Abstract
Post COVID-19, mucormycosis occurred after the SARS-CoV-2 has rampaged the human population and is a scorching problem among the pandemic globally, particularly among Asian countries. Invasive mucormycosis has been extensively reported from mild to severe COVID-19 survivors. The robust predisposing factor seems to be uncontrolled diabetes mellitus, comorbidity and immunosuppression acquired through steroid therapy. The prime susceptive reason for the increase of mucormycosis cases is elevated iron levels in the serum of the COVID survivors. A panoramic understanding of the infection has been elucidated based on clinical manifestation, genetic and non- genetic mechanisms of steroid drug administration, biochemical pathways and immune modulated receptor associations. This review lime-lights and addresses the “What”, “Why”, “How” and “When” about the COVID-19 associated mucormycosis (CAM) in a comprehensive manner with a pure intention to bring about awareness to the common public as the cases are inevitably and exponentially increasing in India and global countries as well. The article also unearthed the pathogenesis of mucormycosis and its association with the COVID-19 sequela, the plausible routes of entry, diagnosis and counter remedies to keep the infection at bay. Cohorts of case reports were analysed to spotlight the link between the pandemic COVID-19 and the nightmare-mucormycosis.
Keywords: COVID-19 induced Mucormycosis, Comorbidity, Hyperglycemia, Ketosis, Dexamethasone, Secondary infections, Diagnosis
Graphical abstract

1. Introduction
With continuous zigzagging in the prognosis, management, sequelae, complications and pathophysiology, the coronavirus disease 2019 (COVID-19) is still pounding the world mercilessly. Initial symptoms included whooping cough and superior pyrexia with problems in the different organs leading to breath deficiency, smell and taste deprivation, generalised discomfort, diarrhoea, drastic cardiac damage and infections. Effective and optimal treatment can be obtained by diagnosing the symptoms at its earlier stage, and Otorhino-laryngological studies are predominantly linked with COVID-19 since the beginning via collecting the swab samples from the nasopharyngeal region to identify the cases, to declare loss of smell like the classic symptom and to pick out the virus from the cavity of the middle ear (Frazier et al., 2020).
Lately, physicians observed the presence of a deadly invasive fungus causing infection mucormycosis in COVID-19 patients. A set of factors interplays like respiratory disorders, nosocomial infection (Kamrul-Hasan and Selim, 2021), diabetes mellitus, immune-suppressing therapy and systemic immune system alteration due to COIVD-19 virus can result in mucormycosis secondary infection (Chen et al., 2020). Furthermore, the hyperproduction of cytokines modulating inflammation and breakdown of immunity mediated by cells due to declined count of a cluster of differentiation (CD) positive cells (both 4 and 8) can make the host susceptible for fungal and bacterial co-infection (G. Song et al., 2020; Y. Song et al., 2020). A prolonged stay on ventilators is another reason for developing fungal infections (Yang et al., 2020). Lastly, excessive use of steroidal drugs for the management of COVID aids in suppressing the immunity of the person and easily exposes them to fungus and other microbial colonies. Thus, the above data collectively implied that, a higher possibility of secondary fungal infections in critically ill COVID- 19 patients can occur anywhere between later days of hospitalisation or after discharge from the hospital (Gangneux et al., 2020).
2. Comprehensive representation of acute and chronic complications in COVID-19 infection
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the viral pathogen accountable for the on-going pandemic coronavirus disease 2019 (COVID-19), progresses as a second wave worldwide and has caused unprecedented mortality and morbidity scale globally. Though the number of affected cases is exponentially increasing, post-COVID symptoms have become areas of medical interest, and several medical centres have been monitoring the aftermath of the infection. Since this disease is novel, its clinical course and possible long-term health consequences remain uncertain; therefore, the doctors depend on the scientific and clinical evidence on the sub-acute and prolonged long-term effects of COVID-19 that hastily affect multiple organ systems (Gupta et al., 2020). However, comparative studies among the previous SARS (2003) and MERS (2012) survivors can provide baseline information on the assemblage of long-term symptoms, persistent complications and sequel of post- pathogenesis of COVID-19. First-hand reports on post-acute consequences and preparedness of COVID-19 are published from selected countries like the USA, China and Europe based on the COVID-19 survivors (Huang et al., 2021). The on-going COVID 19 was designated in two stages: first, the sub-acute phase, which covered the symptoms and disorders between 4 and 12 weeks beyond COVID 19 infection; secondly, the symptoms and complications in the chronic phase are accountable after 12 weeks of COVID 19 manifestation (Nalbandian et al., 2021). Representation of organ-wise sequel of events reported from case reports (Fan et al., 2021; Guan et al., 2020; Guzik et al., 2020; Huang et al., 2020; Nalbandian et al., 2021; Y. Song et al., 2020; Wang et al., 2020; Zheng et al., 2021) are presented as box information (Box 1 ).
Box 1. Sequela of events in organs system in the course of existing and post COVID-19 infection.
| Pulmonary sequela | The post symptoms primarily derived from acute respiratory distress syndrome (ARDS) was observed. Common pulmonary sequela included Dyspnea, hypoxia due to decreased breathing capacity. In contrast, the restrictive pulmonary physiology revealed the parenchymal abnormalities characterised by ground-glass opacity and “cord-like consolidation” (Zhang et al., 2020). Also, muscle weakening due to deconditioning was a subsidising solid factor. In addition, the survivors developed substantial lung fibrosis, which was related to barotrauma secondary to high-pressure ventilation, and minor complications included extracorporeal membrane oxygenation (Fraser, 2020) Recommendations: Monitoring the stability with CT chest and pulmonary angiogram is clinically appropriate to monitor any fluctuations. |
| Renal sequela | Acute kidney injury (AKI) is widely noticed in the later stages of infection, and it is related to proteinuria and hematuria, along with elevated serum creatinine levels and urea nitrogen levels. Severe acute tubular necrosis with an accumulation of SARS-CoV-2 nucleocapsid protein antigens was observed in severe cases of chronic COVID-19, which directly infected kidney tubules. However, it is plausible to speculate that the virologic mechanism provokes complication like glomerulopathy and protein leakage in the bowman's capsule. Recommendations: Monitoring acute functional changes in kidney function suggestive strategies includes lung-protective ventilation, limiting the ventilator-induced hemodynamic effects and the cytokine load in kidneys. |
| Cardiopulmonary sequela | Prolonged problems included cardiometabolic demand, myocardial fibrosis, arrhythmias, tachycardia and autonomic dysfunction. Moreover, common disorders included palpitations, dyspnea and chest pain was also reported. Cardiac dysfunction was diagnosed due to direst virologic events. Systemic inflammation can potentially lead to coronary microcirculation disruption and downstream myocardial ischemic sequela, despite clinical interventions, sudden cardiac arrest in patients with no prior history of ischemic heart disease. Fulminant myocarditis and cardiogenic shock were observed in few cases. Recommendations: Monitored with echocardiogram and electrocardiogram follow-up at pertinent intervals. Continuous monitoring of monitor plasma cTnI and NT-proBNP levels in the acute phase can be a preventive measure to further complications. The transthoracic echocardiographic examination may be recommended for chronic COVID -19 patients. |
| Liver sequela | Close monitoring of liver function routine test with mild to elevated serum transaminases, bilirubin, LDH, and prothrombin time in acute COVID -19 cases. Hypoalbuminemia and prolonged prothrombin were noted in chronic patients, while jaundice was less reported. Chronic liver diseases like liver cirrhosis caused mortality in severely affected patients being the pre-disposing and significant contributing factor for casualty was specifically reported in COVID- 19 patients with prior. Recommendations: A liver transplant can be the least option; however, transplant recipients are highly susceptible to SARS-CoV2 infection due to immunosuppressive state and corticosteroid therapy. Exceptional cases have survived but the development of multiple nosocomial infections and develop septic shock. |
| Gastrointestinal sequela | Contrasting changes in the microbiome of the GI tract is reported post-COVID- 19; the most common complaints include nausea, vomiting and diarrhoea at the onset. Moreover, prolonged viral shedding is noted and speculated to alter the beneficial commensals. Recommendations: Routine faecal negative test is recommended to ensure the recovery of patients in the acute phase. |
| Immunological and haematological sequela | Lymphopenia is commonly observed, acute coagulation disorders, septic shock venous thromboembolism (VTE) and disseminated intravascular coagulation (DIC) proportionally increased with the severity of the disease. Retrospective studies markedly report on the thromboembolic events in the later part for acute infection. Recommendations: Monitoring on D-dimer levels may be precautionary to probe thrombosis and prevent VTE; use of anticoagulants decreased mortality |
| Neuro-psychiatric sequela | Chronic cases reported acute cerebrovascular disease, reduced consciousness, skeletal muscle injury (Herman et al., 2020). Acute phases were accompanied by decreased sensory perceptions like taste, smell, and appetite and neuralgia. Moreover, headache, fatigue, faintness, acute cerebrovascular disease and epilepsy were also widely recorded. At the same time, the psychological dysfunctions included depression, post-traumatic stress and interrupted sleep. |
| Multi-system inflammatory syndrome in children (MIS-C) | MIS-C, commonly referred to as pediatric inflammatory, a multisystem syndrome associated with SARS-CoV-2 under 19 years old (WHO) characterised by cardiovascular impairment such as coronary artery aneurysm and neurological disorders like cranial nerve palsies, muscle weakness, stroke, headache and encephalopathy Recommendations: Serial echocardiographic assessment is advised to post 6 weeks of presentation, and Cardiac MRI is recommended for patients who suffered fibrosis and inflammation during the acute phase of COVID -19 |
| Reproductive sequela | Pregnant women associated with the allied respiratory problem were observed to have poorer perinatal consequences such as spontaneous abortion, maternal death, and pre-term labour (Wang et al., 2020). Unlike SARS, COVID-19 did not lead to any male reproductive impairment like male infertility and viral orchitis; indeed, the latter can lead to severe testicular damages like azoospermia (Xu et al., 2006). |
| Endocrinal sequela | Administration of steroids for chronic cases may trigger DKA and induced diabetes and at the same time worsen the control over the existing diabetes mellitus. Alterations in thyroid secretions were noted with sub-acute thyroiditis and bone demineralisation in fewer cases. |
Alt-text: Box 1
3. The threat of secondary infections along COVID- 19
It is interesting to know the underlying scientific facts that this pandemic has influenced and repelled the infections by microorganism among the population. In recent times, such as social distancing, wearing a face mask, sanitiser, and other protective kits as accelerated protective measures against COVID -19 infections has lessened conventional bacterial infections. At the same time, the administration of chemotherapy to target viral infection has influenced drug resistance for secondary bacterial infection. In parallel, “superinfections”, i.e. the presence of co-infection in COVID 19 patients in hospitals, especially in ICU units, is seen to be increased. Several studies unanimously reported common bacterial respiratory pathogens Streptococcus pneumoniae in most of the COVID -19 (Lecoq et al., 2009). The most commonly isolated pathogens from long-stay patients (with a duration of 9 days) in ICU were S. aureus, S. pneumoniae and H. influenza (Verroken et al., 2020). A similar study by Zhang et al. (2020) reported hospital-acquired infections such as Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa and Enterococcus in COVID- 19 patients.
Along with the more common pathogens enlisted above, Mycoplasma pneumoniae was documented from sputum specimens (Chen et al., 2020). Global data on the secondary pathogen reveals common nosocomial pathogens related to UTIs (urinary tract infections), open wound infections, respiratory pneumonia, bacteremia and gastrointestinal (Hughes et al., 2020). Case reports on the presence of atypical pathogens like Legionella co-infection (Arashiro et al., 2020) and Chlamydia or Mycoplasma infections (Oliva et al., 2020) have been described suspicion of their roles in increasing the lethality along with COVID -19 has also been studied (Wang et al., 2009). Very few cases with Pneumocystis jirovecii pneumonia in COVID -19 patients have been reported worldwide (Feldman and Anderson, 2021). Suggestive use of procalcitonin as a marker can detect secondary infections in the COVID-19 patients and improve the initial antibiotic medications (Han et al., 2020).
4. Mucormycosis “the black fungus infection”
A fungal infection caused by mucorales from Mucoromycota phylum gained importance in the past few years due to their higher incidence in immunocompromised populations as Mucormycosis. It is one of the common mould causing infections and ranked third for causing invasive infections in organ transplant and malignant tumor cases (Baldin and Ibrahim, 2017). The virulent mucorales causing mucormycosis are from Lichtheimia, Rhizopus and Mucor genera (Farmakiotis and Kontoyiannis, 2016), however the most common ones belong to Rhizopus sp. These fungus needs infringed immune system to propagate and seen mostly in neutropenia, transplantation (solid organ/hematopoietic), excessive iron, and predisposition to diabetes mellitus (diabetic ketoacidosis form), malnutrition and any infection breakthrough states in a person, with greater chance in people with weaken immune system in addition to lesions, traumatic injury and burn injury in the skin (Petrikkos and Tsioutis, 2018). Based on the site the mucormycosis is clinically classified as rhino-orbital, cutaneous, pulmonary, disseminated, gastro-intestinal and other forms like renal infections, osteomyelitis, endocarditis and peritonitis with invasive models being invasive and aggressive, growing within the blood vessels causing thrombosis, necrosis of tissues plus hematogenous fungus dissemination (Baldin and Ibrahim, 2017; Petrikkos et al., 2012). To gain maximum survival, rapid treatment with a higher dosage, prior diagnosis and tracing the suspicious candidate at the earliest is recommended, sometimes aggressive therapeutic like amputations and a heavy dosage of liposomal amphotericin B could not increase the longevity of the patient in some instances (Roilides et al., 2014). The degree of severity depends upon several factors such as infection in specific region/part of body, dissemination of fungal spores via blood throughout body, delay in treatment, neutropenia, old age and presence of parallel infections commonly caused by Cunninghamella species (Roilides et al., 2014; Petrikkos and Tsioutis, 2018).
5. Pathogenesis of mucormycosis
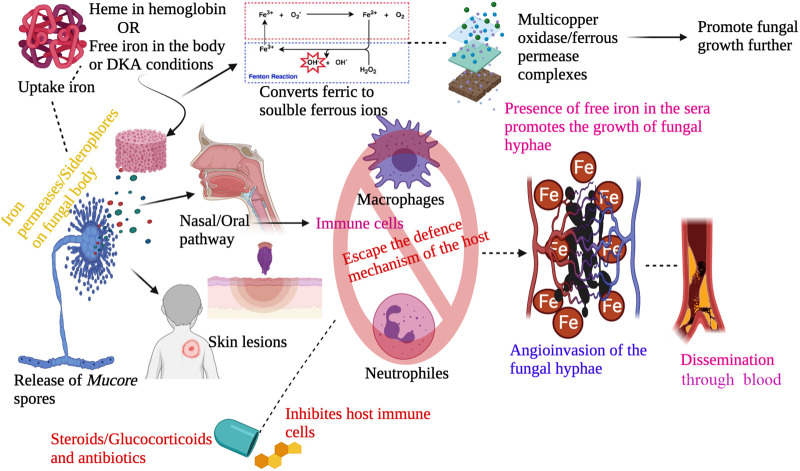
Usually, the spores of the fungi disperse through the air and enter within humans via inhalation, inoculation in some regions like skin lesions, the oral way in the gastrointestinal tract (Ibrahim, 2011). Whatever may be the entry point the fungus propagation involves crucial steps like spore inoculation, escaping phagocytosis from macrophages and neutrophils, hyphae germination, growing using the condition of the host like iron overloads, ketoacidosis, attaching with the endothelium using receptors and via endocytosis damages the endothelium resulting in necrosis of tissue, thrombus formation or haemorrhages as shown in Fig. 1 . This condition contributes to multiorgan dysfunction.
Fig. 1.
The usual pathogenesis of the fungal infection mucormycosis.
The omnipresent fungal spores enter in the body through naso-oral pathway or skin injury, followed by absconding from the host immune cells due to pathophysiological or immunocompromised conditions in the body. After passing the cellular barricade the fungal spores produce hyphae in the blood vessels causing dissemination in various organs and leading to fungal infection at the site of entry.
5.1. Iron role in mucormycosis
Mucorales species can thrive in conditions like iron in the host by utilising iron for its growth, and the presence of unbound iron in the serum contributes to mucormycosis in diabetic ketoacidosis (DKA) predisposing peoples. Usually, iron levels are maintained in the serum by binding with host proteins like transferrin, lactoferrin and ferritin. This mode of maintaining low iron levels in the body is a pervasive defensive mechanism of the host against Mucorales (Ibrahim et al., 2012). The fungus obtains iron through iron permeases or siderophores present in their body and reduces ferric to soluble ferrous ions. This obtained ferrous ion is assimilated in multicopper oxidase/ferrous permease complexes of protein, and the gene encoding these proteins has been shown to influence the virulence of fungal infection in the mucormycosis animal model (Ibrahim et al., 2010). Alternatively, iron is taken from the host using heme (Santos et al., 2003). The Rhizopus oryzae obtains iron from the haemoglobin of the host and explain their angioinvasive nature. Degradation of heme through heme oxygenases releases ferric ion intracellularly and promotes the growth of Mucorales species (Boelaert et al., 1993).
5.2. Plausible entry route of the fungal spores in COVID patients
Substantially, mucormycosis is observed in people with a predisposition for diabetic ketoacidosis and immunosuppression causes with a 100% fatality rate for severe infection states. The localisation of infection for example (Rhino-cerebral) determines the severity of infection. More specifically, Rhino-Orbito-Cerebral Mucormycosis (ROCM) is more dangerous as it causes vision impairment and seizures in brain leading to death (Pal et al., 2021). Mostly the omnipresent spores enter through a various route like ingestion, inoculation in the injured region, inhalation. Apart from these, the fungal contamination in medical supplies like bandages, ostomy adhesive bags, linens (Duffy et al., 2014) can also contribute to the outbreak of invasive fungal growth among the COVID patients admitted in the hospitals. Outbreaks of healthcare-associated mucormycosis are previously reported and the infection portal of entry included surgical instruments and medical devices such as catheters, adhesive tape and bandages, wooden tongue depressors, water circuitry damage and even adjacent building construction. These are more severe in neonatology, haematological, and transplantation units (Foster et al., 2021; Rammaert et al., 2012). There is another possibility of inhalation spores during the hospitalisation period, and due to the immunosuppressive effects of the drugs administered to mitigate COVID, the individual can become a susceptible host after discharge from hospital or while under surveillance for the fungal growth as higher levels of iron is observed in the serum of patients undergoing treatment and COVID survivors. This iron provides a favourable condition for the growth of spores and causes further infections and mucormycosis. As it already knows, the viability of the spores depends on the suitable conditions, here iron supply can make them stay alive for a longer duration.
5.3. Steroids as potential drugs for COVID-19
Natural production of steroids occurs in the adrenal cortex of healthy individuals, while commercially available corticosteroid drugs are supplemented to patients under severe infections. Glucocorticoids and mineralocorticoids are chiefly used to treat a broad spectrum of infections, among them, dexamethasone (glucocorticoid) is mainly used in the treatment of viral pneumonia, chronic obstructive lung disease, inflammation in organs, asthma and in combination therapy to treat tuberculosis (Ramamoorthy and Cidlowski, 2016). The prescribed posology of Dexamethasone dose ranged between 0.5 and 10 mg; however, the dosage varies depending on the severity of infection; at the same time, a lower dose should be used to minimise side effects. The exclusive potential of dexamethasone to limit the inflammation at a higher potency made it the best choice for the effective drug treatment in SARS-CoV-2 patients (Cruz-Topete and Cidlowski, 2015). Ironically, dexamethasone is Janus-faced, it is anti-inflammatory and immunosuppressive in action, and the latter has provoked post-infection complications in the patients. On the contrary, other non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, ibuprofen, etc., are potential candidates for inhibiting the vascular stage of inflammation (Becker, 2013).
5.4. Mechanism of action of dexamethasone
The principal anti-inflammatory effect of dexamethasone inhibited the pro-inflammatory gene that provoked the acute inflammatory response characterised by cytokine storm and production of other chemokines, cell adhesion molecules (CAM). In coronavirus infection, dexamethasone is administered to down-regulate the Aryl hydrocarbon receptors (AhR) and Indoleamine 2, 3-Dioxygenase 1 (IDO1) genes can subsequently reduce the inflammation (Saraya and Amal, 2012). The mechanism of action is dependent on the dosage; low dosages of dexamethasone act via genomic mechanism (Fig. 2 ) (Mitre-Aguilar et al., 2015), which requires more extended time and fewer side effects; conversely, higher dosages can be rapid in action through non-genomic routes (Fig. 3 ) and is accompanied with worse side-effects (Lecoq et al., 2009). Clinical research findings revealed that corticosteroids proved efficient against COVID 19 and pneumonia infections in both in vitro and in vivo activity. The commonest corticosteroid being the dexamethasone was used to treat the infection. However, lower doses reduced mortality and were influential in patients with severe COVID manifestation. However, controlled usage for the treatment was recommended due to potential harm at higher doses (Ahmed and Hassan, 2020).
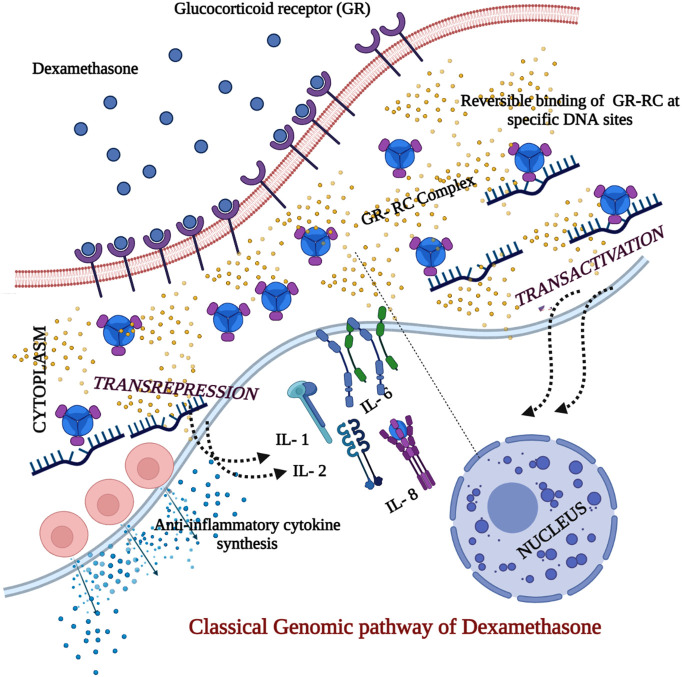
Fig. 2.
Classical genomic pathway of dexamethasone in the corticosteroid therapy for COVID 19.
The dexamethasone (DM) is lipophilic and easily permeable through cell membrane by the process of diffusion and makes entry into cytoplasm, the DM binds to the glucocorticoid receptor (GR) on the cell membrane and forms a Glucocorticoid receptor complex (GR-RC) and mediates corticosteroid into the cell. The GR-GC binds specifically to the sites in DNA and resulting in transactivation and trans-repression in parallel. The interleukins (IL-1, 2, 6 & 8), TNF and IFN-gamma are inhibited while the same activates the anti-inflammatory cytokine synthesis.
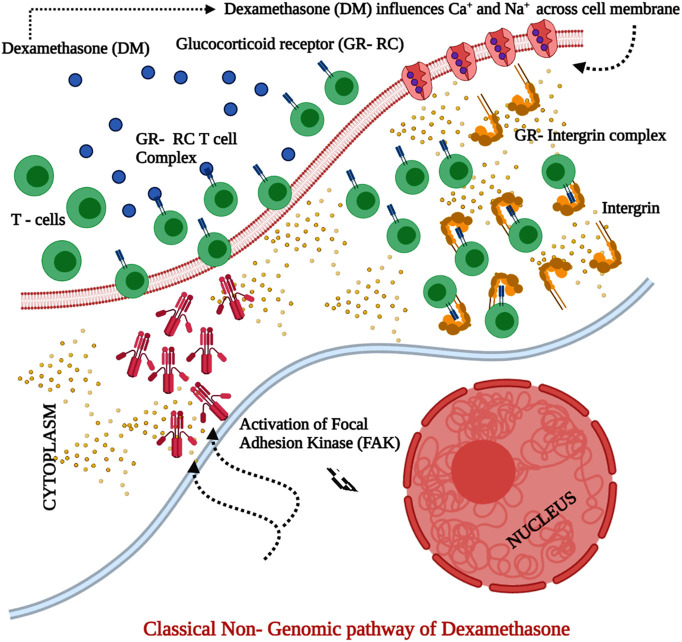
Fig. 3.
Classical Non- genomic pathway of dexamethasone in the corticosteroid therapy for COVID 19.
Severity of the infection warrants high dosage of corticosteroid. At higher dosages, dexamethasone (DM) binds to the membrane-associated glucocorticoid receptor (GR) on the T- lymphocytes which impairs the T lymphocyte–mediated immune response. Simultaneously, the integrin in cytoplasm binds with GR complex and activates focal adhesion kinase (FAK). Moreover, the high dosages of DM also influence active transport of calcium and sodium ions to suppress the inflammation.
5.5. Dexamethasone and immunosuppression in COVID-19
After the randomised clinical controlled trials, the U.K announced that dexamethasone was the first drug shown to reduce the mortality percentage and down the death rate by one-third of critically ill patients who were on life support with ventilators (Roberts, 2020). Observational studies confirmed the advantages of corticosteroid over short-term mortality and a notable reduction in the need for mechanical ventilation. Despite the above facts, the team also underlined that critical clinical data and observations across the period are necessary to conclude, and there might be the onset of secondary infections due to prolonged usage of these steroids (van Paassen et al., 2020). Raju et al. (Raju et al., 2021) performed an extensive search across global clinical trial registries and reported that out of 60 trials, 11 reported better respiratory rates as the outcome after the administration of corticosteroids for COVID-19. Similarly, another study comprising 2104 patients was administered with dexamethasone, which lowered the 28-day mortality among the patients under invasive mechanical ventilation or oxygen alone at randomisation but not among patients with no respiratory support. Therefore, glucocorticoids curb inflammation-mediated lung injury and reduce the progression to respiratory failure and death (Wootton, 2021). Contrastingly, clinical evidence does not suggest using corticosteroids in COVID-19 since they seemingly increased the plasma viral load in the patients post-infection (Russell et al., 2020). The underlying science conveys that dexamethasone is an effective drug when used for short-term therapy, but the hard fact is that the host defence mechanisms will be curtailed severely on long-term administration.
A survey across the 73 reported literature cases with 21,350 COVID-19 patients administered with corticosteroid revealed that the drug aided in the recovery of the severely ill patients; however, it was neither beneficial nor harmful among the high low-dose corticosteroid regimens. Ironically, it was evidenced from the cluster of recent reports that low-dose corticosteroids did not pose a significant impact in the period of SARS-CoV-2 viral shedding (Cano et al., 2021). As a point of conclusion, though multiple therapeutic options are explored, including corticosteroids therapy, the debate on the efficacy of inhibiting the infection is uncertain. Despite its primary role in delimiting the infection relied on dose specificity and severity of illness, the secondary or side effects are potential and have undoubtedly pushed the patients to the bottom line of the immunosuppressive state. COVID-19 is a multi-organ disease that necessitates the fact that not only the causative agent (SARS)- CoV-2 should be focussed, but it is equally important to monitor and provide care to the whole body as well. This is true to the familiar statement put forth by Sir William Osler's (1849–1919) that “The good physician treats the disease; the great physician treats the patient who has the disease” (Bondy, 1980).
6. COVID-19 induced immunosuppression increases susceptibility for mucormycosis infection
Mucormycosis is a rare fungal infection caused by an opportunistic group of filamentous moulds called mucormycetes classified under Mucorales (Lin et al., 2017). Mucormycosis, previously termed as Zygomycosis, is the cohort of invasive fungal infections (IFIs) caused by saprophytic environmental fungi–Rhizopus, Mucor, Cunninghamella, Apophysomyces, Lichtheimia (Absidia), Saksenaea, Rhizomucor (Roden et al., 2005). Generally, the infection is acquired through intake of contaminated food, inhalation of spores or inoculation in open cut wounds. However, recently, many COVID patients are diagnosed with this infection in the pandemic period, and many are susceptible to the same, while several cases have been recorded across the world. However, the top reasons for this infection seem to be the severe immunocompromised state of the hosts (e.g. organ transplantation, malignancy, autoimmune disorders, impairments in immunity or neutropenia), hyperglycemia or poorly controlled diabetes mellitus and immunocompetence in post-traumatic cases. Clinical findings suggest that SARS-CoV-2 infection ultimately affected the integral immune system and altered it by suppressing the activity of immune cells, particularly T lymphocytes, CD4+ and CD8+ T cells, which are potential workforce involved in the pathological process of COVID-19 infection (Liu et al., 2020). This substantial reduction of the T cells and lymphocytes is recorded in the most severe COVID-19 cases and is accompanied by a higher risk of acquiring opportunistic secondary infections (Peng et al., 2019).
Mucormycosis is characterised by a notable propensity to invade the blood vessels leading to necrosis, thrombosis and obstruction of blood vessels. Casualty is observed in cases with 30–50% of infection (Reid et al., 2020). Different organs might be infected, but the most frequently and severely infected are the lungs, and it stands in the second place in terms of clinical manifestation involved (58%) with a mortality rate above 80% (Lin et al., 2017). Potenza described the serology of mucormycosis in haematological patients, and the team reported that Mucorales-specific T-cells (CD4+ and CD8+) were abundant and actively produced IL-4, IL-10, IL-17 and IFN-γ, which directly destroyed the Mucorales hyphae (Potenza et al., 2011). Retrospective findings on the cohort reports of critically ill COVID cases revealed the onset of acute respiratory distress syndrome (ARDS) accompanied with acute invasive pulmonary aspergillosis resulting in immune paralysis (Koehler et al., 2020). In a particular case, either immunosuppressant drugs or corticosteroids were administered, but the patient showed a severe form of multiple organ failure and sustained severe lymphopenia. Therefore, it can be speculated that SARS-CoV-2 infection itself can provoke an immunosuppressive state, that the patient is pushed to a high risk of acquiring opportunistic infections particularly, mucormycosis (Lamoth and Kontoyiannis, 2019). Thus the above data suggest that the parallel link between COVID-19 and Mucormycosis is obvious. Since the major risk factor for mucormycosis is uncontrolled diabetes mellitus, which is also a potent risk factor for COVID-19. Furthermore, the steroids that are administered are known to suppress immunity; unfortunately, they are the only life-saving drugs for treatment, decreasing COVID-19 mortality.
7. Mucormycosis fuelled by hyperglycemia and diabetic ketoacidosis
Diabetic ketoacidosis (DKA) and deferoxamine-treated (i.e. remove excess iron due to multiple blood transfusion) patients are particularly predisposed to mucormycosis. Diabetic patients suffer from raised blood glucose levels, inducing excessive glucosylation of transferrin and ferritin, lowering their iron affinity. In addition, the acidic pH of blood favours the accumulation of ketone bodies like β-hydroxybutyrate [BHB], which strongly deregulates the chelation of iron. Therefore, this erratic combination enhances the growth of mucor in the host (Gebremariam et al., 2016). The host normal host defence mechanism against mucormycosis can be understood with the clinical statement that individuals with phagocytic dysfunction are at a higher risk for mucormycosis.
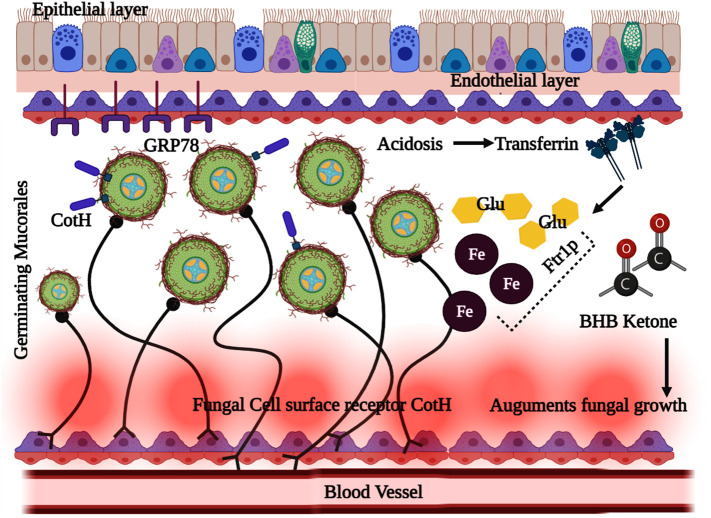
Meanwhile, another study revealed that neutropenia patients are more vulnerable to mucormycosis than AIDS (Sugar, 2005) because the neutrophils, particularly T lymphocytes, are critical for limiting fungal proliferation. Moreover, mononuclear and polymorphonuclear phagocytes can potentially kill the mucor fungi through a cascade of immune reactions modulated by cationic peptides and defensins (Ibrahim et al., 2012). The crucial association of phagocytic impairment with diabetic ketoacidosis (DKA) is reported to increase mucor infections. In the DKA, characterised by hyperglycemia and low pH, the phagocytes deteriorate, resulting in less functional chemotaxis and sub-standard phagocytosis through oxidative and non-oxidative mechanisms. The virulence factor of the Mucorales is boosted by acquiring the free iron content from the host, which exponentially increases the pathogenesis of the disease. This was supported by the clinical data of patients with DKA, where the free iron in the serum was elevated, which supported the fungal growth at acidic pH (Spellberg et al., 2005). Biochemical pathways were studied to understand the fungal pathogenesis, in which the glucose-regulated protein (GRP78) was identified as the receptor to mediate the penetration of Mucorales into endothelial cells. GRP78 is cellular and a chaperone protein present in the endoplasmic reticulum and expressed by glucose starvation. It was apparent that consistently elevated glucose and iron levels are noted in DKA, which improved the GRP78 expression and invasion of Mucorales into endothelial cells through receptor-dependent mechanism (Wang et al., 2009). The Mucorales' overall immunological processes and biochemical interaction in the hyperglycemic condition and DKA are illustrated in Fig. 4 .
Fig. 4.
Complex biochemical associations and immunomodulated interactions in hyperglycemia and ketoacidosis disorder leading to fatality in COVID -19 associated mucormycosis (CAM).
The failure of transferrin protein to sequester free iron in serum results in hyperglycemia and ketoacidosis. The ketone bodies β-hydroxy butyrate [BHB] along with the free iron molecules affects the immune response, while the glucose, free iron and BHB augmented the expression of fungal CotH which resulted in the proliferation of mucor inside the blood vessels.
8. Grimming sequela of COVID-19 with free iron
Higher levels of circulating biomarkers like ferritin, predominantly hyperferritinemia was observed in COVID-19 survivors. To obtain a deeper knowledge of the iron regulation and its impact at the cellular level, Sonnweber et al. (2020) ascertained the level of mRNA levels of important iron homeostasis mediators in the peripheral blood monolymphocyte culture found that the level of hepcidin is higher in patients who have severe to acute symptoms of COVID. Hepcidin sequesters iron in macrophages and enterocytes to promote ferritin movement intracellularly and lead to tissue damages (Daher et al., 2017). Toxicity due to iron overload in patients is usually treated with deferoxamine, a bacterial iron siderophore making them highly prone to infection due to mucormycosis (Boelaert et al., 1988).
9. Case reports on COVID 19 associated mucormycosis (CAM)
The COVID 19 infection has a peculiar threat from mild to fatal pneumonia and associated secondary bacterial or fungal infections. The mortality is high due to associated comorbidity and an immunocompromised condition which has paved the way for mucormycosis (Salehi et al., 2020). Among several other opportunistic infections, such as pulmonary aspergillosis, oropharyngeal candidiasis, jiroveci pneumonia, etc., a cluster of reports is available on rhino-orbital mucormycosis post-COVID-19 disease (Chowdhary et al., 2020). A recent article by (John et al., 2021) reported that most CAM cases are from India, accounting for 71%, with an accurate number of 140 cases/million (Prakash and Chakrabarti, 2019). This is due to several facts like the most significant population size and the second-largest country with adults between the age of 20–79 with proposed diabetes mellitus (IDF, 2019). 50% of the CAM cases were diabetic, 18% diabetic ketoacidosis, and 57% had suffered uncontrolled diabetes mellitus. With reference to the clinical reports, mucormycosis can occur in six different clinical presentations based on the location of fungal proliferation and distribution. They include the–rhinocerebral, pulmonary, cutaneous, gastrointestinal, disseminated (Spellberg et al., 2005). The first and the most common type is the rhinocerebral, followed by the pulmonary and cutaneous type (Petrikkos et al., 2012). Fig. 5 represented various types of mucormycosis observed among the recently reported cases worldwide.
Fig. 5.
Clinical representation of various types of COVID -19 associated mucormycosis (CAM) on the basis of case reports.
Recently, Sarkar and his co-workers from India presented a case report on the incidence of orbital mucormycosis with concurrent COVID-19 illness in 10 patients. All of them had diabetes, while four patients diagnosed with DKA and the five patients developed DKA during the corticosteroid therapy for COVID-19. The medications included intravenous dexamethasone and Liposomal Amphotericin B for mucormycosis. While four received Remdesivir, and nine was on ventilatory support. Among the cluster, four expired within the month, five survived but with irreversible vision loss, while only one patient recovered with favourable ocular and systemic outcomes (Sarkar et al., 2021). Several studies have come up with findings on the fungal superimposition or co-infection in COVID-19 patients. The studies disclosed that the lower levels of T lymphocytes, particularly CD4 and CD8 and higher IL-2 R, IL-6, IL-10, and TNF-α, were markedly notable in COVID 19 cases that favour the proliferation of mucor inside the host (Zhu et al., 2020). COVID-19 is not the predisposing factor for any mucormycosis. According to the case reports discussed in Table 1 , related short-term corticosteroid therapy was the actual influencing factor along with the comorbid conditions for the onset and progression of mucor infection. However, large data sets are still required to confirm the reasons for mucormycosis onset. Notably, the initial symptoms occurred in the period of 2- 28 days, which again can be correlated with the already existing diseases like diabetes, hypertension etc.; the table presented the review of the case reports that were available in the online resources when given the search tag COVID 19 + STERIODS + MUCORMYCOSIS, while the case reports presented in the table belonged to the period 2020–2021.
Table 1.
Representation of case reports on COVID-19 associated Mucormycosis with detailed prognosis.
| S. No. | Gender | Medical history | Stage of COVID -19 infection | Therapy adopted | Predisposing factor for mucormycosis | Time of onset of symptoms & clinical manifestation | Histological examination | Present condition | Country and reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. | M/60 | Diabetes | Severe | High-dose steroid for COVID 19. Methylprednisolone |
Hyperglycemic steroid for COVID-19 | 12 days; Periorbital facial pain, oedema and acute vision loss Diagnosed as Rhino-orbital mucormycosis |
Non-septate fungi | Death | India Mehta and Pandey, 2020 |
| 2. | F/33 | Diabetes Asthma hypertension |
Severe | No steroids Remdesivir Vancomycin |
DKA Diabetic ketoacidosis | 2 days; Necrosis in nasal and palate, left eye ptosis, confused mental status, ophthalmoplegia proptosis Diagnosed as Rhino-orbito-cerebral mucormycosis |
– | Death | USA Werthman-Ehrenreich, 2021 |
| 3. | M/22 | Pancreatitis | Severe | High-dose steroid for COVID Linezolid Meropenem |
Steroid for COVID 19 treatment | 27 days; Disseminated to lymph nodes, heart, brain, and kidney Diagnosed as Pulmonary mucormycosis in lungs |
Non-septate fungi | Death | United Kingdom Hanley et al., 2020 |
| 4. | M/49 | Normal | Severe | Dexamethasone Tocilizumab Remdesivir Ceftriaxone |
Steroid for COVID 19 treatment | 21 days; Necrotic empyema, spontaneous pneumothorax Diagnosed as Pulmonary mucormycosis with Bronchopulmonary fistula |
Non-septate fungi | Death | USA Placik et al., 2020 |
| 5. | M/66 | Hypertension | Severe | Hydroxychloroquine Lopinavir–ritonavir |
Lymphopenia | 21 days; Pulmonary infiltrates and parenchymal thickening of the whole left lung, cavitary lesions and pleural effusion, opacity of the left maxillary sinus Diagnosed as Pulmonary mucormycosis |
Non-septate fungi | Death | Italy Pasero et al., 2020 |
| 6. | M/86 | Hypertension | Severe | Hydrocortisone for COVID 19. Ceftriaxone Azithromycin Oseltamivir |
Steroid for COVID 19 treatment | 5 days; Gastric ulcers, acute diarrhoea, melena, severe anaemia, and fever. Diagnosed as Gastro mucormycosis |
Non-septate fungi | Death | Brazil do Monte Junior et al., 2020 |
| 7. | M/60 | Diabetes, asthma, hypertension, hyperlipidaemia | Severe | Dexamethasone Remdesivir Convalescent plasma therapy (single dose) |
Hyperglycemia, steroid for COVID-19 | 7 days; Oedema of the eyelids and conjunctival chemosis. Extensive opacification of right maxillary, ethmoid, and frontal sinuses Diagnosed as Rhino- orbital mucormycosis |
Non-septate fungi | Death | USA Mekonnen et al., 2021 |
| 8. | 40/W | None | Mild | Remdesivir Levofloxacin Dexamethasone |
Short-term corticosteroid therapy | 8 days; Opacifications of paranasal sinuses Diagnosed as Rhino-orbitocerebral mucormycosis |
Non-septate fungi | Death | Iran Veisi et al., 2021 |
| 9. | 54/M | Non-insulin-dependent diabetes mellitus (DM) | Severe | Remdesivir Levofloxacin Dexamethasone |
Short-term corticosteroid therapy | 12 days Unilateral opacifications of the left orbit and paranasal sinuses Diagnosed as rhino-orbital mucormycosis |
Non-septate fungi | Alive | Iran Veisi et al., 2021 |
| 10. | 38/M | Remdesivir Dexamethasone |
Short-term corticosteroid therapy | 18 days; Mucosal thickening involving left maxillary and ethmoid sinuses Diagnosed as sino-orbital mucormycosis |
No information Confirmed with CT scan |
Alive | India Maini et al., 2021 |
||
| 11. | 72/M | Steroidinduced diabetic, hypothyroid | Severe | Ramdevpir Methyl prednisolone convalescent plasma (2 doses) |
Impaired immune functioning |
9 days; Pneumothorax Diagnosed as pulmonary mucormycosis |
Non-septate hyphae | Alive | India Chennamchetty et al., 2021 |
| 12. | 41/M | Diabetes mellitus | Mild | Steroids and hydroxychloroquine | Developed diabetic ketoacidosis (DKA) | Tissue necrosis from angioinvasion and subsequent thrombosis Diagnosed as rhinocerebral mucormycosis |
Confirmed with CT scan | Alive | USA Alekseyev et al., 2021 |
| 13. | 32/F | Uncontrolled diabetes Left eye complete ptosis and left facial pain |
Mild | Not mentioned | Immunosuppression due to COVID 19 | Opacification of the left ethmoid, maxillary and frontal sinus Diagnosed as paranasal mucormycosis |
Confirmed with CT scan | Alive | India Saldanha et al., 2021 |
| 14. | 68/M | Heart transplant recipient Diabetes mellitus |
Severe | Remdesivir Hydroxychloroquine Convalescent plasma infusion (single dose) Methylprednisolone Prednisone taper |
Previously under immunosuppressive medication for transplantation | 175 days; Purplish skin discoloration with fluctuant swelling was noted in the right axilla. Diagnosed as cutaneous mucormycosis |
Non-septate hyphae | Death | India Khatri et al., 2021 |
The statistical evidence indicated that the developed countries had comparatively fewer chances of acquiring mucormycosis (Jeong et al., 2019) than the developing nations. True to the fact, the prevalence of mucormycosis in India is 0.14%, while the gross rate across the world is 0.005–1.7% (Chander et al., 2018). The cues for suspicion must be focussed on patients with diabetes, renal complications and any immunocompromised states (Werthman-Ehrenreich, 2021). However, the fatality and irreversible medical complications occur in intra-cranial, rhino-orbital cases with 50- 80% (Deutsch et al., 2019). The diagnosis of mucormycosis remains highly challenging, particularly necrosis, which is a visible symptom at the later stage of infection when the fungal dissemination is almost spread throughout the body. Delay or postponement of the treatment for mucormycosis exponentially increases the mortality rate from 35 to 65% (Werthman-Ehrenreich, 2021). The invasive fungal infections (IFI) in COVID 19 patients is increasing; however, the pathophysiology of secondary IFI is uncertain, and several mechanisms have been studied to understand their intervention and clinical findings presented as follows;
Firstly, COVID-19 is extensively connected to pulmonary parenchymal disease (Hanley et al., 2020); autopsy examination of COVID -19 cases showed substantial alveolar necrosis, vascular microthrombi formation, interstitial pulmonary infiltration. These causes weakening of the pulmonary system, and it may take several weeks to be cured, while the system is slowly revamping under immunosuppressive, secondary IFI infections like Aspergillosis, Rhizopus, and Mucormycosis shoot up and proliferate (Kanne et al., 2020).
Secondly, COVID-19 has a strong potential to impair the host immune system and cause significant reduction of CD4+, CD8+ T-lymphocyte counts and simultaneously elevate inflammatory factors like cytokines and interleukins (IL). The nidus or the inactive state of immune cells is utilised by the IFIs (G. Song et al., 2020). Critical COVID-19 patients require prolonged mechanical ventilation and extended stay in ICU, which are significant causes for secondary bacterial infections and IFIs.
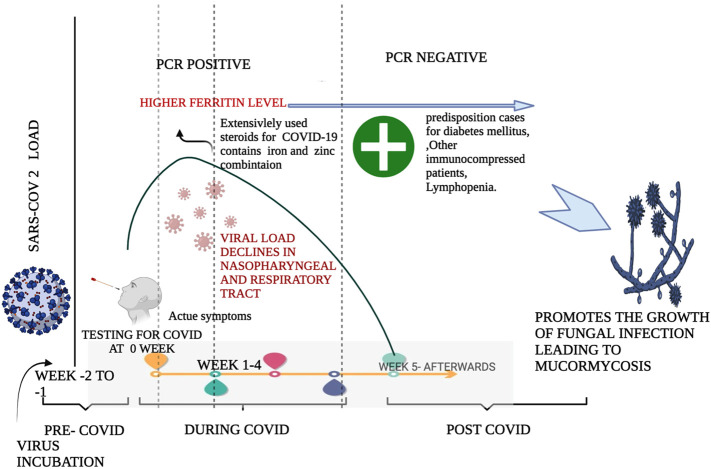
Thirdly, in the DKA and hyperglycemic COVID -19 patients, the Mucorales establish their harmful saprophytic trait of acquiring the iron from the host and utilising it for development. In DKA, free iron is available, taken through siderophores or iron permease, which further modulates the virulence of Mucorales. The corticosteroids further amplify this effect in comorbid conditions, and therefore in such a state of impaired neutrophils and failure of phagolysosome fusion, the host is exceptionally vulnerable to acquire mucormycosis (Hoang et al., 2020). Finally, there is self-immunosuppression entailed by COVID-19 infections in few cases, while the majority became inclined to IFIs like mucormycosis due to steroid-induced immunosuppression. The corticosteroid dexamethasone treatments and anti- IL mediated strategies (Nasir et al., 2020) are like double-edged swords; they have shown the potential to reduce mortality in COVID -19 patients under the high requirement of supplemental oxygen (Group, 2021) and simultaneously cause immunosuppression on long-term administration. Fig. 6 illustrated the timeline intervention of CAM in the post-infectious stage of COVID-19.
Fig. 6.
Illustrates the time line of COVID-19 infection in a person and the causes leading to post COVID-19 horrendous disease Mucormycosis.
Higher load of SARS-COV2 is observed during the 2nd and 3rd week of the COVID-19 pathological state. As higher dosage of steroids containing iron and zinc are administered in the acute symptom phase leads to higher level of iron in the serum of the patients. This higher level of iron and its protein ferritin is seen to be higher even after COVID-19 phase in the survivors' serum. The combination of steroids and iron level lays bedrock for the fungal infection. Since steroids makes the person immunocompromised by reducing the immune cells and higher levels of iron helps the fungus to thrive and migrate effortlessly in the host body.
The trials of RECOVERY published in June 2020 recommended and licenced the use of steroids for COVID-19 treatment. It specifically suggested using low doses for patients with moderate to severe COVID-19 infections for a short duration. However, due to compelling reasons and multiple complications, high doses of steroids can be administered for longer durations in exceptional cases. In such cases, close monitoring of the patients is required and should be promptly evaluated for onset diabetes and secondary infections. To put it in a nutshell, customised medication depending upon the host's heath history should be given, and a cavalier approach to use steroids should be discouraged.
10. Diagnosis and chemotherapy for COVID-19 associated Mucormycosis (CAM)
According to CDC reports, CAM is non-communicable (Jain and Madhu, 2020) but strictly opportunistic. There are no validated specific biomarkers for detecting the mucormycosis infection, and other quick diagnostic methods include histopathological examinations of the samples collected from the infected sites through biopsy (Chegini et al., 2020), KOH mount and Calcofluor stain. Since mucor is endo-saprophytic, it is challenging to culture in vitro. Hence biopsy mediated identification become the mainstay of diagnosis. In a certain presentation of mucormycosis, e.g. in pulmonary mucormycosis, radiological findings help distinguish the pattern of aspergillosis from other IFIs. Since no specific biomarker is available, a rule out technique of adopting negative galactomannan and beta-d-glucan pointers (Rudramurthy et al., 2017) can be used to narrow down identification to a certain limit. COVID-19 associated pulmonary aspergillosis (CAPA) can be detected using galactomannan in bronchoalveolar lavage, while its role in mucormycosis has not been confirmed. A computed tomography (CT) scan and Magnetic Resonance Imaging (MRI) are highly useful in the visualisation of the interstitial opacities, opaqueness in the sinusoidal and ethmoid regions (Sharma et al., 2021). Rhino-orbital mucormycosis is characterised by swelling in the para-sinus area, and rarely CAM can mimic the ophthalmic vein thrombosis (Turbin et al., 2020). A dearth of clinical suspicion and the difficult task of isolating the causative fungi perhaps lead to the erroneous diagnosis of mucormycosis (Garg et al., 2021).
Pasero et al. (2020) observed Mucorales-specific T-cells only in patients affected by invasive mucormycosis and proposed that they can be used as an additional diagnostic marker for IFIs, particularly Mucorales. In an in vivo study, the anti-GRP78 immune serum shielded the DKA mice from mucormycosis; however, we are uncertain about the protective effects of anti-GRP78 immune serum in neutropenic host from mucormycosis, but these experimental observations can provide foundation and clues for the novel therapeutic interventions in future (Ibrahim et al., 2012). The chemotherapeutic options include administering the liposomal amphotericin B as the first-line treatment, Posaconazole and combination therapy of liposomal amphotericin B or amphotericin B lipid complex caspofungin as second-line medications. However, in severe cases in rhino-cerebral mucormycosis, surgery is often required, along with medicinal therapy. Prognosis should be focussed to monitor the reversal of comorbid risk (Skiada et al., 2013). However, the antifungal chemotherapeutics may be continued for a gross of time depending on the recovery time.
11. Present updated commendation guidelines for mucormycosis
In 2017, guidelines for mucormycosis nursing was stated by The European Conference on Infections in Leukemia (ECIL), with an upgrade in 2019 by the European Confederation of Medical Mycology (EMM) recommends liposomal Amphotericin B (L-AmB) are primary treatment among grown-ups. In contrast, ECIL suggested the use of Amphotericin B lipid complex (ABLC) formulation in mucormycosis patients without central nervous system (CNS) involvement (Tissot et al., 2017). Pediatric groups can be administered with L-AmB and ABLC as primitive treatment. The dosage regime by both the society suggests 5–10 mg/kg of L-AmB and 10 mg/kg in CNS inclusion as primordial treatment mode (Cornely et al., 2019). The United States and Europe approve isavuconazole (ISZ) in 2015 as an effective oral and intervenous formulation to treat mucormycosis (Donnelley et al., 2016). Few antifungal drugs like SCY-078, Rezafungin, encochleated amphotericin B and orolofim are under investigation for the treatment (Van Daele et al., 2019). Lately, a peptide Cot H3 observed in the mucormycosis invasion in the endothelium through cell receptor GRP78 is obtained from Mucorales. Antibodies against the peptides act synergistically with other antifungal drugs to effectively protect diabetic/neutropenic animal models from mucormycosis (Gebremariam et al., 2019). Working on the interaction between the receptors and peptide will provide a gateway for effective treatment in mucormycosis in the coming future.
At present, mucormycosis treatment rely on quadruple fundamentals like diagnosing early, administering L-AmB, surgical removal of the infected tissues and increasing immunity(Cornely et al., 2019). The immunity is altered or reversed using IFN-γ and GM-CSF to elevate the production of TNF-α from peripheral mononuclear cells (PMNs) in response to R. microsporus and L. corymbifera or combining interleukin (IL-2 and 7), which stimulates t cells production specific for R. oryzae in healthy peoples suggesting cytokines can spike up immune response by recruiting cells of innate immune responses. However, the mechanism of cytokine-mediated immunity is still under investigation (Montaño and Voigt, 2020).
12. Common over the counter prophylactic measures for fungal infections
Fungal infection is a prevalent infectious disease caused due to inadequate hygiene, warmth and humidity, and compromised immune systems underlying various diseases. Few tabletop preventive measures used for decades to fight fungal infections in general, along with antifungal medications, are enriching the supply of good microbes in our body to prevent fungus growth. The source of good bacteria includes yoghurt and fermented foods rich in probiotics. Hygiene is one of the chief steps to keep infections at bay, washing the affected area with soap and water twice (skin injuries the entry route of fungus). They are using few products possessing antifungal properties like apple cider vinegar, tea tree oil, Oregano oil, coconut oil, turmeric, aloe vera, garlic, neem leaves, baking soda, hydrogen peroxide, ginger, ascorbic acid-rich products can be utilised and included to boost immune response in the body. Preventive measures can be taken to keep the infection at bay after recovering from COVID. In addition, maintain the glycemic index and including physical activities to maintain weight can aid in mitigating mucormycosis.
13. Conclusion
Being relatively rare yet a prime cause of burden in immune-suppressed peoples, mucormycosis accounts for the higher mortality rate. Novel immune-suppressing drugs are suspected as the major contributor to the increasing incidence of the present pandemic conditions, especially in developing countries like India. This is a perilous infection caused by a fungus of various species like Rhizopus, Mucor, and Lichtheimia (former Absidia). Tackling this horrendous infection can be done at its earlier stage, and regularly monitoring the immunocompromised patients can better diagnose the fungal development and immediate prognostic steps to prevent surgical removal of the organs/tissues. Emphasis must be given on health, hygiene, and the food consumed after recovery from COVID can help curb the second affliction.
Ethics committee approval
Not applicable.
Funding
This research received no external funding.
CRediT authorship contribution statement
Conceptualisation, B.B., A.M., K.R.R.R.; Writing-original draft preparation, K.P., H.K., A.M.; Literature search, selected bibliographic sources, K.R.R.R., U.I., M.P., M.E., K.P., V.A.; W.C.; Figures, K.P., A.M., B.B; B.B. and A.M were coordinated the working group; Supervision, Validation, Writing-review & editing, B.B., A.M., All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors were grateful and extended their appreciation to the authorities for their support.
Editor: Lotfi Aleya
References
- Ahmed M.H., Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr. Clin. Med. 2020:1–10. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J. Med. Cases. 2021;12(3):85. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arashiro T., Nakamura S., Asami T., Mikuni H., Fujiwara E., Sakamoto S., Miura R., Shionoya Y., Honda R., Furukawa K. SARS-CoV-2 and Legionella co-infection in a person returning from a Nile cruise. J. Travel Med. 2020;27(3) doi: 10.1093/jtm/taaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin C., Ibrahim A.S. Molecular mechanisms of mucormycosis—the bitter and the sweet. PLoS Pathog. 2017;13(8) doi: 10.1371/journal.ppat.1006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D.E. Basic and clinical pharmacology of glucocorticosteroids. Anesth. Prog. 2013;60(1):25–32. doi: 10.2344/0003-3006-60.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert J., Van Roost G., Vergauwe P., Verbanck J., De Vroey C., Segaert M. The role of desferrioxamine in dialysis-associated mucormycosis: report of three cases and review of the literature. Clin. Nephrol. 1988;29(5):261–266. [PubMed] [Google Scholar]
- Boelaert J.R., de Locht M., Van Cutsem J., Kerrels V., Cantinieaux B., Verdonck A., Van Landuyt H.W., Schneider Y.-J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Invest. 1993;91(5):1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy P.K. What's so special about Osler? Yale J. Biol. Med. 1980;53(3):213. [PMC free article] [PubMed] [Google Scholar]
- Cano E.J., Fuentes X.F., Campioli C.C., O’Horo J.C., Saleh O.A., Odeyemi Y., Yadav H., Temesgen Z. Impact of corticosteroids in COVID-19 outcomes: systematic review and meta-analysis. Chest. 2021;159(3):1019–1040. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander J., Kaur M., Singla N., Punia R., Singhal S.K., Attri A.K., Alastruey-Izquierdo A., Stchigel A.M., Cano-Lira J.F., Guarro J. Mucormycosis: battle with the deadly enemy over a five-year period in India. J. Fungi. 2018;4(2):46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegini Z., Didehdar M., Khoshbayan A., Rajaeih S., Salehi M., Shariati A. Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: a systematic review of case reports and case series. Mycoses. 2020;63(12):1264–1282. doi: 10.1111/myc.13187. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennamchetty V.K., Adimulapu S., Kola B.P., Rao M.R. Post-COVID pulmonary mucormycosis-a case report. IP Indian J. Immunol. Respir. Med. 2021;6(1):62–66. [Google Scholar]
- Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020;26(11):2694. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C., Dannaoui E., Hochhegger B., Hoenigl M., Jensen H.E., Lagrou K., Lewis R.E. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti-and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher R., Manceau H., Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Med. 2017;46(12):e272–e278. doi: 10.1016/j.lpm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Deutsch P.G., Whittaker J., Prasad S. Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina. 2019;55(7):319. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Monte Junior E.S., Dos Santos M.E.L., Ribeiro I.B., de Oliveira Luz G., Baba E.R., Hirsch B.S., Funari M.P., De Moura E.G.H. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin. Endosc. 2020;53(6):746. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelley M.A., Zhu E.S., Thompson G.R., 3rd Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect. Drug Resist. 2016;9:79. doi: 10.2147/IDR.S81416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J., Harris J., Gade L., Sehulster L., Newhouse E., O’Connell H., Noble-Wang J., Rao C., Balajee S.A., Chiller T. Mucormycosis outbreak associated with hospital linens. Pediatr. Infect. Dis. J. 2014;33(5):472–476. doi: 10.1097/INF.0000000000000261. [DOI] [PubMed] [Google Scholar]
- Fan C., Lu W., Li K., Ding Y., Wang J. ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front. Med. 2021;7:1045. doi: 10.3389/fmed.2020.563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmakiotis D., Kontoyiannis D.P. Mucormycoses. Infect. Dis. Clin. N. Am. 2016;30(1):143–163. doi: 10.1016/j.idc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Feldman C., Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;13(1):1–15. doi: 10.1186/s41479-021-00083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C.E., Revell P.A., Campbell J.R., Marquez L. Healthcare-associated pediatric cutaneous mucormycosis at Texas Children’s Hospital, 2012–2019. Paediatr. Infect. Dis. J. 2021;40(8):746–748. doi: 10.1097/INF.0000000000003153. [DOI] [PubMed] [Google Scholar]
- Fraser E. Long term respiratory complications of covid-19. BMJ (Clinical research Ed.) 2020;370 doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- Frazier K.M., Hooper J.E., Mostafa H.H., Stewart C.M. SARS-CoV-2 virus isolated from the mastoid and middle ear: implications for COVID-19 precautions during ear surgery. JAMA Otolaryngol. Head Neck Surg. 2020;146(10):964–966. doi: 10.1001/jamaoto.2020.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangneux J.-P., Bougnoux M.-E., Dannaoui E., Cornet M., Zahar J. Invasive fungal diseases during COVID-19: we should be prepared. J. Mycol. Med. 2020;30(2) doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., Puri G.D., Chakrabarti A., Agarwal R. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021:1–10. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam T., Alkhazraji S., Soliman S.S., Gu Y., Jeon H.H., Zhang L., French S.W., Stevens D.A., Edwards J.E., Filler S.G. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci. Adv. 2019;5(6) doi: 10.1126/sciadv.aaw1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam T., Lin L., Liu M., Kontoyiannis D.P., French S., Edwards J.E., Filler S.G., Ibrahim A.S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Invest. 2016;126(6):2280–2294. doi: 10.1172/JCI82744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, R.C Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Gatheral T., Williams C. Procalcitonin for patient stratification and identification of bacterial co-infection in COVID-19. Clin. Med. 2020;20(3):e47. doi: 10.7861/clinmed.Let.20.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., Thursz M., Manousou P., Corbett R., Goldin R. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C., Mayer K., Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020 Jul 14;95(2):77–84. doi: 10.1212/WNL.0000000000009673. [DOI] [PubMed] [Google Scholar]
- Hoang K., Abdo T., Reinersman J.M., Lu R., Higuita N.I.A. A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well-controlled diabetic patient. Med. Mycol. Case Rep. 2020;29:22–24. doi: 10.1016/j.mmcr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S. Bacterial and fungal co-infection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A.S. Host cell invasion in mucormycosis: role of iron. Curr. Opin. Microbiol. 2011;14(4):406–411. doi: 10.1016/j.mib.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A.S., Gebremariam T., Lin L., Luo G., Husseiny M.I., Skory C.D., Fu Y., French S.W., Edwards J., John E., Spellberg B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010;77(3):587–604. doi: 10.1111/j.1365-2958.2010.07234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012;54(suppl_1):S16–S22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF . 2019. IDF Diabetes Atlas.https://diabetesatlas.org/en/resources/ [Google Scholar]
- Jain N., Madhu S.V. Long term management challenges in diabetic patients with rhino-orbito-cerebral mucormycosis. Clin. Diabetol. 2020;9(2):138–140. [Google Scholar]
- Jeong W., Keighley C., Wolfe R., Lee W., Slavin M., Kong D., Chen S.-A. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. Journal of Fungi. 2021;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Radiological Society of North America; 2020. Essentials for Radiologists on COVID-19: An Update—Radiology Scientific Expert Panel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrul-Hasan A.B., Selim S. Mucormycosis, the deadly new worry to COVID-19 pandemic. Mymensingh Med. J. 2021;30(3):874–880. [PubMed] [Google Scholar]
- Khatri A., Chang K.-M., Berlinrut I., Wallach F. Mucormycosis after coronavirus disease 2019 infection in a heart transplant recipient–case report and review of literature. J. Med. Mycol. 2021;31(2) doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., Hallek M., Jung N., Klein F., Persigehl T. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth F., Kontoyiannis D.P. Therapeutic challenges of non-aspergillus invasive mold infections in immunosuppressed patients. Antimicrob. Agents Chemother. 2019;63(11) doi: 10.1128/AAC.01244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoq L., Vincent P., Lavoie-Lamoureux A., Lavoie J.-P. Genomic and non-genomic effects of dexamethasone on equine peripheral blood neutrophils. Vet. Immunol. Immunopathol. 2009;128(1–3):126–131. doi: 10.1016/j.vetimm.2008.10.303. [DOI] [PubMed] [Google Scholar]
- Lin E., Moua T., Limper A.H. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443–448. doi: 10.1007/s15010-017-0991-6. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int. J. Surg. Case Rep. 2021;82 doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., Simko J.P., Winn B.J. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal. Plast. Reconstr. Surg. 2021;37(2) doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre-Aguilar I.B., Cabrera-Quintero A.J., Zentella-Dehesa A. Genomic and non-genomic effects of glucocorticoids: implications for breast cancer. Int. J. Clin. Exp. Pathol. 2015;8(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Montaño D.E., Voigt K. Host immune defense upon fungal infections with mucorales: pathogen-immune cell interactions as drivers of inflammatory responses. Journal of Fungi. 2020;6(3):173. doi: 10.3390/jof6030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S. Post-acute COVID-19 syndrome. Nat. Med. 2021:1–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir N., Farooqi J., Mahmood S.F., Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63(8):766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Siccardi G., Migliarini A., Cancelli F., Carnevalini M., D’Andria M., Attilia I., Danese V.C., Cecchetti V., Romiti R. Co-infection of SARS-CoV-2 with chlamydia or mycoplasma pneumoniae: a case series and review of the literature. Infection. 2020;48(6):871–877. doi: 10.1007/s15010-020-01483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Singh B., Bhadada S.K., Banerjee M., Bhogal R.S., Hage N., Kumar A. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021 doi: 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L., Simeo R., Buselli A., Rizzo D., Bussu F. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Meng H., Sun Y., Xiao Y., Zhang H., Lv K., Cai B. Clinical features of pulmonary mucormycosis in patients with different immune status. J. Thorac. Dis. 2019;11(12):5042. doi: 10.21037/jtd.2019.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012;54(suppl_1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- Petrikkos G., Tsioutis C. Recent advances in the pathogenesis of mucormycoses. Clin. Ther. 2018;40(6):894–902. doi: 10.1016/j.clinthera.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020;15(11):2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza L., Vallerini D., Barozzi P., Riva G., Forghieri F., Zanetti E., Quadrelli C., Candoni A., Maertens J., Rossi G. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood J. Am. Soc. Hematol. 2011;118(20):5416–5419. doi: 10.1182/blood-2011-07-366526. [DOI] [PubMed] [Google Scholar]
- Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J. Fungi. 2019;5(1):26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Prajith V., Biatris P.S. Therapeutic role of corticosteroids in COVID-19: a systematic review of registered clinical trials. Future J. Pharm. Sci. 2021;7(1):1–18. doi: 10.1186/s43094-021-00217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., Cidlowski J.A. Corticosteroids: mechanisms of action in health and disease. Rheum. Dis. Clin. 2016;42(1):15–31. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammaert B., Lanternier F., Zahar J.R., Dannaoui E., Bougnoux M.E., Lecuit M., Lortholary O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012;54(suppl_1):S44–S54. doi: 10.1093/cid/cir867. [DOI] [PubMed] [Google Scholar]
- Reid G., Lynch J.P., III, Fishbein M.C., Clark N.M. Mucormycosis, Seminars in Respiratory and Critical Care Medicine. Thieme Medical Publishers; 2020. pp. 099–114. [DOI] [PubMed] [Google Scholar]
- Roberts M. BBC News; 2020. Coronavirus: Dexamethasone Proves First Life-saving Drug. [Google Scholar]
- Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., Sein M., Sein T., Chiou C.C., Chu J.H. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Roilides E., Antachopoulos C., Simitsopoulou M. Pathogenesis and host defence against mucorales: the role of cytokines and interaction with antifungal drugs. Mycoses. 2014;57:40–47. doi: 10.1111/myc.12236. [DOI] [PubMed] [Google Scholar]
- Rudramurthy S., Singh G., Hallur V., Verma S., Chakrabarti A. High fungal spore burden with predominance of Aspergillus in hospital air of a tertiary care hospital in Chandigarh. Indian J. Med. Microbiol. 2017;34(4):529–532. doi: 10.4103/0255-0857.195359. [DOI] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha M., Reddy R., Vincent M.J. Paranasal mucormycosis in COVID-19 patient. Indian J. Otolaryngol. Head Neck Surg. 2021:1–4. doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R., Buisson N., Knight S., Dancis A., Camadro J.-M., Lesuisse E. Haemin uptake and use as an iron source by Candida albicans: role of CaHMX1-encoded haem oxygenase. Microbiology. 2003;149(3):579–588. doi: 10.1099/mic.0.26108-0. [DOI] [PubMed] [Google Scholar]
- Saraya M.A., Amal A.E.-A.I. Dexamethasone as adjunctive therapy for treatment of varicella pneumonia. Egypt. J. Chest Dis. Tuberc. 2012;61(3):9–13. [Google Scholar]
- Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021;69(4):1002. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M., Ahmadikia K., Badali H., Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID‐19: A clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185(4):607–611. doi: 10.1007/s11046-020-00472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021:1–6. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiada A., Lanternier F., Groll A.H., Pagano L., Zimmerli S., Herbrecht R., Lortholary O., Petrikkos G.L. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3) Haematologica. 2013;98(4):492. doi: 10.3324/haematol.2012.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Liang G., Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020:1–8. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Liu P., Shi X., Chu Y., Zhang J., Xia J., Gao X., Qu T., Wang M. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69(6):1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., Kurz K., Koppelstätter S., Haschka D., Petzer V. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir. Res. 2020;21(1):1–9. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Edwards J., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar A. In: Principles and Practice of Infectious Diseases. Mandell G.L., B.J., Dolin R., editors. Elsevier; Philadelphia, PA: 2005. Agents of mucormycosis and related species; p. 2979. [Google Scholar]
- Tissot F., Agrawal S., Pagano L., Petrikkos G., Groll A.H., Skiada A., Lass-Flörl C., Calandra T., Viscoli C., Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbin R.E., Wawrzusin P.J., Sakla N.M., Traba C.M., Wong K.G., Mirani N., Eloy J.A., Nimchinsky E.A. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit. 2020;39(4):305–310. doi: 10.1080/01676830.2020.1768560. [DOI] [PubMed] [Google Scholar]
- Van Daele R., Spriet I., Wauters J., Maertens J., Mercier T., Van Hecke S., Brüggemann R. Antifungal drugs: what brings the future? Med. Mycol. 2019;57(Supplement_3):S328–S343. doi: 10.1093/mmy/myz012. [DOI] [PubMed] [Google Scholar]
- van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit. Care. 2020;24(1):1–22. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur. J. Ophthalmol. 2021 doi: 10.1177/11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verroken A., Scohy A., Gérard L., Wittebole X., Collienne C., Laterre P.-F. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit. Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Wey S., Zhang Y., Ye R., Lee A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009;11(9):2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with pre-term delivery. Clin. Infect. Dis. 2020;71(15):844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. e265–264. e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton D. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Qi L., Chi X., Yang J., Wei X., Gong E., Peh S., Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol. Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Li J., Liu H., Han N., Ju J., Kou Y., Chen L., Jiang M., Pan F., Zheng Y. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8(1):1–8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K.I., Feng G., Liu W.Y., Targher G., Byrne C.D., Zheng M.H. Extrapulmonary complications of COVID-19: a multisystem disease? J. Med. Virol. 2021;93(1):323–335. doi: 10.1002/jmv.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B., Zhu F., Zhu B., Cui L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]