Abstract
Melanoma is one of the most aggressive types of cancer wherein resistance to treatment prevails. Therefore, it is important to discover novel molecular targets of melanoma progression as potential treatments. Here we show that paired-like homeodomain transcription factor 1 (PITX1) plays a crucial role in the inhibition of melanoma progression through regulation of SRY-box transcription factors (SOX) gene family mRNA transcription. Overexpression of PITX1 in melanoma cell lines resulted in a reduction in cell proliferation and an increase in apoptosis. Additionally, analysis of protein levels revealed an antagonistic cross-regulation between SOX9 and SOX10. Interestingly, PITX1 binds to the SOX9 promoter region as a positive regulatory transcription factor; PITX1 mRNA expression levels were positively correlated with SOX9 expression, and negatively correlated with SOX10 expression in melanoma tissues. Furthermore, transcription of the long noncoding RNA (lncRNA), survival-associated mitochondrial melanoma-specific oncogenic noncoding RNA (SAMMSON), was decreased in PITX1-overexpressing cells. Taken together, the findings in this study indicate that PITX1 may act as a negative regulatory factor in the development and progression of melanoma via direct targeting of the SOX signaling.
Subject terms: Cancer, Cell biology, Molecular biology, Oncology
Introduction
Melanoma is the most lethal form of skin cancer, and is one of the most aggressive types of human cancer that develops from melanocytes1. Surgical resection is the most common treatment for melanoma with early diagnosis, but the prognosis of highly metastasized melanoma is poor, with only a 5-year survival rate2. The aggressiveness of melanoma is attributed to the combined effects of oncogenic signal pathways and cancer-related transcription factors3. About 50% of all melanomas harbor activating BRAF mutations (over 90% V600E). BRAFV600E has been implicated in different mechanisms underlying melanoma tumorigenesis, most of which are due to deregulated activation of mitogen activating protein kinase (MAPK) pathways4. Thus, BRAF inhibitors are important chemotherapeutic treatments for unresectable or metastatic BRAF mutated melanoma. Although the anti-tumor effects of BRAF inhibitors are impressive, the durability of the response is limited due to drug resistance5. However, immunotherapy is currently changing the landscape of melanoma treatment; immunomodulatory therapies, such as anti-PD-1 and anti-CTLA-4 antibody drugs, are the standard of care for patients with metastatic or unresectable melanoma. Unfortunately, despite the promising results, only 20–40% of the patients with melanoma show long-term benefits, whereas the remaining 80% develop resistance to these immune-checkpoint inhibitors6. Therefore, melanoma treatment requires the development of new targets that are effective against resistance.
Paired-like homeodomain 1 (PITX1), which belongs to the category of bicoid-related homeobox genes, plays a role in the development of the Rathke pouch and adult pituitary gland7. PITX1 was originally found as a transcription factor gene with pituitary specific expression8. On the other hand, PITX1 has multiple tumor suppressive functions that inhibit the RAS pathway9 and induce the activation of p53 transcription10. In addition, we identified PITX1 as a novel suppressor gene for human telomerase reverse transcriptase (hTERT), which mainly regulates telomerase, and is active in most tumors. PITX1 directly binds to specific PITX1 response element sites in the hTERT promoter region, resulting in telomerase inhibition11. Moreover, downregulation of PITX1 is observed in various cancers including malignant melanoma12–16. Collectively, this evidence suggests that PITX1 dysfunction induces activation of oncogenic pathways and promotes cancer development.
SOX9 and SOX10 in the SOX (SRY-box) gene family of transcription factors have a crucial role in neural crest (NC) development during the embryonic stage17. NC cells are a transient embryonic cell population that gives rise to most of the peripheral nervous system, chondrocytes and osteoblasts of craniofacial structures, and melanocytes. Previous studies demonstrated the essential role of SOX10 in the pathogenesis of nevi and melanoma in both mice and humans by regulating cell proliferation and survival of melanocytic cells18,19. Additionally, mouse Sox10 haploinsufficiency fully prevents NrasQ61K-driven formation of melanoma development in vivo, which shows that SOX10 plays a crucial role in melanomagenesis18. In contrast, SOX9 is expressed in normal human melanocytes but its expression is downregulated in nevi and melanoma. Moreover, overexpression of SOX9 in both human and mouse melanoma cell lines inhibits cell proliferation in vitro and in vivo20. Intriguingly, another study showed that SOX9 and SOX10 are functionally antagonistic regulators of postnatal melanocyte and melanoma development. SOX9 protein suppresses SOX10 transcription by directly binding to its promoter21. These findings indicate that downregulation of the SOX9 could promote melanoma proliferation by activating SOX10 expression. Alternatively, the overexpression of SOX9 results in an increased invasion in vitro and increases the number of metastases in a mouse model22. Therefore, it is likely that SOX9 displays oncogenic and tumor-suppressive functions, suggesting that SOX9 plays a role in switching the phenotype of melanoma cells23.
Researchers have found that melanoma cells express two distinct gene expression signatures, and these signatures correlate with in vitro characteristics, which are reversible depending on their cellular microenvironments. One signature is characterized by the low expression of SOX9 and high expression of SOX10. These melanoma cells are highly proliferative and less invasive in vitro; thus, they are named as proliferative phenotype. The other signature is characterized by the high expression of SOX9 and low expression of SOX10. In contrast to the proliferative cells, these cells are highly invasive but have a low proliferative capacity in vitro and are named as invasive phenotype21. These findings suggest that the expression levels of SOX9 and SOX10 are a marker of melanoma subtype.
Survival Associated Mitochondrial Melanoma-Specific Oncogenic Non-Coding RNA (SAMMSON) was identified as being specifically expressed in melanoma by in silico analysis24. SAMMSON was expressed in most malignant melanomas but was barely detectable in normal melanocytes and benign lesions. SAMMSON is a target of the lineage-specific transcription factor SOX10. Importantly, SAMMSON silencing reduced melanoma cell growth and survival independently of the mutation state of BRAF, NRAS, or p53. Furthermore, SAMMSON silencing produced a response in BRAF inhibitor resistant melanoma cells and phenocopied the effects of downregulation of mitochondrial metabolism protein p32, causing dysfunction of mitochondria and resulting in tumor annihilation24. These findings show the potential of SAMMSON as an informative biomarker of malignancy and a novel therapeutic target of melanoma.
Here, we identified PITX1 as a positive regulator of SOX9 gene via direct binding to its promoter. Overexpression of PITX1 in SOX9low/SOX10high-expressing human melanoma cell lines (proliferative phenotype) inhibited cell proliferation and tumor growth in xenografts, but that of SOX9high/SOX10low-expressing human melanoma cell lines (invasive phenotype) did not. Additionally, induction of PITX1 strongly suppressed SOX10 expression and SAMMSON transcription, which act as oncogenic driver genes in melanoma, via up-regulation of SOX9. Moreover, PITX1 mRNA expression level was positively correlated with SOX9 mRNA expression level in human melanoma and normal skin tissues. Taken together, these findings suggest that PITX1 plays a suppressor role in the proliferative phenotype of melanoma cells as an upstream transcription factor of SOX9 and SOX10.
Results
Transient overexpression of PITX1 inhibits melanoma cell proliferation and induces apoptosis
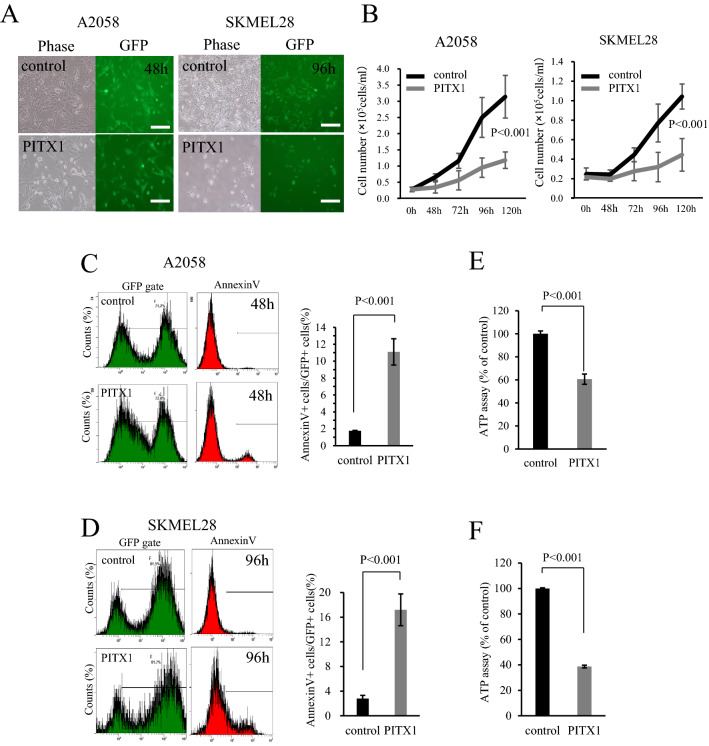
We have previously identified that as a tumor suppressor gene, PITX1 transcription factor directly regulates TERT expression in both mouse and human melanoma11. To investigate whether PITX1 has multiple functions as a tumor suppressor gene, we generated A2058 (PITX1-A2058) and SKMEL28 (PITX1-SKMEL28) cells that transiently overexpress PITX1 or a control vector. As shown in Fig. 1A, a fluorescence microscopy analysis 48 or 96 h after transfection indicated a high infection efficiency for both the control and PITX1 lentiviral vector encoding GFP. The GFP-positive cell rate is shown in Supplementary Fig. S1. PITX1-A2058 and PITX1-SKMEL28 exhibited significantly decreased cell proliferation compared to control cells with the lentiviral vector (Fig. 1B). To further explore the effect of PITX1 on cell proliferation, we analyzed apoptosis through flow cytometry using Annexin V staining and cytostasis through ATP assay. Annexin V positive cells gated on GFP had increased numbers of both PITX1-A2058 and PITX1-SKMEL28 cells compared to control cells (apoptotic cells in PITX1-A2058 were increased 6.6-fold, and 6.1-fold in PITX1-SKMEL28 compared to control cells, respectively) (Fig. 1C,D). Moreover, quantitative ATP levels were reduced in PITX1-induced cells (PITX1-A2058 and PITX1-SKMEL28 cells) compared with the control cells at 96 h (ATP level in PITX1-A2058 and PITX1-SKMEL28 was decreased by 40% and 60%, respectively, compared with the control cells) (Fig. 1E,F). These data indicate that overexpression of PITX1 inhibited melanoma cell proliferation, accompanied by inducing apoptosis and cytostatic effect. On the other hand, reduction of telomerase activity through PITX1 eventually leads to induction of a replication cellular senescence after several cell divisions. These results suggested that tumor suppression by PITX1 may be functionally distinct from TERT repression.
Figure 1.
Overexpression of PITX1 inhibits melanoma proliferation. (A) A2058 and SKMEL28 cell lines were infected with control and PITX1 expression lentivirus vectors. Infection efficiency was monitored after 48 h or 96 h by fluorescence analysis of vector-encoded GFP (right panels). Phase contrast images are shown at left. Scale bars: 100 µm. (B) Number of PITX1 transfected cells and control cells over 5 days. A2058 cells are shown at left, and SKMEL28 cells are shown at right. Bars correspond to means ± S.D. for three independent experiments (P < 0.001). Curve images of growth were created using Excel. (C,D) Flow cytometric analysis of apoptosis in PITX1 overexpressing cells (C: A2058, D: SKMEL28). Quantification of apoptosis as % Annexin V positive cells in GFP positive gate. Bars correspond to means ± S.D. of three independent experiments (P < 0.001). Annexin V-positive cell count images were created using Kaluza software, and the bar graphs were created using Excel. (E,F) ATP assay in PITX1-overexpressing cells (E: A2058, F: SKMEL28). ATP content was measured using CellTiter-Glo 2.0 After Assay Kit at 96 h after infection with PITX1 expressing or control lentivirus vector. Values are expressed as a percentage of control. Bars correspond to the means ± SD of three independent experiments (P < 0.001). These bar graphs were created using Excel.
Identification of active enhancers in PITX1-expressing cells using ChIP-seq
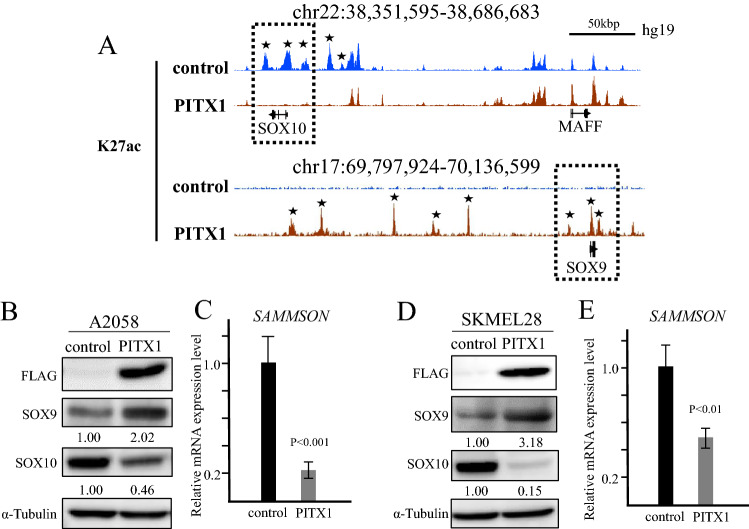
To determine target genes for PITX1 in melanoma cells, we performed ChIP-seq analysis in PITX1 stably transfected A2058 cells (PITX1s-A2058: Supplementary Fig. S2) using anti-Acetyl-Histone H3 Lys27 (H3K27ac) antibody. H3K27ac is one of the active enhancer markers in chromatin. We found that H3K27ac in PITX1s-A2058 cells, but not in control cells, was enriched across the SOX9 region, whereas the peaks around the SOX10 genomic region disappeared. Conversely, MAF BZIP transcription factor F (MAFF), a gene encoding near SOX10, showed no change in the peaks around the gene region in these cells (Fig. 2A). To investigate whether the expression profiles for SOX9 and SOX10 are correlated with the change in status of H3K27ac, we performed a western blotting analysis of PITX1-A2058 cells using anti-SOX9 and SOX10 antibodies. As shown in Fig. 2B, SOX9 and SOX10 expression levels were significantly increased and decreased in PITX1-A2058 cells, respectively. Interestingly, the expression of SAMMSON, which is known as a melanoma-specific long non-coding RNA that is regulated by SOX1024, coincided with a reduction in SOX10 (Fig. 2C). In addition, a similar phenomenon was also observed in PITX1-SKMEL28 cells (Fig. 2D,E). These results suggest that PITX1 could be a vitally important upstream regulator that controls the signaling pathway connecting SOX9 to SAMMSON via SOX10.
Figure 2.
H3K27ac ChIP-seq in PITX1-expressing cells. (A) Integrated Genome Browser (IGB) screenshots of ChIP-seq data shows H3K27ac at the SOX9 and SOX10 loci in PITX1 overexpressing A2058 (red) and control cells (blue). Stars indicates the regions where the peak of H27Kac differs between PITX1-overexpressing cells and control cells. (B) Western blotting analysis of the protein level of PITX1 (Flag tagged), SOX9 and SOX10 in A2058 cells at 48 h after infection with PITX1 expressing or control lentivirus vector. The expression levels of SOX9 and SOX10 were normalized to α-tubulin levels. Cropped blots were used in this figure. Original full-length blots are presented in Supplementary Fig. S8. (C) qRT-PCR analysis of relative SAMMSON RNA expression levels in PITX1 and control lentivirus vector infected A2058 cells. Expression in the vector control cells was arbitrarily set at 1. GAPDH mRNA expression was used as the internal control. Data are presented as means ± S.D. of three independent experiments (P < 0.001). (D) Western blotting analysis of the protein level of PITX1 (Flag tagged), SOX9 and SOX10 in SKMEL28 cells at 48 h after infection with PITX1 expressing or control lentivirus vector. The expression levels of SOX9 and SOX10 were normalized to α-tubulin levels. Cropped blots were used in this figure. Original full-length blots are presented in Supplementary Fig. S8. (E) qRT-PCR analysis of relative SAMMSON RNA expression levels in PITX1 and control lentivirus vector infected SKMEL28 cells. Expression in the vector control cells was arbitrarily set at 1. GAPDH mRNA expression was used as the internal control. Data are presented as means ± S.D. of three independent experiments (P < 0.01). All the bar graphs were created using Excel.
A2058 and SKMEL28 cells are categorized in the proliferative phenotype of melanoma cells (SOX9high/SOX10low). To investigate whether the inhibition of growth by PITX1 was also observed in the invasive phenotype of melanoma cell lines (SOX9high/SOX10low), we performed overexpression analysis of PITX1 in the WM3211 melanoma cell line25. Western blotting showed that WM3211 cells expressed SOX9 at a higher level than A2058. However, the expression level of SOX10 was low in WM3211 cells (Supplementary Fig. S2A). Overexpression of PITX1 in WM3211 did not significantly affect cell proliferation compared with the control cells (Supplementary Fig. S2B,C). Additionally, SOX9 and SOX10 protein levels indicated no change after inducing PITX1 (Supplementary Fig. S2D). These results suggest that the inhibition of cell proliferation by PITX1 is induced in the proliferative phenotype of melanoma cells but not in the invasive phenotype of melanoma cells.
PITX1 directly binds to the SOX9 promoter in vivo
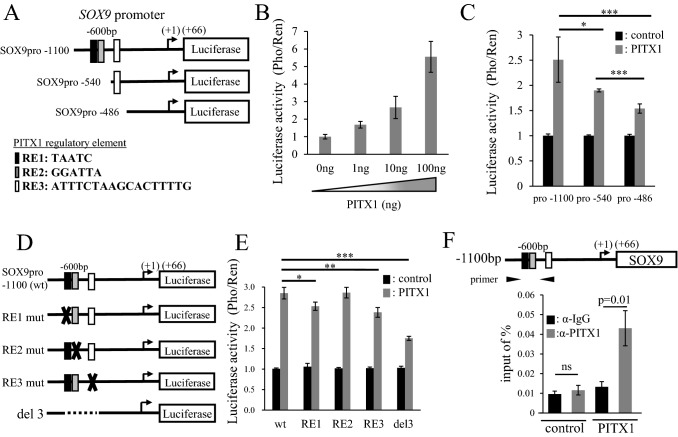
To investigate whether PITX1 upregulates SOX9 mRNA expression through modulation of SOX9 promoter activity, we constructed a SOX9 promoter-luciferase reporter plasmid (pGL3) containing a 1100-bp fragment (SOX9pro-1100) within the SOX9 promoter region (Fig. 3A). Further, to identify potential PITX1 regulatory elements (RE) within the SOX9 gene promoter, we performed in silico analysis using the TRAP web tool26. As a result, we identified three RE sites within the SOX9 promoter. The sites/sequences in the SOX9 promoter region are RE1 (TAATC: − 592/− 588), RE2 (GGATTA: − 578/− 573) and RE3 (ATTTCTAAGCACTTTTG: − 520/− 504) (Fig. 3A and Supplementary Fig. S4). We then investigated the effect of PITX1 co-transfection with the SOX9pro − 1100 Luc reporter into A2058 cells on the transcriptional activity of the SOX9 promoter by measuring Luc reporter activity. The result showed that SOX9 promoter activity was increased by PITX1 overexpression in a dose-dependent manner (Fig. 3B). We next constructed various truncated fragments of the 5′ region of the SOX9 gene (SOX9pro − 540 reporter plasmid including only RE3, and SOX9pro − 486 reporter plasmid without RE) to identify the functional element in RE3 (Fig. 3A). Although SOX9pro − 1100 yielded the strongest promoter activity of these constructs, the promoter activities of SOX9pro − 540 and SOX9pro − 486 were decreased by 30% and 40%, respectively, compared to SOX9pro − 1100. These results suggested that critical positive regulatory elements are present between the − 1100 and − 486 regions. To further explore important REs in the SOX9 promoter, we generated SOX9 mutant promoter vectors containing the RE1 mut (altered TAATC to GTACC), RE2 mut (altered GGATTA to GGTACC), and RE3 mut (altered ATTTCTAAGCACTTTTG to GGTACCGGTACCCAGG) sequences (Fig. 3D). SOX9 promoter activity for the mutation of RE1 and RE3, but not RE2, was reduced by approximately 12% compared to SOX9pro − 1100 (wt) (Fig. 3E). We next constructed reporter plasmids with deletion of RE1, RE2 and RE3 in the SOX9 promoter region (del3) (Fig. 3D). SOX9 promoter activity decreased by 40% in del3 compared to parental SOX9pro − 1100 (Fig. 3E), and showed the same level as that of the truncated fragment SOX9pro − 486 (Fig. 3C). This result suggested that PITX1 could directly access RE1 and RE3 in the SOX9 promoter region.
Figure 3.
Upregulation of SOX9 promoter transcriptional activity by PITX1. (A) The schematic drawing shows the position of the truncation site of each SOX9 reporter plasmid. Black, gray and white boxes indicate PITX1 regulatory elements (RE). (B) PITX1 enhanced relative luciferase activity in a dose-dependent manner. The firefly luciferase activity (Pho) was standardized using Renilla reniformis luciferase activity (Ren) from co-transfected pGL4.70. Luciferase activity in empty-vector-transfected cells (PITX1:0ng) was arbitrarily set at 1. Data are presented as means ± S.D. for three independent experiments. (C) PITX1 expression vectors were co-transfected into the human melanoma cell line A2058 with reporter plasmids containing a full-length promoter (SOX9pro − 1100), or a truncated SOX9 promoter region in which RE sites in the SOX9 promoter region partly or fully eliminated (SOX9pro − 540 and SOX9pro − 486). Luciferase activity in empty-vector-transfected cells was arbitrarily set at 1. Data are presented as means ± S.D. for three independent experiments (*P < 0.05, ***P < 0.001). (D) The schematic diagram shows the luciferase reporter plasmids that encode wild-type and RE mutated versions of the SOX9 promoter region (RE1 mut, RE2 mut and RE3 mut). The cross mark represents mutation. The del3 reporter plasmids are deleted of RE1, RE2 and RE3 in the SOX9 promoter region. (E) PITX1 expression vectors were co-transfected with reporter plasmids containing the wild-type, RE-mutated, or deleted versions of the SOX9 promoter. Luciferase activity in empty-vector-transfected cells was arbitrarily set at 1. Data are presented as means ± S.D. for three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001). (F) Schematic diagram showing the SOX9 promoter and three boxes indicating PITX1 binding regions (upper panel). The black arrows indicate qPCR primers for the RE region in the SOX9 promoter based on a ChIP assay. ChIP assay of RE regions in the SOX9 promoter, showing enrichment with PITX1 antibody compared with IgG controls in A2058 clones. Anti-IgG antibody was used as a negative control. Input represents qPCR for the SOX9 promoter DNA before immunoprecipitation. The data represent the ratio of the target fragment to the input DNA. Bars correspond to means ± S.D. for three independent experiments (P = 0.01, ns: not significant). All the bar graphs were created using Excel.
To determine whether PITX1 directly binds to the SOX9 promoter, we performed a ChIP assay using nuclear extracts prepared from PITX1s-A2058 cells and control cells. The promoter region containing RE sites in the SOX9 promoter exhibited fourfold amplification (qPCR) from chromatin that was precipitated with the anti-PITX1 antibody from PITX1 expressing cells, compared to control cells (Fig. 3F). These findings suggest that PITX1 can directly bind to REs in the SOX9 promoter in A2058 cells.
Expression profile of PITX1, SOX9 and SOX10 in human melanoma tissue
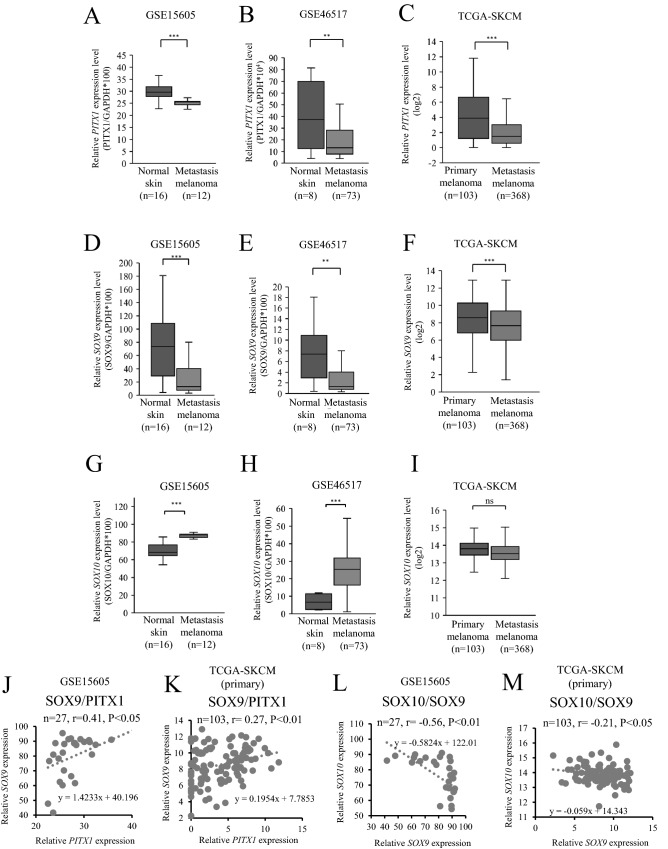
To further investigate the relationship between PITX1, SOX9 and SOX10 expression and clinical prognosis, we used RNA-seq datasets (GSE46517, GSE15606, and the cancer genome atlas [TCGA]-SKCM) from the Gene Expression Omnibus (GEO) and TCGA database to measure the expression level of these genes in melanoma primary, metastatic, and normal skin tissue. As shown in Fig. 4A,B, the relative expression of PITX1 was lower in metastatic tissue compared to normal skin tissue. Additionally, PITX1 expression was lower in metastatic tissue than in primary melanoma tissue (Fig. 4C). Similarly, SOX9 expression was lower in metastatic tissue compared to normal skin tissue (Fig. 4D,E). Moreover, the relative expression of SOX9 was lower in metastatic tissue than in primary melanoma tissue (Fig. 4F). In contrast, SOX10 expression was higher in the metastatic tissue compared to normal skin tissue (Fig. 4G,H). However, there was no sufficient difference in SOX10 expression between primary and metastasis melanoma tissue (Fig. 4I). A possible reason is that SOX9/SOX10 signaling may be disrupted in highly malignant melanoma cells; therefore, it likely plays a more important role in carcinogenesis compared with that in malignancy. Furthermore, in agreement with our result, PITX1 expression levels were positively correlated with SOX9 levels in tumor and normal skin tissues (Fig. 4J). In primary melanoma tissue, we found a positive correlation between SOX9 and PITX1 expression in the TCGA data set (Fig. 4K). According to a previous report21, SOX9 expression levels were negatively correlated with SOX10 levels in tumor and normal skin tissues (Fig. 4L). Expression levels of SOX9 were negatively correlated with those of SOX10 levels in the TCGA primary melanoma tissue data set; however, it is lower than the GEO data set (Fig. 4M). These findings suggested that a loss of PITX1 expression leads to up-regulation of SOX10 by down-regulation of SOX9, eventually promoting the progression of human melanoma tissue.
Figure 4.
PITX1 and SOX9 are positively correlated in clinical melanoma tissues. (A–I) The relative expression levels of PITX1, SOX9, and SOX10 in metastatic melanoma samples and normal skin or in primary and metastasis melanoma samples were determined using the GSE15605, GSE46517, and TCGA-SKCM RNA-seq datasets. The expression levels were normalized to GAPDH in GEO dataset (**P < 0.01, ***P < 0.001). The expression levels showed log2 scale in the TCGA dataset (***P < 0.001, ns: not significant). (J) Relationship between PITX1 mRNA expression level and SOX9 mRNA expression level in metastatic melanoma samples and normal skin. Pearson’s correlation coefficient (r) was calculated for PITX1 and SOX9 mRNA expression levels in the sixteen normal skin and twelve metastatic melanoma samples. (K) Relationship between PITX1 and SOX9 mRNA expression levels in metastatic melanoma samples and normal skin. Pearson’s correlation coefficient (r) was calculated for PITX1 and SOX9 mRNA expression levels in 103 primary melanoma samples. (L) Relationship between SOX10 mRNA expression level and SOX9 mRNA expression level in metastatic melanoma samples and normal skin. Pearson’s correlation coefficient (r) was calculated for SOX10 and SOX9 mRNA expression levels in the sixteen normal skin and twelve metastatic melanoma samples. (M) Relationship between SOX10 and SOX9 mRNA expression levels in metastatic melanoma samples and normal skin. Pearson’s correlation coefficient (r) was calculated for SOX10 and SOX9 mRNA expression levels in 103 primary melanoma samples. Box and scatter plots were created using Excel.
Gene activation of PITX1 inhibits melanoma proliferation in vivo
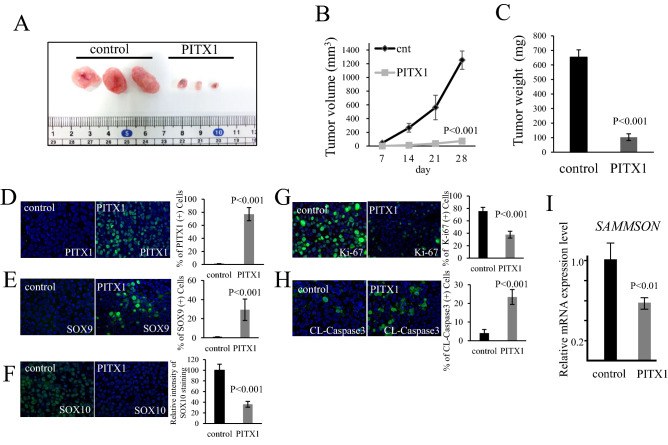
To determine whether PITX1 is important for melanoma growth in vivo, we performed subcutaneous tumor growth analysis using PITX1s-A2058 cells. A2058 cells with PITX1 activation showed significantly smaller tumors than control cells in Bulb-c/nu/nu mice (Fig. 5A). The tumor volume with PITX1s-A2058 was remarkably decreased to 10% of that for the control cells 28 days after subcutaneous inoculation (Fig. 5B). Moreover, the tumor weight was decreased to 15.7% compared to the control cells (Fig. 5C). These results indicate that PITX1 has inhibitory effects on melanoma growth in vivo.
Figure 5.
PITX1 inhibits tumor growth in vivo. (A) Photograph of subcutaneous tumors generated in Balb-c/nu/nu mice with PITX1 transfected A2058 cells and that of the control. The left shows the control (n = 3), and the right shows PITX1 (n = 3). (B,C) Quantification of tumor volume and weight in PITX1 and control samples. The tumor volume curve and bar graph for tumor weight were created using Excel. (D–H) Immunostaining of paraffin sections of the subcutaneous tumors for PITX1, SOX9, SOX10, Ki-67 and cleaved caspase-3 (FITC: Green). DAPI staining for nuclei (blue). Merged FITC and DAPI fluorescent images are shown. Each individual channel’s image is shown in Supplementary Fig. S4. Bar graph shows the number of positive expressing cells (or relative intensity level) for each gene in the PITX1 sample compared to control. Data are presented as means ± S.D. for three independent experiments (P < 0.001). Scale bars: 100 µm. (I) qRT-PCR analysis of relative SAMMSON RNA expression levels in the subcutaneous tumors. Control cells were arbitrarily set as 1. GAPDH mRNA expression was used as the internal control. Data are presented as means ± S.D. for three independent experiments (P < 0.01).
To investigate the gene expression profile of tumors produced from PITX1s-A2058 cells, we performed immunostaining analysis using anti-PITX1, SOX9, SOX10 Ki-67 and cleaved Caspase3 (CL-Caspase3) antibodies. PITX1 and SOX9 showed markedly increased levels in tumors stably expressing PITX1 (Fig. 5D,E). In contrast, SOX10 expression was reduced in this tumor tissue (Fig. 5F). We confirmed these results using qRT-PCR and western blotting (Supplementary Fig. S6). Furthermore, the expression of Ki-67 was significantly reduced in this tumor tissue (Fig. 5G). Conversely, the expression level of CL-Caspase3, which is a known apoptosis marker, was remarkably increased in the same tumor tissue (Fig. 5H). Overexpression of PITX1 induced apoptosis in 23% of the cells in vivo, but this is insufficient to explain the inhibitory effect on melanoma growth (Fig. 5B). Cytostasis may also be thought to be induced by PITX1 expression (Fig. 1E). Intriguingly, downregulation of SOX10 expression in tumor cells was associated with decreased SAMMSON expression, suggesting that PITX1 plays a crucial role as a regulatory factor to directly suppress melanoma growth thorough the SOX9-SOX10 and SAMMSON pathway (Fig. 5I). The findings of this study revealed that PITX1 has a multifunctional role in tumor suppression, such as in the regulation of telomerase activity and the SOX signaling (Fig. 6).
Figure 6.
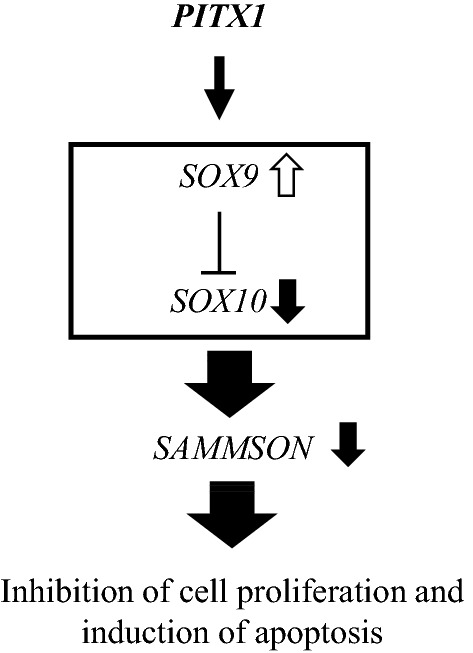
Working model for PITX1 function in melanoma. PITX1 activates SOX9, which suppresses SOX10 and SAMMSON to inhibit melanoma growth and proliferation.
Discussion
We have reported that PITX1 directly regulated SOX9 through targeting the promoter region in melanoma cells. Intriguingly, increased SOX9 expression by PITX1 eventually leads to suppressive effects on cell proliferation and induction of apoptosis via downregulation of SOX10 and SAMMSON, which play an oncogenic role in melanoma. Thus, our findings in this study provide evidence that PITX1 has the ability to act as an upstream regulator of the SOX9-SOX10 and SAMMSON pathways. Interestingly, another group showed that PITX1 activates the SOX9 promoter through a unique binding motif during human astrocyte differentiation27. This suggests that PITX1 functions as an upstream factor of SOX9 during physiological differentiation in normal cells.
Although upregulation of SOX9 expression in SOX10-inhibited melanoma cells was observed as a reduction in cell proliferation and metastasis, high levels of SOX9 expression can restore melanoma progression without an effect on SOX1028, suggesting that this different phenotype depends on the SOX9 expression level. High levels of SOX9 expression induce up-regulation of NEDD9 (Neural Precursor Cell Expressed, Developmentally Down-Regulated 9), a member of the Crk-associated substrate (CAS) family of signal transduction proteins, instead of SOX10 inhibition. NEDD9 has a role as a scaffolding protein in regulating tumor progression in various cancers including melanoma28. In our study, NEDD9 expression was not affected by the overexpression of PITX1 (Supplementary Fig. S7). This data suggested that the control mechanism of SOX9 by PITX1 is different from the regulator of the SOX9-NEDD9 signaling pathway that causes the development of melanoma cells.
In this study, we demonstrated the inhibitory effect of PITX1 in the proliferative phenotype of melanoma cell lines. In contrast, RNA-seq data comparing proliferative and invasive melanoma samples showed that the expression of PITX1 and SOX9 was upregulated in the invasive state23. Therefore, a detailed analysis of the role of PITX1 in the invasive phenotype of melanoma cells is an important issue to be addressed in the future.
We previously found that microRNA-19b (miR-19b) directly inhibits PITX1 mRNA translation through a miR-19b binding site within the 3′ UTR of PITX1 mRNA29. Moreover, the expression of miR-19b depends on the SOX10 expression level in Schwann cells, whose precursors arise from the neural crest. Indeed, reduction of SOX10 expression by siRNA induced a decrease in miR-19b expression30. These results show that SOX10 overexpression may trigger an increase in the levels of miR-19b expression and lead to a decrease in PITX1 expression in melanoma cells. In addition, our present data showed that PITX1 inhibits SOX10 expression. It is therefore likely that a negative feedback relationship exists for the regulation of PITX1 and miR-19b via SOX10 expression in melanoma cells. Further study involving a detailed analysis of the regulation of miR-19b and SOX10 transcription may contribute greatly to the discovery of a novel melanoma inhibition pathway.
We and the other researchers showed that SAMMSON expression is positively correlated with the expression level of SOX10. In this study, SAMMSON expression level was less reduced in SKMEL28 compared with A2058 by the overexpression of PITX1, whereas SOX10 was more efficiently abolished in SKMEL28 compared with A2058 (Fig. 2B,D). Furthermore, another group indicated that sumoylation at K55 in SOX10 is important for its transcriptional activity toward SAMMSON under the treatment of BRAF inhibitor31. Therefore, it is possible that the amount of sumoylated SOX10 protein was more reduced in A2058 compared with in SKMEL28 under the PITX1 overexpression condition.
We previous study showed that PITX1 induced apoptosis in human breast cancer MCF-7 cells as a p53 activator, and forced expression of PITX1 resulted in p53-independent apoptosis in human osteosarcoma MG-63 cells via an unknown mechanism10. In addition, PITX1 expression is downregulated in osteosarcoma tissues and is correlated with the patient survival rate32. In our study, overexpression of PITX1 resulted in apoptosis both in human melanoma A2058 and SKMEL28 cells that carry a dysfunctional p53 mutation33. Therefore, our results indicate that the PITX1-SOX9 signaling pathway could contribute to the regulation of p53-independent apoptosis to prevent development of cancers including melanoma and osteosarcoma.
We previously identified PITX1 as one of the regulatory factors for telomerase activity that controls hTERT transactivation, and as a novel tumor suppressor gene11. Telomerase activity, which is detected in most cancer cells, contributes to cell immortalization via elongation of telomeres. Mutation of the hTERT promoter is reported to occur in over 70% of human melanoma tissues. Mutation hotspots were discovered in the promoter region (− 146C > T and − 124C > T) and are known to generate a new motif for transcription factors, which has been linked to ectopic hTERT reactivation34. We showed that over-expression of PITX1 suppressed hTERT mRNA transcription using A2058 cells with an hTERT promoter mutation (− 124C > T)10. This result indicated that PITX1 has the ability to suppress hTERT expression regardless of the mutation status of the hTERT promoter. PITX1 thereby plays a crucial role in regulating oncogenic signaling pathways, such as telomerase activity and SOX-SAMMSON transcription, suggesting that control of PITX1 may strongly contribute to a novel therapeutic strategy to target melanoma.
BRAF mutations have been identified in 40–50% of melanoma patients4. BRAF inhibitors (e.g., vemurafenib and dabrafenib) contribute to improvements in the clinical status of these patients. Unfortunately, resistance to these agents typically occurs 6–8 months after initiation of therapy in the vast majority of patients treated with vemurafenib and dabrafenib5. Therefore, identification of new targets for melanoma therapy is important to overcome the issue of resistance to drug therapy. SAMMSON silencing reduced cell proliferation and induced apoptosis independently of the BRAF, NRAS, or p53 mutation status in melanoma cells. Importantly, melanoma cells showing resistance to BRAF inhibitors were also sensitive to SAMMSON silencing. SAMMSON, which is one of the novel candidate targets of melanoma therapy24, is regulated by the SOX10 gene. The SOX10 binding consensus sequence is present upstream of the SAMMSON gene loci; moreover, knockdown of SOX10 led to a reduction in SAMMSON expression24. In our study, overexpression of PITX1, which was correlated with suppression of SOX10 expression, reduced SAMMSON expression. On the other hand, sorafenib is a kinase inhibitor that acts on many protein kinases, such as VEGF, PDGFR, and RAF, and is also reported to up-regulate PITX1 by inhibiting the phosphatase activity of PTP1B, which promotes proteasomal degradation of PITX135. Restoration of PITX1 by sorafenib treatment resulted in inhibition of RAS activity in hepatocellular carcinoma (HCC) cell lines. Additionally, inhibition of PTP1B phosphatase activity was accompanied by up-regulation of PITX1 expression in colon cancer cell lines by the receptor tyrosine kinase inhibitor regorafenib36. The Expression Atlas web tool showed that PTP1B expression is positive in melanoma cell lines as well as in colon and HCC cell lines (https://www.ebi.ac.uk/gxa/home). These data suggest the possibility that sorafenib and regorafenib could up-regulate PITX1 in melanoma cells, which may affect the SOX9/SOX10 signaling via restoration of PITX1 expression. Thus, more detailed experiments using combinations of BRAF inhibitors and PITX1 inducing drugs for melanoma treatment will lead to the development of anticancer agents through novel targets.
Materials and methods
Cell culture
Cell culture was performed as described previously37. A2058 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). SKMEL28 cells were obtained from the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University, Japan. A2058 and SKMEL28 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA). WM3211 cells were obtained from the Rockland Immunochemicals Inc (Limerick, PA, USA) and maintained in a culture medium consisting of 80% MCDB153 (Sigma) with 20% Leibovitz’s L-15 (Sigma), 2% FBS (HyClone), and 1.68-mM CaCl2 (Sigma). All cells were cultured at 37 °C in a humidified incubator under 5% CO2. FLAG-PITX1 stable expression A2058 clones were maintained in DMEM supplemented with 10% FBS and G418 (300 μg/ml; Calbiochem, La Jolla, CA, USA). All cell lines were confirmed to be mycoplasma-free using a MycoAlert mycoplasma detection kit (Lonza, Walkersville, MD, USA) and were not passaged more than 20 times from the validated stocks. Fluorescent images were obtained using a KEYENCE BZ-X710 microscope (Keyence, Osaka, Japan) using an objective with a 40 × magnification.
Cell proliferation assay
Cell proliferation assay was performed as described previously29. Cells (2.5 × 104 cells/ml) were seeded in a 6 cm2 culture dish. Cells were counted each day and the average cell number in three dishes was quantified using a Coulter Counter Z2 (Beckman Coulter, Woerden, Netherlands).
Analysis of apoptosis
Analysis of apoptosis was performed as described previously37. Apoptotic cells were measured by Annexin V staining using an APC Annexin V apoptosis detection kit with 7-AAD according to the manufacturer's (BioLegend, San Diego, CA, USA) instructions. Annexin V positive but 7-AAD negative (early apoptotic cells) and Annexin V positive and 7-AAD positive (late-stage apoptosis) cells were determined using a Gallios flow cytometer and analyzed using Kaluza software (Beckman Coulter, Brea, CA, USA).
ATP assay
The ATP assay was performed using CellTiter-Glo 2.0 (Promega, Madison, WI, USA), according to the manufacturer’s protocol.
Chromatin immunoprecipitation sequence analysis
We performed chromatin immunoprecipitation (ChIP) as described in Ref.38. A2058 cells were cross-linked for 10 min by 1% formaldehyde and fragmented by a sonicator (UD-211; TOMY SEIKO, Tokyo, Japan). The samples were immunoprecipitated with 4 μg of antibodies against H3K27ac (05-1334, Merck Millipore, Billerica, MA, USA). We used protein A-sepharose beads and G-sepharose beads (GE Healthcare, Barrington, Ill, USA) to immunoprecipitate the fragments. Chromatin immunoprecipitation sequence (ChIP-Seq) libraries were made using a KAPA Hyper Prep kit (Roche, Wilmington, MA, US) with 1 ng of ChIP DNA. Libraries were size-selected prior to PCR amplification using AMPure XP beads (Beckman Coulter, Brea, CA, USA). Multiplexed libraries were run on an Illumina HiSeq 2500 genome sequencer (Illumina Inc. San Diego, CA, USA) using the 50-base pair single read method. The nucleotide sequence data reported are available in the DDBJ Sequenced Read Archive under the accession numbers DRX230605 and DRX230606.
Western blotting analysis
Western blotting was performed as described previously37. Membranes were blotted with rabbit polyclonal antibody against human PITX1 antigen (ab70273, 1:2,000; Abcam, Cambridge, MA, UK), rabbit monoclonal antibody against human SOX9 antigen (ab185966, 1:2000; Abcam), rabbit monoclonal antibody against human SOX10 antigen (ab155279, 1:2000; Abcam), mouse monoclonal antibody against FLAG antigen (F1804, 1:2000; Sigma), or with polyclonal antibody against α-tubulin (PM054-7, 1:5000; MBL, Tokyo, Japan) and the appropriate standard peroxidase-labeled anti-mouse IgG and anti-rabbit IgG secondary antibodies, according to the manufacturer's instructions (GE Healthcare, Piscataway, NJ, USA). Immunoreactive bands were visualized using the ECL detection system (Pierce, Rockford, IL, USA).
qRT-PCR
RNA isolation and reverse transcriptase (RT)-PCR were performed as described previously37. mRNA expression of SAMMSON was analyzed using specific primers: SAMMSON: forward; 5′-CCTCTAGATGTGTAAGGGTAGT, reverse; 5′-TTGAGTTGCATAGTTGAGGAA. cDNA was amplified using an Applied Biosystems StepOne thermal cycler system and SYBR green PCR kit (Foster City, CA, USA). mRNA levels were normalized against GAPDH mRNA (PCR primers: forward; 5′-AGCCACATCGCTCAGACAC, reverse; 5′-GCCCAATACGACCAAATCC).
ChIP assay
The chromatin immunoprecipitation (ChIP) assay was performed as described previously37. The ChIP assay was performed with a SimpleChIP Plus Sonication Chromatin IP kit (Cell Signaling Technology (CST), Danvers, MA, USA) according to the manufacturer's protocol. Briefly, to cross-link the DNA in chromatin to histones, the cells were incubated in 1% formaldehyde for 10 min at 37 °C. After being washed with cold phosphate-buffered saline (PBS) containing protease inhibitors (CST), the cells were resuspended in cell lysis buffer (CST) containing protease inhibitors. DNA then was broken into 100- to 300-bp fragments using a Covaris S220 sonicator (Woburn, MA, USA). The remainder of the sample was immunoprecipitated using anti-PITX1 antibody (ab70273, Abcam) or anti-rabbit IgG antibody (#2729, CST) for 16 h at 4 °C. Protein G magnetic Beads (CST) were then used to collect the immunoprecipitated complexes, which were eluted using elution buffer (CST) after extensive washing. Cross-linking was then reversed by the addition of 5 M NaCl, which was followed by protease K treatment for 12 h at 65 °C. DNA was recovered using DNA purification columns (CSTs), and was used as a template for PCR amplification of the region of the PITX1 binding sites in the SOX9 promoter. The forward and reverse PCR primers used were: forward, 5′-TTGCAAAAGCGCAGCAGAAT-3′ and reverse, 5′-GTTTTGGTGACTCAACGCCC-3′. DNA was amplified using an Applied Biosystems StepOne thermal cycler system and a SYBR green PCR kit.
Plasmid construction
FLAG-tagged PITX1 expression plasmids were generated as described previously29. FLAG-tagged PITX1 expression lentivirus plasmid pFLAG-PITX1 was constructed by PCR amplification of PITX1 cDNA without the 3′UTR region from genomic DNA using KOD plus DNA polymerase (TOYOBO, Tokyo, Japan) and the following primer sequences: forward primer: 5′-GCTCTAGAATGGACGCCTTCAAGGGGGGCATGAGCCTG, reverse primer: 5′-CGGGATCCTCAGCTGTTGTACTGGCACGCGTTGAGGCC, and was inserted into the XbaI/BamHI digested pLVSIN-EF1α-IRES-ZesGreen1 vector (Takara, Shiga, Japan). The pLVSIN-EF1α-IRES-ZesGreen1 vector was used as a control. Production of lentivirus was performed using a Lentiviral High Titer Packaging Mix (Takara) according to the manufacturer’s protocol.
Various lengths of the SOX9 promoter region (shown in Fig. 3A), which included the transcription start site, were PCR amplified from genomic DNA using KOD plus DNA polymerase (TOYOBO) and inserted into the Acc65I/BglII-digested luciferase (Luc) reporter vector pGL3-basic (Promega). The primer sequence is as follows: for SOX9 pro -1100; forward primer: 5′-GAGGTACCGTGGAGCGTTTTGTCTGCGGTGGTG, reverse primer: 5′-GGAAGATCTTGAAACTGGCGAGTCTCCGCGCCAC, for SOX9 pro − 540; forward primer: 5′-GAGGTACCAACATTTGCTTCAAAAGACTATTTC, reverse primer: 5′-GGAAGATCTTGAAACTGGCGAGTCTCCGCGCCAC, for SOX9 pro − 486; forward primer: 5′-GGTACCGCTACGCATTAAGAAGCGGCTGCTT, reverse primer: 5′-GGAAGATCTTGAAACTGGCGAGTCTCCGCGCCAC.
A PCR-based site-directed mutagenesis kit (Toyobo) was used to generate mutations in a number of nucleotides in the RE site of the SOX9 promoter (shown in Fig. 3D). The PCR primers used were: RE1; forward primer: 5′-GGTACCTATTTATATGGATTA TTACGGAGGA, reverse primer: 5′-ACATGCTCGGGTTCGCCGGGGCTGG, RE2; forward primer: 5′-GGTACCTTACGGAGGAACAGCGGGCGTTGAG, reverse primer: 5′-ATATAAATAGATTAACATGCTCGGG, RE3; forward primer: 5′-GGTACCCAGGCAGGCAGGCTCGCTCCAGGCG, reverse primer: 5′-GGTACCAGTCTTTTGAAGCAAATGTTTTGGT. The sequences of all plasmids were confirmed by DNA sequencing.
Luciferase assay
Luciferase assay was performed as described previously11. The cells were plated in 12-well plates 24 h before transfection. SOX9 promoter reporter plasmids and PITX1 expression plasmids were co-transfected using Lipofectamine LTX reagent (Invitrogen) according to the manufacturer’s protocol. pGL4.70-renilla (Promega) was co-transfected as an internal control. The cells were lysed 48 h after transfection and subjected to a luciferase assay using the Picagene dual Sea Pansy bioluminescence kit (Toyo Ink, Tokyo, Japan) according to standard protocols. All experiments were performed in at least triplicate. Luciferase activity was calculated as the activity of the reporter constructs compared to the Renilla activity.
Expression analysis of human melanoma tissue
Human melanoma tissue mRNA dataset GSE15605, GSE46517 and TCGA-SKCM was downloaded from the GEO (http://www.ncbi.nlm.nih.gov/geo/) and TCGA database (https://portal.gdc.cancer.gov/). GSE15605 included twelve metastatic tumor samples from melanoma patients and sixteen normal skin samples without melanoma as controls, and GSE46517 included seventy metastatic tumor samples from melanoma patients and eight normal skin samples without melanoma as controls. Relative mRNA expression level in the GEO data set was normalized to GAPDH. TCGA-SKCM included 103 primary and 368 metastatic tumor samples from patients with melanoma. Relative mRNA expression level in the TCGA data set was analyzed using the UCSC Xena browser (https://xenabrowser.net/). We used only the mRNA dataset from GEO and TCGA. We have not used any human tissue samples in this study.
Xenograft analysis
All animal experiments were approved by the Institutional Animal Care and Use Committee of Tottori University (Approval number: 19-Y-42). The mice were maintained under specific pathogen-free conditions with a 12-h ligh–dark cycle. Four-week-old female BALB/c-nu/nu (BALB/cSlc-nu/nu) mice were obtained from SLC Japan (Japan, Shizuoka). The animals were injected subcutaneously in the right and left flank regions with 1.0 × 107 A2058 cells expressing FLAG-PITX1 and FLAG-control suspended in 100 ml (PBS). All experiments and methods were performed in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines39. We declared that all methods were carried out in accordance with the relevant guidelines and regulations.
Immunostaining
Luciferase assay was performed as described previously11,16. Dewaxed paraffin sections were used for immunofluorescent staining. The sections were incubated with rabbit polyclonal antibody raised against PITX1 (ab70273, 1:2000; Abcam), SOX9 (ab185966, 1:500; Abcam), SOX10 (ab155279, 1:10,000; Abcam), Ki-67 (pre-diluted, Nichirei, Tokyo, Japan) or cleaved Caspase3 (#9664, 1:400; Cell Signaling Technology) at 4 °C overnight. After being washed in T-TBS, the sections were incubated with secondary Alexa488-conjugated anti-rabbit IgG antibody (#8890, 1:500, Cell Signaling Technology) for 1 h at room temperature. After further washing, coverslips were placed on the glass slides using a water-soluble mounting medium. The slides were observed using fluorescence microscopy. Images were captured using an AxioImagerZ2 fluorescence microscope (Carl Zeiss GmbH, Jena, Germany) with a 40 × objective.
Graphs
Cell growth curves, bar graphs, box plots, and scatter plots were created using Excel (Microsoft Corporation, Redmond, WA, USA). Cell count images of Annexin V-positive cells were created using Kaluza software (Beckman Coulter).
Statistics
Statistics was performed as described previously29. Data from more than three separate experiments are presented as means ± S.D. Significance was established at P-values less than 0.05 using an unpaired two-tailed Student’s t-test. The relationship between the expression levels of genes was calculated by Pearson’s correlation coefficient (r).
Supplementary Information
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid for Early-Career Scientists (Grant Number 18K16030 and 21K16213). This research was partly performed at Tottori Bio Frontier, managed by Tottori Prefecture.
Author contributions
H.K. and T.O. designed the experiments and analyzed the data. T.O., S.N., and J.T. performed the experiments and contributed to discussion. H.K. and T.O. wrote the manuscript. S.N., J.T., and H. A. contributed to data analysis and discussion. H.K. conceived and managed the project. All authors revised and edited the manuscript.
Data availability
All data is provided in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-97791-6.
References
- 1.Uong A, Zon LI. Melanocytes in development and cancer. J. Cell Physiol. 2010;222:38–41. doi: 10.1002/jcp.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandru A, Voinea S, Panaitescu E, Blidaru A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life. 2014;7:572–576. [PMC free article] [PubMed] [Google Scholar]
- 3.Wellbrock C, Arozarena I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015;28:390–406. doi: 10.1111/pcmr.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascierto PA, et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzano JL, et al. Resistant mechanisms to BRAF inhibitors in melanoma. Ann. Transl. Med. 2016;4:237. doi: 10.21037/atm.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szeto DP, et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13:484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamonerie T, et al. Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev. 1996;10:1284–1295. doi: 10.1101/gad.10.10.1284. [DOI] [PubMed] [Google Scholar]
- 9.Kolfschoten IG, et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell. 2005;121:849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Liu DX, Lobie PE. Transcriptional activation of p53 by Pitx1. Cell Death Differ. 2007;14:1893–1907. doi: 10.1038/sj.cdd.4402209. [DOI] [PubMed] [Google Scholar]
- 11.Qi DL, et al. Identification of PITX1 as a TERT suppressor gene located on human chromosome 5. Mol. Cell. Biol. 2011;31:1624–1636. doi: 10.1128/mcb.00470-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, et al. Decreased PITX1 homeobox gene expression in human lung cancer. Lung Cancer. 2007;55:287–294. doi: 10.1016/j.lungcan.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Chen YN, Chen H, Xu Y, Zhang X, Luo Y. Expression of pituitary homeobox 1 gene in human gastric carcinogenesis and its clinicopathological significance. World J. Gastroenterol. 2008;14:292–297. doi: 10.3748/wjg.14.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lord RV, et al. Increased CDX2 and decreased PITX1 homeobox gene expression in Barrett's esophagus and Barrett's-associated adenocarcinoma. Surgery. 2005;138:924–931. doi: 10.1016/j.surg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Nakabayashi M, et al. PITX1 is a reliable biomarker for predicting prognosis in patients with oral epithelial dysplasia. Oncol. Lett. 2014;7:750–754. doi: 10.3892/ol.2013.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osaki M, et al. Decreased PITX1 gene expression in human cutaneous malignant melanoma and its clinicopathological significance. Eur. J. Dermatol. EJD. 2013;23:344–349. doi: 10.1684/ejd.2013.2021. [DOI] [PubMed] [Google Scholar]
- 17.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 18.Shakhova O, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat. Cell Biol. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- 19.Cronin JC, et al. SOX10 ablation arrests cell cycle, induces senescence, and suppresses melanomagenesis. Cancer Res. 2013;73:5709–5718. doi: 10.1158/0008-5472.CAN-12-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passeron T, et al. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J. Clin. Investig. 2009;119:954–963. doi: 10.1172/JCI34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakhova O, et al. Antagonistic cross-regulation between Sox9 and Sox10 controls an anti-tumorigenic program in melanoma. PLoS Genet. 2015;11:e1004877. doi: 10.1371/journal.pgen.1004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng PF, et al. Methylation-dependent SOX9 expression mediates invasion in human melanoma cells and is a negative prognostic factor in advanced melanoma. Genome Biol. 2015;16:42. doi: 10.1186/s13059-015-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verfaillie A, et al. Decoding the regulatory landscape of melanoma reveals TEADS as regulators of the invasive cell state. Nat. Commun. 2015;6:6683. doi: 10.1038/ncomms7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leucci E, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531:518–522. doi: 10.1038/nature17161. [DOI] [PubMed] [Google Scholar]
- 25.Graf SA, Busch C, Bosserhoff AK, Besch R, Berking C. SOX10 promotes melanoma cell invasion by regulating melanoma inhibitory activity. J. Investig. Dermatol. 2014;134:2212–2220. doi: 10.1038/jid.2014.128. [DOI] [PubMed] [Google Scholar]
- 26.Thomas-Chollier M, et al. Transcription factor binding predictions using TRAP for the analysis of ChIP-seq data and regulatory SNPs. Nat. Protoc. 2011;6:1860–1869. doi: 10.1038/nprot.2011.409. [DOI] [PubMed] [Google Scholar]
- 27.Byun JS, et al. The transcription factor PITX1 drives astrocyte differentiation by regulating the SOX9 gene. J. Biol. Chem. 2020;295:13677–13690. doi: 10.1074/jbc.RA120.013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, et al. SOX9 is a dose-dependent metastatic fate determinant in melanoma. J. Exp. Clin. Cancer Res. 2019;38:17. doi: 10.1186/s13046-018-0998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohira T, et al. miR-19b regulates hTERT mRNA expression through targeting PITX1 mRNA in melanoma cells. Sci. Rep. 2015;5:8201. doi: 10.1038/srep08201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokey NG, Srinivasan R, Lopez-Anido C, Krueger C, Svaren J. Developmental regulation of microRNA expression in Schwann cells. Mol. Cell. Biol. 2012;32:558–568. doi: 10.1128/MCB.06270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S, et al. ERK-mediated phosphorylation regulates SOX10 sumoylation and targets expression in mutant BRAF melanoma. Nat. Commun. 2018;9:28. doi: 10.1038/s41467-017-02354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong G, et al. Strong expression of paired-like homeodomain transcription factor 1 (PITX1) is associated with a favorable outcome in human osteosarcoma. Tumour Biol. 2015;36:7735–7741. doi: 10.1007/s13277-015-3512-1. [DOI] [PubMed] [Google Scholar]
- 33.Ji Z, et al. p53 rescue through HDM2 antagonism suppresses melanoma growth and potentiates MEK inhibition. J. Investig. Dermatol. 2012;132:356–364. doi: 10.1038/jid.2011.313. [DOI] [PubMed] [Google Scholar]
- 34.Horn S, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 35.Tai WT, et al. Protein tyrosine phosphatase 1B dephosphorylates PITX1 and regulates p120RasGAP in hepatocellular carcinoma. Hepatology. 2016;63:1528–1543. doi: 10.1002/hep.28478. [DOI] [PubMed] [Google Scholar]
- 36.Teng HW, et al. Protein tyrosine phosphatase 1B targets PITX1/p120RasGAP thus showing therapeutic potential in colorectal carcinoma. Sci. Rep. 2016;6:35308. doi: 10.1038/srep35308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohira T, et al. PITX1 protein interacts with ZCCHC10 to regulate hTERT mRNA transcription. PLoS One. 2019;14:e0217605. doi: 10.1371/journal.pone.0217605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mimura I, et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol. Cell. Biol. 2012;32:3018–3032. doi: 10.1128/MCB.06643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilkenny C, Browne WJ, Cuthi I, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012;41:27–31. doi: 10.1111/j.1939-165X.2012.00418.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is provided in the manuscript.