Abstract
The placenta of hornworts is unique among bryophytes in the restriction of transfer cells that are characterized by elaborate wall labyrinths to the gametophyte generation. During development, cells around the periphery of the sporophyte foot elongate, forming smooth-walled haustorial cells that interdigitate with gametophyte cells. Using immunogold labeling with 22 antibodies to diverse cell wall polymers, we examined compositional differences in the developmentally and morphologically distinct cell walls of gametophyte transfer cells and sporophyte haustorial cells in the placenta of Phaeoceros. As detected by Calcofluor White fluorescence, cellulose forms the cell wall scaffolding in cells on both sides of the placenta. Homogalacturonan (HG) and rhamnogalacturonan I (RG-I) pectins are abundant in both cell types, and haustrorial cells are further enriched in methyl-esterified HGs. The abundance of pectins in placental cell walls is consistent with the postulated roles of these polymers in cell wall porosity and in maintaining an acidic apoplastic pH favorable to solute transport. Xyloglucan hemicellulose, but not mannans or glucuronoxylans, are present in cell walls at the interface between the two generations with a lower density in gametophytic wall ingrowths. Arabinogalactan proteins (AGPs) are diverse along the plasmalemma of placental cells and are absent in surrounding cells in both generations. AGPs in placental cell walls may play a role in calcium binding and release associated with signal transduction as has been speculated for these glycoproteins in other plants. Callose is restricted to thin areas in cell walls of gametophyte transfer cells. In contrast to studies of transfer cells in other systems, no reaction to the JIM12 antibody against extensin was observed in Phaeoceros.
Keywords: AGPs, Bryophytes, Hemicelluloses, Immunocytochemistry, Pectins, Transfer cells, Wall ingrowths
Introduction
Although the hornwort sporophyte is capable of carbon assimilation sufficient for maintenance, carbon uptake for continued growth requires transport of sugar and nutrients from the gametophyte through the placenta, a specialized tissue at the interface between the two generations (Thomas et al. 1978). The hornwort sporophyte elongates from a persistent basal meristem and produces and disperses spores for an extended timespan. Consequently, the placenta is persistent and active throughout the growing season. The sporophyte develops a bulbous foot before emerging from the gametophyte involucre and initiating spore production. During development, cells at the foot periphery elongate to form smooth-walled haustorial cells that penetrate the adjoining gametophyte tissue (Ligrone et al. 1993, Ligrone & Renzaglia 1990, Gambardella & Ligrone 1987). Concomitant with the intrusive growth of haustorial cells, gametophyte cells develop elaborate cell wall labyrinths that are diagnostic of transfer cells and vastly increase the surface area of the plasmalemma (Gambardella & Ligrone 1989, Gambardella & Ligrone 1987, Ligrone & Renzaglia 1990, Ligrone et al. 1993). Transfer cells are widespread across land plants where they are found in strategic locations and facilitate enhanced membrane-mediated nutrient transport from symplast to apoplast or vice versa (Pate & Gunning 1972, Offler et al. 2002, Thompson et al. 2001, Regmi et al. 2017). In the hornwort placenta, transfer cells are restricted to the gametophyte generation, but are highly variable in location in the placenta of mosses and liverworts (Villarreal & Renzaglia 2006, Vaughn & Hasegawa 1993, Ligrone & Renzaglia 1990, Ligrone et al. 1993, Browning & Gunning 1979, Renzaglia 1978).
The degree of integration of placental cells varies across hornwort taxa. In Phaeoceros and most other genera, gametophytic transfer cells and sporophytic haustorial cells are interdigitated with random intergenerational spaces between. Placental spaces in some genera including Phaeoceros contain prominent crystals of proteinaceous material (Gambardella & Ligrone 1987). On the other extreme, exemplified by Anthoceros, the foot contains an orderly palisade of peripheral cells and a zone of crystal-free mucilage separates the foot from transfer cells on the gametophyte side (Frangedakis et al. 2020, Renzaglia 1978, Ligrone et al. 1993).
A key to understanding the complexity of biochemical and cellular processes at the gametophyte-sporophyte junction lies in the composition and organization of the apoplastic interface between the generations. In this zone, the composition and arrangement of constituent cell wall polymers largely prescribe the transport of materials and cellular interactions between generations (Humphrey et al. 2007). In the liverwort Marchantia, which has well-developed transfer cells in both generations, differential expression of cell wall polymers is consistent with gametophyte-to-sporophyte unidirectional movement of materials (Henry et al. 2020). Whereas pectins are widespread on both sides of the placenta in this liverwort, hemicelluloses (xyloglucans) and arabinogalactan proteins (AGPs) show restricted occurrences. Notably, sporophyte transfer cell walls are relatively poor in cellulose and enriched with xyloglucans and a diversity of AGPs not found in the gametophyte counterpart, a finding that is consistent with directional movement. With sparse data on cell wall composition in the placenta of other taxa and transfer cells in general, it is not clear whether there is a common cell wall configuration that supports the function of placental cells. The organization of the placenta of Phaeoceros provides a platform on which to explore the variability of wall composition in morphologically distinct cells that co-operate in unidirectional transport.
In this second study of placental cell walls in bryophytes, we probed two species of Phaeoceros, P. carolinianus (Mischx.) Prosk. from the US and P. laevis (L.) Prosk. from Italy, using immunogold labeling at the TEM level with 22 monoclonal antibodies to cell wall polysaccharides and AGPs. Because they are closely related genetically, these morphologically similar species are recognized by some as subspecies of the genus P. laevis sensu latu (Duff et al. 2007, Bisang et al. 2010). The two cell types of interest in this study of the hornwort placenta are haustorial cells and intermingling gametophyte cells with transfer cell morphology. Focusing on this system, we addressed the following questions: What are the compositional differences and/or commonalities between the labyrinthine cell walls in gametophyte transfer cells and the smooth walls in sporophyte haustorial cells? How does polymer composition in these cells compare with transfer cell wall composition in other systems?
Materials and Methods
Fertile plants of Phaeoceros carolinianus from Makanda, IL and Phaeoceros laevis from Campania, Italy were collected and processed immediately for light and electron microscopy. Histochemistry and immunolabeling were conducted on fully developed placentae with mature cells and developed cell walls.
Preparation for transmission electron microscopy
Plants were prepared for TEM observation using the standard fixation protocol outlined in Renzaglia (2017). Excised portions of gametophytic tissue with embedded feet were fixed in 2.5% v/v glutaraldehyde in 0.05 M Sorenson’s buffer (pH 7.2) for 1 h at room temperature and overnight at 4 °C. Following 2–3 rinses in the same buffer for 15 min each, the samples were post-fixed in 2% buffered osmium tetroxide for 30 min and rinsed in autoclaved, distilled water. The samples were dehydrated in progressively higher ethanol to water concentrations and rinsed twice in 100% ethanol. Infiltration was achieved by progressive placement of material in higher concentrations of LR White resin diluted with ethanol. Once the samples reached 100% LR White, they were placed in gel capsules and heated in an oven at 60 °C for 48 h. The samples were sectioned on an ultramicrotome until the placenta was located. Thin sections (90–100 nm) were collected on 200 mesh nickel grids for immunolabeling.
Thick sections (800 to 1500 nm) were collected on glass slides and stained with 1% toluidine blue in 1% sodium tetraborate to assess the stage of development and the quality of the tissue for histochemical staining and immunogold labeling. Only sporophytes with fully developed feet and good structural preservation were probed further as follows.
Histochemical staining
Cellulose was detected by incubating sections on glass slides for 1 h in a drop of Calcofluor White (Sigma-Aldrich) and a drop of 10% KOH in the dark. Controls were made using only 10% KOH solution. Materials were viewed under a Leica DM5000 B compound microscope using UV fluorescence.
For silver intensification of immunohistochemical tests, 0.5 μm-thick sections were mounted on glass slides coated with BioBond (EMS, USA) to enhance sticking. The slides were placed in a chamber with high relative humidity for the following incubation steps: 1) 30 min in 1% (w/v) bovine serum albumin (BSA) in phosphate-buffered saline (PBS); 2) 4 h in primary antibodies diluted 1:40 with PBS-BSA; 3) three 30 min exchanges of PBS-BSA; gold-conjugated goat-anti-rabbit secondary antibody (BB International, UK) diluted 1:20 in PBS-BSA; 4) three washes of PBS. After a rinse with double-distilled water, the slides were incubated in a freshly prepared solution from the Amersham InstenSe (Amersham Bioscience, UK) silver enhancement kit, which reacts with gold particles and makes them visible under the light microscope as a fine black precipitate. After the silver developed (30 min), slides were washed with distilled water, dried and coverslip mounted with Permount (Polysciences). Serial sections were stained for 1 min with 1% toluidine blue in 1% sodium tetraborate for general histology. The sections were photographed using a Zeiss Axioskop microscope (Carlzeiss, Oberkochen, Germany).
Immunogold labeling
Samples were processed as detailed in Lopez et al. (2017) and Renzaglia et al. (2017). In short, thin sections on nickel grids were floated on drops of BSA/PBS overnight at 4 °C, and then overnight on a primary antibody specific to the desired wall epitope. The grids were then rinsed 4 × 4 min each in 0.05 M BSA/PBS, treated with a secondary antibody with a gold tag attached for 30 min at room temperature, rinsed again in PBS 4 × 4 min each, and then with a jet of sterile H2O.
Grids were observed before and after post-staining with lead citrate and uranyl acetate (these stains allow for better contrast but may obscure the immuno-gold labels in the transmission electron microscope). Control grids were prepared by excluding the primary antibodies. Samples were viewed and digital micrographs were collected with a Hitachi H7650 transmission electron microscope. The monoclonal antibodies used in this study are listed in Table 1.
Table 1.
Primary antibodies used to immunogold label carbohydrates and arabinogalactan proteins in placental cell walls of Phaeoceros.
| Antibody | Antigen (s)/ Epitope | Reference/ Source |
|---|---|---|
| CCRCM38 | homogalacturonan (HG) | Pattathil 2010. Souce: Carbosource Service, University of Georgia |
| JIM5 | Homogalacturonan/ Un-esterified | Knox et al., 1990/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM19 | Homogalacturonan/ Un-esterified | Verhertbruggen et al., 2009a/ J. P. Knox PlantProbes, University of Leeds, UK |
| JIM7 | Homogalacturonan/Partial Methyl-esterified | Willats et al., 2000/ M. Hahn, Complex Carbohydrate Research Center, University of Georgia, USA |
| LM18 | Homogalacturonan/ Methyl-esterified | Verhertbruggen et al.,2009/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM20 | Homogalacturonan/ Methyl-esterified | Verhertbruggen et al., 2009a/ J. P. Knox PlantProbes, University of Leeds, UK |
| CCRCM36 | Rhamnogalacturonan-I | Carbosource Service, University of Georgia |
| CCRCM2 | Rhamnogalacturonan-I MeBSA complex | Pattathil 2010. Souce: Carbosource Service, University of Georgia |
| LM5 | Galactan, rhamnogalacturonan-I/ (1–4)-β-d-galactan | Jones et al., 1997/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM16 | galactosyl residues on rhamnogalacturonan I (RGI) | Vehrertbruggen et al. 2009. Source: PlantProbes, Leeds |
| LM15 | XXXG motif of xyloglucan | Marcus et al., 2008/ J. P. Knox PlantProbes University of Leeds, UK |
| LM21 | Mannan/ β-(1,4)-manno-oligosaccharide | Marcus et al., 2010/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM25 | Galactoxylated xyloglucans | Pedersen et al., 2012/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM28 | Glucuronoxylan | Cornuault et al., 2015/ J. P. Knox PlantProbes, University of Leeds, UK |
| CCRCM1 | Xyloglucan alpha-Fuc-(1,2)-beta-Gal | Pattathil 2010. Souce: Carbosource Service, University of Georgia |
| CCRCM7 | Rhamnogalacturonan I trimer or larger of beta-(1,6)-Gal carrying one or more Ara residues of unknown linkage | Pattathil 2010. Souce: Carbosource Service, University of Georgia |
| JIM8 | Arabinogalactan protein (AGP)/ unknown | Pennell et al., 1991/ J. P. Knox PlantProbes, University of Leeds, UK |
| JIM13 | Arabinogalactan protein (AGP)/ β-d-GlcA-(1,3)-α-d-GalpA-(1,2)-l-Rha (glucuronic acid-galacturonic acid-rhamnose) | Yates et al., 1996/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM2 | Arabinogalactan protein (AGP)/ β-d-GlcA (glucuronic acid) | Smallwood et al., 1996/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM6 | Arabinan, rhamnogalacturonan-I/ (1–5)-α-L-arabinan, also labels arabinogalactan protein | Willats et al., 1998; Verhertbruggen et al., 2009b/ J. P. Knox PlantProbes, University of Leeds, UK |
| JIM12 | Extensin | Smallwood et al., 1994/ J. P. Knox PlantProbes, University of Leeds, UK |
| Anticallose | Callose/ (1,3)-β-linked penta-to-hexa-glucan | Meikle et al., 1991/ Biosupplies Australia |
Quantification of label abundance
Images were analyzed in PhotoScapeX (Mooii Tech) by counting the number of labels in three randomly placed 100 Å~ 100 pixel frames in the cell wall of interest. This process was repeated on 10–15 images for each MAb. The average of all counts was calculated and scored as follows: averages of 1 to 4 labels per frame were assigned a single plus (+), and two pluses (+ +) were assigned to averages between 5 and 9 labels. Any average greater than 9 labels per frame received a triple plus (+ + +). Antibodies with average label density between 0 and 1 were assigned a plus/ minus (+/−).
Results
The placenta of Phaeoceros consists of peripheral foot cells that lack wall ingrowths and elongate to form haustorial cells that interdigitate with gametophyte transfer cells (Fig. 1). Haustorial cells develop early in embryology and penetrate the gametophytic tissue, which in response initiates cell division followed by the development of cell wall ingrowths (Fig. 1A). Gametophyte cells in the fully developed placenta display extensive wall labyrinths that vastly increase the surface area of the cell membrane (Fig. 1B). The wall ingrowths consist of a peripheral electron-lucent area and an inner fibrous core that is continuous with the basal primary wall (Fig. 1B). Haustorial cells have thin walls throughout (Fig.1B–D). The placenta includes a network of intergenerational spaces locally expanding into wide lacunae, which originate from the separation of gametophyte cells and intrusive growth of haustorial cells. The intergenerational zone contains loose fibrillar/granular material and protein crystals of varying size in P. laevis but not P. carolinianus (Fig. 1C). Calcofluor white staining demonstrates that cellulose is a key constituent of placental cell walls including the smooth wall of haustorial cells and the wall labyrinth apparatus of gametophyte cells (Fig. 1D).
Figure 1.
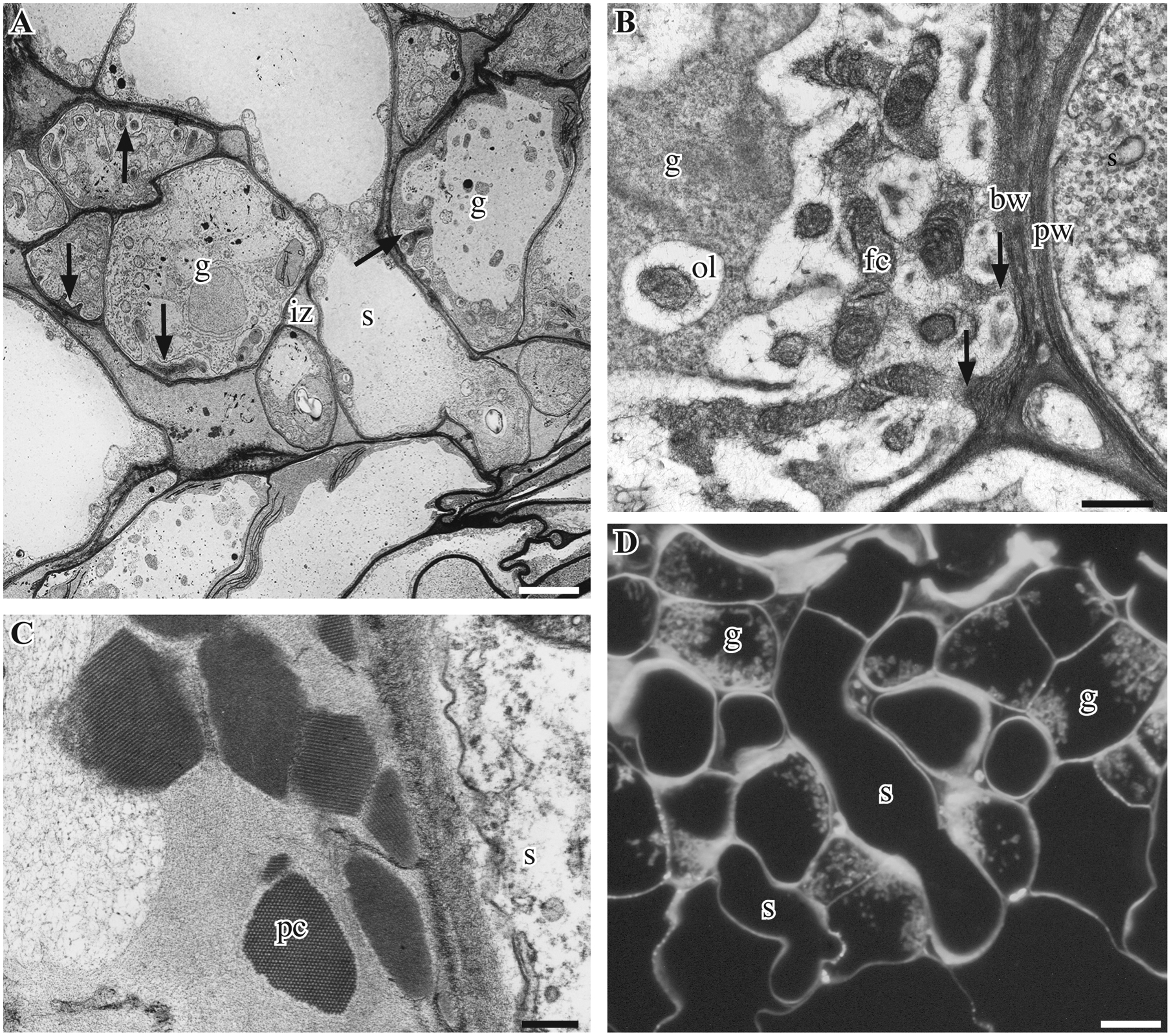
Sporophyte-gametophyte junction in Phaeoceros. A. Transmission electron micrograph (TEM) of developing placenta in P. laevis showing an elongated sporophyte haustorial cell (s) penetrating differentiating gametophytic (g) transfer cells with nascent wall ingrowth (arrows) separated by an intergenerational zone (iz). B. P. carolinianus showing smooth thin primary cell wall (pw) of a sporophyte haustorial cell (s) adjacent to a gametophyte cell (g) with extensive wall ingrowths that are continuous with the basal wall (arrows) and are composed of an electron-lucent outer layer (ol) and fibrous inner core (fc). C. P. laevis. TEM showing sporophyte (s) haustorial cell and adjacent protein crystals (pc) found in the granular to fibrous material between generations. D. P. carolinianus. Calcofluor white fluorescence showing cellulose in cell walls of elongated haustorial sporophyte (s) cells, and in basal wall and extensive wall ingrowths of intermingled gametophyte cells (g) in thick section. Scale Bars: 0.5 μm (A), 2.0 μm (B), 0.25 μm (C), 0.2 μm (D)
In general, the haustorial cells and the fibrous core of wall ingrowths are enriched in pectins relative to neighboring non-placental tissues (Fig. 2). Labelling with monoclonal antibodies targeted to un-esterified HG epitopes (CCRC-M38, JIM5, LM19) gives a positive reaction in placental cells (Fig. 2A–G). Silver enhancement of sections incubated in CCRC-M38 shows even labelling of both gametophyte and sporophyte cells as well as of intercellular spaces (Fig. 2A, B). Immuno-silver labeling with JIM5 demonstrates the presence of un-esterified HGs in all placental cells as well as in adjacent parenchyma cells on both sides of the placenta (Fig. 2C). TEM imaging of CCRC-M38 labeling shows a strong reaction in the core of wall ingrowths (Fig. 2D), the haustorial cell walls and the fibrillar material in intercellular spaces (Fig. 2E). Un-esterified HG epitopes targeted by JIM5 are also aggregated along haustorial cell walls and concentrated in the cores of wall ingrowths (Fig. 2F). LM19 (targeting un-esterified HG) abundantly labels haustorial cell walls and also produces scattered labelling of wall ingrowth cores (Fig. 2G). Placental cells also react with antibodies against methyl-esterified HG pectins (LM18, JIM7, LM20) (Fig. 2H–K). LM18 labels the haustorial cell walls throughout (Fig. 2H) and the wall ingrowth cores (Fig. 2I). JIM7 gives a similar labeling of haustorial cells as LM18, but produces only a sparse labeling of wall ingrowths (Fig. 2J). The LM20 epitope is scattered and restricted to sporophyte cells (Fig. 2K).
Figure 2.
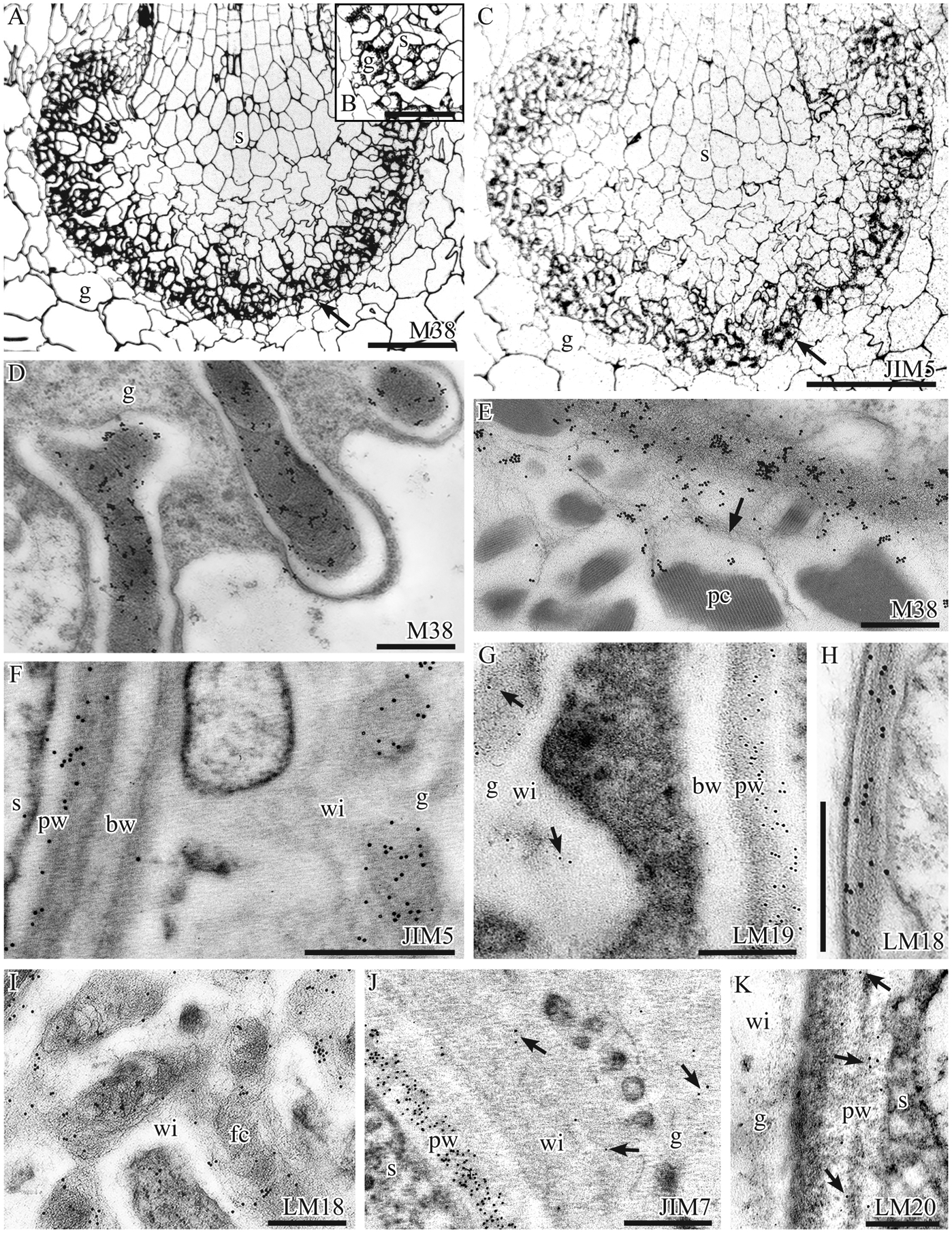
Immunolabeling with monoclonal antibodies to pectin epitopes. A-C P. laevis silver-enhanced immunogold labeling light micrographs. A. CCRC-M38 labels all cell walls in sporophyte (s) and gametophyte (g) and is particularly intense in the placenta (arrow). B. Higher magnification of CCRC-M38 labeling showing silver in haustorial cells of the sporophyte (s), the wall labyrinth in gametophyte (g) cells and intergenerational zone. C. JIM5 labels gametophyte (g) and sporophyte (s) cells with particular intensity in the wall ingrowths of gametophyte cells (arrow). D-K Immunogold labeling TEM micrographs. D, E. P. laevis. CCRC-M38 labelling yields abundant aggregates of gold particles in placental walls. (D) Fibrous core of gametophyte wall ingrowths, (E) Haustorial cell wall and associated fibrous material (arrow) around protein crystals (pc) in the intergenerational zone. F. P. carolinianus. JIM5 labels inner primary wall (pw) of sporophyte (s) haustorial cells and the fibrous core of gametophyte (g) wall ingrowths (wi), but not the basal wall (bw). G. P. carolinianus. LM19 abundantly labels the sporophyte (s) primary wall (pw) but in the gametophyte (g), labeling is sparse (arrows) in the electron dense region of wall ingrowths (wi) and absent in the basal wall (bw). H, I. P. laevis. Strong labeling with LM18. H. Sporophyte haustorial cell wall. I. Fibrous core (fc) of gametophyte wall ingrowths (wi). J. P. carolinianus. JIM7 labels throughout the sporophyte (s) primary cell wall (pw) but is sparse (arrows) in gametophyte (g) wall ingrowths (wi). K. P. carolinianus. LM20 lightly labels the sporophyte (s) primary wall (pw) (arrows) but does not label gametophyte (g) wall ingrowths (wi). Scale bars: 200 μm (A, C), 100 μm (B), 0.1 μm (D, E, G, I, J), 0.5 μm (F, H)
RG-I pectins, labeled with CCRC-M36 are found in all cells and are abundant in wall ingrowths of gametophyte cells and interdigitating haustorial cells (Fig. 3A, B). In the placenta, RGI pectin epitopes recognized by LM5 are restricted to the fibrous core of wall ingrowths on the gametophyte side (Fig. 3C).
Figure 3.
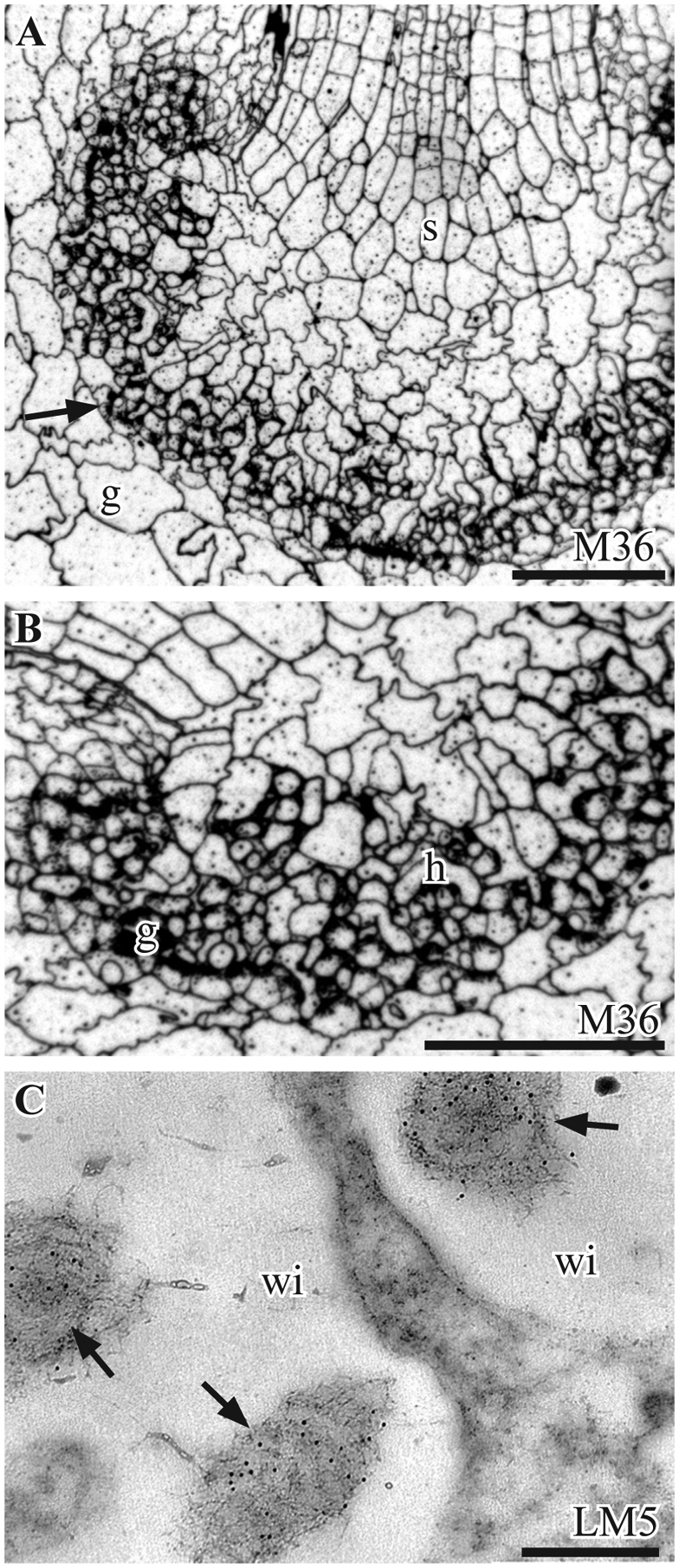
Immunolabeling with monoclonal antibodies to rhamnogalacturonan-I epitopes. A, B. P. laevis. Silver-enhanced immunogold labeling with the CCRC-M36 MAb. A. Labeling occurs in all cell walls in the gametophyte (g) and sporophyte (s) and is particularly intense in the placenta (arrow). B. Higher magnification of the placenta showing abundant staining in haustorial cells (h), gametophyte wall ingrowths (g) and zone between generations. C. P. carolinianus. TEM micrograph of abundant LM5 labels (arrows) in the fibrous cores of gametophyte wall ingrowths (wi). Scale bars: 200 μm (A, B), 0.1 μm (C)
Four antibodies (LM15, LM21, LM25, LM28) were used to target epitopes associated with xyloglucan and glucuronoxylan hemicelluloses, with only LM15 and LM25 epitopes located in the Phaeoceros placenta (Fig. 4). Immunosilver labeling with LM15 identifies xyloglucan epitopes clearly in non-placental gametophyte cells but not in placental cells (Fig. 4A). However, TEM imaging of LM15-treated sections reveals scattered labeling of wall ingrowths (Fig. 4B). Galactosylated xyloglucan epitopes recognized by LM25 are relatively abundant at the interface between the two generations and less so in wall ingrowth cores (Fig. 4C). Tests with LM21 (mannan) and LM28 (glucuronoxylan) produced no appreciable labeling of placental cells (not shown).
Figure 4.
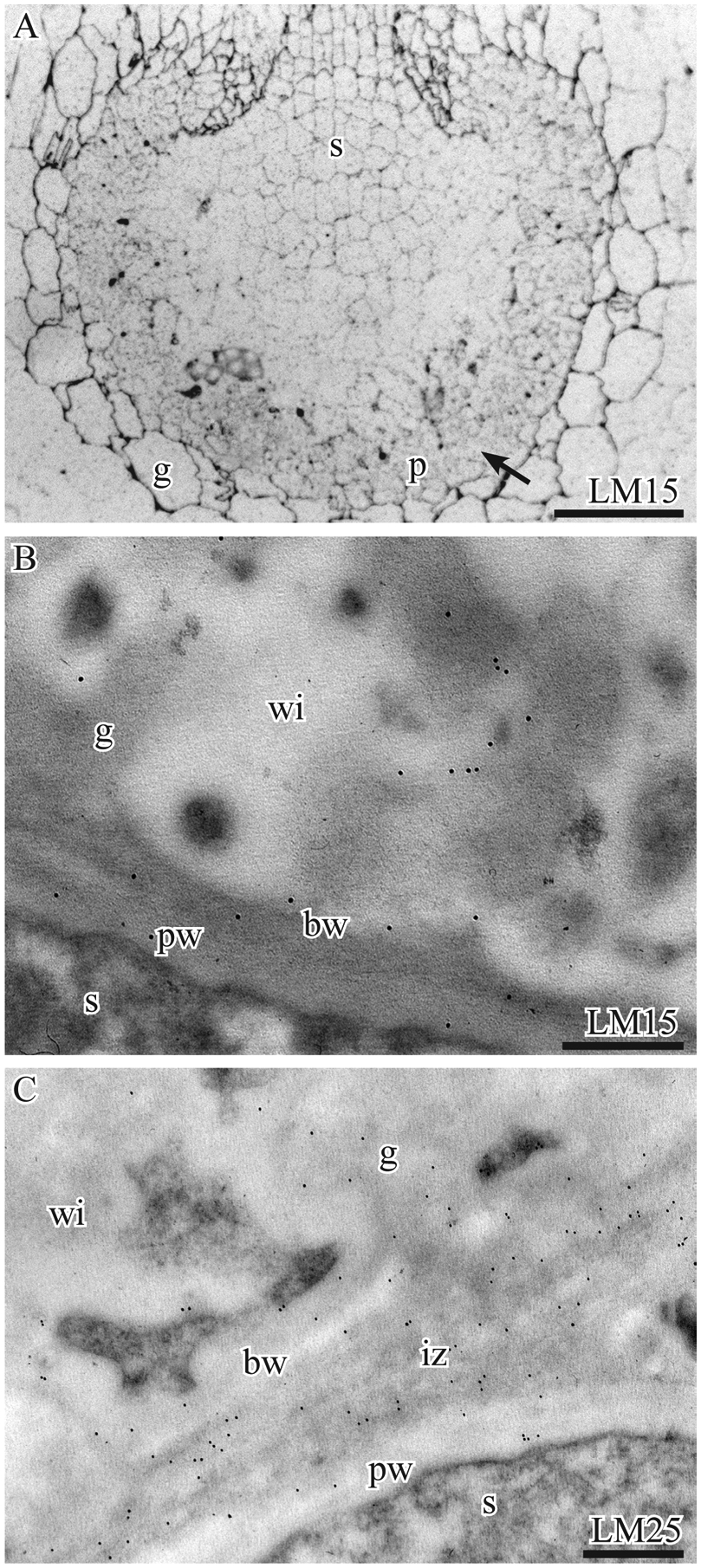
Immunolabeling with monoclonal antibodies to hemicellulose epitopes. A. P. laevis. Silver-enhanced immunogold labeling with LM15 shows strong labeling of gametophyte (g) surrounding the placenta and weak labeling of sporophyte (s) and placental cells (arrow). B, C. P. carolinianus TEMs. B. LM15 labeling is scattered in the basal wall layer (bw) and along the periphery of the fibrous core of wall ingrowths (wi) in gametophyte (g) cells and is sparse in the sporophyte (s) primary wall (pw). C LM25 weakly labels the basal walls (bw) and wall ingrowths (wi) in the gametophyte (g) and the primary cell wall (pw) of the sporophyte (s) but more strongly labels the intergenerational zone (iz). Scale bars: 200 μm (A), 0.1 μm (B, C)
Gametophyte and sporophyte display considerable diversity in the use of AGPs (targeted by JIM13, LM2, LM6, JIM8) in the placenta of Phaeoceros (Fig. 5). AGP epitopes recognized by JIM13 are specific to the placenta (Fig. 5A), where they are localized in the fibrous core of wall ingrowths (Fig. 5B) and in haustorial cell walls (Fig. 5C). Very weak labeling is observed with LM2 in both generations (Fig. 5D). LM6 arabinan epitopes are expressed in the gametophyte along the plasma membrane outlining wall ingrowths (Fig. 5E) and, to a lower extent, in haustorial cells (not shown). JIM8 AGP epitopes are absent from Phaeoceros (not shown). No expression of the extensin protein was detected with JIM12.
Figure 5.
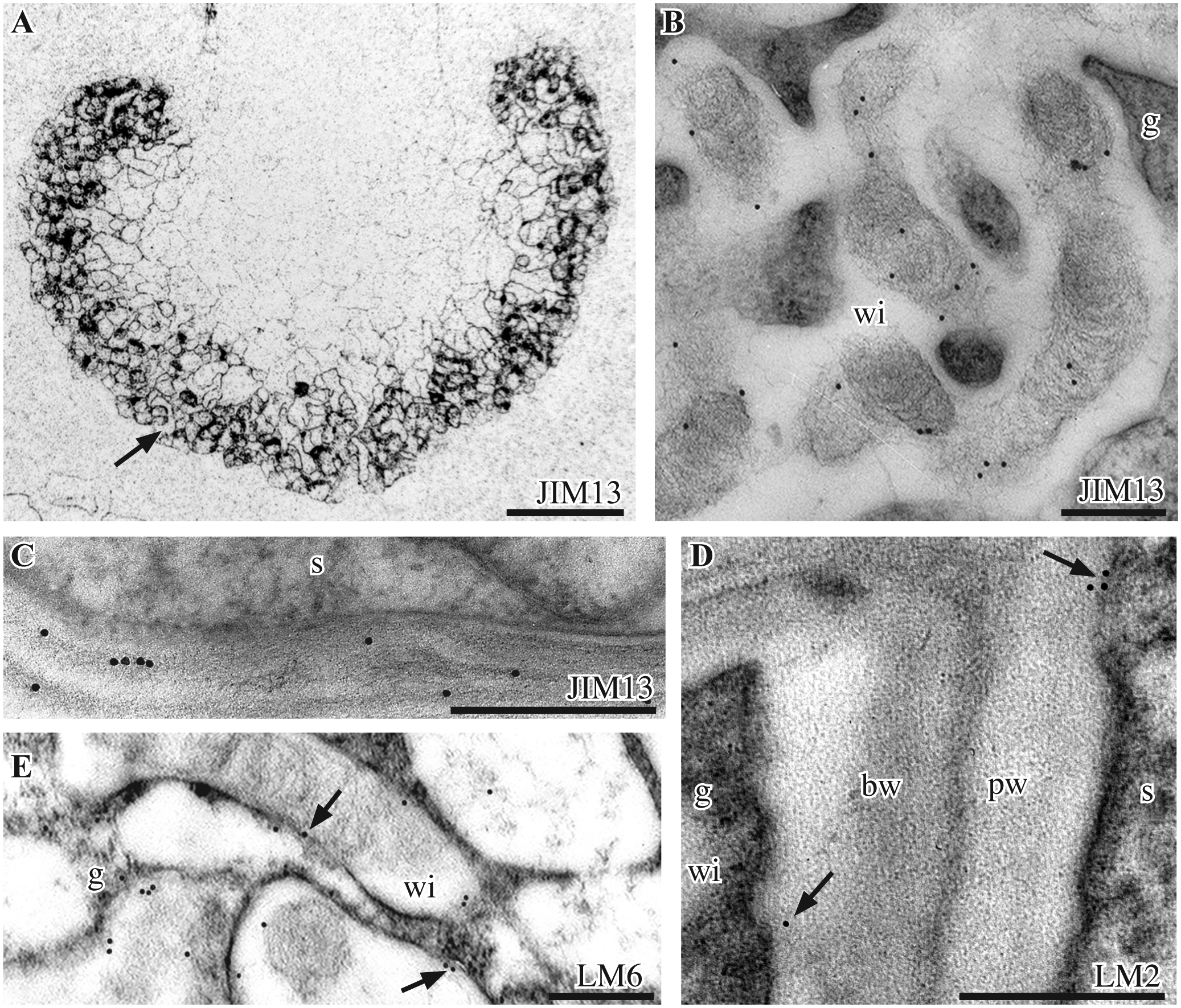
Immunolabeling with monoclonal antibodies to AGPs. A-C. P. laevis. JIM13 immunolabelling. A. Silver-enhanced immunogold labeling shows labeling in the placenta only (arrow). B. TEM shows JIM13 labels the fibrous core of gametophyte (g) wall ingrowths (wi). C. TEM of sporophyte (s) haustorial cell wall labeled. D, E. P. carolinianus. TEMs. D. Few LM2 labels (arrows) occur in the primary cell wall (pw) of the sporophyte (s) and basal wall layer (bw) of gametophyte (g) cells. E. LM6 labels on and near the plasmalemma (arrows) around gametophyte (g) wall ingrowths (wi). Scale bars: 200 μm (A), 0.1 μm (B, E), 0.5 μm (C, D)
Callose, as visualized with an anti-callose monoclonal antibody, occurs in clusters associated with thin cell wall areas away from wall ingrowths in gametophyte transfer cells (Fig. 6).
Figure 6.
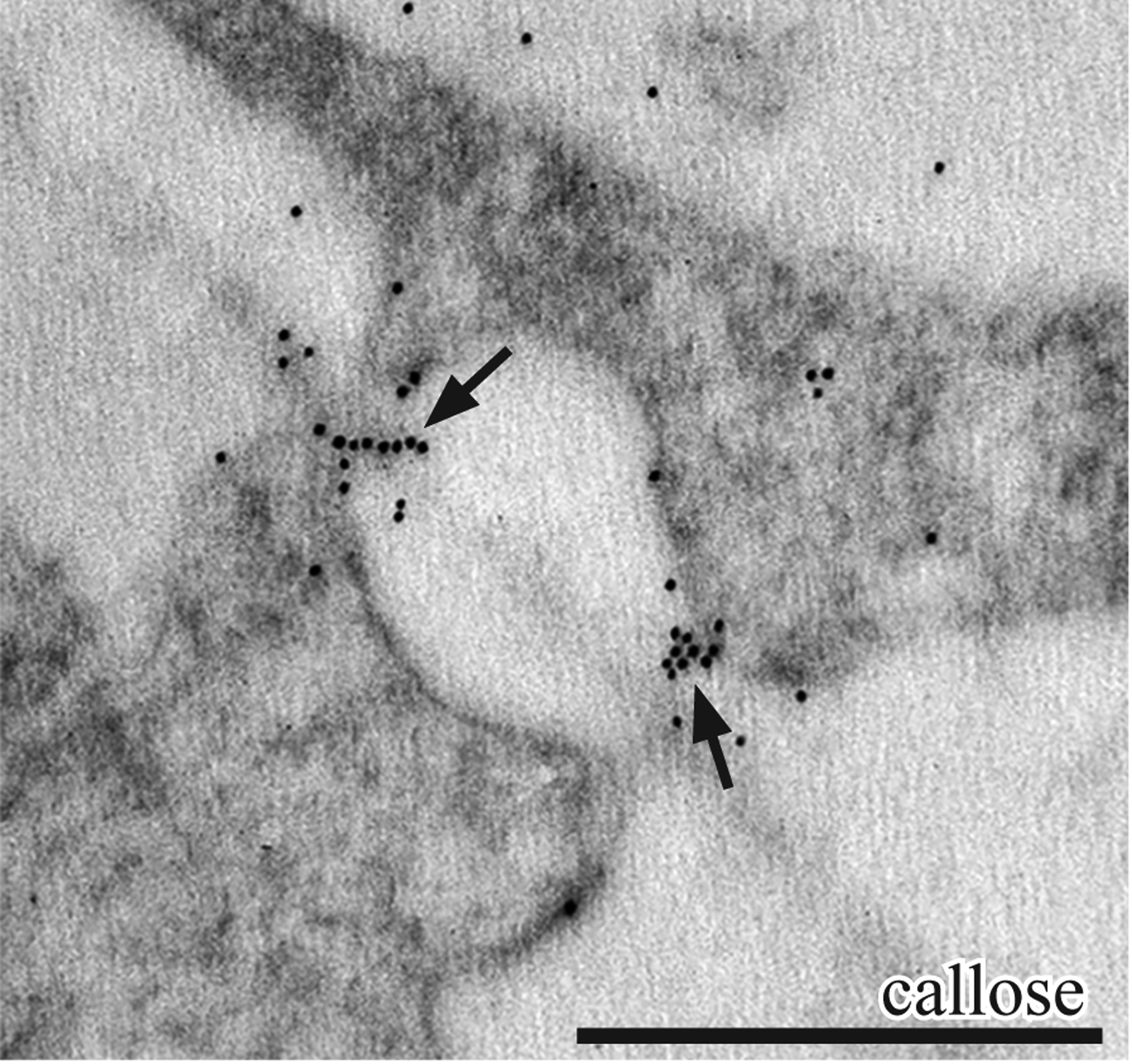
P. carolinianus. TEM immunogold labeling with anti-callose. Gametophyte cells of placenta that show labels are clustered (arrows) in the thin areas of the primary wall at the former site of plasmodesmata. Scale bar: 0.5 μm
Discussion
The primary difference between the placenta of the two hornwort species examined in this study is the occurrence of protein crystals in P. laevis and their absence in P. carolinianus. Gambardella and Ligrone (1987) found that placental crystals in P. laevis are not stained with the PATAg test for carbohydrates, but are readily digested by proteases, suggesting a sugar-free protein composition. In line with these results, the crystals did not label with any of the antibodies used in this study, whereas the fibrillar material around them gave positive response to CCRC-M38. This MAb recognizes a number of HG epitopes and the reaction of material around crystals may indicate middle lamellae material that has become detached during the formation of the placenta. Alternatively, because CCRC-M38 was raised against Arabidopsis seed mucilage, the fibrillar material may represent compositionally similar mucilage in the intercellular spaces of the gametophyte/ sporophyte junction. Placental crystals are restricted to hornworts among bryophytes and have been reported to be taxonomically diagnostic and consistent within genera (Renzaglia et al. 2009, Vaughn & Hasegawa 1993), yet this does not appear to be the case in Phaeoceros. It is possible that differences in the abundance of symbiotic cyanobacteria and the consequent differential availability of assimilated nitrogen in the two taxa may account for this surprising discrepancy.
A cellulosic scaffolding is the foundation of cell walls in the placenta of Phaeoceros. This contrasts with findings in Marchantia, where cellulose is sparse in wall ingrowths, especially on the sporophytic side of the placenta (Henry et al. 2020). As typical of the primary plant cell walls (Cosgrove 2005, Broxterman & Schols 2018), cellulose in Phaeoceros placental cells is embedded in a diverse polysaccharidic network rich in pectins and hemicelluloses, and interspersed with AGPs (Table 2).
Table 2.
Relative intensity of immunogold labeling of placental cells in Phaeoceros.
| Primary Antibody | Sporophyte | Gametophyte |
|---|---|---|
| CCRCM38 ** | ++ | ++ |
| JIM5 *, ** | ++ | + |
| LM19 * | ++ | + |
| JIM7 * | +++ | ++ |
| LM18 ** | ++ | ++ |
| LM20 * | + | − |
| CCRCM36 ** | ++ | ++ |
| LM5 * | + | ++ |
| LM16 ** | − | − |
| LM15 *, ** | ± | + |
| LM21 * | − | − |
| LM25 * | + | + |
| LM28 * | − | − |
| JIM8 * | − | − |
| JIM13 *, ** | + | + |
| LM2 * | ± | ± |
| LM6 *, **,1 | ± | ++ |
| JIM12 Extensin * | − | − |
| Callose *, ** | − | + |
P. carolinianus,
P. laevis.
Notes: +++, very strong; ++, strong; +, weak; ±, present; −, absent;
LM6 binds to arabinan residues in RG-I pectins and AGPs.
Pectins are the most diverse and abundant non-cellulosic polymers in the Phaeoceros placenta, as is true in the primary cell walls of seed plants (O’Neill & York 2018). The arrangement and composition of pectins are hypothesized to control cell wall properties such as porosity and flexibility (Caffall et al. 2009) (Table 3), thus providing a window into their role in placental cells. The antibodies against homogalacturonans (HG) epitopes (JIM7 and LM20) label haustorial cell walls more intensely than gametophyte wall ingrowths (Table 2). Cell walls enriched in methyl-esterified HGs have a lower apoplastic pH, and may facilitate nutrient uptake by membrane transport proteins (Clausen et al. 2003, Willats et al. 2001), all properties arguably of primary importance in placental cells. The abundance of methyl-esterified HGs in haustorial cells, which conspicuously elongate during intrusive growth, is consistent with the role suggested for these polymers in priming cell wall flexibility. In gametophyte placental cells, the bulk of HGs occurs in wall ingrowth cores, a trait consistent with their proposed role in defining cell wall permeability properties. Methyl-esterified HGs, which bind Ca2+ ions to form ridged gels, might enhance cell wall strength while maintaining a high level of porosity (Liners et al. 1989). Methyl-esterified HGs also occur in transfer cell wall ingrowths on both sides of the placenta in the fern Ceratopteris (Table 3) and in the epidermis of Vicia faba (Vaughn et al. 2007). In contrast, both methyl-esterified and de-esterified HG pectins are absent from the wall labyrinth apparatus in Elodea leaf cells, but are abundant in the rest of the cell walls (Ligrone et al. 2011). Both types of HG pectins are widespread in vegetative tissues of Physcomitrium patens, including apical meristem cells (Mansouri 2012), rhizoids and protonemata (Lee et al. 2005, Berry et al. 2016).
Table 3.
Summary of cell wall constituents in the placenta of three land plants: two bryophytes, Phaeoceros and Marchantia and a fern, Ceratopteris.
| MAb╲ Taxon |
Phaeoceros Sporophyte |
Phaeoceros Gametophyte |
Marchantia Sporophyte |
Marchantia Gametophyte |
Ceratopteris Sporophyte |
Ceratopteris Gametophyte |
|---|---|---|---|---|---|---|
| CCRCM38 | P | P | - | - | - | - |
| JIM5 | P | P | P | P | A | A |
| LM19 | P | P | P | P | - | - |
| JIM7 | P | P | P | P | P | P |
| LM18 | P | P | - | - | - | - |
| LM20 | P | A | P | P | - | - |
| CCRCM36 | P | P | - | - | - | - |
| CCRCM2 | - | - | - | - | A | A |
| LM5 | P | P | A | A | A | A |
| LM16 | A | A | - | - | - | - |
| LM15 | P | P | P | P | - | - |
| LM21 | A | A | P | P | - | - |
| LM25 | P | P | P | P | - | - |
| LM28 | A | A | A | A | - | - |
| CCRCM1 | - | - | - | - | P | P |
| CCRCM7 | - | - | - | - | A | A |
| JIM8 | A | A | P | P | A | P |
| JIM13 | A | P | P | P | - | - |
| LM2 | P | P | P | A | P | P |
| LM6 | P | P | A | A | P | P |
| JIM12 | A | A | A | A | - | - |
| Callose | A | P | A | A | - | - |
Notes: P = present, A = absent, - = not tested
Of the four tested antibodies that target rhamnogalacturonan-I pectic domains, two (LM5 and CCRC-M36) label the Phaeoceros placenta, with slightly higher levels of LM5 epitopes on the gametophyte side. Because LM5 targets a pectin domain that is thought to interact with HGs and AGPs to enhance the firmness and porosity of the wall, this pectin might ensure the maintenance of nutrient transport following cessation of growth, when cells must be rigid but porous. The location of the CCRC-M36 epitope (associated with RG-I) in the Phaeoceros placenta may indicate a signaling function as reported in Arabidopsis roots (Cornuault et al. 2018, McCartney et al. 2003). RG-I pectins are variable in occurrence in transfer cells of other plants. They are absent from the placenta of Marchantia (Henry et al. 2020) and C. richardii (Johnson 2008), and present in transfer cells of Vicia (Vaughn et al. 2007) and Elodea (Ligrone et al. 2011). In bryophytes, RG-I pectins are more restricted in occurrence than HG pectins. For example, LM5 does not label primary cell walls in the gametophyte apex of P. patens but it does bind to water-conducting cells, rhizoids and protonemal cells (Mansouri 2012, Ligrone et al. 2002), as well as disparate tissues in liverworts (Berry et al. 2016, Möller et al. 2007, Dehors et al. 2019).
Xyloglucan hemicelluloses (LM15, LM25) occur in both species of Phaeoceros on both sides of the placental junction, but they are not abundant (Table 2). Hemicelluloses are commonly complexed with cellulose and form the structural framework for primary walls across land plants but are generally less abundant in bryophytes than tracheophytes (Cornuault et al. 2018, Popper & Fry 2003, Sarkar et al. 2009). Xyloglucans in hornworts and tracheophytes have similar motifs (Peña et al. 2008) and are known to increase cell wall expansibility by weakening the cellulose network (Whitney et al. 2006, Chanliaud et al. 2002). High levels of xyloglucans in sporophyte wall ingrowths of Marchantia suggest a role in binding pectin and AGPs for ensuring sufficient stiffness despite the low cellulose content (Henry et al. 2020). The low abundance of xyloglucan and the absence of LM21 and LM28 epitopes associated with other hemicelluloses in Phaeoceros suggests that here pectins may play a more significant role in the regulation of wall extensibility and porosity. Hemicelluloses showed high levels of labeling in epidermal transfer cells of Vicia when targeted with polyclonal antibodies (Vaughn et al. 2007), and xyloglucans were found to be evenly distributed in transfer cell walls in pea nodules (Dahiya & Brewin 2000).
Arabinogalactan proteins (AGPs) are common in the placenta of the three land plants hitherto probed but are variable across taxa and across generations (Table 3). Most notable is the restriction of JIM13 AGP epitopes to the placental cells in Phaeoceros. AGPs are proteoglycans made of a heavily O-glycosylated (90% of the overall mass) protein backbone (Dehors et al. 2019). They are ubiquitous cell wall components in land plants, speculated to be involved in a diversity of vital processes such as differentiation, cell to cell recognition, embryogenesis, programmed cell death, and tip-growth (Happ & Classen 2019). AGPs are hypothesized to be involved in pH-dependent signaling by releasing Ca2+ as a secondary messenger that regulates development (Lamport & Várnai 2013, Lamport et al. 2014). Accumulation of cytosolic Ca2+ is important in the development of reticulate wall ingrowths in angiosperms that are similar to those in bryophytes (Offler & Patrick 2020). AGPs are thought to act as positional markers that aid in directing the polarized growth of wall ingrowths (Seifert & Roberts, 2007). The regulated signaling by AGPs might also be critical in the placenta, where the two generations have to interact and transport nutrients in a coordinate way. AGPs are released into the cell wall when they separate from their glycosylphosphatidylinositol anchors in the plasmalemma. In the cell wall, they are believed to function as pectin plasticizers by preventing HG domain crosslinking (Lamport & Kieliszewski 2005).
Blocking of AGP function using β-D-glycosyl Yariv reagents in Tobacco BY-2 cells inhibits cell expansion (Lamport & Kieliszewski 2005). The control of cell expansion by AGPs might be a key-function to the development of the hornwort placenta. The LM2 epitope is the only AGP epitope detected in the placenta of all three taxa so far investigated, being located in sporophyte cell walls and abundantly in Marchantia (Table 3) (Johnson 2008, Henry et al. 2020). Sporophyte transfer cell walls of Marchantia are rich in the JIM13 epitope (Henry et al. 2020), which is also widespread in Phaeoceros but has not yet been searched in Ceratopteris. In contrast, Johnson (2008) documented intense labeling for JIM8 AGPs in gametophyte placental cells, but not in the sporophyte in Ceratopteris. JIM8 weakly labels both generations in Marchantia but gives no signal in Phaeoceros. LM6 specifically binds to (1,5)-α-l-arabinan residues, thus signaling the occurrence of either RG-I pectins or AGPs (Willats et al. 1998). The specific localization of LM6 labeling at the plasmalemma/cell wall interface in Phaeoceros points to an AGP, not pectin. The LM6 epitope is expressed in gametophyte and sporophyte placental cell walls of Phaeoceros and Ceratopteris but not in either generation in Marchantia. Small amounts of LM6 and JIM8 epitopes are expressed in wall ingrowths of cotyledon epidermal cells in Vicia (Vaughn et al. 2007). Treatment of expanding Vicia cotyledons with the AGP inhibitor ß-D-glucosyl Yariv, caused roughly a 50% reduction in the size of the wall labyrinth apparatus in epidermal cells (Vaughn et al. 2007).
AGPs in bryophytes are reported to be associated with water conducting cells (Ligrone et al. 2002), apical cell extension and differentiation of protonemata, (Lee et al. 2005, Kobayashi et al. 2011), water balance (Shibaya et al. 2005), cell wall regeneration in cultured protoplasts (Shibaya & Sugawara 2007), cell plate formation (Shibaya & Sugawara 2009) and hyaline cell wall deposition in Sphagnum (Kremer et al. 2004). Consistent with a role in signaling, AGPs are abundantly expressed during spermatogenesis and oogenesis in Ceratopteris (Lopez & Renzaglia 2014, 2016) and spermatogenesis in Aulacomnium palustre (Lopez-Swalls 2016).
Extensins, as probed with JIM12, are not present in the placenta of the two hornwort species (Table 3). Extensins are hydroxyproline-rich glycoproteins that add strength to cell walls and are involved in cell wall assembly and growth, e.g., in tip growth in pollen tubes and root hairs of Arabidopsis (Diet et al. 2006, Ringli 2010, Velasquez et al. 2012), and in interaction between the cell wall and cytoplasm (Bascom et al. 2018).
Callose is widely used in bryophytes in processes such as tip growth, spore wall development and sperm cell differentiation (Schuette et al. 2009, Cao et al. 2014, Möller et al. 2007, Tang 2007, Berry et al. 2016, Bopp et al. 1991, Renzaglia et al. 2015, Renzaglia & Garbary, 2001). Callose occurrence in thin areas of gametophyte cell walls of Phaeoceros, which were presumably crossed by plasmodesmata before cell separation, suggests employment in the closing of plasmodesmatal pores. Callose was also found at the base of newly formed cell wall ingrowths and adjacent plasmodesmata in transfer cells in pea nodule vascular tissues (Dahiya & Brewin 2000). In contrast, callose was not found in the placenta of Marchantia (Henry et al. 2020). Callose is a regular occurrence in Vicia transfer cells (Samuels et al. 1995,Vaughn et al. 1996) and has been suggested to provide a scaffolding for the assembly of cell wall polymers during the development of wall ingrowths (Vaughn et al. 2007, Meikle et al. 1994). Because we immuno-probed only fully-developed placentae, it is possible that callose was present during the initial phases but was removed during maturation.
Conclusions
Compared with tracheophytes, the primary cell walls of bryophytes are not well characterized (Mansouri 2012, Roberts et al. 2012, Berry et al. 2016). Although the major wall polymers, namely cellulose, pectins, hemicelluloses and AGPs, are present in bryophytes, there are fewer cell wall-related genes in the genomes of bryophytes compared with seed plants (Yokoyama 2020). Special cell walls such as those around sperm cells, spores and in placental cells have modifications in the type, abundance and arrangement of cell wall constituents relative to normal primary walls, which probably reflect specific functional modulation (Cosgrove 2005, Broxterman & Schols 2018). The present study shows that placental cell walls in Phaeoceros are enriched with cellulose that imparts strength, and pectins that may enhance porosity and permeability. Cell wall compositional differences between sporophyte and gametophyte placental cells probably reflect modulation functional to cellular interaction and directional nutrient transport. Notably, the abundance of methyl-esterified HGs in sporophytic haustorial cells likely underpins the intrusive growth of these cells. Likewise, the occurrence of a diversity of AGPs restricted to placental cells suggests a role in calcium binding and release associated with signal transduction and solute movement across generations.
Acknowledgements
This work was supported by grants from the National Science Foundation (NSF 1758497) and the National Institutes of Health (NIH 5R25GM107760-07).
References
- Bascom CS, Winship LJ, & Bezanilla M (2018) Simultaneous imaging and functional studies reveal a tight correlation between calcium and actin networks. Proceedings of the National Academy of Science USA 115: 2869–2878. 10.1073/pnas.1711037115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry EA, Tran ML, Dimos CS, Budziszek MJ Jr., Scavuzzo-Duggan TR, & Roberts AW (2016) Immuno and affinity cytochemical analysis of cell wall composition in the moss Physcomitrella patens. Fronters Plant Science 7: 248. 10.3389/fpls.2016.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisang I, Lüth M, & Hofmann H (2010). Phaeoceros laevis subsp. carolinianus (Michx.) Prosk. In: Swiss bryophytes Working Group (Hrsg.), www.swissbryophytes.ch: Moosflora der Schweiz. [Google Scholar]
- Bopp M, Quader H, Thoni C, Sawidis T, & Schnepf E (1991) Filament disruption in Funaria protonemata: formation and disintegration of tmema cells. Journal of Plant Physiology 137: 273–284. 10.1016/S0176-1617(11)80131-8 [DOI] [Google Scholar]
- Browning A, and Gunning B (1979). Structure and function of transfer cells in the sporophyte haustorium of Funaria hygrometrica Hedw: II. Kinetics of uptake of labelled sugars and localization of absorbed products by freeze-substitution and autoradiography. Journal of Experimental Botany 30, 1247–1264. [Google Scholar]
- Broxterman SE, & Schols HA (2018) Interactions between pectin and cellulose in primary plant cell walls. Carbohydrate Polymer 192: 263–272. 10.1016/j.carbpol.2018.03.070 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Kerry H, & Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research 344: 1879–1900. 10.1016/j.carres.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Cao JG, Dai XL, Zou HM, & Wang QX (2014) Formation and development of rhizoids of the liverwort Marchantia polymorpha. Journal Torrey Botanical Society 1: 126–134. [Google Scholar]
- Chanliaud E, Burrows KM, Jeronimidis G, & Gidley MJ (2002) Mechanical properties of primary plant cell wall analogues. Planta 215: 989–996. 10.1007/s00425-002-0783-8 [DOI] [PubMed] [Google Scholar]
- Clausen MH, Willats WG, & Knox JP (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydrate Research 338: 1797–1800. 10.1016/S0008-6215(03)00272-6 [DOI] [PubMed] [Google Scholar]
- Cornuault V, Buffetto F, Rydahl MG, Marcus SE, Torode TA, Xue J, & Ralet MC (2015) Monoclonal antibodies indicate low-abundance links between heteroxylan and other glycans of plant cell walls. Planta 242: 1321–1334. 10.1007/s00425-015-2375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornuault V, Pose S, & Knox JP (2018) Extraction, texture analysis and polysaccharide epitope mapping data of sequential extracts of strawberry, apple, tomato and aubergine fruit parenchyma. Data in Brief 17: 314–320. 10.1016/j.dib.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ (2005) Growth of the plant cell wall. Nature Reviews Molecular Cell Biology 6: 850–862. 10.1038/nrm1746 [DOI] [PubMed] [Google Scholar]
- Dahiya P, & Brewin NJ (2000) Immunogold localization of callose and other cell wall components in pea nodule transfer cells. Protoplasma 214:210–218. 10.1007/BF01279065 [DOI] [Google Scholar]
- Dehors J, Mareck A, Kiefer-Meyer MC, Menu-Bouaouiche L, Lehner A, & Mollet JC (2019) Evolution of cell wall polymers in tip-growing land plant gametophytes: composition, distribution, functional aspects and their remodeling. Frontiers in Plant Science 10: 441–469. 10.3389/fpls.2019.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diet A, Link B, Seifert GJ, Schellenberg B, Wagner U, Pauly M, & Ringli C (2006) The Arabidopsis root hair cell wall formation mutant lrx1 is suppressed by mutations in the RHM1 gene encoding a UDP-L-rhamnose synthase. The Plant Cell 18: 1630–164. DOI: 10.1105/tpc.105.038653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff RJ, Villarreal JC, Cargill DC, & Renzaglia KS (2007) Progress and challenges toward developing a phylogeny and classification of the hornworts. The Bryologist, 110: 214–243. [Google Scholar]
- Frangedakis E, Shimamura M, Villarreal JC, Li FW, Tomaselli M, Waller M, Sakakibara K, Renzaglia KS & Szövényi P (2020). The Hornworts: Morphology, evolution and development. New Phytologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella R, & Ligrone R (1987) The development of the placenta in the anthocerote Phaeoceros laevis (L.) Prosk. Planta 172: 439–447. 10.1007/BF00393859 [DOI] [PubMed] [Google Scholar]
- Happ K, & Classen B (2019) Arabinogalactan-Proteins from the Liverwort Marchantia polymorpha L., a Member of a Basal Land Plant Lineage, Are Structurally Different to Those of Angiosperms. Plants 8: 460. 10.3390/plants8110460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J, Lopez RA, & Renzaglia KS, (2020) Differential localization of cell wall polymers across generations in the placenta of Marchantia polymorpha. Journal of Plant Research. 10.1007/s10265-020-01232-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, & Goring DR (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytologist 176: 7–21. 10.1111/j.1469-8137.2007.02192.x [DOI] [PubMed] [Google Scholar]
- Johnson GP (2008) Early Embryology of Ceratopteris Richardii and immunocytochemistry of placental transfer cell wall ingrowths. Southern Illinois University Carbondale, Carbondale, Illinois. [Google Scholar]
- Jones L, Seymour GB, & Knox JP (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1−4)-β-D-galactan. Plant Physiology 113: 1405–1412. DOI: 10.1104/pp.113.4.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, & Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181:512–521. 10.1007/BF00193004 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Motose H, Iwamoto K, & Fukuda H (2011) Expression and genome-wide analysis of the xylogen-type gene family. Plant Cell Physiology 52: 1095–1106. 10.1093/pcp/pcr060 [DOI] [PubMed] [Google Scholar]
- Kremer C, Pettolino F, Bacic A, & Drinnan A (2004) Distribution of cell wall components in Sphagnum hyaline cells and in liverwort and hornwort elaters. Planta 219: 1023–1035. 10.1007/s00425-004-1308-4 [DOI] [PubMed] [Google Scholar]
- Lamport DT, & Várnai P (2013) Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist 197: 58–64. 10.1111/nph.12005 [DOI] [PubMed] [Google Scholar]
- Lamport DT, Varnai P, & Seal CE (2014) Back to the future with the AGP–Ca2+ flux capacitor. Annals of Botany 114: 1069–1085. 10.1093/aob/mcu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport DTA, & Kieliszewski MJ (2005). Stress upregulates periplasmic arabinogalactan-proteins. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology, 139: 60–64. [Google Scholar]
- Lee KJ, Sakata Y, Mau SL, Pettolino F, Bacic A, Quatrano RS, & Knox JP (2005) Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell 17: 3051–3065. 10.1105/tpc.105.034413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, & Renzaglia KS (1990) The sporophyte–gametophyte junction in the hornwort, Dendroceros tubercularis Hatt (Anthocerotophyta). New phytologist 114: 497–505. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, & Renzaglia KS (1993) The gametophyte-sporophyte junction in land plants. Advances in Botanical Research 19: 231–318. 10.1016/S0065-2296(08)60206-2 [DOI] [Google Scholar]
- Ligrone R, Vaughn KC, & Rascio N (2011) A cytochemical and immunocytochemical analysis of the wall labyrinth apparatus in leaf transfer cells in Elodea canadensis. Annals of Botany 107: 717–722. 10.1093/aob/mcr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, Vaughn KC, Renzaglia KS, Knox JP, & Duckett JG (2002) Diversity in the distribution of polysaccharide and glycoprotein epitopes in the cell walls of bryophytes: new evidence for multiple evolution of water-conducting cells. New Phytologist 156: 491–508. doi: 10.1105/tpc.105.034413 [DOI] [PubMed] [Google Scholar]
- Liners F, Letesson JJ, Didembourg C, & Van Cutsem P (1989) Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiology 91: 1419–1424. DOI: 10.1104/pp.91.4.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RA, & Renzaglia KS (2014) Multiflagellated sperm cells of Ceratopteris richardii are bathed in arabinogalactan proteins throughout development. American Journal Botany 101: 2052–2061. 10.3732/ajb.1400424 [DOI] [PubMed] [Google Scholar]
- Lopez RA, & Renzaglia KS (2016) Arabinogalactan proteins and arabinan pectins abound in the specialized matrices surrounding female gametes of the fern Ceratopteris richardii. Planta 243: 947–957. 10.1007/s00425-015-2448-4 [DOI] [PubMed] [Google Scholar]
- Lopez RA, Mansouri K, Henry JS, Flowers ND, Vaughn KC, & Renzaglia KS (2017) Immunogold localization of molecular constituents associated with basal bodies, flagella, and extracellular matrices in male gametes of land plants. Bio-Protocol 7: e2599. DOI: 10.21769/BioProtoc.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Swalls RA (2016) The special walls around gametes in Ceratopteris richardii and Aulacomnium palustre: using immunocytochemistry to expose structure, function, and development. Southern Illinois University Carbondale, Carbondale, Illinois. [Google Scholar]
- Mansouri K (2012). Comparative ultrastructure of apical cells and derivatives in bryophytes, with special reference to plasmodesmata. Southern Illinois University Carbondale, Carbondale, Illinois. [Google Scholar]
- Marcus SE, Blake AW, Benians TA, Lee KJ, Poyser C, Donaldson L, & Gilbert HJ (2010) Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant Journal 64:191–203. 10.1111/j.1365-313X.2010.04319.x [DOI] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, & Knox JP (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biology Journal 8:60. 10.1186/1471-2229-8-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney L, Steele-King CG, Jordan E, & Knox JP (2003) Cell wall pectic (1→ 4)-β-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. The Plant Journal 33: 447–454. 10.1046/j.1365-313X.2003.01640.x [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, & Stone BA (1991) The location of (1→ 3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1→ 3)-β-glucan-specific monoclonal antibody. Planta 185:1–8. 10.1007/BF00194507 [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, & Stone BA (1994) A (1→ 3, 1→ 4)-β-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1→ 3, 1→ 4)-β-glucans. The Plant Journal 5: 1–9. 10.1046/j.1365-313X.1994.5010001.x [DOI] [PubMed] [Google Scholar]
- Möller I, Sørensen I, Bernal AJ, Blaukopf C, Lee K, & Øbro J (2007) High-through put mapping of cell-wall polymers within and between plants using novel microarrays. The Plant Journal 50: 1118–1128. [DOI] [PubMed] [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, & Talbot MJ. 2002. Transfer cells: cells specialized for a special purpose. Annual Review of Plant Biology 54: 431–454. [DOI] [PubMed] [Google Scholar]
- Offler CE, & Patrick JW (2020) Transfer cells: What regulates the development of their intricate wall labyrinths?. New Phytologist 228: 427–444. 10.1111/nph.16707 [DOI] [PubMed] [Google Scholar]
- O’Neill MA, & York WS (2018). The composition and structure of plant primary cell walls. Annual Plant Reviews Online 1–54. [Google Scholar]
- Pate JS, Gunning BES. 1972. Transfer cells. Annual Review of Plant Physiology 23: 173–196. [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, & Dong R (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiology 153: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen HL, Fangel JU, McCleary B, Ruzanski C, Rydahl MG, Ralet MC, & Field R (2012) Versatile high-resolution oligosaccharide microarrays for plant glycobiology and cell wall research. Journal of Biological Chemistry 287: 39429–39438. doi: 10.1074/jbc.M112.396598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña MJ, Darvill AG, Eberhard S, York WS, & O’Neill MA, (2008) Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants. Glycobiology 18: 891–904. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, & Roberts K (1991) Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3: 1317–1326. 10.1105/tpc.3.12.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, & Fry SC (2003) Primary Cell Wall Composition of Bryophytes and Charophytes. Annals of Botany 91: 1–12. 10.1093/aob/mcg013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi KC, Li L & Gaxiola RA (2017) Alternate modes of photosynthate transport in the alternating generations of Physcomitrella patens. Frontiers in Plant Science 8:1956. doi: 10.3389/fpls.2017.01956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS (1978) Comparative morphology and developmental anatomy of the Anthocerotophyta. Journal of the Hattori Botanical Laboratory 44: 31–90. [Google Scholar]
- Renzaglia KS, & Garbary DJ (2001) Motile male gametes of land plants: diversity, development, and evolution. Critical Review Plant Science 20: 107–213. 10.1080/20013591099209 [DOI] [Google Scholar]
- Renzaglia KS, Lopez RA, & Johnson EE (2015) Callose is integral to the development of permanent tetrads in the liverwort Sphaerocarpos. Planta 241: 615–627. 10.1007/s00425-014-2199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Lopez RA, Henry JS, Flowers ND, & Vaughn KC (2017) Transmission electron microscopy of centrioles, basal bodies and flagella in motile male gametes of land plants. Bio-Protocols 7. DOI: 10.21769/BioProtoc.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Villarreal JC, & Duff RJ (2009). New insights into morphology, anatomy, and systematics of hornworts. Bryophyte Biology 2: 139–171. [Google Scholar]
- Roberts AW, Roberts EM, & Haigler CH (2012) Moss cell walls: structure and biosynthesis. Frontiers in Plant Science 3: 166–173. 10.3389/fpls.2012.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH, & Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. Journal of Cell Biology 130: 1345–1357. 10.1083/jcb.130.6.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Bosneaga E, & Auer M (2009) Plant cell walls throughout evolution: towards a molecular understanding of their design principles. Journal of Experimental Botany 60: 3615–3635. 10.1093/jxb/erp245 [DOI] [PubMed] [Google Scholar]
- Schuette S, Wood AJ, Geisler M, Geisler-Lee J, Ligrone R, & Renzaglia KS (2009) Novel localization of callose in the spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Annals of Botany 103: 749–756. 10.1093/aob/mcn268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, & Roberts K (2007) The biology of arabinogalactan proteins. Annual Review of Plant Biology 58: 137–161. 10.1146/annurev.arplant.58.032806.103801 [DOI] [PubMed] [Google Scholar]
- Shibaya T, & Sugawara Y (2007) Involvement of arabinogalactan proteins in the regeneration process of cultured protoplasts of Marchantia polymorpha. Physiology Plant 130: 271–279. 10.1111/j.1399-3054.2007.00905.x [DOI] [Google Scholar]
- Shibaya T, & Sugawara Y (2009) Induction of multinucleation by β-glucosyl Yariv reagent in regenerated cells from Marchantia polymorpha protoplasts and involvement of arabinogalactan proteins in cell plate formation. Planta 230: 581–588. 10.1007/s00425-009-0954-y [DOI] [PubMed] [Google Scholar]
- Shibaya T, Kaneko Y, & Sugawara Y (2005) Involvement of arabinogalactan proteins in protonemata development from cultured cells of Marchantia polymorpha. Physiology Plant 124: 504–514. 10.1111/j.1399-3054.2005.00525.x [DOI] [Google Scholar]
- Smallwood M, Beven A, Donovan N, Neill SJ, Peart J, Roberts K, & Knox JP (1994) Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant Journal 5: 237–246. 10.1046/j.1365-313X.1994.05020237.x [DOI] [Google Scholar]
- Smallwood M, Yates EA, Willats WG, Martin H, & Knox JP (1996) Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 198: 452–459. 10.1007/BF00620063 [DOI] [Google Scholar]
- Tang CTC (2007) The wound response in Arabidopsis thaliana and Physcomitrella patens. Rutgers University, New Brunswick, New Jersey. [Google Scholar]
- Thomas RJ, Stanton DS, Longendorfer DH, & Farr ME (1978) Physiological evaluation of the nutritional autonomy of a hornwort sporophyte. Botanical Gazette, 139: 306–311. [Google Scholar]
- Thompson RD, Hueros G, Becker HA, & Maitz M (2001) Development and functions of seed transfer cells. Plant Science 160: 775–783. [DOI] [PubMed] [Google Scholar]
- Vaughn KC, & Hasegawa J (1993). Ultrastructural characteristics of the placental region of Folioceros and their taxonomic significance. Bryologist 112–121. [Google Scholar]
- Vaughn KC, Hoffman JC, Hahn MG, & Staehelin LA (1996) The herbicide dichlobenil disrupts cell plate formation: immunogold characterization. Protoplasma 194: 117–12. 10.1007/BF01882020 [DOI] [Google Scholar]
- Vaughn KC, Talbot MJ, Offler CE, & McCurdy DW (2007) Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of arabinogalactan proteins. Plant Cell Physiology 48: 159–168. 10.1093/pcp/pcl047 [DOI] [PubMed] [Google Scholar]
- Velasquez SM, Salgado SJ, Petersen BL, & Estevez JM (2012) Recent advances on the posttranslational modifications of EXTs and their roles in plant cell walls. Frontiers Plant Science 3: 93–99. 10.3389/fpls.2012.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, & Knox JP (2009) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydrate Research 344: 1858–1862. 10.1016/j.carres.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Villarreal AJC, & Renzaglia KS (2006) Sporophyte structure in the neotropical hornwort Phaeomegaceros fimbriatus: implications for phylogeny, taxonomy, and character evolution. International Journal of Plant Sciences 167: 413–427. [Google Scholar]
- Whitney SE, Wilson E, Webster J, Bacic A, Reid JG, & Gidley MJ (2006) Effects of structural variation in xyloglucan polymers on interactions with bacterial cellulose. American Journal of Botany 93: 1402–1414. 10.3732/ajb.93.10.1402 [DOI] [PubMed] [Google Scholar]
- Willats WG, Limberg G, Buchholt HC, Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, & Knox JP (2000) Analysis of pectic epitopes recognized by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydrate Research 327:309–320. 10.1016/S0008-6215(00)00039-2 [DOI] [PubMed] [Google Scholar]
- Willats WG, Marcus SE & Knox JP (1998) Generation of a monoclonal antibody specific to (1,5)-α-l-arabinan. Carbohydrate Research 308: 149–152. 10.1016/S0008-6215(98)00070-6 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, & Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology 47: 9–27. [PubMed] [Google Scholar]
- Yates EA, Valdor JF, Haslam SM, Morris HR, Dell A, Mackie W, & Knox JP (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6: 131–139. 10.1093/glycob/6.2.131 [DOI] [PubMed] [Google Scholar]
- Yokoyama R (2020). A Genomic Perspective on the Evolutionary Diversity of the Plant Cell Wall. Plants 9: 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]


