Abstract
Colorectal cancer(CRC) is one of the most prevalent malignancies in the Asia-Pacific region, and many countries in this region have launched population CRC service screening. In this study, CRC screening key indicators, including the FIT(fecal immunochemical test) screening rate (or participation rate) and the rate of undergoing colonoscopy after positive FIT in 2019 and 2020, were surveyed in individual countries in the Asia-Pacific region. The impact of the pandemic on the effectiveness of CRC screening was simulated given different screening rates and colonoscopy rates and assuming the pandemic would persist or remain poorly controlled for a long period of time, using the empirical data from the Taiwanese program and the CRC natural history model.
During the COVID-19 pandemic, most of the programs in this region were affected, but to different extents, which was largely influenced by the severity of the local pandemic. Most of the programs continued screening services in 2020, although a temporary pause occurred in some countries. The modeling study revealed that prolonged pauses of screening led to 6% lower effectiveness in reducing CRC mortality.
Screening organizers should coordinate with health authorities to elaborate on addressing screening backlogs, setting priorities for screening, and applying modern technologies to overcome potential obstacles. Many novel approaches that were developed and applied during the COVID-19 pandemic, such as the risk-stratified approach that takes into account personal CRC risk and the local epidemic status, as well as new digital technologies, are expected to play important roles in CRC screening in the future.
Abbreviations: FIT, Fecal immunochemical test; CRC, Colorectal cancer; FHbC, Fecal hemoglobin concentration
1. Introduction
1.1. Current CRC burden and population-based screening in the Asia-Pacific region
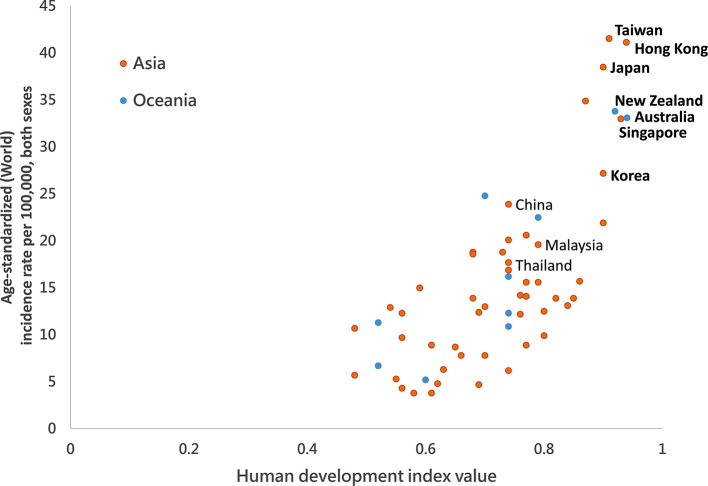
Colorectal cancer (CRC) has become the most prevalent malignancy in the Asia-Pacific area, with an incidence of 17.7/105 and mortality of 8.6/10 (Giorgi Rossi et al., 2015), yielding 1,030,054 incident cases and 514,052 deaths annually. As of 2020, 53% of CRCs are from the Asia-Pacific region and have become one of the major clinical and public health challenges in this area. (Onyoh et al., 2019; Global Cancer Observatory: Cancer Today, n.d.) The incidence of CRC is closely associated with the degree of economic development and the Westernization of lifestyles. (See Fig. 1 ) To mitigate its incidence, screening is the most effective approach to reduce CRC mortality and incidence. The fecal immunochemical test (FIT) is currently the most popular screening modality applied in population-based screens, especially in areas where healthcare resources and colonoscopy capacity are limited, as it may specifically select people at higher risk of CRC from a large population and reduce the demand for colonoscopies. In the Asia-Pacific region, many countries or regions with high CRC incidence have launched population-based CRC screening programs in the past two decades (Schreuders et al., 2015). (See Table 1 ) The effectiveness of FIT screening in reducing CRC mortality and/or incidence has been reported not only in Western countries but also in Asia (Chiu et al., 2015; Giorgi Rossi et al., 2015; Zorzi et al., 2015; Chiu et al., 2021). Japan was the first country in this region to launch a FIT-based population screening, as early as 1992, followed by Korea, Taiwan, and Australia in 2000's, and later by New Zealand in 2017 and Hong Kong in 2020 (rolling out from 2016 to 2019 and full program from 2020 on) (Shim et al., 2010; Saito, 2006; Bowel screening, n.d.; Eligibility of Colorectal Cancer Screening Programme updated, n.d.). There are also several ongoing pilot studies in China, Thailand, and Malaysia (Sarakarn et al., 2017; Li et al., 2018). All the existing programs in Asia-Pacific (Chiu et al., 2021) countries use FIT as the primary screening method, but with different age ranges and interscreen intervals (Onyoh et al., 2019).
Fig. 1.
Correlation between age-standardized colorectal cancer incidence and Human Development Index (HDI) in the Asia-Pacific region.
Bold: Countries with nation- or territory-wide screening programs.
Table 1.
Existent population CRC screening program in the Asia-Pacific region.
| Region/country | Screening modality | Screening interval (year) | Screening program | FIT kit distribution method | Age range | Launch year | |
|---|---|---|---|---|---|---|---|
| Asia | Japan | 2 sample FIT | 1 | Organized | Pick-up or postal maila | 40+ | 1992 |
| Korea | 1 sample FIT | 1 | Organized | Pick-up | 50+ | 2004 | |
| Singapore | 2 sample FIT | 1 | Organized | Pick-up | 50+ | 2009 | |
| Taiwan | 1 sample FIT | 2 | Organized | Pick-up | 50–74 | 2004 | |
| Hong Kong | 1 sample FIT | 2 | Organized | Pick-up | 50–75 | 2020 | |
| Brunei | 1 sample FIT | 2 | Organized | Pick-up | 40+ | 2008 | |
| Thailand | 1 sample FIT | 5 | Pilot | Pick-up | 50–65 | 2011 | |
| Oceania | New Zealand | 1 sample FIT | 2 | Organized | Postal mail | 50–74 | 2011 |
| Australia | 1 sample FIT | 2 | Organized | Postal mail | 50–74 | 2006 | |
In Japan, the way of distributing FIT kits varies across municipalities.
1.2. COVID-19 pandemic and its global impact on CRC screening services
Since its outbreak in China in December 2019, COVID-19 has spread across the world to become a global pandemic. During the pandemic, cancer screening services had to be curtailed in many Western countries, especially in North America and Europe (Delayed Cancer Screenings—A Second Look, n.d.). For example, in the UK, according to the National Endoscopy Database, the reduction in weekly procedure volume, compared with pre-COVID, was 97% for Bowel Cancer Screening Programme (BCSP) colonoscopies and 99% for BCSP flexible sigmoidoscopies, and up to 72% of expected CRCs were not detected during the pandemic (Rutter et al., 2021; Bowel scope screening to stop in England, n.d.). In the UCLA health system in the US, the utilization of CRC screening declined drastically at the beginning of the COVID-19 pandemic, largely driven by a drop in invasive screening modalities such as colonoscopy (Myint et al., 2020). In the Netherlands, during the lockdown, the number of colonoscopies decreased by 45%. After the lifting of lockdown, endoscopy volumes started to return to normal, except for that for CRC screening (Lantinga et al., 2021). Though most COVID-19 cases and deaths were in Asia in the initial stage from January to March 2020, the pandemic stabilized thereafter, though there was a second wave in the summer to autumn seasons and a rising number of cases this past winter; both were much smaller than the first wave. As of January 2021, the overall number of COVID-19 cases and the related deaths were highest in America, followed by Europe, Asia, and Africa. If we look at the existing nationwide or territory-wide organized CRC screening programs in the Asia-Pacific region, Japan and South Korea were more severely affected by the pandemic, whereas Singapore, Australia, New Zealand, and Taiwan succeeded in containing it.
In this study, we survey and compare the status of population CRC screening activities between 2019 and 2020 in the Asia-Pacific region, predict the possible long-term outcomes of the CRC screening program if the pandemic is prolonged, and discuss the future direction of CRC screening.
2. Material and methods
2.1. Survey on the status of CRC screening services in the Asia-Pacific region before and after the COVID-19 outbreak
The status of screening activities in the Asia-Pacific region, including Australia, Hong Kong, Japan, Korea, New Zealand, and Taiwan, was ascertained from the websites of the programs and by personal communication by e-mail with screening organizers or leading experts in each country. We collected the key CRC screening indicators, including the number of FITs done, the screening rate, and the colonoscopy rate, among those with positive FITs in 2019 and 2020. The results of 2020 were compared with those of 2019. The formulas for calculating the screening participation rate, screening rate, and colonoscopy rate were:
The above indicators were calculated as appropriate and when feasible. For example, in countries where FIT kits were sent via postal mail (Australia or New Zealand), the screening participation rate was calculated. In countries where people need to visit clinics or hospitals to obtain FIT kits (such as Taiwan, Japan, Korea, and Hong Kong), it was not feasible to calculate the real participation rate, so the screening rate was calculated instead using the mid-year population of screening age as the size of the target population. Because most of the major screening programs in the Asia-Pacific region advise undergoing diagnostic colonoscopy within 3 to 6 months of a positive FIT, followed by additional time commitments for a clearing colonoscopy to resect large neoplasms or surgery to resect invasive cancer, which may be delayed during the pandemic, the detection rate of CRC by FIT was not collected in this survey, considering the logistic difficulty of collecting complete data.
2.2. Estimation of the impact of the COVID-19 pandemic on the effectiveness of CRC screening in Asia
With minor disruptions to a screening program, significant increases in CRC burden or fatality are unlikely to arise, given the lead time between adenoma, early-stage cancers, and deaths and the possibility of catching up with delayed screening tests after a short pause of the screening program. The risk of more substantive disruptions, such as those caused by the COVID-19 pandemic, however, raises questions about ongoing unfavorable health impacts.
Given the similar disease burden and the way screening services are offered (by FIT screening) in Asian programs, we used empirical data obtained from the Taiwanese CRC Screening Program to estimate the impact of the COVID-19 pandemic on the effectiveness of CRC screening if the pandemic persists or remains poorly controlled (Onyoh et al., 2019). We simulated a cohort of 300,000 subjects with 100 replications based on a five-state stage-based natural history model of CRC (including one CRC-free state and four CRC states stratified by stage [early (stage 0 and I) vs. advanced stages (stage II and above)] and symptoms (asymptomatic vs. symptomatic phases) and the subsequent prognostic model with the parameters based on the empirical data from the Taiwanese program to project the effect of FIT screening on CRC mortality (Chiu et al., 2015; Chiu et al., 2021). We first used a hypothetical cohort without any intervention to simulate or project the expected number of advanced-stage CRCs at diagnosis, and then we changed the screening rate and the colonoscopy rate from the prepandemic rates to the pandemic rates during a 12-year follow-up to estimate CRC deaths. The framework of this natural history model was described in our previous study, and the base-case parameters used in the current simulation are listed in Supplementary Table 1 (Wu, 2006). (Chen et al., 2007) The measurement outcome of this simulation was the effectiveness of reducing CRC death.
3. Results
3.1. CRC screening key indicators in 2020 in the Asia-Pacific and comparison with 2019
There were fewer cases and deaths from COVID-19 per 105 population in this region than in the North and South American and European continents, and most of the programs in the Asia-Pacific region continued their screening services or only temporarily paused them. (See Table 2 ) Collecting parameters of CRC screening during the pandemic is rather difficult because many of the countries prioritized health care resources to contain COVID-19 over screening activities, resulting in delayed collection and sorting of the screening data. The following are the screening facts in the Asia-Pacific during the pandemic by the end of 2020 and their comparison with 2019:
Table 2.
Reported COVID-19 cases and deaths per 105 population in major organized CRC screening programs in the Asia-Pacific region.
| Country | Cases / per 105 | Death / per 105 | CRC screening program |
|---|---|---|---|
| Full program | |||
| Japan | 225 | 3 | Generally continued but paused in some municipalities for different periods |
| South Korea | 133 | 2 | Continued |
| Singapore | 1045 | 1 | Paused from March to August 2020 |
| Hong Kong | 124 | 2 | Continued |
| Australia | 114 | 4 | Continued |
| New Zealand | 45 | 1 | Paused for 3 months from Mar 23 to June 22, 2020 |
| Taiwan | 3 | <1 | Continued |
Data source: Website of Johns Hopkins University Center for Systems Science and Engineering. Accessed on Jan 10, 202143.
3.1.1. Japan (regional)
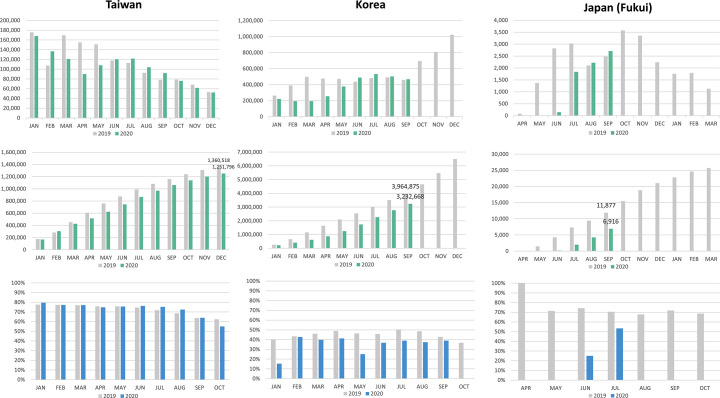
Although two-sample FIT screening was offered, no central screening organizer existed at the national level in Japan, and CRC screening services were managed and funded by individual municipalities. Accordingly, the screening activities were managed independently by each municipality in terms of choosing the invitation list (general population and occupational health check-ups) and the brand of FIT kit to be used, and auditing the program. The government's fiscal year is from 1 April of each year to 31 March of the next year. In 2020, during the period of a state of emergency, which coincided with the start of the fiscal year of 2020, most municipalities curtailed opportunities for cancer screening and routine health check-ups. Even though the screening service was provided without interruption during the COVID-19 pandemic, it was still severely affected. The data from the Fukui Health Promotion Center, where community screening services have been provided for Fukui prefecture residents (population size approximately 780,000), showed that almost no screenings took place during April and May in 2020. The colonoscopy rate after positive FIT was 19.6% in 2020, which was significantly lower than that in 2019 (72.3%, P < 0.001) (Fig. 2A).
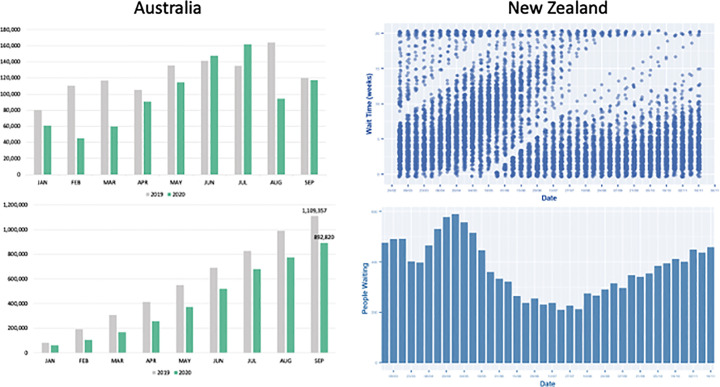
Fig. 2.
Comparison of the key CRC screening indicators in 2019 and 2020 in the Asia-Pacific CRC screening programs.
2A: Asia
Upper: Number of completed FITs by month
Middle: Cumulated number of completed FITs by month
Lower: Colonoscopy rate after positive FIT
2B: Oceania
Australia
Upper: Number of completed FITs by month
Lower: Cumulated number of completed FITs by month
New Zealand
Upper: Waiting time for colonoscopy
Lower: Number of people waiting for colonoscopy
In Japan, the government's fiscal year is from 1 April of each year to 31 March of the next year.
In the metropolitan Setagaya-Tokyo, though the number of subjects undergoing CRC screening in 2020 was nearly equal to that in 2019, the colonoscopy rate was much lower, probably due to the public's perceived risk from endoscopic procedures during the pandemic.
3.1.2. South Korea
In South Korea, the national CRC screening program never stopped during the pandemic. Compared with 2019, the number of screening participants from January to May was much lower in 2020, which reflected the impact of the first surge of the COVID-19 pandemic in March. It recovered to the level of the previous year after July with the temporary subsidence of the pandemic. In 2019, from January to October, a total of 4,659,142 subjects underwent FIT screens. During the corresponding period in 2020, there were only 3,445,660 participants, which was a reduction of 26%. The average colonoscopy rate from January to September 2020 was 35.6%, which was significantly lower than that in 2019 (46.1%, P < 0.0001) (Fig. 2A).
3.1.3. Taiwan
Taiwan successfully contained the COVID-19 outbreak with only a significant peak from March to April in 2020, when there was a surge in the homeward-bound tide from endemic countries, which decreased gradually (Coronavirus disease 2019(COVID-19), n.d.). Though the screening service by the Taiwan CRC Screening Program continued amid the COVID-19 pandemic, screening participation and compliance with colonoscopy after positive FIT were both affected during the first half of the year. A significant reduction in FIT screening participation was observed from March to May, which has been the most popular season for screening in ordinary years, amounting to respective 4.5% and 10% reductions in the FIT screening rate and a 3-month colonoscopy rate after positive FIT (Taiwanese program recommends undergoing colonoscopy within 3 months of positive FIT). Because of this, the Health Promotion Administration of the Taiwanese government held a press conference on July 1, 2020 stressing the importance of screening (FIT) and diagnostic exams (colonoscopy) after a positive screening test. Thanks to the 253-day streak without domestic COVID-19 case from April 12 to Dec 22, the screening participation recovered after June, and the number of FIT screening participants was 1,251,796 (16.7% of the eligible population), which was significantly less than that in 2019 (1,360,518, 18.5% of eligible population) (P < 0.0001). As of December 2020, the total colonoscopy rate of FIT-positive screening participants from January to December was 73.5%, which was similar to that in 2019 (73.9%) (P = 0.95) (Fig. 2A).
3.1.4. Australia
The federally administered National Bowel Cancer Screening Program (NBCSP) has continued to distribute FIT kits without interruption in 2020. The kits were delivered by the postal service, but there was a decrease in kits returned between January and May 2020. The number of participants rose back to the usual level in June and July but declined again after August 2020. Between January and September 2019, a total of 1,109,357 kits were returned, but only 892,820 kits were returned during the corresponding period of 2020, which was a 20% reduction. The overall screening participation rate was 31.1% in 2020, which was significantly lower than that in 2019 (43.9%, P < 0.0001). Colonoscopy services were limited during the start of the pandemic (mainly from the end of March to May), which meant that there were some delays in diagnostic colonoscopy after a positive FIT result. The number of colonoscopy and sigmoidoscopy procedures was 326,442 from January to June 2019, which decreased to 263,093 during the corresponding period in 2020 (decreased by 19.4%) (Review of the impact of COVID-19 on medical services and procedures in Australia utilising MBS data: skin, breast and colorectal cancers, and telehealth services, n.d.) (Fig. 2B).
3.1.5. New Zealand
The national CRC screening program was electively suspended from March 23 to June 22 in New Zealand owing to COVID-19. The main reason was that postal service was hindered during the pandemic, which caused the rate of spoiled kits to exceed 10% due to transit delay. The screening activity resumed after recovery of postal delivery service, and both the waiting time for colonoscopy and the number of people waiting for colonoscopy returned to the pre-COVID lockdown level after September, after which the backlog in cases was erased (Fig. 2B).
3.1.6. Thailand (Khon Kaen pilot)
The CRC screening pilot trial was performed in Khon Kaen Province and was suspended from February to December 2020 because of the COVID pandemic. Though it was initially planned to restart in early 2021, it is still paused due to the upward surge of the pandemic in Thailand in the first 3 months of 2021.
3.1.7. Singapore (National University of Singapore cohort)
The national CRC screening program was suspended from March to August 2020 in Singapore. Though the exact number of FIT screenings in the national program has yet to be formally released by the government, if we compare the volume of colonoscopy performed in the National University Hospital from January to October, the number was 2043 in 2019 and 1377 in 2020 (reduction by 32.6%), leading to decreased detection of adenoma (646 vs. 520) and cancer (22 vs. 15), which was proportional to the reduction in colonoscopy number.
3.1.8. China (Tianjin)
Population-based screening for CRC through a questionnaire and FIT in Tianjin, China, was suspended from January to February 2020 (Li et al., 2018). In 2020, a total of 340,000 subjects aged 40–50 attended the program and completed either FIT or the questionnaire, which was a 50% reduction compared with the ordinary level.
3.2. Simulation of the possible impact of CRC screening delays on cancer detection and related death
Based on the scenario of the Taiwanese screening program in 2019 (assuming a stable 60% screening rate, 7% positivity rate, and 70% colonoscopy rate), biennial FIT screening can reduce CRC mortality by 29.4% over a 12-year follow-up, whereas the decreased screening rate (assuming 50%) and colonoscopy rate (assuming 67%) due to the COVID-19 pandemic may compromise the effectiveness of such screening by 6.3%, resulting instead in a 23.1% mortality reduction during the same time span.
4. Discussion
From the results of this survey of the Asia-Pacific region, it is evident that most countries' screening programs continued providing screening services, though some paused during the COVID-19 pandemic. According to our simulation, such pauses may affect the effectiveness of the screening if the pandemic is prolonged due to insufficient vaccination coverage or the spread of more contagious viral variants. Even so, the pandemic highlighted the problems of current CRC screening and provided an opportunity to rethink what the “new normal” should be after the pandemic and what quick and resilient action we can take if another pandemic happens in the future.
4.1. How to mitigate the impact of COVID-19 on CRC screening programs?
4.1.1. Manage the backlog
As per the screening strategy of CRC screening in the Asia-Pacific region, all the currently existing programs use a FIT-based two-tier screening strategy. The FIT positivity rate using the cutoff of 20 μg Hb/g feces, the level that is most commonly used in Asia-Pacific programs, ranges from 4 to 10%, so the demand for colonoscopy is much lower than that in colonoscopy-based screening programs. Nevertheless, timely colonoscopy for screening test-positive subjects is crucial in FIT screening programs because the FIT-positive population represents a selected high-risk population, 1 in 20 to 25 of them having CRC and 1 in 5 having advanced adenoma, which is 20-fold and 2- to 4-fold the level of the general population, respectively. Delays in the diagnostic examination may let advanced adenoma progress into invasive cancer or from early-stage CRC into advanced-stage CRC. Both would affect the survival of the patients and hence the effectiveness of the entire screening program. Corley et al. reported that if colonoscopy was performed later than the 10th month after a positive FIT, then the risk of CRC and advanced-stage CRC at the time of diagnosis was significantly higher than in those who underwent colonoscopy within one month (Corley et al., 2017). Similarly, in the Taiwanese screening program, Lee et al. reported that the risk of CRC and advanced-stage CRC was 1.3-fold and 2-fold if colonoscopy was performed at 9 to 12 months and 2-fold and 2.8-fold if performed after the 12th month, respectively (Lee et al., 2019). Similar results were also reported from an Italian program (Zorzi et al., 2020).
During the COVID-19 pandemic, managing colonoscopy backlogs has become a challenge, as many endoscopic units are temporally closed or have limited service and only for emergent procedures, as mentioned previously. (Rutter et al., 2021; Lantinga et al., 2021) According to the survey in the current study, colonoscopy services were largely affected in Japan (Fukui), and the rate of colonoscopy after positive FIT dropped to less than half of that of the previous year. Other programs also suffered from either shutdown of the endoscopy service or unwillingness to undergo colonoscopy by the screening participants. To address any accumulating backlog, several issues need to be dealt with. First, it is important to liaise with other healthcare sectors and the health authorities to put FIT-positive cases as a priority, as such cases are mostly asymptomatic and likely to be considered nonessential. Moreover, the screening organizer should be aware of the latest information about available colonoscopy slots and actively keep communication with the hospitals to make the most efficient use of the constrained endoscopy resources. Second, further risk stratification within FIT-positive subjects is warranted when colonoscopy capacity is extremely limited. Current major guidelines on endoscopic practice during the COVID-19 pandemic have advised how to set the priority for colonoscopy and have recommended prioritizing patients with alarming symptoms such as bleeding or significant body weight loss as well as those with a positive FIT. Further stratification within FIT-positive subjects, however, has not been addressed. Studies have demonstrated that 1 in 2 FIT-positive subjects were diagnosed with neoplastic lesions at colonoscopy, but only 1 in 5 had advanced neoplasms, implying that there is still room for further stratification. Previous studies have demonstrated that quantitative measurement of FIT (FHbC, fecal hemoglobin concentration) is positively associated with the likelihood of detecting advanced neoplasms, either advanced adenoma or invasive cancer, at colonoscopy (Chiu et al., 2017; Auge et al., 2014). It is therefore reasonable to use FHbC to stratify the risk and set the priority for colonoscopy. Finally, a prolonged waiting time for surgery for CRC may also compromise the effectiveness of the screening program. A recent modeling study indicated that even modest delays in cancer surgery of 3 to 6 months might significantly impact survival, particularly in stage 2 or 3 cancer patients (Sud et al., 2020). Guaranteeing the treatment flow after diagnosis is crucial in securing the effectiveness of screening.
4.1.2. When to stop and when to restart screening
Implementing and easing social distancing has been an urgent global issue during the COVID-19 pandemic, particularly when considering the revival of economic and social activities and the resumption of ordinary healthcare services, including cancer screening. A simple index for easing social distancing was recently proposed by Chen et al. for the global, country, region, and community levels, taking into consideration the global dynamic changes in three factors: the spread of SARS-CoV-2, the recovery rate, and the case-fatality rate. In short, this social distancing index (SDI) is the ratio of cumulative confirmed cases to cumulative recovered COVID-19 patients, which is captured by (1 – case fatality) – 1 during a fixed time period.
The advantage of using this index is that it takes into account the cumulative number of recovered COVID-19 patients, thereby reflecting the available health care capacity and the ability to accommodate screening services. If we further take the time-varying vaccination rate into account, this index can be modified as:
When the SDI is less than 1, social distancing can be gradually lifted, and medical capacity might be sufficient to provide ordinary healthcare for non-COVID-19 patients and screening services. The sooner the SDI drops below 1, the more likely that we can resume our prepandemic or new normal life and regular healthcare service.
Combining SDI with the risk profile parameters may lead local screening organizers to be resilient to the dynamic change of pandemic and to allocate the available endoscopy capacity. The conceptual framework of such a strategy is demonstrated in Fig. 3 . In this two-dimensional matrix, the horizontal axis refers to the risk profile parameter, which can be FHbC, CRC risk score, or severity of alarming symptoms, and the vertical axis is the SDI. As the SDI is a dynamic and quantitative indicator that could be used at various jurisdiction levels, it can be flexibly applied to various situations during the pandemic period and accommodate regions that have already lifted social distancing but have seen resurgences of outbreaks due to cluster infections. The only premise of using such an approach is that the screening organizers should be fully aware of the most accurate and most up-to-date information on confirmed cases, recoveries, and deaths, which is required for the calculation of the SDI, and the most accurate information on the available healthcare and colonoscopy capacity in its jurisdiction.
Fig. 3.
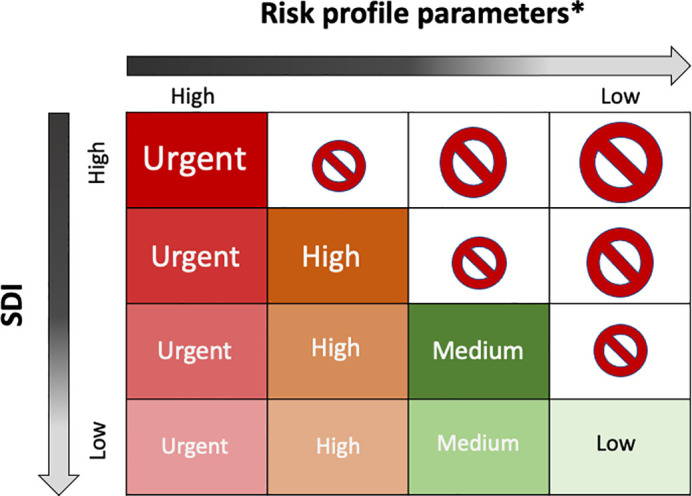
The conceptual framework of using the SDI index and CRC risk profile to resume CRC screening during the pandemic.
Prohibition sign refers to “not to screen”
*Risk profile parameters could be FHbC, risk score
4.1.3. Adopting flexible measures and embracing modern technologies in CRC screening
CRC screening using FIT is one of the most complex cancer screenings and involves multiple steps. Watchful monitoring of the quality of each step is mandatory. In most Asian programs, people should first visit hospitals or screening sites to obtain FIT kits and then revisit to return stool samples. If tests turn out to be positive, those people are required to visit hospitals for scheduling their colonoscopy, then another visit for the colonoscopy, and finally an outpatient visit for consultation about the pathology report and advice for future surveillance should any lesion be resected at colonoscopy. With so many hospital visits, especially during the COVID-19 pandemic, people may be reluctant to participate in CRC screening because it may increase the risk of being exposed to the virus. In some programs, screening kits are distributed by postal mail, which may eliminate the need to visit medical institutions unless their FIT results turn out to be positive. Previous studies have demonstrated that such an approach could effectively improve screening uptake and may be considered a feasible approach for continuing CRC screening during the pandemic given that the postal delivery system is still consistently and smoothly working, because delayed sample return may increase the likelihood of false-negative results, which may lead to interval cancers (van Rossum et al., 2009; Gupta et al., 2020; Coronado et al., 2018). Even after the pandemic, we should also consider using the postal mail to deliver FIT kits, which may both increase screening participation and reduce the congestion of hospitals, which is common in many Asian countries.
Screening organizers may also consider applying modern technology such as a smartphone app or telemedicine at many steps of FIT screening, such as consultation about eligibility for CRC screening, notification of FIT results, scheduling colonoscopy, pathology result notification, and advising about the timing of surveillance colonoscopy, to facilitate CRC screening during the COVID pandemic (Azulay et al., 2019; El Bizri et al., 2021). In fact, telehealth technology has been boosted and widely adopted in the past year owing to the COVID-19 pandemic, and many healthcare systems have observed a tremendously increased number of telemedicine visits (McCall, 2020). As the pandemic went on, the demand for remote consultations increased, and telemedicine was stepped up to help overcome the difficulties of providing consultations in person at the outpatient clinic when the public was asked to keep social distancing and stay home. Discussion on more complex diagnostic or treatment issues of cancer, such as chemotherapy or target therapy, which is rather challenging even in a conventional consultation, may not be feasible by a telemedicine-based approach, but screening, on the contrary, is rather simple and straightforward. Additionally, applying these digital technologies is tremendously helpful to lighten the workload of the already overstretched healthcare services. Implementation of telemedicine, comprising video, telephone, and other electronic communication tools, such as AI-based chatbots, would be very likely to help with CRC screening even after the COVID-19 pandemic (Budd et al., 2020). During the pandemic, face mask distribution has been done via digital rationing and resource allocation systems that use a color-coded system to indicate what percentage of masks are still in stock and display the name of the store, its location, its opening hours, and its contact information (Tai et al., 2021). A GPS-based information system – the Intelligent Electronic Fences System – is another successful application of IT technology to contain the COVID-19 pandemic. Via collaboration between health authorities and mobile phone carriers and based on an individual's mobile phone signal and nearby cell towers, it triangulates the location of quarantined individuals and identifies any potential people whom they might have come into contact with, in real time. The above technologies could also be applied during this pandemic or any future emerging infectious disease outbreak by providing real-time information on available clinical services and colonoscopy capacity, which may be ever-changing during the pandemic, to help avoid hospital congestion and keep sufficient social distancing.
4.2. Advantage of organized over opportunistic screening
Several studies have demonstrated the advantages of organized CRC screening over opportunistic screening in terms of screening uptake and quality control (Chiu et al., 2017; Levin et al., 2018). In contrast to ad hoc opportunistic screening, organized screening focuses more on the quality of each step of the screening process, including follow-up of participants, and reports publicly on cancer screening program performance. In this way, it provides greater protection against the harms of screening, such as poor-quality screening or complications of screening; pays more attention to screening participation and compliance with diagnostic examinations after a positive screening test; better allocates resources; and decreases disparities in screening uptake (Rabeneck et al., 2020; Eisinger et al., 2008).
In this COVID-19 pandemic, it has become clear that both healthcare and public health systems play a crucial role. Taking Taiwan as an example, the healthcare system is a single-payer universal access insurance system that covers 99% of the entire Taiwanese population. Such a high rate of health insurance coverage prevented the gap of epidemic prevention because people could easily obtain access to minimally essential healthcare. The strategy adopted by Taiwan's National Health Insurance Administration (NHIA) to share information through MediCloud, its cloud databank, to control the pandemic was a key factor in Taiwan's success in capping COVID-19 outbreaks and deaths. For CRC screening, the Taiwanese government subsidizes the majority of the expenses on screening and subsequent treatments, population-based CRC screening is delivered in an organized way, and the central government is the main screening organizer that takes the responsibility for invitations, referrals, resource allocation, and the coordination with individual municipalities. By the combined use of the aforementioned information system and the screening database, we can administer the available screening resources, manage the backlog and monitor the key screening indicators, even during the COVID-19 pandemic, highlighting the advantages of the organized screening program. In the Asia-Pacific region, countries such as Korea, New Zealand, and Australia also have single-payer universal access insurance systems, and CRC screening is also publicly funded and managed by the central government. This may be a reasonable explanation why most CRC screening programs in this region can take rather resilient and quick responses to the waxing and waning of COVID-19 outbreaks. Even so, there is still room for improvement. Though the majority of CRC screening programs in Asia-Pacific are nominally organized, they are not completely organized in several aspects, such as in their lack of integration and coordination among regional and central screening organizers, their rather arbitrary screening invitation and colonoscopy referral processes, and their lack of real-time quality monitoring or assurance mechanisms. Further action and elaboration are mandatory.
4.3. “New normal” after the pandemic
Almost all CRC screening programs were affected by the COVID-19 pandemic, and it remains unclear when ordinary screening activities can completely resume. This is a time for reflection, rethinking the current screening system, and figuring out proactive and implementable approaches for the post-COVID era because other emerging infectious diseases can still occur in the future. This is important, as noncommunicable diseases such as cardiovascular diseases, diabetes, their related complications, and cancer still account for the majority of premature deaths in developed countries. The role of screening will become even more important because it can play a role not only in screening high-risk populations but also in shifting the population into a lower-risk category. After all, resection of adenoma can reduce the risk of CRC, thereby reducing the risk profile of the entire population should a higher proportion of the population be exposed to screening, thereby benefiting more people (Rose, 1985).
The current scheme of CRC screening is not without shortcomings. In colonoscopy-based screens, neoplastic lesions were detected in approximately 30% of the screenees, which means that nearly 70% of the colonoscopies were negative but occupied a tremendous proportion of valuable endoscopic capacity. Even in FIT screening, 50% of colonoscopies would lack neoplastic lesions. How to further stratify the target screening population becomes an important issue. Using age- and gender-specific FIT cutoffs or applying a risk scoring system that accommodates common risk factors may become feasible solutions (Chen et al., 2018; Sarkeala et al., 2021; Chiu et al., 2016).
5. Conclusion
During the COVID-19 pandemic, CRC screening programs in the Asia-Pacific region have been less affected than Western programs, and most of them continue providing screening services. Even so, the pandemic exposed the fragility of the healthcare system and the vulnerability of the current CRC screening system. During the pandemic, we have learned how to take resilient actions to resume screening services and to address the backlog to avoid complete shutdown, not only from the successes but also the errors of other countries. Novel approaches that have been developed to contain COVID-19 or deal with the backlog of FIT screening or diagnostic colonoscopy also provide insight into how the efficiency of the current CRC screening system could be improved. We have learned during this unprecedented outbreak how to deal with the impact of the COVID-19 pandemic by addressing the backlog based on risk (local epidemic and individual CRC risk), adjusting the screening logistics (e.g., distribute the FIT kit via postal mail), or applying modern technologies (e.g., telemedicine). These approaches can be applied not only to possible future outbreaks of emerging infectious diseases but also to improve the current scheme of CRC screening in many aspects.
Disclosure
All authors have no potential financial, professional, or personal conflicts to disclose that are relevant to the study.
Funding and grant support
This study was supported by the Health Promotion Administration, Ministry of Health and Welfare (A1081113) of the Taiwanese government. The funding source had no role in the study design; data collection, analysis, or interpretation; report writing; or decision to submit this paper for publication.
Credit author statement
Conception and design of the study: Han-Mo Chiu, Sam Li-Shen Chen, Hsiu-Hsi Chen. Generation, collection, assembly, analysis and/or interpretation of data: Han-Mo Chiu, Chiu-Wen Su, Weng-Feng Hsu, Grace Hsiao-Hsuan Jen, Chen-Yang Hsu. Drafting and revision of the manuscript: Han-Mo Chiu, Hsiu-Hsi Chen. Approval of the final version of the manuscript: all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the researchers who provided the screening facts of the individual countries:
Australia: Erin Symonds, Graeme Young
China (Tianjin): Li-Zhong Zhao
Hong Kong: Yuen Tung Lam, Joseph JJ Sung, Martin CS Wong
Japan (Fukui, Tokyo): Takahisa Matsuda, Kazuo Matsuda, Chisato Hamashima
Korea: Hyun-Soo Kim
New Zealand: Susan Parry
Singapore: Khay Guan Yeoh
Thailand (Khon Kaen): Pongdech Sarakarn
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106622.
Appendix A. Supplementary data
Supplementary material
References
- Auge J.M., Pellise M., Escudero J.M., et al. Risk stratification for advanced colorectal neoplasia according to fecal hemoglobin concentration in a colorectal cancer screening program. Gastroenterology. 2014;147:628–636. doi: 10.1053/j.gastro.2014.06.008. (e1) [DOI] [PubMed] [Google Scholar]
- Azulay R., Valinsky L., Hershkowitz F., et al. Repeated automated mobile text messaging reminders for follow-up of positive fecal occult blood tests: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowel scope screening to stop in England https://scienceblog.cancerresearchuk.org/2021/01/14/bowel-scope-screening-to-stop-in-england/
- Bowel screening https://www.timetoscreen.nz/bowel-screening/about-the-national-bowel-screening-programme/
- Budd J., Miller B.S., Manning E.M., et al. Digital technologies in the public-health response to COVID-19. Nat. Med. 2020;26:1183–1192. doi: 10.1038/s41591-020-1011-4. [DOI] [PubMed] [Google Scholar]
- Chen L.S., Liao C.S., Chang S.H., et al. Cost-effectiveness analysis for determining optimal cut-off of immunochemical faecal occult blood test for population-based colorectal cancer screening (KCIS 16) J. Med. Screen. 2007;14:191–199. doi: 10.1258/096914107782912022. [DOI] [PubMed] [Google Scholar]
- Chen S.L., Hsu C.Y., Yen A.M., et al. Demand for colonoscopy in colorectal Cancer screening using a quantitative fecal immunochemical test and age/sex-specific thresholds for test positivity. Cancer Epidemiol. Biomark. Prev. 2018;27:704–709. doi: 10.1158/1055-9965.EPI-17-0387. [DOI] [PubMed] [Google Scholar]
- Chiu H.M., Chen S.L., Yen A.M., et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121:3221–3229. doi: 10.1002/cncr.29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H.M., Ching J.Y., Wu K.C., et al. A risk-scoring system combined with a fecal immunochemical test is effective in screening high-risk subjects for early colonoscopy to detect advanced colorectal neoplasms. Gastroenterology. 2016;150:617–625. doi: 10.1053/j.gastro.2015.11.042. (e3) [DOI] [PubMed] [Google Scholar]
- Chiu S.Y., Chuang S.L., Chen S.L., et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut. 2017;66:293–300. doi: 10.1136/gutjnl-2015-310256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H.M., Jen G.H., Wang Y.W., et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 doi: 10.1136/gutjnl-2020-322545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley D.A., Jensen C.D., Quinn V.P., et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal Cancer and Cancer stage at diagnosis. JAMA. 2017;317:1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado G.D., Petrik A.F., Vollmer W.M., et al. Effectiveness of a mailed colorectal Cancer screening outreach program in community health clinics: the STOP CRC cluster randomized clinical trial. JAMA Intern. Med. 2018;178:1174–1181. doi: 10.1001/jamainternmed.2018.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease 2019(COVID-19) https://www.cdc.gov.tw/en/Disease/SubIndex/
- Delayed Cancer Screenings—A Second Look https://ehrn.org/articles/delayed-cancer-screenings-a-second-look/
- Eisinger F., Cals L., Calazel-Benque A., et al. Impact of organised programs on colorectal cancer screening. BMC Cancer. 2008;8:104. doi: 10.1186/1471-2407-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bizri M., El Sheikh M., Lee G.E., et al. Mobile health technologies supporting colonoscopy preparation: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eligibility of Colorectal Cancer Screening Programme updated https://www.info.gov.hk/gia/general/202012/30/P2020123000566.htm?fontSize=1
- Giorgi Rossi P., Vicentini M., Sacchettini C., et al. Impact of screening program on incidence of colorectal Cancer: a cohort study in Italy. Am. J. Gastroenterol. 2015;110:1359–1366. doi: 10.1038/ajg.2015.240. [DOI] [PubMed] [Google Scholar]
- Global Cancer Observatory: Cancer Today https://gco.iarc.fr/today
- Gupta S., Coronado G.D., Argenbright K., et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: summary of a Centers for Disease Control and Prevention-sponsored summit. CA Cancer J. Clin. 2020;70:283–298. doi: 10.3322/caac.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantinga M.A., Theunissen F., Ter Borg P.C.J., et al. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53:166–170. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.C., Fann J.C., Chiang T.H., et al. Time to colonoscopy and risk of colorectal Cancer in patients with positive results from fecal immunochemical tests. Clin. Gastroenterol. Hepatol. 2019;17:1332–1340. doi: 10.1016/j.cgh.2018.10.041. (e3) [DOI] [PubMed] [Google Scholar]
- Levin T.R., Corley D.A., Jensen C.D., et al. Effects of organized colorectal Cancer screening on Cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155:1383–1391. doi: 10.1053/j.gastro.2018.07.017. (e5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhao L.Z., Ma D.W., et al. Predicting the risk for colorectal cancer with personal characteristics and fecal immunochemical test. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall B. Could telemedicine solve the cancer backlog? Lancet Digital Health. 2020;2:E456–E457. [Google Scholar]
- Myint A., Hilda O., Lee S, Roh L., Connoly L., et al. Impact of the COVID-19 pandemic on colorectal Cancer screening rates and modalities in a large integrated health system. Am. J. Gastroenterol. 2020;115:S154–S155. [Google Scholar]
- Onyoh E.F., Hsu W.F., Chang L.C., et al. The rise of colorectal Cancer in Asia: epidemiology, screening, and management. Curr. Gastroenterol Rep. 2019;21:36. doi: 10.1007/s11894-019-0703-8. [DOI] [PubMed] [Google Scholar]
- Rabeneck L., Chiu H.M., Senore C. International perspective on the burden of colorectal Cancer and public health effects. Gastroenterology. 2020;158:447–452. doi: 10.1053/j.gastro.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Review of the impact of COVID-19 on medical services and procedures in Australia utilising MBS data: skin, breast and colorectal cancers, and telehealth services https://www.canceraustralia.gov.au
- Rose G. Sick individuals and sick populations. Int. J. Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- Rutter M.D., Brookes M., Lee T.J., et al. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70:537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- Saito H. Colorectal cancer screening using immunochemical faecal occult blood testing in Japan. J. Med. Screen. 2006;13(Suppl. 1):S6–S7. [PubMed] [Google Scholar]
- Sarakarn P., Promthet S., Vatanasapt P., et al. Preliminary results: colorectal Cancer screening using fecal immunochemical test (FIT) in a Thai population aged 45-74 years: a population-based randomized controlled trial. Asian Pac. J. Cancer Prev. 2017;18:2883–2889. doi: 10.22034/APJCP.2017.18.10.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkeala T., Farkkila M., Anttila A., et al. Piloting gender-oriented colorectal cancer screening with a faecal immunochemical test: population-based registry study from Finland. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-046667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuders E.H., Ruco A., Rabeneck L., et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- Shim J.I., Kim Y., Han M.A., et al. Results of colorectal cancer screening of the national cancer screening program in Korea, 2008. Cancer Res. Treat. 2010;42:191–198. doi: 10.4143/crt.2010.42.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud A., Jones M.E., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020;31:1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Y.L., Chi H., Chiu N.C., et al. The effect of a name-based mask rationing plan in Taiwan on public anxiety regarding a mask shortage during the COVID-19 pandemic: observational study. JMIR Form Res. 2021;5 doi: 10.2196/21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum L.G., van Rijn A.F., van Oijen M.G., et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int. J. Cancer. 2009;125:746–750. doi: 10.1002/ijc.24458. [DOI] [PubMed] [Google Scholar]
- Wu G.H., Wang Y.M., Yen A.M., et al. Cost-effectiveness analysis of colorectal cancer screening with stool DNA testing in intermediate-incidence countries. BMC Cancer. 2006;6:136. doi: 10.1186/1471-2407-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi M., Fedeli U., Schievano E., et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64:784–790. doi: 10.1136/gutjnl-2014-307508. [DOI] [PubMed] [Google Scholar]
- Zorzi M., Hassan C., Capodaglio G., et al. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy. 2020;52:871–876. doi: 10.1055/a-1159-0644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material