Abstract
Background:
Cannabis use is increasing among young adults, but its effects on cardiovascular health are poorly understood. We aimed to assess the association between recent cannabis use and history of myocardial infarction (MI) in young adults (aged 18–44 yr).
Methods:
We performed a cross-sectional study using pooled data from the 2017 and 2018 cohorts of the American Behavioral Risk Factor Surveillance System survey of US adults. We analyzed the association between any recent cannabis use and history of MI using a weighted logistic regression model that adjusted for demographic factors, socioeconomic factors, health-related behaviours, concomitant substance use and other comorbidities. We also assessed this association after stratifying by frequency of use and by primary method of consumption.
Results:
Among 33 173 young adults (18.5 million weighted), 4610 respondents (3.2 million weighted) reported recent cannabis use (17.5%, 95% confidence interval [CI] 16.8%–18.2%). A history of MI was more frequent among recent cannabis users (n = 61 of 4610, 1.3%) relative to nonusers (n = 240 of 28 563 [0.8%], adjusted odds ratio [OR] 2.07, 95% CI 1.12–3.82). A history of MI was associated with cannabis use of more than 4 times per month (adjusted OR 2.31, 95% CI 1.18–4.50), and with smoking as a primary method of consumption (adjusted OR 2.01, 95% CI 1.02–3.98).
Interpretation:
Our study provides evidence supporting an association between recent cannabis use and history of MI in young adults. Increasing cannabis use in an at-risk population could have negative implications for cardiovascular health.
Cannabis is one of the most commonly used recreational drugs.1 Recent legalization of cannabis in Canada,2 and decriminalization in multiple jurisdictions in the United States,3 has contributed to its increased availability and social acceptance. Cannabis use is also increasing, particularly among young adults (aged 18 to 44 yr).4,5 Furthermore, when comparing prevalence rates before and after legalization in Canada, use among young adults increased by a larger amount relative to other age groups.6 Despite the widespread use of cannabis, its effects on health remain poorly understood.
The American Heart Association recently issued a recommendation not to smoke or vapourize any product containing cannabis because of its potential harm on cardiovascular health, and called for more research on the epidemiology and trends in cannabis use among youth and high-risk populations.7 The association between recent cannabis use and stroke has been assessed;8 however, its effect on other cardiovascular outcomes remains incompletely characterized. Although heavy cannabis use has been reported to trigger acute myocardial infarction (MI), the current evidence is limited to case–control studies that are prone to bias and studies relying solely on administrative data.9–14 It is also limited in its definition of exposure, as these studies assess patients with heavy cannabis use (cannabis abuse or cannabis use disorder).9–14 Very few studies have assessed the prevalence of recent cannabis use (any use within past 30 days) and its association with MI.7,9,15 Prevalence estimates of the primary method of cannabis consumption and the frequency of cannabis use are incompletely characterized, and the potential impact of these factors on the risk of MI remains undefined.
We aimed to assess the prevalence of recent cannabis use and its association with history of MI in young adults (aged 18 to 44 yr) in the US, using national health survey data.
Methods
Study design and participants
We performed a cross-sectional study using data collected from 2 cycles of the annual Behavioral Risk Factor Surveillance System (BRFSS), a health-related telephone survey conducted by the Centers for Disease Control and Prevention (CDC).16 The BRFSS was designed to collect prevalence data on risk behaviours, chronic health conditions and use of preventive services that may affect health status among adults in the US; it has been shown to produce prevalence estimates with high levels of reliability and validity.17–19 The median response rates for the BRFSS survey were 45.9% for 2017,20 and 49.9% for 2018.21
The BRFSS survey consists of a standard set of questions used by all jurisdictions (i.e., states, District of Columbia and territories), as well as optional BRFSS modules and jurisdiction-specific questions. Information on cannabis use is collected as an optional module, and thus was not available for all jurisdictions. As of 2017, the optional cannabis module was expanded to include information on primary method of cannabis consumption. Therefore, for our analysis, we pooled data from the 2017 and 2018 BRFSS cohorts, representing 12 jurisdictions in 2017,22 and 16 jurisdictions in 2018.23
We included all respondents aged between 18 and 44 years in this study. We did not include participants older than 44 years because the BRFSS does not include information on variables that may confound the relation between cannabis use and cardiac outcomes in older patients, such as degree of atherosclerosis, use of lipid lowering or antithrombotic medications and history of peripheral vascular disease. We excluded respondents who were not asked the optional cannabis module, who refused to disclose or were unsure of their cannabis use or who had missing demographic, comorbidity or outcome data. The study sample size was determined by the cohort, and we did not perform posthoc power calculations.24
We report this study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for reporting cross-sectional studies.25
Exposure
The primary exposure for our study was any recent cannabis use, categorized as a binary outcome. In the BRFSS, participants were asked, “During the past 30 days, on how many days did you use [marijuana or hashish (2017)] [marijuana or cannabis (2018)]?” We defined any recent cannabis use as using cannabis 1 or more time during the past 30 days.
We also generated a frequency variable for cannabis use, and defined less frequent cannabis use as 4 or fewer times during the past 30 days (≤ 1 time per week) and more frequent cannabis use as more than 4 times during the past 30 days (> 1 time per week). We chose this threshold because, in our clinical practice, we frequently encounter patients who consume cannabis once a week, and regular, weekly cannabis use has been associated with adverse cardiac morphological changes.26 We obtained data on the primary method of consumption (i.e., smoking, vapourization or other forms of consumption) directly from the BRFSS survey. Further details on the variables used to define exposure in our study are found in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content.
Outcome
The primary outcome was history of MI, which we defined as a “yes” response to the question: “Has a doctor, nurse, or other health professional ever told you that you had any of the following? … (Ever told) you had a heart attack, also called a myocardial infarction?”
Covariates
Covariates included demographic factors (e.g., sex, race, age), socioeconomic factors (e.g., highest level of education, annual household income), health-related behaviours (e.g., health care coverage status), concomitant substance use (e.g., tobacco and alcohol consumption), other comorbidities (e.g., kidney disease, diabetes status) and jurisdiction-level legalization status of cannabis (Appendix 1).
Statistical analysis
To address potential selection bias and maintain a representative sample, the BRFSS uses a raking methodology to calculate sample weighting.27 This method incorporates detailed race and ethnicity, regions within jurisdictions, education level, marital status, age, gender, homeowner status and telephone source, and reproduces estimates that match national distributions.28 Furthermore, the BRFSS produces prevalence rates that are comparable with other national health surveys, such as the National Health and Nutrition Examination Survey and the National Health Interview Survey.19 Data were stratified and reweighted in accordance with CDC guidelines to reduce nonconvergence and nonresponse bias, and to improve generalizability.29,30 These adjusted sampling weights were used for calculating prevalence estimates of cannabis use (with 2-sided 95% confidence intervals [CIs]).
Our primary analysis was the association between recent cannabis use and history of MI. We used odds ratios (ORs) with 2-sided 95% CIs to express the adjusted treatment effect, which we estimated with a weighted logistic regression model, controlling for all covariates without further covariate selection. Secondary analyses included the assessment of history of MI of recent cannabis users, stratified by frequency and primary method of consumption.
We conducted sensitivity analyses using only the 2017 BRFSS cohort with additional adjustment for hypertension and hypercholesterolemia; data for these variables were not collected in the 2018 BRFSS cohort. Additional sensitivity analyses for missing data included a missing category cohort analysis for variables with more than 5% missing data, and an inverse probability weighted analysis for missing exposure data. We performed negative outcome controls to check for bias from residual confounding in our model. We assessed the associations between any recent cannabis use and skin cancer, and recent cannabis use and blindness, as negative outcome controls, and expected to observe no association between these variables.31 Additionally, we performed a confounder analysis to estimate how large an imbalance between the cannabis user and nonuser groups would need to be in the prevalence of cocaine use (as an unmeasured confounder) to nullify the association with history of MI (Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content).
We performed a posthoc analysis of cannabis use as a continuous variable and its association with history of MI. In addition, we performed a posthoc, propensity-score matched analysis of our primary comparison as an alternative method to adjust for confounding. Further details of this analysis are included in Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content. Furthermore, we performed separate posthoc tests for interactions between cannabis use and combustible cigarette use, and cannabis use and e-cigarette use, with history of MI.
We performed a complete case analysis, and considered a 2-sided p < 0.05 to be statistically significant. We performed all statistical analysis using R Software (version 3.6.3).
Ethics approval
The study protocol was deemed exempt from ethics review, as BRFSS data sets are publicly available.
Results
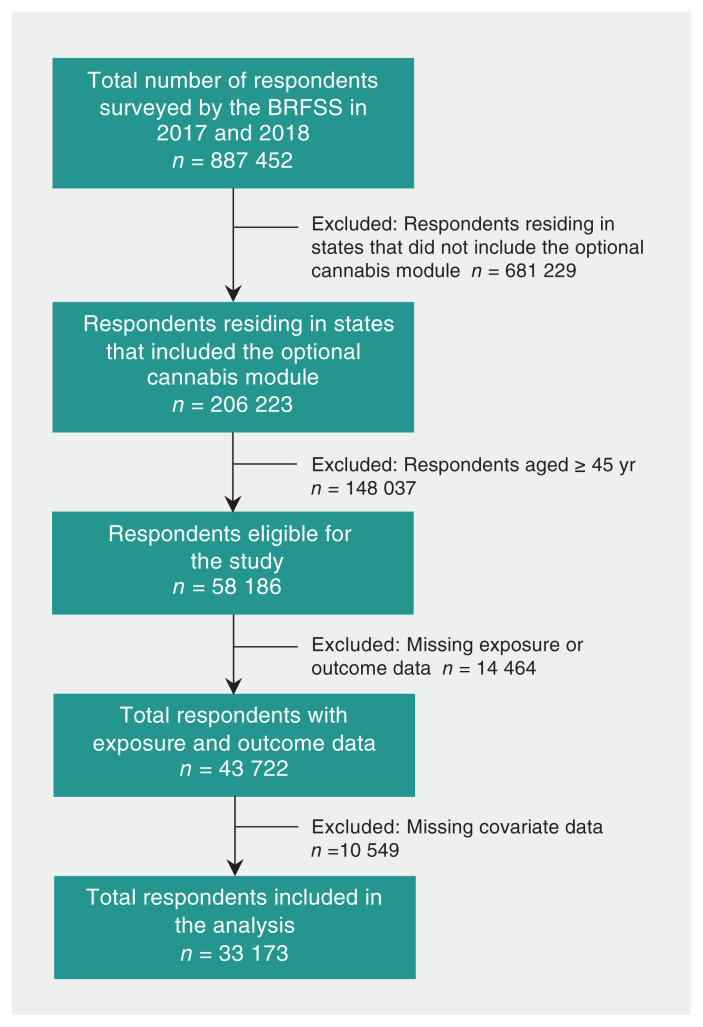
Complete data were available from 33 173 young adults (18.5 million weighted; Figure 1). Of these, 4610 respondents (3.2 million weighted; 17.5%, 95% CI 16.8% to 18.2%) reported recent cannabis use and 28 563 respondents (15.3 million weighted; 82.5%, 95% CI 81.7% to 83.3%) did not report any recent cannabis use. Compared with nonusers, the prevalence of recent cannabis use was higher among males (62.9% v. 49.3%), unmarried respondents (68.0% v. 46.4%), current combustible cigarette users (31.6% v. 13.2%), current e-cigarette users (18.1% v. 5.1%) and heavy alcohol drinkers (17.4% v. 5.2%). Further details on respondent characteristics are reported in Table 1, with a detailed summary of missing data reported in Appendix 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content.
Figure 1:
Flowchart showing creation of the study cohort. Note: BRFSS = Behavioral Risk Factor Surveillance System.
Table 1:
Respondent characteristics of young adults from the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys
| Characteristic | No. of nonusers n = 28 563 |
Weighted proportion of nonusers (95% CI)* | No. of cannabis users n = 4610 |
Weighted proportion of cannabis users (95% CI)* | Standardized difference† |
|---|---|---|---|---|---|
| Sex, male | 13 850 | 49.3 (48.2–50.5) | 2897 | 62.9 (60.4–65.3) | 0.3 |
| Race | 0.2 | ||||
| White | 18 762 | 48.2 (47.2–49.3) | 2957 | 47.5 (45.1–50) | |
| Black | 2275 | 10.5 (9.9–11.2) | 433 | 14.6 (12.7–16.6) | |
| Hispanic | 4593 | 29.3 (28.3–30.3) | 682 | 26.6 (24.2–29) | |
| Other | 2215 | 10.4 (9.6–11.2) | 343 | 8.7 (7.1–10.2) | |
| Multiracial | 718 | 1.6 (1.4–1.8) | 195 | 2.6 (2.0–3.2) | |
| Age group, yr | 0.3 | ||||
| 18–24 | 4696 | 21.8 (20.8–22.8) | 1174 | 29.3 (27.0–31.6) | |
| 25–34 | 10 210 | 37.2 (36.1–38.3) | 1955 | 42.5 (40.1–45.0) | |
| 35–44 | 13 657 | 41.0 (39.9–42.0) | 1481 | 28.2 (25.9–30.5) | |
| Body mass index category | 0.2 | ||||
| Underweight | 495 | 2.1 (1.7–2.5) | 124 | 2.5 (1.8–3.1) | |
| Normal weight | 9559 | 34.8 (33.7–35.8) | 1940 | 43 (40.5–45.5) | |
| Overweight | 9508 | 33.5 (32.5–34.6) | 1445 | 32.3 (29.9–34.6) | |
| Obese | 9001 | 29.6 (28.6–30.6) | 1101 | 22.3 (20.2–24.4) | |
| Highest education level | 0.2 | ||||
| Some high school | 1717 | 12.6 (11.8–13.5) | 342 | 11.8 (9.8–13.7) | |
| Completed high school | 7113 | 27.3 (26.3–28.3) | 1376 | 29.8 (27.5–32.1) | |
| Some college | 8235 | 32.2 (31.2–33.3) | 1496 | 38.4 (35.9–40.9) | |
| Completed college | 11 498 | 27.9 (27.0–28.7) | 1396 | 20.1 (18.4–21.7) | |
| Annual household income, $ | 0.1 | ||||
| < 15 000 | 2667 | 11.9 (11.1–12.6) | 544 | 12.7 (11.1–14.3) | |
| 15 000–25 000 | 4165 | 15.5 (14.7–16.3) | 861 | 16.7 (14.9–18.5) | |
| 25 000–35 000 | 2787 | 10.3 (9.6–10.9) | 544 | 10.9 (9.4–12.5) | |
| 35 000–50 000 | 3738 | 12.4 (11.6–13.1) | 695 | 13.6 (11.9–15.3) | |
| > 50 000 | 15 206 | 50.0 (48.9–51.1) | 1966 | 46.1 (43.5–48.6) | |
| Marital status | 0.5 | ||||
| Unmarried | 11 429 | 46.4 (45.3–47.5) | 2939 | 68.0 (65.6–70.4) | |
| Married | 14 029 | 44.1 (43–45.2) | 1167 | 23.5 (21.3–25.6) | |
| Divorced | 2231 | 6.3 (5.8–6.8) | 368 | 5.9 (4.9–7.0) | |
| Widowed | 176 | 0.5 (0.4–0.6) | 28 | 0.4 (0.1–0.7) | |
| Separated | 698 | 2.7 (2.4–3.0) | 108 | 2.3 (1.5–3.0) | |
| Jurisdiction-level cannabis legalization status | 0.2 | ||||
| Recreational and medical use | 4391 | 44.0 (43.2–44.8) | 1225 | 54.6 (52.3–56.9) | |
| Medical use only | 16 932 | 37.8 (37.1–38.5) | 2484 | 31.5 (29.4–33.6) | |
| Limited medical use only | 7240 | 18.2 (17.8–18.7) | 901 | 13.9 (12.6–15.2) | |
| Current health coverage | 24 932 | 85.5 (84.7–86.3) | 3733 | 82.6 (80.8–84.4) | 0.1 |
| Combustible cigarette use | 0.5 | ||||
| None | 19 265 | 71.7 (70.8–72.7) | 1798 | 46.7 (44.2–49.2) | |
| Current | 4376 | 13.2 (12.5–13.9) | 1770 | 31.6 (29.2–33.9) | |
| Former | 4922 | 15.1 (14.3–15.8) | 1042 | 21.8 (19.8–23.8) | |
| Current smokeless tobacco user | 1616 | 3.7 (3.4–4.0) | 365 | 5.8 (4.5–7.1) | 0.1 |
| E-cigarette use | 0.9 | ||||
| None | 21 001 | 73.5 (72.6–74.5) | 1400 | 33.0 (30.7–35.4) | |
| Current | 1470 | 5.1 (4.6–5.6) | 886 | 18.1 (16.1–20.0) | |
| Former | 6092 | 21.4 (20.5–22.3) | 2324 | 48.9 (46.4–51.4) | |
| Current heavy alcohol drinker‡ | 1691 | 5.2 (4.7–5.7) | 862 | 17.4 (15.5–19.4) | 0.4 |
| Sedentary lifestyle§ | 5684 | 20.2 (19.3–21.1) | 789 | 16.7 (14.7–18.6) | 0.1 |
| Kidney disease | 346 | 1.3 (1.0–1.5) | 59 | 1.3 (0.7–1.8) | 0.001 |
| Diabetes | 976 | 3.5 (3.0–3.9) | 124 | 2.4 (1.6–3.2) | 0.1 |
| Skin cancer | 356 | 0.9 (0.7–1.1) | 54 | 0.8 (0.5–0.12) | 0.01 |
| Blind | 613 | 2.9 (2.6–3.3) | 173 | 3.3 (2.5–4.2) | 0.1 |
Note: CI = confidence interval.
Weighted proportions and 95% confidence intervals were calculated in consideration of the complex sampling design of the Behavioral Risk Factor Surveillance System survey.
Standardized difference values greater than 0.1 are considered clinically significant.
Heavy alcohol drinker defined as > 14 drinks per week for males, and > 7 drinks per week for females.
Sedentary lifestyle defined as no reported physical activity or exercise during past 30 days other than regular job.
Most cannabis users reported frequent cannabis use, defined as more than 4 times during the past 30 days (70.5%, 95% CI 68.3%–72.7%; Table 2). Smoking cannabis was the most prevalent primary method of consumption (76.3%, 95% CI 74.3%–78.4%) relative to vapourization (11.3%, 95% CI 9.8%–12.8%) and other forms of consumption, including edibles (12.4%, 95% CI 10.8%–13.9%).
Table 2:
Characteristics of cannabis use among young adults from the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys
| Characteristic | No. of cannabis users n = 4610 |
Weighted proportion of cannabis users (95% CI)* |
|---|---|---|
| Frequency of use† | ||
| Less frequent | 1457 | 29.5 (27.3–31.7) |
| More frequent | 3153 | 70.5 (68.3–72.7) |
| Primary method of consumption | ||
| Smoke | 3640 | 76.3 (74.3–78.4) |
| Vapourize | 431 | 11.3 (9.8–12.8) |
| Other | 539 | 12.4 (10.8–13.9) |
Note: CI = confidence interval.
Weighted proportions and 95% confidence intervals were calculated in consideration of the complex sampling design of the Behavioral Risk Factor Surveillance System survey
We defined less frequent use as consumption of cannabis ≤ 4 times per month (≤ 1 time per week), and more frequent use as consumption of cannabis > 4 times per month (> 1 time per week).
Primary analysis
In our primary analysis of recent cannabis use, assessed as a binary outcome, a history of MI was reported by 61 of 4610 cannabis users (1.3%) and 240 of 28 563 nonusers (0.8%) (risk difference 0.5%, 95% CI 0.2%–0.8%; unadjusted OR 1.92, 95% CI 1.11–3.34; adjusted OR 2.07, 95% CI 1.12–3.82) (Table 3 and Appendix 5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content). The association between recent cannabis use and MI was similar in magnitude to associations with MI observed for current tobacco smoking (adjusted OR 2.56, 95% CI 1.56–4.21) and current smokeless tobacco use (adjusted OR 1.88, 95% CI 1.00–3.50) (Appendix 6, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content).
Table 3:
Association between cannabis use and myocardial infarction among young adults from the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys
| Characteristic | Unadjusted OR for myocardial infarction (95% CI) | Adjusted OR for myocardial infarction* (95% CI) |
|---|---|---|
| Cannabis use | ||
| No | Reference | Reference |
| Yes | 1.92 (1.11–3.34) | 2.07 (1.12–3.82) |
| Frequency of use† | ||
| No use | Reference | Reference |
| Less frequent | 1.26 (0.46–3.45) | 1.48 (0.52–4.21) |
| More frequent | 2.20 (1.21–3.99) | 2.31 (1.18–4.50) |
| Primary method of consumption | ||
| No use | Reference | Reference |
| Smoke | 2.02 (1.10–3.70) | 2.01 (1.02–3.98) |
| Vapourize | 1.26 (0.33–4.92) | 2.26 (0.58–8.82) |
| Other | 1.91 (0.61–6.01) | 2.36 (0.81–6.88) |
| Sensitivity analyses‡ | ||
| Additionally adjusted model§ | 3.00 (1.19–7.56) | 3.54 (1.13–11.05) |
| Missing category cohort¶ | 1.60 (1.03–2.50) | 1.68 (1.01–2.79) |
| Inverse probability weighting for missing data | 1.88 (1.09–3.25) | 2.04 (1.11–3.76) |
| Negative control analyses | ||
| Skin cancer | 0.93 (0.58–1.48) | 1.02 (0.59–1.75) |
| Blindness | 1.15 (0.86–1.54) | 0.98 (0.71–1.35) |
| Posthoc analyses | ||
| Cannabis as a continuous variable** | 1.02 (1.00–1.04) | 1.02 (1.00–1.04) |
| Propensity-score matched analysis | – | 2.11 (1.12–3.97) |
Note: CI = confidence interval, OR = odds ratio.
Model adjusted for sex, race, age, body mass index, education level, income level, marital status, health care coverage, combustible cigarette use, smokeless tobacco use, e-cigarette use, heavy drinking (> 14 drinks per week for males, and > 7 drinks per week for females), sedentary lifestyle (no reported physical activity or exercise during past 30 days other than regular job), chronic kidney disease, and diabetes.
Less frequent defined as consumption of cannabis ≤ 4 times per month (≤ 1 time per week). More frequent defined as consumption of cannabis > 4 times per month (> 1 time per week).
Sensitivity analyses assess cannabis use as a binary outcome (the reference group is nonusers).
Analysis of only 2017 data including adjustment for hypertension and high cholesterol.
The missing category cohort includes a level for “missing data” for each covariate with > 5% missing data.
The incremental amount for cannabis use as a continuous variable is per additional day of use within the last 30 days.
Secondary analysis
A history of MI was associated with more frequent cannabis use (adjusted OR 2.31, 95% CI 1.18–4.50) relative to nonusers. Less frequent cannabis use was also associated with an elevated, albeit nonsignificant, odds of history of MI (adjusted OR 1.48, 95% CI 0.52–4.21), relative to nonusers.
Smoking cannabis as a primary method of consumption was associated with a higher odds of history of MI (adjusted OR 2.01, 95% CI 1.02–3.98) relative to nonusers. Similarly, a higher odds of history of MI was observed with vapourization as a primary method of cannabis consumption (adjusted OR 2.26, 95% CI 0.58–8.82) and other forms of cannabis consumption, including edibles (adjusted OR 2.36, 95% CI 0.81–6.88) when compared with nonusers; however, these were not statistically significant.
Additional analyses
The association between recent cannabis use and increased odds of history of MI, relative to nonusers, was sustained with additional adjustment for hypertension and hypercholesterolemia, (adjusted OR 3.54, 95% CI 1.13–11.05) (Appendix 6), and across additional sensitivity analyses for missing data (adjusted OR 1.68, 95% CI 1.01–2.79 in missing category cohort analysis; adjusted OR 2.04, 95% CI 1.11–3.76 in inverse probability weighting analysis). We did not observe an association between recent cannabis use and our negative outcome control variables of skin cancer (adjusted OR 1.02, 95% CI 0.59–1.75) and blindness (adjusted OR 0.98, 95% CI 0.71–1.35). Furthermore, the results of our confounder analysis suggest that the prevalence of cocaine use (as an unmeasured confounder) would have to be implausibly large to nullify the association with history of MI (Appendix 2).
In a posthoc comparison of cannabis use as a continuous variable, the association between cannabis use and history of MI was 1.02 (95% CI 1.00–1.04) per additional day of use within the last 30 days. The association between recent cannabis use and history of MI persisted in our propensity-score matched analysis (adjusted OR 2.11, 95% CI 1.12–3.97). The posthoc tests for interaction between cannabis use and combustible cigarette use, and cannabis use and e-cigarette use, with history of MI were not significant (p = 0.3 and p = 0.9, respectively).
Interpretation
We found evidence of an association between recent cannabis use and an increased odds of history of MI in a generalizable population of younger adults. This association was stronger among more frequent users of cannabis. Although a similarly elevated odds of history of MI was observed across methods of recent cannabis consumption, only smoking as a primary method achieved statistical significance.
Our findings add to those of previous studies that have identified an association between heavy cannabis use and MI in medical and perioperative settings.7,15,32 However, previous studies have relied on a sole assessment of cannabis as a binary exposure in patients with heavy cannabis use (i.e., cannabis abuse or cannabis use disorder). One particular advantage of our analysis was our ability to investigate this association with more granular data related to frequency of cannabis use and method of consumption. Notably, the magnitude of the observed association between cannabis use and history of MI was increased among more frequent users. Moreover, the findings of previous studies may not be representative of young adults using cannabis, as they exclusively selected participants within a hospital (noncommunity) setting, and thus may be largely influenced by selection bias or may not accurately reflect the health-related behaviours of members within this cohort. The complex sampling design, weighting methodology and external validation of the BRFSS suggest that our observed association between cannabis consumption and history of MI may be more generalizable to a broader population of young adults.
The impact of exogenous cannabinoids on the cardiovascular system has been well described.7,33 After acute exposure, cannabis induces dose-dependent tachycardia and, in some cases, decreased ventricular contractility, palpitations, atrial fibrillation and arrhythmia. Additionally, Δ-9-tetrahydrocannabinol–mediated activation of the cannabinoid receptor subtype 1 has been shown to increase myocardial oxygen demand, induce platelet activation and cause endothelial dysfunction.7 In addition, normal angiography findings are commonly observed among published cases of acute MI after cannabis use, suggesting cannabis-induced coronary artery or microvascular vasospasm may reduce blood flow to the myocardium after exposure. 34 Frequent and sustained cannabis exposure may contribute to a mismatch between myocardial oxygen supply and demand, and in the context of cannabis-induced myocardial dysfunction and coronary macrovascular or microvascular impairment, may act as a potential mechanism for acute MI. Furthermore, cannabis smoke inhalation has been shown to induce a nearly fivefold increase in carboxyhemoglobin concentrations, and a threefold increase in tar, relative to tobacco smoke inhalation.35 Therefore, smoking cannabis as a primary method of consumption may exacerbate this mismatch between myocardial oxygen supply and demand by reducing oxygen-carrying capacity and impairing myocardial oxygen delivery, potentially leading to MI. However, further research is needed to delineate the mechanism for these associations.
The effect estimates for history of MI were similar across methods of consumption, including among people using other forms of cannabis, including edibles, as a primary method of consumption. This finding is consistent with a case study reporting an acute MI triggered after consumption of a lollipop containing a large dose of Δ-9-tetrahydrocannabinol.36 The Canadian government recently legalized cannabis edibles;2 however, the risks associated with edibles are poorly understood and are of concern to Canadian physicians.37 Edibles are often perceived as a safer alternative for cannabis consumption, but recent evidence suggests that edible cannabis is attributed to a larger proportion of cannabis-related emergency department visits for cardiovascular symptoms compared with inhaled cannabis.38 Further investigation is needed to characterize the mechanism of physiologic and therapeutic effects induced by specific molecules derived from cannabis, and the impact of route of administration on bioavailability.39 Additionally, future studies linking health-related survey and administrative databases,40 or prospectively assessing the impact of cannabinoid use on clinical outcomes, may provide additional insight on this potential causal relationship.
Limitations
Although we analyzed a nationally representative sample, with granular data regarding cannabis consumption and the ability to control for several important confounders, the cross-sectional design of the BRFSS meant that we lacked information on the temporal relationship between cannabis use initiation and onset of MI. We were unable to differentiate between participants who began using cannabis before having an MI, and those who began using cannabis after having an MI. However, the plausibility of our association is strengthened by a similar association between recent cannabis use and history of stroke from the same data set.8 Additionally, MI leading to cannabis use (reverse causation) is unlikely, and the elevated odds observed among more frequent users may provide evidence of a biologic gradient for this association. Regardless, health care professionals need to be aware that a relationship between any recent cannabis use and history of MI exists.
Another limitation of our study is potential bias from missing data in our cohort. To address this, we performed 2 additional sensitivity analyses. We observed a consistent association between recent cannabis use and history of MI across these analyses, suggesting limited bias due to missing data in our complete case analysis.
The retrospective design of the BRFSS may have led to unmeasured confounding in our analysis. Specifically, the BRFSS did not collect information on the use of cocaine and other illicit substances. However, the association between cannabis use and MI has been shown in similar studies that have been able to adjust for cocaine use.10 To further assess this problem, we performed a negative outcome control analysis of skin cancer and blindness, which suggested limited residual confounding in our model. Our confounder analyses suggested that the prevalence of cocaine use (as an unmeasured confounder) would have to be implausibly large to nullify the association between cannabis use and history of MI.
The BRFSS did not include the chemical composition and concentration of cannabinoids used by respondents. The chemical composition of products derived from cannabis varies substantially, and we were unable to determine if our observation could be attributed to a specific compound or grouping of compounds. Also, cannabis obtained through illegal means may contain unregulated, harmful cardiotoxic compounds that may confound our analysis. Laboratory confirmation of cannabis use would provide an ideal measure for our analysis; however, it is not feasible to conduct such a test at the scale of the BRFSS.
Additionally, the BRFSS did not include information on cardiovascular confounders. The prevalence of many cardiovascular confounders, such as history of peripheral vascular disease, are low among young adults (aged 18 to 44 yr), and substantially increase with older age. We therefore restricted our analysis to young adults to minimize potential bias from the absence of adjustment of these confounders.
Lastly, the BRFSS did not collect detailed information on MI, such as type of MI, extent of myocardial necrosis and plasma levels of cardiac biomarkers. This information could be important for understanding differences in clinical outcomes, and would provide greater insight on the potential mechanism(s) leading to cannabis-induced MI.
Conclusion
Recent cannabis use was associated with increased odds of history of MI in young adults (aged 18 to 44 yr). The magnitude of this association increased among more frequent users of cannabis. The large sample size, generalizability and detailed data on cannabis consumption of this cross-sectional study provide unique insight into this growing public health concern. Further studies and more data are needed to confirm these findings and elucidate the mechanisms contributing to cannabis-associated cardiovascular outcomes.
Footnotes
Visual abstract available at: https://www.cmaj.ca/lookup/doi/10.1503/cmaj.202392/tab-related-content
Competing interests: Karim Ladha and Hance Clarke are principal investigators of an observational medical cannabis study funded by Shoppers Drug Mart. Subodh Verma is President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization, and reports research grants and/or speaking honoraria from Boehringer Ingelheim, Eli Lilly, AstraZeneca, Janssen, Merck, Novartis, Novo Nordisk, Amgen, Sanofi, Servier, Sun Pharmaceuticals, HLS Therapeutics, Amarin, Valeant, Bayer, PhaseBio and Pfizer. C. David Mazer reports consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim and Octapharma. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Karim Ladha, Nikhil Mistry, Duminda Wijeysundera, Hance Clarke and C. David Mazer contributed to the conception and design of the work. All of the authors contributed to data acquisition, analysis and interpretation of data for the work. Karim Ladha, Nikhil Mistry, and C. David Mazer drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Karim Ladha and Nikhil Mistry share cofirst authorship.
Funding: Support for this study was provided in part by Merit Awards from the Department of Anesthesiology and Pain Medicine, University of Toronto (Karim Ladha, Duminda Wijeysundera, Hance Clarke, Gregory Hare and C. David Mazer), an Ontario Graduate Scholarship (Nikhil Mistry), an Endowed Chair in Translational Anesthesiology Research at St. Michael’s Hospital and the University of Toronto (Duminda Wijeysundera) and a Tier 1 Canada Research Chair in Cardiovascular Surgery (Subodh Verma).
Disclaimer: The funders had no role in the design, analysis, interpretation, preparation, review or approval of the manuscript.
Data sharing: All data of the Behavioral Risk Factor Surveillance System survey are made available by the Centers for Disease Control and Prevention online at: https://www.cdc.gov/brfss/annual_data/annual_data.htm
References
- 1.World Drug Report. Geneva: World Health Organization; 2018. Available: https://www.unodc.org/wdr2018/ (accessed 2020 Oct. 15). [Google Scholar]
- 2.Cannabis legalization and regulation. Ottawa: Government of Canada, Department of Justice; 2018. Available: https://www.justice.gc.ca/eng/cj-jp/cannabis/ (accessed 2020 Oct. 15). [Google Scholar]
- 3.Hartman M. Cannabis overview: legalization. Denver and Washington (D.C.): National Conference of State Legislatures; 2019. Available: https://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx (accessed 2020 Oct. 15). [Google Scholar]
- 4.Mitchell W, Bhatia R, Zebardast N. Retrospective cross-sectional analysis of the changes in marijuana use in the USA, 2005–2018. BMJ Open 2020;10: e037905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotermann MJHR. Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Health Rep 2019;30:3–13. [DOI] [PubMed] [Google Scholar]
- 6.Rotermann M. What has changed since cannabis was legalized? Health Rep 2020; 31: 11–20. [DOI] [PubMed] [Google Scholar]
- 7.Page RL, II, Allen LA, Kloner RA, et al. Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2020;142:e131–52. [DOI] [PubMed] [Google Scholar]
- 8.Parekh T, Pemmasani S, Desai R. Marijuana use among young adults (18–44 years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke 2020;51:308–10. [DOI] [PubMed] [Google Scholar]
- 9.Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805–9. [DOI] [PubMed] [Google Scholar]
- 10.Chami T, Kim CH. Cannabis abuse and elevated risk of myocardial infarction in the young: a population-based study. Mayo Clin Proc 2019;94:1647–9. [DOI] [PubMed] [Google Scholar]
- 11.Desai R, Fong HK, Shah K, et al. Rising trends in hospitalizations for cardiovascular events among young cannabis users (18–39 years) without other substance abuse. Medicina (Kaunas) 2019;55:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai R, Patel U, Sharma S, et al. Recreational marijuana use and acute myocardial infarction: insights from nationwide inpatient sample in the United States. Cureus 2017;9:e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson-Sasso CP, Tompkins C, Kao DP, et al. Marijuana use and short-term outcomes in patients hospitalized for acute myocardial infarction. PLoS One 2018;13:e0199705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RS, Katta SR, Patel R, et al. Cannabis use disorder in young adults with acute myocardial infarction: trend inpatient study from 2010 to 2014 in the United States. Cureus 2018;10:e3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravi D, Ghasemiesfe M, Korenstein D, et al. Associations between marijuana use and cardiovascular risk factors and outcomes: a systematic review. Ann Intern Med 2018;168:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behavioral Risk Factor Surveillance System. Atlanta: Centers for Disease Control and Prevention. Available: https://www.cdc.gov/brfss/ (accessed 2020 Oct. 15). [Google Scholar]
- 17.Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med Res Methodol 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cossman RE, Cossman JS, James WL, et al. Evaluating heart disease presciptions-filled as a proxy for heart disease prevalence rates. J Health Hum Serv Adm 2008;30:503–28. [PubMed] [Google Scholar]
- 19.Hsia J, Zhao G, Town M, et al. Comparisons of estimates from the Behavioral Risk Factor Surveillance System and Other National Health Surveys, 2011–2016. Am J Prev Med 2020;58:e181–90. [DOI] [PubMed] [Google Scholar]
- 20.The Behavioral Risk Factor Surveillance System. 2017 Summary Data Quality Report. Atlanta: Centers for Disease Control and Prevention; 2018. Available: https://www.cdc.gov/brfss/annual_data/2017/pdf/2017-sdqr-508.pdf (accessed 2020 Oct. 15). [Google Scholar]
- 21.Behavioral Risk Factor Surveillance System. 2018 Summary Data Quality Report Atlanta: Centers for Disease Control and Prevention; 2019. Available: https://www.cdc.gov/brfss/annual_data/2018/pdf/2018-sdqr-508.pdf (accessed 2020 Oct. 15). [Google Scholar]
- 22.2017 BRFSS modules used by category. Atlanta: Centers for Disease Control and Prevention; 2018. Available: https://www.cdc.gov/brfss/questionnaires/modules/category2017.htm (accessed 2020 Oct. 15). [Google Scholar]
- 23.2018 BRFSS modules used by category. Atlanta: Centers for Disease Control and Prevention; 2019. Available: https://www.cdc.gov/brfss/questionnaires/modules/category2018.htm (accessed 2020 Oct. 15). [Google Scholar]
- 24.Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat 2001;55:19–24. [Google Scholar]
- 25.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- 26.Khanji MY, Jensen MT, Kenawy AA, et al. Association between recreational cannabis use and cardiac structure and function. JACC Cardiovasc Imaging 2020;13:886–8. [DOI] [PubMed] [Google Scholar]
- 27.Methodologic changes in the Behavioral Risk Factor Surveillance System in 2011 and potential effects on prevalence estimates. MMWR Morb Mortal Wkly Rep 2012;61:410–3. [PubMed] [Google Scholar]
- 28.Iachan R, Pierannunzi C, Healey K, et al. National weighting of data from the Behavioral Risk Factor Surveillance System (BRFSS). BMC Med Res Methodol 2016;16: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Behavioral Risk Factor Surveillance System: complex sampling weights and preparing 2017 BRFSS module data for analysis. Atlanta: Centers for Disease Control and Prevention; 2018. Available: https://www.cdc.gov/brfss/annual_data/2017/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2017-508.pdf (accessed 2020 Oct. 15). [Google Scholar]
- 30.The Behavioral Risk Factor Surveillance System (BRFSS): complex sampling weights and preparing 2018 BRFSS module data for analysis. Atlanta: Centers for Disease Control and Prevention; 2019. Available: https://www.cdc.gov/brfss/annual_data/2018/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2018-508.pdf (accessed 2020 Oct. 15). [Google Scholar]
- 31.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010; 21: 383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goel A, McGuinness B, Jivraj NK, et al. Cannabis use disorder and perioperative outcomes in major elective surgeries: a retrospective cohort analysis. Anesthesiology 2020;132:625–35. [DOI] [PubMed] [Google Scholar]
- 33.Information for Health Care Professionals. Cannabis (marihuana, marijuana) and the cannabinoids. Ottawa: Health Canada; 2018. Available: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids.html (accessed 2020 Oct. 15). [Google Scholar]
- 34.Patel RS, Kamil SH, Bachu R, et al. Marijuana use and acute myocardial infarction: a systematic review of published cases in the literature. Trends Cardiovasc Med 2020;30:298–307. [DOI] [PubMed] [Google Scholar]
- 35.Wu TC, Tashkin DP, Djahed B, et al. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med 1988;318:347–51. [DOI] [PubMed] [Google Scholar]
- 36.Saunders A, Stevenson RS. Marijuana lollipop-induced myocardial infarction. Can J Cardiol 2019;35:229.e1–.e3. [DOI] [PubMed] [Google Scholar]
- 37.Grewal JK, Loh LC. Health considerations of the legalization of cannabis edibles. CMAJ 2020;192:E1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med 2019;170: 531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ladha KS, Ajrawat P, Yang Y, et al. Understanding the medical chemistry of the cannabis plant is critical to guiding real world clinical evidence. Molecules 2020;25:4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanmartin C, Decady Y, Trudeau R, et al. Linking the Canadian Community Health Survey and the Canadian Mortality Database: An enhanced data source for the study of mortality. Health Rep 2016;27:10–8. [PubMed] [Google Scholar]