Abstract
While p53 activity is critical for a DNA damage-induced G1 checkpoint, its role in the G2 checkpoint has not been compelling because cells lacking p53 retain the ability to arrest in G2 following DNA damage. Comparison between normal human foreskin fibroblasts (HFFs) and HFFs in which p53 was eliminated by transduction with human papillomavirus type 16 E6 showed that treatment with adriamycin initiated arrest in G2 with active cyclin B/CDC2 kinase, regardless of p53 status. Both E6-transduced HFFs and control (LXSN)-transduced cells maintained a prolonged arrest in G2; however cells with functional p53 extinguished cyclin B-associated kinase activity. Down regulation was mediated by p53-dependent transcriptional repression of the CDC2 and cyclin B promoters. In contrast, cells lacking p53 showed a prolonged G2 arrest despite high levels of cyclin B/CDC2 kinase activity, at least some of which translocated into the nucleus. Furthermore, the G2 checkpoint became attenuated as p53-deficient cells aged in culture. Thus, at late passage, E6-transduced HFFs entered mitosis following DNA damage, whereas the age-matched parental HFFs sustained a G2 arrest. These results indicate that normal cells have p53-independent pathways to maintain DNA damage-induced G2 arrest, which may be augmented by p53-dependent functions, and that cells lacking p53 are at greater risk of losing the pathway that protects against aneuploidy.
The inability to arrest or undergo apoptosis in response to negative signals is a hallmark of cancer cells. In some cell types, DNA damage leads to cell cycle arrest, presumably to allow time for repair so that cells do not replicate or segregate damaged DNA (27, 32) or to eliminate damaged cells from the proliferative pool (10). While normal cells are capable of arresting in G1 and G2 in response to a genotoxic stress, cells lacking the commonly mutated tumor suppressor gene, p53 (19, 24), arrest solely in G2. DNA damage leads to stabilization of p53 and consequently transcriptional up regulation of the cyclin-dependent kinase (CDK) inhibitor, p21 (12, 21, 61), resulting in arrest in G1.
Although the necessity for p53 in the DNA damage-induced G2 checkpoint has been ruled out by the fact that cells without p53 function are capable of arresting in G2, a role has been suggested in a variety of experimental systems. Overexpression of p53 in p53-null human fibroblasts led to both G1 and G2 block (1). Rat embryo fibroblasts transfected with human ras and the temperature-sensitive mutant tsp53Val135 arrest in G1 and G2 when shifted to the permissive temperature (40, 56). Expression of wild-type p53 in human ovarian cancer cell line by using tsp53Val135 led to arrest in G2 but not G1 (58). The role of p53 in the G2 checkpoint, however, has yet to be demonstrated from DNA damage induction through to arrest, in one experimental system (reviewed in reference 60), or in primary cells. Furthermore, the general observation that p53-depleted cells are capable of a DNA damage-induced arrest in G2 needs to be reconciled with any mechanism proposed for p53 in the G2 checkpoint.
Studies on the G2 transition and checkpoint have focused on CDC2 and its positive regulatory subunit, cyclin B. The kinase activity of this complex and levels of cyclin B oscillate with the cell cycle (14, 15). After binding with cyclin B, the kinase activity of CDC2 is dependent on the phosphorylation status of CDC2. CDC2 undergoes an activating phosphorylation on threonine 161 by CDC7/cyclin H and immediate inhibitory phosphorylation on tyrosine 15 by Wee1 kinase (39, 47) and threonine 14 by Myt1 kinase (36). Activation of cyclin B/CDC2 kinase activity, and subsequent progression into mitosis, is then dependent on the dephosphorylation of the inhibitory sites by CDC25C (15, 18). When faced with genotoxic stress (44), unreplicated DNA (54), or negative cellular signaling (3), cyclin B/CDC2 kinase activation is inhibited and cells arrest in G2. An increase in tyrosine-phosphorylated forms of CDC2 has been associated with DNA damage (26, 45, 50, 59) and inactive kinase (33). Although DNA damage-induced activation of Wee1 kinase may be involved in the mechanism of this inhibitory phosphorylation of CDC2, much evidence in both fission yeast and human cells (17, 48, 51) points to inhibition or sequestration of CDC25C by the 14-3-3 ς protein. 14-3-3 ς is a member of a family of proteins that is expressed in response to a variety of signals, including epithelial differentiation and DNA damage (reviewed in references 34 and 49). The 14-3-3 proteins show sequence homology with the DNA damage-induced Rad24 and Rad25 proteins of fission yeast and have been demonstrated to bind and possibly sequester the activating CDC25C phosphatase for cyclin B/CDC2, thereby leading to a G2 arrest. A possible mechanism for p53’s role in the G2 checkpoint has been reported to involve p53-mediated transcriptional activation of 14-3-3 ς (23), though that model does not explain how cells depleted of p53 are capable of a DNA damage-induced G2 arrest.
Transcriptional regulation of the cyclin B, CDC2, and CDC25 genes have also been proposed as a means to modulate the G2 checkpoint. Irradiation of HeLa cells resulted in an arrest in G2, the maintenance of which correlated with down regulation of cyclin B mRNA and protein (41, 42). This decrease in mRNA levels was in part due to decreased stability of the cyclin B message (7, 38); CDC2 and CDC25 transcription was also decreased (7). p53 overexpression in p53 null EJ bladder cancer cells led to arrest in both G1 and G2/M, with decreased CDC2 and cyclin B transcript levels (57). The down regulation of CDC2 and cyclin B transcripts was attributed to the senescent phenotype (55) rather than a specific function of p53. Recent evidence shows cyclin B and CDC2 protein and mRNA down regulation in a p53- and possibly p21-dependent manner (2, 9), and p53’s role may lie in the transcriptional repression of the cyclin B promoter (25).
Cells which have initiated a G2/M checkpoint in response to DNA damage can succumb to a variety of fates, including apoptosis (reviewed in reference 13), prolonged permanent arrest (35), recovery after repair of DNA damage (reviewed in reference 43), or adaptation to the damage, allowing progression through the cell cycle with the DNA damage that initially evoked the arrest (52). Although roles for p53 in apoptosis and DNA repair have been described, p53’s role in the G2 checkpoint and adaptation remains to be elucidated. Experimental systems which utilize immortalized cells, tumor cell lines, and cells lacking functional p53 that have been grown in culture for multiple population doublings acquire uncharacterized genetic abnormalities, which can confound the interpretation of p53’s role. By comparing colon carcinoma cell lines that differed in p53 status and utilizing extensively passaged human fibroblasts, Bunz et al. concluded that p53 induction of p21 is necessary to sustain G2 arrest after DNA damage (5). We have used a model system in which p53 is depleted from primary human cells by transduction with the retrovirus LXSN, carrying the human papillomavirus type 16 (HPV 16) E6 oncogene (herein referred to as E6 cells). These cells were monitored throughout their proliferative life span and compared to the vector-transduced controls (herein called LXSN cells). We demonstrate that the initiation of the G2 checkpoint is a p53-independent event and show that both LXSN and E6 cells sustain a prolonged G2 arrest, although their mechanisms to maintain the arrest differ. Finally, as E6 but not LXSN cells undergo multiple population doublings, the ability to sustain G2 arrest is lost, reminiscent of neoplastic progression.
MATERIALS AND METHODS
Cell culture and media.
Primary human fibroblasts derived from neonatal foreskin (HFFs) were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (complete medium) at 37°C and 5% CO2. Cells were transduced with LXSN vector or LXSN-16E6 by infection with amphotropic viruses containing each vector as previously described (20). Population doubling level (PDL) counts began with first plating after G418 selection. Cells were counted at each passage; population doublings were determined and added to the previous value. E6 and LXSN cells were stored in liquid nitrogen, in DMEM with 15% FBS and 10% dimethyl sulfoxide. Mouse embryo fibroblasts (MEFs) derived from p53 null (p53−/−) and p21 null (p21−/−) transgenic mice were a gift from Chris Kemp. MEFs were cultured in DMEM supplemented with 10% FBS.
Cell cycle synchronization.
E6 and LXSN cells were grown to confluence and remained so for 24 h. They were released from density arrest by replating at 1 × 106 to 2 × 106 cells per 150-mm-diameter tissue culture plates, or 5 × 105 to 7.5 × 105 cells per 100-mm-diameter plate, in DMEM 10% FBS with aphidicolin, to allow cell cycle progression and synchronization to the G1/S border. After remaining in aphidicolin (Sigma) at 3 μg/ml for 24 h, the cells were washed twice with phosphate-buffered saline (PBS) and refed DMEM–10% FBS. After 3 to 5 h (enough time for cells to proceed into the cell cycle), the treatment group of cells were pulsed with 2 mM adriamycin (ADR; stock solution in PBS) for 1 h at 37°C. They were washed twice in PBS and received complete medium. The cells were harvested for total cellular protein and total RNA and fixed for flow cytometry at several time points after release from synchronization.
Flow cytometry.
Cells were fixed at variable time points after release from density-aphidicolin synchronization. For each time point, cells were trypsinized and fixed with 70% ethanol. The fixed cells were then stained with propidium iodide (50 μg/ml) with RNase (5 μg/ml). The stained cells were analyzed for DNA content by fluorescence-activated cell sorting (FACS) in a FACScan (Becton Dickinson Instruments). Cell cycle fractions were quantified with CellQuest (version 1.2; Becton Dickinson).
Western blotting.
After trypsinization, cells were washed with cold PBS and lysed with WE 16th lysis buffer (Tris-HCl [50 mM, pH 7.5], NaCl [250 mM], EDTA [5 mM], Nonidet P-40 [1%], glycerol [20%], sodium orthovanadate [0.5 mM], β-glycerophosphate [80 mM], sodium fluoride [50 mM], phenylmethyl sulfonyl fluoride [1 mM], leupeptin [25 μg/ml], aprotinin [10 μg/ml], pepstatin [10 μg/ml]). Lysates were sonicated on ice, clarified by centrifugation at 14,000 rpm and stored at −70°C. Protein concentrations were determined by the DC protein assay (Bio-Rad). Nuclear and cytoplasmic extracts were prepared by hypotonic lysis with Dounce homogenization followed by high-salt extraction of the pelleted nuclei according to the basic protocol (1a); 20 μg of total cell lysates was loaded on sodium dodecyl sulfate (SDS)–10 or 12% polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore). Western blot analyses were performed with mouse monoclonal anti-human cyclin B1 (Pharmingen), anti-cyclin E (Pharmingen), anti-p53 (Oncogene Science Ab6), and anti-histone H1 (Upstate Biotechnology), rabbit polyclonal anti-CDC2 (Oncogene Science), and anti-Raf1 (Santa Cruz) antibodies. Secondary antibodies used were 1:35,000 anti-mouse–horseradish peroxidase (Jackson Immunoresearch Laboratories) and 1:20,000 goat anti-rabbit–peroxidase (Boehringer Mannheim) conjugates. Detection was by chemiluminescence (DuPont NEN Research Products) and exposure to X-Omat-Blue film (Kodak).
Kinase assays.
Immunoprecipitations were performed by incubating 100 μg of whole lysate with a 1:50 (vol/vol) ratio of mouse monoclonal anti-cyclin B1 (PharMingen) on ice for 20 min. Protein G-Sepharose (Pharmacia Biotech), equilibrated 1:1 (vol/vol) with H1 wash buffer (Tris-HCl [25 mM, pH 7.5], NaCl [125 mM], MnCl2 [10 mM], dithiothreitol [1.0 mM]), was added to each sample. The samples were rotated at 4°C for 1 h. Precipitated protein pellets were washed twice in lysis buffer, twice in H1 wash buffer, and once in kinase reaction buffer (Tris-HCl [50 mM], NaCl [70 mM], MnCl2 [10 mM], dithiothreitol [1 mM]). Samples were pelleted and incubated for 30 min (a reaction time which has been determined to be in the linear range of the kinase reaction) at 37°C with 25 μl of reaction mix containing kinase reaction buffer with H1 histone (40 μg), unlabeled ATP (10 μM), and [γ-32P]ATP (10 μCi; 10 μCi/μl). Kinase reactions were stopped with the addition of 25 μl of 4× running buffer (Tris-HCl [0.25 M, pH 6.8], SDS [8%], glycerol [40%], β-mercaptoethanol [20%], bromophenol blue [0.05%]) and boiling for 5 min. Of the resultant reaction mix, 15 μl was loaded onto an SDS–12% polyacrylamide gel, and the proteins were separated by electrophoresis. Gels were stained with Coomassie blue to verify equal loading of histone, dried, exposed to X-Omat film (Kodak), and developed.
Northern blotting.
Total cellular RNA was prepared with a Qiagen RNeasy mini kit and quantified (Beckman DU-64, Nucleic Soft Pac module, Warburg program); 10 μg of RNA was run on 1% agarose–formaldehyde gels, transferred to Hybond-N membranes (Amersham), and hybridized to 32P-labeled DNA probes. Probes for CDC2, cyclin B1, and 36B4 were made by digesting and gel purifying fragments from plasmids pSP73/CDC2 (gift of L. Bonin), pLXSN/cyclin B1 (gift of J. Pines), and pGEM5/36B4 (gift of J. Gudas). The 514-bp PvuII fragment of cyclin B1 and the 445-bp AccI/BglII fragment of CDC2 were labeled by PCR performed with [32P]dCTP and a single antisense primer corresponding to the 3′ sequence of the fragment. The 36B4 fragment was radioactively labeled by a random hexamer priming kit (Boehringer Mannheim).
Cotransfection assays.
Cytomegalovirus (CMV)-driven mammalian expression vector was used, with and without the p53 gene (30). Reporter constructs were created as follows. Sequences 927, 2,239, and 367 bp upstream of the cyclin B, CDC2, and c-fos ATG start sites, respectively, were amplified from human genomic DNA, using oligonucleotides constructed with a KpnI 5′ and NcoI 3′ restriction enzyme recognition sequence overhangs. Each promoter fragment was ligated into pGEMT (Promega), expanded, and subjected to KpnI-NcoI digestion. KpnI/NcoI gel-purified fragments were ligated into pGL3 Basic vector (Promega), containing the luciferase gene in frame with the ATG of the 3′ NcoI site of the ligated promoter. Promoter-luciferase constructs containing the p53 response element (RE) (pCAST2Bluc and pCAST2Hluc [30]) were used as a control. Expression vector (4 μg), either with or without the p53 gene, was cotransfected with 4 μg of cyclin B-pGL3, CDC2-pGL3, cfos-pGL3, or p53 RE-luciferase, using Lipofectamine (Life Sciences), into p53−/− MEFs. p21−/− MEFs were cotransfected with 4 μg of expression vector, with or without p53 and 4 μg of cyclin B-pGL3 or CDC2-pGL3. The untransfected control cells underwent mock transfection with no plasmids added to the Lipofectamine mixture. Luciferase activities were assessed 48 h after transfection, using reagents from Promega, and relative light units (RLU) emitted from 20 μg of cell lysate was quantified for each sample (Monolight 2010 Luminometer; Analytical Luminescence Laboratory). Transfection efficiency was determined by dot blotting, probing for the luciferase gene, using DNA obtained from an aliquot of cells used for luciferase activities. RLU values were corrected for transfection efficiency and protein concentration. Data from three separate trials were arbitrarily normalized with the RLU/microgram of protein values from samples cotransfected with both pCMVp53 and cyclin B-luciferase from each trial.
MI.
Asynchronous cells growing on slides were either treated continuously with 100 nM ADR in complete medium or untreated (placed in complete medium). At 24 h, cells were fixed with 100% ice-cold methanol. Cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and visualized by fluorescence microscopy, and mitotic figures were counted, with oil immersion optics. Over 2,000 cells were counted for each cell type and population doubling level (PDL). Mitotic index (MI) was defined as the percentage of cells in mitosis. Data for adriamycin-treated cells are presented as MI of ADR-treated cells/MI of untreated cells.
RESULTS
The DNA damage-induced arrest in G2 occurs independently of p53 status.
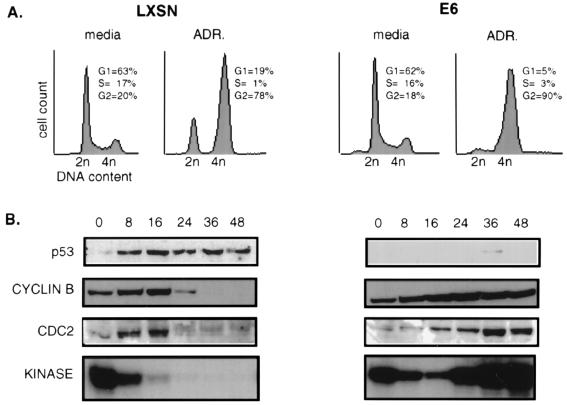
The DNA damage-induced checkpoints, in relation to p53 status, were examined by comparing early-passage HFFs transduced with the LXSN vector alone (LXSN-HFFs) or carrying the HPV 16 E6 oncogene (E6-HFFs). Asynchronously growing cells were treated with the DNA-damaging drug ADR. As anticipated, p53 protein levels rose rapidly in LXSN cells (Fig. 1B), and FACS analysis 24 h postexposure showed arrest in G1 and G2 (Fig. 1A). In contrast, E6 cells were depleted of a G1 population and had a marked increase in the G2 population without any detectable p53. Although both cell types showed an accumulation of cells in G2, examination of cyclin B/CDC2-associated kinase activity showed clear differences between the LXSN and E6 cells. Cyclin B-associated kinase activity decreased by 8 h postexposure in LXSN-HFFs and was extinguished by 16 h, though cyclin B and CDC2 protein levels were unchanged during this time. By 24 h there was a dramatic down regulation of cyclin B and CDC2 protein that persisted over 48 h. E6-HFFs showed a similar pattern of reduced cyclin B-associated kinase activity for the first 16 h postexposure; however, cyclin B-associated kinase activity then increased over time; cyclin B and CDC2 protein levels were stable throughout.
FIG. 1.
DNA damage-induced G2 arrest occurs independently of p53 status. Asynchronous cells were continuously treated with complete medium containing 100 nM ADR. (A) Cell cycle profiles show the LXSN cells to arrest in both G1 and G2, while the E6 cells solely arrest in G2, by 24 h after ADR exposure. (B) Total protein was harvested at the indicated hour. p53, cyclin B, and CDC2 protein levels were determined by Western blotting analysis. The same protein extracts were used for cyclin B-associated kinase activities performed on histone H1. CDC2 protein is represented as two bands; the slower-migrating band represents the phosphorylated inactive form of CDC2, and the faster-migrating band represents the unphosphorylated active form of CDC2.
The active CDC2 kinase in the E6 cells was not associated with apoptosis, as judged by the lack of a sub-G1 population (Fig. 1A) and by lack of the characteristic morphological changes of disrupted nuclear membranes or of nuclear condensation, segmentation, and fragmentation (data not shown). Immunofluorescence with DAPI staining of nuclei and tubulin staining of cytoskeleton confirmed an interphase morphology (data not shown). Therefore, it appeared that the p53-depleted E6 cells remain arrested with 4n DNA content and no evidence of early mitosis or apoptosis, yet with active cyclin B-associated kinase activity.
DNA damage-induced decrease in cyclin B and CDC2 is dependent on p53.
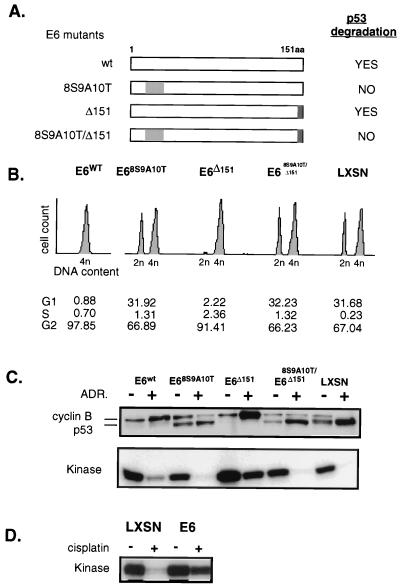
The E6 protein has been shown to have other activities in addition to its ability to target p53 for degradation. To attribute the differences between the LXSN and E6 cells to their p53 status, E6 mutants that vary in the ability to degrade p53 were each transduced into HFFs (Fig. 2A). Three mutants were used; 16E6-8S9A10T fails to bind or degrade p53 (reference 16 and Fig. 2C), 16E6-Δ151 fails to bind the tumor suppressor hDLG (human homologue of the Drosophila discs large tumor suppressor protein) (29) but retains p53 binding and degradation, and the double mutant 16E6-8S9A10T/Δ151 fails to bind either tumor suppressor. Asynchronously growing HFFs expressing wild-type or mutant E6 protein were exposed to ADR and analyzed for cell cycle position, p53 and cyclin B protein levels, and cyclin B/CDC2 kinase activity. Cells expressing the E6 proteins that eliminated p53, i.e., wild-type E6 and E6-Δ151 cells, arrested with a 4n DNA content and substantial levels of cyclin B and B-associated kinase activity (Fig. 2B and C). In contrast LXSN, 16E6-8S9A10T, and 16E6-8S9A10T/Δ151 cells, which are incapable of degrading p53, arrested in G1 and G2 with increased p53 and greatly reduced cyclin B and associated kinase activity. These results confirm that G2 arrest can occur independently of p53 status whereas decreased cyclin B protein and kinase activity is correlated with loss of p53. The persistence of cyclin B was not unique to p53 depleted human fibroblasts, as the same result was obtained in mammary epithelial cells transduced with E6 (data not shown). Examination of the response to other DNA-damaging agents including low-dose actinomycin D (data not shown) extended the generalizability of these findings. Cisplatin exposure results in the addition of adducts to the DNA and leads to induction of p53 in LXSN cells and to G2 arrest in both LXSN and E6 cells (reference 22 and data not shown). Despite the different DNA-damaging mechanism, cyclin B-associated kinase activity was suppressed only in the LXSN cells (Fig. 2D). Thus, the down regulation of cyclin B that occurred with maintenance of prolonged G2 arrest was not dependent on the type of DNA damage or on the cell type but rather on the presence of p53.
FIG. 2.
Down regulation of cyclin B, CDC2, and kinase activity is a function of p53, rather than E6 or agent of DNA damage used. (A) E6 mutants vary in the ability to degrade p53. E6 is a 151-amino-acid protein, depicted in a linear diagram. Amino acids 8 to 10 are responsible for binding to p53, leading to its degradation. The gray shading of this region indicates E6 mutants with replacement of these amino acids such that p53 binding is disrupted and p53 degradation does not occur. The darker shading indicates deletion of the C-terminal amino acid, 151, which would disrupt E6 binding to hDLG, as described in the text. (B) Asynchronous HFFs transduced with the various mutants were treated with complete medium containing 100 nM ADR for 24 h. Cell cycle profiles were analyzed by flow cytometry. (C) Western blotting and kinase assays were performed with proteins harvested from same cells with and without continuous exposure to ADR for 24 h as for panel B. Cyclin B and kinase down regulation occurs only in the cells capable of DNA damage induced up regulation of p53. (D) The p53-dependent down regulation of kinase activity is not a unique finding in cells DNA damaged with ADR. E6-HFFs and LXSN-HFFs were treated with cisplatin (1 μg/ml) continuously for 24 h. Cyclin B-associated kinase activity assays performed with protein lysates obtained from cells treated or untreated, as indicated, show the LXSN cells with extinguished kinase activity, while the E6 cells maintain high kinase activity.
Cyclin B-associated kinase activity during maintenance of the G2 checkpoint varied with p53 status.
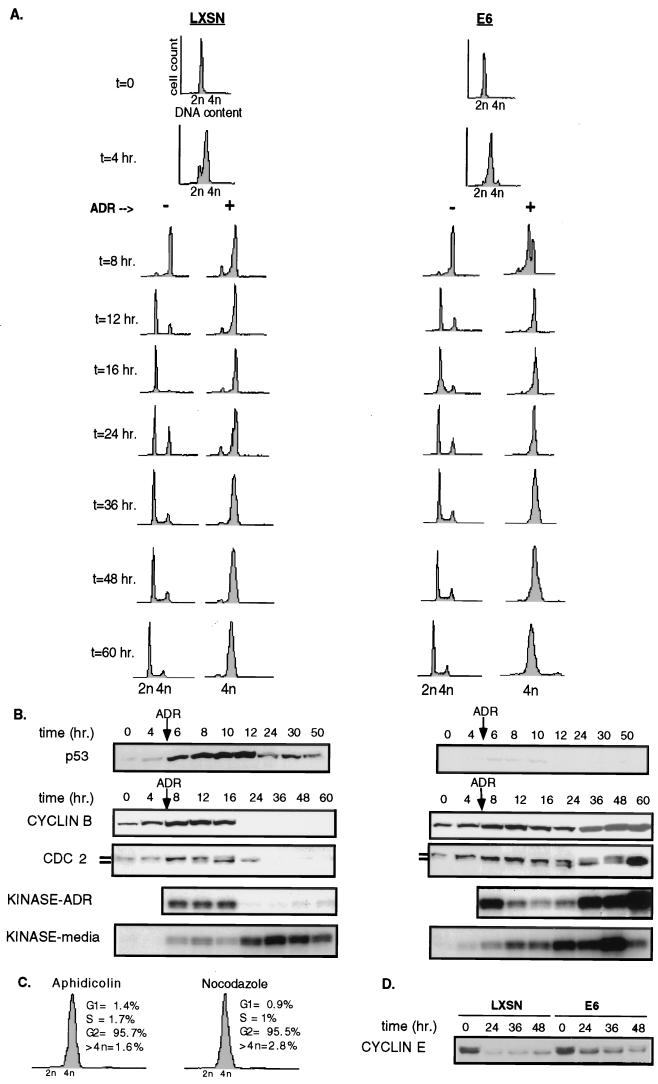
Comparison of the G2 checkpoint between asynchronously growing LXSN and E6 cells is complicated by G1 arrest in cells with functional p53, resulting in a smaller proportion of LXSN cells arrested in G2. The possibility that the differences observed between the LXSN and E6 cells were due to the different proportion of cells in G2 was addressed. Both cell types were synchronized at G1/S by density arrest followed by passage to lower density into medium containing the reversible DNA polymerase inhibitor aphidicolin. Reentry into the cell cycle was achieved with washing and replacing with aphidicolin-free medium. DNA damage pulse treatment with ADR caused both LXSN and E6 cells to arrest predominantly in G2 as the first functional DNA damage-induced checkpoint. Synchronization also allowed an analysis of the kinetics of initiation and maintenance of G2 arrest. Figure 3A shows that both populations were arrested with a 2n content by aphidicolin; untreated cells proceeded through S phase into G2 by 8 h, and the majority of the cells completed mitosis and were in the G1 phase of the cell cycle by 12 h postsynchronization. Most of the ADR-treated LXSN cells also reached G2 by 8 h and remained arrested in G2 for at least 60 h; a small subpopulation remained in G1 due to a greater propensity for the LXSN cells to arrest in G0 with synchronization. The E6 cells reached G2 with generally the same kinetics and also remained arrested in G2. The initiation of the G2 checkpoint can be considered to take place 3 to 5 h after the pulse of ADR, or between 8 and 12 h after release from aphidicolin as shown in Fig. 3A, at the time when cells without DNA damage enter mitosis. Maintenance of the G2 arrest under these conditions of DNA damage is prolonged, extending beyond 60 h.
FIG. 3.
G2 checkpoint is initiated independent of p53 status and cyclin B/CDC2 kinase and maintained without adaptation. A time course experiment was performed on G1/S-synchronized cells as described in Materials and Methods. Time zero (t = 0) indicates the time of release from synchronization. At 5 h after release (t = 5), half of the plates were pulsed with 2 μM ADR in complete medium for 1 h, followed by replacement with complete medium. The other half remained in complete medium and monitored like the untreated control. Flow cytometry (A) and western blotting and kinase assays (B) were performed on cells harvested at the times indicated. (C) To assess the presence of a cycling subpopulation, synchronized ADR-treated E6 cells, treated identically to the cells used for panel A, were reexposed at t = 24 to aphidicolin (3 μg/ml) or nocodazole (0.05 μg/ml) in complete medium for an additional 24 h. Cells were then fixed, stained, and subjected to flow cytometry. (D) E6-HFFs and LXSN-HFFs do not adapt to a DNA damaged-induced G2 arrest. Asynchronous E6 and LXSN cells were pulsed with ADR (2 μM) for 1 h; protein was harvested over a time course and subjected to Western blotting with anti-cyclin E.
p53 accumulated rapidly after exposure to ADR in the LXSN cells (Fig. 3B). Interestingly, cyclin B and CDC2 protein levels and their associated kinase activity increased as the cells accumulated in G2 and at the initiation of the G2 checkpoint (8 to 12 h); cyclin B and CDC2 protein levels fell dramatically between 16 and 24 h (Fig. 3B). Predictably, E6 cells did not show induction or accumulation of p53. As in the LXSN cells, cyclin B and CDC2 levels increased as E6 cells entered G2 and initiated the G2 checkpoint. However, in contrast to the extinction of cyclin B-associated kinase seen in the cells with functional p53, maintenance of G2 arrest in cells lacking p53 occurred with persistent cyclin B and CDC2 and high levels of cyclin B-associated kinase activity. Generally, high levels of cyclin B-associated kinase activity herald entry into mitosis, but no morphologic signs of mitosis were observed, and the cell cycle profile indicated that the cells remained with a 4n DNA content. The high level of kinase activity found in the ADR-treated E6 cells represents a large population of E6 cells containing kinase activity, as the kinase reactions are carried out with excess substrate and for a reaction time within the linear range of the kinase activity (data not shown).
Although there was no evidence of a subpopulation of cycling E6 cells after the G2 checkpoint had been initiated, either by FACS or by microscopic evidence of mitosis (see also Fig. 6), this possibility was formally addressed given that such a cycling population could contribute to cyclin B/CDC2 kinase activity. Synchronized ADR-treated E6 cells that arrested in G2 were reexposed to aphidicolin for an additional 24 h, such that any cells passing the G2/M checkpoint and completing mitosis would be arrested at the subsequent G1/S restriction point with a 2n DNA content. Only 1.4% of E6 cells had a 2n DNA content, and 1.7% had a DNA content between 2n and 4n (Fig. 3C). As a control, synchronized ADR-treated E6 cells were blocked in mitosis with nocodazole; 0.9% of the cells were found in G1, and 1.0% were found in S (Fig. 3C). This indicated that <1% of cells could have passed the DNA damage-induced G2 block and completed mitosis.
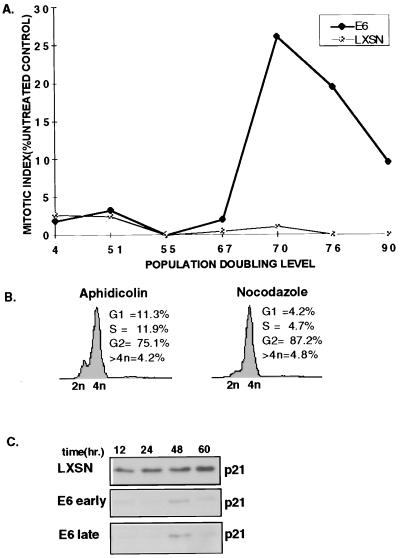
FIG. 6.
Cells lacking p53 attenuate the DNA damage-induced G2 checkpoint after multiple population doublings. (A) Asynchronously growing E6 and LXSN cells varying in PDL were continuously exposed to complete medium containing 100 nM ADR or medium alone for 24 h, fixed, and stained with DAPI, and the MI was determined. (B) Late-passage cells were synchronized by density arrest followed by aphidicolin synchronization. At 4 h after release, they were pulsed with 2 μM ADR for 1 h. At 24 h after release from synchronization, when G2 maintenance would be expected, the medium was replaced with complete medium containing aphidicolin (3 μg/ml) or nocodazole (0.05 μg/ml) for an additional 24 h. Cells were then fixed, stained, and examined by flow cytometry. (C) p21 loss is not involved in the attenuation of the G2 checkpoint in late-passage E6 cells. Synchronized, ADR-pulsed early-passage E6 and late-passage LXSN and E6 cells were harvested at the indicated time points. p21 levels were evaluated by Western blotting.
Another possibility that could account for the reactivation cyclin B/CDC2 kinase activity is that E6 cells progressed through mitosis without cytokinesis. To identify cells with a 4n content that had entered G1, cyclin E levels were assayed (Fig. 3D). Neither the LXSN nor E6 cells showed a dramatic increase in cyclin E protein levels. Furthermore, if E6 cells were to have adapted and entered G1 without cytokinesis, these cells would enter S phase and reduplicate their DNA; however, cells with >4n DNA content never exceeded 3% of the E6-HFF population (Fig. 1, 3A, and 3C).
Taken together, these results indicate that the initiation of a G2 arrest in response to DNA damage was not dependent on p53 function. Inhibition of cyclin B/CDC2 kinase, which has been implicated in DNA damage-induced G2 arrest, did not occur with initiation of the G2 checkpoint but rather occurred with maintenance of G2 arrest in cells with functional p53. Otherwise normal cells, in which p53 has been eliminated, also respond to DNA damage with a sustained G2 arrest, with no more than 3% of cells exiting G2. E6 cells contrast from the parental cells by remaining in G2 despite active cyclin B/CDC2 kinases.
Subcellular localization of cyclin B and CDC2 in ADR-treated E6 cells.
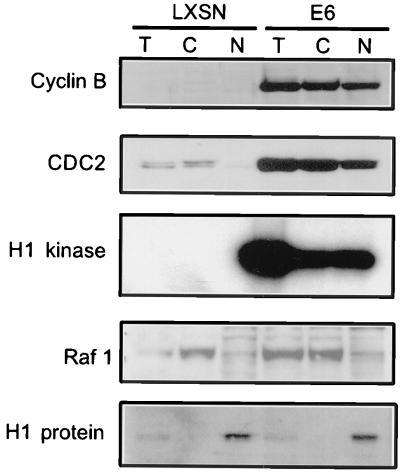
One explanation for the active cyclin B/CDC2 kinase activity in the G2-arrested E6 cells could be that the cyclin B/CDC complexes are excluded from the nucleus, as suggested from the observation that p21 promotes nuclear localization of mitotic cyclin/CDKs (11). To test this hypothesis, nuclear and cytoplasmic extracts were harvested 36 h after treatment with ADR, during maintenance of G2 arrest. Cyclin B and CDC2 were distributed in both the nuclei and cytoplasm of E6 cells, and cyclin B-associated kinase activity was isolated from both cytoplasmic and nuclear fractions (Fig. 4). Thus, at least some active cyclin B/CDC2 complexes translocated into the nuclei of ADR-treated E6 cells. LXSN cells treated with ADR showed no cyclin B, a small amount of cytoplasmic CDC2, and no detectable kinase activity in either fraction. Western blotting to cytoplasmic and nuclear controls (Raf1 and histone H1 proteins, respectively) showed that separation of the cytoplasmic and nuclear components was achieved, as the cytoplasmic fractions did not show histone bands and the nuclear component showed only trace contamination with Raf1 (Fig. 4). These results rule out the possibility that E6 cells maintain their G2 arrest, by excluding active cyclin B/CDC2 kinase from the nucleus.
FIG. 4.
Cyclin B and CDC2 translocate into the nucleus of ADR-treated E6 cells. Asynchronously growing E6-HFFs and LXSN-HFFs were treated continuously with 100 nM ADR for 36 h. Total (T), cytoplasmic (C), and nuclear (N) proteins were harvested 36 h after exposure as described in Methods and Materials; 20 μg of total, cytoplasmic, and nuclear extracts were loaded onto an SDS–12% polyacrylamide gel and transferred. Western blot analyses for cyclin B, CDC2, the cytoplasmic control (Raf1), and the nuclear control (histone H1) were performed. The ADR-treated LXSN cells show no cyclin B and diminished CDC2 protein levels, while the E6 cells show both cyclin B and CDC2 levels, in the cytoplasm and nucleus. Cyclin B-associated H1 kinase assays were performed on 100 μg of total, cytoplasmic, and nuclear extracts.
Transcriptional regulation of cyclin B and CDC2.
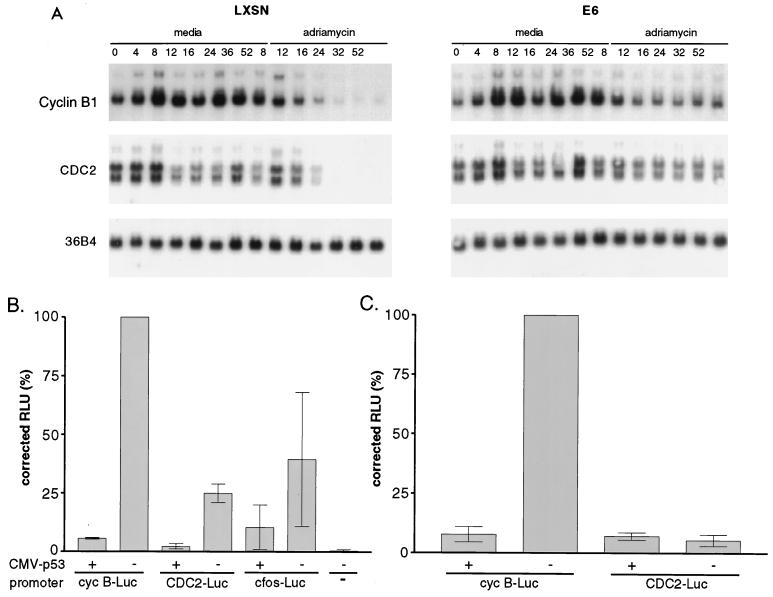
The mechanism involved in down regulation of cyclin B and CDC2 protein levels in the LXSN cells was explored further; Northern blotting was performed as an initial screen to determine whether p53 influenced cyclin B or CDC2 RNA levels (Fig. 5A). ADR-treated LXSN cells showed a decrease in cyclin B and CDC2 transcripts to undetectable levels by 19 h postexposure (24 h after aphidicolin release). E6 cells, however, showed stable levels of cyclin B and CDC2 mRNA. To determine whether the down regulation of RNA was mediated by p53-dependent repression of the cyclin B and CDC2 promoters, transient cotransfection assays were performed in p53−/− MEFs, using cyclin B or CDC2 promoters driving a luciferase reporter, with or without p53. p53 protein expression in the p53−/− MEFs was documented by Western blotting (data not shown). Reproducibly, p53 repressed the CDC2 promoter 5-fold and the cyclin B promoter 10-fold (Fig. 5B). In control transfections, the previously characterized p53-repressible c-fos promoter (31) showed twofold repression by p53 (Fig. 5B). The p53-inducible promoters of the beta interferon and human T-lymphocytic leukemia virus type 1 genes gave 12- and 5-fold-increased luciferase expression, respectively, when cotransfected with the p53 expression vector (data not shown).
FIG. 5.
p53’s role in the G2 checkpoint appears to be mediated by the transcriptional down regulation of cyclin B and CDC2. (A) E6-HFFs and LXSN-HFFs were synchronized and pulsed with 2 μM ADR for 1 h at 5 to 6 h after aphidicolin release. Cells were harvested for RNA at the indicated hour. Northern blotting was performed, and blots were probed with radiolabeled cyclin B, CDC2, and the loading control, 36B4. Lanes 1 to 8 indicate the hour of release from synchronization into complete medium; lanes 12 to 14 indicate the hours after aphidicolin release in the cells pulsed with ADR. (B) Cotransfection experiments show p53 to be a transcriptional repressor of cyclin B and CDC2 promoters. p53−/− MEFs were cotransfected with a CMV-driven expression vector (p53) or without p53 (−) and the various promoter-reporter constructs shown and described in Materials and Methods. Luciferase assays were performed on the protein lysates, and RLU values are normalized for transfection efficiency represented per microgram of protein. Error bars represent standard errors of the means of triplicate experiments. The untransfected control represents p53−/− MEFs that have been mock transfected. (C) Identical cotransfections were performed in p21−/− MEFs.
Overexpression of p53 can lead to p21 induction, with its downstream effects on transcription via E2F, and arrest in G1. To address the possibility that G1 arrest or p21-mediated transcriptional repression can account for the cyclin B and CDC2 promoter down regulation seen with p53 overexpression, transient cotransfection assays were performed with p21-expressing MEFs (Fig. 5C). In these cells, p53 overexpression would not lead to p21-mediated transcriptional regulation; furthermore, p21−/− MEFs have been shown to be significantly deficient in the ability to undergo a G1 arrest in response to DNA damage (4, 8); therefore, G1 arrest alone could be excluded as an explanation of inactive cyclin B and CDC2 promoters. Expression of human p53 in CMV-p53 plasmid-transfected p21−/− MEFs was confirmed by Western blotting (data not shown). The cotransfection assays showed that the cyclin B promoter was repressed by p53 in the p21−/− MEFs to a similar degree as in the p53−/− MEFs. The low basal activity of the CDC2 promoter in p21−/− MEFs made it difficult to analyze p53 repression.
The DNA damage-induced G2 checkpoint becomes attenuated in p53-depleted cells at later population doubling levels.
Previous studies have shown that gamma-irradiated E6 cells showed an attenuation of the G2 checkpoint after multiple PDL, whereas PDL-matched LXSN cells did not (28). To test whether attenuation occurred in chemotherapy-treated E6 cells, the MI was determined 24 h after ADR treatment and compared to the MI of untreated cells (Fig. 6). For most of their life span in culture, both E6-HFFs and LXSN-HFFs displayed an intact G2 checkpoint. By PDL 67, a subpopulation of E6-HFFs entered mitosis after DNA damage, and by PDL 70 to 76, 25 to 30% of the p53−/− cells had lost their G2 checkpoint, whereas the LXSN cells arrested in G2 checkpoint at all PDLs tested. Between PDL 80 and 90, both LXSN and E6 populations showed increased doubling time, crisis, or replicative senescence in culture, and checkpoint function could no longer be measured.
To further test if late-passage E6 cells have lost the ability to sustain a G2 arrest, aphidicolin-synchronized late passage, presenescent E6 cells, which were pulsed with ADR, were then reexposed to aphidicolin to trap cells that had escaped G2 in at the subsequent G1/S phase. Figure 6B shows 11.3% of the E6 cells in G1 and 11.9% in S phase, the latter indicating the percentage of cells cycling through S phase prior to the inhibition of DNA polymerase by aphidicolin. In contrast, only 4.2% of the cells could be found in G1 after nocodazole block, and 4.7% were in S phase. Notably, no increase in the tetraploid population was seen with the late-passage E6 cells that were kept in nocodazole for 24 h. These data with late-passage E6 cells are markedly different from the results with early-passage E6 cells (Fig. 3C), indicating that p53 loss does not directly result in the inability to sustain G2 arrest, but more likely the ensuing genetic instability resulting from p53 loss predisposes cells to lose the pathway that maintains G2 arrest.
A role for p21 in the regulation of the G2 checkpoint has recently received much attention (2, 5, 6, 9). Given that p21 may be induced in a p53-independent manner, it was possible that the sustained G2 arrest seen in early-passage E6 cells was due to p53-independent induction of p21 and that p21 induction was compromised in late-passage E6 cells. As expected, LXSN cells showed high levels of p21 in response to DNA damage throughout their proliferative life span (late-passage LXSN cells are shown in Fig. 6C). Early- and late-passage E6 cells showed equivalent minimal induction of p21 in response to ADR treatment. Therefore, p53-independent induction of p21 did not play a role in sustaining G2 arrest in early-passage E6 cells.
DISCUSSION
Our results demonstrated that initiation of the G2 checkpoint in response to DNA damage was independent of p53 status, as both E6 and LXSN cells arrested in G2 with similar kinetics. Interestingly, initiation of the G2 checkpoint was also independent of inhibition of cyclin B/CDC2 kinase activity. Active cyclin B/CDC2 was present in both E6 and LXSN cells up to 16 h postexposure, the time by which initiation of the G2 arrest had occurred in cells exposed to ADR. In this system, the outcome of DNA damage was a sustained arrest in G2. In HFFs, the complete inhibition of cyclin B/CDC2 kinase activity occurred as a later event related to down regulation of the CDK and cyclin genes and was associated with the presence of functional p53. Cells depleted of p53 were equally capable of maintaining a G2 arrest despite high cyclin B/CDC2 kinase activity that translocates to the nucleus.
In previous models of DNA damage-induced G2 arrest, the common denominator on which all pathways converged was the kinase activity of cyclin B/CDC2. It has been assumed that active cyclin B/CDC2 kinase inevitably results in entry into mitosis and, if inactive, results in arrest at the G2/M border. However, there has been evidence that has shown that although important, cyclin B/CDC2 is not the sole engine driving the cell cycle through G2 and into mitosis. HeLa cells overexpressing a permanently active CDC2 mutant, CDC2AF, showed high levels of cyclin B protein and cyclin B/CDC2 kinase activity yet were still capable of a DNA damaged-induced G2 delay (26). Work with Aspergillus nidulans has demonstrated parallel pathways controlling entry into mitosis. NIMA kinase (a mitotic kinase) as well as cyclin B/CDC2 must be active for Aspergillus to proceed into mitosis (46). NIMA kinase activity is not dependent on active cyclin B/CDC2 kinase, suggesting the existence of an independent and parallel pathway leading to mitosis. There is evidence for a NIMA-like mitotic pathway in vertebrate cells (37), and the closest human NIMA kinase homologue, Nek2 kinase (NIMA-related kinase), has been described (53). Nek2, or a kinase similar to it, may catalyze a G2 transition pathway that is regulated in a p53-independent manner in response to DNA damage.
There is considerable evidence implicating cyclin B/CDC2 in the transition from G2 to mitosis, and clearly, p53 targets cyclin B and possibly CDC2 for transcriptional repression during sustained G2 arrest. However, cyclin B/CDC2 kinase activity either does not contribute to G2 arrest or is not the only pathway mediating G2 arrest, since cells lacking p53 remain arrested in G2 without disrupting cyclin B/CDC2 activity. There are two possible scenarios to explain the sustained G2 arrest: (i) there is a single pathway maintaining G2 arrest which does not involve p53, p21, or cyclin B/CDC2, and the down regulation of cyclin B/CDC2 is unrelated to G2 arrest; or (ii) there are redundant pathways, such that p53+ cells have two mechanisms and p53− cells have one mechanism, and either is sufficient to sustain G2 arrest. This model assumes that one of the pathways in the p53+ cells involves cyclin B/CDC2. A variation of the second scenario is that p53+ and p53− cells use distinct pathways to regulate G2 arrest, either a p53-dependent down regulation of cyclin B/CDC2 or a p53-, CDC2-independent pathway, and that the latter serves as a default pathway if cells lose p53 function. While we have no data to rule out the first scenario, the extensive data linking cyclin B/CDC2 to the G2/M transition make the possibility of redundant pathways an attractive hypothesis.
An important interpretation of our data, which has been experimentally addressed, is that the E6-expressing cells might have adapted and drifted out of G2 and gone back into S phase, and the cell cycle status rather than lack of p53-mediated repression of cyclin B/CDC2 transcription could account for the maintenance of cyclin B and CDC2 activity. This possibility has been ruled out by the following data. First, if the ADR-treated E6 cells were to have proceeded into G1, then an increase in cyclin E levels would be expected. This was not observed (Fig. 3D). Second, if the ADR-treated E6 cells adapted, they would reenter S phase for another round of DNA synthesis, as these cells do not have a G1 checkpoint, leading to an 8n DNA content. A population of cells with >4n DNA was not demonstrated by flow cytometry (Fig. 1 and 3A) up to 60 h or by a comparison of aphidicolin and nocodazole trapping of ADR-treated E6 cells after initiation and maintenance of the G2 checkpoint (Fig. 3C). Third, if there were adaptation and progression through the cell cycle, we would also expect cyclical changes in cyclin B protein and mRNA levels, as we do for the untreated controls that progress through G2/M. We show by Western blot (Fig. 3B) and Northern blot (Fig. 5A) analyses that this clearly does not occur for the ADR-treated E6 cells. This is in contrast to the fluctuation of cyclin B mRNA in the synchronous cycling untreated E6 cells (and untreated LXSN cells) (Fig. 5A). Finally, the promoter-reporter analyses presented in Fig. 5B and C were performed with p53−/− and p21−/− MEFs, to rule out the possibility that the effects are due to a specific phase of the cell cycle.
Attenuation of the DNA damage-induced G2 checkpoint or the ability to sustain G2 arrest occurred during the in vitro life span of HFFs expressing E6, but not HFFs transduced with control vector (see also reference 28). Indeed many established cell lines lack a functional G2 checkpoint. Recently, it was shown that p53− colon carcinoma cell lines and p53 knockout human fetal fibroblasts (a method that requires extensive population doublings) could not sustain DNA damage induced G2 arrest, whereas p53+ lines could (5). Although that finding was used to conclude that p21 inhibition of CDC2 has a central role in sustaining a G2 arrest, our data indicate that loss of G2 arrest is a late event in p53− cells and that more likely an as yet uncharacterized mechanism that sustains G2 arrest in p53− cells is lost due to the genetic instability accompanying prolonged proliferation without a G1 checkpoint.
Unfortunately, knowing that loss of the ability to maintain a sustained G2 arrest is only a secondary event related to p53 inactivation does not clarify which of the above two scenarios for G2 control is correct. In the first model, the G2 arrest mechanism, though not caused by p53, may be prone to loss in cells lacking a G1 checkpoint or other p53-dependent functions. The second model would predict that there is an equal chance of losing either of the two G2 arrest pathways; however, the chance of the p53+ cells encountering inactivating mutations in both pathways is high. This would be the equivalent of familial cancer syndromes; when an inherited allele (or in this case, pathway) is nonfunctional, loss of the second allele results in tumors at an early age, in comparison with tumors in which both alleles need to be inactivated.
The details of the mechanism(s) sustaining G2 arrest await discovery of the genes involved in the p53-independent pathway. Importantly, numerical and structural chromosomal abnormalities developed in the p53− cells only after loss of their G2 checkpoint; loss of G1 alone did not appear to be sufficient for the development of aneuploidy (28). This has important implications for the process of neoplastic progression, as the tolerance of aneuploidy is a feature of cancer cells. Attenuation of the G2 checkpoint response appears to be involved in this tolerance of aneuploidy. This finding underscores the importance of understanding control of the G2 checkpoint, as loss of this checkpoint frees the barrier to genomic instability.
ACKNOWLEDGMENTS
T.M.P. was supported by training grant CA09515, K12 CA76930, and CA79629-01. J.A.B. and L.G. were supported by the Molecular and Cellular Biology Graduate Program. J.A.B. was also supported by an N.S.F. predoctoral fellowship. The work was funded by grant CA64975 from NCI to D.A.G.
We thank Bill Kaufmann and members of the Galloway laboratory for stimulating discussions, and we thank the Flow Cytometry and Image Analysis lab for help with FACS analysis.
REFERENCES
- 1.Agarwal M L, Agarwal A, Taylor W R, Stark G R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 21.1.1–21.1.5. [Google Scholar]
- 2.Azzam E I, de Toledo S M, Pykett M J, Nagasawa H, Little J B. CDC2 is down-regulated by ionizing radiation in a p53-dependent manner. Cell Growth Differ. 1997;8:1161–1169. [PubMed] [Google Scholar]
- 3.Barth H, Hoffmann I, Klein S, Kaszkin M, Richards J, Kinzel V. Role of cdc25-C phosphatase in the immediate G2 delay induced by the exogenous factors epidermal growth factor and phorbolester. J Cell Physiol. 1996;168:589–599. doi: 10.1002/(SICI)1097-4652(199609)168:3<589::AID-JCP11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 5.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 6.Cayrol C, Knibiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- 7.Datta R, Hass R, Gunji H, Weichselbaum R, Kufe D. Down-regulation of cell cycle control genes by ionizing radiation. Cell Growth Differ. 1992;3:637–644. [PubMed] [Google Scholar]
- 8.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 9.de Toledo S M, Azzam E I, Keng P, Laffrenier S, Little J B. Regulation by ionizing radiation of CDC2, cyclin A, cyclin B, thymidine kinase, topoisomerase IIalpha, and RAD51 expression in normal human diploid fibroblasts is dependent on p53/p21 Waf1. Cell Growth Differ. 1998;9:887–896. [PubMed] [Google Scholar]
- 10.Di Leonardo A, Linke S P, Clarkin K, Wahl G M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 11.Dulic V, Stein G H, Far D F, Reed S I. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.el-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 13.Enoch T, Norbury C. Cellular responses to DNA damage: cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem Sci. 1995;20:426–430. doi: 10.1016/s0968-0004(00)89093-3. . (Review.) [DOI] [PubMed] [Google Scholar]
- 14.Evans T, Rosenthal E T, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 15.Ferrell J E, Jr, Wu M, Gerhart J C, Martin G S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster S A, Demers G W, Etscheid B G, Galloway D A. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J Virol. 1994;68:5698–5705. doi: 10.1128/jvi.68.9.5698-5705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furnari B, Rhind N, Russell P. CDC25 mitotic inducer targeted by CHK1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 18.Gould K L, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 19.Greenblatt M S, Bennett W P, Hollstein M, Harris C C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. . (Review.) [PubMed] [Google Scholar]
- 20.Halbert C L, Demers G W, Galloway D A. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins D S, Demers G W, Galloway D A. Inactivation of p53 enhances sensitivity to multiple chemotherapeutic agents. Cancer Res. 1996;56:892–898. [PubMed] [Google Scholar]
- 23.Hermeking H, Lengauer C, Polyak K, He T, Zhang L, Thiagalingam S, Kinzler K, Vogelstein W B. 14-3-3 is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1998;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 24.Hollstein M, Sidransky D, Vogelstein B, Harris C C. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. . (Review.) [DOI] [PubMed] [Google Scholar]
- 25.Innocente S A, Abrahamson J L, Cogswell J P, Lee J M. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin P, Gu Y, Morgan D O. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 28.Kaufmann W K, Schwartz J L, Hurt J C, Byrd L L, Galloway D A, Levedakou E, Paules R S. Inactivation of G(2) checkpoint function and chromosomal destabilization are linked in human fibroblasts expressing human papillomavirus type 16 E6. Cell Growth Differ. 1997;8:1105–1114. [PubMed] [Google Scholar]
- 29.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyono T, Hiraiwa A, Ishii S, Takahashi T, Ishibashi M. Inhibition of p53-mediated transactivation by E6 of type 1, but not type 5, 8, or 47, human papillomavirus of cutaneous origin. J Virol. 1994;68:4656–4661. doi: 10.1128/jvi.68.7.4656-4661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kley N, Chung R Y, Fay S, Loeffler J P, Seizinger B R. Repression of the basal c-fos promoter by wild-type p53. Nucleic Acids Res. 1992;20:4083–4087. doi: 10.1093/nar/20.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane D P. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. . (News; comment.) [DOI] [PubMed] [Google Scholar]
- 33.Lee T H, Solomon M J, Mumby M C, Kirschner M W. INH, a negative regulator of MPF, is a form of protein phosphatase 2A. Cell. 1991;64:415–423. doi: 10.1016/0092-8674(91)90649-j. [DOI] [PubMed] [Google Scholar]
- 34.Leffers H, Madsen P, Rasmussen H H, Honore B, Andersen A H, Walbum E, Vandekerckhove J, Celis J E. Molecular cloning and expression of the transformation sensitive epithelial marker stratifin. A member of a protein family that has been involved in the protein kinase C signalling pathway. J Mol Biol. 1993;231:982–998. doi: 10.1006/jmbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- 35.Linke S P, Clarkin K C, Wahl G M. p53 mediates permanent arrest over multiple cell cycles in response to gamma-irradiation. Cancer Res. 1997;57:1171–1179. [PubMed] [Google Scholar]
- 36.Liu F, Stanton J J, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu K P, Hunter T. Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell. 1995;81:413–424. doi: 10.1016/0092-8674(95)90394-1. [DOI] [PubMed] [Google Scholar]
- 38.Maity A, McKenna W G, Muschel R J. Evidence for post-transcriptional regulation of cyclin B1 mRNA in the cell cycle and following irradiation in HeLa cells. EMBO J. 1995;14:603–609. doi: 10.1002/j.1460-2075.1995.tb07036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGowan C H, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalovitz D, Halevy O, Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990;62:671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- 41.Muschel R J, Zhang H B, Iliakis G, McKenna W G. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res. 1991;51:5113–5117. [PubMed] [Google Scholar]
- 42.Muschel R J, Zhang H B, McKenna W G. Differential effect of ionizing radiation on the expression of cyclin A and cyclin B in HeLa cells. Cancer Res. 1993;53:1128–1135. [PubMed] [Google Scholar]
- 43.O’Connor P M. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997;29:151–182. . (Review.) [PubMed] [Google Scholar]
- 44.O’Connor P M, Ferris D K, Hoffmann I, Jackman J, Draetta G, Kohn K W. Role of the cdc25C phosphatase in G2 arrest induced by nitrogen mustard. Proc Natl Acad Sci USA. 1994;91:9480–9484. doi: 10.1073/pnas.91.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor P M, Ferris D K, White G A, Pines J, Hunter T, Longo D L, Kohn K W. Relationships between cdc2 kinase, DNA cross-linking, and cell cycle perturbations induced by nitrogen mustard. Cell Growth Differ. 1992;3:43–52. [PubMed] [Google Scholar]
- 46.Osmani A H, McGuire S L, Osmani S A. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell. 1991;67:283–291. doi: 10.1016/0092-8674(91)90180-7. [DOI] [PubMed] [Google Scholar]
- 47.Parker L L, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 48.Peng C Y, Graves P R, Thoma R S, Wu Z Q, Shaw A S, Piwnica-Worms H. Mitotic and G(2) checkpoint control—regulation of 14-3-3 protein binding by phosphorylation of CDC25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 49.Prasad G L, Valverius E M, McDuffie E, Cooper H L. Complementary DNA cloning of a novel epithelial cell marker protein, HME1, that may be down-regulated in neoplastic mammary cells. Cell Growth Differ. 1992;3:507–513. [PubMed] [Google Scholar]
- 50.Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez Y, Wong C, Thoma R S, Richman R, Wu R Q, Piwnica-Worms H, Elledge S J. Conseration of the CHK1 checkpoint pathway in mammals—linkage of DNA damage to CDK regulation through CDC25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 52.Sandell L L, Zakian V A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 53.Schultz S J, Fry A M, Sutterlin C, Ried T, Nigg E A. Cell cycle-dependent expression of Nek2, a novel human protein kinase related to the NIMA mitotic regulator of Aspergillus nidulans. Cell Growth Differ. 1994;5:625–635. [PubMed] [Google Scholar]
- 54.Smythe C, Newport J W. Coupling of mitosis to the completion of S phase in Xenopus occurs via modulation of the tyrosine kinase that phosphorylates p34cdc2. Cell. 1992;68:787–797. doi: 10.1016/0092-8674(92)90153-4. [DOI] [PubMed] [Google Scholar]
- 55.Stein G H, Drullinger L F, Robetorye R S, Pereira-Smith O M, Smith J R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci USA. 1991;88:11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart N, Hicks G G, Paraskevas F, Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995;10:109–115. [PubMed] [Google Scholar]
- 57.Sugrue M M, Shin D Y, Lee S W, Aaronson S A. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA. 1997;94:9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vikhanskaya F, Erba E, D’Incalci M, Broggini M. Introduction of wild-type p53 in a human ovarian cancer cell line not expressing endogenous p53. Nucleic Acids Res. 1994;22:1012–1017. doi: 10.1093/nar/22.6.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 60.Weinert T. A DNA damage checkpoint meets the cell cycle engine. Science. 1997;277:1450–1451. doi: 10.1126/science.277.5331.1450. . (Comment.) [DOI] [PubMed] [Google Scholar]
- 61.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]