Abstract
Atypical responses to fearful stimuli and the presence of various forms of anxiety are commonly seen in children with autism spectrum disorder (ASD). The fear potentiated startle paradigm (FPS), which has been studied both in relation to anxiety and as a probe for amygdala function, was carried out in 97 children aged 9–14 years including 48 (12 female) with ASD and 49 (14 female) with typical development (TD). In addition, exploratory analyses were conducted examining the association between FPS and amygdala volume as assessed with magnetic resonance imaging in a subset of the children with ASD with or without an anxiety disorder with available MRI data. While the startle latency was increased in the children with ASD, there was no group difference in FPS. FPS was not significantly associated with traditional Diagnostic and Statistical Manual (DSM) or “autism distinct” forms of anxiety. Within the autism group, FPS was negatively correlated with amygdala volume. Multiple regression analyses revealed that the association between FPS and anxiety severity was significantly moderated by the size of the amygdala, such that the association between FPS and anxiety was significantly more positive in children with larger amygdalas than smaller amygdalas. These findings highlight the heterogeneity of emotional reactivity associated with ASD and the difficulties in establishing biologically meaningful probes of altered brain function.
Keywords: fear conditioning, autism, autistic, anxiety, MRI
Lay summary:
Many children with autism spectrum disorder (ASD) have additional problems such as anxiety that can greatly impact their lives. How these co-occurring symptoms develop is not well understood. We studied the amygdala, a region of the brain critical for processing fear and a laboratory method called fear potentiated startle for measuring fear conditioning, in children with ASD (with and without an anxiety disorder) and typically developing children. Results showed that the connection between fear conditioning and anxiety is dependent on the size of the amygdala in children with ASD.
Introduction
Autism spectrum disorder (ASD) is characterized by impaired social communication and by restricted or repetitive behaviors and commonly affects language and cognitive abilities [American Psychiatric Association, 2013]. Beyond the core features of ASD, affected individuals commonly experience a variety of co-occurring conditions including intellectual disability, which affects at least 40% of individuals with autism [Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators & Prevention, 2012]; epilepsy, in approximately 25% [El Achkar & Spence, 2015]; and gastrointestinal problems in 25%–48% [Buie et al., 2010; The Research Units On Pediatric Psychopharmacology Anxiety Study Group, 2002]. Anxiety disorders are particularly troubling, co-occurring mental health conditions that have been estimated to affect up to 69% of individuals with ASD [Kerns et al., 2014, 2020]. Interestingly, a subset of individuals with ASD demonstrate an absence of adaptive fear in some circumstances and may endanger themselves by engaging in behavior such as elopement [Kiely, Migdal, Vettam, & Adesman, 2016]. Attempting to develop biologically meaningful probes of brain function in order to phenotype the behavioral heterogeneity of ASD has become a major initiative of modern ASD research. We have been focusing on efforts to appropriately diagnose anxiety disorders in individuals with ASD and to establish biological or physiological biomarkers that are associated with increased anxiety. Much of this effort focuses on the potential role of the amygdala, a region of the brain important for mediating emotional responses, particularly to fearful stimuli [LeDoux & Pine, 2016].
The amygdala has long been implicated in the mediation of important aspects of anxiety [Adhikari, 2014; Amaral & Corbett, 2003; Marazziti et al., 2015; Taylor & Whalen, 2015]. There is also a long history of neuropathological and neuroimaging findings suggesting that the structure and function of the amygdala is altered in ASD [Aylward Minshew et al., 1999; Bauman & Kemper, 1985; Herrington et al., 2017; Nordahl et al., 2012; Shen et al., 2016]. Interestingly, Kerns et al. [2014, 2020] have shown that some manifestations of clinically-significant anxiety disorder in children with ASD are quite different from those defined by the DSM-5. For example, children with ASD experience intense anticipatory anxiety related to changes in their routine or when they have reduced access to objects of intense interest. They may develop very unusual and uncommon phobias or develop social anxiety that is less related to embarrassment or fear of negative evaluation but rather anxiety due to social confusion stemming from their autism related social deficits. What is not yet clear is how an anxiety disorder develops in the context of ASD and the degree to which amygdala structure and function play a role in the pathogenesis of these troubling symptoms. Both animal and some clinical research has raised the possibility that fear conditioning tasks such as the fear potentiated startle (FPS) paradigm may be useful tools to examine the potential links between anxiety and amygdala function in ASD.
FPS is defined as an elevated startle reflex response to a sudden probe (such as a loud noise) in the presence of a stimulus (the conditioned stimulus or CS) that was previously paired with an aversive event (the unconditioned stimulus—US, e.g., electric shock). Typical rodent FPS studies start with initial training sessions in which a light (CS) precedes an electric shock (US). Afterward, during the testing session, a whole-body startle reflex is elicited by a loud acoustic stimulus (startle probe) in the presence or absence of the light. The startle reflex is measured by a strain gauge in the apparatus that quantifies the animal’s movement. Potentiation of the startle response is indicated by a larger movement when the CS is delivered prior to the startle stimulus in comparison to without the CS. This response is sensitive enough to detect alterations related to drug administration. In rats, the FPS is dampened by anxiolytic drugs [Chi, 1965; Davis, 1979; Davis, Redmond, & Baraban, 1979] and enhanced by anxiogenic drugs [Bridger & Mandel, 1967; Davis et al., 1979]. These findings have been interpreted as indicating that enhanced startle is due to anticipatory fear (or anxiety) experienced by the animal prior to the startle stimulus.
The FPS effect is also reliably measured in humans (Grillon, Ameli, Foot, & Davis, 1993; Grillon, Ameli, Woods, Merikangas, & Davis, 1991; Grillon et al., 2006; Hamm, Greenwald, Bradley, Cuthbert, & Lang, 1991; Jovanovic et al., 2005; Lissek, Biggs, et al., 2008; Lissek, Levenson, et al., 2008; Norrholm et al., 2006; Ross, 1961; Spence & Runquist, 1958). In human studies, the CS is typically a light, or images presented on a screen. The US can range from an aversive air puff to the neck, to an electric shock, to the sound of a human scream. Once the contingency is learned that the CS predicts an aversive event, the CS sometimes precedes or is paired with the startle probe. In humans, the startle reflex is usually measured by the amplitude of the eye blink startle reflex (i.e., the electromyographic response of the orbicularis oculi muscle) or by electrodermal activity (e.g., skin conductance of the palmar surface).
Consistent findings in animal studies support the notion that the amygdala is an important brain region in the mediation of the FPS response. Studies of rats and rhesus monkeys alike have found that lesions to the amygdala before training effectively block FPS [Antoniadis, Winslow, Davis, & Amaral, 2007, 2009; Campeau & Davis, 1995; Heldt, Sundin, Willott, & Falls, 2000; Hitchcock & Davis, 1986; Lee & Davis, 1997], suggesting that the amygdala is necessary for fear learning and FPS. However, there may be some species differences in this phenomenon. For example, in rats, the amygdala is essential both for the learning and for the expression of FPS, while in rhesus monkeys lesions occurring after training do not block FPS [Antoniadis et al., 2007; Antoniadis et al., 2009], indicating that a functioning amygdala is required for acquiring the FPS response but not for its expression. Moreover, there may be neurodevelopmental alterations to how FPS neuropathology impacts FPS response. Neonatal amygdala lesions appear to attenuate but not abolish FPS when tested in adult rhesus monkeys that received additional training in the CS-US pairings, suggesting that early amygdala damage may induce other brain regions to take over the mediation of FPS [Kazama, Heuer, Davis, & Bachevalier, 2012].
Three previous studies have investigated the FPS response in individuals with ASD. All three studies reported that there were no significant differences in the FPS response in participants with ASD compared to typically developing (TD) controls [Bernier, Dawson, Panagiotides, & Webb, 2005; Chamberlain et al., 2013; Sterling et al., 2013]. One study, however, did find a significant increase in baseline startle amplitudes in participants with ASD [Chamberlain et al., 2013]. This finding is reminiscent of the increased baseline startle amplitude in adults with generalized anxiety disorder [Grillon et al., 2016]. Surprisingly, the Chamberlain et al. [2013] study in individuals with ASD found that total anxiety scores and Generalized Anxiety Disorder scores were negatively correlated with startle amplitudes; greater anxiety was associated with lower startle amplitude. One of the studies [Sterling et al., 2013] correlated anxiety symptoms with magnitude of startle during a threat condition but failed to examine startle potentiation (the degree to which the startle reflex is augmented by the threat condition relative to baseline); thus, the association of anxiety with FPS is unknown in this cohort. It is worth noting, that in all three studies of FPS in ASD, anxiety was assessed using self-report or caregiver-report questionnaires that had not been validated for use in individuals with ASD [Jitlina et al., 2017; Mazefsky, Kao, & Oswald, 2011]. Thus, it is possible that levels of anxiety or presence of co-occurring anxiety disorders were not reliably measured, or they may not cover the full range of anxiety symptom expression within this disorder. For example, in Kerns et al. [2020], we reported that sensitivity was poor for brief anxiety screeners compared to diagnostic interviews for children with ASD, and these anxiety scales are very poor for those with IQs <70. In addition, these three studies were limited to older adolescents and adults, with normal or above average IQ and relatively low ASD symptom severity. Chamberlain et al. [2013] made the important observation that younger and more impaired samples may be needed to further explore this phenomenon, noting as well that the anxiety response in ASD may be related to environmental signals that most people do not find threatening, such as specific types of sounds or lighting.
The primary aim of the current study was to evaluate fear conditioning using a potentiated startle paradigm in a cohort of 9–14-year-old children with ASD who are part of the longitudinal Autism Phenome Project at the UC Davis MIND Institute. These children are a diverse group that run the full spectrum of both autism severity and intellectual functioning. Additionally, brain imaging data are available for most of the children and they have completed comprehensive clinical assessment for the presence of anxiety disorders, including both traditional anxiety as defined by the DSM as well as distinct forms of anxiety disorder that occur in ASD [Herrington et al., 2017; Kerns & Kendall, 2012; Kerns et al., 2014, 2020]. Thus, a secondary aim was to determine potential interrelationships between measures of FPS, anxiety, and amygdala volumes in autism. This would provide important information concerning the neurobiological substrates of anxiety in ASD. We hypothesized that FPS would be increased in the children with ASD relative to those with TD. We also hypothesized there would be a positive association between the magnitude of FPS and the anxiety severity as well as the volume of the amygdala in those with available MRI data.
Materials and methods
Participants
Participants (Table 1) were enrolled in the ongoing University of California, Davis Medical Investigation of Neurodevelopmental Disorders (MIND) Institute Autism Phenome Project. Participants with ASD were recruited through the MIND Institute Massie Clinic, California Regional Centers and local clinics and community events when they were 2–3.5 years of age. TD participants were recruited through community events and health fairs. Data utilized in the current study were acquired during a longitudinal visit when participants were between 9 and 14 years of age. All aspects of the study protocol were approved by the University of California, Davis Institutional Review Board, and informed consent was obtained from the parent or guardian of each participant.
Table 1.
Participant Descriptive Statistics
| TD | ASD − AD | ASD + AD | X2, F, t-test: P-value | |
|---|---|---|---|---|
| Participants, n | 49 | 20 | 28 | |
| Gender (M, F) | 35, 14 | 15, 5 | 21, 7 | 0.92 |
| Age | 11.55 (0.83) | 11.77 (0.74) | 11.47 (0.82) | 0.33 |
| tIQ | 112.98 (12.94) | 90.86 (23.37) | 97.83 (29.25) | 0.001 |
| range = 83–136 | range = 41–131 | 39–170 | ||
| Family income (% above $75 K) | 54.20 | 40.00 | 66.70 | 0.19 |
| Parental education (either parent % college degree) | 66.70 | 61.10 | 52.00 | 0.47 |
| Ethnicity (% Hispanic/Latino) | 28.60 | 25.00 | 21.40 | 0.82 |
| Race (% Minority) | 28.60 | 10.00 | 42.90 | 0.04 |
| Psychoactive medication (%) | 12.20 | 60.00 | 28.60 | <0.001 (ASD − AD >TD, P <0.0001; ASD − AD>ASD + AD, P <0.05) |
| ADOS severity | NA | 7.70 (1.66) | 7.11 (1.52) | 0.20 |
| SRS T Score | 44.61 (5.00) | 71.82 (13.32) | 72.81 (11.46) | <0.001 (ASD − AD versus ASD + AD, ns) |
| FPS (%) | 63.96 (69.02) | 40.17 (66.51) | 95.36 (152.49) | |
| FPS mean rank | 53.00 | 37.95 | 49.89 | 0.13 (Kruskal–Wallis H) |
| Left amygdala volume (cm3) | 1.81 (0.20) | 1.86 (0.25) | 1.90 (0.20) | 0.28 |
| Right amygdala volume (cm3) | 1.97 (0.18) | 2.04 (0.26) | 2.05 (0.21) | 0.26 |
| Total cerebral volume (cm3) | 1143.56 (88.56) | 1165.26 (121.61) | 1181.38 (97.94) | 0.56 |
Diagnostic measures included the Autism Diagnostic Observation Scale—Second Edition (ADOS-2) [DiLavore, Lord, & Rutter, 1995; Lord et al., 2000] and the Autism Diagnostic Interview—Revised (ADI-R) [Lord, Rutter, & Couteur, 1994], conducted by trained, licensed clinical psychologists who specialize in ASD and were research reliable for these tools. These assessments were originally completed when participants were 2–3 years of age. Inclusion criteria for ASD at that time point were based on the diagnostic definition of ASD in young children established by the Collaborative Programs of Excellence in Autism network using DSM-IV [American Psychiatric Association, 1994] criteria. ADOS Calibrated Severity Scores (CSS) were calculated to allow comparison of autism severity across participants tested with different ADOS-G modules [Gotham, Pickles, & Lord, 2009]. Diagnostic confirmation with the ADOS-2 and DSM 5 criteria was carried out again at ages 9–12 years concurrent with the present data. IQ for all participants was measured using the Differential Ability Scales (DAS-2; [Elliott, 2007]. All participants were native English speakers, ambulatory, had no vision or hearing problems, and no other neurological conditions. A characteristic of the Autism Phenome Project is that there are very few exclusions. Thus, children at all levels of autism severity and with and without intellectual disability are included in this study along with an age-matched TD control group.
The Anxiety Disorders Interview Schedule–IV–Parent Interview [ADIS–P; Silverman, Albano, Siebelink, & Treffers, 2001] is a semi-structured interview conducted with parents to assess the presence of childhood anxiety disorders with strong inter-rater and test–retest reliability [Lyneham, Abbott, & Rapee, 2007; Silverman, Saavedra, & Pina, 2001]. We used the ADIS-P modules for OCD, separation, social, and generalized anxiety disorders, and specific phobia. Each module receives a clinical severity rating (CSR), from 0 (no interference) to 8 (severe interference), with 4 representing the cutoff for significant interference, and–when all other criteria are met-diagnosis. The Autism Spectrum Addendum [ASA; Kerns, Renno, Kendall, Wood, & Storch, 2017] is a series of prompts and guidelines that are woven into the ADIS protocol to tailor the instrument for children with ASD. The ADIS/ASA gathers additional information about the child’s developmental level, social functioning and motivation, experiences and environment, sensory sensitivities, and perseverative cognitive style. This information is used to guide differential diagnosis of symptoms and estimations of the impairment due to anxiety as opposed to other difficulties (e.g., communication challenges, ASD-related social deficits). Guidelines for differentiating ASD-related perseveration or repetitive thinking from worry, and specific phobias from sensory sensitivities are also included.
The ADIS-ASA also allows interviewers to assess for “autism distinct” manifestations of anxiety that commonly arise in ASD, but do not match the anxiety disorders specified by the DSM. Distinct anxiety types include uncommon phobia (e.g., fears of toilets, specific sounds, and glasses, beards), social fear which includes fear related to social confusion rather than fear of negative evaluation, special interest fear (i.e., excessive worry or anxiety related to being able to engage in perseverative or intense interest activities), fear of change (i.e., children with anxious anticipation of and distress following changes or novelty), and ambiguous compulsive symptoms which capture compulsions or rituals that were associated with negative rather than positive affect, but which could not be clearly identified as being used to “prevent or neutralize” distress consistent with DSM-IV and DSM-5 OCD criteria. Negative reactions to change (i.e., severe distress following change, but no anticipatory fears of change) are also assessed but are not considered manifestations of anxiety. We applied CSR severity scores for autism distinct manifestations of anxiety using the same scaling and criterion as those applied to DSM anxiety disorders in the ADIS-P. The ADIS-ASA also demonstrates excellent inter-rater and retest reliability [Kerns et al., 2014, 2017].
Magnetic Resonance Imaging Acquisition
MRI was carried out at the UC Davis Imaging Research Center using a 3T Siemens TIM Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with an 8-channel head coil. MRI scans were conducted while participants were awake, using principles of behavior analysis to enable scanning in children at all levels of severity and functioning [Nordahl et al., 2016]. 3D T1-weighted MPRAGE (TR 2,170 ms; TE 3.5 ms; FOV 256 mm; FA 7, 192 sagittal slices, 1.0 mm slice thickness; 5:10 acquisition time) were acquired for each child. T2-weighted images were also collected and reviewed by a pediatric neuroradiologist to rule out clinically significant incidental findings. A calibration phantom (ADNI MAGPHAN, Phantom Laboratory, Inc., Salem, NY) was scanned at the end of each MRI session using an MPRAGE pulse sequence matched to the study sequence and using the same landmark and shim as the corresponding participant to ensure accurate measurement of spatial characteristics of the MRI volume. A 3D image distortion map was derived to correct for hardware-induced geometric distortion [Image Owl, Inc., Salem, NY; Nordahl et al., 2012].
Image Processing
Participants’ distortion corrected structural MRI images were visually inspected slice-wise for motion, grainy images, ringing, or Gibbs artifact that blurred the boundaries between gray matter and white matter tissues. If any of these artifacts were present on at least one 2D image slice, the entire 3D structural MRI did not pass inspection. Eighty of the 98 participants with valid FPS data had sufficient quality structural MRI scans to extract amygdala volumes. Distortion corrected and anonymized MRIs were uploaded to MRICloud [https://mricloud.org; Mori et al., 2016] for segmentation using the fully-automated MRICloud T1-Segmentation pipeline (version 7A) [Ceritoglu et al., 2009; Wang & Yushkevich, 2013]. The whole-brain segmentation output for each participant was visually inspected slice-wise for segmentation quality. Total hemispheric volumes and volumes for right and left amygdala were extracted and used in the reported analyses. Total hemispheric volumes were summed for a total cerebral volume (TCV) measurement.
FPS Paradigm
Participants were evaluated using an FPS paradigm adapted from methods used in a previous FPS study conducted on adolescents with ASD [Sterling et al., 2013]. A social story with photos depicting the steps involved in the protocol and appearance of the laboratory space was used to increase familiarization and understanding for a subset of the ASD sample requiring extra support. Participants were seated in a chair facing a computer screen and tablet (Fig. 1A) within a sound attenuated testing room (ETS-Lindgren, Letchworth, UK). A decorative backboard was located behind the computer to draw attention to the computer and tablet but was not visible once the experiment began and the lights were turned off. In order to keep the participants engaged and looking toward the computer screen, a silent, neutral standard video for all children depicting a walk through the landscape of the game Minecraft played on the tablet located above the computer screen.
Figure 1.
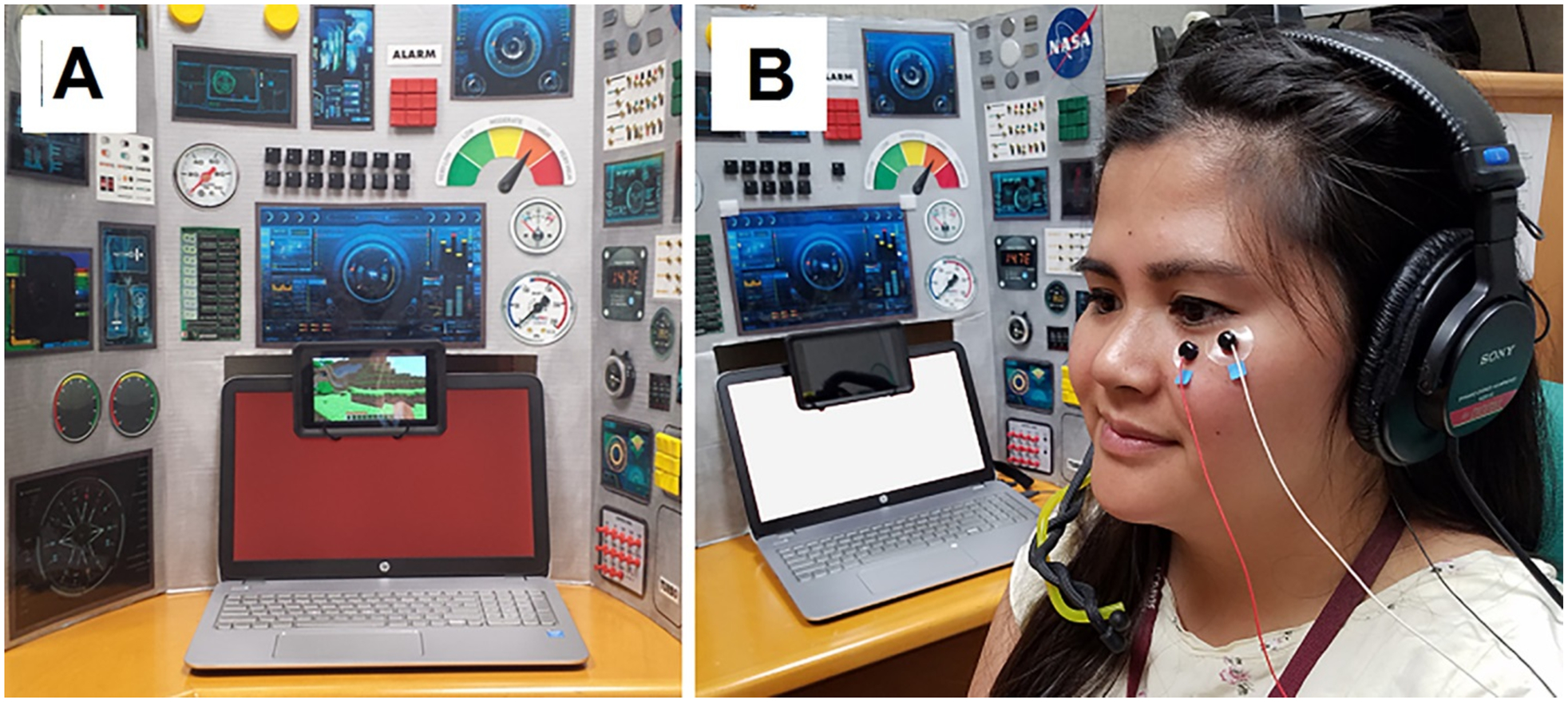
Fear potentiated startle laboratory set-up. (A) Participants are seated facing a spaceship or pilot cockpit themed background and the laptop computer displaying a red (“threat”; CS) or white (“safe”) screen, while a silent video plays on a small tablet to maintain engagement and compliance. (B) Placement of EMG electrodes over the orbicularis oculi muscle measuring the startle response and headphones used to deliver the 50 ms, 100 db white noise startle stimulus.
The FPS protocol (Fig. 2) was administered using a San Diego Instruments (SDI, San Diego, CA, USA) SR-HLAB EMG system. Experimental stimuli included a noxious air puff (the US), a 60 PSI burst of air for 200 ms duration from plastic tubing pointing at the base of the throat and an acoustic startle stimulus (SS), a 100 dB white noise sound lasting 50 ms presented through a set of headphones. The CS was the computer screen in front of the participant turning red for ≤2,000 ms. The FPS paradigm included four phases: (1) habituation I, (2) conditioning, (3) habituation II, and (4) testing (Fig. 2). Habituation I consisted of 12 SS probes paired with a blank white computer screen. The conditioning phase consisted of 20 presentations of the CS paired with the US (the air puff) for 50 ms to facilitate learning of the CS–US association. The air puff was initiated at 0, 160, 280, 710, 1,000, and 1,430 ms after the initiation of the ≤2,000 ms CS so that its onset could not be anticipated; both the CS and US terminated simultaneously. The conditioning phase was followed by a second habituation phase consisting of five SS probes paired with a blank white screen.
Figure 2.
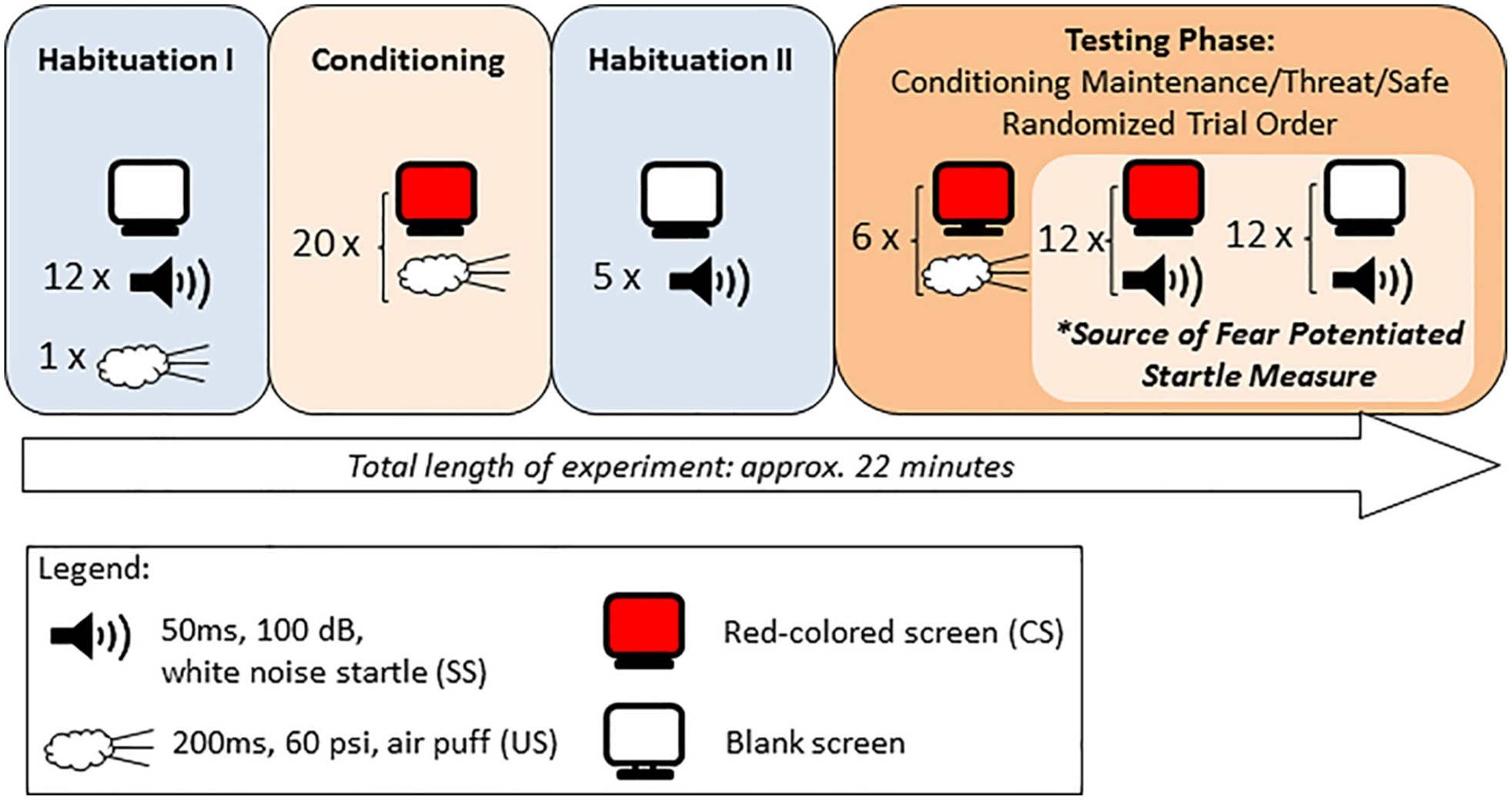
Fear potentiated startle (FPS) paradigm. Habituation to the startle stimulus (SS) is measured during Habituation 1, followed by fear conditioning, during which the aversive air puff to the neck is paired with the red screen background. This is followed by a brief second habituation. Finally, the FPS measurement is derived from the testing phase, during which the percent conditioned increase in startle magnitude is measured by the difference between 12 startle stimulus (SS) trials paired with the CS (red screen; “threat”) and 12 trials paired with the blank/white screen (“safe”). Six additional trials are included to maintain conditioning during the test phase. These 30 test trials are randomly administered.
The final testing phase included 30 randomized trials of three types: (1) 6 CS–US pairings, (2) 12 CS–SS “threat” trials, (3) 12 SS alone “safe” trials. The startle reflex (eyeblink) was measured via electromyographic activity (EMG) recorded using two Ag/AgCl surface electrodes placed under the left eye (orbicularis oculi), and a ground electrode placed behind the left ear (mastoid) (Fig. 1B). Eyeblink amplitude and latency were collected and analyzed using the SRH-LAB software (San Diego Instruments, San Diego, CA). Gain was adjusted to 0.5 (0.5-mV electrode input was amplified to 2,500-mV signal output) and band pass filtered (100–1,000 Hz). A 60-Hz notch filter was used to eliminate 60 Hz interference and sampling rate was 1 kHz.
The FPS response was analyzed from the 12 “threat” and 12 “safe trials”; potentiation was indicated by a greater response in the threat condition compared to the safe condition, measured as percentage startle potentiation. Eyeblink startle amplitude, latency (time taken between stimulus presentation and maximum EMG response), and habituation (measured as the slope of the first 12 startle amplitudes in response to SS presentations during the habituation I phase) were also examined.
FPS Data Review and Cleaning
All FPS protocol trials were visually reviewed by the first author (David Hessl), blind to group or other participant variables, within SRH-LAB software and any trial with excessive EMG noise that obscured an unambiguous startle response within a time window from SS onset to 150 ms postonset was excluded from analysis. A minimum of four acceptable trials per FPS condition (safe and threat) were required [Lieberman et al., 2017] to be considered valid for inclusion in the analyses. Using this criterion, 26 participants with ASD and 7 with TD were excluded from analysis due to noisy EMG data (poor electrode impedance, movement artifacts, and excessive facial EMG activity). An additional nine participants with ASD and one with TD were noncompliant with the protocol and had to be excluded. Two with ASD and two with TD were EMG startle “nonresponders” (i.e., they had no measurable startle response across trials). Finally, data from four with ASD and four with TD were excluded due to equipment problems. Participants who were successful with the FPS protocol and provided valid data for analysis were compared to those who were excluded from further analysis (excluding equipment problems) on variables such as group (ASD vs. TD), presence of an anxiety disorder, family income, ethnicity/race minority status, parental education level, and participant age and IQ (within ASD and TD). Overall, ASD participants were less likely than TD to be successful with providing FPS data (χ2 = 10.86, P = 0.001), and among those with ASD, those providing valid FPS data had higher IQ (mean = 90.79 vs. 69.92; P = 0.003). There were no other significant differences on other variables associated with success with the FPS protocol.
Statistical Analysis
Participants with ASD were assigned to the ASD with anxiety disorder (ASD + AD) subgroup if they met diagnostic criteria for a DSM anxiety disorder or clinically significant distinct anxiety according to ADIS or ADIS-ASA criteria and to the ASD without anxiety disorder (ASD − AD) subgroup if they did not meet threshold criteria by this clinical assessment across all anxiety categories assessed by the instruments. ADIS CSR scores were used as indices of anxiety severity. Anxiety severity was taken for each participant as the highest CSR domain score across all anxiety disorders assessed, including traditional DSM anxiety disorders and ADIS-ASA “autism distinct” disorders. Demographic and clinical group differences were first examined using Chi-square tests or one-way ANOVA. The association between each of these variables and FPS, startle latency and startle amplitude were examined using Pearson’s r or Spearman’s ρ correlation as appropriate. Demographic or clinical variables that demonstrated significant group differences and were correlated with the startle protocol dependent variables were included as covariates in subsequent analyses. Startle amplitude and its potentiation by fear conditioning was examined using a 2 (condition) × 3 (group) repeated measures ANOVA, utilizing a log-transformation of startle amplitude to achieve normality and reduce skew in the data. A repeated measures ANOVA was similarly applied to the startle latency data. For each participant, we calculated degree of fear-potentiated startle (expressed as a percentage) using the following formula: [ln(average “threat” startle amplitude) − ln(average “safe” startle amplitude)/ln(average “safe” startle amplitude)] * 100.
To examine startle habituation across the 12 habituation trials, we conducted a linear mixed effects model of response amplitude (again, log transformed) including fixed effects of group and trial as a continuous covariate, a group by trial interaction, and a random effect including a random intercept and random slope for trial. The mixed effects model was estimated using an unstructured variance–covariance matrix with the nlme package (v. 3.1) in R (v. 3.6.3). Significance of trial, group, and interaction terms was assessed using a likelihood ratio test that contrasted models with and without each term in models estimated using maximum likelihood [Hoffman, 2015].
Direct associations between anxiety severity and FPS, as well as between ADIS anxiety severity and amygdala volume were examined using Spearman’s ρ correlations. Differences in amygdala volume (as a proportion of TCV) by group were examined using a 3 (group) by 2 (hemisphere) ANOVA.
To examine the role of the amygdala as a moderator of associations between ADIS severity scores and FPS response, we conducted two multiple regression analyses in which ADIS CSR scores were regressed onto FPS response, the left (or right) amygdala volume, and interaction thereof. We followed up these analyses by separating anxiety into DSM traditional and autism distinct anxiety in a mixed effects model with type of anxiety as a within-person factor and testing two- and three-way interactions with FPS and left (or right) amygdala volumes. For all these analyses, FPS and amygdala scores were converted into Z-scores. Given limitations in the number of participants with MRI data, we considered analyses pertaining to amygdala volume as exploratory. Finally, since the TD sample had a very limited range of anxiety severity scores, moderation analyses were conducted only within the ASD group.
Results
Demographic and Clinical Data
In all, 97 children aged 9–14 years completed the FPS protocol and provided valid data, including 48 (12 female) with ASD [20 without an anxiety disorder (5 female and 15 male) and 28 with an anxiety disorder (7 female, 21 male)] and 49 with TD (14 female). Descriptive statistics by groups are shown in Table 1. Among the ASD + AD group, 4 met diagnostic criteria for Separation Anxiety Disorder, 4 with Social Anxiety Disorder, 11 with Generalized Anxiety Disorder, 16 with Specific Phobia, 3 with Social Fear, 5 with Uncommon Phobia, 1 with Special Interest Fear, and 8 with Fears of Change (the latter 4 categories are autism-specific derived from the ADIS-ADA). Fifteen of the 28 participants with anxiety met criteria for multiple anxiety disorders, 22/28 met criteria for at least one DSM anxiety disorder and 6/28 had clinically significant autism distinct anxiety without meeting criteria for a DSM anxiety disorder. Among all participants with valid FPS data, 70 had acceptable MRI data for analyses pertaining to amygdala volume (42 TD, 16 ASD − AD, and 22 ASD + AD). The three groups were compared for significant demographic and other potentially confounding differences. The ASD groups, especially ASD − AD, were more likely to be taking a psychoactive medication (Chi-square = 16.52, P <0.001; 12.2% TD, 60% ASD − AD, 28.6% ASD + AD), the most common being stimulants. Participant race differed significantly by group (Chi-square = 6.14, P = 0.046): ASD + AD had a higher proportion of non-Caucasian participants. Finally, IQ differed significantly by group, F(2,80) = 7.85, P = 0.001 (both ASD groups had lower IQ than the TD group). Other demographic variables did not significantly differ between groups. Race (White/Caucasian vs. minority) was significantly correlated with startle amplitude in the TD group and thus was entered as a covariate in the startle amplitude analyses to check for possible confounding effects. No other demographic variables were correlated with outcomes of interest.
Startle Amplitude and Startle Habituation
During the habituation I phase of the protocol, there were no significant mean differences in response amplitude between the three groups (Chi-square (2) = 1.56, P = 0.46). Response amplitude decreased significantly (Fig. 3) across the 12 trials (b = −0.036, SE = 0.004, t = −8.58; Chi-square (1) = 50.8, P <0.0001). However, groups did not significantly differ in their rates of habituation across trials (group by trial interaction: Chi-square (2) = 0.22, P <0.89).
Figure 3.
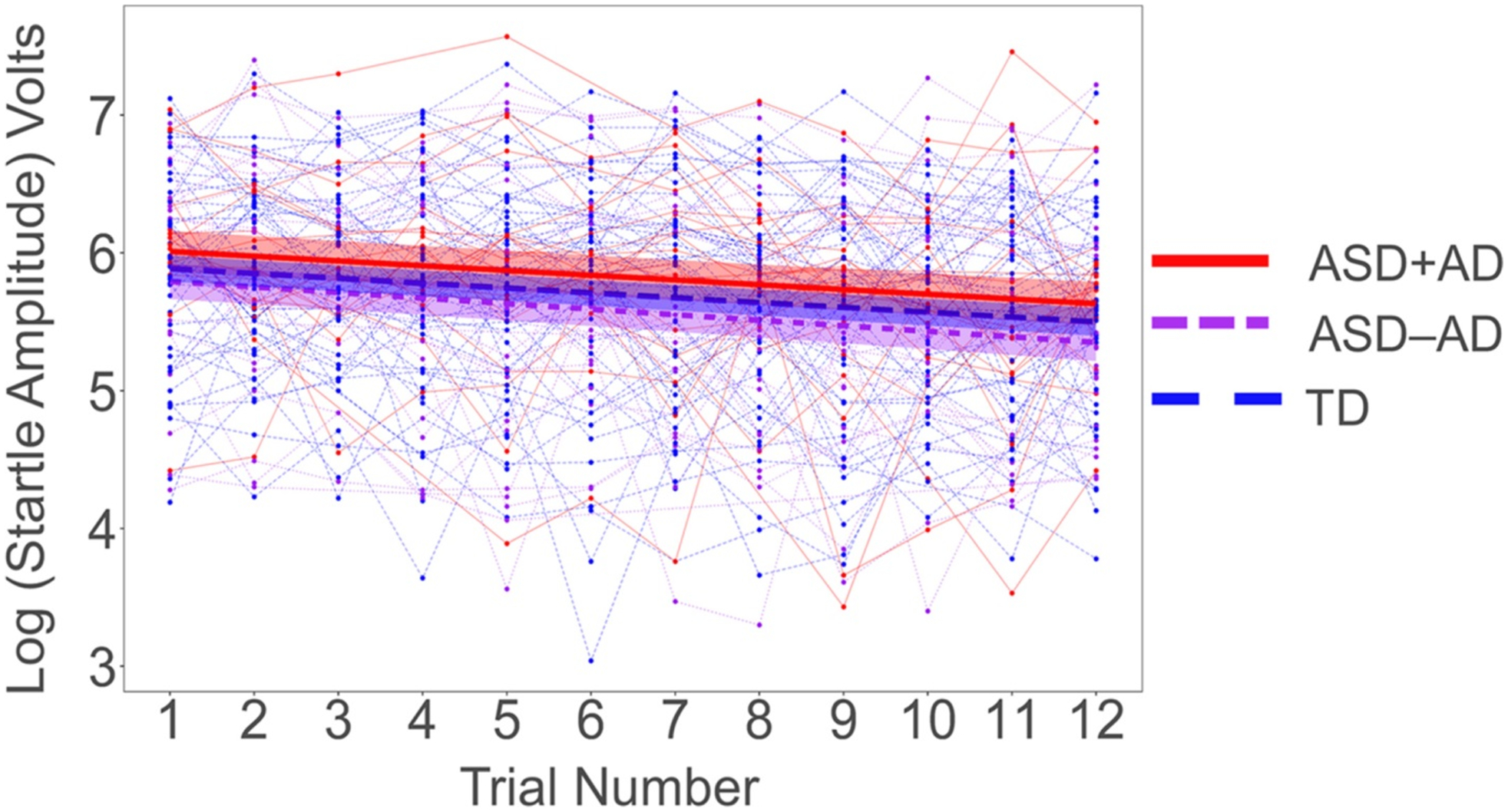
Startle amplitudes across the 12 startle stimulus (SS) trials during Habituation 1, by group. Data shown are individual cases, with mean levels shown by thicker solid and dashed lines for each group. Error bars show SEM.
Startle Latency
Startle latency distributions were generally normal and without skew or significant outliers, and therefore raw latencies were used for analysis. The 3 (group) by 2 (condition:” safe” vs.” threat”) repeated measures ANOVA with startle latency as the dependent variable yielded a main effect of condition [F(94) = 82.00, P <0.001, ηP2 = 0.47; the “threat” condition elicited a shorter latency startle response than the” safe” condition] and a significant main effect of group [F(2,94) = 4.46, P = 0.014, ηP2 = 0.09] but no significant group by condition interaction (P = 0.296; Fig. 4). Follow-up pair-wise group comparisons using Tukey’s HSD showed that the ASD + AD group had longer latencies than TD (P = 0.03) while the difference between ASD − AD and TD approached significance (P = 0.06).
Figure 4.
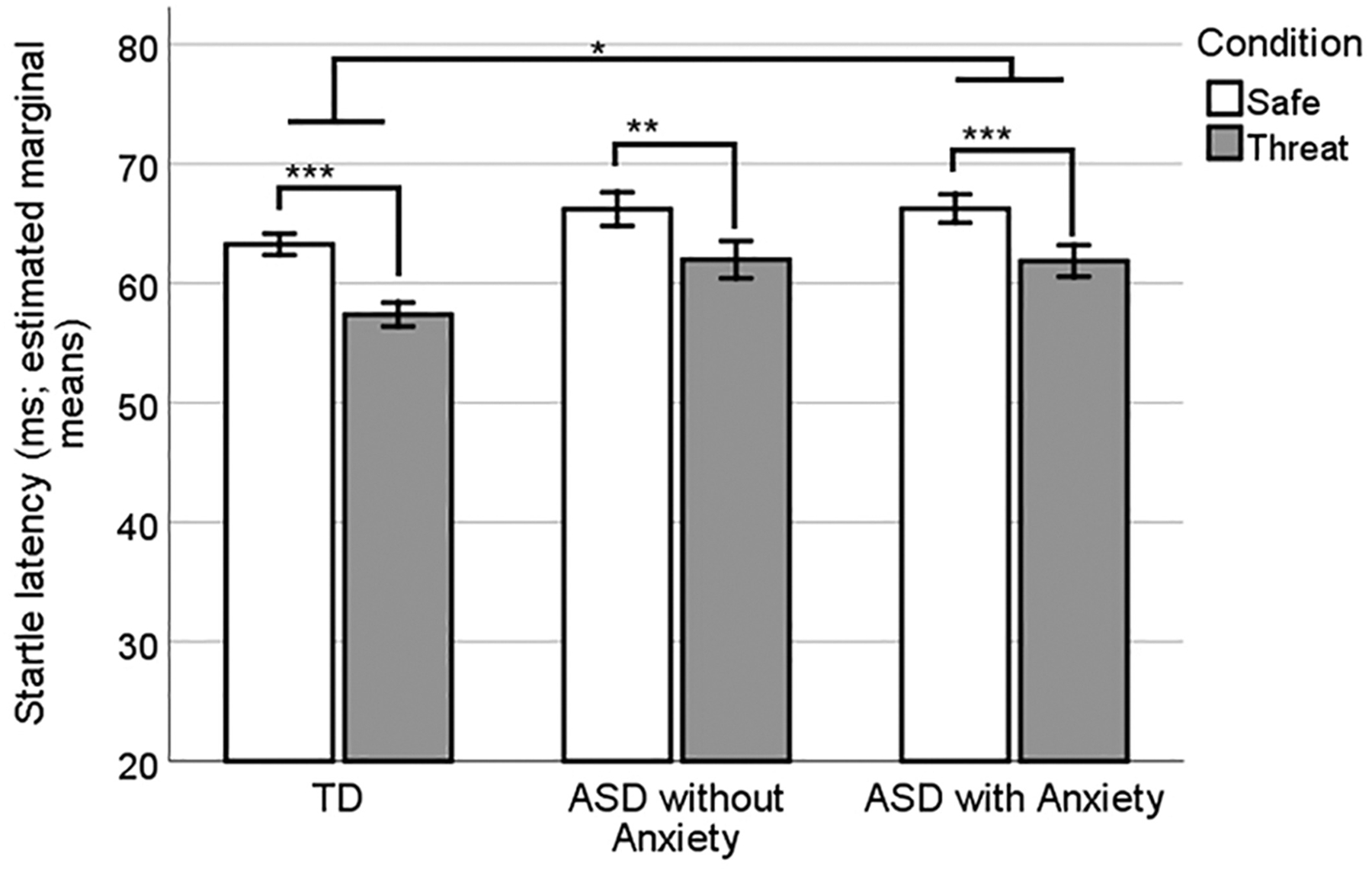
Startle latencies (ms) by group and condition. The threat condition produced significantly shorter latencies overall, and the ASD + AD group had significantly longer latencies than TD. Error bars show SEM.
Fear Potentiated Startle
A 3 (group) by 2 (condition:” safe” vs.” threat”) repeated measures ANOVA yielded a main effect of condition [F(94) = 72.75, P <0.001, ηP22 = 0.44] and no significant main effect of group (P = 0.35) or condition by group interaction (P = 0.15). (Inclusion of the dichotomous race variable as a covariate in the model did not change these results). Estimated marginal means of startle amplitude are shown in Fig. 5, with the expected main effect of condition demonstrating greater startle amplitude during “threat” versus” safe” trials. However, FPS was similar in the ASD and TD groups.
Figure 5.
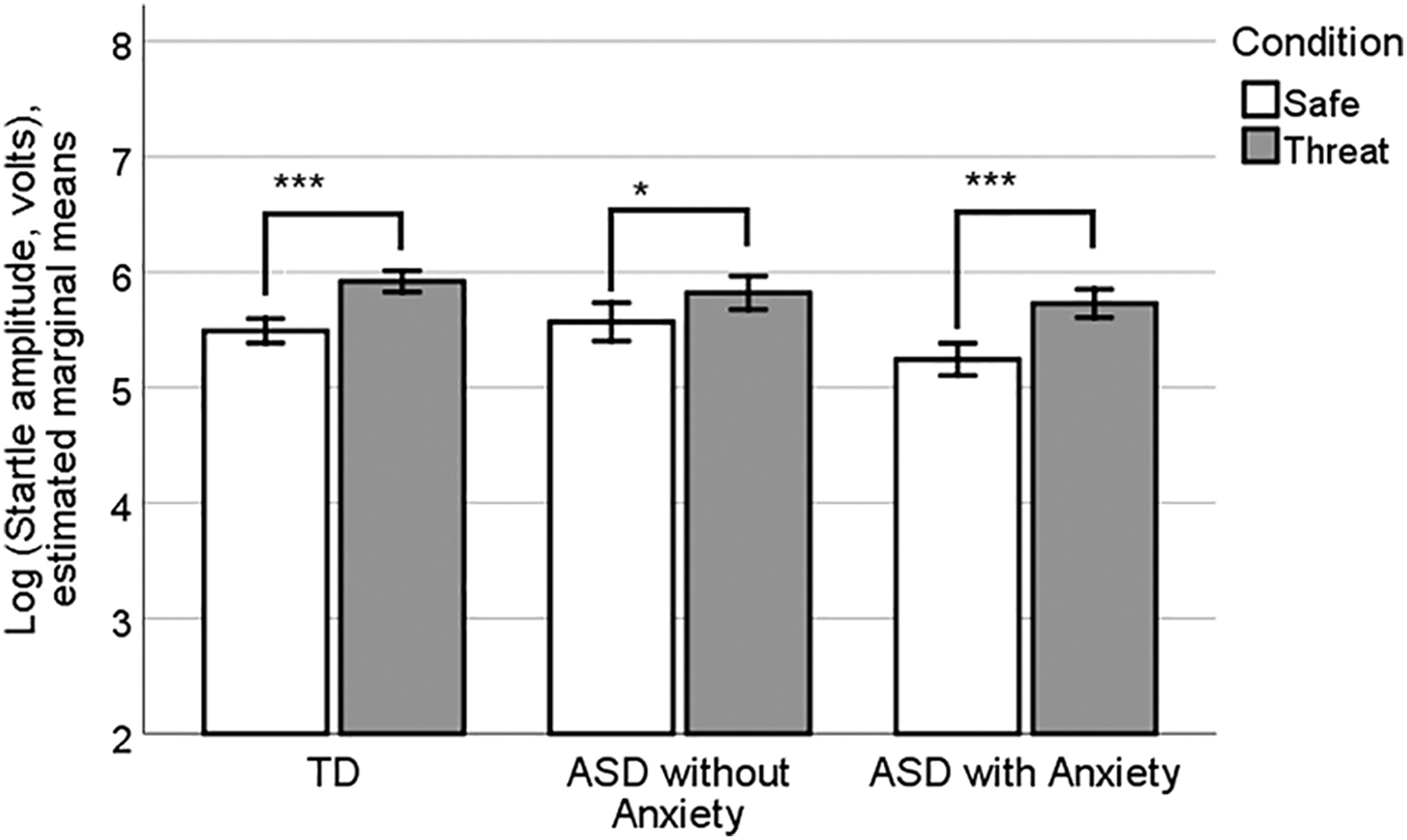
Startle amplitudes during the FPS testing phase. A significant main effect of condition demonstrated greater startle amplitudes during “threat” versus “safe” trials. However, there were no significant group or group-by-condition interactions.
Association Between FPS and Anxiety Severity
The ASD − AD and ASD + AD groups were combined for this analysis to capture a full range of severity because some participants in the ASD − AD group had subclinical anxiety and CSR scores. FPS was not significantly associated with overall (ADIS CSR, ρ = 0.204), traditional (DSM ADIS CSR, ρ = −0.12) or distinct (ADIS-ADA “autism distinct” CSR, ρ = 0.18) anxiety. As the TD group had no significant anxiety disorders and very limited range of CSR scores, correlations were not attempted for this group.
Amygdala Volume
Analysis of variance examining group differences (TD, ASD − AD, ASD + AD) in amygdala volume adjusted for TCV showed a significant main effect of hemisphere (right>left, F = 84.11, P <0.001, ηP2 = 0.52) and no main effect of group and no significant group by hemisphere interaction.
Association Between FPS and Amygdala Volume
Examination of scatterplots showing the association between FPS and amygdala volumes (adjusted for TCV) revealed three bivariate outliers in the ASD group and two in the TD group (Fig. 6). The individual trial startle responses underlying these FPS scores were re-reviewed and confirmed to be valid. Various arithmetic transformations did not achieve satisfactory distributions for Pearson correlations, so Spearman’s ρ was used. In the ASD group, FPS was significantly and negatively correlated with adjusted right amygdala volume (ρ = −0.461, P = 0.004, n = 38) while the correlation with adjusted left amygdala volume approached significance (ρ = −0.305, P = 0.062, n = 38). These correlations in the TD group were small and nonsignificant for the left and right amygdala (ρ = 0.038 and 0.121, respectively).
Figure 6.
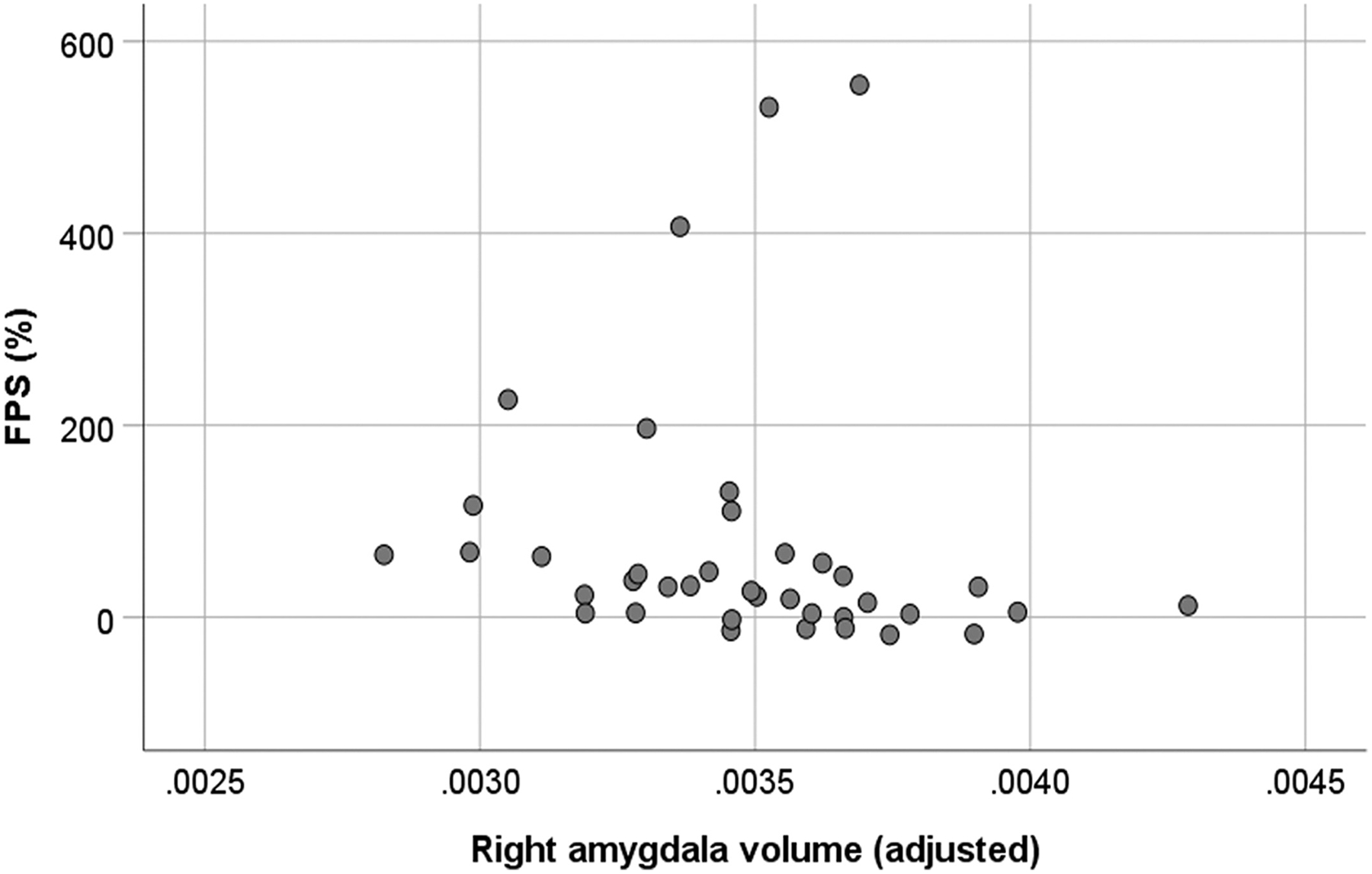
Scatterplot showing the significant negative association between right amygdala volume (adjusted for total brain volume) and fear potentiated startle (FPS) in participants with ASD (with and without anxiety disorder).
Amygdala Volume as a Moderator of the Association Between FPS and Anxiety Severity
The association between FPS (Z-scored) and anxiety severity (CSR scores) was significantly moderated by both left amygdala (b = 0.84, SE = 0.31, t = 2.76, P = 0.009) and right amygdala (b = 0.74, SE = 0.34, t = 2.14, P = 0.040), indicating that for each 1 SD increase in left amygdala volume the slope of the association between FPS and anxiety increased by +0.84 (ADIS CSR points per SDFPS). Similarly, for each 1 SD increase in right amygdala volume the slope increased by +0.74 (ADIS CSR points per SD FPS). These slopes were not significantly different from zero at the average volume of left or right amygdala (P’s ≥0.39). Assessing the interval of significance of the left amygdala moderation, FPS was significantly negatively associated when the left amygdala was −1.39 SD below the mean and significantly and positively associated when left amygdala was +1.38 SD. For the weaker right amygdala moderation, FPS was significantly negatively associated at −3.28 SD and positively associated at +4.02 SD right amygdala volume. Figure 7 depicts the predicted relations between FPS and ADIS CSR while moderated at three selected amygdala volumes: The average, and ± 1 SD from the average. Finally, similar results were observed when anxiety severity scores (CSR) were treated as an ordered factor and subjected to ordinal logistic regression, such that significant interactions between FPS and amygdala volume were observed for both left (χ2(1) = 8.06, P = 0.0045) and right (χ2(1) = 4.98, P = 0.026) amygdala, as assessed by a likelihood ratio test of logistic models with and without this interaction term. The moderation of the association between anxiety and FPS did not significantly differ for DSM and autism distinct forms of anxiety severity for either left (χ2(1) = 0.007, P = 0.94) or right (χ2(1) = 0.11, P = 0.74) amygdala.
Figure 7.
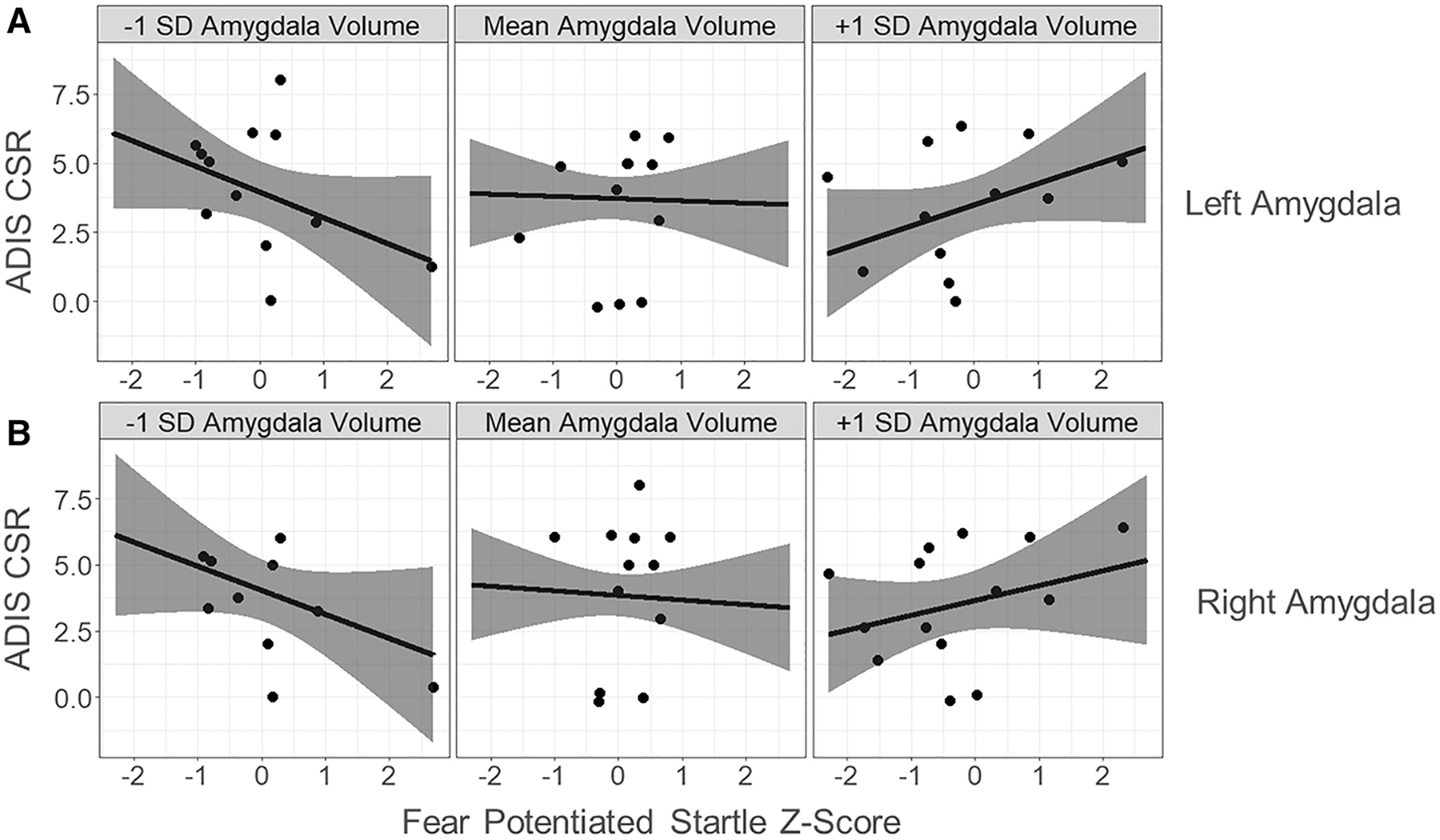
The association between fear potentiated startle (FPS) and anxiety severity, significantly moderated by amygdala volume. The figure depicts the moderating effect of amygdala volumes at −1 SD, the mean, and at +1 SD of (A) left amygdala volume, and b) right amygdala volume. For every 1 SD increase in amygdala volume the slope of the association between FPS and anxiety increased by +0.84 (ADIS CSR per SD FPS) for left amygdala and +0.74 (ADIS CSR per SD FPS) for right amygdala.
Discussion
There have been three previous studies which examined FPS in autism [Bernier et al., 2005; Chamberlain et al., 2013; Sterling et al., 2013]. The current study differed from these in several respects. First, the previous studies involved older individuals (Bernier et al., 12–45 years old; Sterling et al., 13–17.5 years and Chamberlain et al., 15–18 years) and the current study participants were 9–14 years old. Second, the participants in the previous studies all had normal IQ whereas the average IQ for participants in the current study was significantly lower in the ASD group than in TD group and many participants had co-occurring intellectual disability. Third, the numbers of ASD participants were smaller in the previous studies [Bernier et al., 2005; Chamberlain et al., 2013; Sterling et al., 2013] whereas the current study had usable FPS data from 48 individuals with ASD. Fourth, while anxiety was a variable of interest in two of the previous studies, different anxiety assessment tools were utilized (Sterling et al., Revised Children’s Manifest Anxiety Scale Second Edition, RCMAS-2; Chamberlain et al., Spence Children’s Anxiety Scale-Child Report, SCAS-C). These self-report or caregiver-report questionnaires have not been validated for use in individuals with ASD. By comparison, the current study used a semi-structured diagnostic interview, the ADIS, with the Autism Spectrum Addendum (ASA; [Kerns et al., 2017]) which is a series of prompts and guidelines that are woven into the ADIS protocol to tailor the instrument for children with ASD. Finally, the current study is the only one that has additional MRI data on the development of the amygdala that enabled exploratory analysis of the relationship between amygdala volume and FPS. Our hypothesis at the beginning of these studies was that FPS would be increased in our participants with ASD relative to those with TD and that there would be a positive relationship between the magnitude of FPS and the presence of anxiety as well as the volume of the amygdala. As is often the case in ASD research, the outcomes of the study were far more complicated than initially expected.
Consistent with all three previous studies, there was no difference in FPS between TD and ASD groups regardless of whether the individual had a diagnosis of anxiety or not. Moreover, we did not find that there was an association between level of anxiety and FPS. A similar conclusion was reached by Sterling et al. who also did not find any association between FPS and level of anxiety. There were subtle differences observed in ours and in the previous studies. We found, for example, that the ASD group had a longer latency to respond than the TD group. This was not found in the previous studies.
The current report extends prior work by evaluating the structure of the amygdala relative to FPS. The critical question addressed by the present study is whether comorbid anxiety disorder in children with ASD is driven by morphological or functional alterations of the amygdala, a region of the brain necessary for the conditioning of fear and anxiety [LeDoux & Pine, 2016]. Contrary to our initial hypothesis, we found that there was a negative association between amygdala volume (corrected for TCV) and FPS which was significant for the right amygdala and approaching significance for the left—higher FPS level was associated with a smaller amygdala! Interestingly, our results are consistent with the findings of Herrington et al. who studied children in the same age range and found that children with autism and anxiety had a smaller right amygdala relative to ASD participants without anxiety and TD controls. Earlier studies of non-autistic children and adolescents with anxiety suggested that DSM anxiety is associated with an enlarged amygdala [De Bellis et al., 2000]. However, this finding is based on the analysis of a small population of subjects. More recently, larger studies of children in the age range of the current study have shown that children who self-reported higher levels of anxiety have smaller amygdala volume [Warnell, Pecukonis, & Redcay, 2018].
Unlike previous studies, we examined different types of anxiety symptoms in relation to FPS and amygdala volume. We recently reported that while anxiety is found in 69% of the Autism Phenome Project cohort [Kerns et al., 2020], a substantial proportion of the group does not endorse signs of anxiety. Moreover, the types of anxiety observed are also quite heterogeneous, with some children experiencing anxiety in response to social stimuli, some in response to narrow specific situations or objects and others in the form of generalized anxiety and worry. Moreover, we have previously demonstrated that several different trajectories of amygdala growth can be identified in children with ASD [Nordahl et al., 2012]. It is perhaps more reasonable, therefore, to hypothesize that the relationship between FPS and anxiety would depend on the particular trajectory of amygdala development. This perspective prompted us to evaluate whether amygdala size may be a moderator of the relationship between FPS and anxiety.
By carrying out multiple regression analysis examining the moderating effect of amygdala volume (as a proportion of TCV), we have confirmed this hypothesis. In short, we found that in those children with ASD who had a larger amygdala, there was a positive association between the magnitude of FPS and the severity of anxiety. In contrast, in those children with ASD who had a smaller amygdala, there was a negative association between FPS and anxiety severity. This relationship was not observed for children with average size amygdalas, suggesting that the FPS response is not simply a physiological stand-in for anxiety measures. The explanation for these findings is not currently clear. FPS may only serve as an index into anxiety when individuals have an amygdala that is either much larger (or smaller) than average, potentially as a result of more serious or distinct developmental pathologies affecting the amygdala.
Limitations and implications
The present study had several important limitations. First, a relatively high proportion of children with ASD enrolled in the study were unable to complete the FPS protocol and provide valid data for analysis. These children were more likely to have lower intellectual functioning and this may place some limits on the generalizability of findings. The limited sample size may prevent adequate power for detecting group differences and correlations of interest. Similarly, the number of valid FPS protocol trials per condition was relatively low for some participants, which might affect FPS reliability and accuracy. Second, although the anxiety interviews were conducted by very experienced and reliable clinicians, the reports were entirely dependent on caregivers as a high proportion of children with ASD are unable to reliably report their symptoms. This limits access to the internal, lived experience of anxiety in this group. The reliance on external behaviors and emotional expressions may inadequately capture the full range of anxiety, and some unwanted behaviors could be misinterpreted as anxiety driven. Also, the ADIS CSR scores we used to measure anxiety severity do not provide a wide, granular range of scores across all domains of anxiety, which may have limited detection of associations of interest between anxiety severity and FPS or amygdala volume. Although new measures designed for ASD have been recently created, validated, and published (e.g., Parent-Rated Anxiety Scale for Youth with ASD; [Scahill et al., 2019]), the parent-report dimensional anxiety measures available at the time of this project’s design and initiation have poor sensitivity in children with ASD and ID [Kerns et al., 2020]. Third, FPS is a biobehavioral probe of amygdala activity—it is not a direct measure. Although the amygdala has been repeatedly shown to mediate fear conditioning and potentiation, the procedure in the current study involves only one type of startle-inducing stimulus (auditory) and one CS (tactile). There is likely to be considerable variability in how individuals respond to these stimuli and this may be especially true among those with ASD who are known to have atypical responses to sensory stimuli. Finally, MRI data were not available for all participants and as such the results examining associations between amygdala volume and the other study measures should be considered preliminary and require replication in separate samples to confirm the findings.
Conclusions
As with previously published studies, we did not observe group differences in FPS, providing further evidence that fear conditioning is not abnormal in children with ASD when considered as a group. Since more than half of the current participants had an anxiety disorder and it is well established that individuals with anxiety have increased potentiated startle [Grillon et al., 2008; Lissek, Biggs, et al., 2008; Lissek, Levenson, et al., 2008; Lissek et al., 2014], and even these participants did not show altered FPS, this is a surprising finding. Our data provide provocative indications that the relationship between the amygdala, anxiety and FPS may be linked to the heterogeneity of amygdala development in individuals with ASD. This link will need to be confirmed in larger, follow-up studies.
Acknowledgments
The authors are grateful to the many families who have participated in the Autism Phenome Project at the UC Davis MIND Institute. This study has been funded, in part, by NIH grants 1R01MH103371, P50HD093079, R01MH103284, R01MH104438, and U54HD079125.
References
- Adhikari A (2014). Distributed circuits underlying anxiety. Frontiers in Behavioral Neuroscience, 8, 112. 10.3389/fnbeh.2014.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, & Corbett BA (2003). The Amygdala, Autism and Anxiety. Novartis Foundation Symposia, Vol. 251, pp. 177–187; discussion 187–197, 281–197. [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). DSM-IV: Diagnostic and statistical manual. Washington, DC: American Psychiatric Association. [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, & Amaral DG (2007). Role of the Primate Amygdala in Fear-Potentiated Startle: Effects of Chronic Lesions in the Rhesus Monkey. The Journal of Neuroscience, 27(28), 7386–7396. 10.1523/jneurosci.5643-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, & Amaral DG (2009). The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry, 65(3), 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators, & Centers for Disease Control and Prevention. (2012). Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveillance Summaries, 61(3), 1–19. [PubMed] [Google Scholar]
- Aylward Minshew N, Goldstein G, Honeycutt N, Augustine A, Yates K, … Pearlson G (1999). MRI volumes of amygdala and hippocampus in non–mentally retarded autistic adolescents and adults. Neurology, 53(9), 2145–2145, 2150. [DOI] [PubMed] [Google Scholar]
- Bauman M, & Kemper TL (1985). Histoanatomic observations of the brain in early infantile autism. Neurology, 35(6), 866–874. [DOI] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Panagiotides H, & Webb S (2005). Individuals with autism spectrum disorder show normal responses to a fear potential startle paradigm. Journal of Autism and Developmental Disorders, 35(5), 575–583. [DOI] [PubMed] [Google Scholar]
- Bridger WH, & Mandel IJ (1967). The effects of dimethoxyphenylethylamine and mescaline on classical conditioning in rats as measured by the potentiated startle response. Life Sciences, 6(7), 775–781. [DOI] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, … Winter H (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics, 125(Suppl 1), S1–S18. [DOI] [PubMed] [Google Scholar]
- Campeau S, & Davis M (1995). Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience, 15(3), 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceritoglu C, Oishi K, Li X, Chou M-C, Younes L, Albert M, … Mori S (2009). Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. NeuroImage, 47(2), 618–627. 10.1016/j.neuroimage.2009.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain PD, Rodgers J, Crowley MJ, White SE, Freeston MH, & South M (2013). A potentiated startle study of uncertainty and contextual anxiety in adolescents diagnosed with autism spectrum disorder. Molecular Autism, 4(1), 31. 10.1186/2040-2392-4-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi CC (1965). The effect of amobarbital sodium on conditioned fear as measured by the potentiated startle response in rats. Psychopharmacology, 7(2), 115–122. [DOI] [PubMed] [Google Scholar]
- Davis M (1979). Diazepam and flurazepam: Effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology, 62(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Davis M, Redmond DE, & Baraban JM (1979). Noradrenergic agonists and antagonists: Effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology, 65(2), 111–118. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, … Ryan ND (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry, 48(1), 51–57. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, & Rutter M (1995). The pre-linguistic autism diagnostic observation schedule. Journal of Autism and Developmental Disorders, 25(4), 355–379. [DOI] [PubMed] [Google Scholar]
- El Achkar CM, & Spence SJ (2015). Clinical characteristics of children and young adults with co-occurring autism spectrum disorder and epilepsy. Epilepsy & Behavior, 47, 183–190. 10.1016/j.yebeh.2014.12.022 [DOI] [PubMed] [Google Scholar]
- Elliott CD (2007). Differential ability scales (2nd ed.). New York: The Psychological Corporation. [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(5), 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, & Davis M (1993). Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry, 33(8), 566–574. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, & Davis M (1991). Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology, 28(5), 588–595. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, & Levine J (2006). The Benzodiazepine Alprazolam Dissociates Contextual Fear from Cued Fear in Humans as Assessed by Fear-potentiated Startle. Biological Psychiatry, 60 (7), 760–766. 10.1016/j.biopsych.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, & Pine DS (2008). Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. The American Journal of Psychiatry, 165(7), 898–904. 10.1176/appi.ajp.2007.07101581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, O’Connell K, Lieberman L, Alvarez G, Geraci M, Pine DS, & Ernst M (2016). Distinct responses to predictable and unpredictable threat in anxiety pathologies: Effect of panic attack. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(7), 575–581. 10.1016/j.bpsc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Greenwald MK, Bradley MM, Cuthbert BN, & Lang PJ (1991). The fear potentiated startle effect. Integrative Physiological and Behavioral Science, 26(2), 119–126. [DOI] [PubMed] [Google Scholar]
- Heldt S, Sundin V, Willott JF, & Falls WA (2000). Post-training lesions of the amygdala interfere with fear-potentiated startle to both visual and auditory conditioned stimuli in C57BL/6J mice. Behavioral Neuroscience, 114(4), 749–759. [PubMed] [Google Scholar]
- Herrington JD, Maddox BB, Kerns CM, Rump K, Worley JA, Bush JC, … Miller JS (2017). Amygdala volume differences in autism spectrum disorder are related to anxiety. Journal of Autism and Developmental Disorders, 47 (12), 3682–3691. 10.1007/s10803-017-3206-1 [DOI] [PubMed] [Google Scholar]
- Hitchcock J, & Davis M (1986). Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience, 100(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Hoffman L (2015). Longitudinal analysis: Modeling within-person fluctuation and change. New York: Taylor & Francis Group. [Google Scholar]
- Jitlina K, Zumbo B, Mirenda P, Ford L, Bennett T, Georgiades S, … Duku E (2017). Psychometric properties of the spence children’s anxiety scale: Parent report in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(12), 3847–3856. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, & Duncan EJ (2005). Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry, 57(12), 1559–1564. [DOI] [PubMed] [Google Scholar]
- Kazama AM, Heuer E, Davis M, & Bachevalier J (2012). Effects of neonatal amygdala lesions on fear learning, conditioned inhibition, and extinction in adult macaques. Behavioral Neuroscience, 126(3), 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, & Kendall PC (2012). The presentation and classification of anxiety in autism spectrum disorder. Clinical Psychology: Science and Practice, 19(4), 323–347. [Google Scholar]
- Kerns CM, Kendall PC, Berry L, Souders MC, Franklin ME, Schultz RT, … Herrington J (2014). Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(11), 2851–2861. 10.1007/s10803-014-2141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Renno P, Kendall PC, Wood JJ, & Storch EA (2017). Anxiety disorders interview schedule-autism addendum: Reliability and validity in children with autism spectrum disorder. Journal of Clinical Child and Adolescent Psychology, 46(1), 88–100. 10.1080/15374416.2016.1233501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Winder-Patel B, Iosif AM, Nordahl CW, Heath B, Solomon M, & Amaral DG (2020). Clinically significant anxiety in children with autism spectrum disorder and varied intellectual functioning. Journal of Clinical Child and Adolescent Psychology, 1–16. 10.1080/15374416.2019.1703712. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely B, Migdal TR, Vettam S, & Adesman A (2016). Prevalence and correlates of elopement in a nationally representative sample of children with developmental disabilities in the United States. PLoS One, 11(2), e0148337. 10.1371/journal.pone.0148337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using Neuroscience to help understand fear and anxiety: A two-system framework. The American Journal of Psychiatry, 173(11), 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Lee Y, & Davis M (1997). Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience, 17(16), 6434–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Stevens ES, Funkhouser CJ, Weinberg A, Sarapas C, Huggins AA, & Shankman SA (2017). How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. International Journal of Psychophysiology, 114, 24–30. 10.1016/j.ijpsycho.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, & Grillon C (2008). Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy, 46(5), 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, & Grillon C (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75(11), 909–915. 10.1016/j.biopsych.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, & Grillon C (2008). Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. The American Journal of Psychiatry, 165(1), 124–132. 10.1176/appi.ajp.2007.06091513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, & DiLavore PC (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. 10.1023/a:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Couteur A (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. 10.1007/bf02172145 [DOI] [PubMed] [Google Scholar]
- Lyneham HJ, Abbott MJ, & Rapee RM (2007). Interrater reliability of the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent version. Journal of the American Academy of Child and Adolescent Psychiatry, 46(6), 731–736. 10.1097/chi.0b013e3180465a09 [DOI] [PubMed] [Google Scholar]
- Marazziti D, Abelli M, Baroni S, Carpita B, Ramacciotti CE, & Dell’Osso L (2015). Neurobiological correlates of social anxiety disorder: An update. CNS Spectrums, 20(2), 100–111. 10.1017/S109285291400008X [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Kao J, & Oswald DP (2011). Preliminary evidence suggesting caution in the use of psychiatric self-report measures with adolescents with high-functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 5 (1), 164–174. 10.1016/j.rasd.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wu D, Ceritoglu C, Li Y, Kolasny A, Vaillant MA, … Miller MI (2016). MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Computing in Science & Engineering, 18(5), 21–35. [Google Scholar]
- Nordahl CW, Mello M, Shen AM, Shen MD, Vismara LA, Li D, … Rogers S (2016). Methods for acquiring MRI data in children with autism spectrum disorder and intellectual impairment without the use of sedation. Journal of Neurodevelopmental Disorders, 8(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, & Amaral DG (2012). Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: A longitudinal study. Archives of General Psychiatry, 69(1), 53–61. 10.1001/archgenpsychiatry.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, & Duncan EJ (2006). Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory, 13(6), 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LE (1961). Conditioned fear as a function of CS-UCS and probe stimulus intervals. Journal of Experimental Psychology, 61(4), 265–273. [PubMed] [Google Scholar]
- Scahill L, Lecavalier L, Schultz RT, Evans AN, Maddox B, Pritchett J, … Edwards MC (2019). Development of the parent-rated anxiety scale for youth with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 58(9), 887–896 e882. 10.1016/j.jaac.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Shen MD, Li DD, Keown CL, Lee A, Johnson RT, Angkustsiri K, … Nordahl CW (2016). Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Albano AM, Siebelink B, & Treffers PDA (2001). ADIS-C: Anxiety disorders interview schedule for DSM-IV-child version. Lisse: Swets Test Publishers. [Google Scholar]
- Silverman WK, Saavedra LM, & Pina AA (2001). Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent versions. Journal of the American Academy of Child and Adolescent Psychiatry, 40(8), 937–944. 10.1097/00004583-200108000-00016 [DOI] [PubMed] [Google Scholar]
- Spence K, & Runquist W (1958). Temporal effects of conditioned fear on the eyelid reflex. Journal of Experimental Psychology, 55(6), 613–616. [DOI] [PubMed] [Google Scholar]
- Sterling L, Munson J, Estes A, Murias M, Webb SJ, King B, & Dawson G (2013). Fear-potentiated startle response is unrelated to social or emotional functioning in adolescents with autism spectrum disorders. Autism Research, 6(5), 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, & Whalen PJ (2015). Neuroimaging and anxiety: The neural substrates of pathological and non-pathological anxiety. Current Psychiatry Reports, 17(6), 49. 10.1007/s11920-015-0586-9 [DOI] [PubMed] [Google Scholar]
- The Research Units On Pediatric Psychopharmacology Anxiety Study Group. (2002). The Pediatric Anxiety Rating Scale (PARS): Development and Psychometric Properties. Journal of the American Academy of Child and Adolescent Psychiatry, 41(9), 1061–1069. 10.1097/00004583-200209000-00006 [DOI] [PubMed] [Google Scholar]
- Wang H, & Yushkevich PA (2013). Multi-atlas segmentation with joint label fusion and corrective learning-an open source implementation. Frontiers in Neuroinformatics, 7, 27. 10.3389/fninf.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnell KR, Pecukonis M, & Redcay E (2018). Developmental relations between amygdala volume and anxiety traits: Effects of informant, sex, and age. Development and Psycho-pathology, 30(4), 1503–1515. 10.1017/S0954579417001626 [DOI] [PubMed] [Google Scholar]


