Abstract
Background:
Multidrug-resistant Gram-negative bacterial infections are increasingly common among solid organ transplant (SOT) recipients, leading to challenges in the selection of empiric antimicrobial therapy. We sought to develop a clinical tool to predict which SOT recipients are at high risk for extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales (EB) bloodstream infection (BSI).
Methods:
A multicenter case-control study was performed. The source population included SOT recipients with an EB BSI between 2005 and 2018. Cases were those with ESBL-EB BSI; controls were those with non-ESBL EB BSI. The population was subdivided into derivation and validation cohorts based on study site. The predictive tool was developed in the derivation cohort through iterative multivariable logistic regression analyses that maximized the area under the receiver-operating curve (AUC). External validity was assessed using the validation cohort.
Results:
A total of 897 SOT recipients with an EB BSI were included, of which 539 were assigned to the derivation cohort (135, 25% ESBL-EB) and 358 to the validation cohort (221, 62% ESBL-EB). Using multivariable analyses, the most parsimonious model that was predictive of ESBL-EB BSI consisted of 10 variables, which fell into four clinical categories: prior colonization or infection with EB organisms, recent antimicrobial exposures, severity of preceding illness, and immunosuppressive regimen. This model achieved an AUC of 0.81 in the derivation cohort and 0.68 in the validation cohort.
Conclusions:
Though further refinements are needed in additional populations, this tool shows promise for guiding empiric therapy for SOT recipients with EB BSI.
Keywords: bloodstream infection, enterobacterales, extended-spectrum beta-lactamase, predictive tool, transplant
1 ∣. INTRODUCTION
Multidrug-resistant organisms (MDROs) have been increasingly identified as the etiology of infectious complications following solid organ transplantation (SOT).1-5 Such MDRO infections, and particularly MDRO bloodstream infections (BSI), have been associated with significant morbidity and mortality in this population.1,3,6,7 One of the most common causes of MDR Gram-negative (GN) infections among SOT recipients are the extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales (EB) organisms. Prior studies have found that as many as 50% of Escherichia coli (E. coli) and Klebsiella isolates among SOT recipients are ESBL-producing,4 and a history of SOT has been identified in several studies as an independent risk factor for ESBL-EB infection.8-10 This high rate of MDRO, and particularly ESBL-EB, infection is likely related to several unique clinical characteristics of the SOT population, including their frequent contact with the healthcare system, repeated antimicrobial exposures including prolonged prophylactic courses, and chronic immunosuppression.11-14
Following species-level identification of an EB organism on blood culture, most clinical microbiology laboratories require at least 24 h for determination of antimicrobial susceptibility results. While rapid diagnostic assays for identification of select beta-lactamase genes have been developed, they are not yet widely utilized due to their cost and varied ability to detect cephalosporin resistance.15,16 Thus, in the majority of cases, clinicians must select empiric EB BSI therapy without knowledge of the organism's resistance pattern. Due to the higher prevalence of MDROs among SOT recipients, there has been a steady movement toward the empiric usage of carbapenems for these patients, which in turn has increased rates of carbapenem resistance.17-19 Given the need for early effective therapy and the need to limit carbapenem exposure in a population at high risk for MDROs, a predictive tool could potentially assist providers in selection of appropriate empiric therapy. To our knowledge, no such validated tools have been developed for ESBL-EB infections among SOT recipients. Thus, in this study, we developed a clinical prediction tool to determine the risk for ESBL-EB as the etiology of EB BSI at the time of blood culture identification among SOT recipients.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study design and setting
Using a previously described study population,20 a case-control study was conducted at three quaternary care transplant centers: (1) the Hospital of the University of Pennsylvania (HUP), a 776-bed hospital in Philadelphia, Pennsylvania; (2) the University of Maryland Medical Center (UMMC), a 767-bed hospital in Baltimore, Maryland; and (3) the Johns Hopkins Hospital (JHH), an 1,154-bed hospital in Baltimore, Maryland.
2.2 ∣. Study population
The study population was divided into two cohorts: (1) the derivation cohort, which included all adult SOT recipients with an EB BSI at HUP and UMMC between January 1, 2007, and June 30, 2018, and (2) the validation cohort, which included all adult SOT recipients with an EB BSI at JHH between January 1, 2005, and December 31, 2015. The cohorts were divided in this manner because the proportion of EB BSI that were ESBL-producing was significantly higher at JHH than HUP or UMMC, thus allowing us to externally validate the predictive tool in a patient population with a different baseline prevalence of ESBL-EB. Only the first episode of EB BSI was included for each subject. Eligible subjects were identified through the clinical microbiology laboratories at each center. Since these laboratories process both inpatient and outpatient specimens, the cohorts included any SOT recipient with an EB BSI regardless of the location from which the culture was drawn.
In both the derivation and validation cohorts, case patients were defined as those with an ESBL-EB BSI. EB organisms included E. coli, Klebsiella, Enterobacter, Citrobacter, Proteus, and Serratia species. ESBL production was determined by confirmatory testing using the double disk method with both cefotaxime and ceftazidime,21 the ESBL ETEST® (bioMérieux, Durham, North Carolina), or a ceftriaxone MIC of ≥ 8 μg/ml, as this cut point has been shown to have positive and negative predictive values of 100% and 99.5%, respectively, for ESBL production in E. coli, Klebsiella species, and Proteus mirabilis.22 Control patients were those with a non-ESBL-EB BSI, as defined by negative confirmatory testing or a ceftriaxone MIC < 8 μg/ml. Since only the index EB BSI for each SOT recipient was considered, controls did not have a prior ESBL-EB BSI during the study period. All cases and controls in each cohort were included in this study. Approval was obtained from the Institutional Review Board (IRB) at each institution with waivers of informed consent.
2.3 ∣. Data collection
Data on SOT recipients were abstracted from the electronic medical records at each study site using a combination of electronic data extraction, with validation of key fields, and manual chart review. For each EB BSI episode, microbiological data were recorded, including the specific organism identified, results of antibiotic susceptibility testing, and any confirmatory testing performed. Information was also gathered about the subjects' baseline characteristics at the time of EB BSI, including demographics, medical comorbidities, severity of illness in the prior 48 h, type of organ transplant, immunosuppressive regimen, source of EB BSI, colonization or infection with EB organisms in the prior 12 mo (based on growth on a clinical culture from any anatomical site), and antimicrobial exposures in the prior 6 mo (including any agent of which the subject received at least a single dose).
2.4 ∣. Susceptibility testing of EB isolates
All EB isolates identified from study subjects were tested as part of routine care for antibiotic susceptibility at each center's clinical microbiology laboratory. Susceptibility testing was performed using the semi-automated Vitek 2 identification and susceptibility system (bioMerieux, Inc, Durham, NC) at HUP; the Phoenix Automated System (BD Diagnostics, Sparks, Maryland) at JHH; and disc diffusion prior to 2010, and the Vitek 2 system after 2010 at UMMC.
2.5 ∣. Statistical analysis
Using the derivation cohort, case and control subjects were characterized by potential predictors, such as demographics, comorbidities, and prior antibiotic exposures. Continuous variables were compared using the Student's t test or Wilcoxon rank-sum test, and categorical variables were compared using the χ2 or Fisher's exact test. A predictive model was then developed using multivariable logistic regression. First, bivariable analyses were performed to determine the relationship between each predictor and the outcome of interest (ESBL-EB BSI). A multivariable model was then developed, using a forward stepwise procedure to maximize the area under the receiver operating curve (AUC).23,24 Covariates were added to the model until the AUC improved by less than 1%. A simplified scoring system was then developed based on the magnitude of the β coefficient for each variable in the final model. The internal validity of the tool was assessed for calibration by calculating the Hosmer-Lemeshow statistic,25 and for discrimination by calculating the AUC. Next, the performance characteristics (ie, sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) of each possible cut point were evaluated, and a cut-off value was proposed for the tool that optimized the PPV and NPV.
To determine external validity, this scoring system was then applied to the validation cohort, and its performance characteristics were assessed in this population through calculation of the AUC, sensitivity, specificity, PPV, and NPV.
2.6 ∣. Subgroup analyses
(1) Organ transplant type: As the predictors of ESBL-EB BSI may differ by organ transplant type, we performed a subgroup analysis in which we stratified subjects based on whether they had received a thoracic organ transplant (ie, heart or lung(s)), or an abdominal organ transplant (ie, liver, kidney, or pancreas). (2) Transplant era: Since SOT-related practices and rates of ESBL production amongst EB isolates have evolved over the greater than 10-year study period, we performed a subgroup analysis in which we stratified patients based on whether they were transplanted before or after 2010. (3) Time since transplant: Since the predictors of ESBL-EB may differ depending on time since transplant, we performed a subgroup analysis in which we stratified subjects based on whether they were more or less than 1 y post-transplant at the time of their EB BSI. We employed the same statistical methods as described above to develop separate predictive models for each of these subgroups.
3 ∣. RESULTS
3.1 ∣. Study population
A total of 897 SOT recipients were identified with an EB BSI during the study period, of which 356 (40%) were due to an ESBL-producing organism. The derivation cohort consisted of 539 SOT recipients, with 274 subjects from HUP and 265 from UMMC. In this cohort, 135 (25%) of BSIs were due to an ESBL-EB. The median age was 58 y (interquartile range [IQR] 49-65), and 227 (42%) of subjects were female. There were 308 (57%) kidney transplant recipients, 161 (30%) liver transplant recipients, 47 (9%) heart transplant recipients, 39 (7%) lung transplant recipients, and 19 (4%) pancreas transplant recipients. The median time elapsed between transplant and EB BSI was 21 mo (IQR 3-92). Organisms isolated on blood culture in this cohort included E. coli (224, 42%), Klebsiella species (219, 41%), Enterobacter species (51, 9%), Serratia marcescens (21, 4%), Proteus mirabilis (13, 2%), and Citrobacter species (8, 1%). ESBL production was observed in 44 (20%) E. coli isolates and 61 (28%) Klebsiella isolates.
The validation cohort consisted of 358 SOT recipients from JHH, of which 221 (62%) experienced an EB BSI from an ESBL-producing organism. The median age was 54 y (IQR 42-62), and 143 (40%) of subjects were female. There were 216 (60%) kidney transplant recipients, 117 (33%) liver transplant recipients, 22 (6%) heart transplant recipients, 19 (5%) lung transplant recipients, and 8 (2%) pancreas transplant recipients. The median time elapsed between transplant and EB BSI was 22 mo (IQR 4-102). Organisms isolated in this cohort included Klebsiella species (152, 42%), E. coli (114, 32%), Enterobacter species (68, 19%), Serratia marcescens (9, 3%), Proteus mirabilis (9, 3%), and Citrobacter species (6, 2%). ESBL production was observed in 97 (64%) Klebsiella isolates and 74 (65%) E. coli isolates. Additional characteristics of both cohorts are described in Table 1.
TABLE 1.
Baseline characteristics of cases and controls in the derivation and validation cohortsa
| Derivation Cohortb |
Validation Cohort |
|||||
|---|---|---|---|---|---|---|
| Non-ESBL (N = 404) |
ESBL (N = 135) | P value | Non-ESBL (N = 137) |
ESBL (N = 221) | P value | |
| Demographics | ||||||
| Age (median, IQR), years | 58 (48-65) | 59 (51-65) | .26 | 55 (43-63) | 54 (42-62) | .47 |
| Female | 170 (42.1%) | 57 (42.2%) | .98 | 50 (36.5%) | 93 (42.1%) | .29 |
| Medical comorbidities | ||||||
| Congestive heart failure | 43 (10.6%) | 13 (9.6%) | .74 | 14 (10.2%) | 15 (6.8%) | .28 |
| Diabetes mellitus | 228 (56.4%) | 70 (51.6%) | .35 | 53 (38.7%) | 68 (30.8%) | .12 |
| Chronic kidney disease | 64 (15.8%) | 21 (15.6%) | .94 | 3 (2.2%) | 6 (2.7%) | >.99 |
| End stage renal disease on dialysis | 178 (44.1%) | 56 (41.5%) | .60 | 26 (19.0%) | 35 (15.8%) | .44 |
| End stage liver disease | 92 (22.8%) | 39 (28.9%) | .15 | 8 (5.9%) | 12 (5.4%) | .87 |
| Structural lung disease | 31 (7.7%) | 20 (14.8%) | .01 | 7 (5.1%) | 9 (4.1%) | .64 |
| Severity of illness in prior 48 h | ||||||
| Hypotensionc | 94 (24.1%) | 37 (32.5%) | .07 | 81 (59.1%) | 137 (62.0%) | .59 |
| Mechanical ventilation | 35 (9.0%) | 33 (29.0%) | <.01 | 20 (14.6%) | 44 (19.9%) | .20 |
| Type of organ transplant | ||||||
| Heart transplant | 35 (8.7%) | 12 (8.9%) | .94 | 11 (8.0%) | 11 (5.0%) | .24 |
| Lung transplant | 22 (5.5%) | 17 (12.6%) | .01 | 5 (3.7%) | 14 (6.3%) | .34 |
| Liver transplant | 111 (27.5%) | 50 (37.0%) | .04 | 41 (29.9%) | 76 (34.4%) | .38 |
| Kidney transplant | 248 (61.4%) | 60 (44.4%) | <.01 | 84 (61.3%) | 132 (59.7%) | .77 |
| Pancreas transplant | 14 (3.5%) | 5 (3.7%) | >.99 | 3 (2.2%) | 5 (2.3%) | >.99 |
| Immunosuppressive regimen | ||||||
| Corticosteroidsd | 207 (51.2%) | 92 (68.2%) | <.01 | 119 (86.9%) | 198 (89.6%) | .43 |
| Non-corticosteroid immunomodulatore | 179 (44.3%) | 86 (63.7%) | <.01 | 4 (2.9%) | 30 (13.6%) | <.01 |
| Induction regimen | ||||||
| Any induction | 177 (43.8%) | 58 (43.0%) | .86 | 50 (36.5%) | 75 (33.9%) | .62 |
| Induction with antithymocyte globulin (ATG) | 60 (14.9%) | 10 (7.4%) | .03 | 41 (29.9%) | 63 (28.5%) | .77 |
| Allograft rejection in prior 6 mo | 36 (9.0%) | 18 (13.9%) | .11 | 17 (12.4%) | 19 (8.6%) | .24 |
| Neutropenia at time of BSI f | 20 (5.0%) | 8 (5.9%) | .66 | 16 (11.7%) | 22 (10.0%) | .61 |
| Source of EB BSI | ||||||
| Hepatobiliary | 42 (10.4%) | 11 (8.2%) | .49 | 23 (16.8%) | 38 (17.2%) | .92 |
| Central venous catheter | 38 (9.4%) | 10 (7.4%) | .48 | 25 (18.3%) | 36 (16.3%) | .63 |
| Intra-abdominal (eg, abscess) | 32 (7.9%) | 13 (9.6%) | .53 | 22 (16.1%) | 36 (16.3%) | .95 |
| Pulmonary | 18 (4.5%) | 14 (10.4%) | .01 | 7 (5.1%) | 18 (8.1%) | .27 |
| Skin and soft tissue | 12 (3.0%) | 6 (4.4%) | .41 | 3 (2.2%) | 2 (0.9%) | .38 |
| Genitourinary | 182 (45.1%) | 42 (31.1%) | <.01 | 58 (42.3%) | 94 (42.5%) | .97 |
| Hospitalization in prior 6 mo | 270 (66.8%) | 109 (80.7%) | <.01 | 102 (74.5%) | 160 (72.4%) | .67 |
| Antimicrobial exposures in prior 6 mo | ||||||
| Third-generation cephalosporin | 34 (8.4%) | 33 (24.4%) | <.01 | 25 (18.3%) | 73 (33.0%) | <.01 |
| Cefepime | 57 (14.1%) | 42 (31.1%) | <.01 | 46 (33.6%) | 79 (35.8%) | .68 |
| Piperacillin-tazobactam | 94 (23.3%) | 53 (39.3%) | <.01 | 77 (56.2%) | 130 (58.8%) | .63 |
| Fluoroquinolone | 83 (20.5%) | 44 (32.6%) | <.01 | 50 (36.5%) | 87 (39.4%) | .59 |
| Carbapenem | 24 (5.9%) | 36 (26.7%) | <.01 | 47 (34.3%) | 88 (39.8%) | .30 |
| Aminoglycoside | 15 (3.7%) | 17 (12.6%) | <.01 | 13 (9.5%) | 50 (22.6%) | <.01 |
| Trimethoprim-sulfamethoxazole | 107 (26.5%) | 56 (41.5%) | <.01 | 50 (36.5%) | 105 (47.5%) | .04 |
| Colonization or infection with EB organisms in prior 12 mo | ||||||
| Prior EB on any culture | 159 (43.4%) | 72 (55.0%) | .02 | 66 (48.2%) | 127 (57.5%) | .09 |
| Prior E. coli on any culture | 82 (20.3%) | 30 (22.2%) | .63 | 33 (24.1%) | 43 (19.5%) | .30 |
| Prior Klebsiella on any culture | 63 (15.6%) | 40 (29.6%) | <.01 | 34 (24.8%) | 70 (31.7%) | .17 |
| Prior ESBL-EB on any culture | 11 (2.7%) | 58 (43.0%) | <.01 | 12 (8.8%) | 56 (25.3%) | <.01 |
| Prior EB on blood culture | 12 (3.0%) | 2 (1.5%) | .53 | 24 (17.5%) | 45 (20.4%) | .51 |
| Prior EB on lower respiratory culture | 25 (6.2%) | 25 (18.5%) | <.01 | 16 (11.7%) | 27 (12.2%) | .88 |
| Prior EB on urinary culture | 118 (29.2%) | 42 (31.9%) | .56 | 44 (32.1%) | 67 (30.3%) | .72 |
| Prior EB on intra-abdominal culture | 5 (1.2%) | 1 (0.7%) | >.99 | 3 (2.2%) | 29 (13.1%) | <.01 |
Abbreviations: BSI, bloodstream infection; EB, Enterobacterales; ESBL, extended-spectrum beta-lactamase; IQR, interquartile range.
While other characteristics were also assessed, only the variables included in the final predictive model or otherwise felt to be of biologic interest are reported in this table.
All values are reported as number (percentage of total), unless otherwise noted.
Defined by a drop in systolic blood pressure of > 30 mm Hg and diastolic blood pressure of > 20 mm Hg, requirement for vasopressor, or systolic blood pressure < 90 mm Hg.
Assessed at the time of EB BSI, including any corticosteroid dose.
Receipt of any of the following agents in the 30 d preceding EB BSI: abatacept, anakinra, apremilast, azathioprine, cyclophosphamide, cyclosporine, denosumab, hydroxychloroquine, methotrexate, mycophenolate, rituximab, secukinumab, sulfasalazine, tocilizumab, tofacitinib, infliximab, adalimumab, certolizumab, golimumab, or etanercept.
Defined as an absolute neutrophil count of < 500 cells/μl.
3.2 ∣. Derivation of the predictive tool
On multivariable analysis, the most parsimonious model that was predictive of ESBL-EB BSI consisted of 10 variables (Table 2). Of these, eight factors were associated with an increased odds of ESBL-EB: (1) isolation of an ESBL-EB organism on any clinical culture in the prior 12 mo, (2) exposure to a third-generation cephalosporin in the prior 6 mo, (3) exposure to trimethoprim-sulfamethoxazole in the prior 6 mo, (4) exposure to an aminoglycoside in the prior 6 mo, (5) a corticosteroid-containing immunosuppressive regimen (at any dose) at the time of the EB BSI, (6) receipt of a non-corticosteroid immunomodulator in the prior 30 d, (7) mechanical ventilation in the 48 h leading up to the BSI, and (8) presence of hypotension in the 48 h leading up to the BSI. In contrast, two factors were associated with a reduction in the odds of ESBL-EB: (9) isolation of E. coli (rather than another EB organism) on any clinical culture in the prior 12 mo and (10) isolation of an EB organism from a urinary culture (rather than a clinical culture from another anatomic site) in the prior 12 mo.
TABLE 2.
Predictive tool for ESBL-EB BSI among solid organ transplant recipients
| Variable | aOR | 95% CI | P value | Points | |
|---|---|---|---|---|---|
| Colonization or infection with EB organisms in prior 12 mo | ESBL-EB organism isolated on prior culture | 29.70 | 13.10-67.30 | <.01 | 5 |
| E. coli isolated on prior culture | 0.92 | 0.41-2.06 | .85 | −1 | |
| EB organism isolated from prior urinary culture | 0.55 | 0.26-1.18 | .12 | −2 | |
| Antimicrobial exposures in prior 6 mo | Third-generation cephalosporin | 2.87 | 1.46-5.63 | <.01 | 3 |
| Trimethoprim-sulfamethoxazole | 1.81 | 1.01-3.24 | .05 | 2 | |
| Aminoglycoside | 1.16 | 0.38-3.55 | .79 | 1 | |
| Severity of illness in prior 48 h | Mechanical ventilation | 1.72 | 0.81-3.66 | .16 | 2 |
| Hypotensiona | 1.06 | 0.77-1.45 | .72 | 1 | |
| Immunosuppressive regimen | Receipt of non-corticosteroid immunomodulator in prior 30 db | 1.45 | 0.83-2.56 | .19 | 2 |
| Corticosteroid-containing chronic immunosuppressive regimenc | 1.44 | 0.83-2.48 | .19 | 2 |
Abbreviations: aOR, adjusted odds ratio; BSI, bloodstream infection; CI, confidence interval; EB, Enterobacterales; ESBL, extended-spectrum beta-lactamase.
Defined by a drop in systolic blood pressure of > 30 mm Hg and diastolic blood pressure of > 20 mm Hg, requirement for vasopressor, or systolic blood pressure < 90 mm Hg.
Receipt of any of the following agents in the 30 d preceding EB BSI: abatacept, anakinra, apremilast, azathioprine, cyclophosphamide, cyclosporine, denosumab, hydroxychloroquine, methotrexate, mycophenolate, rituximab, secukinumab, sulfasalazine, tocilizumab, tofacitinib, infliximab, adalimumab, certolizumab, golimumab, or etanercept.
Assessed at the time of EB BSI, including any corticosteroid dose.
Based on the adjusted β-coefficients, rounded integer value points were assigned to each predictor. With this scoring system, the maximum attainable score was 18, and the minimum was – 3. This tool showed adequate calibration (Hosmer-Lemeshow P value 0.36) and discrimination (AUC 0.81) for the derivation cohort (Figure 1).
FIGURE 1.
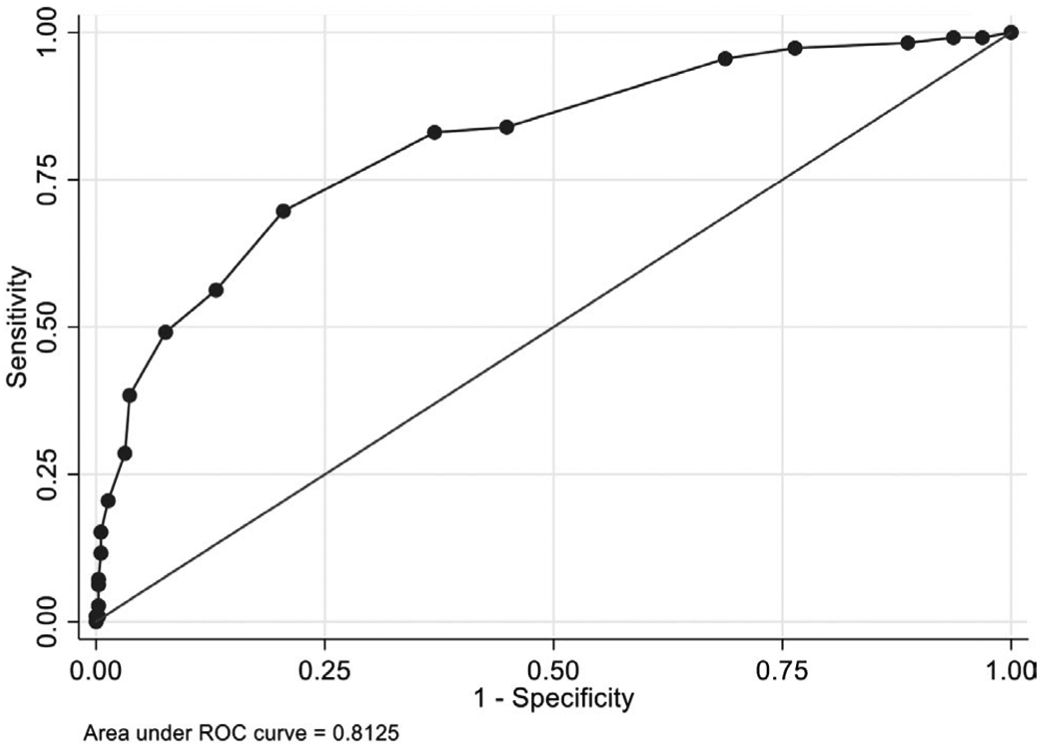
Receiver operator characteristic (ROC) curve for the predictive tool in the derivation cohort
Performance characteristics achieved using different cut points are reported in Table 3. We selected a cut point of 2 to concurrently maximize the PPV and NPV. When this cut point was utilized to define high risk of ESBL-EB BSI in the derivation cohort, the tool achieved a sensitivity of 69.6%, a specificity of 79.5%, a PPV of 50.0%, and a NPV of 89.9%.
TABLE 3.
Performance characteristics of the predictive tool at different cut points in the derivation cohort
| Cut Pointa | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| −3 | 100% | 0% | 22.7% | N/A 2 |
| −2 | 97.3% | 23.6% | 27.3% | 96.8% |
| −1 | 95.5% | 31.3% | 29.0% | 96.0% |
| 0 | 83.9% | 55.1% | 35.5% | 92.1% |
| 1 | 83.0% | 63.0% | 39.7% | 92.7% |
| 2 | 69.6% | 79.5% | 50.0% | 89.9% |
| 3 | 56.3% | 86.9% | 55.8% | 87.1% |
| 4 | 56.3% | 86.9% | 55.8% | 87.1% |
| 5 | 49.1% | 92.4% | 65.5% | 86.1% |
| 6 | 49.1% | 92.4% | 65.5% | 86.1% |
| 7 | 38.4% | 96.3% | 75.4% | 84.2% |
| 8 | 38.4% | 96.3% | 75.4% | 84.2% |
| 9 | 28.6% | 96.9% | 72.7% | 82.2% |
| 10 | 28.6% | 96.9% | 72.7% | 82.2% |
| 11 | 20.5% | 98.7% | 82.1% | 80.9% |
| 12 | 20.5% | 98.7% | 82.1% | 80.9% |
| 13 | 15.2% | 99.5% | 89.5% | 78.0% |
| 14 | 11.6% | 99.5% | 86.7% | 79.3% |
| 15 | 7.1% | 99.8% | 88.9% | 78.5% |
| 16 | 6.3% | 99.7% | 87.5% | 78.4% |
| 17 | 2.7% | 99.7% | 75.0% | 77.7% |
| 18 | 0% | 100% | N/Ab | 77.3% |
Abbreviations: BSI, bloodstream infection; EB, Enterobacterales; ESBL, extended-spectrum beta-lactamase; NPV, negative predictive value; PPV, positive predictive value.
Cumulative number of points on predictive model to be achieved or exceeded to warrant designation as “high risk” of ESBL-EB BSI.
Unable to calculate.
3.3 ∣. External validation of the predictive tool
When the predictive tool was applied to the validation cohort, calibration remained adequate (Hosmer-Lemeshow P value 0.81), but discrimination was reduced (AUC 0.68; Figure 2). Using the selected cut point of 2, the tool achieved a sensitivity of 99.5%, a specificity of 0.7%, a PPV of 61.8%, and a NPV of 50.0%. Performance characteristics for the validation cohort using other cut points are reported in Table S1.
FIGURE 2.
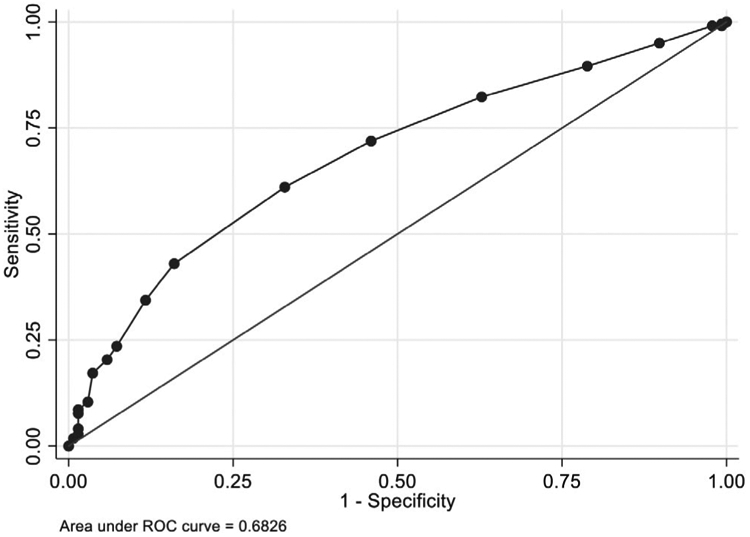
Receiver operator characteristic (ROC) curve for the predictive tool in the validation cohort
3.4 ∣. Subgroup analyses
(1) Organ transplant type: Among abdominal organ transplant recipients, there were 458 subjects in the derivation cohort and 320 in the validation cohort and a total of 308 (40%) ESBL-EB BSIs. The predictive tool developed in this population consisted of seven variables, all of which were also included in the original tool (Table S2). This achieved an AUC of 0.70 in the derivation cohort and 0.71 in the validation cohort. Among thoracic organ transplant recipients, there were 86 subjects in the derivation cohort and 40 in the validation cohort and 53 (42%) ESBL-EB BSIs. The predictive tool developed in this population consisted of five variables (Table S3). In contrast to the original tool, the model for thoracic organ transplant recipients included age > 60 y, as well as prior exposure to cefepime (rather than other antimicrobials). This achieved an AUC of 0.82 in the derivation cohort and 0.70 in the validation cohort. (2) Transplant era: Separate predictive tools were developed based whether the transplant occurred before or after 2010. The predictive variables and performance of these models were not substantially different than the overall model (data not shown). (3) Time since transplant: Separate predictive tools were also developed for those who were less or more than 1 y post-transplant. Again, the predictive variables and performance of these models were not substantially different from the overall model (data not shown).
4 ∣. DISCUSSION
In this study, we present a novel, externally validated clinical tool that predicts the likelihood of an ESBL-producing organism as the etiology of an EB BSI among SOT recipients. To our knowledge, this is the first such model developed in the transplant population. Our prediction tool consists of 10 variables, each of which falls into one of four clinical categories: prior colonization or infection with EB organisms, recent antimicrobial exposures, severity of preceding illness, and immunosuppressive regimen. This scoring system is feasible for use in clinical practice, as it consists of patient factors that may be easily ascertained from the medical record, and could be implemented with minimal cost to health systems. Once the presence or absence of each factor is assessed, the provider can determine whether the threshold has been crossed to warrant the use of empiric carbapenem therapy, or if a narrower agent could be considered, prior to availability of final susceptibility results.
We recommend a cut point of 2 for the tool, such that any patient scoring two or more points should be deemed at high risk of ESBL-EB BSI and considered for empiric carbapenem therapy while awaiting susceptibility testing. However, providers could select different cut points depending on the specific clinical scenario. For example, a lower cut point could be used when the goal is to avoid inadequate initial therapy for EB BSI at any cost, such as in the setting of a critically ill SOT recipient. Conversely, a higher cut point could be utilized when, for example, a SOT recipient is found to have an EB BSI but is clinically stable.
This tool demonstrated excellent discriminatory power in the derivation cohort with an AUC of > 0.8, but only modest discriminatory power in the validation cohort with an AUC of 0.68.26-28 Notably, the derivation and validation cohorts had markedly different proportions of EB BSI that were ESBL-producing (25% and 62%, respectively), which likely contributed to this discrepancy in predictive power. As a result, this tool is likely most applicable to SOT recipients at centers with lower rates of ESBL-EB.
Of the variables included in our model, the most strongly predictive of the outcome was the presence of ESBL-EB on prior culture. This finding mirrors numerous prior studies, which have consistently described prior colonization or infection with ESBL-EB as a strong, independent predictor of future ESBL-EB infection.29,30 For clinicians seeking a rapid method to predict risk for ESBL-producing organisms among SOT recipients presenting with an EB BSI, evaluation of prior microbiologic data may be the most expeditious approach to inform appropriate initial antimicrobial selection. However, in patients without a history of ESBL-EB on prior culture, our tool would be helpful in determining the need for empiric carbapenem therapy based on nuances surrounding their other clinical characteristics.
In addition to prior microbiologic data, there were three other categories of predictors in the clinical tool, including (1) recent antimicrobial exposures, which have been shown in innumerable previous studies to be one of the most significant risk factors for MDRO acquisition across populations31-33; (2) severity of illness immediately preceding detection of the EB BSI, where critical illness was predictive of ESBL-producing organisms, a finding consistent with prior literature linking exposure to intensive care units to MDRO colonization and infection34-36; and (3) immunosuppressive regimen, where corticosteroid-containing regimens were predictive of ESBL-producing organisms, a recently described association in SOT recipients.20
There are several predictive models described in the literature that have sought to determine the risk of ESBL-EB infection among hospitalized patients,15,37-39 though all were developed in the general population and none focused on SOT recipients. Notably, one prior tool did identify presence of immunosuppression as a predictor of ESBL-EB infection,38 but the authors were not able to distinguish which of the different immunosuppressing agents or underlying conditions leading to immunosuppression were significant. Thus, our tool represents an important advancement for immunocompromised hosts.
Our study has several limitations. First, because the study was performed retrospectively at three centers and spanned more than 10 y, the testing methods for ESBL production evolved over the study period, potentially leading to changes in classification over time. However, the largest impact of this change would be seen in organisms with a high prevalence of AmpC, while the majority of organisms in our study were E. coli and Klebsiella species (both of which have a relatively low prevalence of AmpC production). Furthermore, classifications made in our study mirrored the microbiology community's definition over time and are thus reflective of the interpretation of ESBL production at each time point. Second, we were unable to quantify the number of days of antimicrobial usage or distinguish between prophylactic or treatment courses of antibiotics, though we felt that such nuances would also be challenging for providers to distinguish when utilizing the tool at the bedside. Third, the cohorts were not large enough to create separate predictive models for each organ transplant type, though in a subgroup analysis, we were able to evaluate thoracic and abdominal organ recipients independently. However, these subsets were limited by small sample sizes, particularly for thoracic organ transplant recipients, and require further validation. Finally, while our tool was externally validated, it showed only modest discrimination in the validation cohort where the rate of ESBL-producing EB was notably higher than in the derivation cohort, suggesting that the tool may not be generalizable to SOT recipients at dissimilar institutions.
In conclusion, our study demonstrates that the proposed scoring system can be used to predict the presence of an ESBL-producing organism as the etiology of an EB BSI among SOT recipients. This clinical tool can be used to guide empiric therapy prior to availability of antimicrobial susceptibility data, particularly for SOT recipients receiving care at institutions with a lower prevalence of ESBL-EB. Further studies are needed to refine and validate this tool in additional populations, particularly those with disparate ESBL-EB rates, and to assess the clinical and economic impact of its implementation across health systems.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Grant 5 UM 1AI104681-05, with a subaward fellowship grant to JAA); the National Institutes of Health (Grants K24-AI080942 to EL and K01-AI137317 to JAA); and by a Centers for Disease Control and Prevention Cooperative Agreement FOA#CK16-004-Epicenters for the Prevention of Healthcare Associated Infections (to EL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
Emily Blumberg receives research support from Takeda, Merck, and Hologic; is a member of a Data and Safety Monitoring Board (DSMB) for Amplyx; and serves as a member of the scientific advisory committee for Merck. Jennifer Han was affiliated with the University of Pennsylvania during the conduct of this research and is now employed by and holds shares in the GlaxoSmithKline group of companies. Ebbing Lautenbach is a member of a DSMB for Merck and serves as a member of the scientific advisory committees for Paratek and Shionogi. Kevin Alby serves on the scientific advisory boards for Becton Dickinson and Shionogi. Warren Bilker is a member of multiple DSMBs for Genentech. None of these conflicts are relevant to this article. All other authors report no conflicts of interest relevant to this article.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Aguiar EB, Maciel LC, Halpern M, et al. Outcome of bacteremia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae after solid organ transplantation. Transplant Proc. 2014;46(6):1753–1756. [DOI] [PubMed] [Google Scholar]
- 2.Winters HA, Parbhoo RK, Schafer JJ, Goff DA. Extended-spectrum beta-lactamase-producing bacterial infections in adult solid organ transplant recipients. Ann Pharmacother. 2011;45(3):309–316. [DOI] [PubMed] [Google Scholar]
- 3.Shi SH, Kong HS, Xu J, et al. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis. 2009;11(5):405–412. [DOI] [PubMed] [Google Scholar]
- 4.Linares L, García-Goez JF, Cervera C, et al. Early bacteremia after solid organ transplantation. Transplant Proc. 2009;41(6):2262–2264. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Wagener MM, Obman A, Cacciarelli TV, de Vera ME, Gayowski T. Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver Transpl. 2004;10(7):844–849. [DOI] [PubMed] [Google Scholar]
- 6.Candel FJ, Grima E, Matesanz M, et al. Bacteremia and septic shock after solid-organ transplantation. Transplant Proc. 2005;37(9):4097–4099. [DOI] [PubMed] [Google Scholar]
- 7.Oriol I, Sabé N, Melilli E, et al. Factors influencing mortality in solid organ transplant recipients with bloodstream infection. Clin Microbiol Infect. 2015;21(12):1104.e9–1104.14. [DOI] [PubMed] [Google Scholar]
- 8.Martinez JA, Aguilar J, Almela M, et al. Prior use of carbapenems may be a significant risk factor for extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella spp. in patients with bacteraemia. J Antimicrob Chemother. 2006;58(5):1082–1085. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi ZA, Paterson DL, Pakstis DL,et al. Risk factors and outcome of extended-spectrum beta-lactamase-producing Enterobacter cloacae bloodstream infections. Int J Antimicrob Agents. 2011; 37(1):26–32. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Bano J, Picon E, Gijon P, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48(5):1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alevizakos M, Kallias A, Flokas ME, Mylonakis E. Colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae in solid organ transplantation: A meta-analysis and review. Transpl Infect Dis. 2017;19(4):e12718. Epub 2017 Jun 20. [DOI] [PubMed] [Google Scholar]
- 12.Camargo L, Marra AR, Pignatari A, et al. Nosocomial bloodstream infections in a nationwide study: comparison between solid organ transplant patients and the general population. Transpl Infect Dis. 2015;17(2):308–313. [DOI] [PubMed] [Google Scholar]
- 13.Santoro-Lopes G, de Gouvea EF. Multidrug-resistant bacterial infections after liver transplantation: an ever-growing challenge. World J Gastroenterol. 2014;20(20):6201–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LE, D'Agata EM, Paterson DL, et al. Pseudomonas aeruginosa bacteremia over a 10-year period: multidrug resistance and outcomes in transplant recipients. Transpl Infect Dis. 2009;11(3):227–234. [DOI] [PubMed] [Google Scholar]
- 15.Goodman KE, Lessler J, Cosgrove SE, et al. A Clinical Decision Tree to Predict Whether a Bacteremic Patient Is Infected With an Extended-Spectrum Beta-Lactamase-Producing Organism. Clin Infect Dis. 2016;63(7):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spafford K, MacVane S, Humphries R. Evaluation of Empiric Beta-Lactam Susceptibility Prediction among Enterobacteriaceae by Molecular Beta-Lactamase Gene Testing. J Clin Microbiol. 2019;57(10):e00674. Print 2019 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oriol I, Sabé N, Simonetti AF, et al. Changing trends in the aetiology, treatment and outcomes of bloodstream infection occurring in the first year after solid organ transplantation: a single-centre prospective cohort study. Transpl Int. 2017;30(9):903–913. [DOI] [PubMed] [Google Scholar]
- 18.Armand-Lefèvre L, Angebault C, Barbier F, et al. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother. 2013;57(3): 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob Agents Chemother. 2013;57(10):5131–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anesi JA, Lautenbach E, Tamma PD, et al. Risk factors for extended-spectrum beta-lactamase-producing Enterobacterales bloodstream infection among solid organ transplant recipients. Clin Infect Dis. 2020:ciaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing M100-S20. 2010. [DOI] [PMC free article] [PubMed]
- 22.Huang Y, Carroll KC, Cosgrove SE, Tamma PD. Determining the optimal ceftriaxone MIC for triggering extended-spectrum beta-lactamase confirmatory testing. J Clin Microbiol. 2014;52(6):2228–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: Wiley & Sons; 2000. [Google Scholar]
- 26.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. [DOI] [PubMed] [Google Scholar]
- 27.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med. 2008;168(20):2205–2210. [DOI] [PubMed] [Google Scholar]
- 28.Gomila A, Shaw E, Carratalà J, et al. Predictive factors for multidrug-resistant gram-negative bacteria among hospitalised patients with complicated urinary tract infections. Antimicrob Resist Infect Control. 2018;7(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denis B, Lafaurie M, Donay J-L, et al. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis. 2015;39:1–6. [DOI] [PubMed] [Google Scholar]
- 30.Van Aken S, Lund N, Ahl J, Odenholt I, Tham J. Risk factors, outcome and impact of empirical antimicrobial treatment in extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia. Scand J Infect Dis. 2014;46(11):753–762. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Ami R, Rodriguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682–690. [DOI] [PubMed] [Google Scholar]
- 32.Harris AD, McGregor JC, Johnson JA, et al. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis. 2007;13(8):1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao T, Wu Z, Shi Q, et al. A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-lactamase-producing Escherichia coli bloodstream infections. J Glob Antimicrob Resist. 2019;17:147–156. [DOI] [PubMed] [Google Scholar]
- 34.Fridkin SK, Gaynes RP. Antimicrobial resistance in intensive care units. Clin Chest Med. 1999;20(2):303–316.viii. [DOI] [PubMed] [Google Scholar]
- 35.Ma X, Wu Y, Li L, et al. First multicenter study on multidrug resistant bacteria carriage in Chinese ICUs. BMC Infect Dis. 2015;15:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiebaut ACM, Arlet G, Andremont A, et al. Variability of intestinal colonization with third-generation cephalosporin-resistant Enterobacteriaceae and antibiotic use in intensive care units. J Antimicrob Chemother. 2012;67(6):1525–1536. [DOI] [PubMed] [Google Scholar]
- 37.Kengkla K, Charoensuk N, Chaichana M, et al. Clinical risk scoring system for predicting extended-spectrum beta-lactamase-producing Escherichia coli infection in hospitalized patients. J Hosp Infect. 2016;93(1):49–56. [DOI] [PubMed] [Google Scholar]
- 38.Johnson SW, Anderson DJ, May DB, Drew RH. Utility of a clinical risk factor scoring model in predicting infection with extended-spectrum beta-lactamase-producing enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol. 2013;34(4):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tumbarello M, Trecarichi EM, Bassetti M, et al. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55(7):3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


