Abstract
During the Covid-19 pandemic, location of the SARS-CoV-2 infected patients inside the hospital is a major issue to prevent viral cross-transmission. The objective of this study was to evaluate the risk of contamination through aerosol by using a global approach of the multiple environmental parameters to simulate, including seasonal context. A computational fluid dynamic (CFD) simulation based on the Lattice Boltzmann Method approach was used to predict airflow on the entire floor of a private hospital in Paris. The risk of contamination outside the rooms was evaluated by using a water vapor mass fraction tracker. Finally, the air contamination was estimated by a “cough model” producing several punctual emissions of contaminated air from potentially infected patients. In a winter configuration, the simulation showed a well-balanced ventilation on the floor and especially inside the rooms. After cough emissions from COVID-positive rooms, no significant contamination was observed in the circulation area, public waiting space and nurse office. On the contrary, in a summer configuration, the temperature difference due to the impact of the sun radiation between both sides of the building created additional air transport increasing the contamination risk in neighboring rooms and public spaces. Airborne spread was limited to rooms during winter conditions. On the contrary, during summer conditions, market airflow with potentially contaminated air coming from rooms located on the side of the building exposed to solar radiation was evidenced. These observations have implications to locate infected patients inside the building and for the conception of future health care structures.
Keywords: Covid-19, Environment, Viral contamination, Computer Fluid Dynamic simulation, Aerodynamic simulation
1. Introduction
The outbreak of the 2019 coronavirus disease (Covid-19) has caused severe disruptions in hospital organizations throughout the world (Sun et al., 2021). Besides medical problematics, health establishments were urged to create new hospitalization units and new clinical pathways specifically dedicated to the admission and care of patients suffering from the SARS-CoV-2 disease.
The location of the units with infected patients inside the hospital represented an important challenge. Many parameters had to be taken into consideration, such as the type of facilities, the size of the units, accessibility and also the ability to isolate the room in order to prevent viral cross contamination inside the institution. Transmission vectors had been rapidly identified as being mainly the inhalation of virus-laden liquid droplets (WHO, 2020, Chia et al., 2020, Liu et al., 2020). In addition to surface contamination, aerosol transmission represents the main factor of viral infection. Droplets smaller than 5 µm containing viral RNA, exhaled from infected patients, were detected and remained suspended in the air for hours (Liu et al., 2020). Furthermore, it has been demonstrated that SARS-CoV2 could maintain its biological stability in aerosols for days (van Doremalen et al., 2020). In such conditions, the question about the risk of transmission via airborne microdroplets should be seriously considered and has been probably greatly underestimated until now (Morawska and Cao, 2020, Morawska and Milton, 2020; Lewis, 2020). This may have several important implications regarding the potential risk of airborne transmission of viral particles through mechanical aeration and air-conditioning systems inside health establishments (Chirico et al., 2020, Li et al., 2007). Ventilation and air conditioning systems have been clearly involved in the cross transmission of viral infectious diseases such as flu and influenza A (H1N1) diseases (Chirico et al., 2020, Li et al., 2007). Although this way of transmission remains to be firmly established for SARS-CoV-2, international guidelines on air ventilation, based on the assumption of SARS-CoV-2 airborne transmission, have been released (Morawska et al., 2020). These recommendations are mainly to increase the inflow of outdoor air and to stop air recirculation from indoor rooms of infected patients.
Therefore, the challenge for health establishments caring for SARS-CoV-2 patients is to be able to provide adequate ventilation in indoor rooms and at the same time reduce the risk of airborne transmission through recirculating air inside the building (Correia et al., 2020). This is really challenging because air movements inside the building are usually poorly documented and difficult to estimate because it depends on numerous factors related to ventilation characteristics and pressure gradients (Beggs et al., 2008). Moreover, an additional problem is that airflow is under the dependence of temperature gradients and may vary according to seasons. Because this Covid-19 pandemic is going to last a long time, these variations of air circulation inside the hospital according to seasonal temperatures variations, and influence of solar radiation should be considered.
Simulation strategy using Computer Fluid Dynamics (CFD) analysis can be a valuable tool to assess airflow dynamic propagation according to variations on determinant factors (Beggs et al., 2008; Sze-To et al., 2008; Richmond-Bryant et al., 2006; Richmond-Bryant, 2009; Redrow et al., 2011; Kao and Yang, 2006). In this study a 3D CFD model based on Lattice Boltzmann method was used to simulate air flows and the spreading of airborne particles produced by a patient’s cough, related to ventilation at the scale of different sectors on the same floor of a medical institution with SARS-CoV-2 infected patients. Using this methodological approach, the impact of different external temperature conditions, such as a winter and a summer configuration, and more specifically the influence of solar radiation on the building, was evaluated. The final objective of this study was to find solutions to prevent airborne contamination. It could be to better distribute SARS-Cov-2 infected patients according to the characteristics of the ventilation systems and possible impact of the building temperature, but also to sunscreen the building, or to apply insulation on external or internal surfaces. This could improve the safety of non infected next-door patients as well as visitors and health providers working in the facility. Additionally, this could have significant implications for the conception of future health care structures.
2. Methods
This study was undertaken at Institut Mutualiste Montsouris, which is a private hospital located in the southern part of Paris – France. The hospital is a modern building of 7 floors, built in 1999 mainly dedicated to surgical activity and has 420 hospital beds.
The evaluation took place in April 2020, during the first wave of the Covid 19 pandemic in France. During this first wave, the hospital was encouraged to create a unit of 30 beds dedicated to the care of SARS-CoV-2 infected patients. During this period, 180 patients were hospitalized in this unit.
The question about the best location of this unit inside the building was raised initially because the aim was to isolate these patients to avoid cross-contamination. One of the issues was to evaluate the risk of viral spread through air flow. Because this pandemic started during the winter and lasted until summer, it appeared that, in addition to room ventilation, the influence of seasons (external temperature and solar radiation) had to be taken into account.
Evaluating the risk of contamination through aerosol on an entire floor requires a global approach of the multiple environmental parameters to simulate, including the ventilation system, rooms’ layout, windows surfaces and external weather conditions. Since the hospital had no recent thermal insulation treatment, the impact of the sun during a hot summer could significantly affect the air temperature inside the floor. Variations up to 10 °C had been observed during previous heat waves between both sides of the building on the same floor. This temperature gradient could create additional air transport due to buoyancy effect.
2.1. Computer fluid simulation
To understand and anticipate any risk of airborne contamination, we used CFD simulation based on the Lattice Boltzmann Method (PowerFLOW ©) approach, as described elsewhere in details (Chen and Doolen, 1998, Chen et al., 2003, Amiri Delouei et al., 2014). This solver is based on the Lattice-Boltzmann Method (LBM) with a turbulence scheme following a Very Large Eddy Strategy (VLES) approach.
The simulation method solves the flow on a cartesian grid on a mesoscopic scale by describing the statistical distribution of particles and their velocity on an elemental cubic grid (called lattice). A continuous distribution function f(x , t, v) is used to model the evolution of the particles distribution in space and time (i.e. a statistical distribution of particle density). The small scale turbulences are modelled to avoid the resolution of the dissipative small scales, which should induce a refinement of the simulation grid. The LBM-VLES scheme uses also a hybrid wall function to model the wall boundary layer to avoid large computational costs (Teixeira, 1998, Chen et al., 2003, Fares, 2006). This model includes a pressure gradient effect correction.
The challenge behind these simulations is to handle in the meantime the large volume of an entire floor, the ventilation system of each room and the thermal impact of the architecture submitted to solar radiation. To overcome this physical complexity, turbulent flows resolution is a key to handling this issue and resolving large scales provides a more accurate representation of the physics compared to traditional CFD approach.
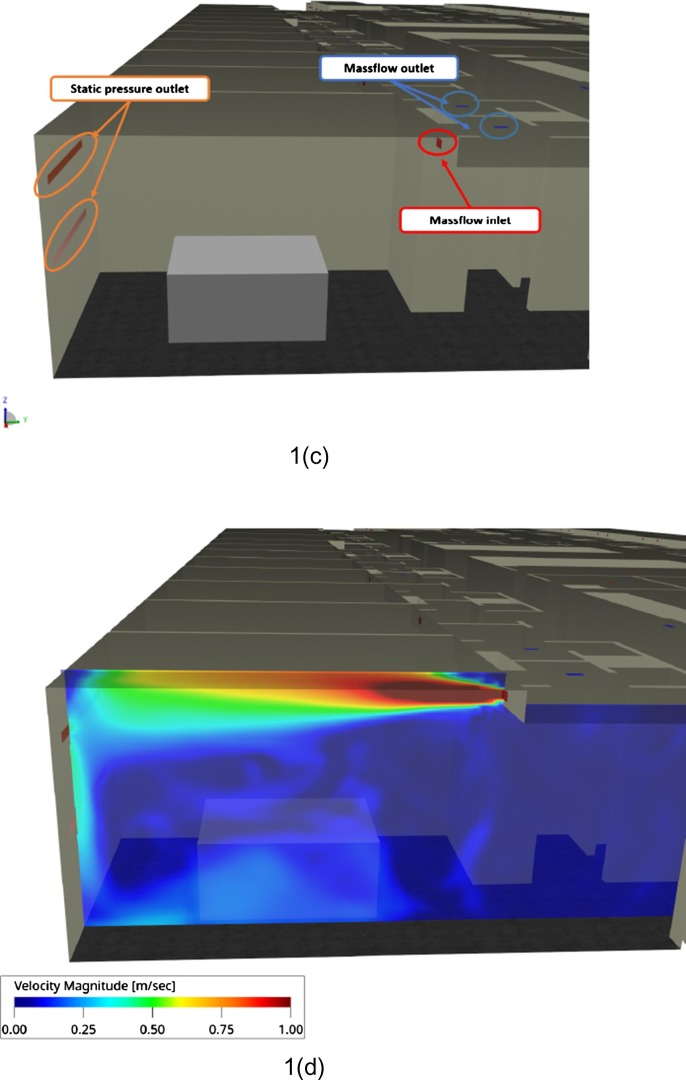
A 3D detailed model of the floor based on the 2D blueprints of the building and the ventilation system was created. Since the floor was half dedicated to COVID, the model included beds representing the contaminated patients as positioned during the first wave (Fig. 1 a). The Orientation of the building according to solar radiation is presented in Fig. 1b. Windowed surface is especially important in this building, corresponding to approximately 50% of the external wall for each patient room and approximately 40% of the overall floor external surface.
Fig. 1.
Schematic representation of the floor under evaluation. (a) COVID positive area in orange and adjacent COVID free area in blue. (b) Orientation of the building. (c) Setup of the boundary conditions inside a room. (d) Instantaneous flow field inside a room. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Since the ventilation system will have a significant impact on the airflow inside the floor, it was included in this simulation. We modeled it as massflow inlets and outlets boundary conditions and positioned them as specified in the blueprints related to the installation of the HVAC system. On top of these boundary conditions, the simulation takes in account the leakages from the windows by adding a surface set as ambient static pressure outlet (Fig. 1c and d). The amount of air blown or extracted by the HVAC system was based on very recent measurements done by the technical staff of the hospital two years ago. This value was set up at 0.04 m3·sec−1 for model calculation. For the calculation, the air temperature coming from the ventilation system was considered as similar during both winter and summer configuration and fixed at 22 °C. This corresponds roughly to the real conditions because it is not an air-conditioning system.
To predict accurately the impact of the summer conditions, we built a thermal model of the floor including material such as concrete for the external walls, glass for the windows and used a weather file representing the conditions of a heat wave in Phoenix (Arizona) (data belonging to Dassault Systèmes and chosen because temperature, hygrometric degrees and the sun’s path were available and close to summer conditions in Paris).
The CFD simulation is coupled with a thermal solver (PowerTHERM ©) calculating the conduction in the material and the radiation due to the solar radiation and the hot surfaces. The convection field is provided by the CFD simulation and is an input for the thermal one. The surface temperature calculated for a heat wave is presented in Fig. 2 .
Fig. 2.
Surface temperatures in summer configuration.
2.2. Assessment of aerosol contamination
In a second step, the risk of contamination outside the rooms was evaluated by using a water vapor mass fraction tracker and the fraction of this contaminated water vapor in the ambient air was analyzed. This water vapor mass fraction is modeled via a Particle Differential Equation (PDE) solver. The current implantation assumes that the volume fraction of the water is small enough so that the transporting medium can still be considered as air. As a result, the air properties are not changed. With this modelisation, both convection and diffusion are considered but the settling is not taken in account. Regarding the diffusion parameters, turbulent diffusion is dominating and a constant Schmidt number is used. The walls can also impact the water vapor since surface allows condensation which can remove water vapor from the air, allowing a tow-way coupling. The air properties change depending on the temperature, accounting for buoyancy, which is a key point for this study.
Finally, the air contamination from COVID was estimated by a “cough model” producing several punctual emissions of 100% contaminated air from a simplified “mouth” on the bed. This air emission corresponds to a cough of 1 s emitted 5 times at 30 s, 60 s, 107 s, 150 s and 204 s. This contaminated air is considered as a pure massless aerosol, similar to a CO2 tracking approach. The result was given in percent of air contamination, corresponding to the air fraction, at a specific location, directly coming from contaminated exhaled air (arbitrarily fixed at 100% contaminated). We evaluated a worst case configuration by leaving all doors open in all the rooms of the floor.
3. Results
In a winter configuration, the simulation showed a well-balanced ventilation in the floor and especially the room. The position of the air inlet in regards of the window and the extraction in the bathroom (close to the entrance) was limiting the airflow outside the room (Fig. 3 ). In the meantime, very low velocities were observed in the floor (<0.2 m/s). In the corridor and the waiting rooms, the air velocities were even lower (<0.1 m/s) (Fig. 3). After five “coughs” emitted in all COVID-positive rooms and 400 s of physical time simulated observation, no significant contamination was observed in the circulation area, public waiting space and nurse’s office (Fig. 4 ). Very close to the COVID-positive rooms, the concentration of contaminated air represented less than 1%.
Fig. 3.
Instantaneous air velocity magnitude in winter configuration.
Fig. 4.
Water vapor mass fraction for the winter configuration after 120 s (a) and 300 s (b) of simulation.
In a summer configuration, the impact of the sun radiation and the convection of hot surfaces became significant. The side exposed to solar radiation presented an increase by 10–20 °C of surface temperature, mainly due to the direct impact of solar radiation (Fig. 5 ). The ventilation system, blowing fresh air directly to the window increased the convection effect and raised the air temperature by 10 °C compared to the cold side (Fig. 5a). This temperature difference between both sides of the building created an additional air transport from the hot to the cold side (Fig. 5b). In this configuration, the contamination risk is strongly raised, as reflected by the water vapor mass fraction at 120 s and 300 s (Fig. 6 ). The evolution of contaminated air was specifically analyzed in four different location of the floor (one room on the sunny side, one on the other side and two rooms in the central area of the floor) (Fig. 7 ). In the room exposed to solar radiation, the early dynamic of contamination was similar between the winter and the summer configuration until 210 s. After the last cough, the summer configuration presents a stronger drop in contamination (from 5% to 2% in 60 s) since the winter configuration presents a slower decrease in contamination (from 5.5% to 5% in 60 s) (Fig. 8 ). The transport of air from the hot side to the cold one explains this phenomenon by propagating the contamination outside the rooms quicker than during winter conditions.
Fig. 5.
Summer configuration. Instantaneous temperature (a) and instantaneous air velocity magnitude (b).
Fig. 6.
Water vapor mass fraction for the summer configuration at 120 s (a) and at 300 s (b).
Fig. 7.
Position of the 4 points of interest in the floor. 1 = corridor and waiting space at the entrance of the floor, 2 = corridor and nurse desk located in the middle of the floor, between the 2 rows of beds, 3 = A room exposed to solar radiation, 4 = A room on the opposite side (non directly exposed to solar radiation).
Fig. 8.
Evolution rate of the contaminated air according to different locations. a = corridor and waiting space at the entrance of the floor, b = corridor and nurse desk located in the middle of the floor, between the 2 rows of beds, c = A room exposed to solar radiation, d = A room on the opposite side (non directly exposed to solar radiation).
In the room on the other side, a similar behavior was observed, with a slower decrease in contamination in the summer compared to the room on the other side. The additional air mouvment due to the buoyancy effect increased the air velocities and mixed contaminated and non-contaminated air.
In the middle of the floor, including waiting rooms and the nurse’s office, almost no air propagation outside of the room was observed during winter conditions (Fig. 8). However, because of the additional transport of air from the hot side to the cold one during summer conditions, an increase in contamination risks was observed. Forty seconds after the first cough, the contaminated air rate increased up to 2% in summer conditions. This behavior did not seem to be dependent on the position analyzed, although a greater peak of contaminated air was observed in position 2 rather than in position 1 (Fig. 8).
4. Discussion
The present evaluation was aimed to highlight airflows inside a hospital floor with SARS-CoV-2 infected patients to prevent airborne viral cross contamination. Among the numerous parameters conditioning air circulation, a specific attention to seasonal temperature variations was paid. Using CFD simulation, it was shown that during summer conditions, potential contaminated air coming from rooms located on the side of the building exposed to solar radiation, could spread toward public areas, waiting room and the nurse’s office and expose neighboring patients, heath care professionals and visitors to viral contamination.
4.1. Viral airborne transmission
The question about viral airborne transmission was raised during previous severe acute respiratory syndrome outbreaks (Chirico et al., 2020). It has been suggested that such contamination could be of major importance regarding the Covid-19 pandemic (Chirico et al., 2020, Li et al., 2007, Morawska et al., 2020, Correia et al., 2020). This was the main motivation to look at airflows in the environment of a hospital floor with infected patients. Airflow patterns are governed by multiple parameters such as velocitie/pressures of the air-supply, exhaust vents of the ventilation systems, pressure gradients and also temperature differences between building sides exposed, or not, to solar radiation. This last parameter raises the question about seasonal variations on airflows, which was the purpose of this research and had never been addressed until now.
Hospital design should integrate these new problematics (Ninomura et al., 2006, Bartley et al., 2010). Infection preventionists play an increasingly important role in preventing health care-associated infection in the physical environment associated with new construction or renovation of health care facilities. It has been shown that a very small amount of pathogen agent’s transmission can cause severe infection in immunocompromised patients. This highlights the critical need for isolation and containment of construction activities from other occupied spaces. The current results support a more proactive involvement of fluid engineering in design of care environment (Farrow and Black, 2009).
4.2. Impact of seasonal variations
One of the important findings highlighted by the current simulation is that in winter conditions, the risk of potential transmission related to the ventilation system appears as negligible, even in a configuration with open doors. This has a potentially important impact. Indeed, the potential roles of ventilation and air conditioning systems in the virus transmission have been mentioned in many reports (Chirico et al., 2020). On the other hand, the limitation of room ventilation and air conditioning systems could create hygiene issues and discomfort in indoor environments. The present results show that under the current conditions of evaluation, and only during winter, it is not necessary to switch off the ventilation system to reduce the risk of virus contamination outside the rooms of infected patients. This is in sharp contrast with the results obtained during summer conditions, where potentially contaminated airflow spread toward public areas and the nurse’s office. There is a large consensus about the role of airborne transmission of SARS-CoV-2, and that the spread of the virus in an indoor environment should be controlled. In a previous simulation that used CFD of airflow similarly as in our study, performed on “ward 8A” in a Hong Kong hospital during the SARS outbreak in 2003, it was found that the air exchange owing to the small temperature differences between cubicles may have played a major role in SARS transmission (Farrow and Black, 2009; Li et al., 2005). Consistently with our results, they showed that a small temperature difference could cause large air exchanges between different areas owing to the relatively large areas of the opening spaces (Chen et al., 2011). This leads to the conclusion that the airflow related to temperature differences between both sides of the building in summer conditions greatly outweighs the influence of ventilation systems on air exchanges. This could be ascribed to several characteristics of the building which is poorly insulated and has a large windowed surfaces. This conclusion should be integrated in future discussions about hospital architectural conception. Because it is difficult to change the framework of erected buildings, other solutions have to be implemented, such as to sunscreen the building, the insulation on external or internal surfaces, the reduction of the open areas between rooms and corridors and the location of patients on the floor side that is less exposed to the sun (Chen et al., 2011).
4.3. Computer modelisation
Computer Fluid Dynamics (CFD) numerical simulations provide a powerful and reliable tool for investigating the variable airflow patterns and air distribution at the scale of a hospital floor (Beggs et al., 2008; Sze et al., 2008; Richmond-Bryant et al., 2006; Richmond-Bryant, 2009; Redrow et al., 2011; Kao and Yang, 2006). Lattice-Boltzmann Method approach enables the simulation of the architecture complexity, a detailed modelization of the HVAC system and the consideration of external weather and especially the impact of thermal gradient. It allows to evaluate each geometrical and ventilation parameter on dispersion of virus-laden particles and many researchers have already used the CFD approach to study hospital-acquired infections (19–22).
4.4. Limitations
This study has several limitations. Firstly, our findings are based on simulations that may differ from the real world conditions. However, an approach using CFD has been applied in a lot of research and is considered as highly reliable (Beggs et al., 2008; Sze et al., 2008; Richmond-Bryant et al., 2006; Richmond-Bryant, 2009; Redrow et al., 2011; Kao and Yang, 2006). Secondly, the time-interval of the evaluation was of short duration. This could have concealed several other parameters that could have influenced airflows and potential viral spread. Thirdly, for simplification purposes, some parameters were not considered in the simulation, such as the potential protection of closed doors from contaminated rooms (Chen et al., 2011). Some differences in the simulation parameters, especially the boundary conditions can significantly change the results. For any new modifications of the HVAC system (ventilation vents, position, etc.) or the volume flow injected or extracted in the hospital, this results could not be applicable anymore. In the condition of the present study, air temperature delivered by the ventilation system was considered fixed at 22 °C. Obviously, air-conditioning with heated/cooled supply air might have had an impact on buoyancy effect, not studied in this analysis. Finally, the estimated risk of airborne viral contamination was grounded on the hypothesis that the cough was a simple exhalation process and that 100% of the droplets arising during cough contain the virus. This is an oversimplification because many factors, such as mouth form, saliva droplets size, relative humidity and temperature could profoundly modify the velocity-time and composition of the exhaled flow during cough (Dbouk and Drikakis, 2020a, Dbouk and Drikakis, 2020b). The contaminated air exhaled by the virtual patient is considered as a contaminated gas without a modelization of the particles themselves. This limitation provides information close to a CO2 measurement test but cannot highlight the deposit of aerosols on surfaces. Nevertheless, despite these limitation factors, the current results appear as sufficiently informative for deciding new locations of SARS-CoV-2 infected patients, or architectural modifications.
4.5. Conclusions
In conclusion, using CFD simulation at the level of a hospital floor with SAR-CoV-2 infected patients, it has been shown that airborne spread was limited to rooms during winter, on condition that there was no temperature difference between the two sides of the structure. On the contrary, in summer conditions, additional airflow with potentially contaminated air coming from rooms located on the side of the building exposed to solar radiation, spreading toward public areas, waiting rooms and the nurse’s office was evidenced. This highlights that temperature difference between both sides of the hospital is a more important factor of airflow contamination than ventilatory systems and should be taken into account before deciding the location of infected patients on the ward. This observation should initiate a reflection on hospital design. In the present situation, it was decided to preferentially locate infected patient on the other side of the hospital ward to reduce the risk of propagation of contaminated air in the middle area of the floor. Alternative solutions could be to rebalance air ventilation to provide more cold air on the sunny side of the building and less cold air on the shaded side of the building, to sunscreen the building, or finally to apply insulation on external or internal surfaces.
CRediT authorship contribution statement
Marc Beaussier: Writing – original draft, Supervision, Validation. Emmanuel Vanoli: Conceptualization, Methodology, Data curation, Software, Formal analysis. Frédéric Zadegan: Conceptualization, Methodology, Validation. Herve Peray: Conceptualization, Methodology, Data curation. Elodie Bezian: Conceptualization, Methodology, Validation. Jonathan Jilesen: Resources. Géraldine Gandveau: Resources. Jean-Michel Gayraud: . : Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Ling MIAO and Nagendra KRISHNAMURTHY (Dassault Systèmes- 3DS) - for the development of the water vapor mass fraction solver used in the present work.
Handling Editor: Thanh Nguyen
References
- Amiri Delouei A., Nazari M., Kayhani M.H., Succi S. Non-Newtonian unconfined flow and heat transfer over a heated cylinder using the direct-forcing immersed boundary-thermal lattice Boltzmann method. Phys. Rev. E: Stat. Nonlinear Soft Matter Phys. 2014;89 doi: 10.1103/PhysRevE.89.053312. [DOI] [PubMed] [Google Scholar]
- Bartley J.M., Olmsted R.N., Haas J. Current views of health care design and construction: practical implications for safer, cleaner environments. Am. J. Infect. Control. 2010;38(5 Suppl 1):S1–S12. doi: 10.1016/j.ajic.2010.04.195. [DOI] [PubMed] [Google Scholar]
- Beggs C.B., Kerr K.G., Noakes C.J., Hathway E.A., Sleigh P.A. The ventilation of multiple-bed hospital wards: review and analysis. Am. J. Infect. Control. 2008;36:250–259. doi: 10.1016/j.ajic.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Chen S., Doolen G.D. Lattice Boltzmann Method for Fluid Flows. Annu. Rev. Fluid Mech. 1998;30:329–364. [Google Scholar]
- Chen H., Kandasamy S., Orszag S., Shock R., Succi S., Yakhot V. Extended Boltzmann kinetic equation for turbulent flows. Science. 2003;301:633–636. doi: 10.1126/science.1085048. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhao B., Yang X., Li Y. Role of two-way airflow owing to temperature difference in severe acute respiratory syndrome transmission: revisiting the largest nosocomial severe acute respiratory syndrome outbreak in Hong Kong. J. R. Soc. Interface. 2011;8:699–710. doi: 10.1098/rsif.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico F., Sacco A., Bragazzi N.L., Magnavita N. Can Air-Conditioning Systems Contribute to the Spread of SARS/MERS/COVID-19 Infection? Insights from a Rapid Review of the Literature. Int. J. Environ. Res. Public Health. 2020;17:6052. doi: 10.3390/ijerph17176052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia G., Rodrigues L., Gameiro da Silva M., Gonçalves T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses. 2020;141 doi: 10.1016/j.mehy.2020.109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk T., Drikakis D. On coughing and airborne droplet transmission to humans. Phys. Fluids. 2020;32 doi: 10.1063/5.0011960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk T., Drikakis D. Weather impact on airborne coronavirus survival. Phys. Fluids. 2020;32 doi: 10.1063/5.0024272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares E. Unsteady ow simulation of the Ahmed reference body using a lattice Boltzmann approach. Comput. Fluids. 2006;35:940–950. [Google Scholar]
- Farrow T.S., Black S.M. Infection prevention and control in the design of healthcare facilities. Healthc. Pap. 2009;3:32–37. doi: 10.12927/hcpap.2009.20924. [DOI] [PubMed] [Google Scholar]
- Kao P.H., Yang R.J. Virus diffusion in isolation rooms. J. Hosp. Infect. 2006;62:338–345. doi: 10.1016/j.jhin.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. Mounting evidence suggests coronavirus is airborne - but health advice has not caught up. Nature. 2020;583:510–513. doi: 10.1038/d41586-020-02058-1. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I.T., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Leung G.M., Tang J.W., Yang X., Chao C.Y., Lin J.Z., et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Milton D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19) Clin. Infect Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomura P., Rousseau C., Bartley J. Updated Guidelines for Design and Construction of Hospital and Health Care Facilities. ASHRAE J. 2006;48:33–37. [Google Scholar]
- Redrow J., Mao S., Celik I., Posada J.A., Feng Z.G. Modeling the evaporation and dispersion of airborne sputum droplets expelled from a human cough. Build. Environ. 2011;146:2042–2051. [Google Scholar]
- Richmond-Bryant J., Eisner A.D., Brixey L.A., Wiener R.W. Transport of airborne particles within a room. Indoor Air. 2006;16:48–55. doi: 10.1111/j.1600-0668.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Richmond-Bryant J. Transport of exhaled particulate matter in airborne infection isolation rooms. Build. Environ. 2009;44:44–55. doi: 10.1016/j.buildenv.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Xie Z., Yu K., Jiang B., Zheng S., Pan X. COVID-19 and healthcare system in China: challenges and progression for a sustainable future. Global Health. 2021;17:14. doi: 10.1186/s12992-021-00665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze-To G.N., Wan M.P., Chao C.Y., Wei F., Yu S.C., Kwan J.K. A methodology for estimating airborne virus exposures in indoor environments using the spatial distribution of expiratory aerosols and virus viability characteristics. Indoor Air. 2008;18:425–438. doi: 10.1111/j.1600-0668.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- Teixeira C.M. Incorporating Turbulence Models into the Lattice-Boltzmann Method. Int. J. Modern Phys. C. 1998;9:1159–1175. [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. Scientific brief on 9 July 2020. Geneva: World Health Organization.