Abstract
As the first authorized COVID-19 vaccine in Japan, the BNT162b2 mRNA COVID-19 vaccine is utilized for mass vaccination. Although efficacy has been proved, real-world evidence on reactogenicity in Japanese personnel is needed to prepare the public. Healthcare workers in a large academic hospital in Japan received two doses of the Pfizer-BioNTech vaccine from March 17 to May 19, 2021. Online questionnaires were distributed to registered recipients following each dose, from day 0 through day 8. Primary outcomes are the frequency of reactogenicity including local and systemic reactions. Length of absence from work was also analyzed. Most recipients self-reported reactogenicity after the first dose (97.3%; n = 3254; mean age [36.4]) and after the second dose (97.2%; n = 3165; mean age [36.5]). Systemic reactions following the second dose were substantially higher than the first dose, especially for fever (OR, 27.38; 95% CI, [22.00–34.06]; p < 0.001), chills (OR, 16.49; 95% CI, [13.53–20.11]; p < 0.001), joint pain (OR, 8.49; 95% CI, [7.21–9.99]; p < 0.001), fatigue (OR, 7.18; 95% CI, [6.43–8.02]; p < 0.001) and headache (OR, 5.43; 95% CI, [4.80–6.14]; p < 0.001). Reactogenicity was more commonly seen in young, female groups. 19.3% of participants took days off from work after the second dose (2.2% after the first dose), with 4.7% absent for more than two days. Although most participants reported reactogenicity, severe cases were limited. This study provides real-world evidence for the general population and organizations to prepare for BNT162b2 mRNA COVID-19 vaccination in Japan and other countries in the region.
Keywords: Reactogenicity, Adverse reaction, BNT162b2, COVID-19 vaccine, Japan
Note
Since the outbreak of the coronavirus (COVID-19) pandemic, Japan's citizens have practiced strict personal hygiene including masks and hand-washing. Japan has declared a nationwide State of Emergency twice since the outbreak, with 10 prefectures currently under a third round as of June 18, 2021. Nonetheless, the disease continues to spread in the country. Vaccination is expected to be a critical step in fighting the disease. Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine (thereafter: Pfizer-BioNTech COVID-19 vaccine) was the first to be authorized for emergency use by the Ministry of Health, Labor and Welfare of Japan on February 14, 2021 [1]. However, due to the high awareness of reactogenicity following vaccines such as Human Papillomavirus (HPV) vaccines in the past, the Japanese people are cautious about COVID-19 vaccination [2]. Vaccine safety has been shown to be important to the Japanese public.
High efficacy of the Pfizer-BioNTech COVID-19 vaccine (95%) was observed in clinical trials and supported in real world vaccination efforts [[3], [4], [5]]. However, previous studies showed reactogenicity following Pfizer-BioNTech COVID-19 vaccines [3,[6], [7], [8]]. Severe allergic reactions after receiving this mRNA-based vaccine were reported as well [9]. Because the frequency and degrees of reactogenicity may vary depending on ethnicity and region, assessment of the adverse reactions is an important step toward preparation of Japan's general population to receive the vaccine. Reactogenicity reflecting differences in age groups and gender is informative, as well as to provide data to organizations anticipating possible absences from work following each worker's dose of the vaccine.
Healthcare workers in a large academic hospital (1051 beds) voluntarily registered to receive COVID-19 vaccines from March 17 to April 23 for the first dose, and April 7 to May 19 for the second dose. Pfizer-BioNTech COVID-19 vaccines were administered. According to a previous study targeting the healthcare workers in the same hospital in the summer of 2020, 0.34% (n = 4147) were found to have antibody evidence of previous SARS-CoV-2 infection [10]. A healthcare questionnaire was distributed prior to the vaccination [11]. Those with medicine/food allergies, previous experience of spasm, or anaphylaxis following vaccination, were asked to remain for 30 min in the vaccination setting.
An online questionnaire survey was conducted with approval of the institutional review board of this academic hospital. (No. 2021055). Questionnaires were distributed to registered vaccine recipients from Day 0 to Day 8 after each dose. Reactogenicity was divided into two groups: injection site reactions such as pain, swelling, burning sensation, redness, induration, and itching; systemic reactions such as fatigue, myalgia (muscle ache), headache, fever (self-reported; ≧37.5 °C), chills, joint pain, and nasal discharge. Anaphylaxis cases were asked to be self-reported, then confirmed by researchers' review of patient treatment records, and subsequently recorded based on Brighton Collaboration's definitions [12]. Consequences of reactogenicity such as any work absences were asked to be self-reported as well.
Primary outcomes are the frequencies of reactogenicity after the first and second dose. Multivariate analysis was performed to assess differences in reactogenicity by sex, age and between the two doses. In addition, the average length of absence from work due to adverse reactions was assessed. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 27 (IBM Japan). Two-sided p-values less than 0.05 were considered statistically significant.
3381 healthcare workers received the first dose of the Pfizer-BioNTech vaccine from March 17 to April 23, 2021. 3354 received the second dose from April 7 to May 19, 2021. After the first dose, 3254 (96.2%; mean age [36.4]) healthcare workers participated in the questionnaire survey; after the second dose, 3165 (94.4%; mean age [36.5]) participated. Characteristics of the participants and reactogenicity are shown in Table 1 . Most participants reported an injection site reaction (91.3% after dose 1; 92.1% after dose 2) or a systemic reaction (75.1% after dose 1; 86.8% after dose 2) during the days 0–8 after vaccination.
Table 1.
Participants’ characteristics and reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine.
| No. (%) |
||
|---|---|---|
| Dose 1 (n = 3254) | Dose 2 (n = 3165) | |
| No. of recipients | 3381 | 3354 |
| No. of respondents | 3254 (96.2) | 3165 (94.4) |
| Demographic characteristics | .. | .. |
| Age Groups | ||
| ≤29 | 1178 (36.2) | 1132 (35.8) |
| 30–39 | 953 (29.3) | 926 (29.3) |
| 40–49 | 632 (19.4) | 624 (19.7) |
| 50–59 | 329 (10.1) | 324 (10.2) |
| 60–69 | 138 (4.2) | 137 (4.3) |
| ≥70 | 24 (0.8) | 22 (0.7) |
| Sex | .. | .. |
| male | 1116 (34.3) | 1091 (34.5) |
| female | 2138 (65.7) | 2074 (65.5) |
| Occupation | ||
| doctor | 926 (28.5) | 899 (28.4) |
| nurse | 1162 (35.7) | 1129 (35.7) |
| pharmacist | 100 (3.1) | 100 (3.2) |
| administrative staff | 1066 (32.8) | 1037 (32.8) |
| Reactogenicity | .. | .. |
| Any | 3165 (97.3) | 3077 (97.2) |
| Injection site reactions (any) | 2972 (91.3) | 2915 (92.1) |
| pain | 2908 (89.4) | 2736 (87.3) |
| swelling | 1001 (30.8) | 1359 (42.9) |
| burning sensation | 930 (28.6) | 1194 (37.7) |
| redness | 494 (15.2) | 840 (26.5) |
| induration | 545 (16.7) | 606 (19.1) |
| itching | 327 (10.0) | 514 (16.2) |
| Systemic reactions (any) | 2445 (75.1) | 2748 (86.8) |
| fatigue | 901 (27.7) | 2304 (72.8) |
| myalgia (muscle ache) | 2132 (65.5) | 2035 (64.3) |
| headache | 463 (14.2) | 1447 (45.7) |
| fever | 95 (2.9) | 1354 (42.8) |
| - low-grade fever (≥37.5°C, but < 38.0°C) | 70/95 (73.7) | 757/1354 (55.9) |
| - high-grade fever (≥38.0°C) | 25/95 (26.3) | 597/1354 (44.1) |
| chills | 119 (3.7) | 1180 (37.3) |
| joint pain | 198 (6.1) | 1092 (34.5) |
| nasal discharge | 49 (1.5) | 69 (2.2) |
| Anaphylaxis | 1 (0.03) | 1 (0.03) |
| Absence from work (no. of days) | .. | |
| no absence | 3183 (97.8) | 2555 (80.7) |
| 1 day | 48 (1.5) | 461 (14.6) |
| 2–3 days | 6 (0.2) | 137 (4.3) |
| 4–5 days | 9 (0.3) | 6 (0.2) |
| ≥6 days | 8 (0.2) | 6 (0.2) |
Regarding adverse reactions after the first dose, pain at the injection site was most commonly reported as a local reaction (89.4%). Approximately 30% self-reported swelling and a burning sensation. For systemic reactions, muscle ache was the top self-reported reaction (65.5%), followed by fatigue (27.7%), headache (14.2%), joint pain (6.1%), chills (3.7%) and fever (2.9%). Regarding adverse reactions after the second dose, pain (87.3%) remained as the top local reaction, but the overall frequency of systemic reactions was higher than after the first dose, especially for fatigue (72.8%), muscle pain (64.3%), headache (45.7%), fever (42.8%), chills (37.3%) and joint pain (34.5%) (Supplementary, Fig. 1).
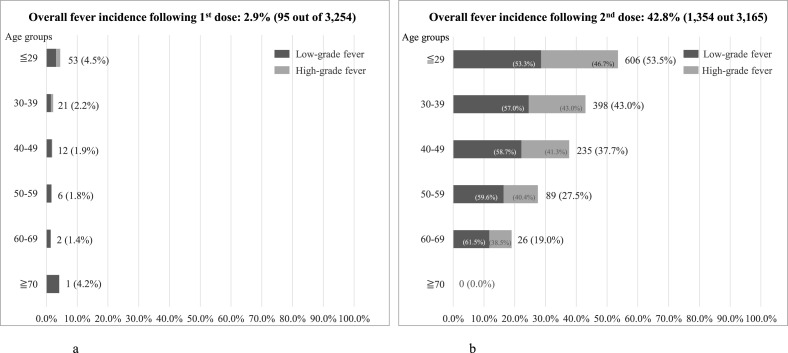
Multivariate analysis was performed to assess the differences in reactogenicity by sex and age, for the first dose and the second dose (Table 2 ). Women were found to have higher rates of reactogenicity in all items, compared to men. Additionally, those with older age tended to have lower rates of reactogenicity. Comparing reactogenicity following the doses, the rates following the second dose were higher, especially for fever (OR, 27.38; 95% CI, [22.00–34.06]; p < 0.001), chills (OR, 16.49; 95% CI, [13.53–20.11]; p < 0.001), joint pain (OR, 8.49; 95% CI, [7.21–9.99]; p < 0.001), fatigue (OR, 7.18; 95% CI, [6.43–8.02]; p < 0.001) and headache (OR, 5.43; 95% CI, [4.80–6.14]; p < 0.001). Incidences of fever by age groups following each dose of vaccine are shown in Fig. 1 a and 1b. Compared to the first dose, healthcare worker participants had substantially higher rates of fever after the second dose, especially for age groups ≦29 years (53.5%). Among participants who reported fever following the second dose, more than 40% reported fever of 38.0 °C or higher in age groups under 60 years old, with the percentage slightly higher among younger groups ( Fig. 1b).
Table 2.
Multivariate analysis for differences in sex, age, and doses; reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine.
| Female (reference: male) |
Age (unit change: one year older) |
Dose 2 (reference: dose 1) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactogenicity | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |||
| pain (injection site) | 1.48 | 1.26 | 1.73 | <0.001 | 0.99 | 0.98 | 1.00 | 0.001 | 0.82 | 0.70 | 0.95 | 0.010 |
| swelling | 1.42 | 1.27 | 1.59 | <0.001 | 0.98 | 0.98 | 0.99 | <0.001 | 1.71 | 1.54 | 1.90 | <0.001 |
| burning sensation | 1.56 | 1.39 | 1.75 | <0.001 | 0.97 | 0.97 | 0.98 | <0.001 | 1.54 | 1.38 | 1.71 | <0.001 |
| redness | 1.58 | 1.38 | 1.82 | <0.01 | 0.98 | 0.98 | 0.99 | <0.001 | 2.04 | 1.80 | 2.32 | <0.001 |
| induration | 1.27 | 1.10 | 1.46 | 0.001 | 1.00 | 1.00 | 1.01 | 0.163 | 1.18 | 1.04 | 1.34 | 0.012 |
| itching | 3.66 | 2.99 | 4.48 | <0.001 | 1.01 | 1.00 | 1.01 | 0.070 | 1.76 | 1.52 | 2.05 | <0.001 |
| fatigue | 1.47 | 1.31 | 1.66 | <0.001 | 0.99 | 0.98 | 0.99 | <0.001 | 7.18 | 6.43 | 8.02 | <0.001 |
| muscle pain | 1.59 | 1.42 | 1.77 | <0.001 | 0.97 | 0.97 | 0.98 | <0.001 | 0.95 | 0.86 | 1.05 | 0.334 |
| headache | 2.46 | 2.16 | 2.81 | <0.001 | 0.99 | 0.98 | 0.99 | <0.001 | 5.43 | 4.80 | 6.14 | <0.001 |
| fever | 1.80 | 1.55 | 2.09 | <0.001 | 0.97 | 0.96 | 0.97 | <0.001 | 27.38 | 22.00 | 34.06 | <0.001 |
| chills | 1.88 | 1.61 | 2.19 | <0.001 | 0.98 | 0.97 | 0.99 | <0.001 | 16.49 | 13.53 | 20.11 | <0.001 |
| joint pain | 2.02 | 1.73 | 2.35 | <0.001 | 0.98 | 0.98 | 0.99 | <0.001 | 8.49 | 7.21 | 9.99 | <0.001 |
| nasal discharge | 1.71 | 1.12 | 2.63 | 0.014 | 1.01 | 1.00 | 1.03 | 0.152 | 1.46 | 1.01 | 2.11 | 0.046 |
Fig. 1.
a: Incidence of fever following 1st dose by age groups. b: Incidence of fever following 2nd dose by age groups.
Notes:
1. Fever is defined as ≧37.5 °C with 2 categories: low-grade fever (≧37.5 °C, but <38.0 °C) and high-grade fever (≧38.0 °C).
2. Percentages of fever incidence by age groups are calculated by numbers of fever incidence of each group as percentage of total number of participating healthcare workers in the associated age groups.
Due to adverse reactions including fever, fatigue, headache, and chills, 610 (19.3%) participating healthcare workers took at least one day off from work after the second dose, compared to 71 (2.2%) after the first dose ( Table 1 ).
One case of anaphylaxis was reported by a woman (32 years old) immediately after receiving the first dose; flushing in the face, itching on the abdominal area, vomiting, and cough were reported; adrenaline injection was administered. No abnormal vital signs were found. Another case of anaphylaxis was reported by a woman (38 years old) 5 min after receiving the second dose. Abnormalities on throat and cough were reported; adrenaline injection was administered. Pharyngeal edema was not found with fiberscope examination. No abnormal vital signs were reported.
This study showed a sample of real-world adverse reactions following Pfizer-BioNTech COVID-19 vaccines among healthcare workers in a large academic hospital in Tokyo, Japan. Most recipients self-reported reactogenicity (including injection site or systemic reactions) after the first dose (97.3%), as well as after the second dose (97.2%). The rate of reactogenicity was higher compared to the findings from Korean studies with 80.1% (n = 277) after the first dose and 89.1% (n = 265) after the second dose [6,7]. These adverse reactions were more commonly seen in female younger participants, which is consistent with the findings of the reports from Korea [6,7].
When compared to a previous large-scale US study (first dose: n = 1,659,724; second dose: n = 971,375) with site injection and systemic reactions assessed separately, our findings were much higher following the first dose (injection site reactions: 91.3% vs 65.4%; systemic reactions: 75.1% vs 48.0%) and second dose (injection site reactions: 92.1% vs 68.6%; systemic reactions: 86.8% vs 64.2%) [8]. Frequency of systemic reactions were substantially greater after the second dose, which was consistent with the findings of the US study. Compared to the US study, we found even higher rates of these systemic reactions in our healthcare workers in Japan: fatigue (72.8% vs 47.8%), muscle ache (64.3% vs 36.8%), and fever (42.8% vs 21.5%) [8]. The large-scale US study also revealed that local and systemic reactions were less commonly reported by participants 65 years and older compared with those younger than 65 years, which is consistent with our findings. Regarding gender differences, no detailed information was available in the US study, but a UK study reported that reactogenicity was higher among women, consistent with the findings of our study [13].
Anaphylaxis was confirmed in one participant after the first dose (0.031%) and one participant after the second dose (0.031%). Both confirmed anaphylaxis cases were Brighton Collaboration level 3; no level 1 or 2 cases were reported in this academic hospital. According to the US Centers for Disease Control and Prevention (CDC), the frequency of anaphylaxis with the Pfizer-BioNTech COVID-19 vaccine was around 11.1 per million doses as of December 2020 [14]. In another study among Mass General Brigham (MGB) employees in America (n = 25,929), anaphylaxis was confirmed in 7 employees after the first dose (0.027%; [95% CI, 0.011–0.056]) [15].
Although most healthcare worker participants in this Japanese academic hospital self-reported local or systemic reactions following each dose of Pfizer-BioNTech vaccine, severe cases were limited. 19.3% of the participants self-reported absence from work after receiving the second dose, but they typically took 1 day off (14.6%), with less than 5.0% needing to take more than 2 days from work.
This study has several limitations worth addressing. First, this questionnaire survey was self-administered online. Although all the participants were healthcare workers, perception regarding adverse reactions may vary, resulting in under-reporting or over-reporting. Second, this study was conducted in a single hospital, which may not fully represent the large number of healthcare workers in Japan.
In conclusion, most healthcare workers reported reactogenicity in this Japanese hospital. The rates of fatigue, headache, fever, chills, and joint pain were greatly increased following the second dose. Higher incidence of adverse reactions was found in women and younger ages. Although adverse reactions were commonly reported, severe cases were limited. Our findings provide real-world evidence on the frequency of reactogenicity after receiving the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Japan, helping clinicians and the general population set realistic expectations for postvaccination adverse reactions in Japan and other countries in the region.
Authorship statement
Prof Naito had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Saita, Yan, Naito.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Saita, Ito, Sasano, Seyama.
Administrative, technical, or material support: Ito, Sasano, Seyama.
Supervision: Naito.
All authors meet the ICMJE authorship criteria.
Declaration of competing interest
The authors declare no conflicts of interests.
Acknowledgement
We thank healthcare workers in Juntendo University Hospital, Tokyo, Japan for their participation. We thank Kristin Thurlby, assistant professor in the Department of Science, Johnson County Community College, Kansas, USA, for her editorial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.09.009.
Ethical statement
Study protocol was approved by the Institutional Review Board (IRB) of Juntendo University Faculty of Medicine, Juntendo University. (No. 2021055) All participants agreed to participate in this study.
Funding
GLAY, a music group, contributed financially to this study. The group plays no role in data interpretation and manuscript preparation.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ministry of Health, Labour and Welfare, Japan. https://www.mhlw.go.jp/stf/newpage_16734.html. Accessed May 29, 2021. [in Japanese)].
- 2.Sakine M., Kudo R., Yamaguchi M., et al. Japan's ongoing crisis on HPV vaccination. Vaccines. 2020;8:362. doi: 10.3390/vaccines8030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., et al. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver S.E., Gargano J.W., Marin M., et al. The advisory committee on immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR (Morb Mortal Wkly Rep) 2020;69:50. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae S., Lee Y.W., Lim S.Y., et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Kor Med Sci. 2021;36(17):e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y.W., Lim S.Y., Lee J.H., et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Kor Med Sci. 2021;36(21):e153. doi: 10.3346/jkms.2021.36.e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapin-Bardales J., Gee J., Myers T. Reactogenicity following receipt of mRNA-Based COVID-19 vaccines. J Am Med Assoc. 2021;325(21):2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 9.Klimek L., Novak N., Hamelmann, et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA. Allergo J Int. 2021;30:51–55. doi: 10.1007/s40629-020-00160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda H., Seyama K., Ito K., et al. SARS-CoV-2 seroprevalence in healthcare workers at a frontline hospital in Tokyo. Sci Rep. 2021;11(1):8380. doi: 10.1038/s41598-021-87688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English version of pre-vaccination screening questionnaire for COVID-19 vaccine. https://www.mhlw.go.jp/content/000759454.pdf. Accessed June 3, 2021.
- 12.Ju Ruggeberg, Gold M.S., Bayas J.M., et al. The Brighton Collaboration Anaphylaxis Working Group. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 13.Powell A.A., Power L., Westrop S., et al. Real-world data shows increased reactogenicity in adults heterologous compared to homologous prime-boost COVID-19 vaccination, March—June 2021, England. Euro Surveill. 2021;26(28) doi: 10.2807/1560-7917.ES.2021.26.28.2100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . 2020. Allergic reactions including anaphylaxis After receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23.https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm [Google Scholar]
- 15.Blumenthal K.G., Robinson L.B., Camrgo C.A., et al. Acute allergic reactions to mRNA COVID-19 vaccines. J Am Med Assoc. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.