Abstract
A previously healthy 39-year-old man presented in cardiogenic shock with evidence of multisystem inflammatory syndrome of adults 2 months after a mild case of coronavirus disease 2019. He was treated with intravenous immunoglobulin and pulse-dose corticosteroids with rapid resolution of his symptoms and normalization of biventricular function. (Level of Difficulty: Intermediate.)
Key Words: acute heart failure, autoimmune, treatment
Abbreviations and Acronyms: IVIG, intravenous immunoglobulin; MIS-A, multisystem inflammatory syndrome in adults; MIS-C, multisystem inflammatory syndrome in children; MRI, magnetic resonance imaging
Central Illustration
History of Presentation
A 39-year-old man with no history of cardiac disease presented to the emergency room with 1 week of fever, right-sided neck swelling, and progressive shortness of breath. Bedside evaluation revealed newly depressed left ventricular function, with hemodynamic and laboratory evidence of cardiogenic shock.
Learning Objectives
-
•
Recognize MIS-A as a rare but important complication of convalescent COVID-19.
-
•
Identify the importance of rapid initiation of immunosuppression in fulminant inflammatory myocardial processes to prevent complications.
-
•
Consider the pathophysiologic implications of response to IVIG in patients with MIS-A.
One week before presentation, he noted the onset of neck swelling accompanied by subjective fevers. He soon developed progressive shortness of breath and orthopnea prompting him to seek care. His initial evaluation in the emergency department revealed a temperature of 101.1ºF, heart rate of 125 beats/min, blood pressure of 103/64 mm Hg, respiratory rate of 33 breaths/min, and oxygen saturation level 94% on 4L nasal cannula.
On physical examination, he was noted to have a regular rate and rhythm without murmurs, rubs, or gallops. His jugular venous pulse was approximately 12 cm of water. His pulmonary exam was normal. He had trace lower extremity edema.
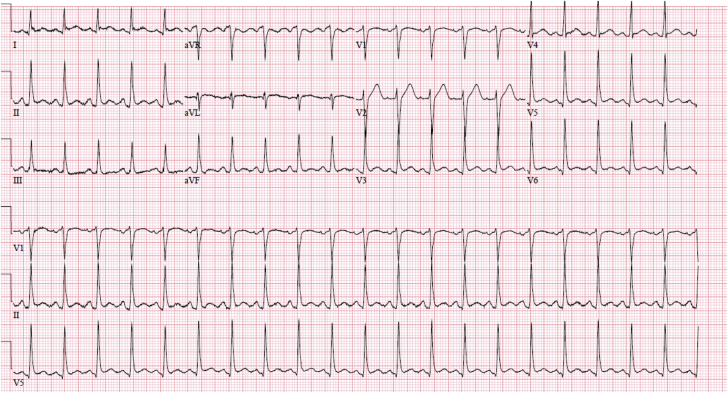
His electrocardiogram revealed diffuse PR depression, 1 mm ST-segment elevations in I, avL, V5, and V6 without reciprocal depressions (Figure 1). A chest x-ray showed moderate pulmonary edema. He was admitted to the cardiac intensive care unit (CCU) for further evaluation.
Figure 1.
Electrocardiogram on Admission
Past Medical History
He had been diagnosed with coronavirus disease-2019 (COVID-19) by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) nasopharyngeal swab real-time polymerase chain reaction (RT-PCR) 8 weeks prior. He had not required hospitalization, received no antiviral or immunomodulatory therapies, and recovered to his usual state of health.
Differential Diagnosis
The differential for this patient’s biventricular failure resulting in cardiogenic shock included nonischemic etiologies such as viral myopericarditis, stress cardiomyopathy, giant cell or eosinophilic myocarditis, a hyperinflammatory syndrome, or possibly acute coronary syndrome.
Investigations
Relevant laboratories included negative nasopharyngeal swab RT-PCRs for SARS-CoV-2 and common respiratory viruses, reactive SARS-CoV-2 nucleocapsid antibodies at index of 46.92 and spike protein antibodies at 104.9 (Roche Elecsys Anti-SARS-CoV-2 total assay). See Table 1 for additional laboratory results.
Table 1.
Laboratory Results During Hospitalization
| Laboratory Result | Reference Values | Admission to CCU (HD1) | Transfer to Step-Down Unit (HD 4) | Discharge From Hospital (HD 8) |
|---|---|---|---|---|
| Sodium, mmol/L | 136-145 | 117 | 134 | 136 |
| Carbon dioxide, mmol/L | 22-31 | 23 | 28 | 25 |
| Blood urea nitrogen, mg/dL | 6-23 | 10 | 25 | 22 |
| Creatinine, mg/dL | 0.50-1.20 | 0.78 | 0.60 | 0.73 |
| White blood cell, K/μL | 4.00-10.00 | 16.50 | 9.97 | 8.62 |
| Hemoglobin, g/dL | 13.5-18.0 | 12.7 | 10.0 | 12.8 |
| Platelets, K/μL | 150-450 | 307 | 447 | 513 |
| Lactic acid, mmol/L | 0.2-2.0 | 2.5 | 1.9 | — |
| NT-proBNP, pg/mL | <450 | 31,877 | — | — |
| CK-MB, ng/mL | 0-7.7 | 7.0 | — | — |
| ESR, mm/h | 0-15 | 100 | — | — |
| PT-INR | 0.9-1.1 | 1.2 | 1.3 | 1.1 |
| Procalcitonin, ng-mL | 0-0.08 | 0.40 | — | — |
| MVO2 saturation, % | 60-80 | 64 | 70.1 | — |
CCU = cardiac care unit, CK-MB = creatine kinase myocardial band; ESR = erythrocyte sedimentation rate; HD = hospital day; NT-pro BNP = N terminal pro B-type natriuretic peptide; MVO2 = mixed venous oxygen; PT-INR = prothrombin time – international normalized ratio.
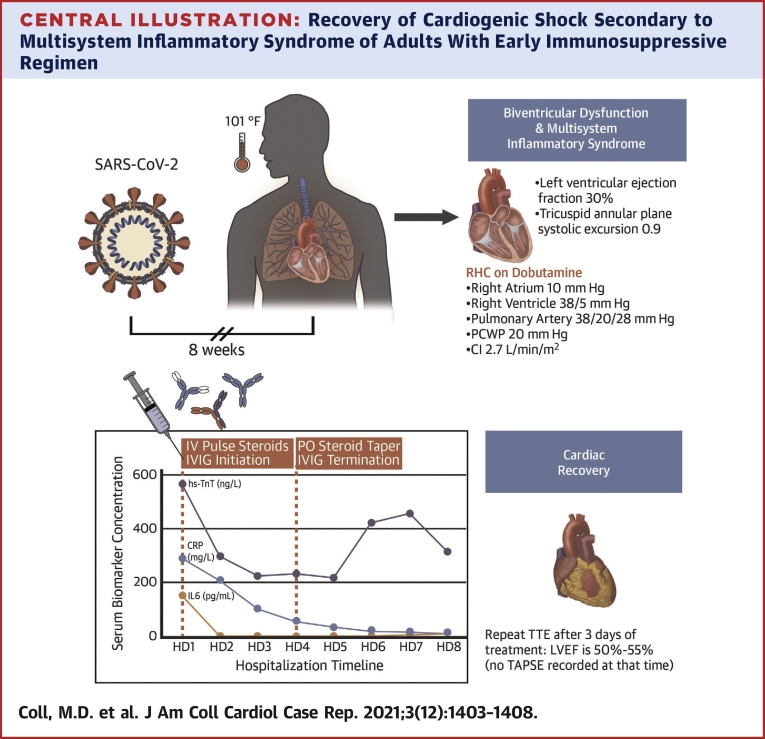
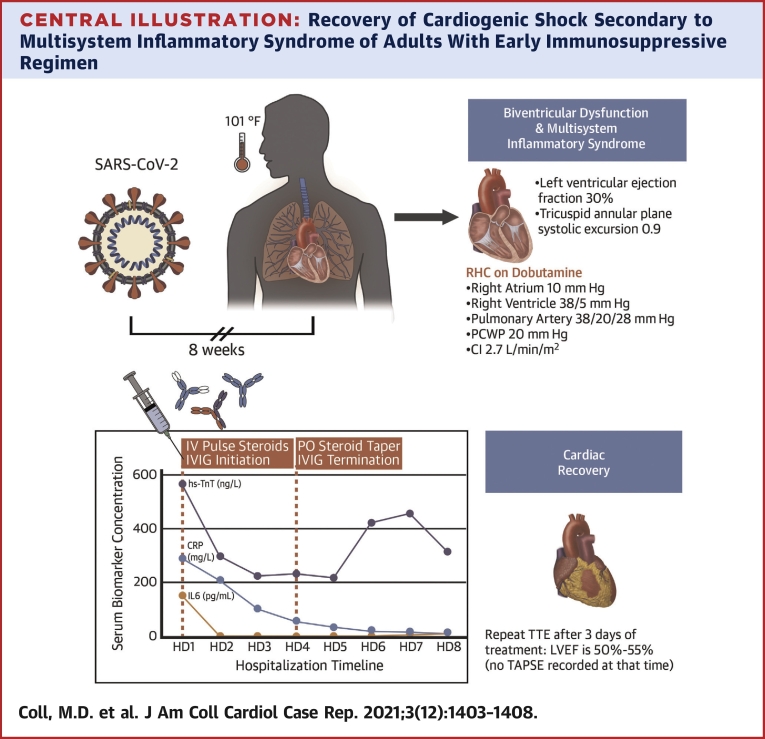
A transthoracic echocardiogram demonstrated left ventricular ejection fraction of 30% (normal: 50%-75%) with global hypokinesis and severely reduced right ventricular function with tricuspid annular plane systolic excursion of 0.92 cm (normal: ≥1.7 cm). and S’ 7.6 cm/s, with inferior vena cava measuring 3 cm (normal: <2.1 cm/s) with no respirophasic variation (Video 1A).
Coronary angiogram revealed no epicardial disease, whereas right heart catheterization revealed mildly elevated right- and severely elevated left-sided pressures with low-normal cardiac index on 2 μg/kg/min/min of dobutamine (right atrium: 10 mm Hg, right ventricle: 38/5 mm Hg, pulmonary artery pressure: 38/20 mm Hg, mean pulmonary artery pressure 28 mm Hg, pulmonary capillary wedge pressure: 20 mm Hg, cardiac index: 2.7 by Fick). Endomyocardial biopsy was deferred.
Management
A systemic hyperinflammatory process was believed to be the most likely etiology of his biventricular failure. He was empirically treated with methylprednisolone 1 g daily for 3 days and intravenous immunoglobulin (IVIG) 0.5 mg/kg per day for 4 days.
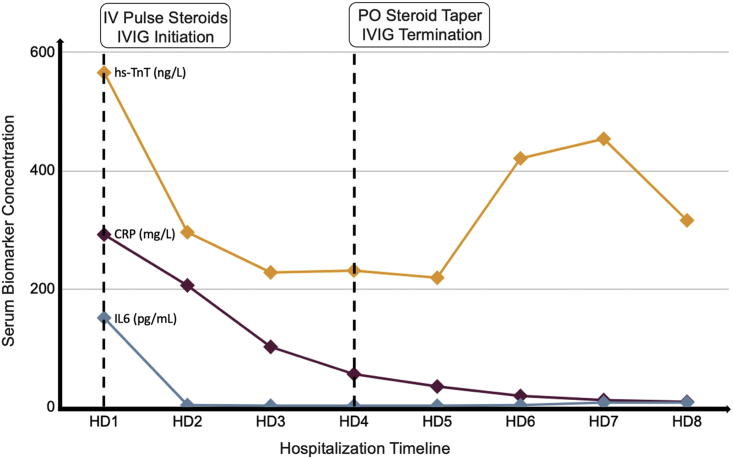
The patient’s symptoms rapidly improved over the course of the first 24 hours after treatment initiation with rapid improvement in biomarkers (Figure 2). Inotropic support with dobutamine was discontinued on day 2 of treatment. Interval limited transthoracic echocardiogram on day 3 of treatment demonstrated normalization of biventricular function (Video 1B).
Figure 2.
Biomarker Trend During Hospitalization
Serum biomarker concentration (vertical axis) as a function of time (horizontal axis). The dotted lines represent therapeutic interventions, as indicated in the text boxes at the top of the graph. CRP = C-reactive protein; HD = hospital day; hs-TnT = high-sensitivity troponin T; IL = interleukin; IV = intravenous; IVIG = intravenous immunoglobulins; PO = per os.
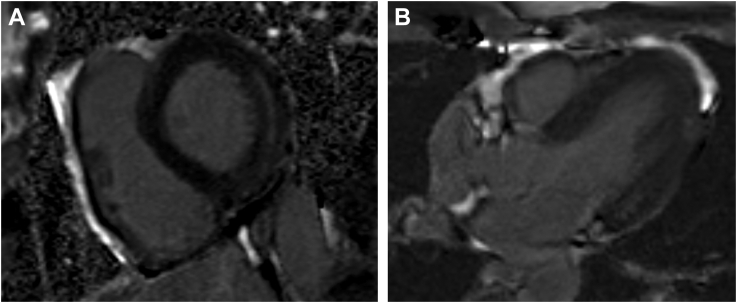
Cardiac magnetic resonance imaging (MRI) performed on hospital day 8 revealed midwall late gadolinium enhancement at the basal lateral left ventricular wall, without evidence of myocardial edema (Video 2, Figure 3).
Figure 3.
Cardiac MRI on Day 8 of Hospital Course
Late gadolinium enhancement short axis view (A) and 4-chamber view (B) showing midwall late gadolinium enhancement at the basal lateral left ventricular wall.
The patient was transferred from the CCU on hospital day 4 and was discharged home 9 days after admission on a prolonged prednisone taper (Central Illustration).
Central Illustration.
Recovery of Cardiogenic Shock Secondary to Multisystem Inflammatory Syndrome of Adults With Early Immunosuppressive Regimen
CI = cardiac index; CRP = C-reactive protein; HD = hospital day; IV = intravenous; IVIG = intravenous immunoglobulin; LVEF = left ventricular ejection fraction; PCWP = pulmonary capillary wedge pressure; PO = per os; RHC = right heart catheterization; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TTE = transthoracic echocardiogram; TAPSE = tricuspid annular plane systolic excursion.
Discussion
Early on during the COVID-19 pandemic, it became clear that SARS-CoV-2 infection was associated with a variety of cardiovascular complications from myocarditis to thromboembolism (1). In fact, 20% of hospitalized patients with COVID-19 have elevated high-sensitivity troponin, reflecting some degree of cardiac injury (2). In the absence of any obstructive coronary disease, the finding of myocardial injury in COVID-19 patients has often led to a diagnosis of myocarditis. However, in many patients with clinically suspected COVID-19 myocarditis, histopathology from endomyocardial biopsy or autopsy has not confirmed the diagnosis (3, 4). Furthermore, it is rare to detect SARS-CoV-2 RNA in cardiac tissue on autopsy (5). Such data reflect our still-limited understanding of the pathogenesis of cardiac injury in patients with SARS-CoV-2.
One emerging mechanism of myocardial damage in COVID-19 is immune system hyperactivation resulting in release of inflammatory molecules which can promote cardiac injury. In the acute phase of COVID-19, this cytokine storm, possibly secondary to innate and adaptive immune overactivation, can result in infiltration of macrophages and T cells into lung or heart tissue resulting in myocardial injury. However, what can account for this patient’s new biventricular dysfunction and cardiogenic shock 8 weeks after COVID-19 infection?
Multisystem inflammatory syndrome in adults (MIS-A) has been recently described in patients who have recovered from COVID-19 in the preceding 12 weeks and present with fever, cardiac dysfunction, shock, skin, and/or gastrointestinal manifestations (6). Similar to this case, SARS-CoV-2 RNA is often not detectable by nasopharyngeal RT-PCR, but SARS-CoV-2 serologies are positive as well as significantly elevated markers of inflammation (C-reactive protein, erythrocyte sedimentation rate, interleukin 6, ferritin, and D-dimer).
A similar syndrome was first recognized in the pediatric population and termed multisystem inflammatory syndrome in children (MIS-C). This entity is characterized by fever, left ventricular dysfunction, mucosal inflammation, and skin changes akin to Kawasaki disease. This clinical similarity has provided some insight into better understanding the pathophysiology of MIS-C. Investigators compared the cytokine profiles in patients with acute COVID-19, MIS-C, and Kawasaki disease, and found that acute COVID-19 profiles were distinct from MIS-C or Kawasaki disease, which shared some overlap, supporting the immune complex formation and deposition as a possible mechanism of endomyocardial injury (7). Another hypothesis is that a robust adaptive immune response to SARS-CoV-2 could result in the generation of cardio-specific autoantibodies as a result of molecular mimicry, although no precise target antigens have been proposed (8). Further investigation into the pathophysiology of both MIS-A and MIS-C will be important in directing future therapies.
Early recognition of MIS-A is important for cardiologists and intensivists, as quick initiation of anti-inflammatory therapy appears crucial to recovery and prevention of lasting end-organ damage (9). As in our case, pulse-dose corticosteroids and IVIG were initiated based on clinical presentation, lab markers of inflammation, and history of recent COVID-19, which are all suggestive of MIS-A or a fulminant myocarditis.
This patient was critically ill in the CCU and weighing the risks and benefits, we did not pursue endomyocardial biopsy as it was unlikely to change management. Furthermore, delay of anti-inflammatory therapy to await biopsy results could have led to irreversible myocardial damage. In fact, we believe that this rapid treatment may explain why the cardiac MRI (performed 8 days after steroid and IVIG initiation) revealed no myocardial edema and minimal scar. This is also consistent with the rapid decline in cardiac and inflammatory biomarkers. Use of IVIG has been reported in several similar cases with favorable outcomes, albeit in a nonrandomized fashion (6). There are no clearly defined recommendations for management of MIS-A, but a prolonged steroid taper appears important. Future study is needed to determine optimal treatment strategies.
Follow-Up
Since being discharged from the hospital, this patient has done well with sustained recovery of left ventricular function. Long-term follow-up and study of these patients will be needed to help determine if there are any lasting sequelae of MIS-A.
Conclusions
This case highlights a late, serious complication of SARS-CoV-2 infection where early recognition and treatment of MIS-A can be lifesaving and lead to quick recovery of myocardial function. Rapid improvement following initiation of immunosuppressive therapy, including IVIG, suggests autoantibody formation and subsequent endomyocardial and pericardial deposition of immune complexes as possible pathophysiological mechanisms; however, important questions remain and will be areas for future study of MIS-A.
Funding Support and Author Disclosures
Dr Mehra has received personal fees from Abbott, Mesoblast, Janssen, Portola, Bayer, Triple Gene, and Baim Institute for Clinical Research; and is an advisory board member for NuPulseCV, Leviticus, and FineHeart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the entire Cardiac ICU care team who helped in caring for this patient including Drs Eric Mills, Katherine Frost, Brittany Ricci, Charlotte Story, and especially the BWH Cardiac ICU director Dr David Morrow. The authors also acknowledge the entire Cardiac ICU nursing team who provide exceptional care for this patient.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic Echocardiogram (TTE) TTE at presentation (A) and day 3 of treatment (B)
Transthoracic Echocardiogram (TTE) TTE at presentation (A) and day 3 of treatment (B)
Cardiac MRI On Day Eight Of Hospital Course MRI 3 Chamber Function View
References
- 1.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindner D., Fitzek A., Brauninger H. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami R., Sakamoto A., Kawai K. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris S.B., Schwartz N.G., Patel P. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consiglio C.R., Cotugno N., Sardh F. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981. doi: 10.1016/j.cell.2020.09.016. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20:339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau V.Q., Giustino G., Mahmood K. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic Echocardiogram (TTE) TTE at presentation (A) and day 3 of treatment (B)
Transthoracic Echocardiogram (TTE) TTE at presentation (A) and day 3 of treatment (B)
Cardiac MRI On Day Eight Of Hospital Course MRI 3 Chamber Function View