Abstract
Objective
Gamma-glutamyl transferase (GGT) to albumin ratio (GAR) has been shown to be helpful to diagnose and determine the severity of coronary artery disease (CAD). Coronary computed tomography angiography (CCTA) is a guide recommended non-invasive test that provides information about the presence, severity, and morphology of coronary plaques. In this study, our main aim was to investigate the relationship between the presence, morphology, and severity of coronary plaques detected via CCTA and GAR in patients with low to moderate risk for undiagnosed CAD.
Methods
Nine hundred and sixty six patients were included who underwent CCTA. The severity of CAD and plaque morphology were investigated. CT-adapted Leaman score (CT-LeSc) was calculated to determine the extent of the CAD. The study population was further evaluated in three groups according to tertiles of GAR.
Results
Atherosclerotic plaques were more common in the male gender and older patients with conventional cardiovascular risk factors. GAR was significantly lower in patients with normal CCTA than in patients with a non-obstructive plaque or obstructive plaque on CCTA. Patients in upper GAR tertiles had a higher coronary calcium score (CACS) and CT-LeSc. GAR was one of the independent predictors to predict severe stenotic plaque and high CACS.
Conclusion
GAR can independently predict the presence, extent, and severity of CAD determined by CT-LeSc. We believe as a cheap, safe, and widely available tool, GAR would be useful in the diagnosis of CAD.
Keywords: gamma-glutamyl transferase, albumin, angiography, tomography, plaque, atherosclerotic
Introduction
Coronary artery disease (CAD) is the most common cause of morbidity and mortality globally (1). Inflammation is one of the major underlying mechanisms for the development of atherosclerosis. Changes in the blood level of certain markers related to inflammation can influence the formation and progression of atherosclerosis as well as possible thrombotic complications. Gamma-glutamyl transferase (GGT) and albumin are known to be associated with the development of CAD and cardiovascular mortality (2, 3).
GGT is located in the cellular membrane and serum and participates in the regulation of glutathione catabolism (G-SH), which is the most important antioxidant for the cell (2). GGT also triggers low-density lipoprotein (LDL) oxidation and reactive oxygen radical formation thus contributing to the atherosclerotic process (4).
Albumin is the principal protein that determines the oncotic pressure of plasma and is also responsible for fluid exchange between body components (5). Low blood albumin level is also associated with CAD, heart failure, stroke, and atrial fibrillation (6, 7) owing to antioxidant, anti-inflammatory, and anti-aggregating effects (8).
Recently, GGT to albumin ratio (GAR) has been shown to be useful in predicting the risk of bleeding and mortality in patients who underwent percutaneous coronary intervention (9). GAR may be helpful to diagnose and determine the severity of the CAD. Coronary computed tomography angiography (CCTA) is a non-invasive test that offers substantially accurate results regarding the anatomy of coronary arteries and provides additional information about the presence, severity, and morphology of coronary plaques (10–14). Guidelines recommend CCTA as the first-line imaging tool to evaluate acute chest pain and stable angina pectoris in patients with mild to moderate risk (15).
It is clinically of value to detect CAD at an early stage and estimating CCTA results with easily obtainable parameters (such as GAR), particularly in patients with mild to moderate risk of CAD. Thus, we sought to investigate the relationship among the presence, morphology, and severity of coronary plaques detected via CCTA and GAR in patients with low to moderate risk for undiagnosed CAD.
Methods
Study population
This retrospective study reviewed records of consecutively 1,453 patients (>18 years old) who presented to our cardiology clinic with stable angina pectoris between 2013 and 2020 and underwent CCTA. Patients with a history of documented CAD or heart failure, hepatic dysfunction, presence of alcohol abuse as well as patients with the neoplastic or systemic inflammatory disease were excluded. Nine hundred sixty six patients were enrolled who met the abovementioned criteria. The study is approved by the Local Ethics Committee.
Risk assessment of CAD and demographics
Clinical data regarding personal and general information such as age, sex, hypertension (HT), diabetes mellitus (DM), hyperlipidemia, smoking status, and family history of CAD were screened for all the patients. Cardiovascular risk factors of the patients were obtained from the patient’s history of diagnosis using hospital records. History of HT, hyperlipidemia, DM, smoking status, and family history of CAD were carefully noted. Smoking status was recorded. Criteria for a positive family history of CAD was having a history of fatal/nonfatal myocardial infarction, or coronary artery bypass surgery or coronary angioplasty in a first-degree relative before the age of 55 and 65, respectively, for men and women.
Coronary computed tomography angiography
All the cardiac exams were performed with one of two multidetector computed tomography (MDCT) system; either a 64 MDCT scanner (Lightspeed vct, General Electric Medical Systems Milwaukee WI, USA) or a dual-source 192 slice MDCT scanner (Somatom Force, Siemens Healthineers Germany). Oral metoprolol (up to 100 mg) was used before the CCTA examination if needed to achieve an optimum heart rate (< 70 beats/minute). Before CCTA, all the patients underwent ECG-gated unenhanced scanning for CCS analysis with a tube voltage of 120 kV, tube current of 80 mAs, and scan thickness of 3 mm. CCTA was performed during full inspiration and breath-hold with a tube voltage of 120 Kv, tube current of 250–650 mAs, and scan thickness of 0.6 mm. An automated dual-rail injector (Medrad Stellant CT injection system, Bayer) was used for contrast injection. Nonionic iodinated contrast material (350 mg I/mL ioversol, Optiray, Guerbet, France) 60–80 mL was administrated intravenously via an 18 gauge catheter inserted in an antecubital vein at a flow rate of 5–6 mL/s followed by a saline flush. A real-time bolus tracking method was used for initiating the scan. Following these procedures, images were processed with software and interpreted by two experienced radiologists according to the Society of Cardiovascular Computed Tomography guidelines (16). As our study was retrospective and designed after the CCTA scans, the radiologists were blind to GAR values of the patients. In addition, no intra/inter observer variation data were present as we performed the study retrospectively.
Coronary computed tomography angiography evaluation
The CCTA data were transferred to a workstation for analyzing coronary plaques with dedicated cardiac assessment software (syngo.via VB10, Siemens Healthineers Germany). Multiplanar reformatted (MPR), maximum intensity projection, 3-D volume-rendered, and curved-MPR images were used for evaluation. Right or left dominancy or co-dominancy of coronary arteries and anatomic variations such as abnormal origin or course and myocardial bridging were recorded. The coronary arteries were evaluated for the presence of atherosclerotic plaque; plaque morphology and the degree of stenosis were recorded according to the Society of Cardiovascular Computed Tomography guidelines (17). All coronary artery segments measuring >2 mm in diameter were included in the evaluation. A plaque is defined as a structure, measuring >1 mm in size, on the wall or in the lumen of the coronary artery, which can be distinguished from pericardial tissue or epicardial fat tissue. Plaques were classified according to the calcification; plaques with no calcification were referred to as non-calcified, whereas those with a calcification rate above 50% (density ≥130 Hounsfield units in native scans) were classified as calcified, and plaques with a calcification rate below 50% were deemed as mixed plaque. Patients were assessed regarding the type of plaque (no plaque, calcified plaque, and non-calcified/mixed plaque), presence or severity of stenosis [no plaque, non-occlusive (luminal stenosis <50%) or occlusive stenosis (luminal stenosis ≥50%)]. Moreover, the study population was further divided into three groups according to tertiles of GAR.
CT-adapted Leaman score (CT-LeSc) was calculated to determine the extent of the CAD as defined elsewhere (18). When CT-LeSc was estimated, a calculation was made for each coronary segment. The score of each segment was calculated by multiplying A (localization of the lesion in the coronary artery) with B (type of plaque) and C (severity of stenosis; >50%). Next, all calculated values were summed up to get patient-based CT-LeSC. Coronary artery calcium score (CACS) was estimated using the Agatston method with dedicated software (19).
Statistical analysis
Categorical variables were presented as numbers and percentages; continuous variables were presented as medians (interquartile range) or means ± standard deviation according to their distribution pattern. The Kolmogorov-Smirnov test was applied to test whether the continuous variables exhibited Gaussian or non-Gaussian distribution. When comparing the difference between two groups, categorical variables were analyzed with the chi-squared test, and continuous variables were analyzed with the Mann-Whitney U test or student’s t-test. When comparing continuous variables for more than two groups, one-way analysis of variance or Kruskal-Wallis test was used. Tukey test or Bonferroni correction was applied for post-hoc tests. Univariate regression analyses were performed to evaluate the individual effect of the variables on the presence of severe stenotic plaque and CACS >100. Possible factors that could potentially affect the presence of these variables were then entered into binary logistic regression analyses. All variables with a p value <0.25 were entered into the multiple models. Hosmer-Lemeshow goodness of fit statistics was used to assess model fits. Two-tailed p value <0.05 was accepted as statistically significant. All statistical analyzes were performed with IBM Statistical Package for Social Sciences version 22 software (IBM Corp, Armonk, NY, USA).
Results
A total of 966 patients (443 patients with and 523 patients without an atherosclerotic plaque on CCTA) were included in the study. Table 1 summarizes the basal characteristics of the study population. Patients who have an atherosclerotic plaque on CCTA were older than those without a coronary plaque. Male gender and classical cardiovascular risk factors like DM and hyperlipidemia were more prevalent in patients with atherosclerosis. Although fasting blood glucose levels, creatinine levels, triglyceride levels, GGT levels and GAR were higher; HDL values, albumin levels and platelet counts were lower in patients with atherosclerotic CCTA compared to patients with normal CCTA (Table 1).
Table 1.
Baseline clinical and laboratory parameters of the patients
| Normal CCTA n=523 | Positive CCTA n=443 | P-value | |
|---|---|---|---|
| Age, years | 50±10 | 59±10 | <0.001 |
| Sex (Male), n (%) | 197 (37.7) | 238 (53.7) | <0.001 |
| Hypertension, n (%) | 315 (60.2) | 278 (62.8) | 0.422 |
| Diabetes Mellitus, n (%) | 72 (13.8) | 89 (20.1) | 0.009 |
| Hyperlipidemia, n (%) | 102 (19.5) | 132 (29.8) | <0.001 |
| Fasting glucose, mg/dL | 91 (83–101) | 96 (86–108) | <0.001 |
| Creatinine, mg/dL | 0.7 (0.6–0.9) | 0.8 (0.7–0.9) | <0.001 |
| Total cholesterol, mg/dL | 215 (185–249) | 216 (185–251) | 0.599 |
| LDL, mg/dL | 133 (108–159) | 141 (109–163) | 0.402 |
| HDL, mg/dL | 51 (43–60) | 47 (41–58) | 0.010 |
| Triglyceride, mg/dL | 131 (92–179) | 138 (108–192) | 0.004 |
| GGT, U/l | 21 (15–32) | 25 (17–36) | <0.001 |
| Albumin, g/dL | 4.4 (4.2–4.6) | 4.4 (4.2–4.6) | 0.001 |
| GGT/albumin | 4.9 (3.4–7.1) | 5.7 (3.9–8.4) | <0.001 |
Continuous variables were represented as mean ± standard deviation or median (Interquartile range 25–75)
CCTA - computed tomography coronary angiography; LDL - low-density lipoprotein; HDL - high-density lipoprotein; GGT - gamma-glutamyl transferase
GAR values regarding atherosclerotic plaque stenosis severity (non-obstructive vs obstructive) and atherosclerotic plaque morphology (calcific vs non-calcific/mixed) are given in Figures 1 and 2, respectively. GAR was significantly lower in patients with normal CCTA than in those with a non-obstructive plaque or obstructive plaque on CCTA. GAR was similar between patients with non-obstructive and obstructive CCTA (Fig. 1). When GAR was evaluated according to plaque morphology, patients who had any type of plaque on CCTA had significantly higher GAR than those with normal coronary arteries. GAR was similar between patients with calcific and non-calcific/mixed plaques (Fig. 2).
Figure 1.
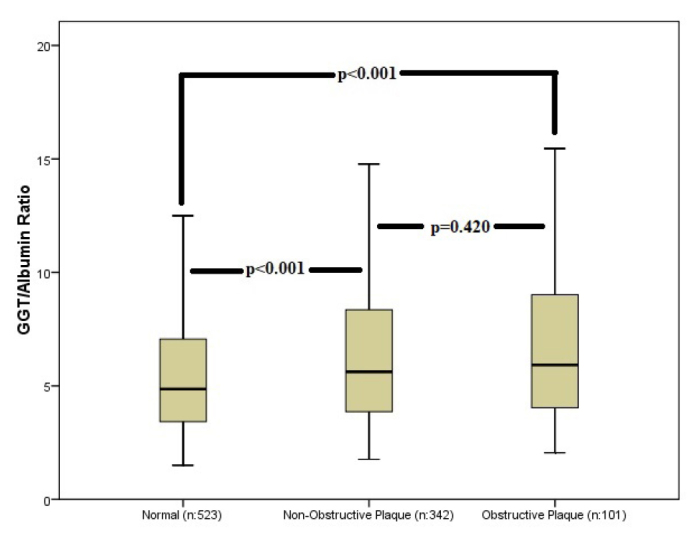
Average gamma-glutamyl transferase/albumin ratio with standard deviations for normal, non-obstructive plaques and obstructive plaque groups. The significance is indicated on the graph
Figure 2.
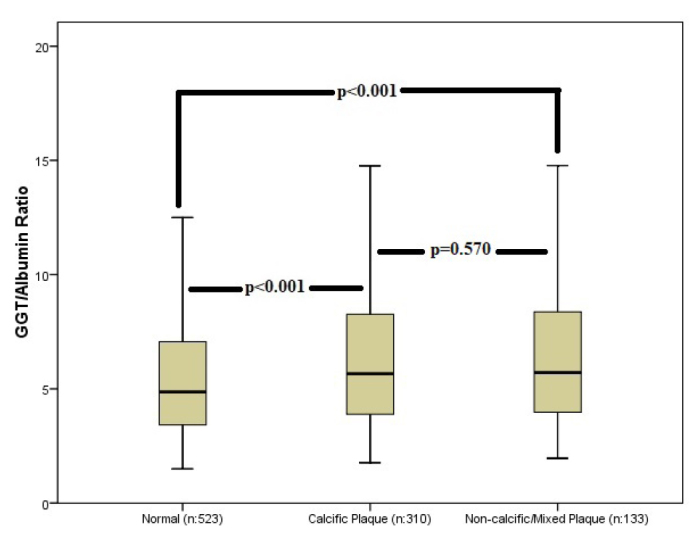
Average gamma-glutamyl transferase/albumin ratio with standard deviations for normal, calcific plaque, and non-calcific/mixed plaque groups. The significance is indicated on the graph
Table 2 shows the clinical features and laboratory parameters according to GAR tertiles. There was a statistically significant difference among the three tertiles in terms of sex, fasting blood glucose levels, creatinine levels, triglyceride levels, GGT levels, GAR, and HDL levels. The male sex ratio was significantly more prevalent with the increasing GAR tertile. HDL levels decreased significantly with the upper tertile, and all the other aforementioned variables steadily increased for the upper tertile. CACS and CT-LeSc were similar between first and second tertiles, whereas the third tertile had significantly higher CACS and CT-LeSc (Table 3). Patients with normal coronary arteries were significantly more prevalent in the first tertile than in the third tertile. Calcific and non-obstructive plaques were significantly more prevalent in the third tertile compared with the first and second tertiles (Table 3).
Table 2.
Clinical and laboratory parameters according to GGT/albumin ratio tertiles
| First tertile (322) | Second tertile (322) | Third tertile (322) | P-value | |
|---|---|---|---|---|
| Age, years | 55±12 | 54±12 | 53±11 | 0.272 |
| Sex (Male), n (%) | 68 (21.1) | 144 (44.7) | 223 (69.3) | <0.001 |
| Hypertension, n (%) | 202 (62.7) | 196 (60.9) | 195 (60.6) | 0.829 |
| Diabetes Mellitus, n (%) | 48 (14.9) | 51 (15.8) | 62 (19.3) | 0.297 |
| Hyperlipidemia, n (%) | 74 (23.0) | 75 (23.3) | 85 (26.4) | 0.535 |
| Fasting glucose, mg/dL | 89 (82–97) | 93 (86–105) | 98 (88–114) | <0.001 |
| Creatinine, mg/dL | 0.7 (0.6–0.8) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | <0.001 |
| Total cholesterol, mg/dL | 218 (186–249) | 215 (183–250) | 215 (185–248) | 0.809 |
| LDL, mg/dL | 135 (107–163) | 135 (109–162) | 132 (108–159) | 0.280 |
| HDL, mg/dL | 55 (46–64) | 49 (42–58) | 45 (39–54) | <0.001 |
| Triglyceride, mg/dL | 115 (82–151) | 138 (101–177) | 159 (118–233) | <0.001 |
| Haemoglobin, g/dL | 13.6±1.3 | 14.3±1.6 | 14.6±1.6 | <0.001 |
| Albumin, g/dL | 4.4 (4.2–4.6) | 4.4 (4.2–4.6) | 4.4 (4.2–4.6) | 0.878 |
| GGT/Albumin | 3.2 (2.7–3.7) | 5.1 (4.7–5.8) | 9.3 (7.8–12.7) | <0.001 |
Continuous variables were represented as mean ± standard deviation or median (Interquartile range 25–75)
GGT - gamma-glutamyl transferase; LDL - low-density lipoprotein; HDL - high-density lipoprotein
Table 3.
Qualitative and quantitative CT angiography information according to the GGT/albumin ratio tertiles
| First tertile (n=322) | Second tertile (322) | Third tertile (322) | P-value | |
|---|---|---|---|---|
| CT-LeSc | 0 (0–2.1) | 0 (0–3.2) | 1.5 (0–4.6)& | <0.001 |
| CACS, AU | 0 (0–10) | 0 (0–11) | 1 (0–70)& | <0.001 |
| CACS Grades, n (%) | ||||
| Zero | 202 (63.1)& | 184 (58.0) | 147 (46.7)& | <0.001 |
| 1–100 | 79 (24.7) | 94 (29.7) | 100 (31.7) | |
| 100–400 | 25 (7.8) | 28 (8.8) | 52 (16.5)& | |
| >400 | 14 (4.4) | 11 (3.5) | 16 (5.1) | |
| CCTA, n (%) | ||||
| Normal | 197 (61.2)& | 181 (56.2) | 145 (45.0)& | 0.001 |
| Non-Obstructive | 99 (30.7) | 104 (32.3) | 139 (43.2)& | |
| Obstructive | 26 (8.1) | 37 (11.5) | 38 (11.8) | |
| Plaque morphology, n (%) | ||||
| No Plaque | 197 (61.2)& | 181 (56.2) | 145 (45.0)& | 0.001 |
| Calcific | 87 (27.0) | 100 (31.1) | 123 (38.2)& | |
| Non-Calcific/Mixed | 38 (11.8) | 41 (12.7) | 54 (16.8) | |
Denotes statistically significant cells after Bonferroni adjustment
Continuous variables were represented as median (Interquartile range 25–75)
AU - Agatston units; CT-LeSc - CT-adapted Leaman score; CACS - coronary artery calcium score; CCTA - coronary computed tomography angiography; HDL - high-density lipoprotein; GGT - gamma-glutamyl transferase
We performed logistic regression analysis to predict severe stenotic plaque and CACS >100. In univariate analysis, GGT levels, albumin levels, and GAR were significantly associated with the presence of severe stenotic plaque and CACS >100. Nevertheless, multivariate regression analysis revealed that GAR but not albumin or GGT was one of the independent predictors in the models (Table 4).
Table 4.
Multivariate logistic regression analyses showing the independent predictors of severe stenotic plaque and CACS >100
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | ||||
|
|
|
||||||||
| Lower | Upper | Lower | Upper | ||||||
| Model 1 for severe plaque | Sex | 1.526 | 1.009 | 2.309 | 0.045 | 2.075 | 1.241 | 3.471 | 0.005 |
| Glucose | 1.007 | 1.003 | 1.012 | 0.001 | |||||
| Age | 1.075 | 1.053 | 1.097 | <0.001 | 1.082 | 1.055 | 1.110 | <0.001 | |
| Total cholesterol | 1.006 | 1.002 | 1.011 | 0.004 | |||||
| LDL | 1.008 | 1.003 | 1.013 | 0.003 | 1.009 | 1.003 | 1.015 | 0.004 | |
| Albumin | 0.266 | 0.151 | 0.467 | <0.001 | |||||
| GGT | 1.007 | 1.000 | 1.014 | 0.044 | |||||
| GGT/albumin ratio | 1.035 | 1.007 | 1.063 | 0.015 | 1.038 | 1.005 | 1.072 | 0.024 | |
| Creatinine | 3.754 | 1.128 | 12.493 | 0.031 | |||||
| Platelet | 0.997 | 0.993 | 1.001 | 0.100 | |||||
| Haemoglobin | 0.910 | 0.804 | 1.030 | 0.136 | |||||
| Monocyte | 1.937 | 0.817 | 4.593 | 0.133 | |||||
| Model 2 for CACS >100 | Sex | 1.461 | 1.026 | 2.081 | 0.035 | ||||
| Hypertension | 1.478 | 1.012 | 2.158 | 0.043 | |||||
| Diabetes mellitus | 1.593 | 1.033 | 2.451 | 0.035 | |||||
| Hyperlipidemia | 1.867 | 1.279 | 2.729 | 0.001 | |||||
| Age | 1.105 | 1.083 | 1.128 | <0.001 | 1.126 | 1.098 | 1.155 | <0.001 | |
| Glucose | 1.007 | 1.003 | 1.011 | 0.001 | |||||
| Creatinine | 4.492 | 1.573 | 12.832 | 0.005 | |||||
| Monocyte | 2.126 | 0.993 | 4.552 | 0.052 | |||||
| Platelet count | 0.997 | 0.994 | 1.000 | 0.032 | |||||
| LDL | 0.997 | 0.993 | 1.002 | 0.234 | |||||
| HDL | 0.990 | 0.976 | 1.003 | 0.141 | 0.972 | 0.955 | 0.989 | 0.002 | |
| Albumin | 0.427 | 0.252 | 0.726 | 0.002 | |||||
| GGT | 1.010 | 1.003 | 1.016 | 0.002 | |||||
| GGT/albumin ratio | 1.044 | 1.018 | 1.071 | 0.001 | 1.051 | 1.018 | 1.086 | 0.002 | |
CACS - coronary artery calcium score; CI - confidence interval; LDL - low-density lipoprotein; GGT - gamma-glutamyl transferase; HDL - high-density lipoprotein
Discussion
Results of our study can be addressed under three main findings; (i) the basal GAR level is an independent predictor of severe CAD and high CACS, (ii) high circulating GAR is linked to well-known cardiovascular risk factors, such as male sex, fasting blood glucose, creatinine, HDL-cholesterol, and high triglyceride; and (iii) the high GAR is related with the factors which determine presence and extent of the CAD (CACS and CT-LeSc). To the best of our knowledge, this study is the first one that investigates the relationship between GAR and coronary atherosclerosis in patients who underwent CCTA.
GGT is an enzyme that is found in the serum and exterior surface of the cell and plays a role in the homeostasis of G-SH, which is the principal antioxidant for the human cell. Cysteine and glycine formed as a result of extracellular hydrolysis of G-SH by GGT are subsequently transported into the cell and used for the synthesis of G-SH that play the principal role in protection against oxidative stress. However, extracellular cysteine-glycine complex inhibits the reduction of Fe3+ to Fe2+. This process results in the formation of peroxide, free oxygen radicals, and oxidized LDL. Thus, aggravation of oxidative stress increases the number of oxidized LDL receptors on the cell surface and facilitates the transportation of LDL/GGT complexes into the plaque. These events lead to the formation and progression of atheromatous plaque. The studies that detect GGT activity inside the atherosclerotic plaque support the hypothesis that GGT has a direct role in atherogenesis (20). Kittleson et al. (21) have found that increased GGT activity, even in the normal range, was associated with increased oxidative stress. Previous studies demonstrated that GGT level correlates with cardiovascular diseases and risk factors. A study by Arasteh et al. (22) have found the relationship between GGT level and severity of the CAD. In a cohort of 163,944 subjects with a 17-year follow-up, Ruttman et al. (23) have found that GGT is related to cardiovascular mortality independently from other cardiovascular risk factors. Lazzeri et al. (24) have determined a relationship between GGT level and mortality in patients undergoing percutaneous coronary intervention for CAD in a three-year follow-up. Similarly, GGT was shown to be an independent predictor of early mortality in patients with ST-elevation myocardial infarction. In another study, serum GGT level was independently associated with coronary stenosis complexity and long-term mortality in patients with stable CAD (21). It seems GGT levels are related not only with the development of atherosclerosis but also with CAD-related mortality.
Albumin contains 80% of thiol groups that play a role in scavenging free oxygen radicals from the plasma. Low albumin is associated with increased oxidative stress, platelet aggregation, and activation (25, 26). The abovementioned mechanisms are factors that increase the risk of atherothrombosis, and low albumin secondary to inflammatory response is related to poor cardiovascular prognosis. In the Framingham offspring study, it was observed that low albumin correlated with increased prevalence of the CAD (27). A prospective study conducted by Chien et al. (28) have found that hypoalbuminemia accelerates cardiovascular events and mortality rates in patients with stable CAD in 18-month follow-up.
As GGT and albumin follow different antioxidant pathways, they may trigger the atherosclerotic process through different mechanisms. Moreover, the proinflammatory effect of high GGT and low albumin also ensures the progression of atherosclerosis. The CARDIA study demonstrated a reverse correlation between GGT and the concentration of circulating antioxidants (29). Considering those studies, increased GGT might aggravate oxidative stress and the need for G-SH at the cellular level. In this study, we evaluated the collective effect of GGT and albumin level on atherosclerosis defined as GAR. Contrary to previous studies, we used CCTA, a non-invasive test, rather than coronary angiography to detect the relationship between CAD and GAR. Therefore, our results suggest that GAR may predict the presence and severity of atherosclerosis before CCTA. Another result of our study is the link of increased blood GAR to well-known risk factors of atherosclerosis, such as male sex, fasting blood glucose, creatinine, and HDL-cholesterol. Although age was significantly higher in the group with atherosclerosis in CCTA, it was found that GAR was associated with atherosclerosis in multivariate analysis independent of age. Although the CCTA positive group had a significantly higher diagnosis of hyperlipidemia, there was no significant difference between the groups regarding LDL levels, and there was a significant difference for triglyceride, suggesting that patients with hyperlipidemia may be receiving statin treatment at a higher rate. The difference in triglyceride levels can be attributed to the lack of initiation of fibrate therapy at moderate levels and the weak triglyceride-lowering effects of statins. CCTA not only determines critical stenosis but also provides a detailed analysis of the extent of coronary atherosclerosis. CACS is a tool for cardiovascular risk assessment (30), however, was unable to detect non-calcified plaques appropriately, which is accepted as more vulnerable for acute coronary syndromes. CT-LeSc offers a better insight regarding the long-term prognosis as it evaluate all parameters, such as localization and type of plaque and severity of stenosis (31, 32). Our study observed that higher GAR was associated with significantly higher CACS and CT-LeSc.
Study limitations
First, our study was a single-center, retrospective trial conducted in a tertiary care university hospital. Therefore, socioeconomic and demographic factors might have influenced our findings. Second, it was not known whether the study patients had subclinical liver disease or non-alcoholic liver disorder, which could have influenced the GGT level. Third, although the patients with habitual alcohol consumption were excluded, we were unable to obtain data about the mild alcohol consumption which might have influenced GGT analyses. Our lack of information about the medications, some demographic and clinical features, and stress test results of the patients could also be listed among the limitations. As our study did not enroll patients with documented CAD and acute coronary syndrome, our results cannot be projected to all CAD groups. However, by including data from almost 1,000 patients, we believe we overcame some limitations mentioned above. Prospective and larger trials are warranted to better understand the relationship between GAR and CAD.
Conclusion
We demonstrated that basal GAR independently predicts the presence, extent, and severity of CAD determined by CT-LeSc and higher CACS on CCTA. GAR is also significantly associated with well-established cardiovascular risk factors. GGT and albumin are both cheap, safe, and easily obtainable parameters; and GAR may help physicians in the diagnosis and early intervention of CAD among patients presented with stable angina pectoris.
HIGHLIGHTS
Since gamma-glutamyl transferase (GGT) and albumin follow different antioxidant pathways, they may trigger the atherosclerotic process through different mechanisms.
We evaluated the collective effect of GGT and albumin level on atherosclerosis defined as GGT-to-albumin ratio (GAR).
Basal GAR independently predicts the presence, extent, and severity of CAD determined by higher coronary artery calcium and Leaman score on coronary computed tomography angiography.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – S.T., E.K., B.S., M.C., S.Ü., M.K., A.N.B., H.K.K., G.E., A.A.; Design – S.T., E.K., B.S., M.C., S.Ü., M.K.; Supervision – S.T., E.K., B.S., M.C., S.Ü., M.K., A.N.B., H.K.K., G.E., A.A.; Fundings – None; Materials – S.T., H.K.K., G.E., A.A.; Data collection &/or processing – S.T., M.K., A.N.B., H.K.K., G.E., A.A.; Analysis &/or interpretation – A.N.B., H.K.K., G.E., A.A.; Literature search – S.T., E.K., B.S., M.C., S.Ü., M.K.; Writing – S.T., E.K., B.S., M.C., S.Ü., M.K., A.N.B., H.K.K., G.E., A.A.; Critical review – S.T., E.K., B.S., M.C., S.Ü., M.K., A.N.B., H.K.K., G.E., A.A.
References
- 1.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. 2013;168:934–45. doi: 10.1016/j.ijcard.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–80. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 4.Pleiner J, Mittermayer F, Schaller G, Marsik C, MacAllister RJ, Wolzt M. Inflammation-induced vasoconstrictor hyporeactivity is caused by oxidative stress. J Am Coll Cardiol. 2003;42:1656–62. doi: 10.1016/j.jacc.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–90. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Münzel T, Gori T, Keaney JF, Jr, Maack C, Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J. 2015;36:2555–64. doi: 10.1093/eurheartj/ehv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–43. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Paar M, Rossmann C, Nusshold C, Wagner T, Schlagenhauf A, Leschnik B, et al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS One. 2017;12:e0182997. doi: 10.1371/journal.pone.0182997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto N, Sato Y, Yoda S, Nakano Y, Kunimasa T, Matsuo S, et al. Prognostic value of non-obstructive CT low-dense coronary artery plaques detected by multislice computed tomography. Circ J. 2007;71:1898–903. doi: 10.1253/circj.71.1898. [DOI] [PubMed] [Google Scholar]
- 12.Öztürk E, Kafadar C, Tutar S, Bozlar U, Hagspiel KD. Non-coronary abnormalities of the left heart: CT angiography findings. Anatol J Cardiol. 2016;16:720–7. doi: 10.14744/AnatolJCardiol.2016.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An L, Liu Q, Feng H, Bai X, Dang Y, Li C, et al. Increased glycoprotein acetylation is associated with high cardiac event rates: Analysis using coronary computed tomography angiography. Anatol J Cardiol. 2018;20:152–8. doi: 10.14744/AnatolJCardiol.2018.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinica G, Chistol RO, Enache M, Leon Constantin MM, Ciocoiu M, Furnica C. Long-term graft patency after coronary artery bypass grafting: Effects of morphological and pathophysiological factors. Anatol J Cardiol. 2018;20:275–82. doi: 10.14744/AnatolJCardiol.2018.51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 16.Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI) J Cardiovasc Comput Tomogr. 2016;10:435–49. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. Society of Cardiovascular Computed Tomography. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–36. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 18.de Araújo Gonçalves P, Garcia-Garcia HM, Dores H, Carvalho MS, Jerónimo Sousa P, Marques H, et al. Coronary computed tomography angiography-adapted Leaman score as a tool to noninvasively quantify total coronary atherosclerotic burden. Int J Cardiovasc Imaging. 2013;29:1575–84. doi: 10.1007/s10554-013-0232-8. [DOI] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 20.Paolicchi A, Minotti G, Tonarelli P, Tongiani R, De Cesare D, Mezzetti A, et al. Gamma-glutamyl transpeptidase-dependent iron reduction and LDL oxidation--a potential mechanism in atherosclerosis. J Investig Med. 1999;47:151–60. [PubMed] [Google Scholar]
- 21.Kittleson MM, Patel JK, Kobashigawa JA. In: Hurst’s the Heart Chapter 72: cardiac transplantation. 14th ed. Fuster V, Harrington RA, Narula J, Eapen ZJ, editors. New York (NY): McGraw-Hill; 2017. [Google Scholar]
- 22.Arasteh S, Moohebati M, Avan A, Esmaeili H, Ghazizadeh H, Mahdizadeh A, et al. Serum level of gamma-glutamyl transferase as a biomarker for predicting stenosis severity in patients with coronary artery disease. Indian Heart J. 2018;70:788–92. doi: 10.1016/j.ihj.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H Vorarlberg Health Monitoring and Promotion Program Study Group. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130–7. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 24.Lazzeri C, Valente S, Tarquini R, Chiostri M, Picariello C, Gensini GF. The prognostic role of gamma-glutamyltransferase activity in non-diabetic ST-elevation myocardial infarction. Intern Emerg Med. 2011;6:213–9. doi: 10.1007/s11739-010-0464-8. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988;37:569–71. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 26.Gresele P, Deckmyn H, Huybrechts E, Vermylen J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. 1984;33:2083–8. doi: 10.1016/0006-2952(84)90577-X. [DOI] [PubMed] [Google Scholar]
- 27.Djoussé L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. 2002;106:2919–24. doi: 10.1161/01.CIR.0000042673.07632.76. [DOI] [PubMed] [Google Scholar]
- 28.Chien SC, Chen CY, Leu HB, Su CH, Yin WH, Tseng WK, et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int J Cardiol. 2017;241:1–5. doi: 10.1016/j.ijcard.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Jacobs DR, Jr, Gross M, Kiefe CI, Roseman J, Lewis CE, et al. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358–66. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]
- 30.Abuzaid A, Saad M, Addoumieh A, Ha LD, Elbadawi A, Mahmoud AN, et al. Coronary artery calcium score and risk of cardiovascular events without established coronary artery disease: a systemic review and meta-analysis. Coron Artery Dis. 2021;32:317–28. doi: 10.1097/MCA.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 31.Andreini D, Pontone G, Mushtaq S, Gransar H, Conte E, Bartorelli AL, et al. Long-term prognostic impact of CT-Leaman score in patients with non-obstructive CAD: Results from the COronary CT Angiography EvaluatioN For Clinical Outcomes InteRnational Multicenter (CONFIRM) study. Int J Cardiol. 2017;231:18–25. doi: 10.1016/j.ijcard.2016.12.137. [DOI] [PubMed] [Google Scholar]
- 32.Butt N, Parajuli S, Ali L, Yacob O, Melaku GD, Hideo-Kajita A, et al. Comprehensive assessment of coronary computed tomography angiography by using Leaman and Leiden score in overweight and obese patients. Int J Cardiovasc Imaging. 2020;36:2377–82. doi: 10.1007/s10554-020-01938-x. [DOI] [PubMed] [Google Scholar]


