Supplemental Digital Content is available in the text.
Keywords: blood coagulation tests, hemostasis, mortality, platelet function, thromboelastography, traumatic brain injury
OBJECTIVES:
Traumatic brain injury is associated with coagulopathy that increases mortality risk. Viscoelastic hemostatic assays such as thromboelastography (Haemonetics SA, Signy, Switzerland) provide rapid coagulopathy assessment and may be particularly useful for goal-directed treatment of traumatic brain injury patients. We conducted a systematic review to assess thromboelastography in the evaluation and management of coagulopathy in traumatic brain injury patients.
DATA SOURCES:
MEDLINE, PubMed Central, Embase, and CENTRAL.
STUDY SELECTION:
Clinical studies of adult patients with traumatic brain injury (isolated or polytrauma) who were assessed by either standard thromboelastography or thromboelastography with platelet mapping plus either conventional coagulation assays or platelet function assays from January 1999 to June 2021.
DATA EXTRACTION:
Demographics, injury mechanism and severity, diagnostic, laboratory data, therapies, and outcome data were extracted for analysis and comparison.
DATA SYNTHESIS:
Database search revealed 1,169 sources; eight additional articles were identified by the authors. After review, 31 publications were used for qualitative analysis, and of these, 16 were used for quantitative analysis. Qualitative and quantitative analysis found unique patterns of thromboelastography and thromboelastography with platelet mapping parameters in traumatic brain injury patients. Patterns were distinct compared with healthy controls, nontraumatic brain injury trauma patients, and traumatic brain injury subpopulations including those with severe traumatic brain injury or penetrating traumatic brain injury. Abnormal thromboelastography K-time and adenosine diphosphate % inhibition on thromboelastography with platelet mapping are associated with decreased survival after traumatic brain injury. Subgroup meta-analysis of severe traumatic brain injury patients from two randomized controlled trials demonstrated improved survival when using a viscoelastic hemostatic assay-guided resuscitation strategy (odds ratio, 0.39; 95% CI, 0.17–0.91; p = 0.030).
CONCLUSIONS:
Thromboelastography and thromboelastography with platelet mapping characterize coagulopathy patterns in traumatic brain injury patients. Abnormal thromboelastography profiles are associated with poor outcomes. Conversely, treatment protocols designed to normalize abnormal parameters may be associated with improved traumatic brain injury patient outcomes. Current quality of evidence in this population is low; so future efforts should evaluate viscoelastic hemostatic assay-guided hemostatic resuscitation in larger numbers of traumatic brain injury patients with specific focus on those with traumatic brain injury-associated coagulopathy.
Coagulopathy occurs in approximately one-third of traumatic brain injury (TBI) patients (1, 2). Post-TBI coagulopathy independently predicts significantly increased rates of death and disability compared with TBI patients without coagulopathy (3, 4). Coagulopathy after TBI may manifest as either hyper- or hypo-coagulability, both of which impact the likelihood and severity of secondary brain injury (5). Anticoagulant and antiplatelet medications are also common in those suffering significant injuries. Thus, quickly identifying abnormal coagulation profiles in acutely injured patients may lead to more appropriate treatment and improved outcomes.
Viscoelastic hemostatic assays (VHAs) such as rotational thromboelastometry (ROTEM, Werfen, Bedford, MA) and thrombo-elastography (Haemonetics SA, Signy, Switzerland) are two guideline-recommended methods to identify coagulopathy after injury (6–8). VHAs provide near real-time information on the coagulation profile, indicate the presence of coagulopathy or fibrinolysis, and can monitor the impact of therapeutic interventions including component transfusion or hemostatic adjuncts such as tranexamic acid (TXA) (5, 9). Furthermore, thromboelastography with platelet mapping (TEG-PM) can rapidly identify abnormalities in platelet function due to either antiplatelet medications or coagulopathy, which are both particularly relevant in TBI patients. In contrast, conventional coagulation assays (CCAs) take much longer to result (10) and only measure the initiation of clot formation or nonfunctional numerical quantities such as platelet count or fibrinogen concentration (11).
Both ROTEM (Werfen) and thromboelastography-guided resuscitation can facilitate the early diagnosis of coagulopathy and may improve outcomes in trauma patients (12, 13). The recent Implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy (ITACTIC) study included a prespecified subgroup of patients with severe TBI, thus bolstering the available data in TBI patients with coagulopathy (14). With this systematic review, we sought to evaluate the role of thromboelastography and TEG-PM in the diagnosis and management of TBI patients.
METHODS
Search Strategy
The review was registered with PROSPERO (CRD42021230876) (15) and was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (16). MEDLINE, PubMed Central, Embase, and CENTRAL databases were searched for articles assessing the role of thromboelastography and TEG-PM in the diagnosis and treatment of TBI patients from January 1999 to June 2021 (Supplemental Table 1, http://links.lww.com/CCX/A764). Titles and abstracts of all identified articles were screened using predefined inclusion and exclusion criteria (Supplemental Table 2, http://links.lww.com/CCX/A764). Included articles were evaluated using checklists from the Scottish Intercollegiate Guidelines Network grading system (17, 18), and randomized controlled trials (RCTs) were further assessed for bias with the Cochrane Risk-of-Bias 2 tool (19) and the evidence quality was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system (20).
Laboratory Measures
For this review, we analyzed the thromboelastography parameters of R-time (R, min); K-time (K, s); alpha angle (α, degrees); maximum amplitude (MA, millimeters); and clot lysis at 30 minutes (LY-30, percent [%] lysis) as well as the rapid thromboelastography (RapidTEG, R-TEG) parameter of activated clotting time (ACT, s) and the TEG-PM parameters of arachidonic acid (AA) and adenosine diphosphate (ADP) % inhibition. Results from both TEG 5000 and TEG 6s analyzers (Haemonetics SA) were included (21). Some of the VHA applications described in this review and the referenced literature fall outside of the indications for use cleared by the U.S. Food and Drug Administration (FDA), including the use of the TEG-PM assay in trauma settings. A listing of the current FDA-cleared uses for thromboelastography and TEG-PM is provided in Supplemental Table 3 (http://links.lww.com/CCX/A764).
Data Analysis
Quantitative results were abstracted by two authors (J.W.C., J.D.D.). RevMan (Cochrane Collaboration, London, United Kingdom, Version 5.4.1) was used for meta-analysis calculations (Supplemental Methods, http://links.lww.com/CCX/A764). Mean difference was calculated using the methodology endorsed by the Cochrane Collaboration (22) and was assessed with R (R Foundation for Statistical Computing, Vienna, Austria, Version 4.0.3) (23). A GRADE evidence profile was created with GRADEpro GDT (20).
RESULTS
Overview of Included Studies
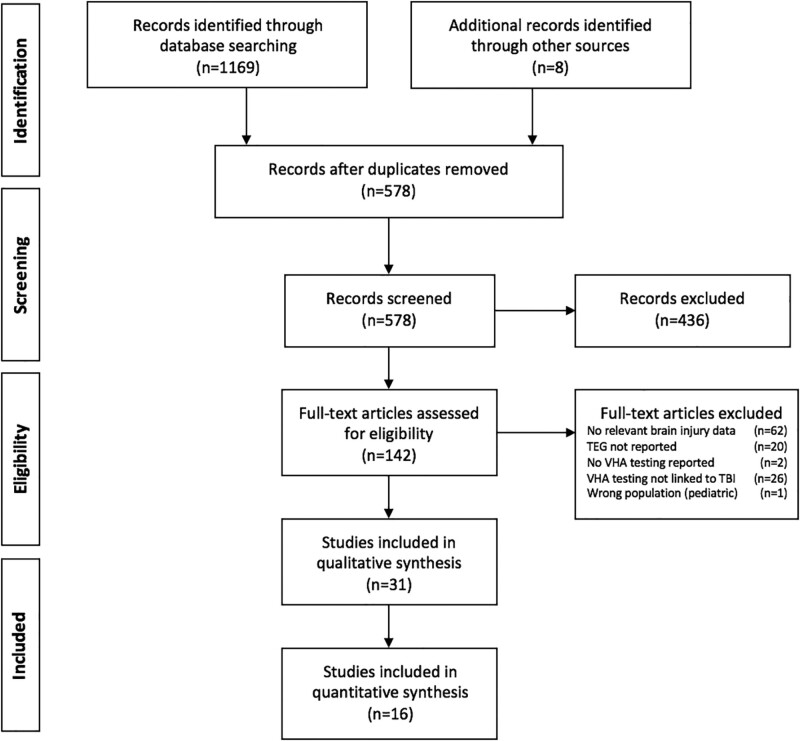
Of 578 unique sources identified, 140 met criteria for full-text review, 31 met criteria for qualitative analysis, and 16 were included in the quantitative analysis (Fig. 1). We grouped the analysis into three focus areas: diagnosis of coagulopathy in TBI, post-TBI prognosis, and management of coagulopathy in TBI patients (30, 31).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram. Additional records identified by other sources included the following: Baksaas-Aasen et al (14), Furay et al (24), Gonzalez et al (13), Sixta et al (25), Kay et al (26), Kumar et al (27), Rao et al (28), and Webb et al (29). TBI = traumatic brain injury, TEG = thromboelastography, VHA = viscoelastic hemostatic assay.
Diagnosis of Coagulopathy in TBI
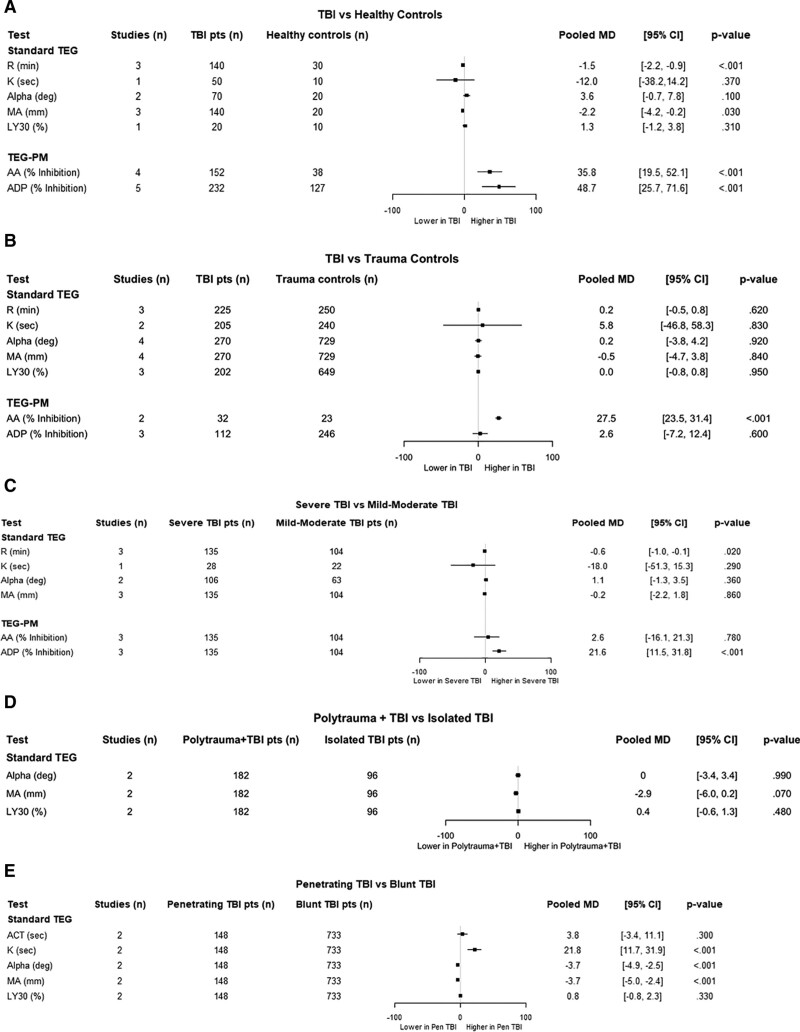
Twelve studies using thromboelastography or TEG-PM to assess coagulopathy in TBI patients were quantitatively analyzed (Fig. 2; Supplemental Figs. 1–5, and Supplemental Table 4, http://links.lww.com/CCX/A764) (26,32–42). An additional four studies were considered for qualitative synthesis (43–46). Thromboelastography patterns in TBI compared with healthy controls, trauma controls, and TBI subgroups are summarized in Supplemental Table 5 (http://links.lww.com/CCX/A764). Compared with healthy controls, TBI patients were hypercoagulable by R-time but also manifested significant platelet dysfunction with elevated % inhibition for both AA and ADP, independent of antiplatelet medication use (Fig. 2A) (32, 33, 36, 41, 42). MA was slightly lower in TBI patients but remained within a normal range. At 96–120 hours, Massaro et al (45) found that TBI patients were relatively hypercoagulable by thromboelastography parameters compared with healthy controls.
Figure 2.
Thromboelastography (TEG) profiles in traumatic brain injury (TBI) patients at admission. A, TBI compared with healthy controls. B, TBI compared with trauma controls without TBI. C, Severe TBI compared with mild to moderate TBI. D, Polytrauma patients with TBI compared with isolated TBI. E, Penetrating TBI compared with blunt TBI. Data are presented as pooled mean difference (MD) for each individual assay obtained on initial patient evaluation. AA = arachidonic acid, ACT = activated clotting time, ADP = adenosine diphosphate, LY30 = clot lysis at 30 minutes, MA = maximum amplitude, pts = patients, TEG-PM = thromboelastography with platelet mapping.
Comparison of admission thromboelastography parameters between TBI patients and non-TBI trauma patients showed no consistent pattern across studies aside from increased AA % inhibition (Fig. 2B), although % AA inhibition may be a nonspecific marker of injury (47). Following thromboelastography values over time, Liu et al (44) found that K, α, and MA values were significantly different from reference ranges in TBI patients at days 1 (hypercoagulability) and 3 (hypocoagulability).
Studies examining TBI subtypes found severe TBI patients were hypercoagulable compared with mild-moderate TBI patients (decreased R-time) (Fig. 2, C and D). Conversely, polytrauma accompanied by TBI resulted in a hypocoagulable state compared with isolated TBI (increased R-time) in one study (37) but a hypercoagulable state in another comparing matched patients (longer ACT and greater α) (35) (Fig. 2, C and D). However, both severe TBI and polytrauma with TBI demonstrated significantly elevated ADP % inhibition. In some studies, patients on ADP-inhibiting medications were either excluded (26, 33, 36) or represented only a small proportion of the patient cohort (39). Patients with penetrating TBI were more hypocoagulable than patients with blunt TBI demonstrating a longer K-time and an abnormally low MA—findings consistent with clinical observations (Fig. 2E) (38, 40). Alpha angle was also lower in penetrating TBI patients but was still well above normal indicating normal clot propagation. No studies have assessed the difference in TEG-PM between patients with penetrating and blunt TBI.
Post-TBI Prognosis
Three studies included data allowing quantitative comparison of thromboelastography and TEG-PM results in TBI nonsurvivors compared with survivors and in patients with progression of TBI by clinical or imaging criteria compared with patients without progression, although a meta-analysis was not possible in comparing survivors to nonsurvivors (28, 29, 36) (Supplemental Figs. 6 and 7 and Supplemental Table 6, http://links.lww.com/CCX/A764). Twelve studies were also considered for qualitative synthesis of thromboelastography and TEG-PM assays results for prognostication in TBI patients for a range of clinical outcomes including mortality, the need for neurosurgical intervention, and bleeding complications (25, 26, 28, 38, 41, 42, 46, 48–52). TEG-PM was generally more reliable in these predictions than standard thromboelastography, particularly regarding mortality. Furthermore, antiplatelet medications (aspirin, cilostazol, dipyridamole, and ADP antagonists) do not appear to impede the ability of TEG-PM to predict mortality in patients with TBI-induced platelet dysfunction (50).
Quantitative analysis of standard thromboelastography assays found that nonsurvivors have a greater K-time and decreased α angle compared with survivors, but in aggregate, neither was outside the normal range in one study (28). However, several studies have found that significantly abnormal thromboelastography parameters portend a poor outcome and may help target early intervention compared with CCAs. Windeløv et al (46) found that admission thromboelastography identified hypocoagulability was associated with higher 30-day mortality, whereas there was no such association for CCAs. Folkerson et al (38) found that coagulopathy after penetrating TBI defined by abnormal R-TEG parameters or platelet count less than 150,000 was independently associated with mortality. Sixta et al (25) found a similar correlation after isolated TBI (80% blunt mechanism). Rao et al (28) noted that increased LY-30 was associated with a need for a neurosurgical procedure and increased K-time was associated with increased mortality. Finally, Kunio et al (52) showed that hypocoagulable thromboelastography parameters in TBI patients were significantly associated with increased mortality risk and longer ICU and hospital length of stay.
Platelet dysfunction leading to reduced clot strength may be more predictive of mortality in TBI patients. Davis et al (36) found ADP inhibition correlated strongly with TBI severity and with mortality. Kay et al (26) similarly reported that platelet dysfunction as indicated by elevated TEG-PM ADP inhibition occurred immediately after isolated blunt TBI and increased with injury severity; the odds of inhospital mortality also increased with greater ADP inhibition (area under the curve [AUC], 0.634 at 80% inhibition; p = 0.041). Daley et al (50) found inhospital mortality was significantly higher in TBI patients with severe (> 60%) ADP inhibition, regardless of antiplatelet medication use, versus TBI patients with normal function.
Stettler et al (42), however, found that TEG-PM did not improve mortality prediction, massive transfusion, or platelet transfusion compared with a combination of clinical and other laboratory measures of injury severity after TBI (55% blunt mechanism). Some of the covariates in the predictive model in this study were not rapidly available, thus limiting broad applicability of these findings.
TBI progression has been assessed in several studies. Folkerson et al (51) found that coagulopathy identified by R-TEG, elevated international normalized ratio (INR), or low platelets was independently associated with intracranial hemorrhage (ICH) exacerbation. Similarly, Webb et al (29) discovered an independent association between increased K-time and ICH progression. Platelet dysfunction has been linked to bleeding complications and ICH progression as well. Nekludov et al (41) found AA inhibition to be higher in TBI patients with any bleeding complication compared with those without complication. Connelly et al (49) found a correlation between TEG-PM AA inhibition and ICH progression (AUC 0.66), whereas there was no correlation for other platelet assays (Multiplate aggregometry, Roche Diagnostics, Indianapolis, IN and VerifyNow, Werfen, Bedford, MA). However, quantitative analysis documented heterogeneous results with regards to standard thromboelastography parameters with no studies assessing TEG-PM in patients with and without bleeding prediction. Thus, further study is warranted to more fully characterize the potential contribution of coagulopathy as identified by VHAs to TBI progression.
Management of Coagulopathy in TBI
Two recent RCTs on VHA-guided resuscitation that included TBI subgroups were used for quantitative analysis (Supplemental Table 7, http://links.lww.com/CCX/A764) (13, 14). In addition, thromboelastography or TEG-PM-guided management in seven articles was considered for qualitative synthesis (24, 27, 33, 39, 53–55).
An R-TEG-based resuscitation strategy evaluated by Gonzalez et al (13) improved survival in a pragmatic RCT compared with resuscitation based on CCAs (INR, activated partial thromboplastin time, fibrinogen, and d-dimer) (81.3% vs 59.6%; log-rank p = 0.032; Wilcoxon p = 0.027). Subgroup analysis of severe TBI patients, however, did not demonstrate a mortality benefit. Furthermore, the TBI subgroup was not specified a priori and was underpowered for mortality assessment as it included only nine TBI patients in the R-TEG group and 12 in the CCA group. Treatment reassignment to the R-TEG group at the request of the treatment team in eight patients also limited the overall strength of this study, although similar outcomes were demonstrated in both intention to treat and as-treated analyses.
Another pragmatic RCT, ITACTIC, recently compared resuscitation guided by CCAs versus VHAs (TEG 6s or ROTEM, Werfen) in trauma patients with major hemorrhage (14). In a prespecified subgroup of severe TBI patients (CCA n = 35 vs VHA n = 39), 64% of patients in the VHA-guided treatment group were alive and free of massive transfusion at 24 hours versus 46% in the CCA-guided treatment group, although this difference was not statistically significant (odds ratio [OR], 2.12; 95% CI, 0.84–5.34; p = 0.148). However, 28-day mortality was significantly reduced in the severe TBI subgroup with VHA-guided intervention (44% mortality) versus CCA-guided intervention (74% mortality) (OR, 0.28; 95% CI, 0.10–0.74; p = 0.016). Patients with TBI in the VHA-guided group were treated with fibrinogen and/or platelets, whereas patients in the CCA-guided treatment group were less likely to receive either treatment, possibly explaining the difference in survival. The potential for VHA-guided interventions to improve mortality risk appeared to increase with injury severity across the subgroups.
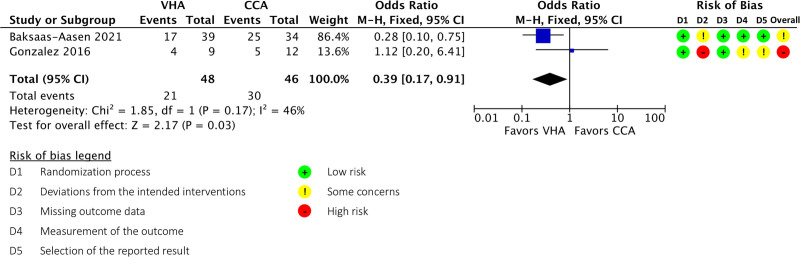
Meta-analysis of TBI patients from these two trials demonstrated a significant 28-day mortality benefit with VHA-guided management (OR, 0.39; 95% CI, 0.17–0.91; p = 0.030) (Fig. 3). Although no publication bias was identified (Supplemental Fig. 8, http://links.lww.com/CCX/A764), overall risk of bias, inconsistency, indirectness, and imprecision resulted in a “very low” quality assessment by the GRADE system (Supplemental Table 8, http://links.lww.com/CCX/A764).
Figure 3.
Outcomes of traumatic brain injury patients managed under a viscoelastic hemostatic assay (VHA)-guided resuscitation protocol compared with conventional coagulation tests. CCA = conventional coagulation assay, df = degrees of freedom, M-H = Mantel-Haenszel.
TEG-PM-guided management may also improve TBI outcomes. In a pragmatic interventional trial, Kumar et al (27) found that a TEG-PM-based protocol significantly decreased platelet transfusions without risking expansion in intracranial bleeding. Similarly, Furay et al (24) found patients with platelet dysfunction (ADP inhibition > 60%) transfused 1 U of apheresis platelets had lower mortality with lower total blood products overall. Holzmacher et al (55) showed platelet transfusion improved AA inhibition in patients with blunt TBI taking antiplatelet medication but did not identify a mortality benefit. A subsequent study compared platelet transfusion versus desmopressin in patients with severe TBI. Although platelet transfusion increased clot strength and thromboelastography standard parameters, both treatments improved ADP inhibition to a similar degree with no difference in mortality.
DISCUSSION
This systematic review synthesizes the available clinical data on thromboelastography-based diagnosis and management coagulopathy in TBI patients and builds upon a 2017 narrative review of all VHAs (2). We demonstrate that thromboelastography and TEG-PM assays reveal coagulation profiles unique to patients with TBI, potentially differentiating them from those with trauma-induced coagulopathy without TBI. Furthermore, abnormalities in thromboelastography and TEG-PM assays are associated with mortality and bleeding complications in TBI patients. Finally, because platelet dysfunction is commonly observed in TBI patients, TEG-PM complements standard thromboelastography in the early identification of patients who may benefit from platelet transfusion or desmopressin therapy. Ultimately, this approach may be associated with improved survival and reduce blood product use similar to other VHAs (4), although the quality of evidence is currently low.
A 2015 Cochrane review of VHAs in trauma found insufficient evidence to recommend their routine use outside of a research protocol (56), while a 2016 Cochrane review highlighted the growing evidence for the use of VHAs to guide component transfusion therapy and reduce morbidity in patients with bleeding (57). More recent experience with VHA-guided management in trauma and TBI patients indicates a favorable view of this approach. Indeed, our analysis identified several studies that found VHAs to identify coagulopathy in a larger proportion of patients than CCAs. In TBI patients, in particular, coagulopathy was felt to be inadequately characterized by CCAs, and the contribution of platelet dysfunction to this coagulopathy was identified as an important knowledge gap. In lieu of high quality clinical data, expert opinion was used to develop early VHA-guided protocols (2), and evaluation of such a protocol by Gratz et al (10) found that VHAs are faster than CCAs and identify coagulopathy in a greater number of patients.
Our review confirmed several unique patterns of coagulopathy in various TBI populations (Supplemental Table 5, http://links.lww.com/CCX/A764) and also found that these patterns change over time. The etiology of TBI-associated coagulopathy is an area of active investigation (58, 59). The observation that patients have preserved platelet counts but paradoxically impaired platelet function has been termed “platelet exhaustion.” This phenomenon is characterized by platelets activated by endothelial release of tissue factor, platelet-activating factor, and von Willebrand factor that are then “spent” and cannot contribute to primary hemostasis or to further platelet stimulation (60). Additionally, platelet function abnormalities may be related to brain-derived extracellular vesicles or immune-thrombotic mechanisms (2). Because of the heterogeneous nature of TBI and TBI-associated coagulopathy and the high risk for mortality in these patients, rapid and accurate assessment is vital to identifying and initiating treatment. Indeed, evidence presented in this review, including results from the recent ITACTIC study (14), suggests that VHA-guided management algorithms may improve survival in patients with TBI. Reported survival enhancement may be linked to reducing secondary injuries following TBI such as intracerebral hemorrhage, cerebral ischemia, or cerebral inflammation realized using a targeted management approach.
Improved survival may also result from early, individualized hemostatic resuscitation. This notion is supported by subgroup analysis of TBI patients in the Prehospital Air Medical Plasma trial that demonstrated improved survival with prehospital plasma infusion (61). Relatedly, although empiric TXA administration does not have a clear benefit in patients with severe TBI, future work should assess the potential for thromboelastography-guided dosing of this and other hemostatic adjuncts (62, 63). Finally, novel blood products such as cold-stored platelets (ClinicalTrials.gov Identifier: NCT04726410) and cold-stored whole blood (ClinicalTrials.gov Identifier: NCT03402035) may have improved hemostatic efficacy over current therapies (64, 65) and warrant evaluation in TBI patients with coagulopathy. The potential benefit of VHA-guided diagnosis and management should be evaluated in larger numbers of TBI patients paying careful attention to underlying baseline coagulation abnormalities and the effect of both empiric and targeted therapies.
Our systematic review has several limitations. First, our search may have excluded important findings from non-English language publications. Second, heterogeneity in patient populations (size and demographics), trauma definitions, comparator groups, timing of assessments, and treatment approaches across included studies limit extrapolation of our findings to more general populations. Finally, most included studies were small and nonrandomized with findings that require validation in larger RCTs.
CONCLUSIONS
Thromboelastography and thromboelastography with platelet mapping characterize coagulopathy patterns in TBI patients, and abnormalities in their respective profiles presage poor outcomes. Accordingly, data-driven and patient-focused treatment protocols designed to normalize these parameters should be considered in the management of TBI patients. Current quality of evidence in this population is low; so future efforts should evaluate this approach in larger numbers of TBI patients with specific focus on those with TBI-associated coagulopathy.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Ms. Katherine Bishop in data abstraction for this article and Zhi Geng, MD, MPH, for his statistical review and assistance with figure creation. We also thank medical librarians Shuna Gould and Abigail Baines from Meridian HealthComms, Plumley, United Kingdom, for their assistance with the literature search and article screening.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Meridian HealthComms, Plumley, United Kingdom, librarian support was funded by Haemonetics SA, Signy, Switzerland, in accordance with Good Publication Practice.
Dr. Cannon is a consultant for CSL Behring and has received honoraria from UpToDate for article authorship unrelated to this work. Drs. Dias and Hartmann are employees of Haemonetics. Dr. Kumar has received honoraria for participation in advisory board meetings from Portola Pharmaceuticals and study grants from Haemonetics. Dr. Cotton has received honoraria for consulting services and participation in scientific advisory meetings for Haemonetics. Dr. Schreiber is a consultant for Haemonetics, CSL Behring, and Tricol and has received grant funding from Haemonetics and CSL Behring. Dr. Zacharowski has received honoraria for participation in advisory board meetings for Haemonetics and Vifor and received speaker fees from CSL Behring and GE Healthcare. He is the Principal Investigator of the European Union-Horizon 2020 project ENVISION (Intelligent plug-and-play digital tool for real-time surveillance of coronavirus disease 2019 patients and smart decision-making in ICUs). Dr. Schöchl has received honoraria for participation in advisory board meetings from Bayer Healthcare, Boehringer Ingelheim, Tem International GmbH, speaker fees from Haemonetics and Vifor, and study grants from CSL Behring. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Simmons JW, Pittet JF, Pierce B: Trauma-induced coagulopathy. Curr Anesthesiol Rep. 2014; 4:189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maegele M, Schöchl H, Menovsky T, et al. : Coagulopathy and haemorrhagic progression in traumatic brain injury: Advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017; 16:630–647 [DOI] [PubMed] [Google Scholar]
- 3.Harhangi BS, Kompanje EJ, Leebeek FW, et al. : Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien). 2008; 150:165–175; discussion 175 [DOI] [PubMed] [Google Scholar]
- 4.Schöchl H, Solomon C, Traintinger S, et al. : Thromboelastometric (ROTEM) findings in patients suffering from isolated severe traumatic brain injury. J Neurotrauma. 2011; 28:2033–2041 [DOI] [PubMed] [Google Scholar]
- 5.Maegele M: Coagulopathy after traumatic brain injury: Incidence, pathogenesis, and treatment options. Transfusion. 2013; 53:28S–37S [DOI] [PubMed] [Google Scholar]
- 6.Spahn DR, Bouillon B, Cerny V, et al. : The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit Care. 2019; 23:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaba K, Rizoli S, Veigas PV, et al. ; Viscoelastic Testing in Trauma Consensus Panel: 2014 Consensus conference on viscoelastic test-based transfusion guidelines for early trauma resuscitation: Report of the panel. J Trauma Acute Care Surg. 2015; 78:1220–1229 [DOI] [PubMed] [Google Scholar]
- 8.Committee on Trauma of the American College of Surgeons: ACS TQIP Guidelines, Massive Transfusion in Trauma. 2015. Available at: https://www.facs.org/quality-programs/trauma/tqip/best-practice. Accessed May 17, 2021
- 9.Cannon JW: Hemorrhagic shock. N Engl J Med. 2018; 378:370–379 [DOI] [PubMed] [Google Scholar]
- 10.Gratz J, Güting H, Thorn S, et al. : Protocolised thromboelastometric-guided haemostatic management in patients with traumatic brain injury: A pilot study. Anaesthesia. 2019; 74:883–890 [DOI] [PubMed] [Google Scholar]
- 11.Ganter MT, Hofer CK: Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008; 106:1366–1375 [DOI] [PubMed] [Google Scholar]
- 12.Veigas PV, Callum J, Rizoli S, et al. : A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016; 24:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez E, Moore EE, Moore HB, et al. : Goal-directed hemostatic resuscitation of trauma-induced coagulopathy a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016; 263:1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baksaas-Aasen K, Gall LS, Stensballe J, et al. : Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med. 2021; 47:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon JW, Kaplan LJ: Use of Thromboelastography in the Evaluation and Management of Patients With Traumatic Brain Injury. 2021. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021230876. Accessed May 17, 2021 [DOI] [PMC free article] [PubMed]
- 16.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scottish Intercollegiate Guidelines Network: A Guideline Developer’s Handbook. (SIGN Publication No. 50). 2019. Available at: https://www.sign.ac.uk/media/1050/sign50_2019.pdf. Accessed May 17, 2021
- 18.Scottish Intercollegiate Guidelines Network: Checklists. 2021. Available at: https://www.sign.ac.uk/what-we-do/methodology/checklists/. Accessed May 17, 2021
- 19.Sterne JAC, Savović J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 20.Schünemann H, Brożek J, Guyatt G, et al. (Eds): GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2013. Available at: guidelinedevelopment.org/handbook. Accessed May 17, 2021
- 21.Neal MD, Moore EE, Walsh M, et al. : A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J Trauma Acute Care Surg. 2020; 88:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan X, Wang W, Liu J, et al. : Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team: R: A Language and Environment for Statistical Computing. 2020. Available at: http://www.r-project.org/. Accessed December 5, 2020
- 24.Furay EJ, Daley MJ, Satarasinghe P, et al. : Desmopressin is a transfusion sparing option to reverse platelet dysfunction in patients with severe traumatic brain injury. J Trauma Acute Care Surg. 2020; 88:80–86 [DOI] [PubMed] [Google Scholar]
- 25.Sixta SL, Cardenas JC, Kitagawa R, et al. : Hypocoagulability in traumatic brain injury as measured by traditional means and thrombelastography. J. Neurol Neurophysiol. 2015; 6:1–526753104 [Google Scholar]
- 26.Kay AB, Morris DS, Collingridge DS, et al. : Platelet dysfunction on thromboelastogram is associated with severity of blunt traumatic brain injury. Am J Surg. 2019; 218:1134–1137 [DOI] [PubMed] [Google Scholar]
- 27.Kumar MA, Schmierer M, Silverman E, et al. : A thromboelastography platelet mapping–guided reversal algorithm limits platelet transfusion in older patients with traumatic brain injury: A pilot study. Neurocrit Care. 2019; 31:S32 [Google Scholar]
- 28.Rao A, Lin A, Hilliard C, et al. : The utility of thromboelastography for predicting the risk of progression of intracranial hemorrhage in traumatic brain injury patients. Neurosurgery. 2017; 64:182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb AJ, Brown CS, Naylor RM, et al. : Thromboelastography is a marker for clinically significant progressive hemorrhagic injury in severe traumatic brain injury. Neurocrit Care. 2021. Apr 12. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Brazinova A, Majdan M, Leitgeb J, et al. ; Austrian Working Group on Improvement of Early TBI Care: Factors that may improve outcomes of early traumatic brain injury care: Prospective multicenter study in Austria. Scand J Trauma Resusc Emerg Med. 2015; 23:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martini WZ: Coagulation complications following trauma. Mil Med Res. 2016; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartels AN, Johnson C, Lewis J, et al. : Platelet adenosine diphosphate inhibition in trauma patients by thromboelastography correlates with paradoxical increase in platelet dense granule content by flow cytometry. Surgery. 2016; 160:954–959 [DOI] [PubMed] [Google Scholar]
- 33.Castellino FJ, Chapman MP, Donahue DL, et al. : Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg. 2014; 76:1169–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valle EJ, Van Haren RM, Allen CJ, et al. : Does traumatic brain injury increase the risk for venous thromboembolism in polytrauma patients? J Trauma Acute Care Surg. 2014; 77:243–250 [DOI] [PubMed] [Google Scholar]
- 35.Samuels JM, Moore EE, Silliman CC, et al. : Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg. 2019; 86:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis PK, Musunuru H, Walsh M, et al. : Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013; 18:201–208 [DOI] [PubMed] [Google Scholar]
- 37.de Oliveira Manoel AL, Neto AC, Veigas PV, et al. : Traumatic brain injury associated coagulopathy. Neurocrit Care. 2015; 22:34–44 [DOI] [PubMed] [Google Scholar]
- 38.Folkerson LE, Sloan D, Davis E, et al. : Coagulopathy as a predictor of mortality after penetrating traumatic brain injury. Am J Emerg Med. 2018; 36:38–42 [DOI] [PubMed] [Google Scholar]
- 39.Guillotte AR, Herbert JP, Madsen R, et al. : Effects of platelet dysfunction and platelet transfusion on outcomes in traumatic brain injury patients. Brain Inj. 2018; 32:1849–1857 [DOI] [PubMed] [Google Scholar]
- 40.Martin G, Shah D, Elson N, et al. : Relationship of coagulopathy and platelet dysfunction to transfusion needs after traumatic brain injury. Neurocrit Care. 2018; 28:330–337 [DOI] [PubMed] [Google Scholar]
- 41.Nekludov M, Bellander BM, Blombäck M, et al. : Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007; 24:1699–1706 [DOI] [PubMed] [Google Scholar]
- 42.Stettler GR, Moore EE, Moore HB, et al. : Platelet adenosine diphosphate receptor inhibition provides no advantage in predicting need for platelet transfusion or massive transfusion. Surgery. 2017; 162:1286–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunham CM, Hoffman DA, Huang GS, et al. : Traumatic intracranial hemorrhage correlates with preinjury brain atrophy, but not with antithrombotic agent use: A retrospective study. PLoS One. 2014; 9:e109473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Li J, Yu J, et al. : Research into the predictive effect of TEG in the changes of coagulation functions of the patients with traumatic brain hemorrhage. Open Med (Wars). 2015; 10:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massaro AM, Doerfler S, Nawalinski K, et al. : Thromboelastography defines late hypercoagulability after TBI: A pilot study. Neurocrit Care. 2015; 22:45–51 [DOI] [PubMed] [Google Scholar]
- 46.Windeløv NA, Welling KL, Ostrowski SR, et al. : The prognostic value of thrombelastography in identifying neurosurgical patients with worse prognosis. Blood Coagul Fibrinolysis. 2011; 22:416–419 [DOI] [PubMed] [Google Scholar]
- 47.Wohlauer MV, Moore EE, Thomas S, et al. : Early platelet dysfunction: An unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012; 214:739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tur Martínez J, Petrone P, Axelrad A, et al. : Comparison between thromboelastography and conventional coagulation test: Should we abandon conventional coagulation tests in polytrauma patients? Cir Esp. 2018; 6:18–20 [DOI] [PubMed] [Google Scholar]
- 49.Connelly CR, Yonge JD, McCully SP, et al. : Assessment of three point-of-care platelet function assays in adult trauma patients. J Surg Res. 2017; 212:260–269 [DOI] [PubMed] [Google Scholar]
- 50.Daley MJ, Enright Z, Nguyen J, et al. : Adenosine diphosphate platelet dysfunction on thromboelastogram is independently associated with increased morality in traumatic brain injury. Eur J Trauma Emerg Surg. 2017; 43:105–111 [DOI] [PubMed] [Google Scholar]
- 51.Folkerson LE, Sloan D, Cotton BA, et al. : Predicting progressive hemorrhagic injury from isolated traumatic brain injury and coagulation. Surgery. 2015; 158:655–661 [DOI] [PubMed] [Google Scholar]
- 52.Kunio NR, Differding JA, Watson KM, et al. : Thrombelastography-identified coagulopathy is associated with increased morbidity and mortality after traumatic brain injury. Am J Surg. 2012; 203:584–588 [DOI] [PubMed] [Google Scholar]
- 53.Hota S, Ng M, Hilliard D, et al. : Thromboelastogram-guided resuscitation for patients with traumatic brain injury on novel anticoagulants. Am Surg. 2019; 85:861–864 [PubMed] [Google Scholar]
- 54.Furay E, Daley M, Teixeira PG, et al. : Goal-directed platelet transfusions correct platelet dysfunction and may improve survival in patients with severe traumatic brain injury. J Trauma Acute Care Surg. 2018; 85:881–887 [DOI] [PubMed] [Google Scholar]
- 55.Holzmacher JL, Reynolds C, Patel M, et al. : Platelet transfusion does not improve outcomes in patients with brain injury on antiplatelet therapy. Brain Inj. 2018; 32:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunt H, Stanworth S, Curry N, et al. : Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015; 2:CD010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wikkelsø A, Wetterslev J, Møller AM, et al. : Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016; 2016:CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Zhang F, Dong JF: Coagulopathy induced by traumatic brain injury: Systemic manifestation of a localized injury. Blood. 2018; 131:2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riojas CM, Ekaney ML, Ross SW, et al. : Platelet dysfunction after traumatic brain injury: A review. J Neurotrauma. 2021; 38:819–829 [DOI] [PubMed] [Google Scholar]
- 60.Moore EE, Moore HB, Kornblith LZ, et al. : Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021; 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gruen DS, Guyette FX, Brown JB, et al. : Association of prehospital plasma with survival in patients with traumatic brain injury: A secondary analysis of the PAMPer cluster randomized clinical trial. JAMA Netw Open. 2020; 3:e2016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CRASH-3 trial collaborators: Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet (London, England). 2019; 394:1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowell SE, Meier EN, McKnight B, et al. : Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020; 324:961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nair PM, Pidcoke HF, Cap AP, et al. : Effect of cold storage on shear-induced platelet aggregation and clot strength. J Trauma Acute Care Surg. 2014; 77:S88–S93 [DOI] [PubMed] [Google Scholar]
- 65.Reddoch KM, Pidcoke HF, Montgomery RK, et al. : Hemostatic function of apheresis platelets stored at 4°C and 22°C. Shock. 2014; 41(Suppl 1):54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.