Abstract
Background
Although SARS-CoV-2 infection often causes milder symptoms in children and adolescents, young people might still play a key part in SARS-CoV-2 transmission. An efficacious vaccine for children and adolescents could therefore assist pandemic control. For further evaluation of the inactivated COVID-19 vaccine candidate BBIBP-CorV, we assessed the safety and immunogenicity of BBIBP-CorV in participants aged 3–17 years.
Methods
A randomised, double-blind, controlled, phase 1/2 trial was done at Shangqiu City Liangyuan District Center for Disease Control and Prevention in Henan, China. In phases 1 and 2, healthy participants were stratified according to age (3–5 years, 6–12 years, or 13–17 years) and dose group. Individuals with a history of SARS-CoV-2 or SARS-CoV infection were excluded. All participants were randomly assigned, using stratified block randomisation (block size eight), to receive three doses of 2 μg, 4 μg, or 8 μg of vaccine or control (1:1:1:1) 28 days apart. The primary outcome, safety, was analysed in the safety set, which consisted of participants who had received at least one vaccination after being randomly assigned, and had any safety evaluation information. The secondary outcomes were geometric meant titre (GMT) of the neutralising antibody against infectious SARS-CoV-2 and were analysed based on the full analysis set. This study is registered with www.chictr.org.cn, ChiCTR2000032459, and is ongoing.
Findings
Between Aug 14, 2020, and Sept 24, 2020, 445 participants were screened, and 288 eligible participants were randomly assigned to vaccine (n=216, 24 for each dose level [2/4/8 μg] in each of three age cohorts [3–5, 6–12, and 13–17 years]) or control (n=72, 24 for each age cohort [3–5, 6–12, and 13–17 years]) in phase 1. In phase 2, 810 participants were screened and 720 eligible participants were randomly assigned and allocated to vaccine (n=540, 60 for each dose level [2/4/8 μg] in each of three age cohorts [3–5, 6–12, and 13–17 years]) or control (n=180, 60 for each age cohort [3–5, 6–12, and 13–17 years]). The most common injection site adverse reaction was pain (ten [4%] 251 participants in all vaccination groups of the 3–5 years cohort; 23 [9·1%] of 252 participants in all vaccination groups and one [1·2%] of 84 in the control group of the 6–12 years cohort; 20 [7·9%] of 252 participants in all vaccination groups of the 13–17 years cohort). The most common systematic adverse reaction was fever (32 [12·7%] of 251 participants in all vaccination groups and six [7·1%] of 84 participants in the control group of the 3–5 years cohort; 13 [5·2%] of 252 participants in the vaccination groups and one [1·2%] of 84 in the control group of the 6–12 years cohort; 26 [10·3%] of 252 participants in all vaccination groups and eight [9·5%] of 84 in the control group of the 13–17 years cohort). Adverse reactions were mostly mild to moderate in severity. The neutralising antibody GMT against the SARS-CoV-2 virus ranged from 105·3 to 180·2 in the 3–5 years cohort, 84·1 to 168·6 in the 6–12 years cohort, and 88·0 to 155·7 in the 13–17 years cohort on day 28 after the second vaccination; and ranged from 143·5 to 224·4 in the 3–5 years cohort, 127 to 184·8 in the 6–12 years cohort, and 150·7 to 199 in the 13–17 years cohort on day 28 after the third vaccination.
Interpretation
The inactivated COVID-19 vaccine BBIBP-CorV is safe and well tolerated at all tested dose levels in participants aged 3–17 years. BBIBP-CorV also elicited robust humoral responses against SARS-CoV-2 infection after two doses. Our findings support the use of a 4 μg dose and two-shot regimen BBIBP-CorV in phase 3 trials in the population younger than 18 years to further ascertain its safety and protection efficacy against COVID-19.
Funding
National Program on Key Research Project of China, National Mega projects of China for Major Infectious Diseases, National Mega Projects of China for New Drug Creation, and Beijing Science and Technology Plan.
Translation
For the Chinese translation of the abstract see Supplementary Materials section.
Introduction
According to WHO, as of July, 2021, the COVID-19 pandemic has caused more than 4 million deaths worldwide. Children are susceptible to SARS-CoV-2 infection, but show milder clinical manifestation of disease.1 This susceptibility raises the possibility of transmission among family members, and risk to elderly members who are more vulnerable to disease.2, 3 Clinical trials of vaccine candidates on different platforms (inactivated,4, 5, 6 protein subunit,7, 8 mRNA9, 10, and vector based11, 12) have shown their safety and efficacy against SARS-CoV-2 in adults, but to date fewer studies have been done in children. An mRNA vaccine candidate BNT162b2 (tozinameran; Pfizer–BioNTech) has shown 100% efficacy in a population aged 12–25 years.13 Another mRNA vaccine candidate mRNA-1273 (Moderna, Cambridge, MA, USA) is reported to be tolerated and to have efficacy against COVID-19 of 100% starting 14 days after the second dose in adolescents aged 12–17 years.14 The safety and immunogenicity of the inactivated vaccine CoronaVac (Sinovac, Beijing, China) is being tested in a phase 1/2 trial in a population aged 3–17 years.15 In addition to CoronaVac, further evaluation of safety and immunogenicity of the inactivated COVID-19 vaccine BBIBP-CorV (Beijing Institute of Biological Products, Beijing, China) is needed in populations aged younger than 18 years.
Research in context.
Evidence before this study
We searched PubMed for research articles published from database inception until April 20, 2021, using the terms “COVID-19” OR “SARS-CoV-2”, “vaccine”, AND “clinical trial” AND “phase”. Article type was set to clinical trial. No language and date restrictions were applied. 34 reports were identified, among which five were for inactivated vaccines, two were for spike protein subunit vaccines, seven were for mRNA vaccines, and eight were for vector-based vaccines. We identified one clinical trial report for the SARS-CoV-2 mRNA vaccine candidate BNT162b2 done in children and adolescents by the time our Article was under revision. On May 25, 2021, Moderna announced that the phase 2/3 study of its mRNA vaccine candidate mRNA-1273 in adolescents had met its primary immunogenicity endpoint.
Added value of this study
The present trial showed that the inactivated COVID-19 vaccine, BBIBP-CorV, was safe and able to elicit a robust humoral response in healthy people younger than 18 years. Seroconversion rate of neutralising antibody was found in all vaccinees on day 28, and the neutralising antibody geometric mean titre was at level comparable to that of adults in earlier trials.
Implications of all the available evidence
Our finding indicates that BBIBP-CorV is safe and immunogenic in healthy individuals aged 3–17 years. Further clinical investigation is needed to evaluate the efficacy of this vaccine candidate for prevention of COVID-19 in the general population.
Our previous phase 1/2 clinical trial interim report of BBIBP-CorV, has shown an acceptable safety profile and immunogenicity in participants aged 18–59 years and 60 years and older.4 Thus, in this study, we further report the full set of safety and immunogenicity data for BBIBP-CorV in phase 1 and 2 clinical trials among healthy people younger than 18 years in China.
Methods
Study design and participants
We did a randomised, double-blind, dose-escalation, controlled phase 1/2 trial of BBIBP-CorV in the Shangqiu City Liangyuan District Center for Disease Control and Prevention in Henan, China. The enrolled participants were healthy individuals aged 3–17 years, who were negative for serum-specific IgM or IgG antibodies against SARS-CoV-2 N and S proteins (tested and verified with a commercial kit from Innovita, China) at the time of screening and vaccine inoculation on the same day. Exclusion criteria were a history of travelling to Hubei, regions outside of China, or regions within China with any reported COVID-19 cases since December, 2019; a history of infection with SARS-CoV; fever (axillary temperature more than 37·3°C if aged older than 14 years, and axillary temperature of more than 37·5°C if aged 14 years or younger), respiratory syndromes, diarrhoea, dyspnoea, or tachypnoea within 14 days before vaccination; abnormal results in laboratory tests (blood biochemistry tests [alanine aminotransferase, aspartate aminotransferase, total bilirubin, creatinine, urea nitrogen], routine blood tests [haemoglobin, white blood cell count], and routine urine tests [protein, urine sugar, blood cells—microscopic examination]); allergy to any ingredient included in the vaccine; a history of seizures or mental illness (defined as a history of convulsion, epilepsy or psychosis); and being unable to comply with the study schedule. Criteria for early suspension of trial are outlined in the protocol (ChiCTR2000032459). Safety was reviewed by a safety monitoring board before proceeding to the next dose group or next age cohort, and for advancement to phase 2.
The trial design was a dose escalation study, in three dose levels (2 μg, 4 μg, and 8 μg per dose) at a 28-day intervals in both adults and children. After an interim analysis showed BBIBP-CorV's safety and immunogenicity in an adult population after two doses of vaccines,4 the adult cohort continued on to receive the third dose of vaccine. These data were obtained according to the original trial protocol. The studies of individuals aged 3–17 years were also done using a three-dose regimen, according to the original protocol.
The protocol and informed consent were approved by the Medical Ethics Committee of Henan Provincial Center for Disease Control and Prevention. Written informed consent from all participants was obtained before screening. This study was done by Henan Provincial Center for Disease Control and Prevention, and implemented in Liangyuan, Shangqiu, in accordance with the Declaration of Helsinki and Good Clinical Practice.
Randomisation and masking
In age de-escalation, dose escalation phase 1 and 2 studies, participants were stratified by age (3–5 years, 6–12 years, or 13–17 years) and were randomly assigned to receive intramuscular injections of 2 μg, 4 μg, 8 μg, or control (1:1:1:1). Within each dose-escalating group of each age cohort in phase 1/2, the ratio of vaccine versus control was 3:1. Participants were sequentially assigned a randomisation number generated by an independent statistician using Stata, version 12.0, and stratified block randomisation (block size eight) by subgroups was adopted. All vaccines used for inoculation were distributed in identical packages with serial numbers to ensure masking of participants. The safety evaluation was masked for all participants. Group allocation was masked from participants, investigators, and outcome assessors for the duration of the study.
Procedures
BBIBP-CorV was developed by Beijing Institute of Biological Products (Beijing, China), and manufactured as previously described.5, 16 Briefly, the strain 19nCoV-CDC-Tan-HB02 (HB02), with optimal replication and highest virus yields in Vero cells, was inactivated by β-propionolactone at a ratio of 1:4000 at 2–8°C. The vaccine was manufactured as a liquid formulation containing 2 μg, 4 μg, or 8 μg of total proteins with aluminium hydroxide adjuvant (0·45 mg/mL) per 0·5 mL in a vial. The control consisted of saline and aluminium hydroxide adjuvant.
In phase 1 and 2, participants in the three groups (3–5 years, 6–12 years, and 13–17 years) received 2 μg, 4 μg, or 8 μg of BBIBP-CorV or control on a three-dose schedule (on days 0, 28, and 56).
For safety assessments, all adverse reactions, including solicited and unsolicited adverse events, from the first dose to 30 days after the full course of vaccinations were collected by the investigator's active visits and spontaneous reports. The investigators had phone calls or face-to-face interviews within 7 days after each dose, and weekly phone calls 8–30 days after each dose. Laboratory safety values (haematology, serum, and chemistry) were measured before vaccination (baseline) and on day 4 after each vaccination. The adverse events and abnormal changes in laboratory test results were graded according to the scales issued by the China State Food and Drug Administration.17 Safety data were collected based on guidance18 issued by the National Medical Products Administration, and monitored by the data safety monitoring board on 8 days after the first vaccination and 30 days after the vaccination schedule had been completed.
Blood samples were collected for serology tests at the scheduled site visits before each vaccination (days 0, 28, and 56), and on day 84. The neutralising antibody responses induced by vaccination were determined by microneuralisation assay with the infectious SARS-CoV-2 virus (strain 19nCoV-CDC-Tan-Strain05, QD01). Serum was diluted 1/4, followed by a series of two-times dilutions to the required concentration, and an equal volume of challenge virus solution was added. After neutralisation in a 37°C incubator for 2 h, a 1·0–2·5 × 105 cells per mL suspension was added to the wells of a 96-well plate (0·1 mL per well) and cultured in a CO2 incubator at 37°C for 4 days. Titres were expressed as the reciprocal of the highest dilution level protecting 50% cells from virus challenge. Human convalescent serum (HCS) was collected from patients at least 15 days after confirmation of SARS-CoV-2 infection. HCS was included as an internal positive control in every assay. Seroconversion was defined as a four-times increase in antibody titre relative to day 0.
Outcomes
The primary outcome for safety was the occurrence of adverse reactions within 7 days after each vaccination. Any abnormal changes in laboratory test results were measured on day 4 after each vaccination, and adverse reactions within 30 days after whole vaccination procedure across all study groups were analysed as secondary safety endpoints. The secondary outcomes for humoral immunogenicity were measured with infectious SARS-CoV-2 neutralising assay (on days 0, 28, 56, and 84) and expressed as neutralising antibody geometric mean titres (GMTs) and seroconversion.
Statistical analysis
The sample size was not determined based on the statistical power calculation. Both phases were designed at the same time. For a sample size of 84 in 2 μg, 4 μg, and 8 μg of each age subgroup (24 in phase 1 and 60 in phase 2), we had an 80% test power to detect a 15% rate difference in immunogenicity with 10% dropout at a 0·05 significance level using a Z test for two independent proportions in PASS13-NCSS10. This result in a planned sample sizes of 24 participants for each vaccination group and eight for each control group in phase 1, and 60 participants for each vaccination group and 20 for each control group in phase 2 were determined.
The safety analysis was based on the safety set, which consisted of participants who had received at least one vaccination after being randomly assigned, and had any safety evaluation information. Humoral immunogenicity analysis was based on the full analysis set, which consisted of enrolled participants who had randomly received a vaccination with blood collection before and after each inoculation, and the compliance set, which consisted of enrolled participants who had randomly received a vaccination with blood sample collection before and after each inoculation and showed no trial protocol violation. From the perspective of trial design, vaccination:control ratio was 3:1 inside the dose-escalating group of each age cohort. The analysis was done by comparing the vaccination group with control within the same dose-escalating group.
Data from phases 1 and 2 were pooled for analysis, since the participants in both studies had similar characteristics and underwent same vaccination regimens and the safety and immunogenicity data of two phases should thus be theoretically comparable. No formal statistical analysis was planned for unsolicited adverse events. The occurrence of laboratory abnormalities was tested by χ2 test, correction χ2 test, or Fisher's exact test between the test group and the control group in each age cohort, among groups who received different doses. GMTs for neutralising antibody with corresponding 95% CIs are reported. Comparison of antibody titres at timepoints after immunisation was done with the two independent sample t tests (normal and homogeneous variance) or corrected t tests (normal but non-homogeneous variance). For abnormally distributed data, a Wilcoxon rank sum test was used to compare the difference between experimental and control groups of different ages, among groups who received different doses. All data were included in the analyses. All analyses were done using GraphPad Prism (version 8.0.1). All statistical tests were two-sided, and the significance level was p values of 0·05 or less for inferential analyses and multiple comparisons were not adjusted.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
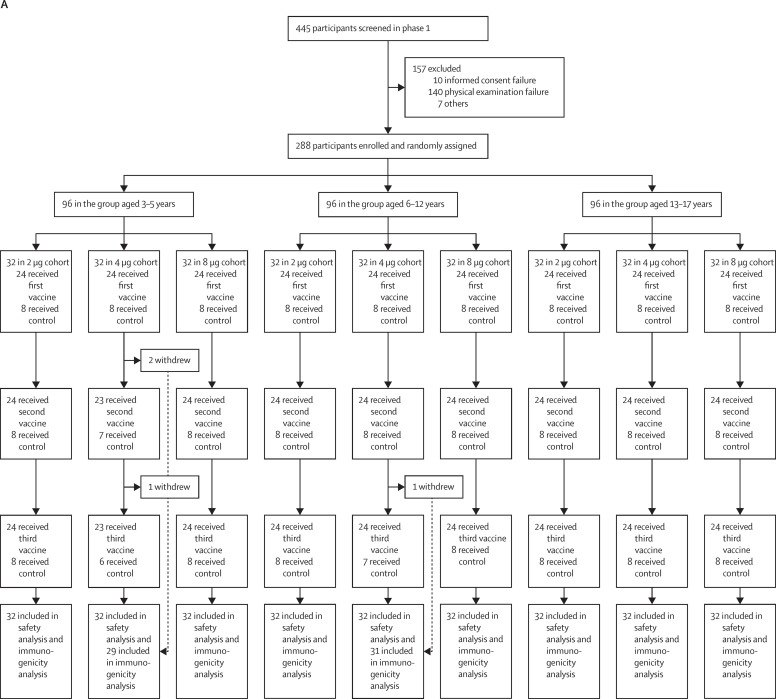
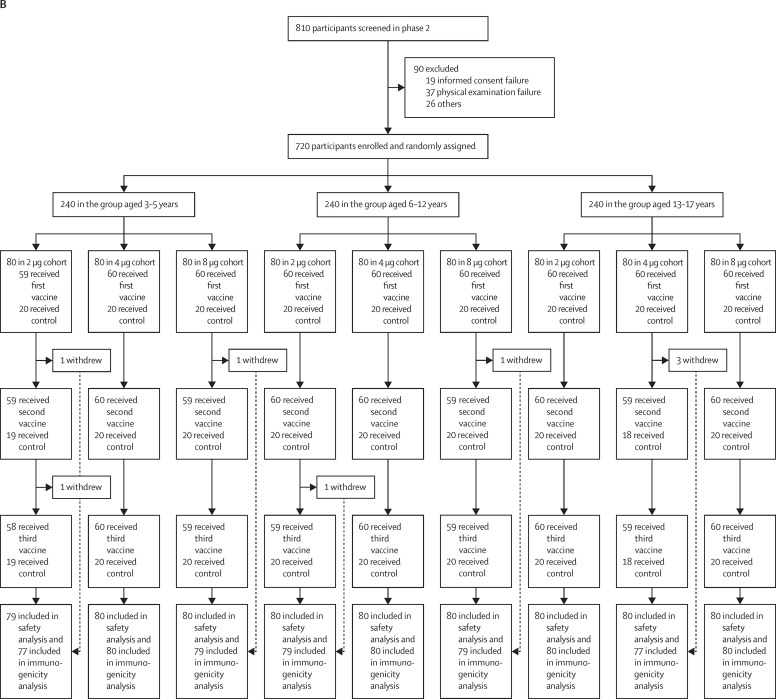
Between Aug 14, 2020, and Sept 24, 2020, 445 participants were screened, and 288 eligible participants were randomly assigned to vaccine or control in the phase 1 study. Enrolled participants were evenly distributed over three age cohorts (3–5 years, 6–12 years, and 13–17 years). Each participant received three shots of either vaccine (at 2 μg, 4 μg, or 8 μg) or control at 28-day intervals. All 288 participants received the first vaccination on day 0, and all but four received the second vaccination on day 28 and the third vaccination on day 56. Exceptions included three participants in the 4 μg 3–5 years cohort and one participant in the 4 μg 6–12 years cohort (figure 1 ). In phase 2, 810 participants were screened and 720 eligible participants were randomly assigned and allocated to vaccine or control. Enrolled participants were evenly distributed over three age groups (3–5 years, 6–12 years, and 13–17 years). Participants in phase 2 underwent identical vaccination procedures to the phase 1 study. All but one of the 720 participants received the first vaccination on day 0, and all but seven received the second vaccination on day 28 and the third vaccination on day 56 (figure 1). Exceptions included one participant in the 2 μg 3–5 years cohort, one participant in the 8 μg 3–5 years cohort, one participant in the 2 μg 6–12 years cohort, one participant in the 8 μg 6–12 years cohort, and three participants in the 4 μg 13–17 years cohort. All withdrawals were requested by participants or their guardian. Detailed demographic characteristics of the participants are listed in Table 1, Table 2 .
Figure 1.
Trial profile for phase 1 (A) and phase 2 (B)
Table 1.
Demographic characteristics of the participants in phase 1
|
3–5 years |
6–12 years |
13–17 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine 2 μg (n=24) | Vaccine 4 μg (n=24) | Vaccine 8 μg (n=24) | Control (n=24) | Vaccine 2 μg (n=24) | Vaccine 4 μg (n=24) | Vaccine 8 μg (n=24) | Control (n=24) | Vaccine 2 μg (n=24) | Vaccine 4 μg (n=24) | Vaccine 8 μg (n=24) | Control (n=24) | |
| Age, years | ||||||||||||
| Mean | 4·6 (0·9) | 4·5 (0·9) | 5·3 (0·7) | 4·7 (0·8) | 9·8 (1·9) | 9·3 (2) | 9·2 (2·1) | 10 (1·6) | 14·7 (1·2) | 15·1 (1·4) | 14·4 (1·1) | 14·9 (1·2) |
| Median | 4·8 (3·7–5·0;3·2–6·0) | 4·6 (3·7–5·3;3·0–5·9) | 5·4 (4·8–5·9;3·8–6·0) | 4·9 (3·9–5·4;3·1–5·8) | 9·8 (8·1–11·6;6·8–13·0) | 9·4 (7·2–10·3;6·3–13·0) | 9·1 (7·0–11·0;6·0–12·5) | 10·4 (9·1–11·1;6·4–12·2) | 14·5 (13·9–15·6;13·2–17·3) | 15·0 (14·3–16·1;13·1–17·8) | 14·2 (13·5–15·1;13·1–16·6) | 14·9 (14·1–15·6;13·2–17·3) |
| Sex | ||||||||||||
| Male | 17 (71%) | 13 (54%) | 11 (46%) | 11 (46%) | 13 (54%) | 14 (58%) | 7 (29%) | 11 (46%) | 12 (50%) | 10 (42%) | 15 (63%) | 17 (71%) |
| Female | 7 (29%) | 11 (46%) | 13 (54%) | 13 (54%) | 11 (46%) | 10 (42%) | 17 (71%) | 13 (54%) | 12 (50%) | 14 (58%) | 9 (37%) | 7 (29%) |
| Ethnicity | ||||||||||||
| Han | 23 (96%) | 24 (100%) | 24 (100%) | 24 (100%) | 23 (96%) | 24 (100%) | 24 (100%) | 23 (96%) | 22 (92%) | 22 (92%) | 23 (96%) | 22 (92%) |
| Hui | 1 (4%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 1 (4%) | 1 (4%) | 2 (8%) | 1 (4%) | 1 (4%) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 1 (4%) |
Data mean (SD), median (IQR; range), or n (%).
Table 2.
Demographic characteristics of the participants in phase 2
|
3–5 years |
6–12 years |
13–17 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine 2 μg (n=60) | Vaccine 4 μg (n=60) | Vaccine 8 μg (n=60) | Control (n=60) | Vaccine 2 μg (n=60) | Vaccine 4 μg (n=60) | Vaccine 8 μg (n=60) | Control (n=60) | Vaccine 2 μg (n=60) | Vaccine 4 μg (n=60) | Vaccine 8 μg (n=60) | Control (n=60) | |
| Age, years | ||||||||||||
| Mean | 4·6 (0·8) | 4·6 (0·8) | 4·4 (0·8) | 4·7 (0·7) | 9·6 (2) | 9·6 (2·2) | 8·8 (2) | 9·9 (1·8) | 15 (1·2) | 14·9 (1·3) | 15 (1·4) | 14·9 (1·4) |
| Median | 4·8 (3·8–5·3;3·0–5·9) | 4·7 (3·9–5·3;3·1–5·9) | 4·4 (3·8–5·1;3·1–5·9) | 4·7 (4·1–5·3;3·2–5·9) | 10·2 (7·7–11·3;6·1–13·0) | 9·4 (7·9–11·5;6·2–13·0) | 8·3 (6·9–10·4;6·2–12·7) | 10·0 (8·6–11·7;6·5–12·9) | 14·7 (14·0–15·9;13·1–17·6) | 14·8 (13·8–15·9;13·0–18·0) | 14·7 (13·7–16·0;13·0–17·9) | 14·8 (13·6–15·7;13·0–18·0) |
| Sex | ||||||||||||
| Male | 31 (52%) | 32 (53%) | 25 (42%) | 31 (52%) | 31 (52%) | 33 (55%) | 32 (53%) | 26 (43%) | 30 (50%) | 32 (53%) | 39 (65%) | 32 (53%) |
| Female | 29 (48%) | 28 (47%) | 35 (58%) | 29 (48%) | 29 (48%) | 27 (45%) | 28 (47%) | 34 (57%) | 30 (50%) | 28 (47%) | 21 (35%) | 28 (47%) |
| Ethnicity | ||||||||||||
| Han | 59 (98%) | 57 (95%) | 60 (100%) | 59 (98%) | 58 (97%) | 56 (93%) | 59 (98%) | 57 (95%) | 60 (100%) | 60 (100%) | 60 (100%) | 60 (100%) |
| Hui | 1 (2%) | 3 (5%) | 0 | 1 (2%) | 2 (3%) | 4 (7%) | 1 (2%) | 3 (5%) | 0 | 0 | 0 | 0 |
Data mean (SD), median (IQR; range), or n (%).
The most common adverse reaction was pain at the injection site, which was reported within 30 days after all three vaccinations by ten (4·0%) of 251 participants in the vaccination groups of the 3–5 years cohort, 23 (9·1%) of 252 participants in the vaccination groups and one (1·2%) of 84 participants in the control group of the 6–12 years cohort, and 20 (7·9%) of 252 participants in the vaccination groups of the 13–17 years cohort (Table 2, Table 3, Table 4 ; appendix 2 p 1). Participants in the vaccine groups in the 6–12 years (p=0·014) and 13–17 years (p=0·0078) cohorts reported significantly more pain than participants in the control groups. A dose-dependent increase in overall injection site adverse reactions in the vaccine groups compared with the control group was detectable in the 6–12 years cohort (table 5 ; appendix 2 p 1). Reported local adverse reactions were all mild (grade 1) and moderate (grade 2) in severity.
Table 3.
Adverse reactions within 30 days after whole vaccination procedure for the group aged 3–5 years in phase 1/2
|
2 μg cohort (n=111) |
4 μg cohort (n=112) |
8 μg cohort (n=112) |
Total (n=335) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination (n=83) | Control (n=28) | p value | Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=251) | Control (n=84) | p value | ||
| Injection site adverse reactions after whole vaccination procedure dose | |||||||||||||
| Any | 1 (1·20%) | 1 (3·57%) | 0·42 | 4 (4·76%) | 0 | 0·24 | 8 (9·52%) | 1 (3·57%) | 0·32 | 13 (5·18%) | 2 (2·38%) | 0·28 | |
| Grade 1 | 1 (1·20%) | 1 (3·57%) | .. | 4 (4·76%) | 0 | .. | 8 (9·52%) | 1 (3·57%) | .. | 13 (5·18%) | 2 (2·38%) | .. | |
| Redness | 1 (1·20%) | 1 (3·57%) | 0·42 | 1 (1·19%) | 0 | 0·56 | 0 | 1 (3·57%) | 0·082 | 2 (0·80%) | 2 (2·38%) | 0·25 | |
| Grade 1 | 1 (1·20%) | 1 (3·57%) | .. | 1 (1·19%) | 0 | .. | 0 | 1 (3·57%) | .. | 2 (0·80%) | 2 (2·38%) | .. | |
| Pain | 0 | 0 | 1 | 3 (3·57%) | 0 | 0·31 | 7 (8·33%) | 0 | 0·11 | 10 (3·98%) | 0 | 0·063 | |
| Grade 1 | 0 | 0 | .. | 3 (3·57%) | 0 | .. | 7 (8·33%) | 0 | .. | 10 (3·98%) | 0 | .. | |
| Swelling | 0 | 0 | 1 | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (0·40%) | 0 | .. | |
| Systemic adverse reactions whole vaccination procedure dose | |||||||||||||
| Any | 14 (16·87%) | 1 (3·57%) | 0·079 | 22 (26·19%) | 10 (35·71%) | 0·34 | 28 (33·33%) | 6 (21·43%) | 0·34 | 64 (25·50%) | 17 (20·24%) | 0·33 | |
| Grade 1 | 3 (3·61%) | 0 | .. | 6 (7·14%) | 2 (7·14%) | .. | 12 (14·29%) | 4 (14·29%) | .. | 21 (8·37%) | 6 (7·14%) | .. | |
| Grade 2 | 11 (13·25%) | 1 (3·57%) | .. | 16 (19·05%) | 8 (28·57%) | .. | 16 (19·05%) | 2 (7·14%) | .. | 43 (17·13%) | 11 (13·10%) | .. | |
| Fever | 5 (6·02%) | 1 (3·57%) | 0·62 | 12 (14·29%) | 3 (10·71%) | 0·76 | 15 (17·86%) | 2 (7·14%) | 0·23 | 32 (12·75%) | 6 (7·14%) | 0·16 | |
| Grade 1 | 0 | 0 | .. | 2 (2·38%) | 0 | .. | 6 (7·14%) | 1 (3·57%) | .. | 8 (3·19%) | 1 (1·19%) | .. | |
| Grade 2 | 5 (6·02%) | 1 (3·57%) | .. | 10 (11·90%) | 3 (10·71%) | .. | 9 (10·71%) | 1 (3·57%) | .. | 24 (9·56%) | 5 (5·95%) | .. | |
| Cough | 8 (9·64%) | 0 | 0·088 | 7 (8·33%) | 5 (17·86%) | 0·17 | 7 (8·33%) | 4 (14·29%) | 0·46 | 22 (8·76%) | 9 (10·71%) | 0·59 | |
| Grade 1 | 2 (2·41%) | 0 | .. | 1 (1·19%) | 1 (3·57%) | .. | 1 (1·19%) | 3 (10·71%) | .. | 4 (1·59%) | 4 (4·77%) | .. | |
| Grade 2 | 6 (7·23%) | 0 | .. | 6 (7·14%) | 4 (14·29%) | .. | 6 (7·14%) | 1 (3·57%) | .. | 18 (7·17%) | 5 (5·95%) | .. | |
| Headache | 1 (1·20%) | 0 | 0·56 | 0 | 0 | 1 | 3 (3·57%) | 0 | 0·31 | 4 (1·59%) | 0 | 0·24 | |
| Grade 1 | 1 (1·20%) | 0 | .. | 0 | 0 | .. | 3 (3·57%) | 0 | .. | 4 (1·59%) | 0 | .. | |
| Diarrhoea | 0 | 0 | 1 | 1 (1·19%) | 1 (3·57%) | 0·42 | 0 | 0 | 1 | 1 (0·40%) | 1 (1·19%) | 0·41 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 1 (3·57%) | .. | 0 | 0 | .. | 1 (0·40%) | 1 (1·19%) | .. | |
| Acute allergic reaction | 0 | 0 | 1 | 0 | 1 (3·57%) | 0·082 | 0 | 0 | 1 | 0 | 1 (1·19%) | 0·083 | |
| Grade 1 | 0 | 0 | .. | 0 | 1 (3·57%) | .. | 0 | 0 | .. | 0 | 1 (1·19%) | .. | |
| Vomiting | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 1 (1·19%) | 0 | 0·56 | 2 (0·80%) | 0 | 0·41 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (1·19%) | 0 | .. | 2 (0·80%) | 0 | .. | |
| Anorexia | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Abnormal skin and mucosa | 0 | 0 | 1 | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (0·40%) | 0 | .. | |
| Fatigue | 0 | 0 | 1 | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (0·40%) | 0 | .. | |
| Overall adverse reactions after whole vaccination procedure | |||||||||||||
| Any | 15 (18·07) | 2 (7·14%) | 0·16 | 27 (32·14%) | 10 (35·71%) | 0·73 | 36 (42·86%) | 8 (28·57%) | 0·26 | 78 (31·08%) | 20 (23·81%) | 0·21 | |
| Grade 1 | 4 (4·82%) | 1 (3·57%) | .. | 11 (13·10%) | 2 (7·14%) | .. | 20 (23·81%) | 5 (17·86%) | .. | 35 (13·94%) | 8 (9·52%) | .. | |
| Grade 2 | 11 (13·25%) | 1 (3·57%) | .. | 16 (19·05%) | 8 (28·57%) | .. | 16 (19·05%) | 3 (10·71%) | .. | 43 (17·13%) | 12 (14·29%) | .. | |
Data are n (%). Any refers to all participants with any grade adverse reactions or reactions. Adverse reactions and reactions were graded according to the scale issued by the China State Food and Drug Administration. Grade 1 is mild, grade 2 is moderate, and grade 3 is severe.
Table 4.
Adverse reactions within 30 days after whole vaccination procedure for group aged 6–12 years in phase 1/2
|
2 μg cohort (n=112) |
4 μg cohort (n=112) |
8 μg cohort (n=112) |
Total (n=336) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=252) | Control (n=84) | p value | ||
| Injection site adverse reactions after whole vaccination procedure dose | |||||||||||||
| Any | 8 (9·52%) | 1 (3·57%) | 0·32 | 19 (22·62%) | 0 | 0·0057 | 11 (13·10%) | 0 | 0·044 | 38 (15·08%) | 1 (1·19%) | 0·0006 | |
| Grade 1 | 6 (7·14%) | 1 (3·57%) | .. | 18 (21·43%) | 0 | .. | 10 (11·90%) | 0 | .. | 34 (13·49%) | 1 (1·19%) | .. | |
| Grade 2 | 2 (2·38%) | 0 | .. | 1 (1·19%) | 0 | .. | 1 (1·19%) | 0 | .. | 4 (1·59%) | 0 | .. | |
| Pain | 5 (5·95%) | 1 (3·57%) | 0·63 | 10 (11·90%) | 0 | 0·056 | 8 (9·52%) | 0 | 0·090 | 23 (9·13%) | 1 (1·19%) | 0·014 | |
| Grade 1 | 5 (5·95%) | 1 (3·57%) | .. | 10 (11·90%) | 0 | .. | 8 (9·52%) | 0 | .. | 23 (9·13%) | 1 (1·19%) | .. | |
| Redness | 1 (1·19%) | 0 | 0·56 | 6 (7·14%) | 0 | 0·15 | 3 (3·57%) | 0 | 0·31 | 10 (3·97%) | 0 | 0·056 | |
| Grade 1 | 0 | 0 | .. | 5 (5·95%) | 0 | .. | 2 (2·38%) | 0 | .. | 7 (2·78%) | 0 | .. | |
| Grade 2 | 1 (1·19%) | 0 | .. | 1 (1·19%) | 0 | .. | 1 (1·19%) | 0 | .. | 3 (1·19%) | 0 | .. | |
| Itching | 1 (1·19%) | 0 | 0·56 | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 2 (0·79%) | 0 | 0·41 | |
| Grade 1 | 1 (1·19%) | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 2 (0·79%) | 0 | .. | |
| Swelling | 1 (1·19%) | 0 | 0·56 | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 2 (0·79%) | 0 | 0·41 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Grade 2 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Induration | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Systemic adverse reactions whole vaccination procedure dose | |||||||||||||
| Any | 6 (7·14%) | 3 (10·71%) | 0·55 | 6 (7·14%) | 1 (3·57%) | 0·50 | 14 (16·67%) | 0 | 0·021 | 26 (10·32%) | 4 (4·76%) | 0·12 | |
| Grade 1 | 2 (2·38%) | 2 (7·14%) | .. | 4 (4·76%) | 0 | .. | 8 (9·52%) | 0 | .. | 14 (5·56%) | 2 (2·38%) | .. | |
| Grade 2 | 3 (3·57%) | 1 (3·57%) | .. | 2 (2·38%) | 1 (3·57%) | .. | 6 (7·14%) | 0 | .. | 11 (4·37%) | 2 (2·38%) | .. | |
| Grade 3 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Fever | 2 (2·38%) | 0 | 0·41 | 1 (1·19%) | 1 (3·57%) | 0·44 | 10 (11·90%) | 0 | 0·056 | 13 (5·16%) | 1 (1·19%) | 0·12 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 5 (5·95%) | 0 | .. | 6 (2·38%) | 0 | .. | |
| Grade 2 | 2 (2·38%) | 0 | .. | 0 | 1 (3·57%) | .. | 5 (5·95%) | 0 | .. | 7 (2·78%) | 1 (1·19%) | .. | |
| Diarrhoea | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Constipation | 0 | 0 | 1 | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Vomiting | 0 | 1 (3·57%) | 0·082 | 0 | 0 | 1 | 2 (2·38%) | 0 | 0·41 | 2 (0·79%) | 1 (1·19%) | >0·99 | |
| Grade 1 | 0 | 1 (3·57%) | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (0·40%) | 1 (1·19%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (0·40%) | 0 | .. | |
| Cough | 2 (2·38%) | 1 (3·57%) | 0·74 | 4 (4·76%) | 0 | 0·24 | 2 (2·38%) | 0 | 0·41 | 8 (3·17%) | 1 (1·19%) | 0·46 | |
| Grade 1 | 1 (1·19%) | 1 (3·57%) | .. | 2 (2·38%) | 0 | .. | 2 (2·38%) | 0 | .. | 5 (1·98%) | 1 (1·19%) | .. | |
| Grade 2 | 1 (1·19%) | 0 | .. | 2 (2·38%) | 0 | .. | 0 | 0 | .. | 3 (1·19%) | 0 | .. | |
| Headache | 0 | 1 (3·57%) | 0·082 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 (1·19%) | 0·083 | |
| Grade 2 | 0 | 1 (3·57%) | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (1·19%) | .. | |
| Acute allergic reaction | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 3 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Overall adverse reactions after whole vaccination procedure | |||||||||||||
| Any | 15 (17·86%) | 4 (14·28%) | 0·66 | 25 (29·76%) | 1 (3·57%) | 0·0045 | 25 (29·76%) | 0 | 0·0011 | 65 (25·79%) | 5 (5·95%) | 0·0001 | |
| Grade 1 | 9 (10·71%) | 3 (10·71%) | .. | 22 (26·19%) | 0 | .. | 18 (21·43%) | 0 | .. | 49 (19·44%) | 3 (3·57%) | .. | |
| Grade 2 | 5 (5·95%) | 1 (3·57%) | .. | 3 (3·57%) | 1 (3·57%) | .. | 7 (8·33%) | 0 | .. | 15 (5·95%) | 2 (2·38%) | .. | |
| Grade 3 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
Data are n (%). Any refers to all the participants with any grade adverse reactions or reactions. Adverse reactions and reactions were graded according to the scale issued by the China State Food and Drug Administration. Grade 1 is mild, grade 2 is moderate, and grade 3 is severe.
Table 5.
Adverse reactions within 30 days after whole vaccination procedure for group aged 13–17 years
|
2 μg cohort (n=112) |
4 μg cohort (n=112) |
8 μg cohort (n=112) |
Total (n=336) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=84) | Control (n=28) | p value | Vaccination (n=252) | Control (n=84) | p value | ||
| Injection site adverse reactions after whole vaccination procedure dose | |||||||||||||
| Any | 10 (11·90%) | 4 (14·29%) | 0·74 | 13 (15·48%) | 0 | 0·027 | 6 (7·14%) | 1 (3·57%) | 0·50 | 29 (24·52%) | 5 (5·96%) | 0·14 | |
| Grade 1 | 9 (10·71%) | 4 (14·29%) | .. | 13 (15·48%) | 0 | .. | 6 (7·14%) | 1 (3·57%) | .. | 28 (11·11%) | 5 (5·96%) | .. | |
| Grade 2 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Pain | 5 (5·95%) | 1 (3·57%) | 0·63 | 12 (14·29%) | 0 | 0·034 | 3 (3·57%) | 0 | 0·31 | 20 (7·94%) | 0 | 0·0078 | |
| Grade 1 | 5 (5·95%) | 1 (3·57%) | .. | 12 (14·29%) | 0 | .. | 3 (3·57%) | 0 | .. | 20 (7·94%) | 0 | .. | |
| Redness | 4 (4·76%) | 2 (7·14%) | 0·63 | 0 | 0 | 1 | 3 (3·57%) | 1 (3·57%) | 1 | 7 (2·78%) | 3 (3·57%) | 0·72 | |
| Grade 1 | 3 (3·57%) | 2 (7·14%) | .. | 0 | 0 | .. | 3 (3·57%) | 1 (3·57%) | .. | 6 (2·38%) | 3 (3·57%) | .. | |
| Grade 2 | 1 (1·19 %) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Itches | 1 (1·19%) | 0 | 0·56 | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 2 (0·79%) | 0 | 0·41 | |
| Grade 1 | 1 (1·19%) | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 2 (0·79%) | 0 | .. | |
| Swelling | 0 | 1 (3·57%) | 0·082 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 (1·19%) | 0·41 | |
| Grade 1 | 0 | 1 (3·57%) | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (1·19%) | .. | |
| Systemic adverse reactions whole vaccination procedure dose | |||||||||||||
| Any | 28 (33·33%) | 0 | 0·0004 | 17 (20·24%) | 1 (3·57%) | 0·038 | 12 (14·29%) | 9 (10·71%) | 0·036 | 57 (22·62%) | 10 (11·90%) | 0·033 | |
| Grade 1 | 24 (28·57%) | 0 | .. | 17 (20·24%) | 1 (3·57%) | .. | 9 (10·71%) | 8 (28·57%) | .. | 50 (19·84%) | 9 (10·71%) | .. | |
| Grade 2 | 4 (4·76%) | 0 | .. | 0 | 0 | .. | 3 (3·57%) | 1 (3·57%) | .. | 7 (2·78%) | 1 (1·19%) | .. | |
| Fever | 11 (13·10%) | 0 | 0·044 | 9 (10·71%) | 1 (3·57%) | 0·25 | 6 (7·14%) | 7 (0·25%) | 0·011 | 26 (10·32%) | 8 (9·52%) | 0·83 | |
| Grade 1 | 9 (10·71%) | 0 | .. | 9 (10·71%) | 1 (3·57%) | .. | 5 (5·95%) | 7 (0·25%) | .. | 23 (9·13%) | 8 (9·52%) | .. | |
| Grade 2 | 2 (2·38%) | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 3 (1·20%) | 0 | .. | |
| Fatigue | 4 (4·76%) | 0 | 0·24 | 0 | 0 | 1 | 0 | 0 | 1 | 4 (1·59%) | 0 | 0·24 | |
| Grade 1 | 3 (3·57%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 3 (1·20%) | 0 | .. | |
| Grade 2 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | .. | |
| Nausea | 2 (2·18%) | 0 | 0·41 | 0 | 0 | 1 | 2 (2·18%) | 0 | 0·41 | 4 (1·59%) | 0 | 0·24 | |
| Grade 1 | 2 (2·18%) | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 3 (1·20%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 1 (0·40%) | 0 | .. | |
| Cough | 1 (1·19%) | 0 | 0·56 | 3 (3·57%) | 0 | 0·31 | 2 (2·18%) | 1 (3·57%) | 0·79 | 6 (2·38%) | 1 (1·19%) | 0·51 | |
| Grade 1 | 0 | 0 | .. | 3 (3·57%) | 0 | .. | 1 (1·19%) | 1 (3·57%) | .. | 4 (1·59%) | 1 (1·19%) | .. | |
| Grade 2 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 1 (1·19%) | 0 | .. | 2 (0·79%) | 0 | .. | |
| Dyspnoea | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | 1 (0·40%) | 0 | .. | ||
| Muscle pain | 4 (4·76%) | 0 | 0·24 | 0 | 0 | 1 | 0 | 0 | 1 | 4 (1·59%) | 0 | 0·41 | |
| Grade 1 | 4 (4·76%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 4 (1·59%) | 0 | .. | |
| Vomiting | 2 (2·18%) | 0 | 0·41 | 0 | 0 | 1 | 2 (2·18%) | 0 | 0·41 | 4 (1·59%) | 0 | 0·41 | |
| Grade 1 | 2 (2·18%) | 0 | .. | 0 | 0 | .. | 2 (2·18%) | 0 | .. | 4 (1·59%) | 0 | .. | |
| Headache | 2 (2·18%) | 0 | 0·41 | 1 (1·19%) | 0 | 0·56 | 0 | 1 (3·57%) | 0·082 | 3 (1·20%) | 0 | 0·31 | |
| Grade 1 | 2 (2·18%) | 0 | .. | 1 (1·19%) | 0 | .. | 0 | 1 (3·57%) | .. | 3 (1·20%) | 1 (1·19%) | .. | |
| Dysphagia | 1 (1·19%) | 0 | 0·56 | 0 | 0 | 1 | 0 | 0 | 1 | 1 (0·40%) | 0 | 0·56 | |
| Grade 1 | 1 (1·19%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (0·40%) | 0 | ||
| Diarrhoea | 0 | 0 | 1 | 4 (4·76%) | 0 | 0·24 | 0 | 1 (3·57%) | 0·082 | 4 (1·59%) | 1 (1·19%) | 0·79 | |
| Grade 1 | 0 | 0 | .. | 4 (4·76%) | 0 | .. | 0 | 0 | .. | 4 (1·59%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (3·57%) | .. | 0 | 1 (1·19%) | .. | |
| Overall adverse reactions after whole vaccination procedure | |||||||||||||
| Any | 38 (45·24%) | 4 (14·29%) | 0·0034 | 30 (3·57%) | 1 (3·57%) | 0·0010 | 18 (21·43%) | 10 (35·71%) | 0·13 | 86 (34·13%) | 15 (17·86%) | 0·0049 | |
| Grade 1 | 33 (39·29%) | 4 (14·29%) | .. | 30 (3·57%) | 1 (3·57%) | .. | 15 (17·86%) | 9 (32·14%) | .. | 78 (30·95%) | 14 (16·67%) | .. | |
| Grade 2 | 5 (5·95%) | 0 | .. | 0 | 0 | .. | 3 (3·57%) | 1 (3·57%) | .. | 8 (3·17%) | 1 (1·19%) | .. | |
Data are n (%). Any refers to all the participants with any grade adverse reactions or reactions. Adverse reactions and reactions were graded according to the scale issued by the China State Food and Drug Administration. Grade 1 is mild, grade 2 is moderate, and grade 3 is severe.
The most common systemic reactions reported after each vaccination in all three age cohorts were mild to moderate fever and cough. After the vaccination schedule was completed, fever was reported by 32 (12·7%) of 252 participants in all three dose groups, and by six (7·1%) of 84 participants in the control group of the 3–5 years cohort; 13 (5·2%) of 251 participants in all three dose groups, and one (1·2%) of 84 participants in the control group of the 6–12 years cohort; and 26 (10·3%) of 252 participants in all three dose groups, and eight (9·5%) of 84 participants in the control group of the 13–17 years cohort (table 3–4; appendix 2 p 1). The frequency of fever was lower after the second and third doses compared with the first dose (appendix 2 pp 2–10). The second most common systematic reaction was cough, and no signs of upper respiratory tract infection were detected during follow-up. Cough was reported by 22 (8·7%) of 252 participants in the vaccination groups and nine (10·7%) of 84 participants in the control group of the 3–5 years cohort; eight (3·2%) of 252 participants in the vaccination groups and one (1·2%) of 84 participants in the control group of the 6–12 years cohort; and six (2·4%) of 252 participants in the vaccination group and one (1·2%) of 84 participants in the control group of the 13–17 years cohort. Neither of two common systematic adverse reactions were significantly different between vaccination and control groups (Table 2, Table 3, Table 4). An increase in overall systematic adverse reactions in the vaccine group compared with the control group was detectable independent of dose level in the 13–17 years cohort (table 5; appendix 2 p 1) There was one grade 3 systematic acute allergic reaction reported 4 days after the second vaccination in the 2 μg group of 6–12 years cohort.
Laboratory abnormalities were mild to moderate in severity after each vaccination dose. Elevated white blood cell count (reference range 4–10 × 109 cells per L) occurred in the 3–5 years cohort (17 [23·6% of 72 in the vaccination group and six [25·0%] of 24] in the control group) and the 6–12 years cohort (11 [15·3%] of 72 in the vaccination group and five [20·8%] of 24 in the control group). The 13–17 years cohort reported fewer elevated white blood cell counts (two [2·8%] of 72 in the vaccination group and two [8·3%] of 24 in the control group). An additional haemoglobin abnormality was observed in the 13–17 years cohort (seven [9·7%] of 72 in the vaccination group and one [4·2%] of 24 in the control group). Laboratory abnormalities of grade 3 were all white blood cell count value changes, and were reported in the 3–5 years 2 μg group (two [2·8%] of 72) and 6–12 years 4 μg group (one [1·4%] of 72). Grade 3 laboratory abnormalities were transient (ie, resolved within 10 days).
One vaccine-related unsolicited adverse event (grade 3 allergic purpura) was reported after the second dose of 2 μg in the cohort aged 6–12 years. Throughout the course of hospitalisation, this participant was diagnosed as allergic and intolerant to a variety of food allergens. This participant made a complete recovery after a 5-day hospitalisation, but withdrew from the third dose vaccination and is still under follow-up monitoring due to safety concerns.
Neutralising antibody titres against infectious SARS-CoV-2 were assessed 28 days after every vaccination (days 0, 28, 56, and 84). Neutralising antibodies for all participants were tested negative before vaccination as a requirement of the enrolment and remained negative throughout the studies in the control groups.
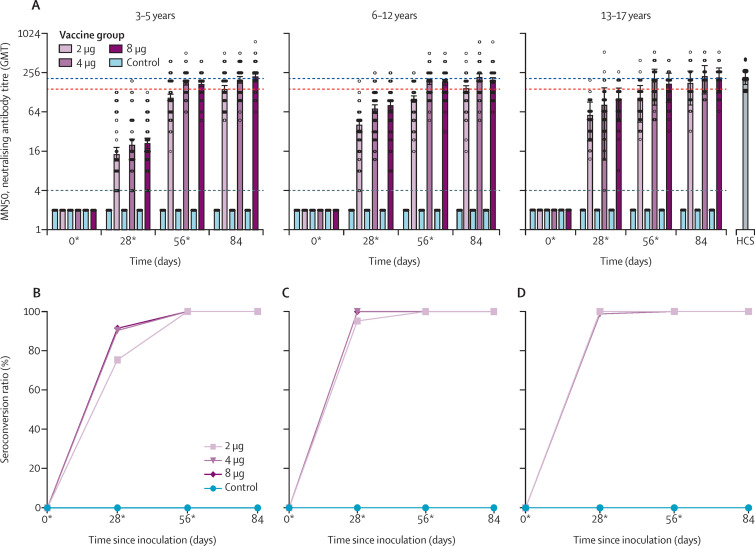
In the cohort aged 3–5 years, 61 (75%) of 81 participants in the 2 μg group, 75 (90%) of 83 participants in the 4 μg group, and 75 (91%) of 82 participants in the 8 μg group were seroconverted on day 28 (figure 2 ). In the cohort aged 6–12 years, 80 (95%) of 84 participants in the 2 μg group, all 84 (100%) participants in the 4 μg group, and all 83 (100%) participants in the 8 μg group were seroconverted on day 28 (figure 2). In the cohort aged 13–17 years, all 83 (100%) participants in the 2 μg group, 82 (99%) of 83 participants in the 4 μg group, and all 82 (100%) participants in the 8 μg group were seroconverted on day 28 (figure 2). Seroconversion rates reached 100% on day 56 in all three dose levels of all three age cohorts.
Figure 2.
Immunogenicity of BBIBP-CorV in participants aged 3–17 years
(A) Titres in the different study groups measured by infectious SARS-CoV-2 neutralising assay. Circles show the individual neutralisation titres. Bars represent the geometric mean titres of neutralisation antibody. Error bars refer to the 95% CI. The negative in neutralisation antibody detection is represented as GMT=2. The seroconversion rates of participants aged 3–5 years (B), 6–12 years (C), and 13–17 years (D) were defined as an increase of at least four-times post-vaccination titre from baseline. The black dotted line shows the lower limit of the assay (values beyond the lower limit were set to 2). The blue and red dashed lines indicate the average GMT in 18–59 years and 60 years or older cohorts on day 42 (14th day post the second vaccination). HCS=human convalescent serum. *Days of vaccination.
Dose-level dependent immunogenicity was observed on day 56 (28 days after the second vaccination) in all three age cohorts. The 4 μg and 8 μg groups showed significantly higher antibody response compared with the 2 μg group (appendix pp 16, 20, 24).
In the cohort aged 3–5 years, the neutralising antibody of all three dose levels were detectable on day 28, at a GMT of 14·5 (95% CI 11·2–18·7) in the 2 μg group, 20·2 (16·3–25·0) in the 4 μg group, and 21·3 (17·5–26·0) in the 8 μg group. By day 28, there were no significant differences in neutralising antibody GMT among the 2 μg (p=0·50), 4 μg (p=0·80), and 8 μg (p=0·85) groups. By day 56 (28 days after the second vaccination), the neutralising antibody GMT increased to 105·3 (95% CI 95·4–116·2) in the 2 μg group, 180·2 (163·4–198·8) in the 4 μg group, and 170·8 (202·2–249·1) in the 8 μg group. The 2 μg group neutralising antibody GMT was significantly lower than that of the 4 μg (p<0·001) and 8 μg (p<0·001) groups, while no significant difference in neutralising antibody GMTs between the 4 μg group and 8 μg group was observed (p=0·71). By day 84 (28 days after the third vaccination), the 2 μg group neutralising antibody GMT was significantly lower than that of the 4 μg (p=0·0018) and 8 μg (p=0·0013) groups, and no significant difference in neutralising antibody GMT was detected between the 4 μg and 8 μg groups (p=0·85; figure 2; appendix 2 pp 14–17).
Unlike the 3–5 years cohort, the neutralising antibody GMT of the 2 μg group in both the 6–12 years (30·0, 95% CI 25·0–35·9) and 13–17 years (48·0, 42·5–54·3) cohorts significantly differed to that of the 4 μg groups (6–12 years 54·1, 45·6–64·1 [p<0·001]; 13–17 years 61·4, 52·1–72·3 [p=0·0053]) and 8 μg groups (6–12 years 55·8, 45·3–68·7 [p<0·001]; 13–17 years 80·2, 69·3–92·9 [p<0·001]) within same age cohort on day 28. Neutralising antibody GMTs of the 4 μg and 8 μg groups in the cohorts aged 6–12 years (p=0·80) and 13–17 years (p=0·53) were comparable at day 56 (figure 2; appendix 2 pp 18–24).
All dose levels across all age cohorts showed comparable neutralising antibody GMTs compared with HCS (198·0, 95% CI 250·2–156·7) on day 84, except the 2 μg group in the 6–12 years cohort, which showed significantly lower neutralising antibody GMT (127·0, 95% CI 144·2–111·8; p=0·02) compared with HCS on day 84.
Discussion
In this phase 1/2 clinical trial of participants younger than 18 years, the three-dose regimen of 2 μg, 4 μg, and 8 μg of BBIBP-CorV inactivated vaccine had an acceptable safety profile, and was able to elicit robust humoral response against SARS-CoV-2.
Local and systemic adverse reactions were mostly mild to moderate in severity. Adverse reactions occurred predominantly after the first dose and showed similar frequency after the first vaccination in participants aged 3–17 years and participants aged 18–59 years or 60 years or older.5 The most common adverse reactions were pain and fever, which were transient or resolved in few days. There were three grade 3 white blood cell count abnormal changes in the vaccination group, but all were clinically insignificant.
One unsolicited adverse event was reported in the 2 μg group in the cohort aged 6–12 years during phase 2 study. This participant was later diagnosed as being allergic to various food allergens, but considering this adverse event occurred on the second day after the second vaccination dose, we recorded it as an adverse event possibly related to the vaccine. Therefore, in future studies, more attention should be paid to vaccinees with a history of allergy, and more safety data for large-scale clinical or post-marketing application are needed.
The BBIBP-CorV inactivated vaccine was immunogenic and induced robust humoral responses, with the seroconversion ratio of 100% in all vaccination groups. By day 56 (28 days after the second vaccination), the neutralising antibody elicited by BBIBP-CorV ranged from 105·3 to 180·2 in the cohort aged 3–5 years, 84·1 to 168·6 in the cohort aged 6–12 years, and 88·0 to 155·7 in the cohort aged 13–17 years, which were similar to the BBIBP-CorV-elicited antibody level in adult participants (figure 2).4 We found a lower seroconversion rate and lower neutralising antibody titre on day 28 in the cohort aged 3–5 years than that of other age cohorts. Lower antibody response after the first vaccination was also observed in the cohort aged 60 years and older in our previous study.4 We reason the lower neutralising antibody responses might be caused by undertrained or atrophy of the immune system of younger children (3–5 years) or older individuals (≥60 years).19
A report of a phase 1/2/3 study of BNT162b2 has shown its safety and 100% efficacy in individuals aged 12–25 years.9, 13 Another mRNA vaccine candidate, mRNA-1273, is reported to be tolerated and to have efficacy against COVID-19 of 100%, starting 14 days after the second dose, in children aged 12–17 years.14 Efforts are also being made to further evaluate safety, immunogenicity, and efficacy of mRNA vaccines (NCT04816643, NCT04649151, and NCT04796896) or an inactivated vaccine in s population younger than 12 years.15 These clinical trials, along with ours, will further address the feasibility of safe and efficacious vaccines in preventing disease in children and adolescents.
There are several limitations of our clinical trial, including: a short duration of follow-up (84 days), safety profile, and need for a longer follow-up period to evaluate antibody persistence; participants had limited racial and ethnic diversity as compared with the general population; cellular immunity elicited by BBIBP-CorV, especially T-cell response, was not evaluated (planned for follow-up studies); an absence of data for cross-protection efficacy of neutralising antibody elicited in the cohort aged 3–17 years against newly emerged variants (eg, B.1.1.720 and B.1.61721); and the neutralising antibody elicited by BBIBP-CorV could inhibit SARS-CoV-2 infection in cell culture, but the protection efficacy in people younger than 18 years is unknown. Although phase 3 studies of two inactivated SARS-CoV-2 vaccine candidates (BBIBP-CorV and CoronaVac) have demonstrated their efficacies in a general population of adults,22, 23, 24 an efficacy study of BBIBP-CorV in people younger than 18 years is necessary. An immune-bridging phase 3 study is planned for populations aged 3–17 years and 18 years and older using the 4 μg dose, with a two-shot regimen 21 days apart for additional safety data, immunogenicity, and efficacy; the trial will be done in the UAE. Additionally, although we included HCS as internal positive control in every assay, there was still no standardised HCS authorised by WHO with a universal distribution of donor characteristics (eg, disease severity and collection timing).
In conclusion, we found that the inactivated COVID-19 vaccine BBIBP-CorV is tolerable and immunogenic in individuals aged 3–17 years. Humoral responses against SARS-CoV-2 were elicited after the first inoculation of the vaccine and 100% seroconversion was achieved in all participants by day 56. These findings support the evaluation of this vaccine candidate in phase 3 trials with populations aged 3–17 years to further ascertain its safety and protection efficacy against SARS-CoV-2.
Data sharing
We support data sharing of the individual participant data. The individual participant data that underlie the results reported in this Article, after de-identification (ie, text, tables, figures, and appendix 2) will be shared. Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents, including the study protocol, statistical analysis plan, and the informed consent form, will be available immediately after publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to wangyanxia99@163.com or nvsiclinicaltrials@163.com. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.
Declaration of interests
XMY, YTZ, YKY, HW, WW, NL, XJZ, LD, YXZ, JZ, MM, YLQ, SHZ, JJC, QQL, HF, YX, and XTZ are employees of Beijing Institute of Biological Products, which developed the vaccine and funded the trial. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the National Program on Key Research Project of China (2020YFA0707500, 2016YFD0500301, 2017YFC0840300, 2020YFC0842100), National Mega projects of China for Major Infectious Diseases (2016ZX10004001–003), National Mega Projects of China for New Drug Creation (2018ZX09734–004), and Beijing Science and Technology Plan (Z201100005420014). The China National Biotec Group and the Beijing Institute of Biological Products provided the study product, and oversaw all trial operations. The funders used contract clinical research organisations to coordinate interactions with regulatory authorities and oversee clinical site operations. Data were collected by the clinical site research staff, managed by a blinded contract research organisation data management team, monitored by a contract research organisation, and overseen by the funder and an independent data and safety monitoring board. The analysis was done by an independent statistician who was not involved in the trial after the data were collected, checked, and locked for the specific groups before unblinding. Manuscript preparation was done by the study authors and the decision to submit the manuscript for publication was made by the study authors.
Contributors
The study was designed by SLX, YTZ, YKY, and YXW. XMY and WSG provided regulatory oversight. WW provided project management.
WZ, ZQX, WYY, and LLH collected study data and oversaw participant visits. Safety data analysis and interpretation were done by JZ, XZS, GXX, YX, and HF. Immunogenicity testing was done by BYH, PPL, MM, YLQ, SHZ, JJC, QQL, WLW, and XTZ. Immunogenicity data collected and analysed by YLY, NL, LD, YXZ, and XJZ, and was interpreted by GFG, HW, WBX, GZW, WJT, MX, and WJH. GXX and ZYL wrote the manuscript. Data were accessed and verified by YXW, YLY and WW. All authors contributed to the reviewing and editing of the report and approved the final version.
Supplementary Materials
References
- 1.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moghadas SM, Fitzpatrick MC, Shoukat A, Zhang K, Galvani AP. Identifying silent COVID-19 infections among children is critical for controlling the pandemic. medRxiv. 2021 doi: 10.1101/2021.01.06.21249349. published online Jan 8. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenck RW, Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moderna Moderna announces TeenCOVE Study of its COVID-19 vaccine in adolescents meets primary endpoint and plans to submit data to regulators in early. June. 2021. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-teencove-study-its-covid-19-vaccine
- 15.Han B, Song Y, Li C, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Zhang Y, Huang B, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182:713–721. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Medical Products Administration 2019. http://www.nmpa.gov.cn/WS04/CL2138/373037.html
- 18.Chinese National Medical Products Administration Guideline for grading standards of adverse events in clinical trials of preventive vaccines. 2019. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460.html
- 19.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 20.Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherian S, Potdar V, Jadhav S, et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. bioRxiv. 2021 doi: 10.3390/microorganisms9071542. https://www.biorxiv.org/content/10.1101/2021.04.22.440932v1 published online April 24. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021 doi: 10.1056/NEJMoa2107715. published online July 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We support data sharing of the individual participant data. The individual participant data that underlie the results reported in this Article, after de-identification (ie, text, tables, figures, and appendix 2) will be shared. Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents, including the study protocol, statistical analysis plan, and the informed consent form, will be available immediately after publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to wangyanxia99@163.com or nvsiclinicaltrials@163.com. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.