To the Editor:
The current massive COVID-19 immunization campaign has initiated a change in the course of the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The duration of postvaccination protection can be estimated from longer-term monitoring of the persistence of naturally acquired protection (since November 2019). Therefore, this rapid meta-analysis was conducted to evaluate the reinfection rates in post–COVID-19 patients as the primary endpoint to predict protection not only in the general population but also in vulnerable long-term care (LTC) recipients.
Methods
A search of the relevant literature was carried out in the MEDLINE, EMBASE, Web of Science, MedRxiv, and BioRxiv databases on June 7 and August 20, 2021, to identify any original studies on reinfections in post–COVID-19 patients. Eligible studies had to include the measure of association of acquired SARS-CoV-2 infection in post–COVID-19 individuals with previously uninfected ones. Key findings [ie, numbers of infected and reinfected individuals, measures of association including the 95% confidence intervals (95% CIs), viral variant of concern, follow-up period of ≥180 days, etc] were extracted.
This was a rapid and pragmatic meta-analysis to estimate reduction in the risk of reinfection in post–COVID-19 patients, expressed by the efficacy of naturally acquired protection, that is, (1 – measure of association) × 100%. Given the nonhomogeneity of the studies identified, the outcome was assessed using the random effects model (DerSimonian-Laird method). Analysis were performed using Stata, version 17 (StataCorp, College Station, TX), at a significance level of α = 0.05 with a 2-tailed 95% CI. The protocol of this study was not registered.
Results
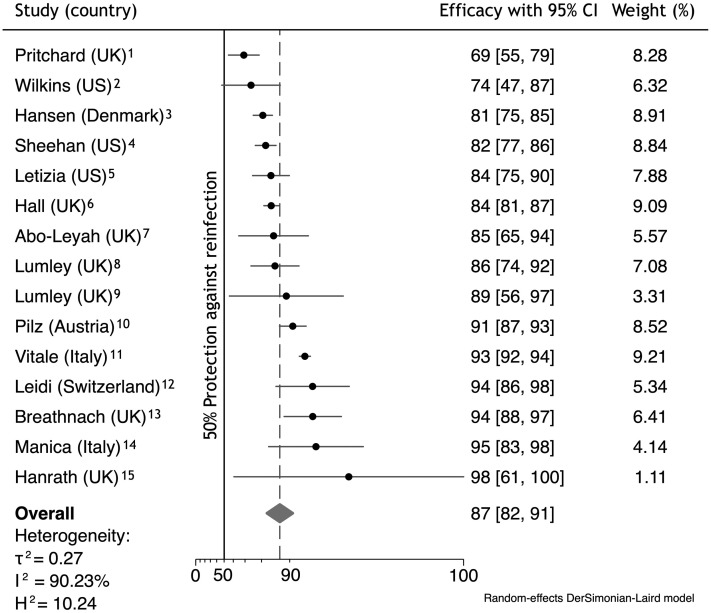
A total of 15 eligible publications were identified to assess the risk of reinfection in post–COVID-19 patients. These publications reported the results of cohort studies conducted in the general population (8 studies), in the population of health care workers (6) or in the military (1) including either only adults or individuals regardless of age (ie, also children and adolescents). The mean or median age of the study populations ranged from 19 to 59 years. Among the 10,123,319 subjects enrolled in the studies (42.8% of males), 67,124 were polymerase chain reaction (PCR)–positive (4), seropositive (7), or PCR/seropositive (4) with documented reinfection in 0.6% of cases beyond 60-120 days after complete resolution of the first infection. The mean follow-up time for reinfection was 234 days (range, 180-360 days) between March 2020 and May 2021.
The pooled efficacy of naturally acquired immunity determined using a random effects method achieved 87.1% (95% CI 82.4%, 90.6%) against any PCR-confirmed COVID-19 independently of the presence or absence of symptoms (Figure 1 ).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Publication bias, the effect of small studies, as well as omission of studies were not found. If the meta-analysis had included only adjusted measures of association (9 studies), the pooled efficacy would have achieved 85.0% (95% CI 77.0%, 90.2%). When considering only symptomatic reinfections (4 studies), the efficacy of naturally acquired protection increased to 91.7% (95% CI 84.6%, 95.5%).
Fig. 1.
Forest plot of efficacy of naturally acquired protection after COVID-19 against any reinfection (results are from a meta-analysis). H2, heterogeneity; I2, inconsistency; τ2, variance; UK, United Kingdom; US, United States of America. Particulars of the forest plot: the diamonds indicate overall efficacy, with lateral points indicating the 95% CI; the dashed lines indicate the point of pooled efficacy.
Discussion
This meta-analysis confirmed a high (87%) level of protection acquired after COVID-19. When assessing the efficacy in symptomatic patients only, their 92% level of protection was close to that seen postvaccination.
As naturally acquired immunity to SARS-CoV-2 infection can dramatically reduce the risk of reinfection within at least 1 year, one may reasonably expect that vaccine-induced protection could confer a similar level of protection against COVID-19 for the first year.
Unfortunately, only 3 studies investigated the impact of SARS-CoV-2 variants of concern (B.1.1.7) on the risk of reinfection, making it impossible to quantitatively assess the efficacy of naturally acquired immunity against new variants.1 , 6 , 8 The current more frequent failure of postvaccination protection, especially against the Delta variant, should also be taken very seriously as an issue related to post–COVID-19 protection.
Although all the studies were designed and conducted as cohort ones, the risk of bias was not the same across the studies as documented by the different pooled efficacies from adjusted and unadjusted measures of association. The published outcomes ranged between 69% and 98%, and the lower limit of the 95% CI was higher than 47%. One may assume that the efficacy results obtained from studies with a moderate or high risk of bias did not critically impact the final pooled efficacy in this meta-analysis. It should not be forgotten that the level of protection afforded by naturally acquired immunity depends on disease severity.16 Therefore, the effectiveness determined from cohort studies could be skewed as a result of not taking into account mild or subclinical primary infections.
Although no study focused specifically on LTC recipients, they could be protected against reinfections the same as the general population in the context of current vaccination.17
In conclusion, the persistence of post–COVID-19 protection suggests a similarly durable post-vaccination efficacy within the first year. Even if naturally acquired protection against COVID-19 can reduce the risk of reinfections no less than vaccination, the risk of impact of new circulating variants, especially the Delta variant should by no means be underestimated.
Footnotes
The author declares no conflicts of interest.
Supplementary Data
References
- 1.Pritchard E., Matthews P.C., Stoesser N. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27:1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkins J.T., Hirschhorn L.R., Gray E.L. Serologic status and SARS CoV-2 infection over 6-months of follow-up in healthcare workers in Chicago: A cohort study. Infect Control Hosp Epidemiol. 2021 Aug 9 doi: 10.1017/ice.2021.367. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen C.H., Michlmayr D., Gubbels S.M. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheehan M.M., Reddy A.J., Rothberg M.B. Reinfection rates among patients who previously tested positive for COVID-19: A retrospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab234. ciab234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letizia A.G., Ge Y., Vangeti S. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: A prospective cohort study. Lancet Respir Med. 2021;9:712–720. doi: 10.1016/S2213-2600(21)00158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall V.J., Foulkes S., Charlett A. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abo-Leyah H., Gallant S., Cassidy D. The protective effect of SARS-CoV-2 antibodies in Scottish healthcare workers. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00080-2021. 00080-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumley S.F., Rodger G., Constantinides B. An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis. 2021:ciab608. doi: 10.1093/cid/ciab608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumley S.F., O'Donnell D., Stoesser N.E. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilz S., Chakeri A., Ioannidis J.P. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51:e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale J., Mumoli N., Clerici P. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021:e212959. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leidi A., Koegler F., Dumont R. Risk of reinfection after seroconversion to SARS-CoV-2: A population-based propensity-score matched cohort study. Clin Infect Dis. 2021:ciab495. doi: 10.1093/cid/ciab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breathnach A.S., Riley P.A., Cotter M.P. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J Infect. 2021;82:e11–e12. doi: 10.1016/j.jinf.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manica M., Pancheri S., Poletti P. The risk of symptomatic reinfection during the second COVID-19 wave in individuals previously exposed to SARS-CoV-2. medRxiv. Apr 20 2021 doi: 10.1101/2021.04.14.21255502. [DOI] [Google Scholar]
- 15.Hanrath A.T., Payne B.A.I., Duncan C.J.A. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect. 2021;82:e29–e30. doi: 10.1016/j.jinf.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthmiller J.J., Stovicek O., Wang J. SARS-CoV-2 infection severity is linked to superior humoral immunity against the spike. mBio. 2021;12 doi: 10.1128/mBio.02940-20. e02940-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salcher-Konrad M., Smith S., Comas-Herrera A. Emerging evidence on effectiveness of COVID-19 vaccines among residents of long-term care facilities. J Am Med Dir Assoc. 2021;22:1602–1603. doi: 10.1016/j.jamda.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.