Abstract
Ras is an essential component of signal transduction pathways that control cell proliferation, differentiation, and survival. In this study we have examined the cellular responses to high-intensity Ras signaling. Expression of increasing amounts of the oncogenic form of human HRas, HRasV12, results in a dose-dependent induction of apoptosis in both primary and immortalized cells. The induction of apoptosis by HRasV12 is blocked by activated Rac and potentiated by dominant interfering Rac. The ability of Rac to suppress Ras-induced apoptosis is dependent on effector pathway(s) controlled by the insert region and is linked to the activation of NF-κB. The apoptotic effect of HRasV12 requires the activation of both the ERK and JNK mitogen-activated protein kinase cascade and is independent of p53. These results demonstrate a role for Rac in controlling signals that are necessary for cell survival, and suggest a mechanism by which Rac activity can confer growth advantage to cells transformed by the ras oncogene.
The Ras GTPase functions as a transducer of signals from cell surface receptors to intracellular pathways that control cell growth, differentiation, and survival. Through genetic and biochemical studies, it has been established that Ras interacts with multiple downstream effectors controlling distinct signaling cascades (for reviews, see references 3 and 19). These include the Ser/Thr kinase Raf, the p110 catalytic subunit of phosphoinositide 3-kinase (PI 3-kinase), and Ral-GDS, the exchange factor for Ral GTPase. Raf regulates the activity of a kinase cascade that includes the MEK and mitogen-activated protein (MAP) extracellular-regulated kinases (ERKs). Activation of PI 3-kinase leads to the activation of the Rac GTPase, which functions downstream of Ras in signaling pathways that control actin polymerization, transcriptional activation, and cell proliferation.
The relative contribution of Ras-dependent signaling pathways to its biological effects has been analyzed in studies using Ras effector binding loop mutants that are specifically defective in the activation of a single effector pathway (51). Based on these analyses, it is now well accepted that the mitogenic and oncogenic properties of Ras depend on the coordinated activation of multiple effector pathways (18, 23, 40, 51). Specifically, in some cell types, Ras-mediated cell proliferation and transformation depend on the synergistic activation of the Rac/Rho and ERK MAP kinase cascades (18).
An additional level of regulation that contributes to the signaling properties of Ras relates to the cellular context within which Ras operates. Several lines of evidence support this mode of regulation. First, the expression of activated Ras in immortal cell lines leads to oncogenic transformation, whereas in primary cells activated Ras can induce a permanent cell cycle arrest (43, 49). Second, overexpression of Ras in proliferating Drosophila imaginal tissue promotes proliferation and subsequent apoptosis (21). In contrast, Ras activation leads to the suppression of apoptosis in postmitotic imaginal tissue (25). Last, Ras function is critical for proliferation in established fibroblast cell lines but for differentiation in neuronal cells (15, 34).
The outcome in response to Ras is also dictated by the relative levels of activation of different effector pathways as well as the timing of activation. For example, expression of activated Ras typically promotes mitogenesis in fibroblasts. In contrast, activation of Ras induces apoptosis in these cells if protein kinase C activity is suppressed (4). Moreover, the preferential activation by Ras of the PI 3-kinase effector pathway protects fibroblasts from c-Myc-induced apoptosis, whereas the selective activation of the MAP kinase effector pathway potentiates c-Myc-induced apoptosis (22). Finally, the ability of Ras to induce neuronal differentiation is dependent on the duration of activation of the MAP kinase cascade (7, 47).
In this study we have investigated the relationship between the level of Ras activity and the biological consequences. We show that high levels of Ras activity induce an apoptotic response which is p53 independent and requires the activation of both the JNK and ERK MAP kinase cascades. We further demonstrate that Rac-mediated signals are necessary and sufficient to protect against Ras-induced apoptosis through a pathway that involves NF-κB activation. These findings implicate Rac in controlling signals that are necessary for cell survival and suggest a mechanism by which Rac can contribute to the mitogenic and oncogenic potential of Ras.
MATERIALS AND METHODS
Cell culture and microinjection.
The following cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with antibiotics and maintained in either fetal calf (FCS) or calf serum (CS) at 37°C with an atmosphere of the indicated percent CO2: REF-52 (10% FCS, 7% CO2), COS-1 (5% FCS, 5% CO2), HEK-293 (10% FCS, 5% CO2), Swiss-3T3 (10% FCS, 7% CO2), Rat-1 (5% CS, 7% CO2), NRK 1570 (fibroblasts) and 1571 (epithelial) (5% CS, 5% CO2), MDCK (10% FCS, 5% CO2), and MEF p53WT and p53−/− mouse embryo fibroblasts expressing wild-type p53 and null for p53, respectively) (10% FCS, 10% CO2). NRK 1570 and 1571 and HEK-293 cells were obtained from the American Type Culture collection. MDCK and MEF cells were kindly provided by Morag Park (McGill University) and Martine Roussel (St. Jude Children’s Research Hospital), respectively. For microinjection, cells were plated onto gridded glass coverslips and cultured in DMEM supplemented with the indicated concentration of FCS or CS. The cells were grown to confluence and then placed in DMEM with 0.5% FBS for 24 h before microinjection. A solution containing the indicated plasmid in microinjection buffer (50 mM HEPES [pH 7.2], 100 mM KCl, 5 mM Na2HPO4) was microinjected into cell nuclei.
Immunofluorescence microscopy.
For monitoring protein expression, injected cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) and then permeabilized with 0.1% Triton X-100 for 3 min at room temperature. The coverslips were incubated first with mouse antibodies to T7 (anti-T7 monoclonal; Novagen) or hemagglutinin (HA) (12CA5 monoclonal; American Type Culture Collection) epitopes in PBS containing 2% albumin and then with fluorescein-conjugated goat antibody to mouse immunoglobulin G (IgG). For monitoring NF-κB dependent transcription, cells were stained with polyclonal antibodies to chloramphenicol acetyltransferase (CAT) (5′→3′ Inc.) followed by staining with rhodamine-conjugated goat antibody to rabbit IgG. The cells were photographed with a Zeiss Axiophot fluorescence microscope.
Detection of apoptotic cells.
Apoptosis was monitored by using an ApoAlert annexin V apoptosis detection Kit (Clontech Laboratories, Inc.). Briefly, cells were gently washed once with PBS and then incubated in buffer containing fluorescein isothiocyanate (FITC)-annexin V and propidium iodide for 15 min at 37°C to determine phosphatidylserine content in the outer leaflet of the plasma membrane and membrane integrity, respectively. The cells were then washed with PBS and fixed in PBS containing 3.7% formaldehyde. At 16 h after microinjection with expression plasmids and before any gross morphological changes were apparent, cells were positive for FITC-annexin V staining and negative for propidium iodide staining, indicating that these cells were apoptotic rather than necrotic. At 24 h after microinjection, cells stained positively for both FITC-annexin V and propidium iodide, indicating the loss of membrane integrity which is characteristic of late apoptotic cells. To visualize nuclear condensation, REF-52 cells were microinjected and fixed as described above, permeabilized with 0.1% Triton X-100, and then incubated in PBS containing 1.5 μg of propidium iodide per ml for 30 min at 37°C.
Plasmids.
Unless otherwise indicated, plasmid pCGT, which is derived from pCGN with a replacement of the HA epitope by the T7 epitope (17), was used as a mammalian expression vector to express the Ras and Rac mutants used in this study. pCGT RacV12,H40 and pCGT RacV12,L37 were created as described elsewhere (17). pCGT RacV12,ΔIns was created as described elsewhere (20). Constructs were kindly provided by the following: pCDNA3 c-Rel and the NF-κB–CAT by Paula Enrieto (State University of New York at Stony Brook), Myr-Akt by Nissim Hay (The University of Chicago), MKP-3 by Kathleen Kelly (National Institutes of Health), MKK7 by Christoph Reinhard (Chiron Corp.), RhoN19, pEVX RhoV14, CDC42V12, and CDC42N17 by Alan Hall (University College, London), insulin receptor by Robert Lewis (University of Nebraska); epidermal growth factor (EGF) receptor by Joseph Schlessinger (New York University), dominant interfering JNK by Roger Davis (University of Massachusetts), and dominant interfering NIK by Ken Marcu (State University of New York at Stony Brook).
Jun kinase assay.
COS-1 cells were cotransfected with 10 μg of FLAG-tagged JNK1 and the indicated concentrations of expression plasmids encoding RasV12, MKP-1, MKP-3, RacV12, or MKK7, using the CaPO4 method. After a 12-h incubation with the DNA-CaPO4 precipitates, cells were incubated in medium containing FCS (5%) for 6 h and then incubated for 12 h in serum-free medium. Cells were lysed in lysis buffer (10 mM HEPES [pH 7.5], 10% glycerol, 150 mM NaCl, 1 mM Na3VO4, 0.6% Triton X-100, 50 mM NaF, 1 mM okadaic acid, 1 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, leupeptin [10 μg/ml], aprotinin [10 μg/ml]). FLAG-tagged JNK1 was immunoprecipitated with 5 μg of monoclonal antibody to FLAG M2 (Eastman Kodak). Immune complexes were collected by incubation with protein G-Sepharose, washed extensively with lysis buffer, and then incubated for 30 min at 37°C in kinase assay buffer (20 mM HEPES [pH 7.6], 20 mM MgCl2, 20 mM β-glycerol phosphate, 0.1 mM Na3VO4, 2 mM dithiothreitol [DTT], 20 μM ATP containing 10 μCi of [γ-32P]ATP and glutathione S-transferase fused to the NH2 terminus of c-Jun (3 μg per reaction) as the substrate. The reaction products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by autoradiography. Fold activation was determined with a Storm 860 PhosphorImager in combination with ImageQuant version 1.1 software (Molecular Dynamics).
MAP kinase assay.
COS-1 cells were transfected with 1 μg of HA-tagged ERK2 and the indicated constructs as described above. Cells were lysed in lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 0.5% Nonidet P-40, 5 mM EDTA, 1 mM DTT, 1 mM Na3VO4, 1 μM okadaic acid, 1 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, leupeptin [10 μg/ml], aprotinin [10 μg/ml]), and lysates were clarified by centrifugation. HA epitope-tagged ERK2 was immunoprecipitated with monoclonal antibody 12CA5. Immune complexes were collected by binding to protein A-Sepharose, washed extensively in lysis buffer and then assayed for 10 min at 37°C in kinase assay buffer (20 mM HEPES [pH 7.5], 20 mM MgCl2, 1 mM Na3VO4, 20 μM ATP, [γ-32P]ATP at ∼2,000 cpm/pmol) containing 0.2 mg of myelin basic protein per ml. The reaction products were analyzed as described above for the Jun kinase assay.
Akt kinase assay.
HEK-293 cells were transfected with 8 μg of HA-tagged myr-Akt (see Results) by a standard CaPO4 transfection protocol. Cells were lysed in lysis buffer (20 mM Tris [pH 7.4], 140 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM Na3VO4, 1 μM okadaic acid, 1 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, leupeptin [10 μg/ml], aprotinin [10 μg/ml]), and lysates were clarified by centrifugation. HA epitope-tagged myr-Akt was immunoprecipitated with monoclonal antibody 12CA5. Immune complexes were collected by binding to protein A-Sepharose, washed extensively in lysis buffer, and then assayed for 10 min at 37°C in kinase assay buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM vanadate, 1 mM DTT, 10 mM ATP, 20 μM [γ-32P]ATP) containing 1 mg of histone H2B (Sigma) per ml. The reaction products were analyzed as described above for the Jun kinase assay.
RESULTS
High-intensity Ras signaling induces apoptosis.
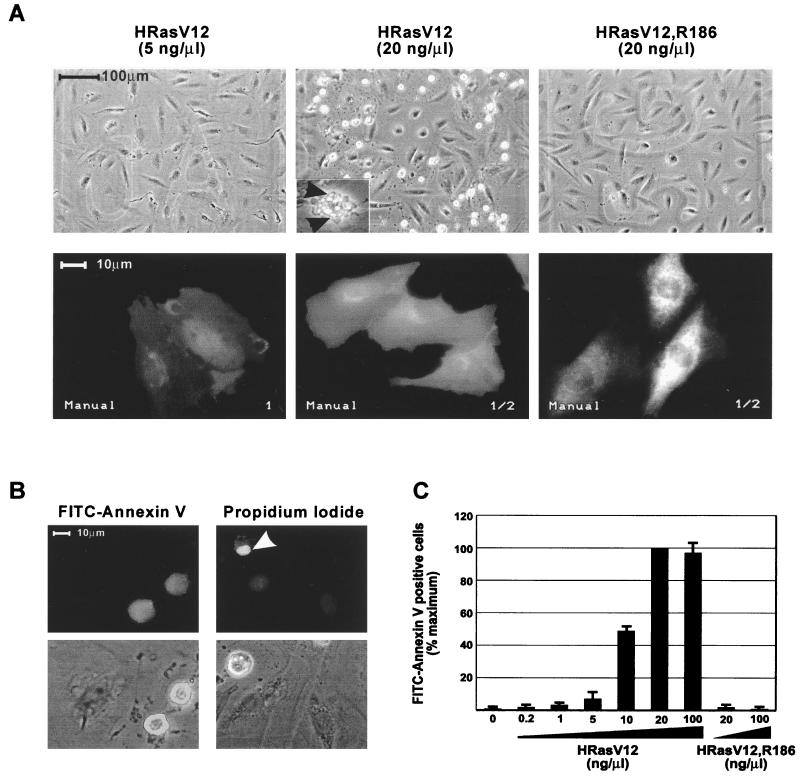
The microinjection approach is uniquely suitable for the introduction of well-defined amounts of macromolecules into living cells. We have used this approach to investigate the dependence of cellular responses on the levels of Ras activity. Microinjection of increasing concentrations (1 to 100 ng/μl) of an expression plasmid encoding activated Ras (HRasV12) into quiescent serum-starved rat embryo fibroblasts (REF-52) caused the dose-dependent appearance of morphological changes that are characteristic of apoptosis (Fig. 1). These include retraction of cellular processes, blebbing of plasma membranes, and loss of adherence (Fig. 1A, inset). That these morphological changes truly reflect apoptosis was confirmed by staining the injected cells with the apoptosis marker annexin V or with propidium iodide (Fig. 1B). To quantitate the apoptotic effect of HRasV12, the number of cells which stained positively for annexin V within the injected area was determined. HRasV12-induced apoptosis was maximal at 20 ng of injected plasmid per μl, and under these conditions approximately 40% of the cells within the injected area were identified as apoptotic (Fig. 1C). Immunofluorescence staining of the injected cells revealed no significant differences between the subcellular distribution of HRasV12 in cells injected with high or low levels of the expression plasmid (Fig. 1A).
FIG. 1.
High-intensity Ras signaling induces apoptosis. (A) Serum-starved REF-52 cells were microinjected with the indicated expression plasmids. (Top panels) Phase-contrast micrographs of the injected areas visualized 24 h after injection. Apoptotic cells appear as highly refractile, loosely adherent spheres. The inset shows a high-magnification image of an HRasV12-expressing REF-52 cell undergoing apoptosis. Note the convolution of the cellular surface and the presence of membrane-bound apoptotic bodies (arrowheads). (Bottom panels) Immunofluorescence micrographs of REF-52 cells injected with the indicated expression plasmids. Cells were fixed and stained 5 h after injection. Differences in the levels of protein expression are indicated by the differences in the intensity of the fluorescence signal. The nuclear exclusion staining pattern in cells expressing HRasV12R186 indicates cytosolic localization. Numbers at lower right denote exposure time. (B) Apoptotic responses induced by HRasV12. Serum-starved REF-52 cells were microinjected with HRasV12 expression plasmid (20 ng/μl) and stained with FITC-annexin V or propidium iodide 24 h after injection. For each stain, the fluorescence micrographs and the corresponding phase-contrast micrographs are shown in the lower panels. The propidium iodide staining shows a condensed apoptotic nucleus (arrowhead). (C) Dose-response relationship between expression levels of HRasV12 and induction of apoptosis. Serum-starved REF-52 cells were microinjected with the indicated concentrations of expression plasmids. Twenty-four hours after injection, cells were incubated with FITC-annexin V. The percentage of annexin V-positive cells was determined by counting FITC-annexin V-positive cells as a proportion of the total number of cells in the injected area. The results are the means of three independent experiments in which at least 100 cells were scored for each condition. Error bars represent standard deviations. Typically, 60% of the cells in the injected areas expressed the exogenous protein as determined by immunofluorescence staining.
To establish the specificity of the Ras-dependent apoptotic response, REF-52 cells were microinjected with high concentrations of expression plasmids encoding a mutated form of HRasV12, HRasV12,R186, which is defective for membrane localization and as a result is biologically inactive (52). The expression of this mutant did not affect cell viability (Fig. 1A and B), indicating that the apoptotic response detected at high levels of HRasV12 expression is related to its signaling capacity. The interval between microinjection and the appearance of apoptotic cells was typically 14 to 16 h. Thus, the apoptotic effect of Ras appears to be mediated by long-term signaling events. It should be noted that the addition of serum growth factors had no effect on Ras-induced apoptosis. Therefore, all of the experiments described in this study were carried out in serum-starved cells.
To assess the significance of the cellular background for the Ras-mediated apoptotic response, we compared the effects of high levels of HRasV12 expression among a number of cell types. As illustrated in Table 1, most (seven of nine) of the cell types tested were induced to undergo apoptosis following microinjection of 20 ng of HRasV12 per μl. Notably, the apoptotic response was detected both in primary MEFs and established human and murine cell lines. MEFs from p53−/− homozygous embryos maintained sensitivity to the apoptotic effects of Ras indicating that in these cells Ras-induced apoptosis occurs via a p53-independent mechanism. Furthermore, the capacity of HRasV12 to induce apoptosis was exhibited both in fibroblast (e.g., Rat-1 and Swiss 3T3) and epithelial (NRK 1571) cell lines. These results suggest that induction of apoptosis by high levels of Ras activity represents a conserved cellular response.
TABLE 1.
Effects of Ras on apoptosis in various cell typesa
| Cell type | Apoptotic cells (%) |
|---|---|
| REF-52 | 44.3 ± 5.3 |
| MEF | 38.0 ± 2.2 |
| MEFp53−/− | 34.4 ± 2.4 |
| Rat-1 | 42.3 ± 2.8 |
| Swiss 3T3 | 32.0 ± 2.6 |
| NRK 1570 | 0.0 |
| COS-1 | 52.0 ± 2.5 |
| NRK 1571 | 48.3 ± 3.0 |
| MDCK | 0.0 |
The indicated cell lines were injected with HRasV12 (20 ng/μl). Twenty-four hours after injection, cells were stained with annexin V, and the number of apoptotic cells was determined as described in the legend to Fig. 1C. Values represent means ± standard deviations of three independent experiments in which at least 100 cells were injected. In cells injected with vector alone, the number of apoptotic cells was negligible (1 to 3%). HRasV12 expression was verified in all cell types by indirect immunofluorescence staining.
Rac signaling protects against Ras-induced apoptosis.
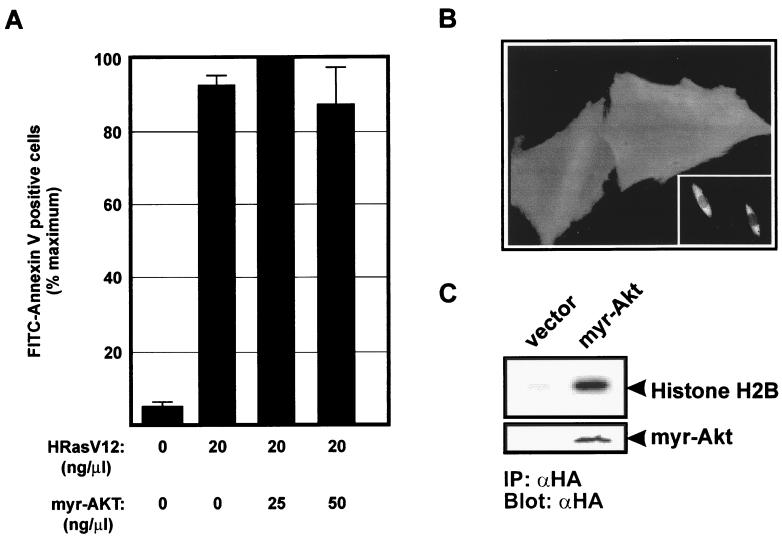
The apoptotic response induced by high intensity of Ras signaling stands in marked contrast to the well-documented growth-promoting effects of activated Ras. One possible explanation for this apparent discrepancy is that Ras can trigger the activation of both pro- and antiapoptotic pathways. At low levels of Ras signaling, the antiapoptotic pathway is activated to an extent which is sufficient to counteract the apoptotic signals. At high levels of Ras signaling, this balance is tilted in favor of the proapoptotic signals presumably because the component(s) of the antiapoptotic pathway are limiting. To test this idea, we first sought to determine the identity of the Ras-dependent antiapoptotic pathway. A plausible candidate is the PI 3-kinase pathway which functions as an effector pathway of Ras and has been implicated in the regulation of cell survival (for reviews, see references 8 and 10). It has been shown that both Rac and protein kinase B (PKB)/Akt are downstream targets of PI 3-kinase (48, 50). To investigate whether PKB/Akt has a role in preventing Ras-induced apoptosis, REF-52 cells were coinjected with HRasV12 and a membrane-targeted form of HA-tagged Akt containing the Src myristoylation signal fused to its N terminus (myr-Akt). Immunofluorescence staining with anti-HA antibodies verified the expression of myr-Akt in the injected cells, and the staining pattern was consistent with its predicted membrane localization (Fig. 2B). When immunoprecipitated from transiently transfected serum-deprived HEK-293 cells and assayed by immune complex kinase assay using histone H2B as a substrate, myr-Akt exhibited substantial kinase activity, confirming that the expressed protein is constitutively active (Fig. 2C). Coexpression of myr-Akt did not alter the extent of apoptosis resulting from HRasV12 expression, indicating that Akt-dependent signals are not sufficient to suppress apoptosis that is induced by Ras (Fig. 2A).
FIG. 2.
Akt is not sufficient to protect against HRasV12-induced apoptosis. (A) REF-52 cells were injected with HRasV12 (20 ng/μl) alone or with increasing concentration of myr-Akt (25 and 50 ng/μl). Twenty-four hours after injection, cells were stained with annexin V and the number of apoptotic cells was determined as described in the legend to Fig. 1C. (B) Immunofluorescence micrographs of REF-52 cells injected with HA-tagged myr-Akt or HA-tagged wild-type Akt (inset) demonstrating plasma membrane and cytosolic localization, respectively. Cells were fixed 12 h after injection and stained with anti-HA antibodies. (C) myr-Akt is constitutively active. HEK-293 cells were transfected with HA epitope-tagged myr-Akt, and kinase activity was determined by immune complex kinase assay using histone 2B as a substrate. IP, immunoprecipitation; αHA, anti-HA antibody.
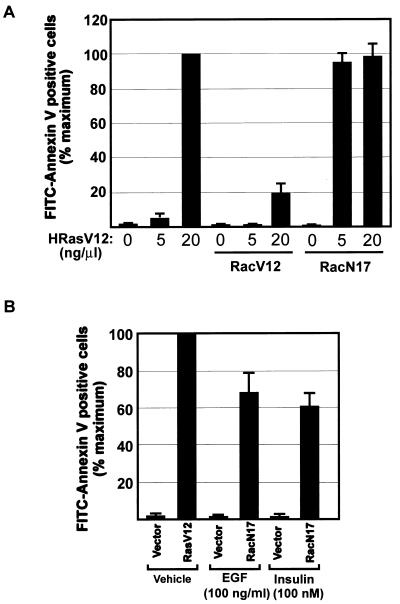
To examine the role of Rac in cell survival, REF-52 cells were microinjected with activated Rac (RacV12) or dominant interfering Rac (RacN17) expression plasmids together with low (5 ng/μl) and high (20 ng/μl) concentrations of HRasV12 expression plasmid. The apoptotic response induced by the expression of high levels of HRasV12 was blocked by coexpression of RacV12, indicating that Rac-mediated signals are sufficient to antagonize the proapoptotic signals elicited by Ras (Fig. 3A). Furthermore, the apoptotic capacity of Ras was potentiated in the absence of Rac activity, as evident from the observation that low levels of HRasV12 expression which normally would not affect cell viability induced a nearly maximal apoptotic response when coexpressed with RacN17. Together, these results suggest a critical role for Rac in the transduction of cell survival signals that provide protection against Ras-induced apoptosis.
FIG. 3.
Rac is necessary and sufficient for suppression of apoptosis. (A) Effect of constitutively active (RacV12) and dominant interfering (RacN17) Rac on Ras-induced apoptosis. Expression vectors encoding the indicated Rac mutants (25 ng/μl) were microinjected together with the indicated concentrations of HRasV12 expression plasmid. (B) Role of Rac in growth factor-mediated cell survival. Serum-starved REF-52 cells were injected with plasmid mixtures containing expression vectors for the growth factor receptor (50 ng/μl) or HRasV12 (20 ng/μl) with or without Rac N17 (50 ng/μl). Six hours after injection, cells were stimulated with EGF (100 ng/ml) or insulin (100 nM). Twenty-four hours after injection, cells were stained with annexin V and the number of apoptotic cells was determined as described in the legend to Fig. 1C. Results are the means of three independent experiments in which at least 100 cells were scored for each condition. Percent maximum corresponds to 47% ± 3.7% (A) 56% ± 9.8% (B) of the cells in the injected area. Error bars represent standard deviations.
Growth factor-dependent cell survival signals require Rac.
Rac proteins are important intermediates of growth factor receptor-mediated signal transduction pathways. The involvement of Rac in these pathways can be either Ras dependent or independent (39). To investigate the significance of the cell survival signals emitted by Rac for the cellular responses induced by growth factors, serum-starved REF-52 cells were injected with expression plasmids encoding various growth factor receptors with or without RacN17. Addition of insulin or EGF to REF-52 cells expressing the insulin or EGF receptor, respectively, had no apparent effect on cell morphology or viability. However, insulin and EGF induced apoptosis when added to cells coexpressing their cognate growth factor receptor and RacN17 (Fig. 3B). This apoptotic response was observed approximately 18 h after incubation with the growth factor and was dependent on the ectopic expression of the growth factor receptor. These results indicate that Rac activity might be essential for counteracting growth factor-dependent proapoptotic signals.
Suppression of Ras-induced apoptosis requires the Rac insert region and is correlated with NF-κB activation.
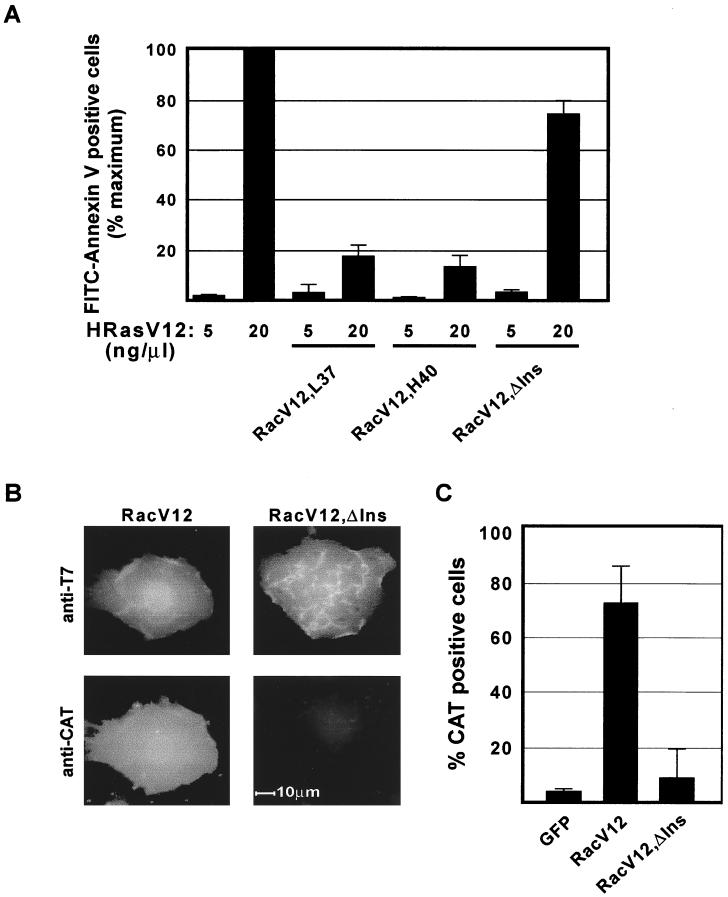
Rac proteins regulate actin polymerization and activation of Jun kinase through distinct effector pathways (17, 27). To examine whether these effector pathways are involved in suppressing apoptosis, we have used partial loss-of-function mutants containing specific amino acid substitutions within the effector binding loop in an activated V12 background. The RacV12,L37 mutant activates JNK but is defective in inducing actin polymerization, whereas the RacV12H40 mutant induces actin polymerization but is defective in JNK activation (17, 27). When coexpressed with HRasV12, both mutants were as effective as RacV12 in blocking Ras-induced apoptosis, indicating that Rac-mediated actin polymerization and JNK activation are not essential components of the anti-apoptotic activity of Rac (Fig. 4A).
FIG. 4.
The insert region of Rac is required for suppression of Ras-induced apoptosis and NF-κB activation. (A) Role of Rac effector pathways in the protection against Ras-induced apoptosis. Serum-starved REF-52 cells were microinjected with a mixture of plasmids containing a high (20 ng/μl) or low (5 ng/μl) concentration of HRasV12 in combination with or the indicated Rac mutants (25 ng/μl). FITC-annexin V-positive cells were scored 24 h after injection. Values correspond to the means of three independent experiments, and the error bars represent the standard deviations. At least 100 cells were scored per condition in each experiment. Percent maximum corresponds to 39% ± 4.9% of the cells in the injected area. (B) Serum-starved REF-52 cells were microinjected with a mixture of expression plasmids containing an NF-κB–CAT reporter construct (50 ng/μl) and the indicated T7 epitope-tagged Rac mutants or with CMV-GFP as a negative control (50 ng/μl). The injected cells were fixed 16 h postinjection and costained with a mixture of mouse antibodies to T7 epitope-tagged Rac mutants and rabbit antibodies to CAT followed by fluorescein-conjugated antibodies to mouse IgG and rhodamine-conjugated antibodies to rabbit IgG. (C) Quantitation of NF-κB dependent transcription. Cells expressing the proteins as determined by immunofluorescence or autofluorescence in the case of GFP were scored for CAT costaining. Results shown are the means of three independent experiments in which at least 50 cells were scored, and the error bars represent the standard deviations.
We have recently shown that the insert region of Rac, an effector binding site located at residues 124 to 135 (11), is essential for Rac-dependent superoxide generation and mitogenic stimulation in fibroblasts (20). To examine whether the Rac insert region controls signaling pathways that are critical for promoting cell survival, we used a Rac mutant lacking the insert region in an activated V12 background (RacV12ΔIns). We have previously demonstrated that this mutant is defective in superoxide production but retains the ability to induce actin polymerization and JNK activation (20). Elimination of the insert region rendered Rac ineffective in protecting against Ras-induced apoptosis (Fig. 4A), indicating that the effector functions that are mediated by this region are crucial for the suppression of apoptosis.
To obtain insights into the downstream events that couple Rac activation to protection against apoptosis, we examined the involvement of the transcription factor NF-κB. NF-κB is a critical regulator of gene expression in response to a variety of signals, and activation of NF-κB has been implicated in antagonizing proapoptotic signals (for reviews, see references 1 and 2). Furthermore, Rac has been implicated in the regulation of NF-κB activation (35, 44). To assay for Rac-dependent NF-κB activation, serum-starved REF-52 cells were microinjected with a CAT reporter plasmid containing three copies of NF-κB binding sites along with expression plasmids encoding T7 epitope-tagged RacV12 or RacV12,ΔIns. Sixteen hours after injection, cells were fixed and analyzed for Rac and CAT expression by double immunofluorescence staining. Consistent with the documented ability of Rac to activate NF-κB-dependent transcription, we observed that RacV12 induced the stimulation of NF-κB activity (Fig. 4B and C). In contrast, the RacV12,ΔIns mutant failed to stimulate NF-κB activity. Thus, the ability of Rac to provide protection against Ras-induced apoptosis seems to correlate with its ability to trigger the activation of NF-κB.
NF-κB activation is necessary and sufficient for suppression of Ras-induced apoptosis.
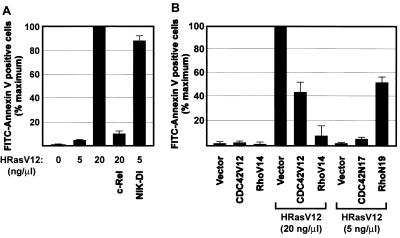
If, as indicated by the results presented above, Rac provides protection against Ras-induced apoptosis by virtue of its ability to activate NF-κB, then the capacity of Ras to induce apoptosis should be inversely correlated with NF-κB activation. To test this prediction, we examined the consequences of upregulation and downregulation of NF-κB activity on Ras-induced apoptosis. In its cytosolic form, NF-κB consists of two subunits, p50 and p65, bound to the inhibitory protein IκB. Activation of NF-κB involves the phosphorylation of IκB by a cascade of specific kinases which results in the targeting of IκB to proteolytic degradation by the proteosome (for reviews, see references 2 and 46). The free NF-κB then translocates to the nucleus, where it binds to κB sites of specific genes. Overexpression of c-Rel, a member of the NF-κB family of proteins (2), abolished the apoptotic effect of HRasV12 (Fig. 5A). To inhibit NF-κB activation, we used a dominant interfering mutant of NIK, the kinase that activates the IκB kinase IκK (6, 31, 38, 53). This mutant sequesters IκK and therefore blocks the phosphorylation-dependent degradation of IκB (38). Expression of the dominant interfering mutant of NIK potentiated the Ras-mediated apoptotic response (Fig. 5A). Thus, activation of NF-κB is a critical component of the Ras and Rac-dependent antiapoptotic pathway. This finding is in agreement with earlier studies demonstrating a role for NF-κB in the suppression of apoptosis in response to oncogenic Ras expression (29).
FIG. 5.
Effects of NF-κB activation and Rho family GTPases on Ras-induced apoptosis. (A) REF-52 cells were injected with the indicated concentrations of HRasV12 in combination with the NF-κB subunit (c-Rel) (50 ng/μl) or a dominant interfering mutant of the IκB kinase kinase (DI-NIK) (50 ng/μl). (B) REF-52 cells were coinjected with the indicated constructs and with the indicated constitutively activated or dominant interfering mutant of Rac, RhoA, or CDC42 (50 ng/μl). Twenty-four hours after injection, annexin V-positive cells were counted. Results are the means of three independent experiments in which at least 100 cells were scored for each condition. Percent maximum corresponds to 42% ± 3.7% of the cells in the injected area (A and B). Error bars represent standard deviations.
Suppression of Ras-induced apoptosis by Rho and Cdc42.
In addition to Rac, members of the Rho family of GTPases include Rho and Cdc42. In certain cell types, these three GTPases function in series to promote actin cytoskeleton rearrangement (28). Moreover, signaling pathways controlled by Rho and Cdc42 lead to NF-κB activation (35) and contribute to the transforming activity of Ras (24, 36, 37). Therefore, we tested the involvement of Cdc42 and Rho in Ras-mediated apoptosis. Expression of either activated Cdc42 (Cdc42V12) or activated RhoA (RhoV14) protected against Ras-induced apoptosis, with RhoV14 being consistently more effective (Fig. 5B). We next examined the effects of expressing dominant interfering mutants of Cdc42 (Cdc42N17) and RhoA (RhoN19) on Ras-mediated apoptosis. Whereas RhoN19 potentiated the apoptotic response induced by Ras, albeit to a lesser extent than RacN17, expression of Cdc42N17 was without an effect. These observations suggest that Rho is a crucial downstream component in the Ras-dependent signaling cascade that promotes cell survival.
Activation of JNK and ERK MAP kinase cascades is required for Ras-induced apoptosis.
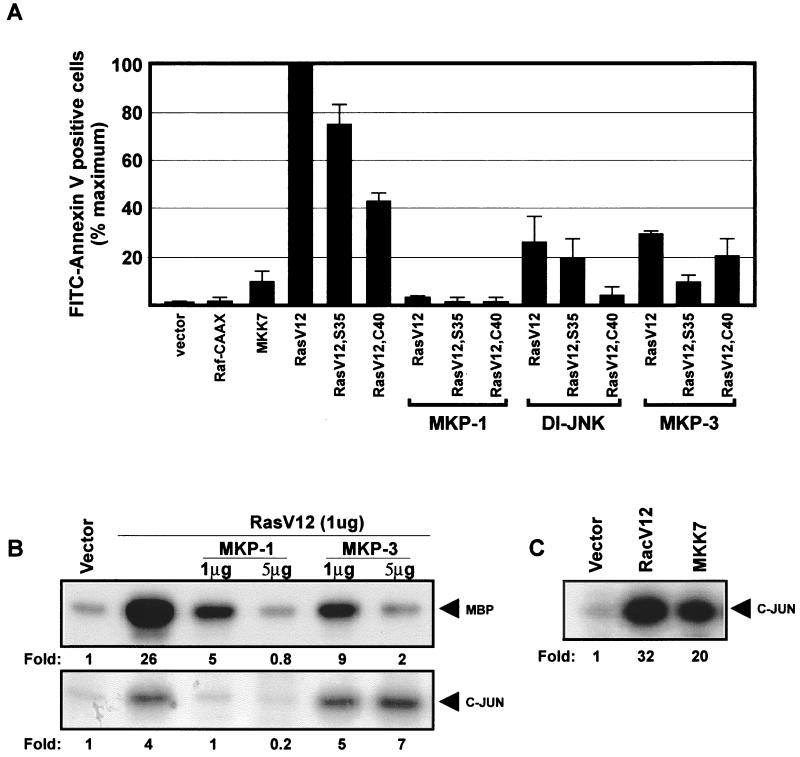
To identify the signaling events which mediate the proapoptotic effects of Ras, we used effector binding loop mutants of Ras which are selectively defective in the activation of specific effector pathways. The HRasV12,S35 mutant cannot activate the Rac pathway but retains the ability to stimulate the ERK MAP kinase pathways. On the other hand, the HRasV12,C40 mutant is defective for ERK MAP kinase activation but is an effective activator of the Rac cascade (18). Both mutants maintained the ability to induce apoptosis under conditions of high-intensity signaling (Fig. 6A), indicating that activation of the MAP kinase cascade is not sufficient for the induction of apoptosis. Consistent with this interpretation, overexpression of a membrane targeted form of the Raf kinase Raf-CAAX had no effect on cell viability. To determine if activation of ERK MAP kinase is necessary for Ras-mediated apoptosis, we used the dual-specificity MAP kinase phosphatase MKP-3 (14, 33). When coexpressed with HRasV12, MKP-3 blocked the activation of ERK MAP kinase but had no effect on Ras-dependent activation of JNK (Fig. 6B). The Ras-induced apoptotic response was significantly inhibited by MKP-3 expression, indicating that ERK activation is an important component of the Ras-dependent proapoptotic pathway.
FIG. 6.
ERK and JNK MAP kinase cascades are both required for Ras-induced apoptosis. (A) REF-52 cells were microinjected with an expression plasmid for HRasV12, HRasV12,S35, or HRasV12,C40 (20 ng/μl), Raf-CAAX (50 ng/μl), or MKK7 (50 ng/μl). Each Ras mutant was also coinjected with phosphatases specific for MAP kinase family members: MKP-1, MKP-3, or a kinase-defective mutant of JNK1 (50 ng/μl). Twenty-four hours after injection, cells were stained with annexin V and the number of apoptotic cells was determined as described in the legend to Fig. 1C. Results are the means of three independent experiments in which at least 100 cells were scored for each condition. Error bars represent standard deviations. (B) Effects of MKP-1 and MKP-3 on Ras-induced JNK and ERK kinase activity. COS-1 cells were transfected with 1 μg of expression vector for HRasV12 and either 10 μg of FLAG epitope-tagged JNK1 or 1 μg HA epitope-tagged ERK2 with either 1 or 5 μg of MKP-1 or MKP-3. Kinase activity was determined by immune complex kinase assay using MBP and c-Jun as substrates. (C) MKK7 is a potent activator of JNK. COS-1 cells were transfected with 10 μg of FLAG-tagged JNK1 and 10 μg of RacV12 or 5 μg of MKK7.
Since HRasV12 and the effector binding loop mutants HRasV12,S35 and HRasV12,C40 all activate the JNK MAP kinase cascade (23), we next examined the contribution of JNK activation to the proapoptotic effect of Ras. Expression of the Jun kinase kinase MKK7 (9) resulted in a robust stimulation of JNK (Fig. 6C) but had no effect on cell viability. On the other hand, expression of a dominant interfering mutant of JNK blocked the apoptotic response induced by Ras, suggesting that the proapoptotic Ras-mediated signals require Jun kinase activation (Fig. 6A). Together, these results suggest that the capacity of Ras to trigger apoptosis is dependent on its ability to activate both the ERK and Jun MAP kinase cascades. This conclusion is further supported by the observations that inactivation of both ERK and JNK by the expression the dual-specificity phosphatase MKP-1 (16) abolished the apoptotic effect of Ras (Fig. 6A and B). It should be pointed out that the simultaneous activation of ERK and JNK MAP kinase cascades is not sufficient to promote apoptosis, as indicated by our observation that coexpression of the Raf-CAAX and MKK7 did not elicit apoptosis (not shown). Thus, the Ras-dependent proapoptotic signal is likely to be mediated by additional effector pathway(s). Although the identity of these pathways remains to be determined, they appear to be uniquely controlled by Ras because Rho GTPases which feed into the Ras signaling cascade failed to induce apoptosis in our experimental system even under conditions where activation of NF-κB was blocked (not shown).
DISCUSSION
The decision of cells to undergo cell cycle arrest or apoptosis in response to oncogenic signals is thought to represent a safeguard mechanism to limit uncontrolled cell proliferation associated with tumorigenesis. Consistent with this idea, we have demonstrated that strong constitutive stimulation of the Ras pathway can induce apoptosis in fibroblasts. From a functional standpoint, this response might be analogous to the permanent cell cycle arrest induced by the expression of oncogenic Ras in primary fibroblasts (43). From a mechanistic standpoint, however, Ras-mediated cell cycle arrest appears to differ from Ras-induced cell death in that the former is p53 dependent whereas the latter is not. It is not clear what determines whether a cell will die or arrest in response to persistent Ras activity. Presumably, the apoptotic response would be triggered if a cell fails to engage cell cycle checkpoints. Indeed, the long interval between Ras expression and the induction of apoptosis (16 h) might reflect impairment in late G1 checkpoints. It is also possible that apoptosis occurs as a result of sustained induction of Ras-dependent signals at an inappropriate time during the cell cycle. The latter scenario would be favored under the experimental conditions used in this study because we used a synchronized cell population in which Ras expression was acutely triggered.
We reasoned that the apoptotic response induced by high intensity of Ras signaling is due to a perturbance in the balance between the activation of pro- and antiapoptotic signals and therefore exploited this response to investigate the identity of these signals. The proapoptotic effects of Ras have been shown to be mediated, at least in some cases, by the ERK MAP kinase cascade (12, 13, 22). Our findings indicate that activation of the ERK MAP kinase cascade is not sufficient to induce apoptosis. Rather, the concerted activation of the ERK and the JNK MAP kinase cascades is necessary to elicit the apoptotic response. JNK activation has been implicated in the induction of apoptosis in some circumstances (54). Significantly, the activation of JNK by Ras is relatively poor but becomes pronounced at high levels of Ras expression (5, 26). This may account in part for the dose-dependent relationship between the intensity of Ras signaling and the extent of the apoptotic response. Since constitutive activation of the ERK and JNK MAP kinase cascades failed to induce apoptotic response, we conclude that there are additional Ras-dependent effector mechanisms that contribute to the apoptotic response.
PI 3-kinase has been shown to regulate signaling events that promote cell survival (for reviews, see references 8 and 10). Rac and PKB/Akt are independent downstream targets of PI 3-kinase, each controlling a distinct effector pathway (48, 50). Although several studies have implicated PKB/Akt as the downstream component of survival signaling through PI 3-kinase (30), our results clearly show that PKB/Akt activation is not sufficient to protect against Ras-induced apoptosis. Rather, we have found that Rac-mediated signals are both necessary and sufficient to suppress the apoptotic effect of Ras. Moreover, our observations suggest a role for Rac in the antiapoptotic function of certain serum growth factors at least under conditions where receptor levels are amplified.
The mechanism by which Rac protects fibroblasts from Ras-induced apoptosis is unclear. It has been shown recently that Rac proteins can induce the transcriptional activity of NF-κB through mechanisms that involve phosphorylation of IκBα (35) and the Rac target POSH (45). The role of NF-κB in apoptosis is complex and appears to vary depending on the cell type and the signaling system (2). However, in the context of Ras signaling, NF-κB function is required to protect against the apoptotic effects of oncogenic Ras (29). Thus, it is possible that the antiapoptotic effects of Rac are mediated by NF-κB activation. This hypothesis is supported by the observation that a Rac mutant which is unable to activate NF-κB is defective in suppressing Ras-induced apoptosis. Additionally, all Rho GTPases can activate NF-κB (35), which could explain their common ability to display antiapoptotic function despite having different signaling activities.
Since the antiapoptotic effects of Rac are dependent on the insert region, it is likely that effector function(s) controlled by this region is necessary for the regulation of cell survival. The insert region of Rac has been shown to regulate the Rac-dependent activation of NADPH oxidase in phagocytic cells (11). Recently we have shown that Rac-induced superoxide production in fibroblasts is also dependent on the insert region (20). Significantly, an increase in the intracellular levels of reactive oxygen species leads to NF-κB activation (32, 41, 42), and the generation of reactive oxygen species is necessary for Rac-dependent stimulation of NF-κB transcriptional activity (44). Taken together, these results suggest that the antiapoptotic component of Ras function might be mediated by an effector pathway in which Rac-regulated production of reactive oxygen species leads to the activation of NF-κB. However, since Rho and Cdc42 can activate NF-κB in the absence of superoxide production, it is conceivable that additional mechanisms contribute to the control NF-κB activation.
The function of Rac protein is critical for Ras-dependent cell proliferation and oncogenic transformation (18, 36). The capacity of Rac to regulate signals that are required for protection against Ras-induced apoptosis provides a potential mechanism by which Rac could contribute to the mitogenic potential of Ras. Our results suggest that Ras transmits two classes of signals, one eliciting apoptosis and another, dependent on Rac, that protects against apoptosis presumably by superoxide-mediated induction of gene expression. Since Ras signaling is frequently amplified in a variety of human cancers, Rac-mediated superoxide generation might have a therapeutical significance for the development of effective treatments against cancer.
ACKNOWLEDGMENTS
We thank Amy Walsh for help with the Akt kinase assay, and we thank Laura Taylor and Song Nimnual for helpful comments on the manuscript.
This work was supported by National Institutes Health grant CA55360 and American Heart Association grant 9650340 to D.B.-S.
REFERENCES
- 1.Baichwal V R, Baeuerle P A. Apoptosis: activate NF-κB or die? Curr Biol. 1997;7:R94–R96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-kappaB and I kappaB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 4.Chen C Y, Faller D V. Direction of p21ras-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene. 1995;11:1487–1498. [PubMed] [Google Scholar]
- 5.Derijard M, Hibi, Wu I, Barrett T, Su B, Deng T, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 6.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 7.Dikic I, Schlessinger J, Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994;4:702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 8.Downward J. Ras signaling and apoptosis. Curr Opin Genet Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 9.Foltz I N, Gerl R E, Wieler J S, Luckach M, Salmon R A, Schrader J W. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 10.Franke T F, Kaplan D R, Cantley L C. PI3K: Downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 11.Freeman J, Abo A, Lambeth J. Rac “insert region” is a novel effector region that is implicated in the activation of NADPH oxidase, but not PAK65. J Biol Chem. 1996;271:19794–19801. doi: 10.1074/jbc.271.33.19794. [DOI] [PubMed] [Google Scholar]
- 12.Fukasawa K, Rulong S, Resau J, Pinto da Silva P, Van de Woude G F. Overexpression of Mos oncogene product in Swiss 3T3 cells induces apoptosis preferentially during S-phase. Oncogene. 1995;10:1–8. [PubMed] [Google Scholar]
- 13.Fukasawa K, Van de Woude G F. Synergy between the Mos/mitogen-activated protein kinase pathway and loss of p53 function in transformation and chromosome instability. Mol Cell Biol. 1997;17:506–518. doi: 10.1128/mcb.17.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groom L A, Sneddon A A, Alessi D R, Dowd S, Keyse S M. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 15.Hagag N, Halegoua S, Viola M. Inhibition of growth factor induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986;319:680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch D D, Stork P J S. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. J Biol Chem. 1997;272:4568–4575. doi: 10.1074/jbc.272.7.4568. [DOI] [PubMed] [Google Scholar]
- 17.Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. Rac regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 18.Joneson T, White M, Wigler M, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 19.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 20.Joneson T, Bar-Sagi D. A Rac1 effector site controlling mitogenesis through superoxide production. J Biol Chem. 1998;273:17991–17994. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- 21.Karim F D, Rubin G M. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 23.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Aelst L V, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosrovi-Far R, Solkki P, Clark G, Kinch M, Der C. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 27.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 28.Mackay D J G, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 29.Mayo M, Wang C-Y, Cogswell P, Rogers-Graham K, Lowe S, Der C, Baldwin A. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 30.Marte B M, Downward J. PKB/Akt: connecting PI3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NG-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 32.Meyer M, Schreck R, Baeuerle P A. H2O2 and antioxidants have opposite effects on activation of NF-kappaB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 34.Mulchay L S, Smith M R, Stacey D W. Requirement for ras protooncogene function during serum stimulated growth of NIH 3T3 cells. Nature. 1985;313:241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- 35.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Local J C. Activation of the nuclear factor-κB by Rho, CDC42 and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 36.Qui R, McCormick F, Symons M. The GTPase Rac1 controls cell proliferation and cooperates with the MAP kinase pathway in fibroblast transformation. Nature. 1995;374:457–459. [Google Scholar]
- 37.Qui R-G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an I kappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 39.Ridley A J, Paterson H F, Johnston C, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 41.Schenk H, Klein M, Erdbrugger W, Droge W, Schulze-Osthoff K. Distinct effects of thioredoxin and other antioxidants on the activation of NF-κB and AP-1. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreck R, Baeuerle P A. Assessing oxegen radicals as mediators in activation of inducible eukaryotic transcription factor NF-κB. Methods Enzymol. 1994;234:151–163. doi: 10.1016/0076-6879(94)34085-4. [DOI] [PubMed] [Google Scholar]
- 43.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16ink4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 44.Sulciner D J, Irani K, Yu Z-X, Ferrans V J, Goldschmidt-Clermont P, Finkel T. Rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tapon N, Nagata K, Lamarche N, Hall A. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signaling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thanos D, Maniatis T. NF-kappaB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 47.Traverse S, Seedorf K, Paterson H, Marshall C J, Cohen P, Ulrich A. EGF triggers neural differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 48.Van Weering D H J, de Rooij J, Marte B, Downward J, Box J L, Burgering B M T. Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–1811. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg R A. The cat and mouse games that genes, viruses, and cells play. Cell. 1997;88:573–575. doi: 10.1016/s0092-8674(00)81897-8. [DOI] [PubMed] [Google Scholar]
- 50.Welch H, Eguinoa A, Stephens L R, Hawkins P T. Protein kinase B and Rac are activated in parallel within a phosphatidylinositide 3OH-kinase-controlled signaling pathway. J Biol Chem. 1998;273:11248–11256. doi: 10.1074/jbc.273.18.11248. [DOI] [PubMed] [Google Scholar]
- 51.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 52.Willumsen B, Christensen A, Hubbert N, Papageorge A, Lowy D. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 53.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 54.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]