Dear Editor,
The primal 2021 witnessed the roll-out of a slew of vaccines against the coronavirus disease 2019 (COVID-19) all over the world. These include the novel messenger RNA, DNA, and viral vector vaccines, as well as the classic ones like inactivated and attenuated viruses or subunit vaccines [1], which are inoculated by the intramuscular route. There are 108 vaccines under clinical development in the various phases of evaluation status and 184 vaccines in pre-clinical development. Out of these, just six vaccines offered via an intranasal route of administration are available in the clinical development phase (Table 1 ).
Table 1.
Intranasal COVID-19 vaccines in clinical development phase.
| Vaccine platform | Type of candidate vaccine | Number of doses | Schedule (days) | Developer | Phase |
|---|---|---|---|---|---|
| Viral vector: Replicating (VVr) | DelNS1-2019-nCoV-RBD-OPT1 (Intranasal flu-based-RBD) | 2 | 0 + 28 | University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | 2 |
| Live attenuated virus (LAV) | COVI-VAC | 1–2 | 0 or 0 + 28 | Codagenix/Serum Institute of India | 1 |
| Protein subunit (PS) | CIGB-669 (RBD + AgnHB) | 3 | 0 + 14+28 or 0 + 28+56 | Center for Genetic Engineering and Biotechnology (CIGB) | 1/2 |
| Viral vector: Non-replicating (VVnr) | BBV154, Adenoviral vector COVID-19 vaccine | 1 | 0 | Bharat Biotech International Limited | 1 |
| Live attenuated virus (LAV) | MV-014-212, a live attenuated vaccine that expresses the spike (S) protein of SARS-CoV-2 | 1 | 0 | Meissa Vaccines, Incorporated | 1 |
| Viral vector: Non-replicating (VVnr) | PIV5 vector that encodes the SARS-CoV-2 spike protein | 1 | 0 | Cyanvac LLC | 1 |
Respiratory pathogens like the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, enter the host through the nose and upper respiratory tract. The nasal compartment is more susceptible to SARS-CoV-2 infection and can act as the virus's first reservoir for seeding the lungs. As the first line of defense against viral invasion, pre-existing protection within the respiratory system plays a salient role. Despite the mucosal immunity's well-known importance, most of the vaccinations have been designed to induce circulating humoral immunity rather than mucosal immunity. However, the intranasal vaccination for the COVID-19 is capable of preventing the SARS-CoV-2 transmission at the source and is an attractive aspect to encourage systemic as well as mucosal immunity [2]. The hypothesis of the protection accorded by the intranasal vaccination is not new, since the US Food and Drug Administration (FDA) had approved the live attenuated influenza vaccine (LAIV) in 2003. The salient properties of the intranasal COVID-19 vaccines reflecting their advantageous and disadvantageous aspects have been delineated below.
1. Advantages of intranasal COVID-19 vaccines
-
1.
Broad immune response: The intranasal vaccines induce both innate and adaptive components of the immune system, including antigen-specific memory T and B cells which are helpful in the induction of a broad immune response-neutralizing IgG, mucosal IgA, and T cell responses [3].
-
2.
Needle-free administration: Intranasal inoculation is a minimally invasive, simple and needle-free mode of administration. This hassle-free method contributes to further compliance and fewer medical complications (localized infection and/or pain) as opposed to traditional methods that involve needle-based skin puncture. Furthermore, the risk of spreading blood-borne infections is also subdued, as can occur with contaminated injection needles. In addition, injection pain is one of the reasons for vaccine hesitancy among people thus; the non-invasive intranasal vaccines would reduce the vaccine hesitancy and improve the rate of immunization against SARS-CoV-2 globally [4].
-
3.
Antigen delivery to the site of infection: These vaccines deliver antigen to the site of virus entry and infection (nasal passage), leading to the elicitation of mucosal immunity in the respiratory tract. Mucosal immunity protects the mucosal surfaces of the lungs and upper airways, which are common sites for invasion by the SARS-CoV-2 [3] (Fig. 1 ).
-
4.
More convenient: These vaccines would be easier to store, transport, and administer. Some of these vaccines would even be stable at room temperature, making them easier to ship and potentially improving the vaccine coverage in remote areas, especially at the village level.
-
5.
Easy administration: It is easier to administer the vaccine in the form of a nasal spray, and being non-invasive and needle-free, children and people unable to cope with the sight of a needle may benefit from this. Most of these vaccines do not require double-dose administration like other intramuscularly administered COVID-19 vaccines, such as Covishield (Oxford-AstraZeneca), Covaxin (BBV152), Sputnik V (Gam-COVID-Vac) etc. Further, waiving off the need of trained staff, these can be administered at any small healthcare unit.
-
6.
Additional mode of protection: In the respiratory mucosa, vaccine-elicited IgA and resident memory B and T cells provide an effective barrier to infection; and, indeed if the infection does take place, perhaps by a viral variant, the cross-reactive resident memory B and T cells, which encounter antigen earlier and respond more quickly than systemic memory cells, would be able to obstruct viral replication and reduce viral shedding and transmission [5].
-
7.
High compliance: As per the past experiences, these intranasal vaccines may give good results as compared to the intramuscular vaccines in the children as observed in the live attenuated influenza vaccine (LAIV), which is generally superior to the intramuscular vaccination in the children [4]. Therefore, the intranasal vaccines may ideally suit children as well as adults.
-
8.
Global roll-out: These vaccines would also be able to fulfill the global demand for COVID-19 vaccines, as many of the countries like India, being more populous, face a shortage of COVID-19 vaccines.
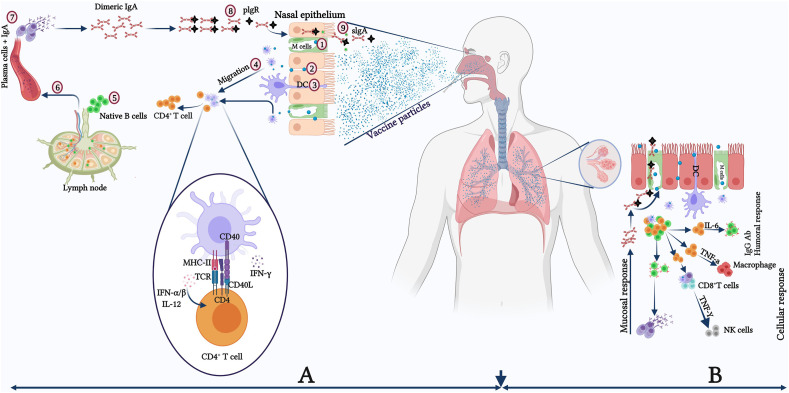
Fig. 1.
Schematic model of the potential effect of nasal vaccines on the upper and lower respiratory tract for the generation of mucosal and systemic immunity.(A) Protective immune responses in the nasopharynx-associated lymphoid tissue (NALT), with the virus-mediated reaction resulting mainly from secretory IgA antibodies generated by mucosal epithelial cells. (1) M cells actively take up antigens or penetrate epithelial junctions passively (2), DCs that extend into the lumen also take up antigens. (3). DCs acquire antigens and transport them to nearby lymphoid follicles or lymph nodes, where they are presented to CD4+ T-cells (4). CD4+ T cells activated by DCs activate naive B cells, causing them to switch isotype and become antigen-specific IgA+ committed B cells (5). These IgA+ B cells migrate from the lymph node to the blood circulations (6). Finally, IgA+ B cells exit the blood and enter towards the effector site. They undergo final differentiation and maturations producing IgA+ plasma cells (process enhanced by IL-5 and IL-6, a subset of Th2 cells) and ultimately form dimeric or polymeric IgA (7). The dimeric or polymeric IgA binds with pIgR in the basolateral region to form sIgA (8) and further transcytoses towards the apical side of the luminal surface (9). (B) Humoral immune response in the lower respiratory tract with bronchus-associated lymphoid tissue (BALT) having humoral as well as mucosal/local immune responses. DC: Dendritic cell; IL: Interleukin; M cell: Microfold cell; pIgR: Polymeric immunoglobulin receptor; sIgA: Secretory IgA; Th: T-helper cell.
2. Disadvantages of intranasal COVID-19 vaccines
-
1.
Sterilizing immunity: Though the intranasal vaccines induce both IgA and IgG antibodies, thereby potentially providing “sterilizing immunity” in the upper respiratory tract (local immunity), it is seen with some candidates that systemic IgG antibodies are not as robust in the lower respiratory tract as at the upper airways.
-
2.
Ineffective long-lasting immunity: Another drawback is their inability to produce effective, long-lasting immunity, thus resulting in a faster waning of immunity. The sticky mucous, which serves as a barrier for pathogens in the respiratory system, may interfere with vaccine access and immune activation resulting in poor immunogenicity and faster diminishing protective immunity
-
3.
Child administration problems: Though this route seems more suitable for the administration of vaccines to young children, however in practice, it has been noticed in the case of the intranasal flu vaccines that kids sneeze right after administration, thus making it far too risky an attempt for something so virulent and lethal. Seasonal allergies in young children also resist smooth administration.
-
4.
Safety-related issues: Another issue is the safety of these vaccines. Since the live attenuated virus (a virus weakened so that it cannot produce disease in an individual) is employed in these vaccines, there is a rare risk of reversion of the attenuation, so the virus can regain its ability to cause disease in the individual. This phenomenon, although extremely rare, has also been observed in the oral polio vaccine. It takes a substantially long time to prove the safety of live attenuated vaccines. An intranasal flu vaccine by a Swiss company, Berna Biotech, was discontinued after being linked with a higher risk of Bell's palsy [6].
In conclusion, the advantages of intranasal COVID-19 vaccines conceivably outweigh its drawbacks. The mucosal and systemic immunity elicited by the intranasal immunization against SARS-CoV-2 can serve as all-round protection against infection as well as transmission. As the global efforts in the form of scientific and frontline involvements are underway to restrict the ongoing COVID-19 pandemic, the intranasal vaccines may serve as a boon by sharing the burden of injectable double-dose vaccines, by serving as an alternative in the present time of limited and inequitable vaccine supplies, as well as a booster to intramuscular vaccines when the supply limitations would be overcome in the coming future.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Sources of funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
This article does not require any human/animal subjects to acquire such approval.
Trial registry number
Name of the registry: Not applicable.
Unique Identifying number or registration ID: Not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Author contributions
Om Prakash Choudhary: Conceptualization, Data Curation, Visualization, Writing - Original Draft, Writing - review & editing. Priyanka: Conceptualization, Supervision, Writing - Original Draft, Writing - review & editing. Teroj A. Mohammed : Writing - Original Draft, Writing - review & editing. Indraj Singh: Writing - Original Draft, Writing - review & editing. All authors critically reviewed and approved the final version of the manuscript.
Guarantor
Om Prakash Choudhary, Assistant Professor, Department of Veterinary Anatomy and Histology, College of Veterinary Sciences and Animal Husbandry, Central Agricultural University (I), Selesih, Aizawl-796015, Mizoram, India.
Declaration of competing interest
All authors report no conflicts of interest relevant to this article.
Acknowledgements
All the authors acknowledge and thank their respective Universities and Institutes. The figure has been created with BioRender (https://biorender.com/).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijsu.2021.106119.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kyriakidis N.C., López-Cortés A., Vásconez González E., Barreto Grimaldos A., Ortiz Prado E. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gamerra M., de Corso E., Cantone E. COVID-19: the crucial role of the nose. Braz. J. Otorhinolaryngol. 2021;87(1):118–119. doi: 10.1016/j.bjorl.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K., Agnihothram S., Baric R.S., David C.S., Perlman S. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44(6):1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wake A.D. The willingness to receive COVID-19 vaccine and its associated factors: “vaccination refusal could prolong the war of this pandemic”- a systematic review. Risk Manag. Healthc. Pol. 2021;14:2609–2623. doi: 10.2147/RMHP.S311074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lund F.E., Randall T.D. Scent of a vaccine. Science. 2021;373(6553):397–399. doi: 10.1126/science.abg9857. [DOI] [PubMed] [Google Scholar]
- 6.Mutsch M., Zhao W., Rhodes P., Bopp M., Chen R.T., Linder T., Spyr C., Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.