Abstract
The effects of cyclic AMP (cAMP) on cell proliferation are cell type specific. Although the growth-inhibitory effects of cAMP have been well studied, much less is known regarding how cAMP stimulates proliferation. We report that cAMP stimulates proliferation through both protein kinase A (PKA)-dependent and PKA-independent signaling pathways and that phosphatidylinositol 3-kinase (PI3K) is required for cAMP-stimulated mitogenesis. In cells where cAMP is a mitogen, cAMP-elevating agents stimulate membrane ruffling, Akt phosphorylation, and p70 ribosomal S6 protein kinase (p70s6k) activity. cAMP effects on ruffle formation and Akt were PKA independent but sensitive to wortmannin. In contrast, cAMP-stimulated p70s6k activity was repressed by PKA inhibitors but not by wortmannin or microinjection of the N-terminal SH2 domain of the p85 regulatory subunit of PI3K, indicating that p70s6k and Akt can be regulated independently. Microinjection of highly specific inhibitors of PI3K or Rac1, or treatment with the p70s6k inhibitor rapamycin, impaired cAMP-stimulated DNA synthesis, demonstrating that PKA-dependent and -independent pathways contribute to cAMP-mediated mitogenesis. Direct elevation of PI3K activity through microinjection of an antibody that stimulates PI3K activity or stable expression of membrane-localized p110 was sufficient to confer hormone-independent DNA synthesis when accompanied by elevations in p70s6k activity. These findings indicate that multiple pathways contribute to cAMP-stimulated mitogenesis, only some of which are PKA dependent. Furthermore, they demonstrate that the ability of cAMP to stimulate both p70s6k- and PI3K-dependent pathways is an important facet of cAMP-regulated cell cycle progression.
Cyclic AMP (cAMP) exerts differential effects on cell proliferation. In many cells, including CHO cells, aortic smooth muscle cells, and Rat-1 fibroblasts, cAMP inhibits the mitogenic response to growth factors (8). Growth-inhibitory effects of cAMP are mediated partly through activation of cAMP-dependent protein kinase A (PKA), which interferes with Raf activation and signaling (23). Less is known regarding how cAMP stimulates growth, although accumulating evidence has dissociated the mitogenic effects of cAMP from effects on mitogen-activated protein kinase (MAPK) (37, 42, 76). In contrast, the effects of cAMP on p70s6k correlate with effects on proliferation, and inhibition of p70s6k activation abolishes cAMP-stimulated DNA synthesis (10). These results prompted us to examine the role of phosphatidylinositol 3-kinase (PI3K)-dependent signaling pathways in cAMP-stimulated proliferation.
Multiple isoforms of PI3K that vary in lipid substrate specificity and subunit structure have been identified (reviewed in reference 71). Typically, mitogens that activate receptor tyrosine kinases stimulate PI3Kα/β whereas those that activate G-protein-coupled receptors stimulate PI3Kγ, although exceptions have been noted (36, 52, 66, 67). PI3K is required for the mitogenic activity of many growth factors, including platelet-derived growth factor, epidermal growth factor, and insulin. Deletion of the platelet-derived growth factor receptor p85 binding site (22, 31), treatment with pharmacological inhibitors (70, 73), or microinjection of PI3K-specific inhibitory antibodies or proteins (25, 39, 56) impairs growth factor-stimulated mitogenesis. For most growth factors shown to require PI3K activity, growth factor treatment stimulates lipid kinase activity. Although only inhibitory effects of cAMP on PI3K lipid kinase activity have been reported, these studies were performed in differentiated cells, i.e., adipocytes (48) and neutrophils (1), or in cells where cAMP fails to stimulate proliferation, such as bovine airway smooth muscle cells (61), B16 melanoma cells (9), and lymphoid cells (45). Whether cAMP requires PI3K activity in cells where it is a mitogen was examined here.
Studies were conducted in a continuous line of Wistar rat thyroid (WRT) cells. The physiologic regulator of these cells, thyrotropin (TSH), stimulates proliferation through cAMP-mediated pathways that require PKA activity (34). Elevation of intracellular cAMP following treatment with cholera toxin, forskolin, or cell-permeable cAMP analogs is sufficient to stimulate DNA synthesis in these cells. Paradoxically, microinjection of the PKA catalytic subunit failed to stimulate DNA synthesis (21, 40). Our results indicate that PI3K is required for a mitogenic response to TSH or cAMP-elevating agents acting downstream from the TSH receptor. The biological effects of PI3K are mediated through downstream kinases such as PDK1 (reviewed in references 3, 15, and 20), Akt (5, 14, 26), and p70s6k (reviewed in references 12 and 53). Rac1 is also activated downstream from PI3K, where it contributes to p70s6k activation (13) and stimulates membrane ruffling (55, 57). Rac1 is required for Ras-mediated transformation (32, 54) as well as cell proliferation (27, 46, 49), including that stimulated by cAMP as shown here. We discovered that cAMP-elevating agents stimulate membrane ruffling, Akt, and p70s6k activity. While the effects of cAMP on membrane ruffling and Akt are PI3K dependent, cAMP-stimulated p70s6k activity is PI3K independent. Furthermore, PKA is required for the effects of cAMP on p70s6k, but not on membrane ruffling or Akt phosphorylation. Therefore, while multiple pathways are required for cAMP-stimulated cell cycle progression, only some of these pathways are PKA dependent.
MATERIALS AND METHODS
Reagents.
Rapamycin, wortmannin, and H89 were purchased from Calbiochem. LY294002, PD98059, and Rp-adenosine-3′,5′-cyclic monophosphothioate (Rp-cAMPS) were obtained from BIOMOL Research Laboratories, New England Biolabs, and Research Biochemicals International, respectively. Fetal calf serum (FCS) was from GIBCO Life Technologies. Bovine serum albumin (BSA) was from Bayer Scientific. All other reagents, including crude bovine TSH (1 U/ml), forskolin, 8-bromo-cAMP (8BrcAMP), cholera toxin, and insulin were purchased from Sigma (St. Louis, Mo.).
Cell culture.
WRT cells and WRT.CRE cells (WRT cells stably transfected with a cAMP response element [CRE]-regulated lacZ gene) were maintained in 3H medium as previously described (35) and rendered quiescent by starvation for 48 to 72 h (WRT) or 24 h (WRT.CRE) (41) in basal medium (Coon’s modified Ham’s F-12 medium containing 0.3% BSA). For DNA synthesis assays, basal medium was supplemented with insulin (0.5 μg/ml) to enhance the mitogenic effects of TSH as described previously (10). Early-passage WRT cells were cotransfected with pSG5 encoding Myc-tagged p110α-CAAX (33) and pDCR, which confers neomycin resistance. Transfected cells were selected and maintained in 3H containing G418 at 300 and 150 μg/ml, respectively. Clonal populations were established from individual G418-resistant colonies, and pools were generated from mass populations of drug-resistant cells. NIH 3T3 and REF52 cells were propagated in Dulbecco’s modified Eagle medium containing 10% FCS and rendered quiescent by starvation in serum-free medium for 24 h. Swiss 3T3 cells were maintained as described previously (59), grown to 90% confluence, and starved in serum-free medium for 16 h. In all studies, the following concentrations were used: 1 mU/ml for TSH, 1 mM for 8BrcAMP, 10 μg/ml for cholera toxin, 10 μM for forskolin, and 0.5 μg/ml for insulin. Inhibitors were used as follows: Rp-cAMPS, 250 to 500 μM; H89, 25 to 50 μM; LY294002, 5 to 15 μM; wortmannin, 25 to 200 nM; rapamycin, 1 nM; and PD98059, 25 μM.
Microinjection.
Glutathione S-transferase (GST) fusion constructs encoding Rac1N17, Cdc42N17, Grb2, the Cdc42/Rac1-interactive binding (CRIB) region of PAK (GST-PAKCRIB; residues 67 to 150), and the N-terminal SH2 domain of human p85α (GST-p85-N-SH2; residues 321 to 440) were expressed and purified from Escherichia coli by affinity chromatography on glutathione agarose (42). GST-Rac1 and Cdc42 proteins were expressed and purified in parallel and then cleaved with thrombin, and the purified G proteins were injected into the cytoplasm at 2.0 mg/ml. Other proteins were injected at the following concentrations: Grb2, 2.4 mg/ml; PAK-CRIB, 8.0 mg/ml; p85-N-SH2, 2.4 mg/ml; and GST, 3 mg/ml. Affinity-purified polyclonal antibodies to the N-terminal SH2 domain (39) of human p85α (2.3 mg/ml) or the C terminus (residues 1054 to 1068) of bovine p110α (2.1 mg/ml) generated by McIlray et al. (39) or kindly provided by S. Courtneidge (56) were injected at the concentrations indicated. All proteins were coinjected with immunoglobulin G (IgG) to identify injected cells. Based on an injection volume of 2 × 10−14 liter, the number of molecules injected per cell is approximately 220,000 to 390,000 (polyclonal antibodies), 850,000 (GST-p85-N-SH2), 1.1 × 106 (Rac1N17, Cdc42N17, and Grb2), 1.4 × 106 (GST), or 1.6 × 106 (GST-PAKCRIB).
DNA synthesis and S6 phosphorylation.
For microinjected inhibitors, cells were treated with cAMP-elevating agents at 1 h postinjection. Cell-permeable inhibitors were added 10 min prior to treatment with cAMP-elevating agents or microinjection of the p85-stimulatory antibody. Wortmannin experiments were conducted in BSA-free basal medium, and the labile inhibitor was readded 12 to 16 h after cAMP treatment. For DNA synthesis studies, cells were labeled with bromodeoxyuridine (BrdU) for 48 h, and DNA synthesis was monitored by immunostaining (34). For S6 phosphorylation studies, cells were fixed for 20 min in 5% acetic acid-ethanol, permeabilized in phosphate-buffered saline (PBS)–0.1% Tween, and stained with fluorescein isothiocyanate-conjugated anti-sheep IgG to detect injected cells and with an affinity-purified rabbit polyclonal antibody raised to a phosphorylated peptide of S6 (amino acids 232 to 249) followed by Texas red-conjugated anti-rabbit IgG.
For DNA synthesis studies in cells expressing p110-CAAX, cells were plated for 16 h, transferred to insulin- and BSA-deficient basal medium for 48 h to remove TSH effects, and then labeled with BrdU for 48 h. Inhibitors were added 2 h prior to BrdU labeling. Wortmannin was readded at 12 to 16 h following addition of BrdU.
CRE-regulated gene expression.
WRT.CRE cells were stimulated with TSH (1 mU/ml) and 3-isobutyl-1-methylxanthine (1 mM) for 6 h and then fixed in 3.7% formaldehyde–PBS for 5 min at room temperature. After fixation, the cells were stained in a mixture of 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, and 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml in PBS for 16 h at 37°C to detect β-galactosidase.
Immunoblotting.
Cells were lysed at 4°C for 20 min in a mixture of 10 mM KPO4, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 50 mM β-glycerophosphate, 2 mM dithiothreitol, 1% Nonidet P-40, 1 mM Na3VO4, 1 mM Pefabloc, and 10 μg each of aprotonin and leupeptin per ml. For detection of Myc-tagged p110-CAAX, cell lysates (300 μg) were subjected to immunoprecipitation with a monoclonal antibody to c-Myc (1 μg/sample; Calbiochem product no. OP10). Immunoprecipitated or total protein lysates were denatured by boiling in Laemmli sample buffer, resolved on sodium dodecyl sulfate–6.75% (p70s6k, Akt, and Myc) or 10% (phospho-MAPK, phospho-Akt, and phospho-S6) polyacrylamide gels, and transferred to polyvinylidene fluoride membranes. Membranes were blocked in PBS–5% (wt/vol) milk–0.1% Tween and then incubated for 2 h with a monoclonal antibody to c-Myc (10 μg/ml; Calbiochem product no. OP10) or polyclonal antibodies raised to p70s6k (1:250; Santa Cruz sc-230), the C terminus of Akt-2 (peptide CDQTHFPQFSYSASIRE), phospho-specific Akt-1 (Ser473) (1:1,000; New England Biolabs product no. 9271), phospho-specific MAPK (Thr183/Tyr185) (1:1,250; Promega product no. V6671), or a phosphorylated peptide of S6. To compare relative effects on Akt and p70s6k, lysates were run on the same gel and transferred to a polyvinylidene fluoride membrane, and the membrane was cut in half and immunoblotted for phosphorylated Akt and S6.
Ruffle formation.
To monitor effects on the actin cytoskeleton, cells plated onto laminin-treated coverslips were incubated in basal medium for 48 h and then treated with cAMP-elevating agents. When used, inhibitors were added for 1 h before stimulation. After treatments, the cells were fixed in 3.7% formaldehyde–PBS and stained with rhodamine-phalloidin essentially as described in reference 28.
RESULTS
cAMP activates Akt and p70s6k.
The second messengers in cAMP-stimulated proliferation have not been well defined. TSH is a cAMP-dependent mitogen for thyroid cells. cAMP-elevating agents acting downstream of the TSH receptor, including cholera toxin (activation of Gαs), forskolin (activation of adenylyl cyclase), and the cAMP analog 8BrcAMP, mimic the mitogenic activity of TSH. We previously reported that p70s6k is essential for cAMP-stimulated cell cycle progression (10). Because p70s6k functions downstream from PI3K in many cells, we explored the role of additional PI3K-dependent signals in cAMP-stimulated mitogenesis.
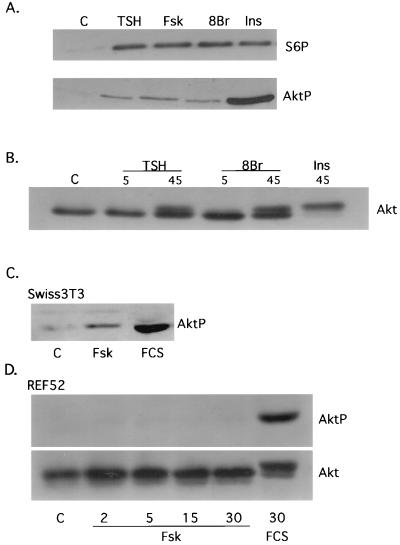
Akt is a serine/threonine-specific protein kinase that mediates many of the effects of PI3K. To elucidate the effects of cAMP on Akt activity, Akt phosphorylation was assessed following treatment with TSH, forskolin, or 8BrcAMP. All cAMP-elevating agents stimulated Akt activity, as determined by immunoblotting with an antibody that specifically recognizes Akt1 phosphorylated at Ser473 (65), a major growth factor-regulated phosphorylation site required for activity (2) (Fig. 1A). To confirm these results, Akt phosphorylation was assessed by the appearance of an Akt2 mobility shift in cAMP-treated cells (Fig. 1B). In comparison with insulin, the effects of cAMP on Akt phosphorylation were modest. In contrast, cAMP-stimulated p70s6k activity, as assessed by S6 phosphorylation (Fig. 1A) or a p70s6k mobility shift (data not shown), was comparable to that of insulin. cAMP effects on Akt were not observed at 5 (Fig. 1B) or 15 (data not shown) min, and maximal effects were observed at 30 to 45 min (Fig. 1B). Although these kinetics are delayed compared to growth factor-stimulated Akt activity in other cells, they are similar to the time course for cAMP-stimulated p70s6k activation in thyroid cells (10). Additionally, in these cells, serum and insulin stimulated Akt and p70s6k activity with kinetics similar to those for the cAMP-elevating agents (data not shown).
FIG. 1.
cAMP activates Akt in a cell-type-specific manner. (A) Quiescent WRT cells (C) were treated for 45 min with TSH (1 mU/ml), forskolin (Fsk; 10 μM), 8BrcAMP (8Br; 1 mM), or insulin (Ins; 0.5 μg/ml). Lysates were analyzed by immunoblotting with phospho-specific S6 and Akt antibodies. Three to five experiments were performed with similar results. (B) Quiescent WRT cells (C) were treated for 5 or 45 min with TSH (1 mU/ml) or 8BrcAMP (1 mM), and lysates were analyzed by immunoblotting with a polyclonal Akt2 antibody. Cells treated with insulin (0.5 μg/ml) for 45 min were included as a control. Three experiments were performed with similar results. (C) Quiescent Swiss 3T3 cells (C) were treated for 30 min with forskolin (10 μM) or 20% FCS, and phosphorylated Akt1 was detected by immunoblotting. Similar results were obtained with a polyclonal Akt2 antibody (not shown). (D) Quiescent REF52 cells (C) were treated for 2, 5, 15, or 30 min with forskolin (10 μM) or for 30 min with 20% FCS, and lysates were analyzed by immunoblotting with a phospho-specific Akt1 antibody or a polyclonal Akt2 antibody. cAMP also failed to stimulate Akt phosphorylation or an Akt mobility shift in NIH 3T3 cells. Four to five experiments were performed with similar results. All studies were conducted in insulin-deficient basal medium.
In Swiss 3T3 fibroblasts, where cAMP also stimulates proliferation, cAMP-elevating agents stimulated Akt1 phosphorylation (Fig. 1C) and an Akt2 mobility shift (data not shown). In contrast, cAMP failed to stimulate Akt1 phosphorylation or an Akt2 mobility shift in REF52 (Fig. 1C) or NIH 3T3 (data not shown) fibroblasts, cells which do not respond to cAMP with proliferation. In close agreement with these results, cAMP stimulated p70s6k activity in thyroid, Swiss 3T3, and rat Schwann cells but not in NIH 3T3 or REF52 cells (10). These results suggest that the ability of cAMP-elevating agents to stimulate proliferation correlates with their ability to activate Akt and p70s6k.
Differential sensitivity of Akt and p70s6k to PI3K inhibitors.
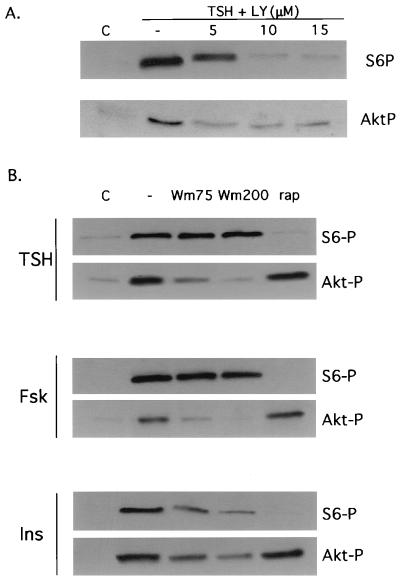
To determine whether cAMP effects on Akt and p70s6k were mediated by PI3K, the effects of LY294002 and wortmannin on TSH- and forskolin-stimulated Akt and S6 phosphorylation were examined. Akt phosphorylation was reduced by the PI3K inhibitors LY294002 (Fig. 2A; data not shown for forskolin) and wortmannin (Fig. 2B), with maximal inhibition at 5 μM for LY294002 and 200 nM for wortmannin. In striking contrast, at the same inhibitor concentrations, cAMP-stimulated S6 phosphorylation was largely unaffected by wortmannin and only partially reduced by LY294002. The apparent dispensability of PI3K was specific to cAMP-stimulated p70s6k activation, since insulin-stimulated S6 phosphorylation was significantly and progressively reduced by increasing concentrations of wortmannin (75 to 200 nM). Marked inhibition of cAMP-mediated S6 phosphorylation was observed with higher concentrations of LY294002 (10 to 15 μM), while higher concentrations of wortmannin (200 nM) had little effect. The effects of LY294002 at higher concentrations may reflect inhibition of cellular targets other than PI3K (7, 17, 19, 47). In vitro, the reported 50% inhibitory concentration for inhibition of mammalian target of rapamycin (mTOR) autokinase activity by LY294002 is 5 μM (7). Treatment with the mTOR inhibitor rapamycin abolished cAMP-stimulated S6 phosphorylation, indicating that cAMP-mediated activation of p70s6k is mTOR dependent (Fig. 2B). In contrast, rapamycin failed to inhibit cAMP-stimulated Akt phosphorylation; therefore, LY294002 effects on Akt are not mediated by mTOR.
FIG. 2.
cAMP effects on p70s6k and Akt are differentially sensitive to wortmannin. (A) Quiescent WRT cells (C) were treated for 45 min with TSH (1 mU/ml) alone or following pretreatment with 5, 10, or 15 μM LY294002 (LY). Lysates were analyzed by immunoblotting with phospho-specific Akt and S6 antibodies. Three to four experiments were performed with the same results. Similar results were obtained with 1 mM 8BrcAMP (data not shown). (B) Quiescent WRT cells (C) were treated for 45 min with TSH (1 mU/ml), forskolin (10 μM), or insulin (0.5 μg/ml) alone or following pretreatment with wortmannin (Wm; 75 or 200 nM), or rapamycin (rap; 1 nM), and lysates were analyzed by immunoblotting with phospho-specific Akt1 and S6 antibodies. Three to five experiments were performed with the same results. All studies were conducted in insulin-deficient basal medium.
To further examine the role of PI3K in cAMP-stimulated S6 phosphorylation, we conducted microinjection studies using GST-p85-N-SH2. S6 phosphorylation was monitored by immunostaining with a phospho-specific S6 antibody. In the absence of growth factors, less than 1% of the cells expressed phosphorylated S6 protein. Following treatment with TSH, phosphorylated S6 was detected in 95.3% ± 1.7% (mean ± standard error [SE]) of the cells (n = 4), and this was unaffected by injection of GST-p85-N-SH2 (95.8% ± 0.96% injected cells express phosphorylated S6, n = 4 representing a total of 905 injected cells), although injection of GST-p85-N-SH2 abolished TSH-stimulated DNA synthesis (see below). Together, these results demonstrate that cAMP-stimulated Akt activation is PI3K-dependent but that cAMP effects on p70s6k activation are primarily PI3K independent. Importantly, these data also show that activation of p70s6k can be uncoupled from Akt.
cAMP stimulates PI3K-dependent membrane ruffling.
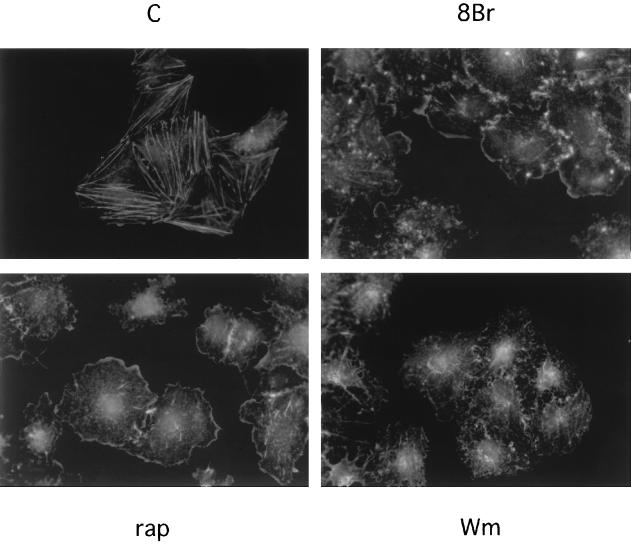
To further explore the role of PI3K in cAMP-mediated signaling, we investigated cAMP effects on membrane ruffling, a PI3K-dependent accumulation of cortical actin filaments induced by many growth factors. As reported previously (43), WRT cells exhibit abundant actin stress fibers in the absence of all growth factors (Fig. 3). Treatment with TSH (data not shown) or 8BrcAMP (Fig. 3) for 45 min resulted in the elaboration of membrane ruffles at cell-cell junctions and at the cell periphery. At this time, actin stress fibers were no longer apparent in cAMP-treated cells. Similar results were observed following forskolin treatment (data not shown). The kinetics of ruffle formation were similar to those for cAMP-stimulated Akt and p70s6k activation.
FIG. 3.
cAMP stimulates PI3K-dependent membrane ruffling. Quiescent WRT cells (C) were treated for 45 min with 8BrcAMP (8Br; 1 mM) alone or following pretreatment with wortmannin (Wm; 75 nM), or rapamycin (rap; 1 nM). Membrane ruffles were visualized by phalloidin staining. Four to seven experiments, performed in duplicate, yielded similar results. Similar results were obtained with TSH (not shown). All studies were conducted in insulin-deficient basal medium.
Membrane ruffles are known to occur through the activation of the small GTPase, Rac1, and Rac1 activation in response to many growth factors is PI3K dependent. To determine if cAMP-stimulated ruffle formation was PI3K dependent, cells were pretreated with wortmannin prior to stimulation with TSH (data not shown) or 8BrcAMP (Fig. 3). Wortmannin blocked cAMP-stimulated ruffle formation but not the dissolution of stress fibers. In contrast, rapamycin did not impair ruffle formation and often enhanced ruffling. These results demonstrate that cAMP activates PI3K-dependent signals leading to Akt activation and membrane ruffling.
PI3Kα/β is required for cAMP-mediated DNA synthesis.
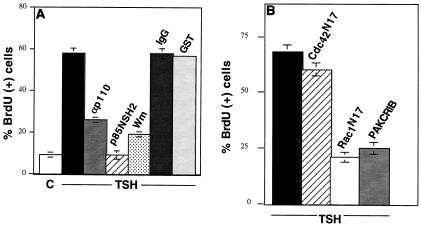
DNA synthesis stimulated by TSH or other cAMP-elevating agents is repressed by wortmannin (Fig. 4A), implicating a role for PI3K (10). Although a role for PI3Kγ downstream from the G-protein-coupled TSH receptor could be envisioned, there are no data at present to support a role for βγ subunits in TSH signaling. Therefore, we examined the role of PI3Kα/β isoforms in cAMP-stimulated cell cycle progression. Microinjection of GST-p85-N-SH2 markedly reduced DNA synthesis stimulated by TSH (Fig. 4A), 8BrcAMP, and cholera toxin (data not shown). These effects were not observed following microinjection of GST or of GST-Grb2, which contains two SH2 domains (Fig. 4A and data not shown). cAMP-stimulated DNA synthesis was also impaired by microinjection of a polyclonal antibody raised against the C terminus of p110α (anti-p110α) but not by nonspecific IgG (Fig. 4A). p85-N-SH2 was a more effective inhibitor than was the p110α antibody. Whether this reflects a contribution from p110β, which is inhibited by p85-N-SH2 but not by anti-p110α, a difference in the molar ratios of these inhibitors to their respective cellular targets, or other effects is not clear.
FIG. 4.
PI3K and Rac1 are required for cAMP-stimulated DNA synthesis. (A) Quiescent WRT cells (C) were treated with TSH (1 mU/ml) alone (unlabeled black bar) or following injection of anti-p110 (αp110) or GST-p85-N-SH2 (p85NSH2) or pretreatment with Wortmannin (Wm; 25 nM). IgG- and GST-injected cells are shown as controls. Inhibitor results are mean ± SE (SE of <1.0 are not shown) of two to six experiments representing 1,060 to 2,108 injected or pretreated cells per condition. Similar results were obtained with 8BrcAMP and cholera toxin (430 to 1,007 cells per condition). (B) BrdU incorporation in TSH-treated WRT cells either alone (unlabeled black bar) or following injection of Rac1N17, GST-PAKCRIB, or Cdc42N17. Results are mean ± SE of two to eight experiments (SE of <1.0 are not shown) representing 952 to 1,236 injected cells per condition. Levels of BrdU incorporation in quiescent and vector-transfected cells were 8% ± 4% and 9% ± 2%, respectively. Similar results were obtained with 8BrcAMP (360 to 1,011 injected cells per condition). In parallel studies, Cdc42N17 reduced FCS-stimulated DNA synthesis by approximately 50% (from 85% BrdU-positive uninjected cells to 43% BrdU-positive injected cells).
To further evaluate the role of PI3K in cAMP-stimulated mitogenesis, other downstream targets of PI3K were examined. As described above (Fig. 3), cAMP induced membrane ruffling, and presumably Rac1 activation, through a PI3K-dependent pathway. Rac1 is required for Ras- and growth factor-stimulated proliferation in other cells where it functions downstream from PI3K. To determine whether Rac1 was required for cAMP-initiated DNA synthesis, a dominant negative Rac1 mutant protein was used. Microinjection of Rac1N17 markedly reduced DNA synthesis stimulated by TSH, cholera toxin, and 8BrcAMP (Fig. 4B and data not shown). Microinjection of GST-PAKCRIB also reduced cAMP-mediated DNA synthesis. In contrast, injection of Cdc42N17 had no effect on DNA synthesis (Fig. 4B). These results indicate that the α and/or β isoforms of PI3K, as well as one of its downstream targets, Rac1, are required for cAMP-stimulated mitogenesis.
cAMP stimulates ruffle formation, Akt, and p70s6k through multiple signaling pathways.
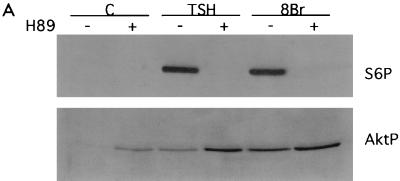
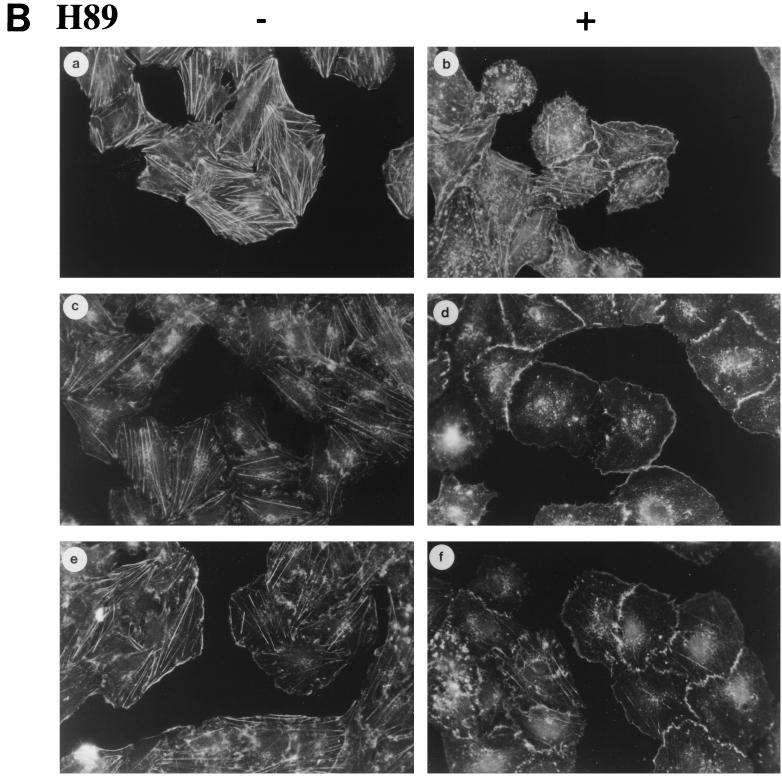
Although cAMP-elevating agents stimulate PKA-dependent DNA synthesis in thyroid cells (34), microinjection of the PKA catalytic subunit failed to stimulate cell cycle progression (21, 40). Although many cAMP effects are elicited through PKA, cAMP has additional targets. cAMP binds directly to and activates ion channels and Rap-specific guanine nucleotide exchange factors (GEFs) (18, 29). We therefore examined whether cAMP effects on ruffle formation and Akt and p70s6k activation were PKA dependent. Treatment with the PKA inhibitor H89 blocked cAMP-stimulated S6 phosphorylation (Fig. 5A) as well as p70s6k mobility shifts (data not shown). Basal levels of S6 phosphorylation in quiescent cells (seen in longer exposures) were also reduced by the PKA inhibitor. In contrast, treatment with H89 did not reduce cAMP-stimulated ruffle formation or Akt phosphorylation, although it abolished CRE-regulated gene expression (Table 1) and DNA synthesis (data not shown). Rather, treatment with H89 augmented basal and cAMP-stimulated Akt phosphorylation levels (Fig. 5A). Comparable effects were observed with Rp-cAMPS (data not shown), a PKA inhibitor that acts by a mechanism distinct from that of H89. Similarly, H89 enhanced membrane ruffling in cells in basal medium (Fig. 5B, panel b) and enabled the detection of membrane ruffles after only 5 min treatment with TSH or 8BrcAMP (panels d and f), when membrane ruffling is not normally observed (panels c and e). These results suggest that cAMP exerts stimulatory effects on Akt and membrane ruffling which are PKA independent. Moreover, PKA activity, even at basal levels, inhibits cAMP-stimulated Akt activation and ruffling, and the outcome is a balance between these two competing pathways. Together with the differential effects of wortmannin on membrane ruffling and Akt versus p70s6k, these results demonstrate a divergence in cAMP-mediated signaling pathways. cAMP effects on membrane ruffling and Akt are PKA independent but PI3K dependent, while those on p70s6k require PKA but not PI3K activity.
FIG. 5.
cAMP effects on Akt and membrane ruffling are PKA independent. (A) Quiescent WRT cells (C) were treated for 45 min with TSH (1 mU/ml) or 8BrcAMP (8Br; 1 mM) alone (−) or following pretreatment with H89 (50 μM; +), and lysates were analyzed by immunoblotting with phospho-specific Akt and S6 antibodies. Two to four experiments were performed with similar results. H89 also increased Akt phosphorylation in cells treated for 5 min with TSH or 8BrcAMP (not shown). (B) Quiescent WRT cells (a) were treated for 5 min with TSH (1 mU/ml; c and d) or 8BrcAMP (1 mM; e and f) alone (−) or following pretreatment with H89 (50 μM; +) and stained with rhodamine-phalloidin. Five experiments were performed with similar results. All studies were conducted in insulin-deficient basal medium.
TABLE 1.
Effects of PKA inhibitors on CRE-regulated gene expression
| Treatment | % β-Galactosidase-positive cellsa |
|---|---|
| Basal | 0 |
| TSH-IBMX | 31.0 |
| TSH-IBMX + H89 (25 μM) | 10.0 |
| TSH-IBMX + H89 (50 μM) | 0 |
| TSH-IBMX + Rp-cAMPS (250 μM) | 0 |
| TSH-IBMX + Rp-cAMPS (500 μM) | 0 |
Quiescent WRT.CRE cells were treated for 6 h with TSH (1 mU/ml) and 3-isobutyl-1-methylxanthine (IBMX) (1 mM) alone or following pretreatment with H89 or Rp-cAMPS and stained for β-galactosidase expression. Results shown are from one representative experiment (at least 400 cells scored per condition) of two to four experiments performed in duplicate with similar results.
Direct elevation of PI3K activity confers hormone-independent DNA synthesis.
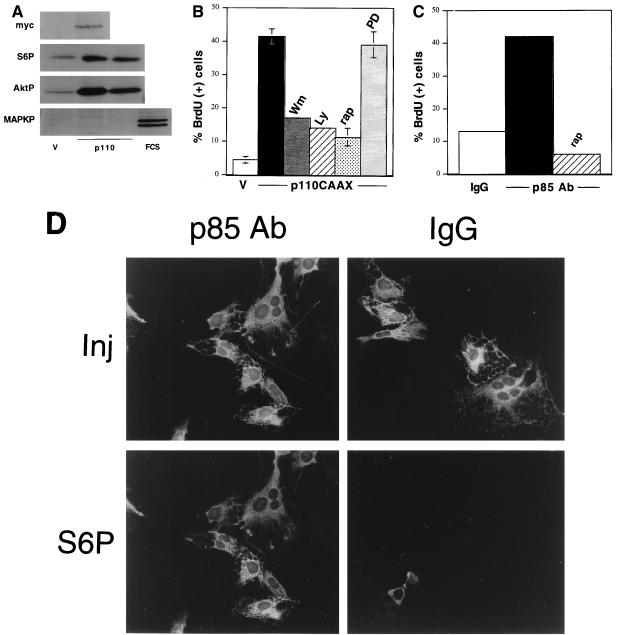
Together, these results suggest that PKA-independent, PI3K-dependent signaling pathways are required for cAMP-stimulated mitogenesis. Two approaches were used to examine whether activation of PI3K was sufficient to stimulate proliferation. First, multiple pools of WRT cells stably expressing a membrane-localized constitutively active form of PI3K (p110-CAAX) (33) were isolated. Transgene expression was documented by immunoprecipitating p110-CAAX with an antibody raised to the Myc epitope tag followed by immunoblotting with the Myc antibody (Fig. 6A). Immunoblotting with anti-p110 antibodies revealed that p110-CAAX was expressed at levels below the endogenous protein in all drug-resistant pools analyzed (data not shown). Chronic expression of p110-CAAX has been reported to be toxic in some cells, perhaps accounting for its low-level expression.
FIG. 6.
Activated PI3K confers TSH-independent DNA synthesis. (A) To analyze expression of myc-p110-CAAX, lysates from p110-CAAX (p110) and vector-transfected control cells (V) were immunoprecipitated and immunoblotted with a monoclonal c-Myc antibody (myc). Results for pool D are shown; similar results were obtained with clone B4-1. To demonstrate activation of p70s6k and Akt, but not MAPK, in p110-CAAX cells, p110-CAAX and vector-transfected control cells were transferred to insulin-deficient basal medium for 24 h, and lysates were analyzed by immunoblotting with phospho-specific antibodies to S6 (S6P), Akt (AktP), or MAPK (MAPKP). For MAPK studies, FCS-treated vector-transfected cells (FCS) were included as a control. Results for pool D and clone B4-1 are shown. Three to four experiments were performed with similar results. (B) TSH-independent DNA synthesis in p110-CAAX cells. Cells were transferred to insulin-deficient basal medium for 48 h, and BrdU incorporation was measured alone (unlabeled black bar) or in the presence of wortmannin (Wm; 100 nM), LY294002 (LY; 5 μM), rapamycin (rap; 1 nM), or PD98059 (PD; 25 μM). Vector-transfected control cells (V) are included as a control. Results are the mean ± SE of four to seven experiments. (C) BrdU incorporation in cells injected with a stimulatory p85 antibody (p85 Ab) alone (black bar) or following pretreatment with rapamycin (rap). Results for control injections with sheep IgG did not differ significantly from those for uninjected cells. Results shown are from one representative experiment of five to eight experiments (894 to 1,060 injected cells per condition) performed with similar results. For comparison, TSH stimulated BrdU incorporation in 53% of treated cells in this experiment. (D) Representative fields of cells injected with the p85 antibody. Immunostaining of injected cells (Inj) and S6 phosphorylation (S6P) are shown. The S6 photomicrographs were exposed for identical times. Phosphorylated S6 was not observed in control cells injected with sheep IgG. Two experiments (549 p85 antibody-injected cells) were performed with similar results.
To determine if stable expression of p110-CAAX was sufficient to confer TSH-independent proliferation, DNA synthesis was measured over 48 h in the absence of all growth factors. Strikingly, despite the modest level of p110-CAAX expression, DNA synthesis was consistently greater in p110-CAAX cells than in parental or vector-transfected controls (Fig. 6B). Treatment with 100 nM wortmannin or 5 μM LY294002 (Fig. 6B) abolished DNA synthesis, confirming that proliferation was PI3K dependent. Like parental cells, proliferation in p110-CAAX cells was inhibited by rapamycin, indicating a requirement for mTOR/p70s6k. Consistently, p110-CAAX cells exhibited basally elevated levels of S6 phosphorylation (Fig. 6A) and a p70s6k mobility shift (data not shown) in addition to elevated levels of phosphorylated Akt1 (Fig. 6A). MAPK activity, as assessed by immunoblotting with a phospho-specific antibody, was not detectably elevated in p110-CAAX cells (Fig. 6A). In addition, treatment with PD98059, a MEK1 inhibitor, had no effect on p110-CAAX-stimulated DNA synthesis (Fig. 6B) although it abolished serum-stimulated MAPK activity in parental cells (data not shown).
To examine acute effects of PI3K on cell proliferation, we used a polyclonal antibody raised to p85-N-SH2 that increases the activity of recombinant p85-p110 dimers threefold and stimulates DNA synthesis in CHO cells (39). Microinjection of the p85 antibody into quiescent cells consistently stimulated a four- to fivefold increase in DNA synthesis (Fig. 6C). Like cells expressing p110-CAAX, cells microinjected with the p85 antibody exhibited elevated levels of p70s6k activity (Fig. 6D). Injection of control IgG had no effect on either DNA synthesis (data not shown) or S6 phosphorylation. Treatment with rapamycin impaired both S6 phosphorylation (data not shown) and DNA synthesis (Fig. 6C) in anti-p85-N-SH2-injected cells. Together, these results demonstrate that stable or acute activation of PI3K and its target p70s6k is sufficient to confer hormone-independent DNA synthesis in thyroid cells.
DISCUSSION
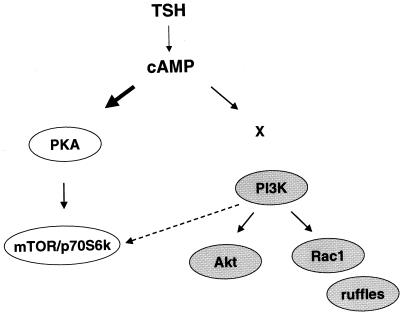
Many hormones regulate proliferation and differentiation through cAMP-mediated signaling pathways. Although cAMP exerts many effects through PKA, cAMP also binds directly to cardiac pacemaker and other ion channels and to Rap1-specific GEFs (18, 29, 30). We demonstrate that mitogenic signals initiated by cAMP diverge to include PKA-dependent pathways leading to p70s6k and PKA-independent pathways that regulate Akt and membrane ruffling (Fig. 7).
FIG. 7.
cAMP stimulates p70s6k and Akt through distinct pathways. cAMP robustly activates p70s6k (bold arrow) through a PKA-dependent pathway. In contrast, cAMP exerts modest effects on Akt and membrane ruffling (thin arrow) which are PKA independent. Both PKA-dependent and -independent effectors contribute to cAMP-stimulated mitogenesis, including mTOR/p70s6k, PI3K, and Rac1. cAMP effects on Akt and membrane ruffling are PI3K dependent, although the mechanism of PI3K activation by cAMP has not been elucidated (X). In contrast, cAMP effects on p70s6k are primarily PI3K independent, although a weak PI3K-dependent contribution (dashed arrow) potentially mediated by PDK1 or Akt may be present.
In WRT cells, cAMP-stimulated mitogenesis requires PKA (34) and mTOR/p70s6k activity (10) whether stimulated by activation of the TSH receptor or intracellularly following treatment with cholera toxin, forskolin, or cell-permeable cAMP analogs. Interference with PKA activity via microinjection of protein kinase inhibitor (34) or with p70s6k activation by treatment with rapamycin (10) impaired cAMP-stimulated DNA synthesis. Paradoxically, microinjection of the PKA catalytic subunit failed to stimulate proliferation (21, 40), suggesting that additional PKA-independent signals contribute to cAMP-stimulated cell cycle progression. Consistent with this hypothesis, cAMP-elevating agents stimulate Akt activity through a PI3K-dependent pathway distinct from the PKA-dependent pathway leading to p70s6k. Treatment with H89 or Rp-cAMPS, two independently acting PKA inhibitors, failed to impair cAMP-stimulated Akt activity, although these inhibitors abolished S6 phosphorylation in the same lysates. Rather, PKA inhibitors consistently enhanced cAMP-stimulated Akt phosphorylation. Moreover, while wortmannin abolished cAMP effects on Akt, it had no effect on cAMP-stimulated S6 phosphorylation. Consistently, microinjection of GST-p85-N-SH2 also failed to reduce cAMP-stimulated S6 phosphorylation although it abolished cAMP effects on DNA synthesis. The differential effects of inhibitors of PKA and PI3K on Akt and S6 phosphorylation provide strong support for a divergence in cAMP-initiated signals through PKA-dependent pathways to p70s6k and PI3K-dependent pathways to Akt. This view is further supported by the finding that cAMP exerts robust effects on S6 phosphorylation and only modest effects on Akt phosphorylation. Therefore, although published reports place Akt upstream from mTOR and p70s6k (6, 62), in thyroid cells these pathways appear to be regulated independently by cAMP-elevating agents.
The effects of cAMP on PI3K-dependent signaling were further investigated by examining effects on membrane ruffling. cAMP-elevating agents stimulated the dissolution of actin stress fibers and the elaboration of prominent membrane ruffles. The ruffles formed following treatment with TSH, forskolin, and 8BrcAMP were qualitatively similar. Most commonly, cAMP-stimulated ruffles were flat, scalloped ruffles that encircled the entire cell and were found at cell-cell and cell-matrix junctions. On occasion, cAMP-treated cells exhibited cytosolic retraction without apparent membrane ruffling. Wortmannin, at concentrations that abolished Akt phosphorylation, prevented ruffle formation in response to cAMP, evidence that cAMP effects on ruffle formation are PI3K dependent. Importantly, PKA inhibitors had no effect on cAMP-stimulated membrane ruffling. Similar to the effects on Akt, pretreatment with H89 enhanced ruffle formation to a small extent in cells in basal medium and to a larger extent in cAMP-treated cells.
We previously reported that cAMP-stimulated DNA synthesis was impaired by interference with either PKA (34) or p70s6k (10) activity. We now demonstrate that PI3K-dependent signals are also required for cAMP-dependent mitogenesis. Microinjection of highly specific inhibitors of either PI3K or Rac1 impaired cAMP-stimulated DNA synthesis. These findings indicate that PI3K-dependent pathways to Akt and Rac1, as well as PKA-dependent pathways to mTOR and p70s6k, contribute to the mitogenic activity of cAMP. These findings may explain the paradox that PKA is insufficient to stimulate thyroid cell proliferation, while cAMP-elevating agents are mitogenic.
The important role of PI3K and p70s6k in thyroid cell proliferation was underscored in experiments where PI3K was overexpressed. Stable expression of an activated form of p110α or acute microinjection of an antibody to p85α that elevates PI3K activity in vitro (39) was sufficient to confer TSH-independent DNA synthesis. However, in both cases, DNA synthesis was repressed by rapamycin, indicating an important contribution of p70s6k to cell proliferation. Indeed, elevated levels of phosphorylated S6 were detected both in p110-CAAX cells and in cells injected with the p85α antibody. Although these findings place PI3K upstream from p70s6k, our results indicate that cAMP stimulates these activities through separable pathways. While we cannot exclude the possibility that cAMP stimulates a modest PI3K-dependent activation of p70s6k comparable in magnitude to its effects on Akt (Fig. 7), such an effect would be obscured by the more robust p70s6k activation elicited by a PI3K-independent pathway (Fig. 7). Both PI3K-dependent and -independent pathways to p70s6k have been reported (38, 44, 45, 64, 75). PI3K-independent pathways to Akt have also been noted (60). Indeed, growth factor-stimulated activation of PI3K does not necessarily lead to a similar activation of all its downstream effectors (72). As in the case for cAMP, increased intracellular calcium stimulates a robust p70s6k activation accompanied by only minor effects on PI3K and Akt activity. In this instance, unlike cAMP effects in thyroid cells, calcium effects on p70s6k were abolished by wortmannin (16). Where activation of p70s6k is PI3K independent, phospholipase Cγ, PKC (reviewed in references 12 and 53), and Raf-1 (38) have been implicated in the regulation of p70s6k activity. In thyroid cells, cAMP-stimulated p70s6k activity was not affected by the MEK inhibitor; therefore, we do not believe that Raf-1 contributes to p70s6k activity in these cells.
The PI3K-dependent effects of cAMP on Akt and membrane ruffles suggest that cAMP regulates PI3K activity and/or localization. Although previous studies have reported only inhibitory effects of cAMP-elevating agents on lipid kinase activity, these studies were conducted in differentiated cells (1, 48) or in cells where cAMP fails to stimulate mitogenesis (9, 45, 61). Our data indicating that both PI3K and Rac1 are required for cAMP-stimulated proliferation, together with the stimulatory effects of cAMP on Akt and membrane ruffling, strongly support the idea that cAMP stimulates PI3K activity. cAMP-elevating agents stimulated Akt phosphorylation in thyroid and Swiss 3T3 cells, where cAMP is a mitogen, but not in NIH 3T3 or REF52 cells, where cAMP lacks mitogenic activity. These results agree with our previous findings that cAMP stimulates p70s6k activity selectively in cells where it is mitogenic (10). These results imply the existence of cell-type-specific mechanisms for the activation of p70s6k, Akt, and presumably PI3K. Therefore, it is likely that the mechanism through which cAMP regulates PI3K activity is distinct from that used by serum growth factors. Interestingly, unlike its effects on Akt and p70s6k, cAMP effects on ruffle formation were not restricted to cells where cAMP is mitogenic. Treatment of REF52 cells with forskolin or 8BrcAMP stimulated ruffles qualitatively similar to those stimulated by cAMP in thyroid cells. Both stimulatory (24, 74) and inhibitory (51) effects of cAMP on membrane ruffling have been described.
Insulin is an important cofactor for the mitogenic activity of cAMP, although insulin alone is not a mitogen in WRT cells. The DNA synthesis studies in parental cells were performed in cells arrested in the presence of 0.5 μg of insulin per ml, which raises the possibility that the inhibitory effects on DNA synthesis observed in the presence of the PI3K and Rac inhibitors are a consequence of effects on basal levels of insulin-stimulated PI3K activity. While this issue cannot be rigorously addressed at the level of DNA synthesis in parental thyroid cells, it is important to note that cAMP effects on all of the signaling molecules examined here were observed in cells arrested in the absence of insulin. Therefore, insulin does not appear to be required for cAMP effects on ruffle formation or Akt phosphorylation. These results are consistent with our earlier report that cAMP stimulates p70s6k activity in an insulin-independent manner (10). Furthermore, in cells expressing p110-CAAX, proliferation was insulin independent, demonstrating that elevations in PI3K and p70s6k activity are sufficient to stimulate cell cycle progression in the absence of exogenous insulin.
How cAMP stimulates cell-type-specific effects on proliferation remains enigmatic. The ability of cAMP to stimulate Akt through a PKA-independent, cell-type-dependent manner suggests the existence of unique signaling circuits in these cells that couple cAMP to the activation of PI3K-dependent signals. Whether these include cAMP-regulated Rap GEFs (18, 29) is not yet known, although it is intriguing that cAMP-GEFI is highly expressed in thyroid tissue. Furthermore, cAMP activates Rap1 in many cells, including thyroid cells (69) and cAMP-responsive Swiss 3T3 cells, where Rap1 acts as a mitogen (77) and an oncogene (4). It is possible that cAMP-regulated GEFs for other small G proteins, perhaps expressed in a cell-type-dependent manner, remain to be discovered. cAMP effects on p70s6k, which are also cell type dependent (10), are PKA dependent. Cell-type-dependent PKA effects could be exerted through the expression of specific PKA isozymes (58) and/or through differential effects on PKA localization mediated by specific anchoring proteins (reviewed in reference 50), activated cell surface receptors (63, 68), and other protein kinases, including PDK1 (11). Thus, there may be multiple ways in which cAMP elevation is translated into cell-type-specific effects on proliferation.
ACKNOWLEDGMENTS
We thank Margaret Chou for valuable advice and discussions and Svetlana Savina for providing technical support.
This work was supported by PHS grant DK45696 to J.L.M. L.A.C. was supported by NSF grant BIR9413215.
REFERENCES
- 1.Ahmed M U, Hazeki K, Hazeki O, Katada T, Ui M. Cyclic AMP-increasing agents interfere with chemoattractant-induced respiratory burst in neutrophils as a result of the inhibition of phosphatidylinositol 3-kinase rather than receptor-operated Ca2+ influx. J Biol Chem. 1995;270:23816–23822. doi: 10.1074/jbc.270.40.23816. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 4.Altschuler D L, Peterson S N, Ostrowski M C, Lapetina E G. Cyclic AMP-dependent activation of Rap1b. J Biol Chem. 1995;270:10373–10376. doi: 10.1074/jbc.270.18.10373. [DOI] [PubMed] [Google Scholar]
- 5.Bellacosa A, Testa J R, Staal S P, Tsucgkus P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing a SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 6.Boudewijn M, Burgering T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 7.Brunn G J, Williams J, Sabers C, Wiederrecht G, Lawrence J J C, Abraham R T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 8.Burgering B M T, Bos J L. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- 9.Busca R, Bertolotto C, Ortonne J-P, Ballotti R. Inhibition of the phosphatidylinositol 3-kinase/p70s6-kinase pathway induces B16 melanoma cell differentiation. J Biol Chem. 1996;271:31824–31830. doi: 10.1074/jbc.271.50.31824. [DOI] [PubMed] [Google Scholar]
- 10.Cass L A, Meinkoth J L. Differential effects of cyclic adenosine 3′,5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology. 1998;139:1991–1998. doi: 10.1210/endo.139.4.5880. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Ma Y, Moore M, Hemmings B A, Taylor S S. Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc Natl Acad Sci USA. 1998;95:9849–9854. doi: 10.1073/pnas.95.17.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou M M, Blenis J. The 70 kDa S6 kinase: regulation of a kinase with multiple roles in mitogenic signalling. Curr Opin Cell Biol. 1995;7:806–814. doi: 10.1016/0955-0674(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 13.Chou M M, Blenis J. The 70kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 14.Coffer P J, Woodgett J R. Molecular cloning and characterization of a novel protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen P, Alessi D R, Cross D A E. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 1997;410:3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- 16.Conus N M, Hemmings B A, Pearson R B. Differential regulation by calcium reveals distinct signaling requirements for the activation of Akt and p70s6k. J Biol Chem. 1998;273:4776–4782. doi: 10.1074/jbc.273.8.4776. [DOI] [PubMed] [Google Scholar]
- 17.Cross M J, Stewart A, Hodgkin M N, Kerr D J, Wakelam M J O. Wortmannin and its structural analogue demethoxyviridin inhibit stimulated phospholipase A2 activity in Swiss 3T3 cells. J Biol Chem. 1995;270:25352–25355. doi: 10.1074/jbc.270.43.25352. [DOI] [PubMed] [Google Scholar]
- 18.de Rooij J, Zwartkruis F T, Verheijen M H G, Cook R H, Nijman S M B, Wittinghofer A, Bos J L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 19.Downing G J, Kim S, Nakanishi S, Catt K J, Balla T. Characterization of a soluble adrenal phosphatidylinositol 4-kinase reveals wortmannin sensitivity of type III phosphatidylinositol kinases. Biochemistry. 1996;35:3587–3594. doi: 10.1021/bi9517493. [DOI] [PubMed] [Google Scholar]
- 20.Downward J. Lipid-regulated kinases: some common themes at last. Science. 1998;279:673–674. doi: 10.1126/science.279.5351.673. [DOI] [PubMed] [Google Scholar]
- 21.Dremier S, Pohl V, Poteet-Smith C, Roger P, Corbin J, Doskeland S O, Dumont J E, Maenhaut C. Activation of cyclic AMP-dependent protein kinase is required but may not be sufficient to mimic cyclic AMP-dependent DNA synthesis and thyroglobulin expression in dog thyroid cells. Mol Cell Biol. 1997;17:6717–6726. doi: 10.1128/mcb.17.11.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fantl W J, Escobedo J A, Martin G A, Turck C W, de Rosario M, McCormick F, Williams L T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992;69:413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- 23.Hafner S, Adler H S, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994;14:6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J D, Rubin C S. Regulation of cytoskeleton organization an dpaxillin dephosphorylation by cAMP. Studies on murine Y1 adrenal cells. J Biol Chem. 1996;271:29211–29215. doi: 10.1074/jbc.271.46.29211. [DOI] [PubMed] [Google Scholar]
- 25.Jhun B H, Rose D W, Seely B L, Rameh L, Cantley L, Saltiel A R, Olefsky J M. Microinjection of the SH2 domain of the 85-kilodalton subunit of phosphatidylinositol 3-kinase inhibits insulin-induced DNA synthesis and c-fos expression. Mol Cell Biol. 1994;14:7466–7475. doi: 10.1128/mcb.14.11.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger family. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joneson T, Bar-Sagi D. A Rac1 effector site controlling mitogenesis through superoxide production. J Biol Chem. 1998;273:17991–17994. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- 28.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki H, Springett G M, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman D E, Graybiel A M. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki H, Springett G M, Toki S, Canales J J, Harlan P, Blumenstiel J P, Chen E J, Bany I A, Mochizuki N, Ashbacher A, Matsuda M, Housman D E, Graybiel A M. A Rap guanine nucleotide exchange factor enriched highly in basal ganglia. Proc Natl Acad Sci USA. 1998;95:13278–13283. doi: 10.1073/pnas.95.22.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazlauskas A, Kashishian A, Cooper J A, Valius M. GTPase-activating protein and phosphatidylinositol 3-kinase bind to distinct regions of the platelet-derived growth factor receptor beta subunit. Mol Cell Biol. 1992;12:2534–2544. doi: 10.1128/mcb.12.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khosravi-Far R, Solski P A, Clark C J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kupperman E, Wen W, Meinkoth J L. Inhibition of thyrotropin-stimulated DNA synthesis by microinjection of inhibitors of cellular ras and the cyclic AMP-dependent protein kinase. Mol Cell Biol. 1993;13:4477–4484. doi: 10.1128/mcb.13.8.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupperman E, Wofford D, Wen W, Meinkoth J L. Ras inhibits thyroglobulin expression but not cyclic adenosine monophosphate-mediated signaling in Wistar rat thyrocytes. Endocrinology. 1996;137:96–104. doi: 10.1210/endo.137.1.8536648. [DOI] [PubMed] [Google Scholar]
- 36.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 37.Lamy F, Wilkin F, Baptist M, Posada J, Roger P P, Dumont J E. Phosphorylation of mitogen-activated protein kinases is involved in the epidermal growth factor and phorbol ester, but not in the thyrotropin/cAMP, thyroid mitogenic pathway. J Biol Chem. 1993;268:8398–8401. [PubMed] [Google Scholar]
- 38.Lenormand P, McMahon M, Pouyssegur J. Oncogenic Raf-1 activates p70 S6 kinase via a mitogen-activated protein kinase-independent pathway. J Biol Chem. 1996;271:15762–15768. doi: 10.1074/jbc.271.26.15762. [DOI] [PubMed] [Google Scholar]
- 39.McIlroy J, Chen D, Wjasow C, Michaeli T, Backer J M. Specific activation of p85-p110 phosphatidylinositol 3′-kinase stimulates DNA synthesis by Ras- and p70 S6 kinase-dependent pathways. Mol Cell Biol. 1997;17:248–255. doi: 10.1128/mcb.17.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meinkoth, J. L. Unpublished data.
- 41.Meinkoth J L, Goldsmith P K, Spiegel A M, Feramisco J R, Burrow G N. Inhibition of TSH-induced DNA synthesis in thyroid follicular cells by microinjection of an antibody to the stimulatory G protein of adenylyl cyclase Gs. J Biol Chem. 1992;267:13239–13245. [PubMed] [Google Scholar]
- 42.Miller M J, Prigent S, Kupperman E, Rioux L, Park S-H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 43.Miller M J, Rioux L, Prendergast G V, Cannon S, White M A, Meinkoth J L. Differential effects of protein kinase A on Ras effector pathways. Mol Cell Biol. 1998;18:3718–3726. doi: 10.1128/mcb.18.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ming X-F, Boudewijn M, Burgering T, Wennstrom S, Claesson-Welsh L, Heldin C-H, Bos J L, Kozma S C, Thomas G. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature. 1994;371:426–429. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- 45.Monfar M, Lemon K P, Grammer T C, Cheatham L, Chung J, Vlahos C J, Blenis J. Activation of pp70/85 S6 kinases in interleukin-2-responsive lymphoid cells is mediated by phosphatidylinositol 3-kinase and inhibited by cyclic AMP. Mol Cell Biol. 1995;15:326–337. doi: 10.1128/mcb.15.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore K A, Sethi R, Doanes A M, Johnson T M, Pracyk J B, Kirby M, Irani K, Goldschmidt-Clermont P J, Finkel T. Rac1 is required for cell proliferation and G2/M progression. Biochem J. 1997;326:17–20. doi: 10.1042/bj3260017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakanishi S, Catt K J, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pool of inositolphospholipids. Proc Natl Acad Sci USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohsaka Y, Tokumitsu Y, Nomura Y. Suppression of insulin-stimulated phosphatidylinositol 3-kinase activity by the β3-adrenoceptor agonist CL316243 in rat adipocytes. FEBS Lett. 1997;402:246–250. doi: 10.1016/s0014-5793(97)00007-0. [DOI] [PubMed] [Google Scholar]
- 49.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 50.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 51.Poggi A, Panzeri M C, Moretta L, Zocchi M R. CD31-triggered rearrangement of the actin cytoskeleton in human natural killer cells. Eur J Immunol. 1996;26:817–824. doi: 10.1002/eji.1830260414. [DOI] [PubMed] [Google Scholar]
- 52.Ptasznik A, Prossnitz E R, Yoshikawa D, Smrcka A, Traynor-Kaplan A E, Bokoch G M. A tyrosine kinase signaling pathway accounts for the majority of phosphatidylinositol 3,4,5-triphosphate formation in chemoattractant-stimulated human neutrophils. J Biol Chem. 1996;271:25204–25207. doi: 10.1074/jbc.271.41.25204. [DOI] [PubMed] [Google Scholar]
- 53.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 54.Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 55.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 56.Roche S, Koegl M, Courtneidge S A. The phosphatidylinositol 3-kinase α is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994;91:9185–9189. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 58.Roger P P, Reuse S, Maenhaut C, Dumont J E. Multiple facets of the modulation of growth by cAMP. Vitam Horm. 1995;51:59–191. doi: 10.1016/s0083-6729(08)61038-9. [DOI] [PubMed] [Google Scholar]
- 59.Rozengurt E. Synergistic stimulation of DNA synthesis by cyclic AMP derivatives and growth factors in mouse Swiss 3T3 cells. J Cell Physiol. 1982;112:243–250. doi: 10.1002/jcp.1041120213. [DOI] [PubMed] [Google Scholar]
- 60.Sable C L, Filippa N, Hemmings B, VanObberghen E. cAMP stimulates protein kinase B in a wortmannin-insensitive manner. FEBS Lett. 1997;409:253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 61.Scott P H, Belham C M, Al-Hafidh J, Chilvers E R, Peacock A J, Goulds G W, Plevin R. A regulatory role for cAMP in phosphatidylinositol 3-kinase/p70 ribosomal S6 kinase-mediated DNA synthesis in platelet-derived-growth-factor-stimulated bovine airway smooth-muscle cells. Biochem J. 1996;318:965–971. doi: 10.1042/bj3180965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott P H, Brunn G J, Kohn A D, Roth R A, Lawrence J C., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skalhegg B, Tasken K, Hansson V, Huitfeldt H S, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- 64.Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi J K, Trotta R, Wlodarski P, Perrotti D, Chan T O, Wasik M A, Tsichlis P N, Calabretta B. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3K/Akt-dependent pathway. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Summers, S. A., E. L. Whiteman, H. Cho, L. Lipfert, and M. J. Birnbaum. Differentiation-dependent suppression of PDGF signaling. J. Biol. Chem., in press. [DOI] [PubMed]
- 66.Thelen M, Didichenko S A. G-protein coupled receptor-mediated activation of PI 3-kinase in neutrophils. Ann N Y Acad Sci. 1997;832:368–382. doi: 10.1111/j.1749-6632.1997.tb46265.x. [DOI] [PubMed] [Google Scholar]
- 67.Thomason P A, James S R, Casey P J, Downes C P. A G-protein βγ-subunit-responsive phosphoinositide 3-kinase activity in human platelet cytosol. J Biol Chem. 1994;269:16525–16528. [PubMed] [Google Scholar]
- 68.Tortora G, Damiano V, Bianco C, Baldassarre G, Bianco A R, Lanfrancone L, Pelicci P G, Ciardiello F. The Rla subunit of protein kinase A (PKA) binds to Grb2 and allows PKA interaction with the activated EGF-receptor. Oncogene. 1997;14:923–928. doi: 10.1038/sj.onc.1200906. [DOI] [PubMed] [Google Scholar]
- 69.Tsygankova, O. M., and J. L. Meinkoth. Submitted for publication.
- 70.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 71.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 72.VanWeering D H J, DeRooij J, Marte B, Downward J, Bos J L, Burgering B M T. Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–1811. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 74.Wade W F, Dickman D K, Peterson D, McCluskey J, Khrebtukova I. Class II cytoplasmic and transmembrane domains are not required for class II-mediated B cell spreading. Immunol Lett. 1995;44:67–74. doi: 10.1016/0165-2478(94)00178-t. [DOI] [PubMed] [Google Scholar]
- 75.Weng Q-P, Andrabi K, Klippel A, Kozlowski M T, Williams L T, Avruch J. Phosphatidylinositol 3-kinase signals activation of p70 S6 kinase in situ through site-specific p70 phosphorylation. Proc Natl Acad Sci USA. 1995;92:5744–5748. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Withers D J, Bloom S R, Rozengurt E. Dissociation of cAMP-stimulated mitogenesis from activation of the mitogen-activated protein kinase cascade in Swiss 3T3 cells. J Biol Chem. 1995;270:21411–21419. doi: 10.1074/jbc.270.36.21411. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida Y, Kawata M, Miura Y, Musha T, Sasaki T, Kikuchi A, Takai Y. Microinjection of smg/rap1/Krev-1 p21 into Swiss 3T3 cells induces DNA synthesis and morphological changes. Mol Cell Biol. 1992;12:3407–3414. doi: 10.1128/mcb.12.8.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]