Abstract
Absence seizures (AS), presenting as short losses of consciousness with staring spells, are a common manifestation of childhood epilepsy that is associated with behavioral, emotional, and social impairments. It has also been suggested that patients with AS are more likely to suffer from mood disorders such as depression and anxiety. This systematic review and meta-analysis synthesizes human and animal models that investigated mood disorders and AS. Of the 1019 scientific publications identified, 35 articles met the inclusion criteria for this review. We found that patients with AS had greater odds of developing depression and anxiety when compared to controls (odds ratio = 4.93, 95% confidence interval = 2.91–8.35, p < .01). The included studies further suggest a strong correlation between AS and depression and anxiety in the form of a bidirectional relationship. The current literature emphasizes that these conditions likely share underlying mechanisms, such as genetic predisposition, neurophysiology, and anatomical pathways. Further research will clarify this relationship and ensure more effective treatment for AS and mood disorders.
Keywords: behavioral disorders, childhood absence epilepsy, GAERS, mood disorders, WAG/Rij
1 |. INTRODUCTION
Absence seizures (AS) are characterized by recurrent 5–10-s episodes of unconsciousness and staring spells with distinct rhythmic 3–4-Hz spike-and-wave discharges (SWDs) on electroencephalogram.1–3 AS are relatively common in the pediatric population, with about 10% of seizures in children with epilepsy considered typical AS.4 Although absence epilepsy (AE) and childhood AE (CAE), which are characterized by AS, were initially thought to be benign epilepsy disorders without developmental or long-term sequelae, this notion has since been challenged. Several studies over the past 20 years have described relationships between CAE and developmental, cognitive, mood, and psychosocial impairments.5–8
For decades, numerous studies have described higher rates of psychiatric disorders observed in pediatric epilepsy.9 In an early study of 146 children aged 5–16 years with one of three types of childhood epilepsy disorder, Ott et al. observed that 51% of their study cohort met criteria for a psychiatric disorder, including mood disorders in 13% and suicide ideation in 18% of the population.10 In two separate studies, Caplan et al. reported anxiety rates of 22%–24% and depression rates of 4%–6% in patients with CAE, compared to anxiety rates of around 7% and depression rates of around 3% in children between the ages of 3 and 17 years in the general population.7,11,12 Moreover, a prevalence of anxiety and depressive-like behaviors has also been reported in animal models, including in Wistar Albino Glaxo From Rijswijk (WAG/Rij),13,14 Long–Evans (LE),15 and Genetic Absence Epilepsy From Strasbourg (GAERS) rats.16
Young adults with CAE have more behavioral, emotional, and psychiatric difficulties, which can lead to avoidance of large gatherings, problematic family relationships, and poor performance at school.17,18 Fastenau et al. found that AS are associated with less proficient language, attention/executive skills, and verbal memory.19 These deficits have long-term sequelae and considerably impact the quality of life even after seizure management or remission.18 Behavioral outcomes are significantly worse in patients with poor seizure control.18
The high rates of mood disorders in humans with AE and in animal models suggest an integral relationship between mood disorders and AS. However, it is unknown whether AS and mood disorders share an underlying pathology, or whether mood disorders are a consequence of AS. Furthermore, the therapeutic effects of antiepileptic drugs (AEDs) and antidepressants on AS are unclear. The relatively small number of studies likely explains the inconsistent findings and speaks to the need for a meta-analysis and a systematic review. To better understand the relationship between anxiety, depression, and AS, we conducted a systematic literature review of both human and animal studies that evaluated depression and anxiety in AS. Clarifying the relationship between AS, neuropsychiatric disorders, and treatment modalities is crucial to mitigating the impacts of this complex disorder.
2 |. MATERIALS AND METHODS
2.1 |. Literature search
We performed a systematic search of the literature to identify studies that reported on depression and anxiety in patients with a history of AS or in animal models of AE. The review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.20
An experienced medical librarian (M.C.F.) consulted on methodology and ran a Medical Subject Headings analysis of known key articles provided by the research team (https://mesh.med.yale.edu/). In each database, we ran scoping searches and used an iterative process to translate and refine the searches. To maximize sensitivity, the search used controlled vocabulary terms and synonymous free-text words to capture the concepts of “psychiatric illness” and “epilepsy.” On September 4, 2019, and again on June 24, 2020, the librarian performed a comprehensive search of multiple databases: MEDLINE (Ovid), Embase (Ovid), PsycINFO (Ovid), Web of Science Core Collection (Clarivate), Scopus (Elsevier), CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCOhost), and the Cochrane Library (Wiley). The search strategy for Ovid MEDLINE is included in Appendix S1.
2.2 |. Inclusion and exclusion criteria
This systematic review included original preclinical studies and retrospective or prospective clinical studies that examined the association between depression or anxiety and AS. We excluded studies without the right study design, including conference abstracts, editorials, case reports, case series, and reviews. Studies on other types of seizures without a clear separation of the cohort with AS and studies that did not explicitly study depression or anxiety were excluded due to wrong study parameters. Articles were limited to the English language. No date limit was applied.
2.3 |. Study selection
Two authors (B.F.G. and M.R.S.S.) independently evaluated the titles and abstracts of the identified articles. Abstracts that did not include sufficient information to determine eligibility for inclusion were retrieved for full-text evaluation. The final search retrieved a total of 1552 references. This set was uploaded to Covidence (https://www.covidence.org/) for screening, which identified duplicates and left 1019 for screening. Authors B.F.G. and M.R.S.S. screened the titles and abstracts, and then reviewed the full text of the 121 eligible articles. Thirty-five articles met the inclusion criteria for extraction. The selected articles were categorized as human or animal studies. The animal studies were further classified based on the models used in the study. Any disagreements were resolved by consensus. A PRISMA flow diagram is illustrated in Figure 1.
FIGURE 1.
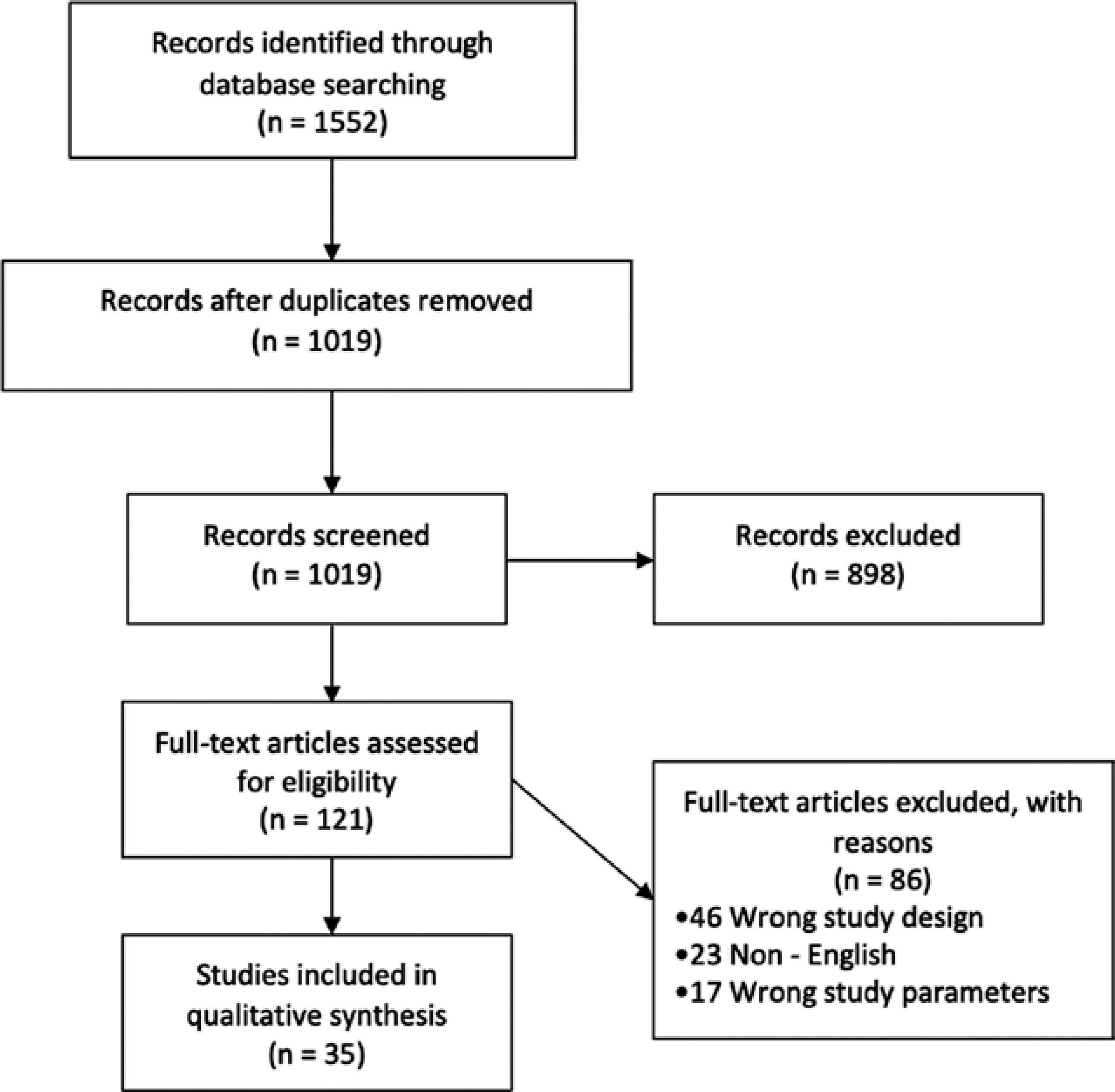
Study flow chart. Of 1019 screened records, 35 studies met the inclusion criteria. Seven studies were done on human populations, and the rest of the studies (n = 28) were conducted on animal models of absence epilepsy. Of 86 excluded articles, 46 articles were either conference abstracts, reviews, editorials, or case reports (wrong study design); 23 articles were published in languages other than English (non-English); and 17 studies did not specifically study absence seizures and depression or anxiety as an outcome (wrong study parameters)
2.4 |. Data extraction
Four reviewers (R.A.O.B., T.G.O.B., A.S., and A.K.) conducted study evaluation and data extraction. The data extracted included a general description of the study population, sample size, type of animal model, interventions, experiments, results, and conclusions. A qualitative summary composed of these descriptive properties was created for each study.
3 |. RESULTS
3.1 |. Human studies
Seven human studies describing the association of AS with depression and anxiety were included. With two exceptions,21,22 all studies were conducted in a pediatric population with the Kiddie Schedule for Affective Disorders and Schizophrenia as the most commonly used diagnostic tool. The participants’ characteristics, diagnostic tools, and outcomes are described in Table 1.
TABLE 1.
Human studies demographics, methods of assessment, and results
| Article | Year of publication | Age range, years | Male, n (%) | Groups (n) | Diagnostic tools | Anxiety/depression/both,n | Main findings |
|---|---|---|---|---|---|---|---|
| Caplan et al.11 | 2005 | 5–16 | 125 (47) | CAE (71) CPS (100) Control (93) |
K-SADS | CAE: 17/3/0 Control: 6a |
Children with CPS and CAE had higher rates of suicidal ideation, and affective and anxiety disorders, with higher rate of anxiety and lower of depression in the CAE group compared to CPS |
| Caplan et al.7 | 2008 | 6.7–11.2 | 76 (44) | CAE (69) Control (103) |
K-SADS, CBCL | CAE: 15/4/1 Control: 9a |
Significantly more CAE subjects had a psychiatric diagnosis, with 31% having affective or anxiety disorder against 9% in the control group |
| Schreibman Cohen et al.24 | 2009 | 6.6–15.18 | 21 (43) | CAE (26) Control (23) |
K-SADS | CAE: 7b Control: 4 |
Psychiatric diagnosis prevalence was higher in children with CAE, of whom 20% had affective-anxiety disorder and 24% had ADHD |
| Vega et al.23 | 2011 | 6–16 | 33 (38) | CAE (45) Control (41) |
BASC | CAE: 5/11/0 Control: 1/1/0 |
CAE children scored significantly higher on the anxiety and depression subscales |
| Precenzano et al.25 | 2016 | 8–11 | 19(31) | CAE (18) Control (43) |
SAFA-A scale, CDI test | N/R | Higher scores of anxiety and depression symptoms were found in the CAE group compared to control |
| Abarrategui et al.21 | 2018 | 18–55 | 27 (44.3) | CAE/JAE (20) Control (21) |
ISRA, NDDI-E, BDI-II | CAE/JAE: 4c Control: 0 |
Patients with CAE/JAE presented the highest anxiety scores; however, it was not statistically significant; 17% of patients had major depression compared to none in the control group |
| Gabriel et al.22 | 2020 | 21–43 | 19(41.3) | JAE (46) General population as control |
Clinical notes | JAE: 8/9 | JAE had more mood disorders and anxiety than general population, however, not statistically significant |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BASC, Behavior Assessment System for Children; BDI-II, Beck Depression Inventory II; CAE, childhood absence epilepsy; CBCL, Child Behavior Checklist; CDI, Children's Depression Inventory; CPS, complex partial seizure; ISRA, Inventory of Anxiety Situations and Responses; JAE, juvenile absence epilepsy; K-SADS, Kiddie Schedule for Affective Disorders and Schizophrenia; N/R, not reported; NDDI-E, Neurological Disorders Depression Inventory for Epilepsy; SAFA-A, Self-Administrated Psychiatric Scales for Children and Adolescents–Anxiety scale.
Diagnosis of anxiety and depression not discriminated.
Anxiety, depression, and ADHD.
Only depression included.
All studies showed a higher prevalence of depression and anxiety in patients with AE. Using structured psychiatric interview and mood self-report scales, Caplan et al.11 showed that children with complex partial seizures (CPS) and CAE had a higher prevalence of suicidal ideation (20% vs. 9%, p < .02) and affective/anxiety disorders (33% vs. 6.5%, p < .01) than unaffected children. Moreover, higher rates of anxiety (50% vs. 27%, p < .02) and lower rates of depression (15% vs. 21.6%, p < .02) were found in the CAE group when compared to CPS. A subsequent study from Caplan et al.7 revealed that 61% of the children with CAE had a psychiatric diagnosis, 31% of whom were diagnosed with an affective or anxiety disorder, compared with 9% in the control group with these diagnoses (p = .01). Girls with CAE were 5.8× more likely to have an anxiety disorder diagnosis than boys (95% confidence interval [CI] = 1.05–31.95). For every additional year of CAE diagnosis, there was a 1.35× greater chance of having a psychiatric disorder (95% CI = 1.05–1.73, p < .02), and children with 10 or more seizures per year were 1.2× more likely to have a psychiatric diagnosis (95% CI = 1.02–1.38, p < .03).
Vega et al.23 assessed depression and anxiety symptoms with the Behavior Assessment System for Children (BASC) Anxiety and Depression subscales, a parent-report questionnaire. Clinically significant symptoms were defined as one and a half standard deviations from the BASC normative sample mean. In the CAE group, 11% of the patients had Anxiety and 25% had Depression subscale scores in the clinically significant range, whereas only one control patient (2.4%) met this criterion for each subscale. Statistical analysis revealed significantly higher questionnaire scores in the CAE group for anxiety (50.7 1; 9.3 vs. 43.3 ± 7.5, p = .001) and depression (50.4 ± 12.9 vs. 43.3 ± 7.7, p = .007) when compared to healthy controls. Contrary to the results from Caplan et al.,7 no association between seizure frequency or disease duration was found with the development of anxiety or depression.
A statistically significant difference (p < .003) was found by Schreibman Cohen et al.24 regarding the presence of a psychiatric diagnosis between healthy children (17%) and a CAE cohort (60%), with 20% diagnosed with affective–anxiety disorder and 24% with attention-deficit/hyperactivity disorder. Similarly, Precenzano et al.25 showed higher scores on anxiety (59.2 ± 7.3 vs. 48.9 ± 10, p < .001) and depression symptoms (15 ± 7.5 vs. 8.7 ± 1.88, p < .001) in the CAE group compared to the control group.
Abarrategui et al.21 found that patients with CAE and juvenile absence epilepsy (JAE) persisting into adulthood presented the highest anxiety scores on ISRA (Inventory of Anxiety Situations and Responses) when compared to controls and to other idiopathic generalized epilepsy syndromes. The CAE/JAE group also showed a higher prevalence of depression (17%) when compared to control (0%); however, both disorders failed to show statistical significance. Another study by Gabriel et al.22 found that patients with JAE had more mood disorders (19.6%), but less anxiety (17.4%) than the general population (19.3% and 25.8%, respectively), although this difference failed to reach significance.
Finally, a meta-analysis was conducted on five studies. Two studies were not included because they did not have a control group22 or there were too few subjects with a diagnosis of anxiety and/or depression.25 Patients with AS had greater odds of developing depression and anxiety when compared to controls (odds ratio = 4.93, 95% CI = 2.91–8.35, p < .01; Figure 2).
FIGURE 2.
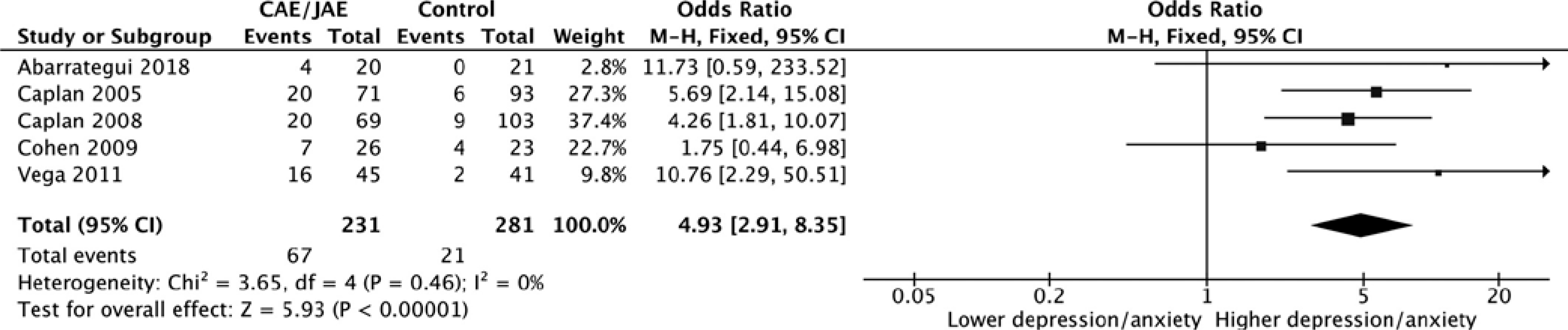
Forest plot assessing the odds of developing anxiety and/or depression in patients with absence epilepsy. The analysis of five studies showed that patients with absence seizures had higher odds of developing depression and/or anxiety (odds ratio = 4.93, 95% confidence interval [CI] = 2.91–8.35, p < .01). CAE, childhood absence epilepsy; JAE, juvenile absence epilepsy; M-H, Mantel-Haenszel
3.2 |. Animal studies
We divided the studies based on the animal models. We further divided the studies based on interventions, including pharmacological, environmental, and nutritional. The animal studies are described in Tables 2 and 3.
TABLE 2.
Animal studies reporting an association between absence seizures and depression or anxiety without any intervention
| Article | Year of publication | Model | Behavioral tests | Analysis type | Main findings |
|---|---|---|---|---|---|
| Bouilleret et al.97 | 2009 | GAERS | Open field | Volumetric MRI (region-of-interest analysis) | Increase in amygdala, cortices, and ventricular volumes when compared to NEC rats. HDM-LD shows hippocampal volume loss. Increase in anxious behavior due to open field test. |
| Jones et al.16 | 2008 | GAERS | Open field, elevated plus maze, sucrose preference | NA | Increase in depressive and anxious behavior due to behavioral tests. |
| Marks et al.53 | 2016 | GAERS | Elevated plus maze, open field | NA | Increase in anxious behavior due to behavioral tests. |
| Marques-Carneiro et al.98 | 2014 | GAERS | Open field, elevated plus maze | Stereological analysis | Increase in anxious behavior due to behavioral tests. Decrease in amygdala and increased thalamic volume when compared to NEC rats. Hippocampal volume was similar in all strains. |
Abbreviations: GAERS, Genetic Absence Epilepsy From Strasbourg; HDM-LD, large-deformation high-dimensional mapping; MRI, magnetic resonance imaging; NA, not available; NEC, nonepileptic controls.
TABLE 3.
Animal studies reporting an association between absence seizures and depression or anxiety along with interventions
| Article | Year of publication | Model | Behavioral tests | Intervention | Administration | Effect of intervention on seizures | Effect of intervention on behavioral disorders |
|---|---|---|---|---|---|---|---|
| Sarkisova et al.37 | 2003 | WAG/Rij | Forced swim, sucrose consumption, open field, light-dark choice, elevated plus maze | Imipramine | Subchronic (15 days) | NA | ↓ depressive behavior; no effect on anxious behavior |
| Sarkisova et al.32 | 2008 | WAG/Rij | Forced swim, sucrose consumption, sucrose preference, open field, light-dark choice | Imipramine, parlodel | Subchronic (15 days) | NA | ↓ depressive behavior; no effect on anxious behavior |
| Shaw et al.52 | 2009 | Long-Evans | Forced swim, sucrose consumption, elevated plus maze, open field | Ethosuximide | Acute | ↓ number of seizures | ↓ depressive behavior; no effect on anxious behavior |
| Sarkisova et al.13 | 2010 | WAG/Rij | Forced swim, sucrose consumption, sucrose preference, light-dark choice, open field | Ethosuximide | Early chronic (P21, 17 days), prolonged early chronic (P21, 152 days) |
↓ number of SWDs | ↓ depressive behavior; no effect on anxious behavior |
| Russo et al.41 | 2011 | WAG/Rij | Forced swim | Carbamazepine, ethosuximide, levetiracetam, zonisamide | Early chronic (P30, 119 days) | ↓ development of seizures and sharp-wave discharges with ethosuximide, levetiracetam, and zonisamide; no effect with carbamazepine | ↓ depressive behavior with ethosuximide; ↑ depressive behavior with levetiracetam; no effect with carbamazepine and zonisamide |
| Russo et al.42 | 2011 | WAG/Rij | Forced swim | Vigabatrin | Subchronic (5 days), early chronic (P30, 119 days) | ↑ number of seizures with subchronic; ↓ number of seizures with early chronic | ↓ depressive behavior |
| Huang et al.15 | 2012 | Long-Evans | Forced swim, sucrose consumption, sucrose preference, elevated plus maze, open field | Lamotrigine | Chronic (35 days) | ↓ number and duration of SWDs | ↓ depressive behavior and anxious behavior |
| Kovacs et al.34 | 2012 | WAG/Rij | Sucrose preference | Clomipramine | Early subchronic (<P21, 14 days) | ↓ sharp-wave discharge activity | ↑ depressive behavior |
| Russo et al.43 | 2013 | WAG/Rij | Forced swim, sucrose preference, elevated plus maze, open field | Aripiprazole | Subchronic (>14 days) | ↓ number and duration of SWDs | ↓ depressive and anxious behavior |
| Russo et al.44 | 2013 | WAG/Rij | Forced swim, sucrose preference | Rapamycin | Acute, subchronic (7 days), early chronic (P45, 119 days) | ↓ number and duration of seizures; ↓ seizure development with early chronic | ↓ depressive behavior with subchronic; ↑ depressive behavior with early chronic; no effect with acute |
| van Luijtelaar et al.40 | 2013 | WAG/Rij | Forced swim, sucrose preference | Ethosuximide | Two months treatment and 4 months treatment | ↓ development of SWDs | ↓ immobility score on forced swim test and no effect on sucrose preference |
| Citraro et al.30 | 2014 | WAG/Rij | Forced swim, open field | Statins | Acute, chronic (119 days), early chronic (<P45, 119 days) | ↓ development of seizures with early chronic; no effect with acute or chronic | ↓ depressive and anxious behavior with early chronic; no effect with acute or chronic |
| Russo et al.46 | 2014 | WAG/Rij | Forced swim | Lipopolysaccharide | Acute | ↑ number and duration of seizures | ↑ depressive behavior |
| Citraro et al.33 | 2015 | WAG/Rij | Forced swim | Haloperidol, risperidone, quetiapine, fluoxetine, duloxetine | Subchronic (49 days), chronic (119 days), early chronic (<P45, 119 days) | ↑ seizure incidence with chronic haloperidol and risperidone; no effect on seizure incidence with chronic quetiapine; no effect on seizure development with early chronic haloperidol, risperidone, or quetiapine; ↓ incidence of nonestablished seizures with early chronic fluoxetine and duloxetine; ↓ incidence of established seizures with chronic duloxetine | ↑ depressive behavior with early chronic haloperidol and risperidone; no effect with quetiapine and duloxetine; ↓ depressive behavior with early chronic fluoxetine |
| Ari et al.31 | 2016 | WAG/Rij | Elevated plus maze | Ketone supplements | Subchronic (7 days), chronic (83 days) | NA | ↓ anxious behavior |
| Citraro et al.47 | 2017 | WAG/Rij | Forced swim, sucrose preference, open field | Perampanel | Acute, subchronic (7 days), early chronic (<P30, 152 days) | No effect with acute and subchronic; ↓ seizure development with chronic | ↓ depressive behavior with early chronic; no effect on anxious behavior |
| Moyanova et al.49 | 2018 | WAG/Rij | Sucrose preference, forced swim | Melatonin | Acute, subchronic (18 days) | No effect on incidence or circadian rhythm of seizures | ↓ depressive behavior with subchronic |
| Sarkisova & Gabova50 | 2018 | WAG/Rij | Forced swim | Maternal care | Prolonged early chronic (>212 days) | ↓ number and duration of seizures | ↓ depressive behavior |
| Leo et al.35 | 2019 | WAG/Rij | Forced swim | Ethosuximide | Chronic (17 days) | NA | ↓ depressive behavior |
| Aygun36 | 2020 | WAG/Rij | forced swim, open field | trazodone | Acute | ↑ number and duration of SWDs | ↑ depressive and anxious behavior |
| Citraro et al.48 | 2020 | WAG/Rij | Forced swim, elevated plus maze | Valproic acid, sodium butyrate | Early chronic (P30, 152 days), prolonged early chronic (P30, 212 days) | ↓ number and duration of SWDs | ↓ depressive behavior with early chronic; ↓ depressive behavior with prolonged early chronic combined administration only; no effect on anxious behavior |
| Dezsi et al.8 | 2013 | GAERS | Open field | Ethosuximide | Early chronic (P21, 154 days) | ↓ frequency of seizures | ↓ anxious behavior |
| Dezsi et al.56 | 2016 | GAERS | Open field, elevated plus maze, light-dark box | Enriched vs. standard environment | Chronic (42 days), early chronic (P21, 77 days) prolonged early chronic (P30, 140 days) | ↓ development and frequency of seizures | ↓ anxious behavior |
| Marks et al.55 | 2019 | GAERS | Open field locomotion, elevated plus maze | Z944, pan-T-type calcium channel antagonist | Acute | NA | ↑ anxious behavior |
Note: Imipramine: antidepressant; parlodel, dopamine agonist; ethosuximide, antiepileptic; carbamazepine: antiepileptic; levetiracetam: antiepileptic; zonisamide: antiepileptic; vigabatrin: antiepileptic; lamotrigine: antiepileptic; clomipramine: antidepressant; aripiprazole: antipsychotic; rapamycin: mammalian target of rapamycin inhibitor; statins: cholesterol synthesis inhibitor; haloperidol: antipsychotic; risperidone: antipsychotic; quetiapine: antipsychotic; fluoxetine: antidepressant; perampanel: antiepileptic; trazodone: antidepressant; valproic acid: antiepileptic.
Abbreviations: GAERS, Genetic Absence Epilepsy From Strasbourg; NA, not available; P, postnatal day; SWD, spike-and-wave discharge; WAG/Rij, Wistar Albino Glaxo From Rijswijk.
3.2.1 |. WAG/Rij rats
The WAG/Rij strain is an inbred rat strain that spontaneously emits cortical SWDs together with clinical manifestations of seizures. WAG/Rij rats begin to show SWDs at the age of 2–3 months.26,27 WAG/Rij rats behave similarly to nonepileptic rats in terms of spontaneous activity, reproductive behaviors, feeding, social interactions, and learning positively or negatively reinforced tasks.28,29 It has been suggested that WAG/Rij rats display more depressive-like and anxious behaviors than control rats.30–35 Depressive-like behavior and anxiety in rats is standardly evaluated through two different behavioral tests, the forced swim test and the sucrose preference test, and anxiety is evaluated through tests involving an open field or an elevated plus maze. Studies have shown an increase in immobility time in forced swim tests, reduced sucrose intake in WAG/Rij rats compared to Wistar control rats,33–35 and less exploration of the field and elevated plus maze.30–32
Antidepressants
A series of pharmacological interventions have been evaluated to explore new avenues of treatment that link depression and anxiety to AS in WAG/Rij rats. Antidepressants have been used to explore the interplay between AS and depressive-like behaviors in rat models of AS.32,36,37 Imipramine, a tricyclic antidepressant (TCA) noted for its antiepileptogenic effects, was found to decrease depressive behavior by reducing immobility times during the forced swim test and by increasing the preference for sucrose in the sucrose preference test.37 This was particularly evident with subchronic administration (e.g., 15 days). However, imipramine had no significant effects on anxiety as measured by elevated plus maze.37 The mechanism of action was explored further through the introduction of parlodel, a dopamine agonist, and raclopride, a dopamine antagonist.32 It was found that raclopride was able to block antidepressant effects of imipramine as suggested by longer duration of immobility in a forced swim test, suggesting that dopamine was involved in mediating depressive-like behaviors in the WAG/Rij model.32
The effects of clomipramine, a TCA, were investigated under a similar premise. It was found that early subchronic administration of clomipramine significantly reduced SWDs. However, clomipramine also evoked behavioral symptoms of depressive-like behaviors in the rat model, indicated by a decreased score on the sucrose preference test as compared to the control group. One possible explanation could be that early clomipramine treatment may restrain the normal development of serotonergic, monoaminergic, and cholinergic systems.34 An alternative explanation is that clomipramine may have been administered for an insufficient duration to reduce depressive behaviors. Citraro et al. attempted to address the latter possibility by introducing different timelines of treatment.33 It was found that early chronic treatment with selective serotonin reuptake inhibitor antidepressants, such as fluoxetine and duloxetine, had antiepileptogenic effects when administered before seizure onset in WAG/Rij rats. Early chronic treatment with fluoxetine also significantly decreased immobility times during forced swim tests.33 These findings reinforce the importance of intervention windows and length of treatment to developmental and behavioral outcomes. Results from these diverse studies suggest that antidepressants have potential benefits in terms of treatment of AS and its behavioral comorbidities, depending on dose, time of intervention, and length of treatment.
Anticonvulsants
Ethosuximide (ESX) has been an extensively studied anticonvulsant therapy due to its clear clinical benefits in AS treatment.8,38,39 Several studies showed that early and chronic treatments with ESX prevented the occurrence of AS and comorbid depressive-like symptoms by blocking the occurrence of SWDs in a WAG/Rij model.13,40 Van Luijtelaar et al.40 demonstrated a significant increase in sucrose preference, whereas Sarkisova et al.13 showed complete resolution of SWDs and a considerable increase in sucrose preference. These findings suggest that it is possible that full resolution of AS is required for improvement of behavioral symptoms. The beneficial effects of ESX were further confirmed through an early intervention, which reduced both epileptic and depressive features in comparison to other anticonvulsants.41 ESX has also been shown to significantly decrease immobility time during forced swim tests as compared to controls.35 However, whereas ESX intervention seemed to be successful in reducing depressive symptoms, it did not have any significant effects on anxious behaviors.
Other anticonvulsant interventions have also been shown to reduce behavioral markers of depression and anxiety. Although less applicable from a clinical perspective, it was found that early chronic intervention with vigabatrin, an AED and a γ-aminobutyric acid (GABA) analogue, decreases the incidence of AS and reduces immobility time during forced swim tests.42 The authors attributed the antidepressant effect of vigabatrin to its effects on seizure development.42 This result motivated further research on aripiprazole,43 an atypical antipsychotic, and rapamycin, a mammalian target of rapamycin,44 which were observed to decrease the number of seizures and depressive behavior when administered at subchronic doses (7 days).
Although early chronic (119 days) intervention with rapamycin decreased the development and number of seizures, it also evoked depressive behaviors, which could be due to the direct effects of rapamycin on appetite.44,45 Furthermore, the beneficial antidepressant and anticonvulsant effects of subchronic rapamycin administration were reversed through lipopolysaccharide administration.46 Lipopolysaccharide was later also found to have convulsant and depressive outcomes even upon acute administration.46 These studies support earlier claims that the presence of AS and depressive symptoms are interconnected.
The optimal duration of intervention may vary among anticonvulsant medications, with some antipsychotic medications ineffective with early chronic administration,33 and other early chronic AED treatments successful.47 Similarly, only early chronic treatments with combined valproic acid and sodium butyrate reduced the incidence of both AS and depressive behaviors, and a shorter duration of treatment increased the incidence of depressive behaviors.48 However, anxiety behaviors were not affected by any combination of anticonvulsant administration.
Nutrition and environment
Although the development of AS is believed to be partially genetically determined, there is evidence that nutrition and other environmental factors have an impact on seizure development. Early chronic administration of statins significantly reduced the occurrence of AS and produced both antidepressant and anxiolytic effects on behavior.30 A high ketone diet was also noted to have antiepileptogenic and anxiolytic effects.31 Chronic and subchronic ketone supplements reduced AS and anxiety-related behavior in WAG/Rij rats that were tested on the elevated plus maze.31
It was also found that defective hippocampal melatoninergic systems could contribute to the presence of depressive symptoms in WAG/Rij rats.49 Lower levels of melatonin in the hippocampus correlated with lower sucrose preference test scores and increased immobility during forced swim tests.49 Chronic intervention (18 days) with melatonin after pinealectomy caused an increase in the sucrose preference test score.49 There has also been evidence that rearing affects the development of AS. WAG/Rij rats had fewer seizures and displayed fewer depressive behaviors when fostered with control mothers immediately after birth.50
3.2.2 |. LE rats
The LE rat strain is characterized by high-voltage rhythmic spike discharges accompanied by immobility and whisker twitching. These rhythmic spike discharges share similarities with SWDs in AS.51
Anticonvulsants
As with other AS rat models, anticonvulsant medication has been the primary vehicle of pharmacological intervention for LE rats. Lamotrigine (LTG), an AED, was found to decrease the frequency and duration of SWDs in an LE model.15 Chronic administration of LTG effectively reduced AS and ameliorated anxious and depressive behaviors.15 Similarly, acute administration of ESX effectively reduced depressive-like symptoms 30 min after treatment in LE rats but did not have any effect on anxious behaviors.52
3.2.3 |. GAERS rats
The GAERS model is a strain of rats that exhibit the spontaneously occurring AE phenotype, which is similar to human AE.53 GAERS display recurrent generalized nonconvulsive seizures based on bilateral and synchronous SWDs that are accompanied by staring, behavioral arrest, and at times twitching of the whiskers.54
Anticonvulsants
Similar to its application for WAG/Rij and LE models, ESX has been used as one of the main pharmacological interventions in the GAERS model. In GAERS rats, ESX has been observed to reduce the frequency of seizures and anxious behavior.8 Another intervention that has been studied is Z944, a pan-T-type calcium channel antagonist, which has been shown to increase anxious behavior.55
Environment
The effect of environmental exposures on epilepsy development and severity has been studied in multiple models. For GAERS rats with environmental enrichment or standard housing before or after the onset of epilepsy, a decrease in the development and frequency of seizures, along with a decrease in anxious behavior, was observed.56
4 |. DISCUSSION
The bidirectional relationship between epilepsy and psychiatric disorder, including depression and anxiety, is not a new concept.16,57 Patients with depression and anxiety are at increased likelihood of a diagnosis of epilepsy later in life, and epilepsy is associated with a higher incidence of various psychiatric disorders such as depression, anxiety, and suicidal ideation.58,59 A recent study conducted using 10 million patient health records suggested a relationship between depression and epilepsy, with each condition considered a risk factor for the other.59 Similarly, a longitudinal cohort study conducted using the UK General Practice Research Database and several other worldwide studies have described the bidirectional association between anxiety and epilepsy.58,60–65 Another study reported higher rates of depressive disorders and anxiety disorders in children with recent onset epilepsy, along with the observation that in some cases, psychiatric disorder antedates epilepsy onset.9 The presence of mood disorders before seizures, therefore, suggests common neurobiological influences and challenges the observation that mood disorders result from AS.
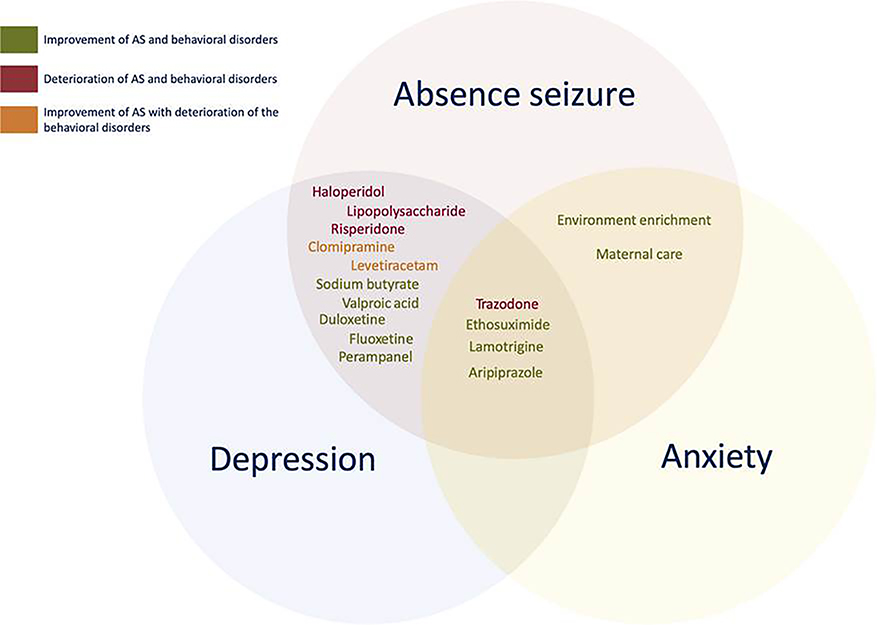
Our systematic review of the literature presents evidence describing a robust association between AS and depression and anxiety. All of the human studies included in this review showed a significant interaction between these behavioral comorbidities. The meta-analysis also revealed significantly higher odds of a diagnosis of depression or anxiety in patients with AE. The bidirectionality was further supported by studies of animal models, which showed the time-dependent and dose-dependent antidepressant effects of AEDs as well as the anticonvulsive effects of antidepressants. Figure 3 illustrates the effects of various AEDs and antidepressants on AS and associated behavioral disorders. These findings suggest a bidirectional relationship between AE and psychiatric disorders, which hints at the possibility of shared factors, such as similar genetic predisposition, neurobiological pathways, and anatomical connections that have an effect on both AE and psychiatric disorders.
FIGURE 3.
Effect of different interventions on absence seizures (AS) and associated depression and anxiety in the genetic models of absence epilepsy. Each circle represents AS or associated behavioral disorders (depression or anxiety). The drugs in each circle were administered to the animals to test their effects on seizures along with respective behavioral disorder(s). The color of the text illustrates the type of effect for each drug: improvement of both seizures and associated behavioral disorder(s) (green), improvement of seizures with the deterioration of the behavioral disorder(s) (orange), or deterioration of both seizures and behavioral disorder(s) (red)
In animal models of AE, depressive-like behaviors exist before and after the emergence of seizures, suggesting that psychiatric comorbidities are seen not only secondarily to AE.16 Although the exact pathogenesis of AE in humans and animal models is not clearly understood, there is strong evidence for a genetic component. Current consensus holds that AE has a complex genotype, with a 16%–45% positive family history.66 Concordances of 70%–85% and 33% have been reported in monozygotic twins and first-degree relatives, respectively.66,67 Mutation in the GABAA receptor has been associated with CAE.68 Also, alteration in the GABAA receptor has been shown to contribute to the etiology of major depressive disorder.69 Deficits in GABAA neurotransmission in GABAA receptor-deficient mice caused behavioral and cognitive disorders.70 Similarly, deficits in GABA neurotransmission are also implicated in anxiety spectrum disorders.71–73
Disturbances in several key neurotransmitter systems are implicated in AE and depression, notably GABAergic and glutamatergic systems. Impaired functioning of glutamatergic and GABAergic pathways has been recognized in AS74,75 as well as in depression and anxiety.76 Although the actual mechanisms are complex and not fully understood,77,78 many pharmacologic and neuroimaging studies have described the role of these neurotransmitters in depression. Specifically, using proton magnetic resonance spectroscopy, abnormal concentrations of glutamate79 and GABA80,81 were identified in patients with depression. The possible therapeutic role of glutamate receptor modulators in AS82 and depression77 also suggests bidirectionality between the two conditions.
Neuroanatomically, various cortical and thalamic areas are implicated in AS, depression,83,84 and anxiety disorders.85 Specifically, Holmes et al. demonstrated that the spikes in AS are generated unilaterally in the dorsolateral frontal region and spread to other frontal cortical areas before evolving into spike-and-wave cycles.86 Additionally, cortical spike generation requires an intact thalamocortical network,87 implicating corticothalamic circuits in pathogenesis of AS. The corticothalamic circuit also plays a central role in cognition88 and depression.89 Disruption of the corticostriatal–thalamic circuit has been shown to impair cognitive control and regulation of emotions in patients with depression.89 Furthermore, animals exhibiting AS have increased serotonin metabolism in the prefrontal cortex and decreased metabolism in the thalamus.90 Ventrolateral prefrontal cortex involvement85 and impaired 5-hydroxytryptamine metabolism7,91 are also found in anxiety disorders.
Furthermore, several studies have suggested that limbic structures, such as the nucleus accumbens, and hypothalamic–brain stem changes may play an important role in both AS epileptogenesis and its behavioral comorbidities.92 Vega et al. found that a few subsets of affective symptoms including social isolation and low self-esteem are more prevalent in children with AE than their peers.23 Interestingly, a recent study showed that a prefrontal–paraventricular thalamus circuit is involved in juvenile social isolation.93 Moreover, a study conducted by van Luijtelaar et al. showed that the chronic treatment of ESX, a thalamic calcium channel blocker and the drug of choice for AE, decreases immobility score on the forced swim test and has no effect on the sucrose preference test.40 The forced swim test is despair related,94 likely involving corticolimbic regions, whereas the sucrose preference test reflects the hedonic pleasure experienced by the animal,95 thus primarily involving the striatal circuits. Selective modulation of depressive symptoms by anticonvulsants and selective subsets of affective symptoms in AE highlight the finding that the behavioral deficits associated with AE appear to follow specific circuits common to both diseases rather than being a global or distributed effect of epileptogenesis.
Limitations of this review include the difficulty of isolating factors that contribute to AS and depression. The most recent literature has not been able to determine conclusively the underlying mechanisms of conditions such as AS and depression due to their complex neuronal network integrations, which makes their secondary effects difficult to compare. In addition, although AE is primarily a pediatric disorder,4 previous studies with both GAERS and WAG/Rij rats have also reported age-dependent phenotypic features of SWDs, although they differ from what is observed in clinical AE.96 Although it has been established that long-term psychiatric comorbidities are worse in patients with longer duration of illness and persist even after seizures subside,18 the possible effects of age on the relationship between seizures and mood disorders have not yet been reported. Another limitation is the relatively small size in the clinical studies, which could lead to publication bias. Further extensive and well-designed studies are still required to understand these integral associations better. Similarly, the review is limited by fewer studies on anxiety than depression, as anxiety is an understudied topic that is harder to diagnose, especially in animal models.
4.1 |. Conclusion
Genetic, neuropathological, neuroanatomic, environmental, and neurotransmitter disturbances associated with both AS and behavioral comorbidities support a bidirectional relationship between these psychiatric and neurological disorders. However, it is essential to emphasize that not all associations and findings observed in animal studies will translate to humans. Therefore, rigorous testing of these hypotheses with additional experiments in different animal models and humans is necessary. Nevertheless, there is diverse and strong evidence that suggests a bidirectional causality in behavioral and seizure phenotypes of AE. As the shared causal antecedents of these disorders continue to be elucidated, they may be utilized to study the pathogenesis of AE, depression, and anxiety, and to provide new therapeutic options.
Supplementary Material
Key Points.
The meta-analysis shows that patients with absence seizures had greater odds of developing depression and anxiety
The known genetic animal models of absence seizures exhibit behavioral disorders
Antiepileptic drugs and antidepressant drugs affect both absence seizures and mood disorders, suggesting common psychopathology
This systematic review suggests bidirectionality between absence seizures and associated depression and anxiety
ACKNOWLEDGMENTS
This work was supported by a grant from the Foundation for Anesthesia Education and Research (FAER) awarded to B.F.G., who completed his PhD in the Investigative Medicine Program at Yale University which is supported by Clinical and Translational Science Award grant number UL1 TR001863 from the National Center for Advancing Translational Science, a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Funding information
Foundation for Anesthesia Education and Research (FAER); National Center for Advancing Translational Science, Grant/Award Number: UL1 TR001863; National Institutes of Health (NIH)
Footnotes
CONFLICT OF INTEREST
G.S. has provided consulting services to Allergan, Axsome Therapeutics, Biohaven Pharmaceuticals, Boehringer Ingelheim International, Bristol-Myers Squibb, Celexio Biosciences, Denovo Biopharma, Epiodyne, Intra-Cellular Therapies, Janssen, Lundbeck, Minerva Pharmaceutical, Navitor Pharmaceuticals, Neurocrine, NeuroRX, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis, Seelos Therapeutics, and Vistagen Therapeutics in the past 12 months. He has received funds for contracted research from Janssen Pharmaceuticals, Merck, and the Usona Institute. He holds equity in Biohaven Pharmaceuticals and has received royalties from Yale University from patent licenses with Biohaven Pharmaceuticals. None of the other authors has any conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Tenney JR, Glauser TA. The current state of absence epilepsy: can we have your attention? Epilepsy Curr. 2013;13:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niedermeyer E Primary (idiopathic) generalized epilepsy and underlying mechanisms. Clin Electroencephalogr. 1996;27:1–21. [DOI] [PubMed] [Google Scholar]
- 3.Panayiotopoulos CP. Typical absence seizures and their treatment. Arch Dis Child. 1999;81:351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posner E Absence seizures in children. BMJ Clin Evid. 2008;2008:0317. [PMC free article] [PubMed] [Google Scholar]
- 5.Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43(Suppl 3):27–32. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JR. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav. 2009;15:404–12. [DOI] [PubMed] [Google Scholar]
- 7.Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–46. [DOI] [PubMed] [Google Scholar]
- 8.Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O’Brien TJ, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia. 2013;54:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JE, Watson R, Sheth R, Caplan R, Koehn M, Seidenberg M, et al. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–7. [DOI] [PubMed] [Google Scholar]
- 10.Ott D, Caplan R, Guthrie D, Siddarth P, Komo S, Shields WD, et al. Measures of psychopathology in children with complex partial seizures and primary generalized epilepsy with absence. J Am Acad Child Adolesc Psychiatry. 2001;40:907–14. [DOI] [PubMed] [Google Scholar]
- 11.Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005;46:720–30. [DOI] [PubMed] [Google Scholar]
- 12.Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256–67.e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkisova KY, Kuznetsova GD, Kulikov MA, van Luijtelaar G. Spike-wave discharges are necessary for the expression of behavioral depression-like symptoms. Epilepsia. 2010;51:146–60. [DOI] [PubMed] [Google Scholar]
- 14.Sarkisova K, Kulikov MA, Shatskova AB. Are WAG/Rij rats with genetic absence epilepsy anxious? [in Russian]. Zh Vyssh Nerv Deiat Im I P Pavlova. 2005;55:253–61. [PubMed] [Google Scholar]
- 15.Huang HY, Lee HW, Chen SD, Shaw FZ. Lamotrigine ameliorates seizures and psychiatric comorbidity in a rat model of spontaneous absence epilepsy. Epilepsia. 2012;53:2005–14. [DOI] [PubMed] [Google Scholar]
- 16.Jones NC, Salzberg MR, Kumar G, Couper A, Morris MJ, O’Brien TJ. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp Neurol. 2008;209:254–60. [DOI] [PubMed] [Google Scholar]
- 17.Olsson I, Campenhausen G. Social adjustment in young adults with absence epilepsies. Epilepsia. 1993;34:846–51. [DOI] [PubMed] [Google Scholar]
- 18.Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. sometimes a wolf in sheeps’ clothing. Arch Pediatr Adolesc Med. 1997;151:152–8. [DOI] [PubMed] [Google Scholar]
- 19.Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abarrategui B, Parejo-Carbonell B, Garcia Garcia ME, Di Capua D, Garcia-Morales I. The cognitive phenotype of idiopathic generalized epilepsy. Epilepsy Behav. 2018;89:99–104. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel D, Ventura M, Samões R, Freitas J, Lopes J, Ramalheira J, et al. Social impairment and stigma in genetic generalized epilepsies. Epilepsy Behav. 2020;104:106886. [DOI] [PubMed] [Google Scholar]
- 23.Vega C, Guo J, Killory B, Danielson N, Vestal M, Berman R, et al. Symptoms of anxiety and depression in childhood absence epilepsy. Epilepsia. 2011;52:e70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreibman Cohen A, Daley M, Siddarth P, Levitt J, Loesch IK, Altshuler L, et al. Amygdala volumes in childhood absence epilepsy. Epilepsy Behav. 2009;16:436–41. [DOI] [PubMed] [Google Scholar]
- 25.Precenzano F, Lombardi RM, Parisi L, Salerno M, Maltese A. Internalizing symptoms in children affected by childhood absence epilepsy: a preliminary study. Acta Med Mediterr. 2016;32:1749–53. [Google Scholar]
- 26.Coenen AM, Van Luijtelaar EL. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1987;1:297–301. [DOI] [PubMed] [Google Scholar]
- 27.van Luijtelaar ELJM, Coenen AML. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci Lett. 1986;70:393–7. [DOI] [PubMed] [Google Scholar]
- 28.Coenen AML, Drinkenburg WHIM, Peeters BWMM, Vossen JMH, van Luijtelaar ELJM. Absence epilepsy and the level of vigilance in rats of the WAG/Rij strain. Neurosci Biobehav Rev. 1991;15:259–63. [DOI] [PubMed] [Google Scholar]
- 29.Vergnes M, Marescaux C, Boehrer A, Depaulis A. Are rats with genetic absence epilepsy behaviorally impaired? Epilepsy Res. 1991;9:97–104. [DOI] [PubMed] [Google Scholar]
- 30.Citraro R, Chimirri S, Aiello R, Gallelli L, Trimboli F, Britti D, et al. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55:1284–91. [DOI] [PubMed] [Google Scholar]
- 31.Ari C, Kovács Z, Juhasz G, Murdun C, Goldhagen CR, Koutnik AM, et al. Exogenous ketone supplements reduce anxiety-related behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front Mol Neurosci. 2016;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkisova KY, Kulikov MA, Midzyanovskaya IS, Folomkina AA. Dopamine-dependent nature of depression-like behavior in WAG/Rij rats with genetic absence epilepsy. Neurosci Behav Physiol. 2008;38:119–28. [DOI] [PubMed] [Google Scholar]
- 33.Citraro R, Leo A, De Fazio P, De Sarro G, Russo E. Antidepressants but not antipsychotics have antiepileptogenic effects with limited effects on comorbid depressive-like behaviour in the WAG/Rij rat model of absence epilepsy. Br J Pharmacol. 2015;172:3177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacs Z, Czurko A, Kekesi KA, Juhasz G. Neonatal tricyclic antidepressant clomipramine treatment reduces the spike-wave discharge activity of the adult WAG/Rij rat. Brain Res Bull. 2012;89:102–7. [DOI] [PubMed] [Google Scholar]
- 35.Leo A, Citraro R, Tallarico M, Iannone M, Fedosova E, Nesci V, et al. Cognitive impairment in the WAG/Rij rat absence model is secondary to absence seizures and depressive-like behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109652. [DOI] [PubMed] [Google Scholar]
- 36.Aygun H Trazodone increases seizures in a genetic WAG/Rij rat model of absence epilepsy while decreasing them in penicillin-evoked focal seizure model. Epilepsy Behav. 2020;103:106847. [DOI] [PubMed] [Google Scholar]
- 37.Sarkisova KY, Midzianovskaia IS, Kulikov MA. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav Brain Res. 2003;144:211–26. [DOI] [PubMed] [Google Scholar]
- 38.Hwang H, Kim H, Kim SH, Kim SH, Lim BC, Chae J-H, et al. Long-term effectiveness of ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. Brain Dev. 2012;34:344–8. [DOI] [PubMed] [Google Scholar]
- 39.Leo A, Caro CD, Nesci V, Palma E, Tallarico M, Iannone M, et al. Antiepileptogenic effects of ethosuximide and levetiracetam in WAG/Rij rats are only temporary. Pharmacol Rep. 2019;71:833–8. [DOI] [PubMed] [Google Scholar]
- 40.van Luijtelaar G, Mishra AM, Edelbroek P, Coman D, Frankenmolen N, Schaapsmeerders P, et al. Anti-epileptogenesis: electrophysiology, diffusion tensor imaging and behavior in a genetic absence model. Neurobiol Dis. 2013;60:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo E, Citraro R, Scicchitano F, De Fazio S, Perrotta I, Di Paola ED, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011;52:1341–50. [DOI] [PubMed] [Google Scholar]
- 42.Russo E, Citraro R, Scicchitano F, Urzino A, Marra R, Rispoli V, et al. Vigabatrin has antiepileptogenic and antidepressant effects in an animal model of epilepsy and depression comorbidity. Behav Brain Res. 2011;225:373–6. [DOI] [PubMed] [Google Scholar]
- 43.Russo E, Citraro R, Davoli A, Gallelli L, Di Paola ED, De Sarro G. Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology. 2013;64:371–9. [DOI] [PubMed] [Google Scholar]
- 44.Russo E, Citraro R, Donato G, Camastra C, Iuliano R, Cuzzocrea S, et al. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology. 2013;69:25–36. [DOI] [PubMed] [Google Scholar]
- 45.Scarpace PJ, Matheny M, Strehler KYE, Toklu HZ, Kirichenko N, Carter CS, et al. Rapamycin normalizes serum leptin by alleviating obesity and reducing leptin synthesis in aged rats. J Gerontol A Biol Sci Med Sci. 2016;71:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo E, Andreozzi F, Iuliano R, Dattilo V, Procopio T, Fiume G, et al. Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav Immun. 2014;42:157–68. [DOI] [PubMed] [Google Scholar]
- 47.Citraro R, Leo A, Franco V, Marchiselli R, Perucca E, De Sarro G, et al. Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive like behavior. Epilepsia. 2017;58:231–8. [DOI] [PubMed] [Google Scholar]
- 48.Citraro R, Leo A, De Caro C, Nesci V, Cantafio MGE, Amodio N, et al. Effects of histone deacetylase inhibitors on the development of epilepsy and psychiatric comorbidity in WAG/Rij rats. Mol Neurobiol. 2020;57:408–21. [DOI] [PubMed] [Google Scholar]
- 49.Moyanova S, De Fusco A, Santolini I, Celli R, Bucci D, Mastroiacovo F, et al. Abnormal hippocampal melatoninergic system: a potential link between absence epilepsy and depression-like behavior in WAG/Rij rats? Int J Mol Sci. 2018;19:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarkisova KY, Gabova AV. Maternal care exerts disease-modifying effects on genetic absence epilepsy and comorbid depression. Genes Brain Behav. 2018;17:e12477. [DOI] [PubMed] [Google Scholar]
- 51.Shaw FZ. Is spontaneous high-v oltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J Neurophysiol. 2004;91:63–77. [DOI] [PubMed] [Google Scholar]
- 52.Shaw FZ, Chuang SH, Shieh KR, Wang YJ. Depression-and anxiety-like behaviors of a rat model with absence epileptic discharges. Neuroscience. 2009;160:382–93. [DOI] [PubMed] [Google Scholar]
- 53.Marks WN, Cavanagh ME, Greba Q, Cain SM, Snutch TP, Howland JG. The Genetic Absence Epilepsy Rats From Strasbourg model of absence epilepsy exhibits alterations in fear conditioning and latent inhibition consistent with psychiatric comorbidities in humans. Eur J Neurosci. 2016;43:25–40. [DOI] [PubMed] [Google Scholar]
- 54.Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg—a review. J Neural Transm Suppl. 1992;35:37–69. [DOI] [PubMed] [Google Scholar]
- 55.Marks WN, Zabder NK, Cain SM, Snutch TP, Howland JG. The T-type calcium channel antagonist, Z944, alters social behavior in Genetic Absence Epilepsy Rats From Strasbourg. Behav Brain Res. 2019;361:54–64. [DOI] [PubMed] [Google Scholar]
- 56.Dezsi G, Ozturk E, Salzberg MR, Morris M, O’Brien TJ, Jones NC. Environmental enrichment imparts disease-m odifying and transgenerational effects on genetically-determined epilepsy and anxiety. Neurobiol Dis. 2016;93:129–36. [DOI] [PubMed] [Google Scholar]
- 57.Cardamone L, Salzberg MR, O’Brien TJ, Jones NC. Antidepressant therapy in epilepsy: can treating the comorbidities affect the underlying disorder? Br J Pharmacol. 2013;168:1531–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72:184–91. [DOI] [PubMed] [Google Scholar]
- 59.Josephson CB, Lowerison M, Vallerand I, Sajobi TT, Patten S, Jette N, et al. Association of depression and treated depression with epilepsy and seizure outcomes: a multicohort analysis. JAMA Neurol. 2017;74:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ettinger A, Reed M, Cramer J. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63:1008–14. [DOI] [PubMed] [Google Scholar]
- 61.Gaitatzis A, Carroll K, Majeed A, Sander JW. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45:1613–22. [DOI] [PubMed] [Google Scholar]
- 62.Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110:207–20. [DOI] [PubMed] [Google Scholar]
- 63.Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. community study. Epilepsia. 1996;37:148–61. [DOI] [PubMed] [Google Scholar]
- 64.Kobau R, Gilliam F, Thurman DJ. Prevalence of self-reported epilepsy or seizure disorder and its associations with self-reported depression and anxiety: results from the 2004 HealthStyles Survey. Epilepsia. 2006;47:1915–21. [DOI] [PubMed] [Google Scholar]
- 65.Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–44. [DOI] [PubMed] [Google Scholar]
- 66.Berkovic S, Newton M. Epilepsy: a comprehensive textbook. Philadelphia, PA: Lippincott-Raven; 1998. p. 217–24. [Google Scholar]
- 67.Bianchi A, Italian League Against Epilepsy. Studies of concordance of syndromes in families with absence epilepsies. In: Duncan J, Panayiotopoulos CP, editors. Typical absence seizures and related epileptic syndromes. London, UK: Churchill Livingstone; 1995. p. 328–37. [Google Scholar]
- 68.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, et al. Mutant GABA A receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49. [DOI] [PubMed] [Google Scholar]
- 69.Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol. 2015;73:97–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pham X, Sun C, Chen X, van den Oord EJCG, Neale MC, Kendler KS, et al. Association study between GABA receptor genes and anxiety spectrum disorders. Depress Anxiety. 2009;26:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hettema JM, An SS, Neale MC, Bukszar J,van den Oord EJCG, Kendler KS, et al. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006;11:752–62. [DOI] [PubMed] [Google Scholar]
- 73.Olexova L, Stefanik P, Krskova L. Increased anxiety-like behaviour and altered GABAergic system in the amygdala and cerebellum of VPA rats—an animal model of autism. Neurosci Lett. 2016;629:9–14. [DOI] [PubMed] [Google Scholar]
- 74.Touret M, Parrot S, Denoroy L, Belin M-F, Didier-Bazes M. Glutamatergic alterations in the cortex of genetic absence epilepsy rats. BMC Neurosci. 2007;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lőrincz ML, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kugaya A, Sanacora G, Kugaya A, Sanacora A. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–19. [DOI] [PubMed] [Google Scholar]
- 78.Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA Jr. Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr Pharm Des. 2009;15:1595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanacora G, Fenton LR, Fasula MK, Rothman DL, Levin Y, Krystal JH, et al. Cortical γ-aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry. 2006;59:284–6. [DOI] [PubMed] [Google Scholar]
- 81.Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology. 2006;186:425. [DOI] [PubMed] [Google Scholar]
- 82.Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry. 2010;11:165–80. [DOI] [PubMed] [Google Scholar]
- 84.Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–52. [DOI] [PubMed] [Google Scholar]
- 85.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–7. [DOI] [PubMed] [Google Scholar]
- 86.Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–79. [DOI] [PubMed] [Google Scholar]
- 87.Meeren HKM, Pijn JPM, Van Luijtelaar ELJM, Coenen AML, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bora E, Harrison BJ, Davey CG, Yücel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2012;42:671–81. [DOI] [PubMed] [Google Scholar]
- 90.Midzyanovskaya IS, Kuznetsova GD, van Luijtelaar ELJM, van Rijn CM, Tuomisto L, MacDonald E. The brain 5HTergic response to an acute sound stress in rats with generalized (absence and audiogenic) epilepsy. Brain Res Bull. 2006;69:631–8. [DOI] [PubMed] [Google Scholar]
- 91.Bercovici E, Cortez MA, Wang X, Snead OC 3rd. Serotonin depletion attenuates AY-9944-mediated atypical absence seizures. Epilepsia. 2006;47:240–6. [DOI] [PubMed] [Google Scholar]
- 92.Onat FY, van Luijtelaar G, Nehlig A, Snead OC 3rd. The involvement of limbic structures in typical and atypical absence epilepsy. Epilepsy Res. 2013;103:111–23. [DOI] [PubMed] [Google Scholar]
- 93.Yamamuro K, Bicks LK, Leventhal MB, Kato D, Im S, Flanigan ME, et al. A prefrontal–paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat Neurosci. 2020;23(10):1240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012;(59):e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffman KL. What can animal models tell us about depressive disorders? In: Hoffman KL, editor. Modeling neuropsychiatric disorders in laboratory animals. Sawston, UK: Woodhead Publishing; 2016. p. 35–86. [Google Scholar]
- 96.Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–55. [DOI] [PubMed] [Google Scholar]
- 97.Bouilleret V, Hogan RE, Velakoulis D, Salzberg MR, Wang L, Egan GF, et al. Morphometric abnormalities and hyperanxiety in genetically epileptic rats: a model of psychiatric comorbidity? Neuroimage. 2009;45:267–74. [DOI] [PubMed] [Google Scholar]
- 98.Marques-Carneiro JE, Faure J-B, Cosquer B, Koning E, Ferrandon A, de Vasconcelos AP, et al. Anxiety and locomotion in Genetic Absence Epilepsy Rats From Strasbourg (GAERS): inclusion of Wistar rats as a second control. Epilepsia. 2014;55:1460–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.