Abstract
New variants of SARS-CoV-2 Alpha (B.1.1.7); Beta (B.1.351) Gamma (P.1) and Delta (B.1.617.2) quickly spread in the UK, South Africa, Brazil and India, respectively. To address whether mutations in SARS-CoV-2 RBD spike protein could affect virus infectivity, peptides containing RBD amino acids mutations have been constructed and interacted with human ACE2 by computational methods. Our results suggest that mutations in RBD amino acids K417, E484, L452, T478 and N501 are expressively increasing the affinity of this protein with human angiotensin-converting enzyme 2 (ACE2), consequently, variants Alpha (B.1.1.7), Beta (B1.351), Gamma (P.1) and Delta (B.1.617.2) could be more infective in human cells compared with SARS-CoV-2 isolated in Wuhan-2019 and the Gamma and Delta variants could be the most infective among them.
Keywords: SARS-CoV-2, New variants, Spike protein, Receptor binding domain, Human ACE2
1. Introduction
In 2002–2003, the outbreak of severe acute respiratory syndrome occurred due to SARS-CoV in the Guangdong Province of China and quickly spread to twenty-seven countries.1, 2, 3 One decade later, in 2012, MERS-CoV caused a severe respiratory disease that emerged in the Middle East with 2494 confirmed cases of human infection and 858 deaths.4, 5, 6, 7 The rate of human-to-human transmission of SARS-CoV-2 is higher than earlier outbreaks of Coronavirus via cough and/or sneezing droplets emitted from infected people.1,8 Due to this rapidly spreading and no efficient repairing mechanisms for RNA mutations, SARS-CoV-2 is susceptible to several and constant mutations. Notably, a single SARS-CoV amino acid change, Spike D480 A/G in the receptor binding-domain (RBD), arose in infected humans and civets and became the dominant variant among 2003/2004 viruses. SARS-CoV-2 is a positive single-stranded RNA virus whose genome encodes four structural proteins: spike (S), small protein (E), matrix (M), and nucleocapsid (N). The Spike protein is a type I fusion protein that forms trimers on the surface of the virion9,10,11,.12, 13, 14 It is composed of two subunits, with S1 responsible for receptor-binding and S2 for membrane fusion. SARS-CoV-2 utilizes angiotensin-converting enzyme 2 (ACE2) as the receptor for entry into target cells.15, 16, 17, 18, 19, 20, 21, 22, 23 Therefore, the S protein determines the infectivity of the virus and its transmissibility in the host. According to several studies, receptor-binding domain (RBD) from Spike protein of SARS-CoV-2 contain six most important amino acids residues (L455, F486, Q493, S494, N501, and Y505) that mediate virus entry into the host cells.12,14,17,26, 27, 28, 29, 30, 31 However, mutations in other amino acids from Spike protein (S) could affect virus infection despite his interaction with human ACE2. Understanding the effect of amino acid substitutions in S protein from new variants comparing the strains on the transmissibility and virulence of SARS-CoV-2 is of broad and immediate interest. In the present study, we assess the impact of the RBD associated amino acid substitutions in the UK (Alpha, B.1.1.7), South Africa (Beta, B.1.351), Brazil (Gamma, P.1) and India (Delta, B.1.617.2) SARS-CoV-2 variants.
2. Methods
2.1. Amino acid sequence alignment
Complete genome from SARS-CoV-2 and Spike protein sequence isolated in Wuhan was downloaded from GenBank (NC_045512.2). SARS-CoV-2 amino acid substitutions in the Spike protein were obtained from CDC (https://www.cdc.gov/coronavirus) and WHO (https://www.who.int/) web sites and global reports platform (https://cov-lineages.org). SARS-CoV-2 spike protein amino acids (Wuhan) were grouped in peptides of 60 residues and compared with 60 residues peptides of Spike protein from SARS-CoV-2 variants. Receptor binding domain (RBD) regions are highlighted.
2.2. Computational methods
We performed our analyses using the crystallographic models of the SARS-CoV-2 Spike protein available in Protein Data Bank (PDBID 6LZG) and PyMol software to analyses the structures. PDB peptides sequences have been built with Swiss Model on line software and 3D structures constructed by PyMol. Docking analyses was performed by ClusPro 2.0 24,.25
3. Results
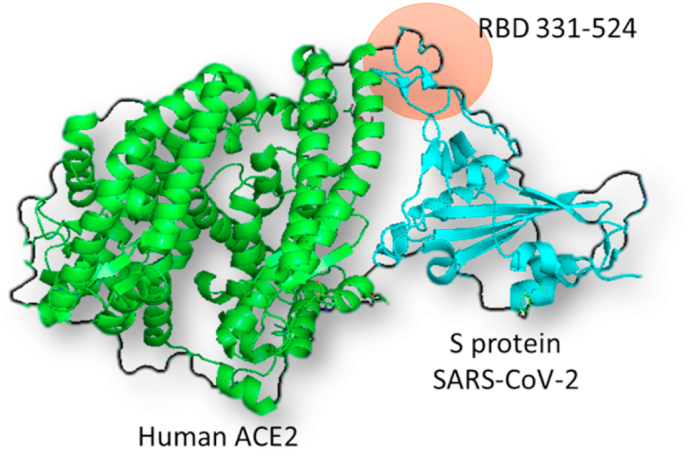
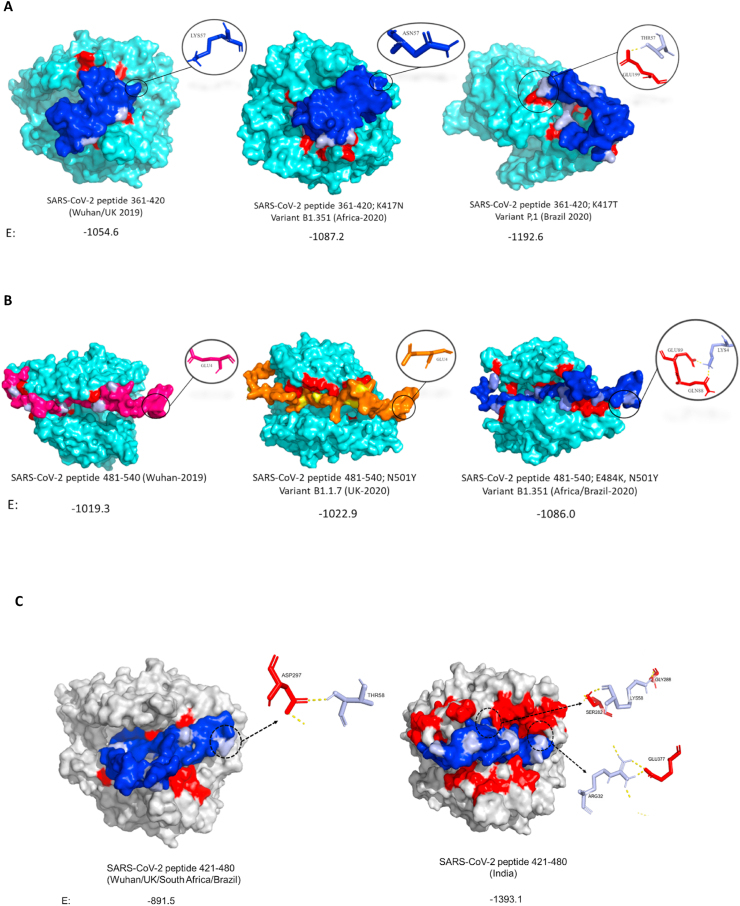
Using the crystal structure from Human ACE2-SARS-CoV-2 S protein deposited in Protein Data Bank (PDBID 6LZG -Fig. 1) and the genome sequence from SARS-CoV-2 (NC_045512.2) we identified and aligned amino acids residues and 3D structures have been built from all mentioned strains (Fig. 2, Fig. 3). Peptides containing RBD mutant regions were built using Swiss online software (Fig. 4) and interacted with human ACE2 using ClusPro 2.0 (Fig. 5), where differences in amino acids interactions from peptides with ACE2 have been identified. Amino acids substitutions Alpha (UK) – N501Y; A570D; P681H; T716I; S982A and D1118H, Beta (South Africa) - D80A; D215G; K417 N; E484K; N501Y; A701V; Gamma (Brazil) - L18F; T20 N; P26S; D138Y; R190S; K417T; E484K; N501Y; H655Y; T1027I and Delta (India) – T19R; L452R; T478K; P681R; D950 N showed that virus mutants presented different regions of mutations compared with SARS-CoV-2 from Wuhan. However, Alpha, Beta and Gamma variants presented the same change N501Y while the Delta variant did not show changes in this amino acid. Interestingly, among RBD amino acids (residues 331–524), Alpha presents only N501Y substitution while Beta presents K417 N; N501Y; E484K, Gamma presents K417 N; E484K; N501Y and Delta presents L452R; T478K. Peptides containing residues 361–420 (E: Wuhan/UK: 1054.6; South Africa: 1087.2 and Brazil: 1192.6), 481–540 (E: Wuhan: 1019.3; UK: 1022.9 and South Africa/Brazil: 1086,0) and 421–480 (E: Wuhan/UK/South Africa/Brazil: 891.5; India: 1393.1) residues showed different binding energy with ACE2 showing that amino acid substitutions at positions 417, 452, 478, 484 and 501 from Spike protein could affect SARS-CoV-2 infectivity.
Fig. 1.
Spike protein from SARS-CoV-2 (blue) interaction with Human angiotensin-converting enzyme 2 (ACE2-green). RBD 331–524 (orange) means reception binding domain amino acids from 331 to 524. PDBID 6LZG. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
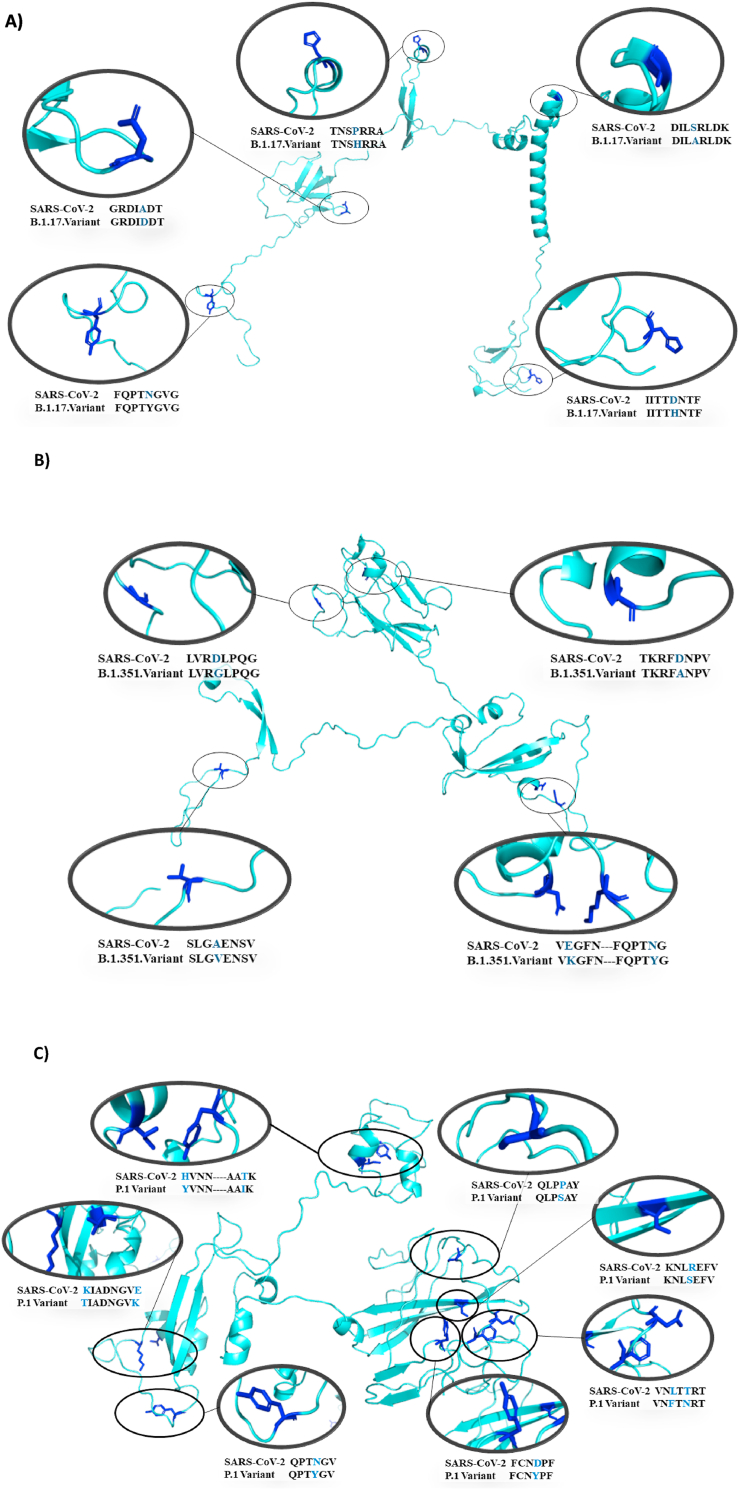
Mutation regions from Spike protein amino acid sequences: SARS-CoV-2 and A) Alpha (B.1.1.7), B) Beta (B.1.351), C) Gamma (P1) and D) Delta (B.1.617.2) variants highlighting RBD regions. Picture shows the alignment of 60 consecutive amino acids residues from S protein where mutations have been occurred.
Fig. 3.
Mutation regions from the Spike protein amino acid sequences: SARS-CoV-2 and A) Alpha (B.1.1.7), B) Beta (B.1.351), C) Gamma (P.1) and D) Delta (B.1.617.2). The picture highlights the mutation regions in the Spike protein. Alpha - N501Y; A570D; P681H; T716I; S982A and D1118H, Beta- D80A; D215G; K417 N; E484K; N501Y; A701V, Gamma- L18F; T20 N; P26S; D138Y; R190S; K417T; E484K; N501Y; H655Y; T1027I and Delta- T19R; L452R; T478K; P681R; D950 N.
Fig. 4.
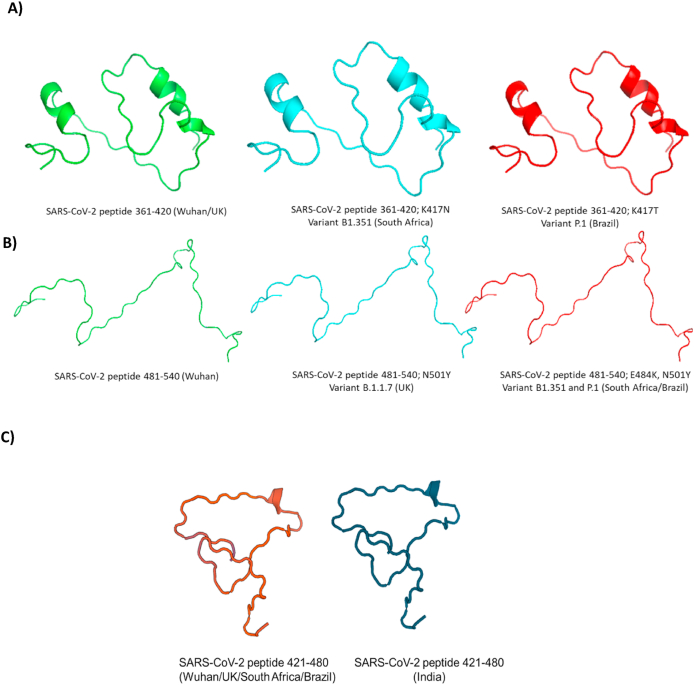
Peptides from SARS-CoV-2 containing amino acids residues A) 361–420 from Wuhan/UK, South Africa and Brazil; B) 481–540 from Wuhan, UK, South Africa/Brazil and C) 421–480 from Wuhan/UK/South Africa/Brazil and India.
Fig. 5.
Peptides of SARS-CoV-2 spike protein interaction with human ACE2. A) Peptides 361–420 from Wuhan/UK (B.1.1.7), South Africa (B1.351), Brazil and B) 481–540 from Wuhan, UK (B.1.1.7), South Africa (B1.351)/Brazil (P.1) and C) 421–480 from Wuhan/UK/South Africa/Brazil and India. E means binding affinity energy between peptides and ACE2 obtained by docking analysis. Interactions of amino acids at position 417 (Lys57, Asn57 and Thr57), 484 (Glu4 and Lys4), 452 (Leu32 and Arg32) and 478 (Thr58 and Lys58) are highlighted. Light Blue (A, B) and gray (C): ACE2, Dark Blue, pink and orange: peptides, Red: Regions of interaction between peptides and ACE2. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Variants are characterized by multiple mutations in the Spike protein (S protein). Under the current public health emergency, it is imperative to understand the mutations that occurred in SARS-CoV-2 since 2019 and investigate if they could bring consequences to virus infection and human disease development. To address these important issues, amino acid sequences of Spike protein from different strains of SARS-CoV-2 (Fig. 1) have been compared and mutations have been highlighted following deposited data from Protein Data Bank (PDB) and Global reports, WHO and CDC (Fig. 2). According to global reports and Health agencies, in comparison with SARS-CoV-2 from Wuhan-2019, the new variants present the following mutations in S protein: Alpha (6 mutations) - N501Y; A570D; P681H; T716I; S982A and D1118H, Beta (6 mutations) - D80A; D215G; K417 N; A701V; N501Y; E484K, Gamma (10 mutations) - L18F; T20 N; P26S; D138Y; R190S; K417T; E484K; N501Y; H655Y; T1027I and Delta (5 mutations) - T19R; L452R; T478K; P681R; D950 N.
Among all mutations, N501Y is of major concern because it involves one of the six key amino acid residues determining a tight interaction of the SARS-CoV-2 receptor-binding domain (RBD) with its cellular receptor angiotensin-converting enzyme 2 (ACE2). However, there are additional mutations in the RBD from Beta and Gamma variants that also could affect the infectivity of SARS-Cov-2 in human cells. As shown, variant Beta carries mutations in the spike protein named as K417 N and E484K while variant Gamma carries K417T and E484K which are not found in the variant Alpha (Fig. 3). To assess if those mutations are able to affect virus infection, 60 residues peptides containing RBD regions and mutations (peptides containing residues 361–420 from Wuhan/UK, South Africa and Brazil and 481–540 from Wuhan, UK and South Africa/Brazil) have been drawn by Swiss model online software (Fig. 4). The interaction with human ACE2 was modeled using software ClusPro 2.0 (Fig. 5). Our results suggest that mutations in spike protein are increasing the interaction with human ACE2 and the amino acid substitutions at positions 417, 484 and 501 are directly contributing to this increase. Additionally, cumulative mutations in the same variants expressively increase the binding energy with ACE2. The variant first identified in Manaus-Brazil (Gamma) presented the highest interaction with ACE2 in both peptides (361–420 – E: 1192.6; 481–540 – E: 1086.0) and could be the most infectious variant among them. While UK (Alpha) variant presents N501Y substitution, the variant from Africa (Beta) presents additional substitutions K417 N and E484K. Lys484 interacts with Glu89 and Gln88 from ACE2 while E484 did not present interactions with amino acids from this receptor. K417 N substitution increased the binding energy compared with Wuhan and UK lineages (E: 1054.6 to −1087.2), but the amino acid does not interact with the receptor in all peptides. The Brazilian variant (Gamma) carries peculiar substitutions K417T additionally to E484K and N501Y. Our results suggest that T417 interacts with Glu199 from ACE2 while Lys417 (Wuhan) and N417 (South Africa) do not present interaction with amino acids from ACE2. Interestingly, the two modifications in the RBD region in the variant from India (L452R and T478K), expressively increased the interaction with ACE2 showing the highest binding energy of all compared peptides.
5. Conclusion
In conclusion, mutations in Spike protein from SARS-CoV-2 are expressively increasing the affinity of this protein with human angiotensin-converting enzyme 2 (ACE2), consequently variants Alpha, Beta, Gamma and Delta could be more infective in human cells compared with SARS-CoV-2 isolated in Wuhan-2019 and the Gamma and Delta variants could be the most infective among them.
Financial support
This project is supported by São Paulo Research Foundation, FAPESP, Brazil with grants: FAPESP 20/12519–4 and FAPESP 20/05761–3.
Declaration of competing interest
The authors declare that there is no interest conflict.
References
- 1.Anderson R.M. Epidemiology, transmission dynamics and control of SARS: the 2002-2003 epidemic. Philos. Trans. R. Soc. B Biol. Sci. 2004;359:1091–1105. doi: 10.1098/rstb.2004.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger A., Preiser W.S.A.R.S. 2019. In Encyclopedia of Environmental Health. [DOI] [Google Scholar]
- 3.Peiris J.S.M., Poon L.L.M. Severe acute respiratory syndrome (SARS) Encyclopedia of Virology. 2008 doi: 10.1016/B978-012374410-4.00780-9. [DOI] [Google Scholar]
- 4.Memish Z.A., Perlman S., Van Kerkhove M.D., Zumla A. Middle East respiratory syndrome. Lancet. 2020 doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015 doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghinai I. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Z. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020 doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 10.Lu R. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr A.R., Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses: Methods and Protocols. 2015 doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega J.T., Serrano M.L., Pujol F.H., Rangel H.R. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: an in silico analysis. EXCLI J. 2020 doi: 10.17179/excli2020-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang J. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020 doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamming I. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004 doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020 doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheblawi M. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020 doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020 doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler C.G.K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020 doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comeau S.R., Gatchell D.W., Vajda S., Camacho C.J. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004 doi: 10.1093/nar/gkh354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozakov D. The ClusPro web server for protein-protein docking. Nat Protoc. 2017 doi: 10.1038/nprot.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi C. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F., Li W., Farzan M., Harrison S.C. Structural biology: structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;80 doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 28.Tian X. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect. 2020 doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020 doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan R. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;80– doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tai W. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]