Abstract
Objective:
With the increasing numbers of COVID-19 vaccinations available there are some reports of new onset of otologic symptoms. We present our experience in recently vaccinated patients over a 30-day time frame.
Study Design:
Retrospective chart review.
Setting:
Tertiary otology ambulatory practice.
Patients:
All patients with available diagnostic codes, COVID-19 questionnaires and clinical notes.
Interventions:
Observational recordings.
Main Outcome Measures:
Within the same 30-day time period in 2019, 2020, and 2021, 1.6, 2.4, and 3.8% respectively, of all office visits were for patients with the diagnosis of new onset idiopathic sensorineural hearing loss (SNHL) without other underlying otologic diagnoses. In this time frame in 2021, 30 patients out of the 1,325 clinical visits had new or significantly exacerbated otologic symptoms that began shortly after COVID-19 vaccination. Specifically, 18 patients received Moderna and 12 patients received Pfizer vaccine. Their mean age was 60.9±13.8 years old; 11 were women and 19 men. The mean onset of symptoms was 10.18 ± 9 days post-vaccination. Symptoms included 25 patients (83.3%) with hearing loss, 15 (50%) with tinnitus, eight (26.7%) with dizziness, and five (16.7%) with vertigo. Eleven patients had previous otologic diagnoses, including six patients with Menière's disease, two with autoimmune inner ear disease (AIED), and three having both.
Conclusions:
There are no definite correlations to the COVID-19 pandemic or vaccination and new or worsened otologic symptoms. Vaccinated patients with new or exacerbated otologic symptoms should be promptly referred for evaluation. Suspected cases of post-vaccination otologic symptoms should be reported to the Center for Disease Control (CDC) vaccine adverse event reporting system (VAERS).
Keywords: COVID-19, pandemic, sudden hearing loss
Our understanding of Coronavirus disease 2019 (COVID-19) has expanded exponentially since the onset of the pandemic during the spring of 2020. It is now accepted that the COVID-19 pandemic is a highly contagious zoonosis resulting from an infection by SARS-CoV-2, spread through respiratory secretions from human-to-human. Many areas of the medical field have established successful guidelines and protocols for safe patient care. On December 11, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for the BNT162b2 mRNA Pfizer-BioNTech COVID-19 in individuals 16 years of age and older (1). Shortly after, on December 18, 2020, the mRNA-1273 SARS-CoV-2Moderna COVID-19 vaccine also received emergency use authorization by the FDA (2). Both vaccines use a novel messenger (mRNA) model which facilitate the production of a spike protein unique to SARS-CoV-2, which stimulates COVID-19 specific immune responses, resulting in the production of immunoprotective antibodies (3). Since vaccination introduction, the daily case numbers are decreasing and the percentage of vaccinated population is steadily increasing (4).
As the number of vaccinations given nationally increased in late January, unsurprisingly, so did apparent post-vaccination adverse events (AE) reports. During pre-release clinical trials, hearing loss and other otologic symptoms were not listed as a common potential AE. Shortly after vaccination began to be available in December 2020, physicians in our clinic as well as some other centers, noticed an increased frequency of patients presenting with new onset of hearing loss, especially sudden sensorineural hearing loss (SSNHL), tinnitus, aural fullness, and/or the exacerbation of previously stable Menière's and autoimmune inner ear disease (AIED). A query to professional online forums, found other otolaryngologists listing individual cases and suspected causations. For reference, we turned to the centralized vaccine adverse event reporting system (VAERS), established by the Center for Disease Control (CDC) and Food and Drug Administration (FDA), an early warning system monitoring vaccine safety after their authorized use in the United States. Reports from the beginning of March noted 27 cases of hearing loss out of 78.6 million COVID-19 vaccine doses given; by the end of March these numbers had increased to 171 cases of hearing loss occurring within 14 days of vaccination out of a total of 127 million vaccinations given. Of these 171 reported, four patients had a history of either some form of inner ear disease or Menière's disease. Thirty-four cases (20%) had recovered by the time the report was filed. In addition to hearing loss, all reported cases noted additional symptoms, including tinnitus (36%), ear discomfort (15%), headache (14%), and nausea (12%) (5). Currently there are only few published case reports regarding the manifestation of otologic symptoms after COVID-19 infection, and no published correlations between hearing loss or other otologic symptoms after immunization with any available COVID-19 vaccines (6).
The House Ear Clinic is an otologic specialty center, and as such is able to monitor the number of patient office visits within a defined time period, specific otologic history, audiometric findings, diagnosis, and additionally record any relationship patients report to a given symptom onset including possible temporal relationship to having received a COVID-19 vaccine. This report of our data is intended to make aware to other professionals of a possible correlation and stimulate discussion regarding most appropriate treatment. It is in no way intended to discourage patients with or without previous otologic diagnoses from obtaining available vaccination.
METHODS
This study was approved by the Institutional Review Board before retrospectively reviewing patient charts. Using a comprehensive electronic medical records search function, we identified all patients seen at the House Clinic in a 30-day interval, from February 21 to March 21 in 2019, 2020, and 2021. We recorded the total number of clinic visits per 30-day period, and then performed a search of specific diagnosis given by the identification of what codes were used, following guidelines established by the International Classification of Diseases-10 diagnostic (ICD-10) codes specific for SSNHL, namely H91.2, H91.21, H91.22, H91.23. Additionally, we used the CPT procedure code 69801 to identify any additional patients who received intratympanic (IT) steroid injections and fit the diagnosis of idiopathic hearing loss. All identified charts were independently reviewed by the authors assuring the criteria for idiopathic sudden hearing loss was met, with no additional underlying diagnosis. Additionally, COVID-19 screening questionnaires were reviewed to document if individuals had received the vaccine and the approximated timing to symptom onset.
For reporting of COVID-19 associated hearing loss a centralized data depository within the House Clinic was created in late February and all physicians were asked to report any patients who noted onset of new otologic symptoms post-vaccination. To ensure inclusive reporting, hearing loss was not required as long as other otologic symptoms were present. Due to local availability, at the time of manuscript submission reported patients were only receiving Pfizer or Moderna vaccines. Data were extracted from patient charts and COVID-19 screening questionnaires, filled as part of the check-in process for each visit. Reported data included demographics, relevant medical history, date of vaccination, onset of otologic symptoms, audiometric data, and treatment. All patients presented were also reported to the CDC by using the VAERS website.
RESULTS
Using the ICD-10 code H91.2, a total of 34, 40, and 51 patients with the diagnosis of SSNHL were identified in a 30-day period from February 21 to March 21 in 2019, 2020, and 2021, respectively. Demographic and clinical data are listed in Table 1. Using the total number of clinic visits for the same 30-day period each year, idiopathic SSNHL was the diagnosis for 1.6, 2.4, and 3.8% of all patient visits during the specified timeline in 2019, 2020, and 2021, respectively.
TABLE 1.
Demographics data for sudden idiopathic hearing loss in a 30-day period
| Year | Sudden Idiopathic Hearing loss Diagnosis | Age at Diagnosis | Total No. of Clinic Visits | % of Sudden Idiopathic Hearing Loss |
| 2019 | 34 (21 F/13 M) | 56.5 ± 14.6 | 2132 | 1.60% |
| 2020 | 40 (18 F/22 M) | 55.2 ± 16.2 | 1641 | 2.44% |
| 2021 | 51 (21 F/30 M) | 53.9 ± 16.8 | 1325 | 3.85% |
The House Clinic centralized depository was then queried, identifying 30 patients with post-vaccination otologic symptoms. The mean age is 60.9 ± 13.8 years old, with a men predominance of 19 men to 11 women (Table 2). The mean time of symptom onset was 10.18 ± 9 days post vaccination, with a range of 1 to 42 days. Overall, 18 patients received the Moderna and 12 patients received the Pfizer vaccine. In the cohort reported, 3 patients experienced symptoms after each dose, 15 patients had symptoms only after the first dose, and 12 patients had symptoms only after the second dose. Table 2 depicts the specific breakdown of symptoms within this cohort; 25 patients (83.3%) complained of hearing loss, 15 (50%) of tinnitus, eight (26.7%) of dizziness, five (16.7%) of vertigo, and nine (30%) had other symptoms, of which the most common was aural fullness. The mean pure tone average (PTA) was 52.2 ± 30.6 dB HL for the affected ear, and 21.2 ± 12.5 dB HL for the unaffected ear. The word recognition score (WRS) was 60.6 ± 38% for the affected ear, and 90 ± 23.0% for the unaffected ear. Currently we have not yet received and recorded a sufficient number of posttreatment audiograms to determine long-term effects, or any possible relationship to a particular treatment given. These data are actively being tracked.
TABLE 2.
Individual patients with reported otologic symptoms after COVID-19 vaccination with specific details and onset of symptoms
| Age | Sex | Prior Oto Hx | Ear | Vaccine | Vaccine Dose No. | Days to Onset of Symptoms | Hearing Loss | Vertigo | Dizziness | Tinnitus | Other Symptoms | Details |
| 74 | F | Active | AU | Moderna | After dose 1 | 7 | X | X | ||||
| 73 | M | Active | AD | Moderna | After each dose | 2–3 | X | |||||
| 53 | F | Active | AU | Pfizer | After dose 1 | 10 | X | |||||
| 51 | M | Active | AD | Pfizer | After dose 1 | 14 | X | X | X | X | ||
| 83 | M | Stable | AS | Moderna | After dose 2 | 10 | X | |||||
| 77 | F | Stable | AS | Moderna | After dose 2 | 30 | X | |||||
| 69 | M | Stable | AS | Pfizer | After dose 1 | 7 | X | X | X | Aural fullness | ||
| 67 | F | Stable | AD | Pfizer | After each dose | 8 | X | X | ||||
| 60 | M | Stable | AS | Pfizer | After dose 2 | 7–14 | X | |||||
| 55 | M | Stable | AD | Pfizer | After dose 1 | 10–14 | X | X | X | X | Aural fullness | |
| 54 | F | Stable | AS | Moderna | After dose 2 | 14–21 | X | X | ||||
| 53 | M | Stable | AS | Moderna | After dose 1 | 2 | X | X | Aural fullness | |||
| 49 | M | Stable | AD | Moderna | After dose 1 | 4–5 | X | |||||
| 43 | M | Stable | AD | Moderna | After dose 1 | 14 | X | X | X | X | ||
| 86 | M | No | AD | Pfizer | After dose 2 | 42 | X | |||||
| 78 | F | No | AD | Pfizer | After dose 2 | 1–2 | X | |||||
| 76 | M | No | AS | Moderna | After dose 2 | 14 | X | X | ||||
| 72 | F | No | AU | Moderna | After dose 2 | 10 | X | |||||
| 71 | M | No | AS | Pfizer | After dose 2 | 1–2 | X | |||||
| 67 | M | No | AS | Moderna | After dose 2 | 7 | X | X | X | |||
| 66 | F | No | AS | Pfizer | After dose 2 | 7–10 | X | X | Aural fullness, bilateral AOM | |||
| 64 | M | No | AS | Moderna | After dose 2 | 7–10 | X | X | X | Aural fullness | ||
| 61 | F | No | AD | Pfizer | After dose 1 | 12 | X | X | X | X | Nausea | |
| 59 | M | No | AD | Moderna | After dose 1 | 6 | X | X | X | |||
| 58 | F | No | AU | Pfizer | After dose 1 | 10 | X | X | X | Aural fullness | ||
| 51 | F | No | AD | Moderna | After each dose | 21 | X | |||||
| 48 | M | No | AS | Moderna | After dose 1 | 2–3 | X | X | ||||
| 44 | M | No | AU | Moderna | After dose 1 | 2–3 | X | X | Aural fullness | |||
| 39 | M | No | AD | Moderna | After dose 1 | 10–14 | X | X | X | Aural fullness | ||
| 25 | M | No | AU | Moderna | After dose 1 | 1–2 | X |
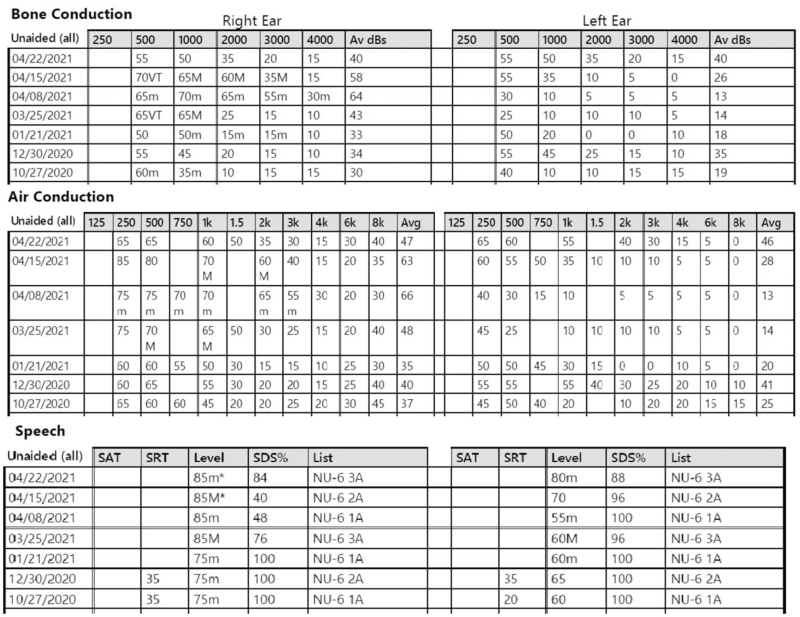
As 36.7% of the patients had a known previous underlying inner ear disorder; this population was further studied. Table 3 summarizes patients with a previous diagnosis of Menière's disease or AIED. Six patients were identified with a previous diagnosis of Menière's disease, two had known AIED, and three patients had both Menière's disease and AIED. An additional three patients had previous stable otologic diagnoses, of which two had presbycusis and one exostosis. Disease activity was based on presence or absence of exacerbations within the previous 6 months. The majority had stable courses despite a mean of 9.25 years from original diagnosis. Of the 11 patients with a known diagnosis of Menière's disease and/or AIED, four had symptomatically active disease before vaccination. Figure 1 shows an audiogram of one patient with underlying AIED, who noted fluctuation shortly after her first vaccination. She suffered a second decreased hearing shortly after her second vaccination dose and is currently undergoing IT steroid injections.
TABLE 3.
Details for patients with previous Menière's or AIED with noted otologic symptoms after COVID-19 vaccine
| Patient Age | Sex | Primary Diagnosis | Hx Duration (yrs) | Prior Oto Hx | Vaccine | Vaccine Dose No. |
| 74 | F | AIED | 5+ years | Active | Moderna | After dose 1 |
| 53 | F | AIED | 7+ years | Active | Pfizer | After dose 1 |
| 83 | M | AIED and Menière's | 12 years | Stable | Moderna | After dose 2 |
| 67 | F | AIED and Menière's | 3+ years | Stable | Pfizer | After each dose |
| 49 | M | AIED and Menière's | 22 years | Stable | Moderna | After dose 1 |
| 73 | M | Menière's | 5+ years | Active | Moderna | After each dose |
| 69 | M | Menière's | 2 years | Stable | Pfizer | After dose 1 |
| 55 | M | Menière's | 5+ years | Stable | Pfizer | After dose 1 |
| 53 | M | Menière's | 20+ years | Stable | Moderna | After dose 1 |
| 51 | M | Menière's | 5 years | Active | Pfizer | After dose 1 |
| 43 | M | Menière's | 5+ years | Stable | Moderna | After dose 1 |
FIG. 1.
Serial audiograms of patient with underlying stable AIED who received first dose of Pfizer vaccine on March 10, with noted exacerbations stating 10 days after first vaccination. She underwent right ear IT steroid injections on March 25 and noted significant improvement to her hearing. She received second dose of vaccine on March 31 and again noted increased activity bilaterally. She then underwent right ear IT steroid injection on March 31 and left ear IT steroid injection on April 15 and 22. AIED indicates autoimmune inner ear disease; IT, intratympanic.
DISCUSSION
Studies published before the COVID-19 pandemic, none with data regarding the novel mRNA vaccines, reviewed over 20 million vaccine doses given, and did not find any association between vaccination and sudden hearing loss (7). We present our data from a busy otologic practice after noticing an increase in patients presenting with SSNHL, or increased exacerbations of inner ear disease in formerly stable patients. Using the standard definition, SSNHL is hearing loss of at least 30 dB over three consecutive frequencies that develops within 72 hours (8). The estimated incidence of SSNHL is between five and 20 cases per 100,000. In a recent publication from an otologic practice, this diagnosis accounted for 1.5 to 1.7% of new patient visits (9,10). Our clinical data shows similar percentages with 1.60% of total visits within a 30-day period being new-onset SSNHL in 2019. However, following the pandemic onset in early 2020 to the present, there is a clear increase in this diagnosis, with more than two-fold increase to 2.44 and 3.85% in 2020 and 2021, respectively. While an increased incidence does not by itself prove causation, the trend here does bring up concern that in some patients, there may be a post-vaccination change in hearing.
Previous reports suggest a link between COVID-19 infection and audio-vestibular symptoms, but no similar links were reported after the vaccine (6,11–13). A recent systematic review analyzed audio-vestibular symptoms after COVID-19 infection and found 28 case reports/series and 28 cross-sectional studies that fit the criteria with an overall reported prevalence of 7.6% for hearing loss, 14.8% for tinnitus, and 7.2% for rotatory vertigo (14). Similarly, our data (Table 2) of post-vaccination symptoms reported hearing loss as the most common manifestation followed by tinnitus and dizziness. Several hypotheses for hearing loss associated with COVID-19 infection and COVID-19 vaccination are proposed on online otologic forums and within our practice. In our cohort, the mean time to onset of symptoms was 10.2 ± 9 days after vaccination, with a broad range of 1 to 42 days. Some of this data is an estimate as patients gave a range of suspected symptoms onset. Interestingly, three patients experienced symptoms after each dose, 15 patients had symptoms only after the first dose, and 12 patients had symptoms only following the second dose. This time frame may coincide with onset of Immunoglobulin G (IgG) production, as IgG antibodies to the vaccine protein antigens first appear 10 to 14 days after priming (15). Since IgG binds more effectively to the antigen and aids in opsonization, one might expect a more aggressive systemic response and manifestations, potentially including hearing loss (15). The mean timing of symptom onset, 10.5 days, did not significantly change in the sub-analysis for patients with previous otologic diagnosis. In this subgroup, both AIED and Menière's disease are thought to have immunologic factors that can contribute to exacerbations (16,17). Therefore a potential systemic immune response and a spike of disease specific IgG could intensify disease activity. This hypothesis is further supported by the fact that some patients in our practice with underling otologic diagnosis experiences similar exacerbations after COVID-19 infection.
Additional hypotheses for why the COVID-19 vaccine could cause sudden SNHL include: viral reactivation, a scaled-down clinical course of COVID-19 with known underlying risk of otologic symptoms, and migraine exacerbation (11,14,18–20). Both the Pfizer and Moderna vaccines use a RNA adenovirus vector which has a high seropositivity rate in the general population, resulting in an expected immune response or more likely, causing possible reactivation of previous latent viruses resulting in response similar to Ramsey-Hunt or Bell's Palsy (11,21). Alternatively, Abouzari et al. highlight the common link between migraines and hearing loss, noting increased headache symptoms in the Pfizer vaccine trials (51% after second dose in older patients) compared with placebo (14% after second dose in older patients) (1,22). Headaches were also the most common side effect after the second dose of Moderna vaccine, with more than 50% of participants having symptoms compared with about 25% in the control group (2).
Despite the unknown etiology of a possible exacerbation of pre-existing otologic symptoms and/or new onset of idiopathic SNHL, a prompt otolaryngology referral is warranted. In all cases, our patients were treated using the American Academy of Otolaryngology–Head and Neck Surgery guidelines regarding sudden onset idiopathic hearing loss (23). Most of our patients are still undergoing treatment, which may include oral, IT steroids, and/or antivirals. In all cases magnetic resonance imaging of the internal auditory canals was also ordered. Our pre-treatment audiograms, with the PTA and WRS recorded, have given us a good baseline with which to document any treatment effect, as well as review outcome versus specific treatment given, to be reported in the future. The extent of hearing recovery and/or symptom resolution is currently unknown.
The situation becomes more complicated with patients having post-vaccination exacerbations in previously stable Menière's or AIED. Our initial treatment consists discussing the use of high-dose oral steroids and/or IT steroid injections; previous responsiveness to both therapies is taken into consideration (24). Figure 1 demonstrates the complexity of AIED patients with noted exacerbation after the first COVID-19 vaccination, subsequent IT steroid treatment with a subjective improvement, yet a documented further decrease in hearing at the time of follow-up following the second COVID vaccine dose. At this time, when asked by patients whether to delay a second vaccination dose or if we recommend a specific manufacturer, we recommend that all patients, whether or not having known underlying inner ear disease adhere to the CDC recommendations and proceed with their vaccination when available.
There are several limitations to our current study. The percentages of sudden hearing loss visits were obtained by a systematic electronic medical records search; however, the presentation of post-vaccination symptoms was monitored by a physician driven data depository. There may be patients who, due to the nature of data collection, were not recorded into the database. Additionally, our clinical data regarding vaccination is limited to the intake questionnaire and details that were documented during a clinic visit. Unquestionably, the presented data are a very small sample size compared with the data available through VAERS. VAERS data looks at adverse events limited to a 14-day post-vaccination period, whereas our dataset includes any patient that noted onset of symptoms post-vaccination. This included one patient with onset of SSNHL 6 weeks after the vaccination. This broadly inclusive data is presented as an observation to encourage discussion of appropriate treatments for otologic exacerbations without discouraging patients from obtaining their vaccine. Given these findings, we strongly encourage all physicians to ask their patients about COVID-19 vaccination and to report any noted change in or new onset of otologic symptoms temporally related to recent vaccination to CDC VAERS, at info @ VAERS.org or 1–800–822-7967.
CONCLUSION
Currently, there are no known definite correlations between sudden hearing loss or other otologic manifestations with COVID-19 vaccination. Our otologic clinic has noticed a two-fold increase in diagnosis of idiopathic SNHL in a 30-day period in 2021 compared with the same interval 2 years ago, but cannot say if this specifically correlates to the pandemic or may represent an uncommon, but real AE not reported before this. We present our patient cohort with noted symptoms temporally presenting shortly after COVID-19 vaccination to promote awareness, as well to recommend a prompt otolaryngic evaluation and treatment. Patients should be encouraged to follow published CDC guidelines regarding COVID vaccination. Physicians should consider asking patients presenting with SSNHL or significant exacerbations of other otologic symptoms, regarding their vaccination status and symptom onset. Any potential relationship should be reported to the CDC via the VAERS website.
Footnotes
There are no relevant disclosures.
The authors disclose no conflicts of interest.
REFERENCES
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Understanding and Explaining mRNA COVID-19 Vaccines. [Google Scholar]
- 4. Public Health. COVID-19 VACCINATIONS IN LA COUNTY. 2021; http://www.publichealth.lacounty.gov/media/coronavirus/vaccine/vaccine-dashboard.htm. Accessed April 1, 2021. [Google Scholar]
- 5. Vaccine Adverse Event Reporting System (VAERS). 2021. https://vaers.hhs.gov/index.html. Accessed April 1, 2021. [Google Scholar]
- 6.Perret M, Bernard A, Rahmani A, Manckoundia P, Putot A. Acute labyrinthitis revealing COVID-19. Diagnostics (Basel) 2021; 11:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter R, Lewis N, Bohrer P, Harrington T, Aukes L, Klein NP. Sudden-onset sensorineural hearing loss after immunization: a case-centered analysis. Otolaryngol Head Neck Surg 2016; 155:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol 1980; 106:772–776. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif 2011; 15:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes GB, Freedman MA, Haberkamp TJ, Guay ME. Sudden sensorineural hearing loss. Otolaryngol Clin N Am 1996; 29:393–405. [PubMed] [Google Scholar]
- 11.Aasfara J, Hajjij A, Bensouda H, Ouhabi H, Benariba F. A unique association of bifacial weakness, paresthesia and vestibulocochlear neuritis as post-COVID-19 manifestation in pregnant women: a case report. Pan Afr Med J 2021; 38:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep 2020; 13:e238419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narozny W, Tretiakow D, Skorek A. In reference to the challenges of pharmacotherapy of SARS-CoV-2 infection in patients with sudden sensorineural hearing loss due to COVID-19. Laryngoscope 2021; 131:E2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol 2021; 22:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis 2011; 3:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciorba A, Corazzi V, Bianchini C, et al. Autoimmune inner ear disease (AIED): a diagnostic challenge. Int J Immunopathol Pharmacol 2018; 32:2058738418808680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derebery MJ. Allergic and immunologic aspects of Meniere's disease. Otolaryngol Head Neck Surg 1996; 114:360–365. [DOI] [PubMed] [Google Scholar]
- 18.Halford WP, Kemp CD, Isler JA, Davido DJ, Schaffer PA. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J Virol 2001; 75:6143–6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stallings CL, Duigou GJ, Gershon AA, Gershon MD, Silverstein SJ. The cellular localization pattern of Varicella-Zoster virus ORF29p is influenced by proteasome-mediated degradation. J Virol 2006; 80:1497–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper ID, Crofts CAP, DiNicolantonio JJ, et al. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: rationale for clinical management. Open Heart 2020; 7:e001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011; 29:5203–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abouzari M, Goshtasbi K, Chua JT, et al. Adjuvant migraine medications in the treatment of sudden sensorineural hearing loss. Laryngoscope 2021; 131:E283–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg 2019; 161: 1_suppl: S1–S45. [DOI] [PubMed] [Google Scholar]
- 24.Alexander TH, Weisman MH, Derebery JM, et al. Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol Neurotol 2009; 30:443–448. [DOI] [PubMed] [Google Scholar]