| Initial BNP, pg/mL | 536.0 (183.6, 1200.0) | 559.0 (194.0, 1500.0) | 184.0 (152.0, 672.0) | 0.14 |
| Peak BNP, pg/mL | 1130.0 (194.0, 2118.0) | 1130.0 (194.0, 2275.0) | 1027.5 (370.0, 1671.5) | 0.75 |
| Echocardiogram | ||||
| Pre-ICI LVEF, % | 60.6 ± 4.9 | 59.9 ± 4.8 | 63.5 ± 4.0 | 0.021 |
| Lowest LVEF at presentation, % | 51.8 ± 14.9 | 50.0 ± 15.8 | 58.2 ± 8.8 | 0.034 |
| Change of LVEF, % | 11.0 ± 13.5 | 12.3 ± 14.3 | 5.3 ± 7.1 | 0.11 |
| LVEF<50% at presentation | 27 (31.4) | 25 (37.3) | 2 (10.5) | 0.026 |
| LVIDD, mm | 46.9 ± 6.0 | 47.8 ± 5.7 | 44.2 ± 6.1 | 0.040 |
| LA size, mm | 37.5 (34, 42) | 37.5 (34, 45) | 38.0 (35.5, 40) | 0.91 |
| Pericardial effusion | 16 (27.6) | 13 (29.6) | 3 (21.4) | 0.24 |
| Global longitudinal strain by echo, % | −14.3 (−16.8, − 12.7) | −14.1 (−16.8, − 12.4) | −15.7 (−16.7, − 15.1) | 0.27 |
| CMR | ||||
| Time from admission to CMR | 4 (2, 8) | 4 (2, 8) | 3 (2, 5) | 0.34 |
| Time from start of ICI therapy to CMR | 58 (28, 118) | 64 (34, 119) | 38 (20, 103) | 0.13 |
| Corticosteroids use before CMR | 54 (72.0) | 42 (71.2) | 12 (75.0) | 1.00 |
| 1.5-T Siemens | 60 (69.8) | 45 (67.2) | 15 (79.0) | 0.14 |
| 1.5-T Philips | 4 (4.7) | 2 (3.0) | 2 (10.5) | 0.14 |
| 3-T Siemens | 22 (25.5) | 20 (29.8) | 2 (10.5) | 0.14 |
| LVEDV, ml | 142.5 (129, 159) | 142 (128, 160) | 143 (129, 151) | 0.76 |
| LV mass index, g/m2 | 70.6 (60.9, 92.0) | 69.0 (59.0, 84.3) | 78.0 (63.7, 119.0) | 0.11 |
| LVEF by CMR, % | 51.3 ± 13.8 | 49.6 ± 14.2 | 57.2 ± 10.6 | 0.034 |
| LVEF<55% | 36 (41.9) | 30 (44.8) | 6 (31.6) | 0.30 |
| LGE, % | 48 (55.8) | 35 (52.2) | 13 (68.4) | 0.30 |
| Edema by T2-weighted STIR / SPAIR | 22 (34.4) | 18 (34.0) | 4 (36.4) | 1.00 |
| Native T1 value (1.5T Siemens), ms | 1070.1 ± 50.6 | 1086.2 ± 46.3 | 1021.9 ± 26.3 | <0.001 |
| Native T1 value (3T Siemens), ms | 1212.5 ± 73.6 | 1212.3 ± 76.7 | 1214.5 ± 44.5 | 0.97 |
| Average T2 value (1.5T Siemens), ms | 56.3 ± 4.9 | 57.0 ± 5.0 | 54.0 ± 3.7 | 0.041 |
| Average T2 value (3T Siemens), ms | 48.9 ± 8.3 | 50.4 ± 7.9 | 39.0 ± 0§ | 0.070 |
| Extracellular volume, % | 33.2 ± 2.1 | 33.2 ± 2.2 | 33.3 ± 0.6 | 0.91 |
| Corticosteroid treatment | ||||
| Time from admission to treatment | ||||
| ≤24 hours | 43 (55.8) | 30 (50.9) | 13 (72.2) | 0.019 |
| 24–72 hours | 17 (22.1) | 12 (20.3) | 5 (27.8) | |
| >72 hours | 17 (22.1) | 17 (28.8) | 0 (0) | |
| Initial corticosteroids dose | ||||
| Low (<60mg/day) | 12 (19.1) | 9 (17.7) | 3 (25.0) | 0.28 |
| Intermediate (60–500mg/day) | 28 (44.4) | 21 (41.2) | 7 (58.3) | |
| High (501–1000mg/day) | 23 (36.5) | 21 (41.2) | 2 (16.7) |
Values are mean ± SD, n (%), median (interquartile range), or n(%).
Percentages are represented as percentage of available data.
Abnormal T1 values were defined as values >2 standard deviation above the site, CMR vendor/field strength specific reference ranges.
Comparison between patients with abnormal and normal T1 values were performed using the Student’s t-tests or Wilcoxon Rank Sum tests for continuous variables, as appropriate based on their normality and the Chi-squared test for categorical variables.
Only 2 patients with identical values. Anti-CTLA4 = anti-cytotoxic T-lymphocyte-associated protein 4; anti-PD1 = anti-programmed cell death protein 1; anti-PDL1 = anti-programmed death-ligand 1; BNP = Brain natriuretic peptide; CMR = cardiovascular magnetic resonance; CV = cardiovascular; DBP = diastolic blood pressure; ICI = immune checkpoint inhibitors; LA = left atrium; LGE = late gadolinium enhancement; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVIDD = left ventricular internal diameter end diastole; LVIDS = left ventricular internal diameter end systole; LV mass = left ventricular mass; MACE = major adverse cardiac events; N/A = not applicable; SBP = systolic blood pressure; STIR = short tau inversion recovery; SPAIR: Spectral attenuated inversion recovery.
Association between ICI myocarditis and T1/T2 values
For the entire cohort, the mean±SD z-scores for T1 and T2 values were 2.9±1.9 (p<0.001) and 2.2±2.1 (p<0.001) respectively. The native T1, and T2 values in our patients were higher than the reference values regardless of the field strength and vendor (e.g. 1.5T Siemens T1: 1079.0±55.5 versus 1000.3±22.1ms, p<0.001; T2: 56.2±4.9 versus 49.8±2.2ms, p<0.001) (Table 3). Amongst the cohort, 67 (78%) and 34 (43%) patients had abnormal T1 and T2 values respectively. The mean T1 values in patients with LGE and T2 values in patients with abnormal T2 STIR/SPAIR are summarized in Supplement Table 5.
Table 3.
T1 and T2 mapping values in comparison to reference ranges and also dichotomized based on those with and without MACE. Data are presented a z-scores and for specific MRI magnets and field strength
| ICI myocarditis patients | Reference Ranges | p* | MACE | No MACE | p† | |
|---|---|---|---|---|---|---|
| T1 z-score (n=86) | 2.9 ± 1.9 | - | - | 4.2 ± 1.0 | 2.3 ± 1.9 | <0.001 |
| T1 value - 1.5T Siemens (n=67) | 1079.0±55.5 | 1000.3±22.1 | <0.001 | 1114.7±40.9 | 1061.6±53.6 | <0.001 |
| T1 value - 1.5T Philips (n=4) | 1014.0±34.0 | 961.5±23.0 | 0.007 | 1013 ± 0 | 1014.3 ± 41.6 | N/A‡ |
| T1 value - 3.0T Siemens (n=15) | 1239.3±72.1 | 1097.3±144.6 | <0.001 | 1244.0±64.0 | 1237.5±77.7 | 0.88 |
| T2 z-score (n=79) | 2.2 ± 2.1 | - | - | 3.2 ± 2.4 | 1.8 ± 1.7 | 0.003 |
| T2 value - 1.5T Siemens (n=67) | 56.2±4.9 | 49.8±2.2 | <0.001 | 57.9±6.5 | 55.4±3.7 | 0.045 |
| T2 value - 1.5T Philips (n=4) | 55.0±4.1 | 51.9±0.6 | 0.28 | 54.0±0 | 55.3 ± 4.9 | N/A‡ |
| T2 value - 3.0T Siemens (n=8) | 42.9±4.6 | 39.3±0.1 | 0.063 | 46.0±0 | 42.4±4.8 | N/A‡ |
Student’s t-test comparing T1 or T2 values of patients with immune checkpoint inhibitor associated myocarditis with site- and magnet-specific normal mean values and standard deviation.
Student’s t-test comparing T1 or T2 values of patients with and without major adverse cardiovascular events.
analysis could not be performed due only one patient in the MACE group.
When patients were dichotomized into those with normal and abnormal T1 values (Tables 1 and 2), patients with abnormal T1 values were more likely to be male, have paroxysmal nocturnal dyspnea, and peripheral edema, have lower pre-ICI LVEF, and lower LVEF at diagnosis and during admission. There were no differences between the groups in the time from admission to CMR or the proportion of patients treated with corticosteroids prior to CMR. Patients with normal T1 values were more likely to have received corticosteroids early, within 24 hours, of hospital admission. In the 19 patients with ECV measurements, the mean value was 33.2±2.1% compared to the site-specific normal reference value of 26.0±1.6%.
Association between Histopathology and T1/T2 maps
Among the 38 patients with histologically-confirmed myocarditis, a lymphocytic infiltration was observed in 36 patients (95%), among whom 29 patients (80.6%) had abnormal T1 values. T2 maps were available in 30 of the 36 patients, amongst whom, T2 values were abnormal in 15 patients (50.0%). Twenty-three patients had pathological fibrosis, of whom 19 (82.6%) had abnormal T1 values. Amongst the 6 patients with ECV within this latter group, 83.3% had abnormal ECV values.
Lake Louise Criteria and ICI myocarditis
We applied the modified Lake Louise Criteria to the patients with both T1 and T2 maps (N=79) (7). Data are presented for all patients and the subgroup with pathology (Table 5). When considering abnormal T1 or T2 values along with T2 weighted imaging and LGE, 95% of patients met the “non-ischemic myocardial injury criteria”, 53% met the “myocardial edema” criteria, and 48% met both these main criteria. At least one of the main modified Lake Louise Criteria for myocarditis was present in 100% of the patients. A clinical example is shown in Figure 1.
Table 5:
Major adverse cardiovascular events (MACE) in all patients and in those dichotomized based on normal versus abnormal T1 values.
| All patients (n=86) | Abnormal T1 values* (n=67) | Normal T1 values (n=19) | P value | |
|---|---|---|---|---|
| Follow-up time for MACE†, days | 158 (78, 333) | 161 (78, 333) | 154 (68, 407) | 0.92 |
| MACE‡ | 27 (31.4) | 27 (40.3) | 0 (0) | <0.001 |
| • Complete heart block | 8 (9.5) | 8 (12.1) | 0 (0) | 0.19 |
| • Cardiogenic shock | 10 (12.1) | 10 (15.4) | 0 (0) | 0.11 |
| • Cardiac arrest | 12 (14.5) | 12 (18.5) | 0 (0) | 0.072 |
| • Cardiovascular death | 12 (14.0) | 12 (17.9) | 0 (0) | 0.061 |
Abnormal T1 values were defined as values >2 standard deviation above the site, CMR vendor/field strength specific reference ranges
Time of the MACE was defined by the date of the earliest event when multiple MACE happened.
Patients may have multiple MACE.
Figure 1: Multi-contrast and parametric CMR imaging in patient with confirmed ICI myocarditis (1.5T).
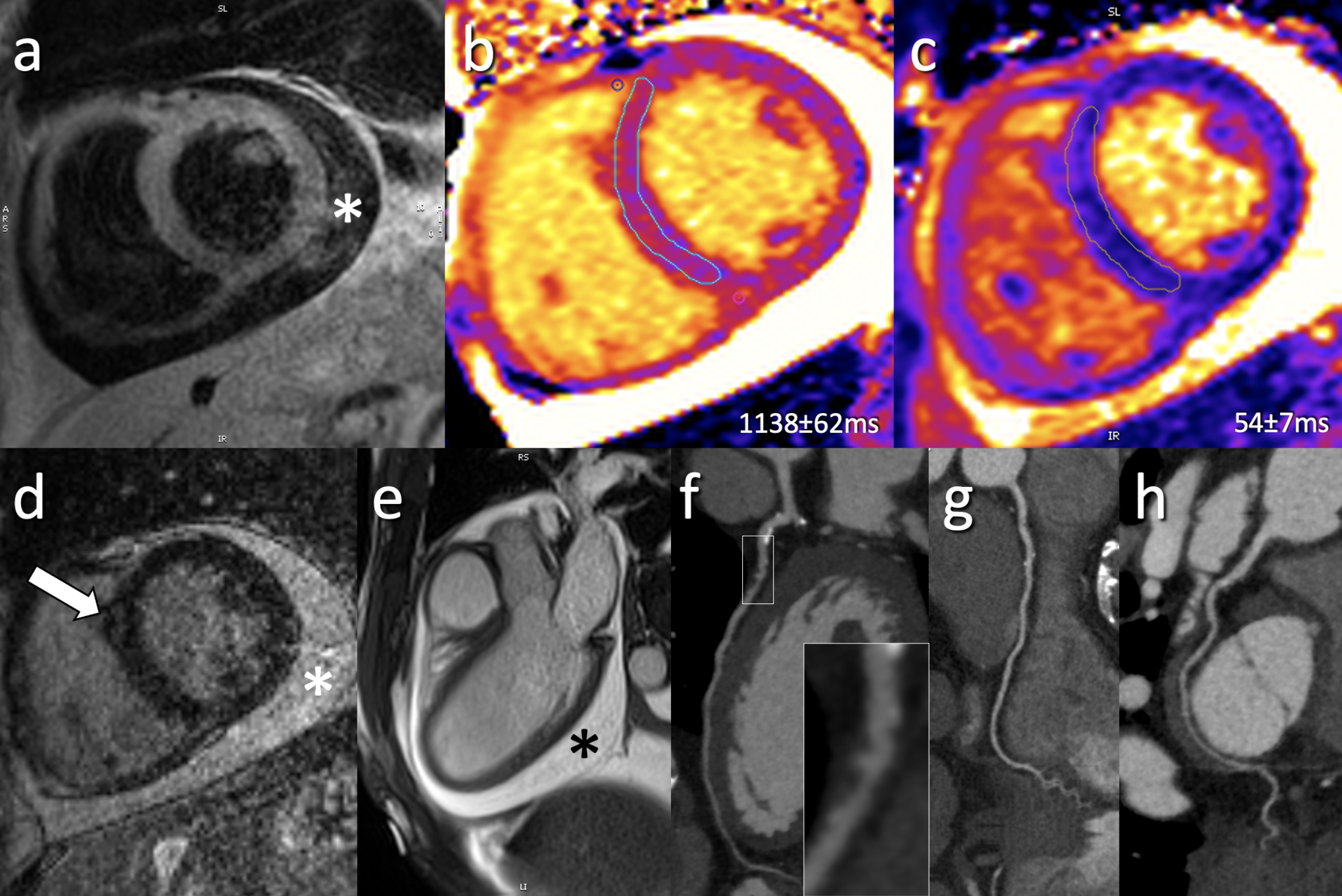
(a) Representative short axis T2-weighted SPAIR without focal signal abnormality; (b) Mid-ventricular short axis MOLLI T1-map demonstrates diffusely elevated T1 values (local normal reference: 1006±24ms) while (c) same slice T2-map demonstrate normal global T2 values (local normal reference: 52±3ms); (d) Post-contrast LGE imaging demonstrates faint mid-myocardial enhancement (arrow) in the mid-ventricular anteroseptum; (e) 3-chamber cine bSSFP image demonstrates a pericardial effusion (*); (f-h) coronary CT angiography performed for exclusion of possible coronary artery disease demonstrated calcified and non-calcified changes (predominately in LAD; f) without significant coronary artery stenosis.
Major Adverse Cardiovascular Events
During a median follow-up time of 158 days, 27 patients (31.4%) developed MACE (Table 6). Patients who developed MACE had higher T1 and T2 z-scores (Table 3). Similarly, in the subgroup imaged with a 1.5T Siemens magnet, those who developed MACE had higher T1 and T2 values (Table 3). The incidence of MACE in patients with normal versus abnormal T1 and T2 values were 0.0% vs. 40.3% (p<0.001, Table 5) and 19.5% vs. 42.1% (p=0.029), respectively. The MACE-free survival was significantly lower in patients with abnormal compared to normal T1 values (Figure 2A) but no significant difference was seen with T2 values (Figure 2B). Using z-scores, an ROC curve for T1 and T2 values demonstrated an AUC of 0.91 (95% CI 0.84–0.98) and 0.70 (95% CI 0.56–0.83) for MACE, respectively (Figure 3).
Table 6.
Univariable and multivariable analysis of association between cardiovascular magnetic resonance imaging T1 and T2 maps and major adverse cardiovascular events.
| Univariable model | Multivariable model 1* | Multivariable model 2† | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Native T1 value, per 1 unit increase in z score | 1.31 (1.14, 1.50) | <0.001 | 1.54 (1.26, 1.87) | <0.001 | 1.44 (1.12, 1.84) | 0.004 ‡ |
| Native T1 value (1.5T Siemens), per 10ms increase | 1.10 (1.03,1.17) | 0.004 | 1.14 (1.05, 1.23) | 0.001 | 1.11 (1.02, 1.21) | 0.017 ¶ |
| Abnormal T1 values§ | - | - | - | - | - | - |
| T2 value, per 1 unit increase in z score | 1.36 (1.12, 1.65) | 0.002 | 1.32 (1.07, 1.61) | 0.008 | 1.22 (0.98, 1.52) | 0.077 |
| T2 value (1.5T Siemens only), per 1ms increase | 1.10 (1.00, 1.20) | 0.042 | 1.07 (0.98, 1.18) | 0.143 | 1.05 (0.96, 1.15) | 0.312 |
| Abnormal T2 values | 2.86 (1.17, 6.99) | 0.022 | 2.48 (1.00, 6.13) | 0.049 | 2.19 (0.81, 5.91) | 0.122 |
CMR = cardiovascular magnetic resonance
Multivariable Model 1: Cox proportional hazard model adjusting for age, sex.
Multivariable Model 2: Cox proportional hazard model adjusting for age, sex, number of cardiovascular risk factors, and LVEF by CMR during the index hospitalization.
In sensitivity analysis when presence of LGE was added to multivariable model 2, the HR per 1 unit increase in z score remained significantly associated with MACE: HR 1.45 (95% CI: 1.14, 1,84), p=0.003.
Similarly when LGE was added to multivariable model 2, native T1 values on the 1.5T magnet remained significanlty associated with MACE: HR per 10ms increase being 1.12 (95% CI: 1.02, 1.22), p=0.013. When assessing the association of LVEF by CMR with outcomes, LVEF was removed from the model.
Since all MACE occurred in patients abnormal with T1 value s (i.e. >mean+2SD) a HR could not be calculated.
Figure 2. Kaplan–Meier curves of MACE free survival in patients with normal and abnormal T1 values (left panel) and T2 values (right panel).
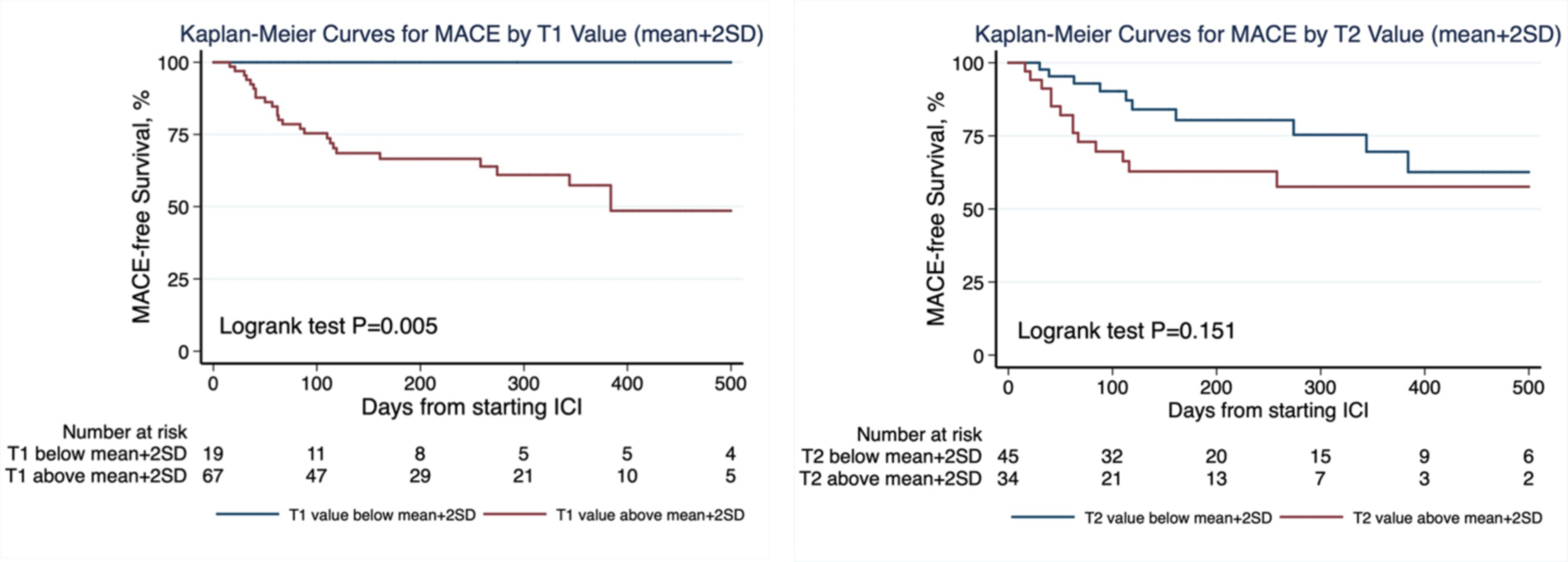
Abnormal values were defined as above mean+2SD of the site, CMR vendor/field strength specific reference ranges.
Figure 3. Receiver-Operating-Characteristic (ROC) curves for major adverse cardiovascular events for cardiovascular magnetic resonance T1 and T2 mapping.
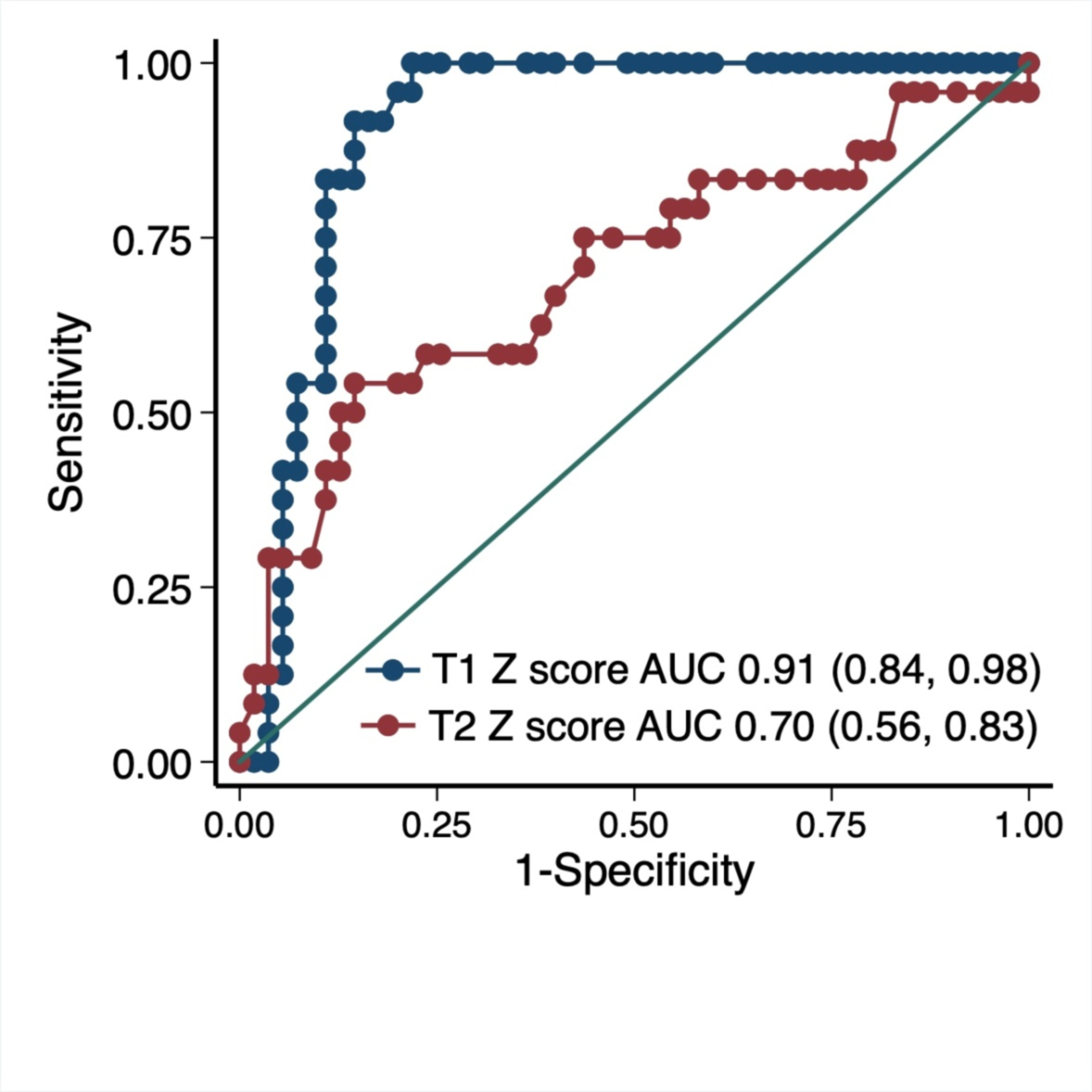
T1/T2 values were converted to z-scores for combined analysis.
Using the T1 z-score as a continuous parameter, regression analysis indicated that the z-score was associated with greater hazards of MACE (Table 6) even after adjusting for age, sex, number of cardiovascular risk factors, and LVEF (HR 1.44, 95% CI 1.12, 1.84, p=0.004). This association was similar when T1 values were examined only on a 1.5T Siemens magnet. When T1 values were dichotomized into normal vs. abnormal values, all MACE events occurred in patients with abnormal T1 values (Table 6). Finally, in our sensitivity analysis T1 remained significantly associated with MACE after additional adjustment for presence of LGE (Table 6, footnote). Higher T2 z-score was also associated with MACE and remained associated after adjusting for age and sex, but became non-significant after additional adjustment for cardiovascular risk factors and LVEF (Table 6).
DISCUSSION
In this report, we provide the first comprehensive data on the use of CMR parametric mapping techniques in patients with ICI myocarditis. We report the following novel findings: 1) Myocardial T1 and T2 values were significantly elevated in 78% and 43% of patients with ICI myocarditis respectively. 2) Patients with abnormal T1 values were more symptomatic and had lower cardiac function. 3) The association between histopathological changes and T1 measurements was stronger than the association with T2 measurements. 4) All patients in our study met at least one of the two main modified Lake Louise Criteria. 5) Higher T1 values had independent prognostic value for the subsequent development of MACE.
The clinical application of CMR for the diagnostic workup of patients with suspected myocarditis is based on the Lake Louise Criteria (27). The updated criteria incorporate parametric mapping techniques and require the presence of myocardial edema (based on T2 maps or T2-weighted imaging) and non-ischemic myocardial injury (based on T1 maps, ECV quantification or LGE) as the two main criteria (7). The addition of parametric mapping improves the diagnostic yield for non-ICI acute myocarditis from an area under the curve of 84% for the original Criteria to as high as 96% with the use of a combination of T1 mapping and LGE images (7). Amongst the individual techniques, T1 mapping provides the greatest diagnostic yield for non-ICI acute myocarditis with a diagnostic odds ratio of 44.1 (7,28). The literature on CMR tissue characterization techniques in patients with ICI myocarditis is limited. We recently demonstrated that, among patients with ICI myocarditis presenting with a preserved LVEF, that LGE and abnormalities on T2 weighted imaging were only present in 48% and 28% of the patients, respectively (2). The application of T1 and/or T2 mapping in these patients has been limited to a case report (12) and our prior report in a small group of patients (2).
Using our ICI registry, we identified abnormal native T1 and T2 values in 78% and 43% of the patients, respectively. When we applied the modified Lake Louise Criteria (combining T1/T2 mapping with T2 weighted imaging and LGE), 48% of the patients met both main criteria for myocarditis, 95% met the “non-ischemic myocardial injury” criteria and 100% met either of the two main criteria of myocarditis. Due to the lack of patients without ICI myocarditis for comparison, we are unable to provide data on the diagnostic performance of the modified Lake Louise Criteria or thresholds of T1 and T2 values for the diagnosis of ICI myocarditis. Therefore, although further investigation is needed, our data suggest that in the appropriate clinical setting, the presence of non-ischemic myocardial injury criteria maybe the most prevalent finding in patients with ICI myocarditis.
The greater prevalence of T1, compared to T2 elevation, in patients with ICI myocarditis is consistent with reports of better diagnostic accuracy of native T1 values in patients with non-ICI myocarditis (28–30). In acute myocarditis, both T1 and T2 elevations reflect inflammation and edema (20). However, T1 mapping can also identify fibrosis which can be present early during myocarditis (31). In our subgroup with histopathology, 95% had lymphocytic infiltration, and the majority had elevated T1 values with only 50% having elevated T2 values. Furthermore, 61% of the patients had myocardial fibrosis, amongst whom the majority had elevated in T1 values. Therefore, although our patients likely had inflammation/edema at the onset of myocarditis, this may have improved at the time of CMR in some patients given that 72% of our patients received corticosteroids before the CMR study and may explain the lower prevalence of T2 abnormalities. However, our findings reflect the real-world use of CMR in these patients. Alternatively, the difference in prevalence of T2 versus T1 abnormalities may reflect lower sensitivity of the T2 mapping sequences to detect myocardial inflammation (28) or the fact that patchy areas of myocardial edema may have been missed due to the use of septal measurements from a single slice. However, for practical purposes, single-slice T2 maps are common clinical practice. It is also likely that the higher prevalence of T1 abnormalities in our cohort reflects the greater degree of myocardial injury from myocarditis and the presence of early myocardial fibrosis. Alternatively, given the time from initiation of ICI therapy to CMR of ~58 days, there could have been ongoing indolent myocardial inflammation that contributed to the total burden of fibrosis. Therefore, until further studies of CMR parametric mapping techniques are available, our study suggests that it is more likely to identify elevated T1 than elevated T2 values in patients with acute ICI myocarditis. Based on our data we have proposed a potential approach to using CMR to assess patients with suspected ICI myocarditis (Central illustration). This will however require further validation.
Central Illustration: A proposed approach to CMR assessment of ICI myocarditis with incorporation of parametric mapping techniques.
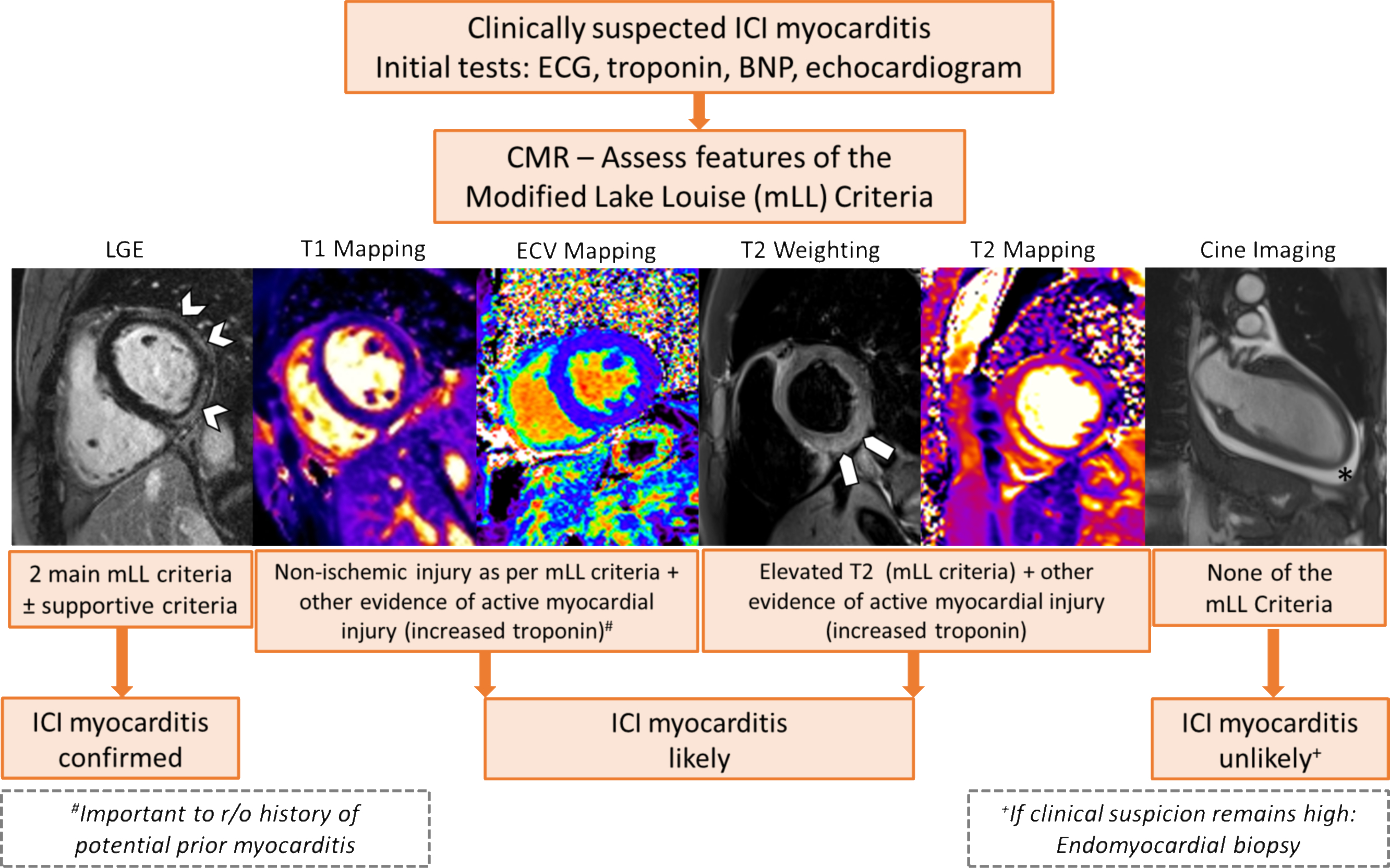
Arrowheads delineate band-like subepicardial late gadolinium enhancement (LGE), block arrows highlight elevated signal intensity on T2 weighted image, and (*) demonstrates the presence of pericardial effusion on a cine bSSFP image.
Approximately 30–40% of patients with ICI myocarditis develop MACE with a mortality that ranges from 15–25% (1–3,13). Therefore, robust prognostic markers are necessary to guide corticosteroid dosing, the need for intensification of immunosuppression beyond corticosteroids, duration of immunosuppression, frequency of cardiac monitoring, and the potential re-initiation of ICI therapy (32). Previously identified prognostic measures in these patients include elevated troponin levels (13) and lower echocardiography global longitudinal strain (14). In prior work, neither LGE nor T2-weighted imaging was prognostic for MACE. In this study, T1 values had good discriminatory and prognostic value for subsequent MACE with100% of the MACE occurring in patients with abnormal T1 values. This association remained significant even after adjusting for relevant covariates and the presence of LGE. Although T2 values were also associated with MACE, this lost significance after adjusting for cardiovascular risk factors and LVEF. Other than a small sample size, one potential rationale for the lack of independent relationship with T2 values may relate to the challenges of reliably measuring the extent of inflammation/edema in this patient population as described above. Alternatively, T2 changes may demonstrate reversible edema which may not be as prognostically important as changes in T1, the latter based on the subgroup with pathology, seems to relate to myocardial injury and fibrosis. This may be the rationale for the presence of an independent relationship between T1 values and MACE. It is also possible that the presence of elevated T1 reflects a pre-existing underlying cardiomyopathy, differences in cardiovascular risk factors or cancer treatment that may have driven prognosis. However, we did not identify such differences in these factors between patients with and without elevated T1 values.
There are limited prior data on the prognostic value of T1 and T2 mapping in patients with non-ICI myocarditis (33). In a single-center study of 46 patients, elevated T2 values >4 SD above the mean and a T2 time >80ms had an odds ratio of 6.3 (95% CI 1.2–24.9) and 4.9 (95% CI 1.1–18.9), respectively, for MACE and hospitalization for heart failure. However, due to the use of different sequence/scanner techniques, average T2 values in the patients with myocarditis in that study were 68.1ms, which is markedly higher than the average value in our patients with ICI myocarditis (~56ms) (11). This may reflect differences in the mechanisms and degree of myocardial injury or alternatively the use of steroids prior to CMR in our patients. Furthermore, T2 values were based on average of 3 slices as compared to a single slice in our study. In a separate larger study of 670 patients with acute or subacute non-ICI myocarditis, 179 patients had ECV measurements. Every 10% increase in ECV was associated with a HR of 2.09 (95% CI 1.07–4.08) and 3.93 (1.11–13.86) for MACE and death respectively (34,35). Unfortunately, we did not have an adequate number of patients with ECV values to assess its prognostic value.
Strengths and Limitations
Strengths of this study include a relatively large sample size of patients with ICI myocarditis with ~45% of the patients having histopathology and CMR data providing a unique opportunity to determine associations. However, this was a retrospective multi-center study and institutional standards were employed, with a non-pre-specified CMR protocol, different magnet strengths, and local site reads. To address this limitation and enable a combined analysis, including data from all centers, respective data were translated into z scores. Although z-scores are recommended by the SCMR for clinical routine (20), they can be challenging to comprehend. Therefore, we also divided and analyzed our cohort based on site, CMR vendor, and field strength normal/abnormal T1/T2 values defined as mean+2SD (20). We used T1/T2 measurements obtained at the individual sites as opposed to a core-lab read. Although this may contribute to inter-observer variability, we believe that this pragmatic approach adds to the strength and clinical relevance of our findings. Additionally, we only had a single short-axis slice to measure T1 and T2 values. Furthermore, the majority of our patients received corticosteroids prior to their CMR study. Therefore, it is possible that with a CMR performed prior to corticosteroids/immunosuppression, more complete imaging of the myocardium (i.e. multiple slices), and consideration of regional changes in T1 or T2 values, the diagnostic yield of these approaches may be higher. However, prior studies in non-ICI myocarditis suggest that a single slice provides similar diagnostic accuracy to multiple slices (36). Furthermore, there are no data to suggest that ICI myocarditis is regional. Although this is the largest report of T1/T2 mapping in ICI myocarditis, the statistical power is still likely limited, thus the lack of stronger association between T2 mapping and MACE needs to be tested in future studies. One set of our multivariable models included 5 variables. This may result in over-fitted models; however, we chose to adequately adjust for confounders given that these are association models (37). Finally, we also did not have patients with negative biopsies to allow the calculation of sensitivity/specificity for T1 and T2 mapping for the diagnosis of ICI-myocarditis.
CONCLUSIONS
In patients with ICI myocarditis, elevated native-T1 values were more common than elevated T2 values. Patients with higher native T1 had signs of greater myocardial injury. Using the modified Lake Louise Criteria, the non-ischemic myocardial injury criteria were seen almost uniformly in our patients with only 53% meeting the edema criteria. Although the latter was higher than the prevalence of abnormalities on qualitative T2-weighted imaging in our prior work (2), it still appears that it is best to rely on the presence of non-ischemic myocardial injury for the diagnosis of ICI myocarditis. In follow-up, higher T1, but not T2 values, were independently associated with MACE. Overall, CMR measured myocardial native T1 value was the most robust parameter to identify myocarditis and its prognosis in patients receiving ICI therapy.
Supplementary Material
Table 4:
Proportion of patients meeting the various components of the modified Lake Louise Criteria in all included patients and those with biopsy proven ICI myocarditis. Abnormal T1 and T2 values were defined as mean + 2SD above site, CMR vendor and field strength specific reference ranges.
| All cases N (%) | Biopsy proven cases N (%) | |
|---|---|---|
| Main Criteria | Total N =79* | Total N=31* |
| • Non-Ischemic Myocardial Injury (Abnormal T1, ECV†, or LGE) | 75 (95%) | 28 (100%) |
| • Myocardial Edema (T2-mapping or T2W images) | 42 (53%) | 19 (63%) |
| Supportive Criteria | ||
| • Pericarditis | 14 (18%) | 8 (26%) |
| • Systolic LV dysfunction (<55%) | 33 (42%) | 16 (52%) |
| Combinations | ||
| • Patients with both main criteria | 38 (48%) | 16 (52%) |
| • Patients with either main criteria | 79 (100%) | 31 (100%) |
| • Patients without T1 or T2 elevation or supportive criteria | 0 (0%) | 0 (0%) |
These numbers refer to patients who had both T1 and T2 maps.
ECV was only available in 19 patients in the entire cohort of 79 patients, and 10 patients amongst those with biopsy proven disease. ECV=extracellular volume fraction; LGE=late gadolinium enhancement; T2W = T2 weighted images; LV=left ventricular
Perspectives.
Competency in Patient Care and Procedural Skills:
The vast majority of patients with myocarditis associated with immune checkpoint inhibitor (ICI) chemotherapy undergoing cardiac magnetic resonance imaging (CMR) with T1 and T2 mapping satisfy the modified Lake Louise Criteria for non-ischemic myocardial injury. Higher myocardial T1 values are associated with more severe myocardial injury and a higher risk of major adverse cardiovascular events.
Translational Outlook:
Further studies are needed to fully characterize and improve the diagnostic performance of CMR mapping for diagnosis of ICI-associated myocarditis.
Sources of Funding:
Dr. P. Thavendiranathan was supported, in part, through the Canadian Institutes of Health Research New Investigator Award (FRN 147814) and a Canada Research Chair in Cardiooncology. This work is supported by the New York Academy of Medicine’s Glorney-Raisbeck Award to Dr S. S. Mahmood. Dr. R.J. Sullivan was supported, in part, through the National Institutes of Health (NIH)/ National Cancer Institute (NCI) (RO1CA229851, UH2CA207355, RO1CA193970). Dr. C.L. Chen, and Dr. D. Gupta were supported, in part, through the National Institutes of Health (NIH)/National Cancer Institute (NCI) P30CA008748. Dr. Neilan was supported, in part, through the Kohlberg Foundation, the NIH/NHLBI (RO1HL130539, RO1HL137562, and K24HL150238), and the NIH/Harvard Center for AIDS Research (P30 AI060354).
Disclosures:
Dr. Thavendiranathan has received speaker’ bureau fees from Amgen, Takeda, and BI. Dr. Mahmood has received consultancy fees from OMR Globus, Alpha Detail, and Opinion Research Team. Dr. Nohria has received research support from Amgen; and has been a consultant for Takeda Oncology. Dr. Heinzerling has received consultancy, advisory board, and speaker fees from MSD, BMS, Roche, Novartis, Amgen, and Curevac. Dr. Sullivan has been a consultant to Merck and Novartis. Dr. Groarke has received research support from Amgen. Dr. Neilan has received advisory fees from Parexel, BMS, H3 Biomedicine, AbbVie, and Intrinsic Imaging. Dr. Neilan has received grant support from Astra Zeneca. Dr. Wintersperger has received research support and speaker’s honorarium from Siemens Healthineers. The University Health Network (UHN) has a master research agreement (MRA) with Siemens Healthineers. Dr. Wintersperger is an inventor of the IG fitting method owned by UHN (US10314548B2). Dr. Yang has received research funding from CSL Behring. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations:
- anti-CTLA4
anti-cytotoxic T-lymphocyte-associated protein 4
- anti-PD1
anti-programmed cell death protein 1
- anti-PDL1
anti-programmed death-ligand 1
- BNP
b-type natriuretic peptide
- CMR
cardiovascular magnetic resonance
- GBCA
gadolinium-based contrast agent
- LGE
late gadolinium enhancement
- ICI
immune checkpoint inhibitor
- MACE
major adverse cardiac events
- STIR
short tau inversion recovery
- SPAIR
Spectral attenuate inversion recovery
- bSSFP
balanced steady state free precession
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. The lancet oncology 2018;19:e447–e458. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Awadalla M, Mahmood SS et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. European heart journal 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang DY, Salem JE, Cohen JV et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA oncology 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaca MP, Olenchock BA, Salem JE et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesbroek PS, Hirsch A, Zweerink A et al. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. European heart journal cardiovascular Imaging 2018;19:1397–1407. [DOI] [PubMed] [Google Scholar]
- 6.Caforio AL, Pankuweit S, Arbustini E et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European heart journal 2013;34:2636–48, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira VM, Schulz-Menger J, Holmvang G et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 8.Thavendiranathan P, Walls M, Giri S et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging 2012;5:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira VM, Piechnik SK, Dall’Armellina E et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048–58. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira VM, Piechnik SK, Dall’Armellina E et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spieker M, Haberkorn S, Gastl M et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson 2017;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monge C, Maeng H, Brofferio A et al. Myocarditis in a patient treated with Nivolumab and PROSTVAC: a case report. Journal for immunotherapy of cancer 2018;6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awadalla M, Mahmood SS, Groarke JD et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J Am Coll Cardiol 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awadalla M, Golden DLA, Mahmood SS et al. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. Journal for immunotherapy of cancer 2019;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone O, Veinot JP, Angelini A et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology 2012;21:245–74. [DOI] [PubMed] [Google Scholar]
- 17.Hinojar R, Foote L, Arroyo Ucar E et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015;8:37–46. [DOI] [PubMed] [Google Scholar]
- 18.von Knobelsdorff-Brenkenhoff F, Schuler J, Doganguzel S et al. Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 19.Giri S, Chung YC, Merchant A et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messroghli DR, Moon JC, Ferreira VM et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neilan TG, Coelho-Filho OR, Shah RV et al. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging 2013;6:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks KA, Tcheng JE, Bozkurt B et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 23.Neilan TG, Farhad H, Mayrhofer T et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging 2015;8:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008;117:686–97. [DOI] [PubMed] [Google Scholar]
- 25.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Statistics in medicine 1995;14:1707–23. [DOI] [PubMed] [Google Scholar]
- 26.Schoenfeld D Partial Residuals for The Proportional Hazards Regression Model. Biometrika 1982;69:239–241. [Google Scholar]
- 27.Friedrich MG, Sechtem U, Schulz-Menger J et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan JA, Lee YJ, Salerno M. Diagnostic Performance of Extracellular Volume, Native T1, and T2 Mapping Versus Lake Louise Criteria by Cardiac Magnetic Resonance for Detection of Acute Myocarditis: A Meta-Analysis. Circ Cardiovasc Imaging 2018;11:e007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radunski UK, Lund GK, Saring D et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clinical research in cardiology : official journal of the German Cardiac Society 2017;106:10–17. [DOI] [PubMed] [Google Scholar]
- 30.Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging 2018;11:1583–1590. [DOI] [PubMed] [Google Scholar]
- 31.Jeuthe S, Wassilew K, D OHI et al. Myocardial T1 maps reflect histological findings in acute and chronic stages of myocarditis in a rat model. J Cardiovasc Magn Reson 2016;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DH, Armanious M, Huang J, Jeong D, Druta M, Fradley MG. Case of pembrolizumab-induced myocarditis presenting as torsades de pointes with safe re-challenge. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners 2020:1078155220904152. [DOI] [PubMed] [Google Scholar]
- 33.Blissett S, Chocron Y, Kovacina B, Afilalo J. Diagnostic and prognostic value of cardiac magnetic resonance in acute myocarditis: a systematic review and meta-analysis. The international journal of cardiovascular imaging 2019;35:2221–2229. [DOI] [PubMed] [Google Scholar]
- 34.Grani C, Eichhorn C, Biere L et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol 2017;70:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grani C, Biere L, Eichhorn C et al. Incremental value of extracellular volume assessment by cardiovascular magnetic resonance imaging in risk stratifying patients with suspected myocarditis. The international journal of cardiovascular imaging 2019;35:1067–1078. [DOI] [PubMed] [Google Scholar]
- 36.Bohnen S, Radunski UK, Lund GK et al. T1 mapping cardiovascular magnetic resonance imaging to detect myocarditis-Impact of slice orientation on the diagnostic performance. Eur J Radiol 2017;86:6–12. [DOI] [PubMed] [Google Scholar]
- 37.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American journal of epidemiology 2007;165:710–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


