Abstract
Purpose
Genomic prognostic signatures are used on prostate biopsy tissue for cancer risk assessment, but tumor heterogeneity and multi-focality may be an issue. We evaluated the variability in genomic risk assessment from different biopsy cores within the prostate using three prognostic signatures (Decipher, CCP, GPS).
Materials and Methods:
Men in this study came from two prospective prostate cancer trials of patients undergoing mpMRI and MRI targeted biopsy with genomic profiling of positive biopsy cores. We explored the relationship between tumor grade, MRI risk, and genomic risk for each signature. We evaluated the variability in genomic risk assessment between different biopsy cores, and assessed how often MRI targeted biopsy or the current standard of care (profiling the core with the highest grade) resulted in the highest genomic risk level.
Results:
224 positive biopsy cores from 78 men with prostate cancer were profiled. For each signature, higher biopsy grade (p<0.001) and MRI risk level (p<0.001) were associated with higher genomic scores. Genomic scores from different biopsy cores varied with risk categories changing by 21-62% depending on which core or signature was used. MRI targeted biopsy and profiling the core with the highest grade resulted in the highest genomic risk level in 72-84% and 75-87% of cases, respectively, depending on the signature used.
Conclusions:
There is variation in genomic risk assessment from different biopsy cores regardless of the signature used. MRI directed biopsy or profiling the highest grade core resulted in the highest genomic risk level in most cases.
Keywords: Prostate Cancer, Genomics, Biomarkers, MRI
Introduction
Treatment decision-making in localized prostate cancer is primarily based on the grade and volume of cancer found on prostate biopsy.1 However, prostate cancer is heterogeneous and multi-focal, and biopsy often under-samples the tumor, leading to an underestimation of risk and misguided management.2 Multiparametric MRI (mpMRI) of the prostate results in more accurate targeting of aggressive tumors, and less underestimation of cancer risk,3 but has a significant false negative rate.4 Tissue based genomic prognostic markers are helpful tools to facilitate treatment decision making,5-8 but evidence has emerged to suggest that genomic findings from different tumors within the prostate yield differing results.9,10 This has important implications when making decisions based on findings from a single biopsy core, which is the current standard of care for these tests.
To better evaluate the degree of variability in genomic risk assessment between different biopsy cores within the prostate we investigated men from two ongoing prospective trials at the University of Miami, where mpMRI targeted and template biopsies of the prostate are sent to Decipher Biosciences for gene expression analysis.
Materials and Methods
Patient Population
Patients for this study came from two ongoing trials at the University of Miami Sylvester Comprehensive Cancer Center. The first trial includes men with low to favorable intermediate risk prostate cancer on active surveillance in a prospective protocol entitled the “Miami MRI selection for Active Surveillance versus Treatment (MAST)” trial (NCT02242773). Men undergo mpMRI and confirmatory biopsy of the prostate within 12 months of diagnosis, and annually thereafter for three years. Men are eligible if they have four or less cores of cancer, allowing up to 2 of those cores to be Grade Group 2. There is no exclusion based on the volume of cancer in each core, however any Grade Group 3 or higher cancer is excluded. The second trial is a phase II randomized trial called the “MRI-Guided Prostate Boosts Via Initial Lattice Stereotactic versus Daily Moderately Hypofractionated Radiotherapy (BLaStM)” trial (NCT02307058). The BLaStM trial includes men with intermediate to high-risk prostate cancer who undergo an mpMRI and mpMRI targeted biopsy at the time of fiducial marker placement to select areas for dose escalation during radiotherapy. Patients from both trials provided written informed consent to have their tissue sent for genomic testing and their data included in this research. The cohort for this study includes consecutive men who underwent biopsies either on the MAST or BLaStM trial during the initial phase of these trials (2016-2017) when positive cores with more than 1mm of cancer were prospectively sent to Decipher Biosciences (San Diego, CA) for gene expression analysis. If available, positive cores from the patient’s diagnostic biopsy were also sent for genomic analysis.
mpMRI and Biopsy of the Prostate
mpMRI and biopsy of the prostate followed a standardized protocol. mpMRI exams were performed on 3T magnets [3T Discovery (GE, Waukesha, WI), MR Magnetom Trio or Skyra (Siemens, Erlagen, Germany)], in accordance with the Prostate Imaging-Reporting and Data System (PIRADS) recommendations. mpMRI was acquired within three months of prostate biopsy and interpreted by one of three fellowship trained radiologists using the most current version of PIRADS at that time. The prostate and any PIRADS 3 or higher regions of suspicion were outlined in Dynacad (InVivo, Gainsville, FL). mpMRI targeted biopsy of the prostate was conducted using a trans-rectal mpMRI-ultrasound fusion platform UroNav (InVivo, Gainsville, FL) with 2 cores taken per target. For MAST patients an additional 12 core extended template biopsy of the prostate was performed. For the BLaStM trial only the suspicious lesions were targeted during fiducial marker placement. All biopsy cores were interpreted by a single pathologist (ONK) with fellowship training in genitourinary pathology.
Specimen Processing and Genomic Analysis
Tumor processing and RNA extraction was performed at Decipher Biosciences with quality control and normalization methods following a standardized clinical-grade microarray assay.8 The assay measures transcriptome-wide expression and calculates the Decipher score, a 22-gene locked signature that ranges from 0.0 to 1.0 with higher scores related to an increased risk of prostate cancer metastasis. The score is categorized into low, intermediate and high-risk groups using cut-points of 0.45 and 0.6, respectively. Microarray derived scores were also generated for the Cell Cycle Progression (CCP)11 and Genomic Prostate Score (GPS)7 signatures and extracted from the Decipher Grid database. While the exact derivation of the commercial CCP and GPS gene expression signatures are proprietary and performed using quantitative RT-PCR, the component RNA transcripts have been published and independent derivations of these signatures have been previously reported.12 For each signature the genes are mapped to an array feature to measure their expression and the features in each signature were then used to train a random forest model in an independent data set with metastasis as the endpoint.13 CCP and GPS signatures are available in the Decipher grid as continuous scores and categorized into low, intermediate, and high risk levels based on the distribution of values observed in a reference population of nearly 2,500 patients.
Statistical Analysis
Biopsy cores with successful gene expression profiling were categorized by mpMRI targeted or template sampling. Each core had a Grade Group assignment (1-5) and a genomic risk assessment based on the Decipher score, as well as the CCP and GPS signatures, which were reported as continuous scores from 0.0 to 1.0 and grouped into categories of low, intermediate, and high risk. Patients in the MAST and BLaStM trials were compared using the Mann-Whitney test for continuous variables and Fisher’s exact test for categorical variables. We evaluated trends in genomic risk by increasing grade group and mpMRI risk level (template/PIRADS) for each signature, using Spearman’s correlation coefficient for continuous genomic risk scores and proportional trend test for genomic categories of risk. We investigated the relationship between mpMRI risk and genomic risk after accounting for cancer grade using ordinal logistic regression with random effects to account for multiple biopsies from a single patient.
We measured variability in genomic risk classification between biopsy cores for each signature by evaluating how often differing levels of genomic risk were represented in each biopsy. We assessed how often the current standard of care of profiling the core with the largest volume of highest grade tumor identified the highest genomic risk level in the biopsy for each signature. We also compared mpMRI targeted and template biopsy cores in their ability to identify the highest genomic risk level for each signature. McNemar test was used to compare signatures in these metrics. Lastly, risk level categorization for each signature was compared in each core to evaluate how often they disagreed in risk assignment and patient-level Spearman’s correlation coefficients were calculated between Decipher, CCP, and GPS scores. All statistical analyses were performed using SAS version 9.4 and R version 3.6.3. All tests were two-sided and statistical significance was considered when p<0.05.
Results
The 78 (MAST=46, BlaStM=32) men enrolled in MAST or BlaStM during the aforementioned time period make up the current study cohort, of which 49% had Grade Group 2 or higher cancer at diagnosis and 67% had a PIRADS 3 or higher target on mpMRI (Table 1). From these 78 men, 376 biopsy cores (212 for MAST and 164 from BlaStM) were submitted to Decipher Biosciences for genomic profiling, of which 224 (MAST=124, BlaStM=100) were successfully profiled (Supplemental Table 1). Among these 224 biopsy cores, 45% had Grade Group 2 or higher cancer and 41% were from mpMRI targets (Table 2).
Table 1.
Patient characteristics for both study cohorts organized by clinical trial.
| TOTAL | MAST | BLASTM | ||
|---|---|---|---|---|
| Patients | N = 78 | N = 46 | N = 32 | p-value a |
| Variable | ||||
| Median (IQR)b | ||||
| Age, years | 66 (59-70) | 64 (57-68.8) | 67.5 (59.8-70.3) | 0.237 |
| PSA, ng/mL | 6.1 (4.5-10.5) | 4.9 (4.3-6.7) | 9.7 (6.2-17.4) | 0.0004 |
| N (%) | ||||
| Race/ethnicity | 0.219 | |||
| Non-Hispanic White | 37 (47.4) | 25 (54.4) | 12 (37.5) | |
| Non-Hispanic Black | 11 (14.1) | 6 (13.0) | 5 (15.6) | |
| Hispanic/Latino | 28 (35.9) | 15 (32.6) | 13 (40.6) | |
| Others | 2 (2.6) | 0 (0.0) | 2 (6.3) | |
| Grade Group | <.0001 | |||
| 1 | 40 (51.3) | 36 (78.3) | 4 (12.5) | |
| 2 | 15 (19.2) | 7 (15.2) | 8 (25.0) | |
| 3 | 10 (12.8) | 3 (6.5) | 7 (21.9) | |
| 4-5 | 13 (16.7) | 0 (0.0) | 13 (40.6) | |
| T stage | <.0001 | |||
| T1 | 58 (74.4) | 46 (100) | 12 (37.5) | |
| T2 | 13 (16.7) | 0 (0.0) | 13 (40.6) | |
| T3 | 7 (8.9) | 0 (0.0) | 7 (21.9) | |
| DRE | <.0001 | |||
| Negative | 55 (70.5) | 46 (100) | 9 (28.1) | |
| Nodule | 18 (23.1) | 0 (0.0) | 18 (56.3) | |
| Extra-capsular disease | 5 (6.4) | 0 (0.0) | 5 (15.6) | |
| PIRADS score on MRI | 0.0002 | |||
| Negative | 28 (35.9) | 22 (47.8) | 6 (18.8) | |
| 3 | 8 (10.3) | 7 (15.2) | 1 (3.1) | |
| 4 | 23 (29.5) | 12 (26.1) | 11 (34.3) | |
| 5 | 19 (24.3) | 5 (10.9) | 14 (43.8) | |
p-values from Mann-Whitney test for continuous variables or Fisher’s exact test for categorical variables
Interquartile range
Table 2.
Biopsy characteristics for both study cohorts organized by clinical trial.
| TOTAL | MAST | BLASTM | ||
|---|---|---|---|---|
| Biopsy cores | N = 224 | N = 124 | N = 100 | |
| Variable | N (%) | N (%) | N (%) | p-value a |
| Grade Group | <.0001 | |||
| 1 | 90 (40.2) | 79 (63.7) | 11 (11.0) | |
| 2 | 62 (27.7) | 35 (28.2) | 27 (27.0) | |
| 3 | 22 (9.8) | 6 (4.8) | 16 (16.0) | |
| 4-5 | 50 (22.3) | 4 (3.3) | 46 (46.0) | |
| Biopsy Type | <.0001 | |||
| Diagnostic | 75 (33.5) | 23 (18.5) | 52 (52.0) | |
| Trial | 149 (66.5) | 101 (81.5) | 48 (48.0) | |
| Targeted Approach | <.0001 | |||
| Template | 132 (58.9) | 79 (63.7) | 53 (53.0) | |
| Targeted | 92 (41.1) | 45 (36.3) | 47 (47.0) | |
| PIRADS among Targeted Biopsy | 0.0004 | |||
| 3 | 17 (18.5) | 14 (31.1) | 3 (6.4) | |
| 4 | 39 (42.4) | 22 (48.9) | 17 (36.2) | |
| 5 | 36 (39.1) | 9 (20.0) | 27 (57.4) |
p-values from Mann-Whitney test for continuous variables or Fisher’s exact test for categorical variables.
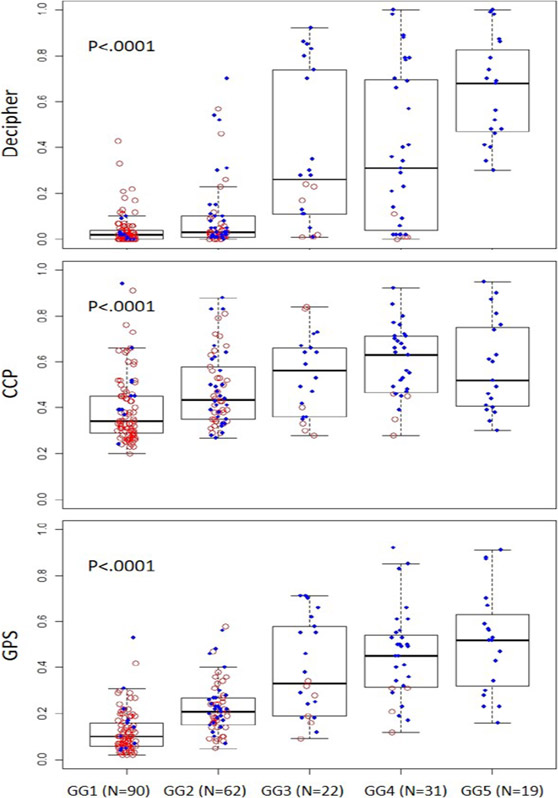
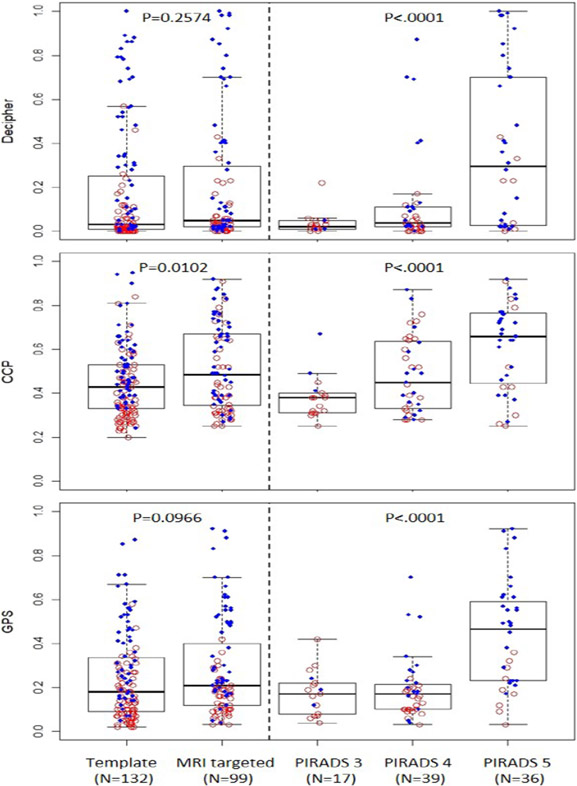
We witnessed a trend towards higher genomic risk scores with higher Grade Groups for each signature (p<.0001) (Figure 1). When comparing MRI targeted and template cores we found higher CCP scores in the MRI targeted cores compared to templates (p=0.01), however no difference between Decipher and GPS scores (Figure 2). However, within MRI targeted cores we found that higher PIRADS levels were associated with higher genomic scores for all three signatures (p<0.001). Similar trends were seen using categorical levels of genomic risk compared to raw genomic scores (Supplemental Figure 1 and 2). We used a linear mixed model to control for the impact of tumor grade on the association between PIRADS and genomic risk level and found that higher PIRADS scores were associated with higher Decipher risk regardless of tumor grade (p=0.015), but for CCP (p=0.16) and GPS (p=0.065) the association lost significance.
Figure 1:
Box plot of genomic risk scores by grade group for each tissue-based genomic prognostic signature (Decipher, CCP, GPS) showing higher score in higher grade groups.
Red circles depict biopsies from MAST trial; Blue dots – from BlastM.
Figure 2:
Boxplot of genomic risk scores by template and MRI guided biopsies for each tissue-based genomic prognostic signatures (Decipher, CCP, GPS) showing higher CCP scores in MRI targeted compared to template biopsies, but no difference in GPS and Decipher scores. Within MRI targeted biospies all signatures showed higher scores with higher PIRADS levels.
Red circles depict biopsies from MAST trial; Blue dots – from BlastM.
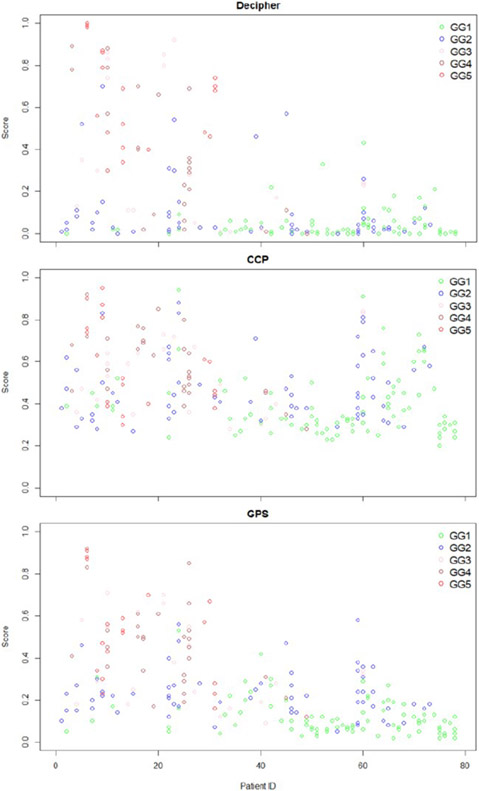
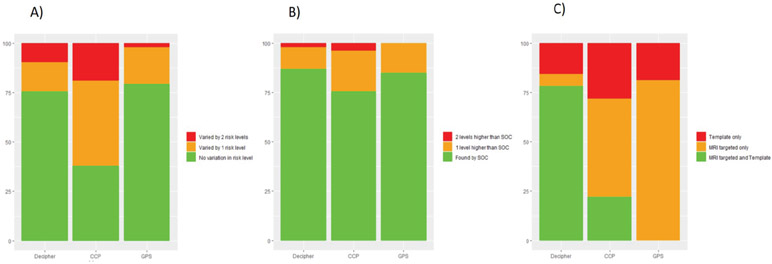
Figure 3 plots genomic risk scores from each biopsy core by patient and grade group for each signature. Among the 57 men with more than one core positive we found significant variability in genomic risk assessment between different biopsy cores, with the level of risk changing by 21-62% depending on which signature was used and which core was profiled (Figure 4a). In most cases risk assessment differed by only one risk level, however 2-19% of cases differed by 2 levels of risk, representing a more meaningful variance. Decipher and GPS appeared similar in their variance, but both were different from CCP (p<0.001). We found that profiling the core with the largest volume of highest grade cancer, resulted in the highest genomic risk level in 75-87% of cases, depending on the signature used (Figure 4b). In those cases where the highest genomic risk level was found in a different core, the risk assessment was only one risk level higher in 11-21% of cases, and two risk levels higher in less than 4% of cases. We did not see a significant difference between the signatures in this distribution. Among the 32 men who had both mpMRI targeted and template cores with a genomic result, we found the highest genomic risk level in the MRI targeted cores in 72- 84% of cases, but there was a significant difference between the signatures in this distribution (p<0.01) (Figure 4c).
Figure 3:
Plot of genomic risk scores per biopsy core and patient for each genomic prognostic signature. Grade group of biopsy core is reflected by the different color circles.
Figure 4:
a) shows how often the genomic risk level assignment for each signature was consistent among the biopsy cores and how often they differed by one or two risk levels. Variance in Decipher and GPS scores appeared similar (p=0.77), while they both differed from variance in CCP scores (p<0.01). b) shows how often the core with largest volume of highest grade cancer found the highest genomic risk level among the biopsy cores and how often one or two risk levels higher are found in a different core for each signature. There was no difference between signatures in this distribution (p>0.1). c) shows the how often MRI guided and template biopsy identified the highest genomic risk level among the biopsy cores for each signature. There was a significant difference between all the signatures in this distribution (p<0.01).
When comparing the three signatures across all 224 biopsy cores we found agreement in the level of risk in 46% of cases, while 35% of the cases disagreed by only one level of risk and 19% of cases disagreed by two levels of risk. We found that Decipher and GPS risk levels did not appear to differ significantly (Decipher vs GPS, p=0.1), however they had both differed from CCP (Decipher vs CCP and GPS vs CCP, p<0.001).
Discussion
Typically, prostate biopsy interpretation is variable, and limited by sampling only a small, often random, fraction of the prostate. Evidence suggests that tissue based genomic markers, like the Decipher test, may be more prognostic than grade for lethal endpoints such as metastasis.14 However, it is unclear how much these tests are impacted by prostate cancer heterogeneity and multi-focality; the Achilles heel of prostate cancer risk assessment. This is an important question since these tests are often used for clinical decision-making regarding the need for treatment in active surveillance, or intensity of treatment (need or duration of ADT) during radiotherapy. However, the standard of care in the biopsy setting is to profile only a single core with different companies having different criteria for which core they select (highest grade core versus highest volume core).
A previous retrospective study conducted genome expression analysis on 26 gross biopsy cores from four radical prostatectomy specimens and reported sizeable differences in derived Decipher, CCP, and GPS scores between biopsy cores.9 Another study retrospectively evaluated 120 prostate cancer samples (RP and biopsy) from 44 patients and used a multiplexed targeted RNA-sequencing assay to derive Decipher, CCP and GPS scores and reported that low and high grade prostate cancer foci exhibit distinct expression profiles.10 Similar to ours, these studies suggest that different tumors within the prostate have distinct expression profiles and variability in genomic risk assessment occurs. However, our study went one step further by estimating the frequency and magnitude of this variability. We discovered that the level of genomic risk can change by as much as 60% depending on the core and signature used, but most of these changes were within one level of risk and more meaningful differences occurred much less frequently.
We found that MRI directed biopsies identified the highest genomic risk level in 71-85% of cases, however the distribution of genomic scores between MRI targeted and template biopsy differed significantly between the signatures. Given the numerous reports showing a benefit of MRI targeting for improving the detection of aggressive pathology, we hypothesized that MRI targeted biopsies would enhance the detection of tumors with more aggressive genomic features. 3,15,16 While higher PIRADS scores were associated with higher genomic scores, this association appeared to be mediated by the grade of the tumor, and after controlling for it the relationship between PIRADS and genomic scores disappeared for the CCP and GPS signature, but remained for Decipher. This finding requires further validation.
This study has several strengths. Foremost, while previous studies have utilized retrospective radical prostatectomy specimens and generalized findings to the biopsy setting, these protocols evaluate mpMRI targeted biopsy and tissue based genomic prognostic markers prospectively, making it directly applicable to men diagnosed with prostate cancer who are trying to decide on the need for treatment or treatment intensification based on biopsy findings. Secondly, while previous studies only looked at the relative expression of target genes within each signature, our study evaluated the actual Decipher test, as it would be performed in clinical practice. However, for the GPS and CCP signatures we assessed the relative expression of genes in each signature, similar to previous studies. Therefore, any results related to CCP and GPS may not be accurate as we used a different platform (microarray versus qtPCR) and did not incorporate proprietary algorithms. Despite this concern, we believe these findings can be conceptually applied to any signature informing genomic risk assessment on heterogeneous biopsy specimens. Another limitation is that we only evaluated men in the initial phase of these trials when cancer positive biopsy cores were being sent for genomic profiling, creating a potential for selection bias. However, to our knowledge this is the largest series looking at variability in genomic risk assessment and the impact of tumor heterogeneity and multi-focality, making it a significant contribution to the literature. Furthermore, both of these trials are still ongoing and will allow further validation of these preliminary findings and relate it’s impact to relevant outcomes like cancer progression.
Conclusion
We evaluated men undergoing mpMRI targeted and template biopsy of the prostate with cancer positive cores undergoing gene expression profiling for three commercially available prognostic signatures. We found considerable variability in genomic risk assessment from different biopsy cores despite the signature used. MRI targeted biopsy and selecting the core with the largest volume of highest grade tumor, which is the current standard of care, identified the highest genomic risk within the biopsy in the majority of cases.
Supplementary Material
Trends in genomic risk categories for each signature by grade group with higher risk categories in higher grade groups (trend test for proportion for each signature had p < 0.001). Low-green, Intermediate – orange, High – red.
Trends in genomic risk categories for each signature by biopsy location based on template biopsy or MRI targeted biopsy with increasing PIRADS scores. We found no difference in genomic risk classification between MRI targeted and template biopsy for any signature, but all signatures had higher risk categories in higher PIRADS levels (trend test for proportion for each signature had p < 0.001). Low-green, Intermediate – orange, High – red.
ACKNOWLEDGEMENTS:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA240139, RO1CA189295, R01CA190105 and U01CA239141 and Paps Corps Champions for Cancer Research Endowed Chair in Solid Tumor Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health”.
KEY ABBREVIATIONS:
- CCP
Cell Cylcle Progression
- GPS
Genomic Prostate Score
- mpMRI
Multi-parametric MRI
- PIRADS
Prostate Imaging-Reporting and Data System
- MAST
Miami MRI selection for Active Surveillance versus Treatment trial
- BLaStM
MRI-Guided Prostate Boosts Via Initial Lattive Stereotactic versus Daily Moderately Hypofractionated Radiotherapy
REFERENCES
- 1.Dall’Era MA, Dall’Era MA, Albertsen PC, et al. : Active Surveillance for Prostate Cancer: A Systematic Review of the Literature. Eur Urol. 2012: 62: 976–83 [DOI] [PubMed] [Google Scholar]
- 2.Patel HD, Tosoian JJ, Carter HB, et al. : Adverse Pathologic Findings for Men Electing Immediate Radical Prostatectomy. JAMA Oncol 2018; 4: 89–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahdoot M, Wilbur AR, Reese SE, et al. : MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 2020; 382: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moldovan PC, Van den Broeck T, Sylvester R, et al. : What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017: 72: 250–266. [DOI] [PubMed] [Google Scholar]
- 5.Moschini M, Spahn M, Mattei A, et al. : Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med 2016: 14: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Simko JP, Simko JP, et al. : Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J. Clin. Oncol 2013; 31: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 7.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. : A 17-gene Assay to Predict Prostate Cancer Aggressiveness in the Context of Gleason Grade Heterogeneity, Tumor Multifocality, and Biopsy Undersampling. Eur Urol. 2014; 66: 550–560. [DOI] [PubMed] [Google Scholar]
- 8.Klein EA, Haddad Z, Yousefi K, et al. : Decipher Genomic Classifier Measured on Prostate Biopsy Predicts Metastasis Risk. Urology 2016; 90: 148–152. [DOI] [PubMed] [Google Scholar]
- 9.Wei L, Wang J, Lampert E, et al. : Intratumoral and Intertumoral Genomic Heterogeneity of Multifocal Localized Prostate Cancer Impacts Molecular Classifications and Genomic Prognosticators. Eur Urol. 2017: 71: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salami SS, Hovelson DH, Kaplan JB, et al. : Transcriptomic heterogeneity in multifocal prostate cancer. JCI Insight 2018; 3: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuzick J, Cuzick J, Berney DM, et al. : Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br. J. Cancer 2012; 106: 1095–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross AE, Johnson MH, Yousefi K, et al. : Tissue-based Genomics Augments Post-prostatectomy Risk Stratification in a Natural History Cohort of Intermediate- and High-Risk Men. Eur Urol. 2016; 69: 157–165. [DOI] [PubMed] [Google Scholar]
- 13.Erho N, Crisan A, Vergara IA, et al. : Discovery and Validation of a Prostate Cancer Genomic Classifier that Predicts Early Metastasis Following Radical Prostatectomy. PLoS ONE 2013; 8: e66855–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan VM: A critical appraisal of biomarkers in prostate cancer. World J Urol 2020: 38: 547–554. [DOI] [PubMed] [Google Scholar]
- 15.Moore CM, Robertson NL, Robertson NL, et al. : Image-Guided Prostate Biopsy Using Magnetic Resonance Imaging–Derived Targets: A Systematic Review. Eur Urol. 2013; 63: 125–140. [DOI] [PubMed] [Google Scholar]
- 16.Radtke JP, Takhar M, Bonekamp D, et al. : Transcriptome Wide Analysis of Magnetic Resonance Imaging-targeted Biopsy and Matching Surgical Specimens from High-risk Prostate Cancer Patients Treated with Radical Prostatectomy: The Target Must Be Hit. Eur Urol Focus 2018: 4: 540–546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trends in genomic risk categories for each signature by grade group with higher risk categories in higher grade groups (trend test for proportion for each signature had p < 0.001). Low-green, Intermediate – orange, High – red.
Trends in genomic risk categories for each signature by biopsy location based on template biopsy or MRI targeted biopsy with increasing PIRADS scores. We found no difference in genomic risk classification between MRI targeted and template biopsy for any signature, but all signatures had higher risk categories in higher PIRADS levels (trend test for proportion for each signature had p < 0.001). Low-green, Intermediate – orange, High – red.