KEY POINTS
Use of sodium-glucose co-transporter 2 (SGLT-2) inhibitors is currently classified as off-label in individuals less than 18 years of age in Canada.
Use of SGLT-2 inhibitors may result in euglycemic diabetic ketoacidosis (DKA) in patients who are insulin dependent and have intercurrent illness.
Education about self-managing diabetes when sick or during periods of decreased insulin dosing is critical for all patients with type 1 diabetes, especially those using SGLT-2 inhibitors.
Stopping SGLT-2 inhibitors during intercurrent illness mitigates the risk of DKA.
A 17-year-old male with known type 2 diabetes (T2DM) presented to the emergency department with lethargy, tachypnea and severe abdominal pain that followed a 5-day history of nausea and vomiting.
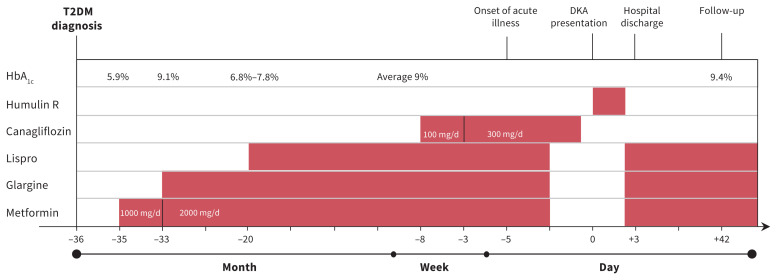
Three years earlier, the patient received a diagnosis of T2DM based on Diabetes Canada criteria.1 At the time of diagnosis, he had intermittent hyperglycemia, a high 2-hour glucose reading on his oral glucose tolerance test (20.5 [normal < 11.1] mmol/L) and a negative GAD65 antibody level (< 3.0 [normal < 5.0] U/mL). His high body mass index (31.9, > 97th percentile), the presence of acanthosis nigricans and an elevated fasting insulin level (277 [normal 35–140] pmol/L), although not diagnostic, were supportive of a diagnosis of T2DM. The patient began dietary and lifestyle interventions. Medical therapy escalated since diagnosis to include metformin and insulin owing to suboptimal glycemic control. A timeline of events is shown in Figure 1.
Figure 1:
Timeline of events and treatment of diabetic ketoacidosis (DKA) in a 17-year-old male with type 2 diabetes mellitus (T2DM). Note: HbA1c = hemoglobin A1c.
The patient completed diabetes self-management education that included information on nutrition, insulin, hypoglycemia, hyperglycemia, management during an intercurrent illness and ketone management according to standard-of-care clinical practice guidelines. Despite this, his hemoglobin A1c (HbA1c) averaged 9%. His diabetes self-management was characterized by finger-stick glucose monitoring 0–2 times daily, frequent insulin omission and suboptimal adherence with metformin. His GAD65 antibody level remained negative. He had had no episodes of diabetic ketoacidosis (DKA) or severe hypoglycemia.
Two months before presentation, the patient’s primary care provider prescribed canagliflozin (a sodium-glucose co-transporter 2 [SGLT-2] inhibitor) starting at 100 mg/d and titrated to 300 mg/d. His prescribed therapy also included metformin (1500 mg at bedtime), insulin glargine (30 units at bedtime) and insulin lispro (1 unit for 3 g of carbohydrate with meals and snacks).
At the time our patient presented to hospital he reported that 5 days earlier, on day 1 of his illness, he had decreased his prandial insulin on his own volition because of “low” blood glucose (about 8 mmol/L). He was fearful of hypoglycemia because of decreased intake and vomiting, which led him to stop taking his insulin and metformin; he continued to take canagliflozin. He had not monitored blood glucose or ketones for the 5 days before his presentation to hospital, nor used the telephone assistance service provided by the diabetes centre.
On initial assessment, the patient had the following vital signs: heart rate of 130 beats/min, blood pressure of 146/92 mm Hg, respiratory rate of 32 breaths/min with Kussmaul respiration, oxygen saturation of 99% on room air, body temperature of 36.7°C and a Glasgow Coma Scale score of 15. He was pale, with sunken eyes, dry mucous membranes and abdominal tenderness with no features of peritonitis.
The patient had a serum glucose level of 17.4 (normal 3.3–11.0) mmol/L. Venous blood gas analysis showed pH 6.93 (normal 7.30–7.40), anion gap 20 (normal 4–16) mmol/L, bicarbonate 4.8 (normal 20–32) mmol/L and lactate 2.4 (normal 0.5–2.2) mmol/L. His β-hydroxybutyrate level was elevated at 4.3 (normal < 0.4) mmol/L, as was serum osmolality at 310 (normal 280–300) mmol/kg. There were no indicators of substance abuse or infection on history or physical examination; urine and blood cultures were not requested.
We diagnosed DKA and provided treatment according to the institutional standard pediatric DKA protocol. We stopped canagliflozin as it was thought to be a substantial contributing factor for our patient’s presentation with DKA. We reviewed his self-management strategies; he stated he was unaware of the risk of DKA associated with SGLT-2 inhibitors and did not have a sick day protocol. He resumed metformin and insulin. At 6-week follow-up, our patient had made a full recovery with improved frequency of blood glucose and ketone testing, but he remained resistant to suggestions for changes in insulin dose and nutritional management.
This case has been reported to the Vigilance Canada Program. Causality is possible.
Discussion
Health Canada has not approved the use of SGLT-2 inhibitors in patients under 18 years of age. These medications are not recommended in national or international clinical practice guidelines for type 1 or T2DM management in pediatrics,1–3 although randomized controlled trials (RCTs) involving pediatric cohorts are underway. Few medical options for the treatment of T2DM in this age group are available, and SGLT-2 inhibitors have proven efficacy for glycemic control in adults.4,5 These 2 factors may contribute to off-label use, especially in older adolescents such as our patient. Sodium-glucose co-transporter 2 inhibitors have been associated with a higher risk of DKA, which may be euglycemic.6,7
Pediatric type 2 diabetes
The annual incidence of T2DM in Canadian children is 1.54 per 100 000 (range 0.4 to 12.54 across the provinces).8 The diagnostic criteria1 do not differ from those in adults except that isolated HbA1c is not independently diagnostic in pediatric populations.1 The Diabetes Canada guideline for the management of pediatric T2DM involves lifestyle modification and pharmacologic therapy primarily with metformin.1 Insulin therapy is advised if there is inadequate glycemic control or evidence of metabolic decompensation (HbA1c > 9%, DKA or symptoms of severe hyperglycemia such as polyuria or polydipsia).1 Doses are titrated to achieve glycemic control to target a HbA1c level of 7.0% or less.1 In our experience, it is not uncommon for young people with T2DM to require both metformin and insulin with dose intensification over time. This may be due to the natural history of T2DM, difficulty with lifestyle modifications or poor adherence to medical treatment.
Few medical treatments for patients with T2DM, however, are at the physician’s disposal. Antihyperglycemic medications commonly used in adults, including glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors and SGLT-2 inhibitors have not been well studied in children and are rarely mentioned in practice guidelines.1–3 Liraglutide is a GLP-1 receptor agonist that was approved in 2020 for treatment of T2DM in children older than 10 years of age in the United States.2 None of these drugs have been approved by Health Canada for use in pediatrics.
Pathophysiology of ketosis with sodium-glucose co-transporter 2 inhibitors
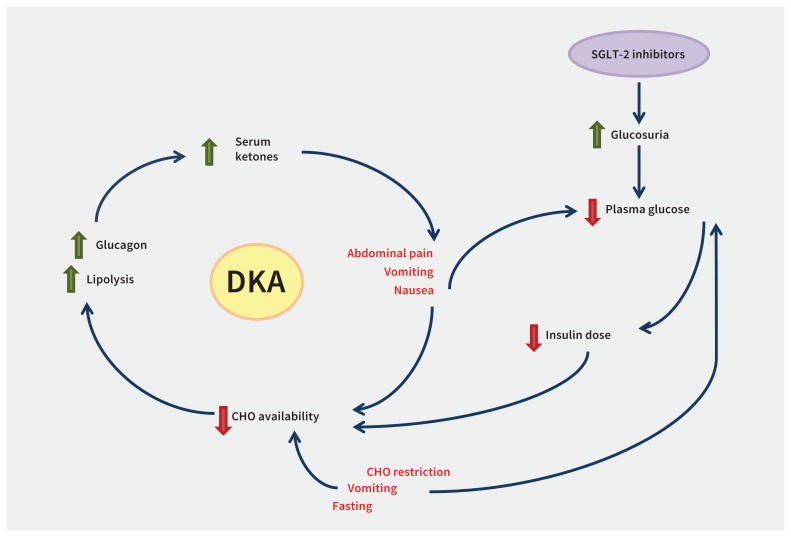
The mechanism by which SGLT-2 inhibitors increase the risk of DKA is shown in Figure 2. Sodium-glucose co-transporters are responsible for 97% of glucose reabsorption6 and their inhibition lowers blood glucose by increasing renal glucose excretion.4,5 Using SGLT-2 inhibitors increases the risk of DKA6,7 by decreasing daily carbohydrate availability by 20%–45%,9 lowering endogenous insulin production and stimulating lipolysis that leads to ketogenesis.6 Patients who also receive insulin may decrease insulin doses in response to lower blood glucose patterns, leading to insulin deficiency. Any additional factor resulting in decreased carbohydrate intake (e.g., sickness, vomiting or fasting), lowering of insulin levels or doses10 (e.g., missed insulin) or increased insulin demand (e.g., stress or infection) can further increase the risk of DKA in the context of SGLT-2 inhibition.7
Figure 2:
Factors contributing to development of diabetic ketoacidosis (DKA) in the setting of insulin-dependent diabetes and sodium-glucose co-transporter 2 (SGLT-2) inhibition. Note: CHO = carbohydrate.
Sodium-glucose co-transporter 2 inhibitors and diabetic ketoacidosis
Few reports of DKA associated with SGLT-2 inhibitors in children exist. A meta-analysis of RCTs in adults with type 1 diabetes showed an increased probability of DKA with SGLT-2 inhibitor therapy compared with placebo (odds ratio 3.38).5 A 2016 review of the existing evidence by an expert panel in Canada found that in T2DM trials, the incidence of DKA was estimated to be 0.16–0.76 per 1000 patient-years and patients could present with normal blood glucose levels.7 A case series found that about 70% of patients presenting with DKA were euglycemic.7 Overall, data from trials showed that DKA asssociated with SGLT-2 inhibitors occurred more commonly in patients who were dependent on insulin.7 Adverse event databases have also shown that DKA is associated with use of SGLT-2 inhibitors, with over 200 DKA events in adults reported with canagliflozin in Canada11 and 8968 cases of DKA associated with canagliflozin, dapagliflozin or empagliflozin reported in the US,12 of which 15 involved patients under age 18, of whom 3 had T2DM.12 More cases of DKA associated with use of SGLT-2 inhibitors may have gone unreported, and further study in the pediatric population is needed.
Risk factors for diabetic ketoacidosis
Our patient was at high risk of DKA because he had an elevated HbA1c level, did not follow diabetes self-management routines and did not monitor his blood glucose and ketone levels when he was sick. Additional risk factors in our patient included decreased intake of carbohydrates, reduction of insulin doses, possible metabolic decompensation of diabetes and increased insulin resistance associated with intercurrent illness. No social factors clearly contributed to poor adherence. Suboptimal adherence is a common challenge to diabetes care in adolescents and is associated with intrinsic (e.g., motivation, anxiety or fear of hypoglycemia) and external factors (e.g., time demands, family support or medical support).1 In our patient, use of a SGLT-2 inhibitor was unlikely to be the sole cause of his DKA; however, the drug’s glucose-lowering effects may have further decreased the perceived importance of ketone testing and it was likely a major contributor.
Patient education
By presenting this case, we aimed to highlight that treatment with a SGLT-2 inhibitor increases the risk of DKA in situations of illness, decreased carbohydrate intake or reduced insulin doses. Before they start SGLT-2 inhibitors, patients should be advised to avoid carbohydrate-restricted and ketogenic diets. They should be informed of the increased risk of DKA and given information on protocols for when they are sick to mitigate this risk.13 Patients should also be aware that ketosis and acidosis can develop even when their blood glucose level is normal. Patients should be taught how to recognize symptoms (i.e., polyuria, polydipsia, nausea, vomiting and abdominal pain). They should stop the SGLT-2 inhibitor at the onset of illness or fasting and start monitoring the level of blood ketones. Risk mitigation also includes increased intake of fluids and carbohydrates. Adjustments to insulin dose are recommended and can be personalized based on the patient’s insulin requirements. These adjustments and ketone surveillance should continue until ketones are cleared and the patient is feeling well.
Sodium-glucose co-transporter 2 inhibitors should be restarted after ketones have cleared and the patient is able to tolerate food and maintain oral hydration. Patients must have the ability to understand and perform these protocol steps. They should seek medical attention if they are unable to maintain hydration or clear ketones despite additional insulin.13 Although the importance of risk-mitigation strategies for DKA is recognized, systematic evaluation and validation of such protocols is still needed.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: Manpreet Doulla has received travel funding from Novo Nordisk, unrelated to this work. Mary Jetha is the local principal investigator for a clinical trial of teplizumab, which is sponsored by Provention Bio Inc., with funding provided to the University of Alberta, unrelated to this work. She is also the local principal investigator for a surveillance study of youth with type 2 diabetes, sponsored by the Canadian Institutes of Health Research, with funding provided to the University of Alberta, unrelated to this work. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed equally to the writing and editing of this manuscript, reviewed it critically for intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2018;42(Suppl 1):S1–325.29650079 [Google Scholar]
- 2.American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes — 2020. Diabetes Care 2020;43(Suppl 1):S163–82. [DOI] [PubMed] [Google Scholar]
- 3.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes in youth. Pediatr Diabetes 2018;19(Suppl 27):28–46. [DOI] [PubMed] [Google Scholar]
- 4.Tang H, Cui W, Li D, et al. Sodium-glucose co-transporter 2 inhibitors in addition to insulin therapy for management of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 2017;19:142–7. [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Shojima N, Noma H, et al. Sodium-glucose co-transporter-2 inhibitors as add-on therapy to insulin for type 1 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab 2018;20:1755–61. [DOI] [PubMed] [Google Scholar]
- 6.Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 2017;33:e2886. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther 2016;38:2654–2664.e1. [DOI] [PubMed] [Google Scholar]
- 8.Amed S, Dean HJ, Panagiotopoulos C, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: a prospective national surveillance study. Diabetes Care 2010;33:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38:1638–42. [DOI] [PubMed] [Google Scholar]
- 10.Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canada Vigilance adverse reaction online database. Ottawa: Government of Canada. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database.html (accessed 2019 Apr. 30). [Google Scholar]
- 12.FDA Adverse Event Reporting System (FAERS) Public Dashboard. Silver Spring (MD): US Food & Drug Administration. Available: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard (accessed 2019 Apr. 30). [Google Scholar]
- 13.Goldenberg RM, Gilbert JD, Hramiak IM, et al. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA protocol. Diabetes Obes Metab 2019;21:2192–202. [DOI] [PubMed] [Google Scholar]