Background:
Thyroid eye disease (TED) causes orbital soft-tissue expansion. Recent studies have suggested that brow and temple changes may also occur. Teprotumumab, a monoclonal antibody to the insulin-like growth factor 1 receptor reduces soft-tissue swelling in TED. In this study, we quantified the changes to pan facial soft-tissue volumes and eyelid position, following treatment with teprotumumab.
Methods:
In this prospective study, consecutive patients who were treated with teprotumumab were appraised for study eligibility. All patients had 3D facial imaging using the Vectra H2. Soft-tissue volume changes in the upper face, periorbita, temples, midface, and lower face were quantified before and after teprotumumab therapy. Furthermore, the marginal reflex distance (MRD)1, MRD2, and intercanthal distance were also measured pretreatment and posttreatment.
Results:
Twenty-three patients were included in the study. The mean duration of TED was 29 months (38). Following teprotumumab therapy, the mean (SD) decrease in volume for each region was 0.75 mL (0.84) in the upper face, 1.8 mL (1.3) in the periorbital region, 0.17 mL (0.5) in the temples, 1.62 mL (3.16) in the midface, and 2.67 mL (4.6) in the lower face. The mean (SD) decrease in the volume of the full face was 8.9 mL (8.7). There was also a significant reduction in MRD1, MRD2, and the intercanthal space following treatment. There was no relationship between previous steroid use and total body weight reduction and changes in facial volume.
Conclusion:
TED may cause significant tissue expansion across the entire face and this may be reduced following teprotumumab therapy.
INTRODUCTION
Thyroid eye disease (TED) is a complex autoimmune condition that manifests acutely with signs of inflammation affecting orbital and periorbital soft tissues. At this stage, patients may present with disfiguring proptosis, diplopia, and eyelid retraction. In the chronic stages, inflammation decreases, but changes to orbital tissues typically become permanent. Soft-tissue changes are not restricted to the orbit; several studies have demonstrated increased retro-orbicularis fat volume and eyebrow volume in patients with TED.1,2 Orbital imaging studies have shown brow changes unique to TED patients and distinct from those caused by aging.3 Hwang et al demonstrated increased soft-tissue volume and fat within the temporal fossa in patients with TED, suggesting that the facial changes occurring in TED may not be limited to the periorbital region.4 Changes to appearance and vision have been shown to impact patients’ self-confidence and ability to carry out daily tasks and, therefore, quality of life.5
Soft-tissue expansion in TED may be explained by overexpression of the insulin-like growth factor 1 receptor (IGF-1R) and its interaction with the thyrotropin receptor (TSHR), a key pathogenic feature of this condition.6 These receptors form a physical and functional complex on the cell membrane of orbital fibroblasts (OFs), and professional immune cells (B cells and T cells7). Binding of autoantibodies to this receptor complex leads to increased production of proinflammatory cytokines,8 hyaluronan, and the differentiation of OFs into myofibroblasts or adipocytes causing soft-tissue expansion.9
Teprotumumab, a fully human monoclonal immunoglobulin to the IGF-1R, has recently been approved for the treatment of TED in the United States.10 Teprotumumab binds to the IGF-1R and inhibits downstream pathways.11 Recent phase 2 and 3 randomized, double-masked clinical trials (NCT01868997 and NCT03298867)12,13 have demonstrated marked reduction in proptosis in patients with active TED. Further work has quantified a significant decrease in orbital soft-tissue volume in patients with TED.14
Given the potential for TED to affect the extraorbital tissue on the face, and the impact of teprotumumab on orbital soft-tissue volume, we hypothesize that treatment with teprotumumab may have a similar impact on the soft tissue of other regions of the face. The primary aim of this study was to quantify facial soft-tissue changes following treatment with teprotumumab in patients with TED. Secondary outcomes included characterization of eyelid changes.
METHODS
This study adhered to the tenets of the Declaration of Helsinki, was performed in accordance with the Health Insurance Portability and Accountability Act, and was approved by the institutional review board at our institution. All patients provided written consent for participation.
PATIENTS AND STUDIES
In this prospective study, all patients presenting at our institution for the treatment of TED were considered for study eligibility. Patients who were currently on any other medical therapy for TED or had received rituximab or tocilizumab in the past were excluded. Furthermore, patients who had any plans to embark on a weight loss regime, or medications that might cause weight loss were excluded. Patients received infusions of teprotumumab (10 mg/kg for the first infusion and 20 mg/kg for subsequent infusions) every 3 weeks with the intention to complete 8 infusions over 24 weeks.
Measurement of Clinical Outcomes
The primary outcome measure was a change in soft-tissue volume of the face, from baseline (preinfusion) to within 3 weeks of the final infusion. Secondary outcome measures included eyelid position: marginal reflex distance (MRD) 1, MRD2, and the intercanthal distance of the same orbit. Other secondary outcomes included the mean change in proptosis, a change in the clinical activity score (CAS) and changes in body weight.
Proptosis was assessed using the same Hertel exophthalmometer by the same person at each visit. The CAS assesses inflammation on a seven-point scale,15 noting the presence of each of the following signs: retrobulbar eye pain, pain on eye movement, eyelid erythema, eyelid swelling, conjunctival redness, chemosis, and inflammation of the caruncle or plica. A CAS of 1 or less is indicative of disease inactivation.15
3D Image Acquisition
The use of 3D imaging systems and more pertinently, the Vectra H system, to quantify volumetric change in facial soft tissue have been shown to be reliable and accurate.16 In the present study, each patient was scanned with the H2 Vectra 3D imaging system (Canfield Scientific, Fairfield, N.J.) at each visit. The patients were seated, asked for a neutral facial expression and to look ahead into the distance at a fixed object. All images were acquired under clinical lighting by an experienced technician familiar with the camera.
The Vectra H2 system guides the user with visual prompts to ensure that the camera is the correct distance from the facial target, with two projected green dots functioning as a guide. Correct distance is achieved when both green dots converge on the surface of the patient’s face.
3D Image Processing: Landmarks
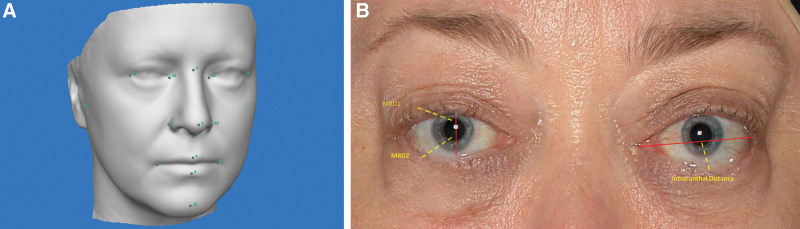
Landmarks were used to register pretreatment and posttreatment images, based on published data on reliability17 (Fig. 1). These landmarks were chosen to make sure the pretreatment and posttreatment reconstructions were correctly aligned and orientated in the x, y, and z axis.
Fig. 1.
Position of landmarks placed on the face. A, Landmarks used were the bilateral medial canthus, lateral canthus, glabella, nasal tip, subnasale, right and left alar, columella, philtral crest, labrale inferious, right and left oral commissure, and menton. B, examples of the markings used for the MRD1, MRD2, and the intracanthal distance on a patient.
Volume Assessment
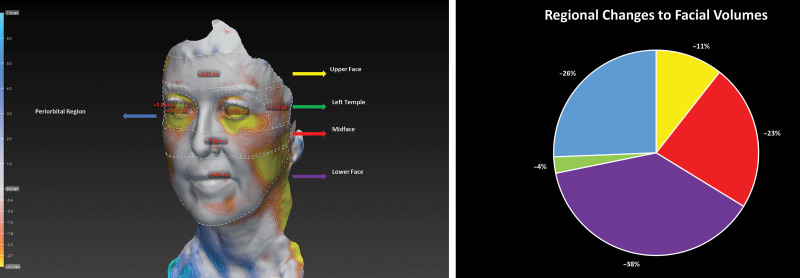
Following 3D reconstruction of the images, the Vectra analysis module was used to calculate differences in volume within predefined regions of the face between the pretreatment and posttreatment images. The face was divided into regions according to the current consensus of dividing the face into thirds as suggested by previous studies on aging and the fat compartments of the face.18,19 The divisions of the face used in this study are shown in Figure 2.
Fig. 2.
The upper face was defined as the region from the hairline superiorly to the upper eyebrows inferiorly (A). The periorbital region was designated the area immediately beneath the eyebrows, extending in a circular shape around each eye (but not including the eyes), limited to the boundaries of the orbitomalar ligament (tear trough inferiorly). The space lateral to the periorbital region (respecting the upper and lower borders of the periorbital region, horizontally) up to the hairline was defined as the temple. The midface was defined as the region below the periorbital space and the temples, down to a line drawn horizontally from ear to ear, crossing the subnasale. The lower face was designated the region below this space up to the outline of the jaw. Decrease in volume across the upper face, midface, temples, periorbita and lower face in a single patient. Colors of the arrows correspond to parts of the attached chart (B).
Eyelid Measurements
The MRD1, MRD2, and intercanthal distances were measured on the 3D images. The Vectra camera has a fixed focal point for each image capture, thereby permitting measurement of distances directly on captured images.
Patient Self-assessment of Cosmetic Results
Following the last infusion, each patient was asked three questions: (1) Do you feel your facial appearance (eyes and rest of the face included) has gone back to how it was before TED?; (2) Are you happy with your appearance currently?; and (3) Would you take the treatment again?
Patients were asked to respond on a four-point scale: 0 = strongly disagree, 1 = undecided, 2 = agree, and 3 = strongly agree.
Statistical Analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS, Inc, Chicago, Ill.). The difference between eyelid measurements for the pretreatment and posttreatment conditions was calculated using a dependent t-test. Relationships between continuous variables were analyzed using a Pearson correlation. Statistical significance was defined as P value less than 0.05. Intraobserver variability (repeatability) was defined by the coefficient of variation, expressed as a percentage. Intraobserver variability was calculated by both observers doing all calculations twice on two separate days. The variability was defined by the coefficient of variation, expressed as a percent. For interobserver variability, both observers completed all volume and eyelid measurements, intraclass correlation coefficients, and their 95% confidence intervals were calculated. The magnitude of the measurement error between the observers was calculated using the Bland–Altman method.20 Given the asymmetric nature of TED,21 each orbit was treated as an independent entity, allowing measurements from both sides in the same patient without bias.
RESULTS
Patient Characteristics
A total of 23 patients (three men and 20 women) were included in the study. Demographic and clinical details are provided in Table 1. The mean (SD) age of patients was 51 (17), whereas the mean duration of TED before treatment with teprotumumab was 29 months (38). None of the patients were current smokers and all patients were euthyroid at the time of treatment.
Table 1.
Demographic and Clinical Details
| Age | Gender | Ethnicity | Smoking History | Duration of TED before First Infusion (mo) | Previous Treatments for TED | Thyroid Status at Time of Initial Infusion | No. of Infusions* | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 70 | Female | Caucasian | No | 5 | Nil | Euthyroid | 6 |
| Case 2 | 69 | Female | Caucasian | No | 36 | IV corticosteroids, OS decompression 3 y prior | Euthyroid | 7 |
| Case 3 | 49 | Female | Caucasian | No | 25 | Oral corticosteroids | Euthyroid | 7 |
| Case 4 | 69 | Female | Black | No | 25 | Nil | Euthyroid | 6 |
| Case 5 | 53 | Female | Hispanic | No | 2 | Nil | Euthyroid | 7 |
| Case 6 | 53 | Female | Caucasian | No | 9 | Nil | Euthyroid | 8 |
| Case 7 | 29 | Female | Caucasian | No | 82 | IV corticosteroids | Euthyroid | 8 |
| Case 8 | 64 | Female | Caucasian | No | 21 | IV corticosteroids | Euthyroid | 7 |
| Case 9 | 57 | Female | Asian | No | 17 | OS Decompression 3 y prior, IV Corticosteroids | Euthyroid | 6 |
| Case 10 | 59 | Female | Asian | No | 10 | Nil | Euthyroid | 6 |
| Case 11 | 38 | Male | Caucasian | Former | 3 | IV corticosteroids | Euthyroid | 7 |
| Case 12 | 15 | Female | Caucasian | No | 6 | Nil | Euthyroid | 8 |
| Case 13 | 38 | Female | Asian | No | 23 | Nil | Euthyroid | 8 |
| Case 14 | 52 | Female | Caucasian | No | 20 | Nil | Euthyroid | 7 |
| Case 15 | 61 | Female | Caucasian | No | 6 | Nil | Euthyroid | 8 |
| Case 16 | 48 | Female | Caucasian | No | 67 | Decompression OU 4 y prior, IV corticosteroids | Euthyroid | 8 |
| Case 17 | 68 | Female | Black | No | 118 | Nil | Euthyroid | 8 |
| Case 18 | 51 | Female | Caucasian | No | 144 | Nil | Euthyroid | 6 |
| Case 19 | 31 | Male | Caucasian | No | 3 | Nil | Euthyroid | 6 |
| Case 20 | 82 | Male | Caucasian | No | 5 | Nil | Euthyroid | 6 |
| Case 21 | 19 | Female | Black | No | 2 | Nil | Euthyroid | 6 |
| Case 22 | 57 | Female | Black | No | 36 | Nil | Euthyroid | 6 |
| Case 23 | 38 | Female | Caucasian | No | 6 | Nil | Euthyroid | 8 |
*At the time of analysis.
Clinical Characteristics
The mean (SD) CAS before therapy was 3.4 (2.2) and 0.5 (0.9) posttherapy (P < 0.01). The mean (SD) exophthalmometry measurements for each orbit (n = 46) were 21.2 mm (3.1) before therapy and 17.5 (2.3) following therapy (P < 0.01). Mean (SD) weight before therapy was 72 kg (15) and 70 kg (16) following therapy (P < 0.05) (Table 2).
Table 2.
Clinical Characteristics of Patients Pretherapy and Posttherapy
| Case | CASODPre | CAS OD Post | % Decrease | CAS OSPre | CAS OS Post | % Decrease | Hertel OD Pre | Hertel OD Post | % Decrease | Hertel OS Pre | Hertel OS Post | % Change | WeightPre(kg) | Weight Post (kg) | % Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 0 | 100 | 6 | 0 | 100 | 17 | 15 | 12 | 19 | 16 | 16 | 70.6 | 65.8 | −6.8 |
| 2 | 2 | 0 | 100 | 4 | 0 | 100 | 18 | 17 | 6 | 19 | 17 | 11 | 57.3 | 54.2 | −5.4 |
| 3 | 7 | 1 | 86 | 7 | 1 | 86 | 29 | 21 | 28 | 25 | 21 | 16 | 72.4 | 72.5 | +0.1 |
| 4 | 3 | 2 | 33 | 4 | 2 | 50 | 23 | 20 | 13 | 22 | 18 | 18 | 83.7 | 84.6 | +1.1 |
| 5 | 4 | 0 | 100 | 3 | 1 | 67 | 20 | 17 | 15 | 20 | 16 | 20 | 77.4 | 75.7 | −2.2 |
| 6 | 7 | 0 | 100 | 7 | 0 | 100 | 24 | 19 | 21 | 26 | 19 | 27 | 99.4 | 93.8 | −5.6 |
| 7 | 1 | 0 | 100 | 3 | 1 | 67 | 20 | 17 | 15 | 25 | 19 | 24 | 61.7 | 58.5 | −5.2 |
| 8 | 7 | 0 | 100 | 7 | 0 | 100 | 18 | 13 | 28 | 18 | 12 | 33 | 52.8 | 48.8 | −7.6 |
| 9 | 2 | 2 | 0 | 2 | 2 | 0 | 17 | 15 | 12 | 17 | 14 | 18 | 66.6 | 57.7 | −13.4 |
| 10 | 7 | 2 | 71 | 7 | 4 | 43 | 23 | 18 | 22 | 22 | 17 | 23 | 87.7 | 82 | −6.5 |
| 11 | 4 | 0 | 100 | 1 | 0 | 100 | 19 | 17 | 11 | 22 | 18 | 18 | 65.9 | 63.3 | −3.9 |
| 12 | 1 | 0 | 100 | 1 | 0 | 100 | 19 | 15 | 21 | 19 | 16 | 16 | 50.3 | 52.5 | +4.4 |
| 13 | 5 | 1 | 80 | 5 | 1 | 80 | 22 | 16 | 27 | 19 | 17 | 11 | 68.7 | 78.6 | +14.4 |
| 14 | 5 | 0 | 100 | 5 | 0 | 100 | 24 | 21 | 13 | 24 | 21 | 13 | 69.5 | 74.2 | +6.8 |
| 15 | 3 | 0 | 100 | 3 | 0 | 100 | 24 | 21 | 13 | 23 | 19 | 17 | 75.2 | 73 | −2.9 |
| 16 | 2 | 0 | 100 | 2 | 0 | 100 | 21 | 19 | 10 | 24 | 19 | 21 | 71.2 | 69.7 | −2.1 |
| 17 | 3 | 0 | 100 | 3 | 0 | 100 | 24 | 19 | 21 | 24 | 18 | 25 | 42.2 | 40.9 | −3.1 |
| 18 | 1 | 0 | 100 | 2 | 0 | 100 | 20 | 18 | 10 | 20 | 18 | 10 | 82.7 | 71.6 | −13.4 |
| 19 | 3 | 0 | 100 | 3 | 0 | 100 | 20 | 18 | 10 | 20 | 18 | 10 | 83.4 | 83.9 | +0.6 |
| 20 | 4 | 1 | 75 | 4 | 1 | 75 | 22 | 17 | 23 | 21 | 17 | 19 | 108.9 | 111.9 | +2.8 |
| 21 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 17 | 15 | 19 | 16 | 16 | 74.3 | 68.5 | −7.8 |
| 22 | 1 | 1 | 0 | 1 | 1 | 0 | 25 | 22 | 12 | 27 | 22 | 19 | 78.5 | 70 | −10.8 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 14 | 0 | 16 | 14 | 13 | 56.3 | 53.4 | −5.2 |
| Mean | 3.3 | 0.4 | 88 | 3.5 | 0.6 | 83 | 21 | 17.7 | 16 | 21.3 | 17.5 | 18 | 72 | 69.8 | −3.1 |
| SD | 2.3 | 0.7 | 70 | 2.3 | 1 | 57 | 3.3 | 2.4 | 27 | 3 | 2.3 | 23 | 15.4 | 15.8 | +2.6 |
Facial Volume Changes
Following teprotumumab therapy, the mean (SD) decrease in the upper face volume was 0.75 mL (0.84), whereas the mean (SD) change in the periorbital region was 1.8 mL (1.3). The mean (SD) decrease in volume in the temples was 0.17 mL (0.5), whereas the mean (SD) decrease in midface volume was 1.62 mL (3.16) and the mean (SD) decrease in the lower face volume was 2.67 mL (4.6). The mean (SD) decrease in the volume of the full face was 8.9 mL (8.7) (Table 3; Fig. 2). There was no significant correlation between the change in total body weight and changes in full face volume (P = 0.1). Furthermore, there was a significant correlation between decrease in periorbital fat and proptosis reduction (P < 0.01, R = −0.45).
Table 3.
Change in Volume (mLs) in Defined Facial Regions following Treatment with Teprotumumab
| Case | Upper Face (mL) |
Midface (mL) |
Lower Face (mL) |
Temple Right (mL) |
Temple Left (mL) |
R Orbit (mL) |
L Orbit (mL) |
Full Face (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | −1.7 | −7.9 | −7.2 | −0.2 | 0.5 | −2.6 | −3.4 | −22.5 |
| 2 | −0.5 | 2.6 | 4.2 | 0.0 | 0.1 | −0.4 | −2.6 | 3.5 |
| 3 | −1.2 | −0.2 | −1.6 | −0.3 | 0.0 | −2.2 | −2.0 | −7.4 |
| 4 | −2.1 | −2.3 | −0.2 | −0.2 | −0.1 | −4.0 | −2.1 | −11.0 |
| 5 | −0.5 | 3.3 | 5.5 | 0.1 | 0.0 | 0.4 | 0.3 | 9.1 |
| 6 | −0.1 | −5.6 | −0.4 | −0.3 | −1.1 | −4.0 | −4.7 | −16.1 |
| 7 | −0.9 | −0.8 | −5.0 | −0.3 | −1.3 | −3.0 | −0.1 | −11.3 |
| 8 | −2.6 | −4.5 | −5.0 | −0.5 | −0.2 | −4.4 | −3.3 | −20.5 |
| 9 | 0.0 | −2.5 | 0.7 | −0.4 | −0.2 | −1.7 | −1.7 | −5.7 |
| 10 | 0.0 | −8.0 | −12.4 | −0.9 | −0.6 | −3.7 | −2.2 | −27.9 |
| 11 | −0.5 | 1.1 | −1.4 | 1.2 | 1.5 | −0.8 | −2.5 | −1.5 |
| 12 | 0.7 | −0.7 | −4.1 | −0.3 | −0.2 | −1.1 | −0.7 | −6.3 |
| 13 | 0.2 | 4.2 | 4.1 | −0.2 | −0.2 | −2.3 | −2.0 | 3.9 |
| 14 | −0.4 | 1.3 | −5.5 | −0.1 | −0.1 | −2.8 | −2.8 | −10.3 |
| 15 | −0.4 | −1.5 | −8.1 | −0.1 | 0.0 | −1.0 | 0.8 | −10.5 |
| 16 | 0.0 | −2.1 | −1.8 | −0.1 | 0.0 | −1.1 | −1.0 | −6.1 |
| 17 | −0.7 | 1.7 | 0.1 | 0.0 | 0.0 | −1.7 | −1.4 | −2.1 |
| 18 | −1.9 | −3.8 | −8.4 | −0.9 | −0.2 | −1.2 | −1.4 | −17.8 |
| 19 | −0.9 | −2.8 | −3.5 | −0.5 | −0.4 | −0.6 | −0.5 | −9.0 |
| 20 | −1.6 | −2.3 | −5.6 | −0.1 | 0.0 | −3.2 | −1.3 | −14.2 |
| 21 | −0.3 | −2.6 | −7.4 | −0.3 | −0.1 | −1.2 | −1.1 | −13.0 |
| 22 | −1.5 | −1.8 | 3.1 | 0.0 | −0.4 | −1.9 | −1.7 | −4.3 |
| 23 | −0.2 | −2.2 | −1.8 | −0.5 | −0.5 | −0.7 | 1.0 | −4.8 |
| Mean | −0.7 | −1.6 | −2.7 | −0.2 | −0.2 | −2.0 | −1.6 | −8.9 |
| SD | 0.8 | 3.2 | 4.6 | 0.4 | 0.5 | 1.3 | 1.4 | 8.7 |
There was a significant correlation between the decrease in CAS and change in orbital volume (P < 0.01, R = −0.67). No correlation was found between a reduction in CAS and a change in full face volume (P = 0.12).
There was no significant correlation between the duration of TED before treatment and change in full face volume (P = 0.9). Four cases had TED for more than 36 months (mean 102 mo, SD 35). Within this subgroup, there was a mean (SD) reduction of 9.3 mL (6.8) volume across the full face. Finally, there was no significant relationship between the number of infusions received and a change in facial volume (P = 0.08).
Subgroup Analysis: The Impact of Prior Steroid Use
Seven patients had previously been treated with corticosteroids (one had oral corticosteroids and six had IV corticosteroids). None of the patients had used corticosteroids within 6 weeks of starting teprotumumab. The mean (SD) age of the corticosteroid group was 51 (19) and 48 (13) for the noncorticosteroid group (P = 0.9). The mean (SD) duration of TED was 36 months (31) for the steroid group and 26 (42) for the noncorticosteroid group (P = 0.6). The mean (SD) number of infusions received by the corticosteroid group was eight (0.5) and seven (1) for the noncorticosteroid group (P = 0.1). The mean (SD) CAS before therapy in the corticosteroid group was 3.6 (2.4) and 3.3 (2.2) in the noncorticosteroid group (0.7). The mean (SD) change in CAS was −3 (2.5) in the corticosteroid group and −2.8 (2) in the noncorticosteroid group (P = 0.6). The mean change in total body weight following treatment was 1.8 kg (5.3) for the corticosteroid group and 3.4 kg (3) for the noncorticosteroid group (P = 0.5).
The mean (SD) decrease in the volume of the full face for the corticosteroid group was 8.8 mL (6.7) and 9.8 mL (9.3) for the noncorticosteroid group (P = 0.5). There were no differences between the corticosteroid group and the noncorticosteroid group for changes in volume in the upper face, midface, lower face, temples, and the periorbital region (P = 0.7, 0.5, 0.4, 0.3, and 0.6, respectively).
For eyelid measurements, there were no differences for changes in MRD1, MRD2, and the intercanthal distance between the corticosteroid and noncorticosteroid groups (P = 0.3, 0.9, and 0.9, respectively).
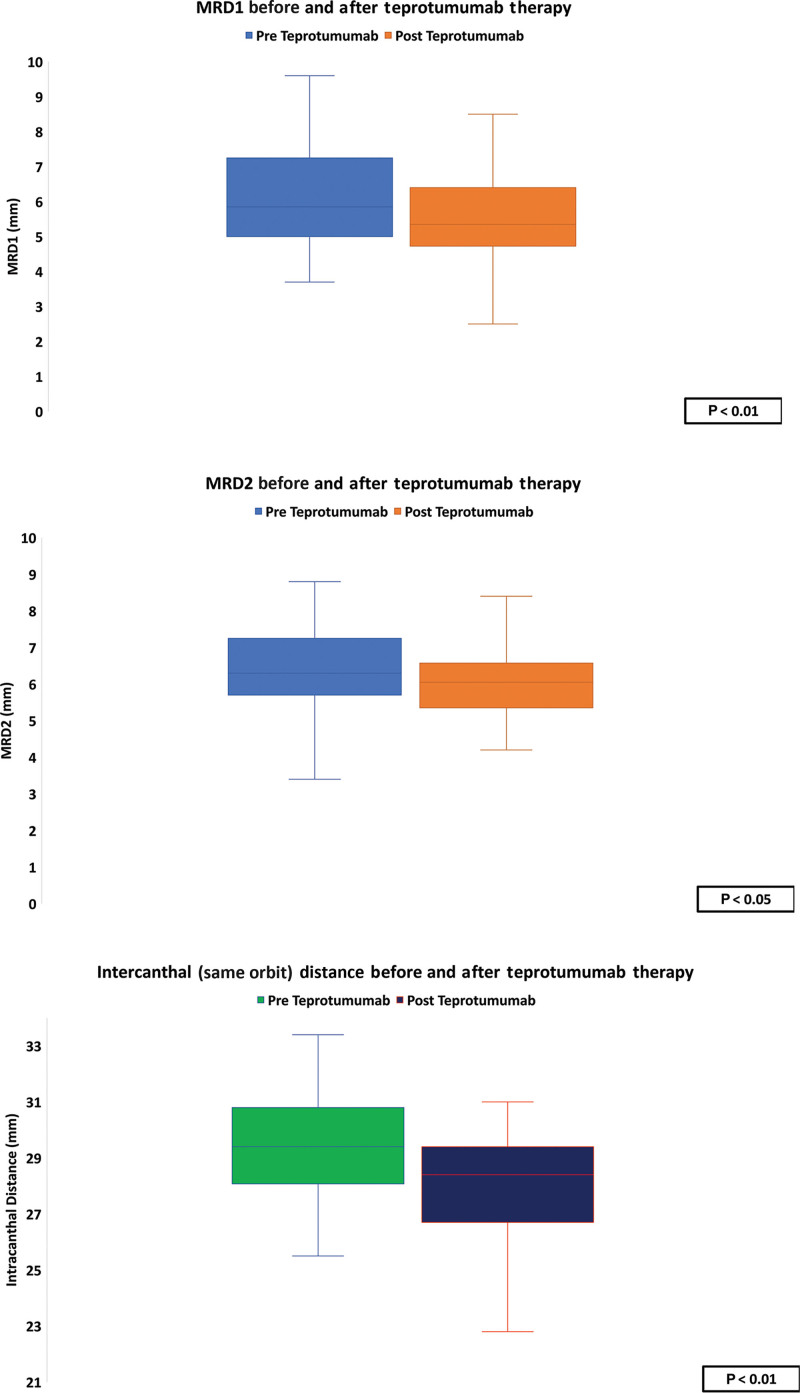
Eyelid Measurements
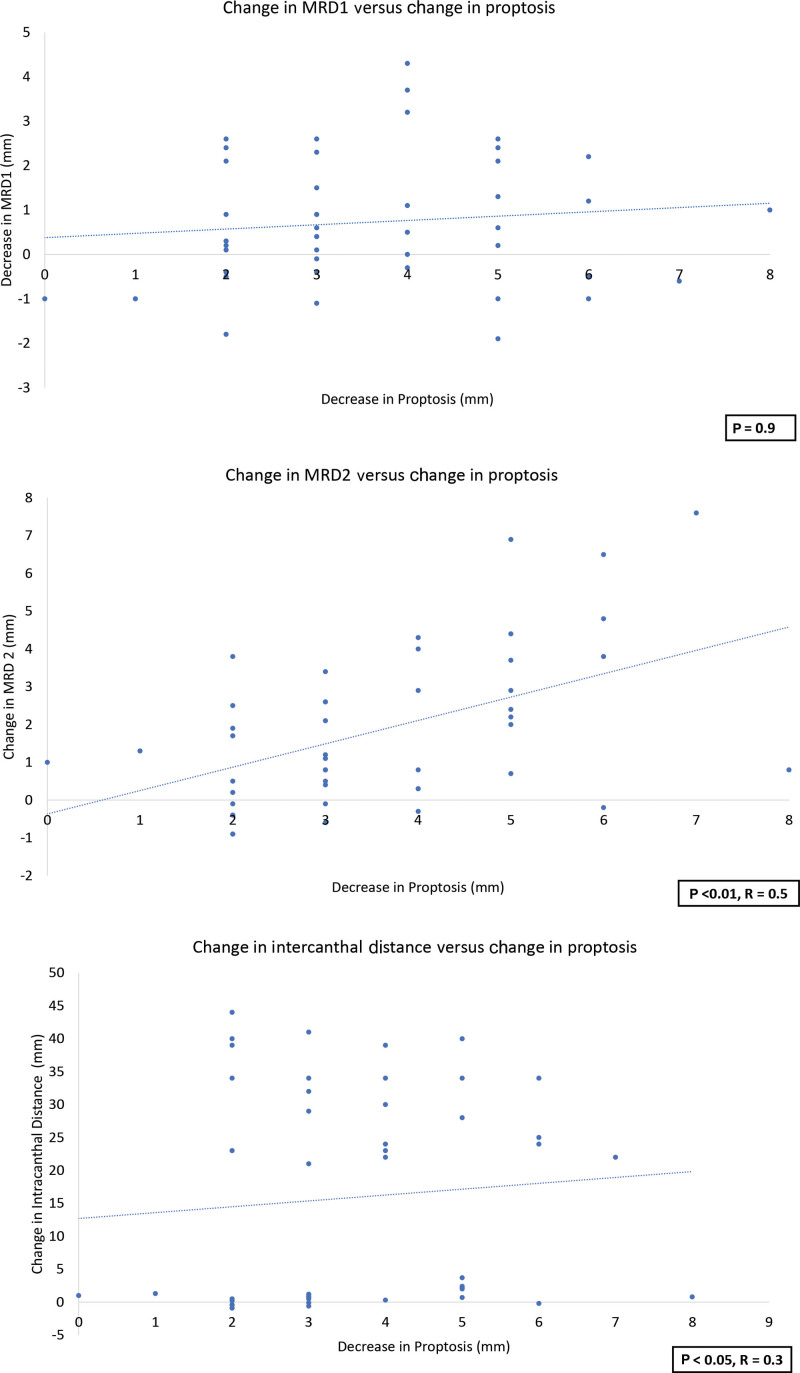
Before therapy, the mean (SD) MRD1 was 5 mm (2) and 4.3 mm (1.2) following therapy (P < 0.01). Mean (SD) MRD2 was 6.3 mm (1.3) before therapy and 5.9 mm (1.3) posttherapy (P < 0.05). The mean (SD) intercanthal distance was 29.5 (1.8) before therapy and 28 (1.8) following therapy (P < 0.01) (Table 4). Although there was no significant relationship between the change in MRD1 and proptosis following teprotumumab therapy (P = 0.9), there was a significant relationship between the change in proptosis and reduction in MRD2 (P < 0.01, R = 0.5). There was a significant correlation between the decrease in intercanthal distance and proptosis (P < 0.05, R = 0.3) (Figs. 3 and 4).
Table 4.
Eyelid Measurements Pre- and Post Teprotumumab Therapy
| Case | MRD1 OD(mm) | MRD1 OD (mm) | % Change | MRD1 OS (mm) | MRD1 OS (mm) | % Change | MRD2 OD (mm) | MRD2 OD (mm) | % Change | MRD2 OS (mm) | MRD2 OS (mm) | % Change | Intercanthal Distance OD (mm) | Intercanthal Distance OD (mm) | % Change | Intercanthal DistanceOS (mm) | Intercanthal Distance OS (mm) | % Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.6 | 2.8 | −50 | 6.7 | 5.4 | −19 | 4.5 | 4.5 | 0 | 3.4 | 5.6 | +65 | 29.1 | 25.1 | −14 | 31.5 | 26.7 | −15 |
| 2 | 4.5 | 5.4 | +20 | 4.4 | 4.9 | +11 | 5.2 | 4.5 | −13 | 5.4 | 4.4 | −19 | 27.9 | 26.4 | −5 | 28.3 | 26.6 | −6 |
| 3 | 4.7 | 5.3 | +13 | 6.7 | 3.5 | −48 | 7.4 | 6.6 | −11 | 7.7 | 7.2 | −6 | 33.4 | 30.6 | −8 | 31.7 | 30 | −5 |
| 4 | 7.4 | 3.4 | −54 | 5.3 | 3.3 | −38 | 6.1 | 6.2 | +2 | 7.7 | 4.3 | −44 | 32.2 | 27.3 | −15 | 30.8 | 29 | −6 |
| 5 | 2.1 | 3.2 | +52 | 2.8 | 2.5 | −11 | 4.9 | 5.4 | +10 | 4.9 | 4.6 | −6 | 28.3 | 28.8 | +2 | 28.9 | 26.5 | −8 |
| 6 | 4.2 | 4.5 | +7 | 3 | 3.8 | +27 | 8 | 5 | −38 | 7.3 | 5.3 | −27 | 30.8 | 24.8 | −19 | 29.2 | 26.7 | −9 |
| 7 | 3.4 | 4 | +18 | 5.6 | 4.6 | −18 | 6 | 5 | −17 | 6.8 | 6.4 | −6 | 31.1 | 29.2 | −6 | 32.4 | 30.3 | −6 |
| 8 | 7.8 | 6.4 | −18 | 7.8 | 6.8 | −13 | 4.2 | 2.8 | −33 | 5.5 | 2.6 | −53 | 27.8 | 22.8 | −18 | 27.3 | 24.2 | −11 |
| 9 | 6.5 | 5.6 | −14 | 6.3 | 5.8 | −8 | 5.1 | 6.9 | +35 | 5.6 | 5.3 | −5 | 25.5 | 26.6 | +4 | 27.1 | 28.3 | +4 |
| 10 | 9.4 | 5.5 | −41 | 6.8 | 5.7 | −16 | 6.2 | 3.7 | −40 | 6.2 | 4.2 | −32 | 31.1 | 29.1 | −6 | 29.4 | 26.6 | −10 |
| 11 | 5.6 | 5.3 | −5 | 8.2 | 7.1 | −13 | 6.1 | 6.5 | +7 | 6.9 | 6.4 | −7 | 30.5 | 29.7 | −3 | 30.8 | 29.2 | −5 |
| 12 | 3.9 | 3.4 | −13 | 6.3 | 4.4 | −30 | 7.7 | 7.3 | −5 | 6.8 | 7.5 | +10 | 28.3 | 29.5 | +4 | 29.1 | 29.3 | +1 |
| 13 | 2.5 | 3.5 | +40 | 3.7 | 3.3 | −11 | 6 | 6.4 | +7 | 5.9 | 6 | +2 | 31.2 | 29.4 | −6 | 30.2 | 28.5 | −6 |
| 14 | 5.9 | 4.2 | −29 | 6.3 | 3.2 | −49 | 6.9 | 7.4 | +7 | 5.4 | 6.2 | +15 | 30.7 | 29.9 | −3 | 32.3 | 30.2 | −7 |
| 15 | 2.4 | 2.9 | +21 | 3.3 | 3.5 | +6 | 5.7 | 6.9 | +21 | 5.9 | 5.9 | 0 | 26.6 | 28.8 | +8 | 27.1 | 28.7 | +6 |
| 16 | 2.2 | 2.8 | +27 | 2.3 | 2.9 | +26 | 6 | 7 | +17 | 7.7 | 7.5 | −3 | 27.5 | 27.9 | +1 | 29.4 | 27.7 | −6 |
| 17 | 4 | 3.3 | −18 | 4.4 | 4.8 | +9 | 9.4 | 8.8 | −6 | 8.4 | 6.7 | −20 | 28.1 | 27 | −4 | 28.8 | 28.2 | −2 |
| 18 | 4.7 | 3.9 | −17 | 4.8 | 4.6 | −4 | 5.2 | 5.6 | +8 | 4.2 | 5 | +19 | 27.7 | 26.8 | −3 | 25.8 | 26.9 | +4 |
| 19 | 2.6 | 2.5 | −4 | 3.3 | 2.8 | −15 | 6.4 | 5.9 | −8 | 6.3 | 6.5 | +3 | 30.5 | 29.9 | −2 | 30.8 | 30.5 | −1 |
| 20 | 7.6 | 5.3 | −30 | 9.8 | 6.1 | −38 | 8.5 | 4.9 | −42 | 8 | 5.5 | −31 | 32.5 | 28.6 | −12 | 30.8 | 26.3 | −15 |
| 21 | 4.8 | 5 | +4 | 3 | 2.3 | −23 | 7.9 | 7.3 | −8 | 8 | 8.2 | +2 | 30 | 31 | +3 | 29.2 | 29.4 | +1 |
| 22 | 4 | 4.8 | +20 | 4.3 | 3.6 | −16 | 4.7 | 5.9 | +26 | 5.8 | 6 | +3 | 29.6 | 28.6 | −3 | 28.3 | 28 | −1 |
| 23 | 3.3 | 3.9 | +18 | 4.4 | 3.8 | −14 | 6.5 | 6.1 | −6 | 6 | 5.9 | −2 | 29.8 | 27.9 | −6 | 28 | 28.3 | +1 |
| Mean | 4.7 | 4.2 | −2.3 | 5.2 | 4.3 | −13.3 | 6.3 | 5.9 | −3.9 | 6.3 | 5.8 | −6.2 | 29.6 | 28.1 | −4.8 | 29.4 | 28.1 | −4.4 |
| SD | 2.0 | 1.1 | 28.0 | 2.0 | 1.4 | 20.1 | 1.4 | 1.4 | 20.6 | 1.3 | 1.3 | 23.8 | 2.0 | 2.0 | 7.3 | 1.8 | 1.6 | 5.7 |
Intercanthal distance is the distance between the medial and lateral canthus of the same orbit.
Fig. 3.
Changes to MRD1, MRD2, and the intercanthal space before and after teprotumumab therapy.
Fig. 4.
The relationship between proptosis and MRD1, MRD2, and the intercanthal distance.
Patient Self-assessment of Cosmetic Results
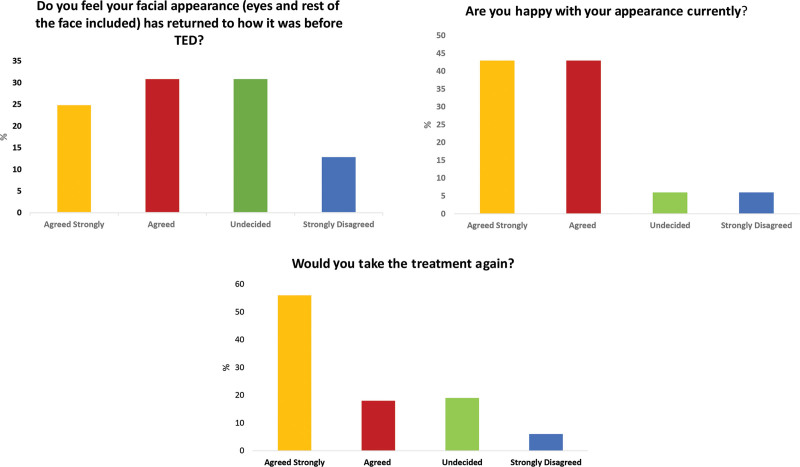
When asked “do you feel your facial appearance (eyes and rest of the face included) has gone back to how it was before TED?,” 25% strongly agreed, 31% agreed, 31% were undecided, and 13% strongly disagreed.
When asked “are you happy with your appearance currently?”, 43% strongly agreed, 43% agreed, 6% were undecided, and 6% strongly disagreed.
When asked “would you take the treatment again?” 56% strongly agreed 18% agreed, 19% were undecided, and 6% strongly disagreed (Fig. 5).
Fig. 5.
Responses to the patient satisfaction questionnaire.
Intraobserver and Interobserver Variability
Mean intraobserver variability calculations expressed in percentages were 0.7% for the upper face, 0.9% for the temples, 1.9% for the periorbital regions, 1.7% for the midface, and 2.1% for the lower face. For eyelid measurements, intraobserver variability was 0.2% for the MRD1, 0.3% for the MRD2, and 0.5% for the intercanthal distance.
Interobserver Variability
Interobserver variability revealed a strong correlation between two observers for all measurements (Table 5).
Table 5.
Intraclass Correlation Coefficient
| Measurement | Intraclass Correlation Coefficient |
|---|---|
| Upper Face | 0.97 |
| Periorbital Region | 0.94 |
| Temples | 0.98 |
| Midface | 0.96 |
| Lower Face | 0.95 |
1 signals perfect agreement; 0 signals no agreement.
Descriptive Case
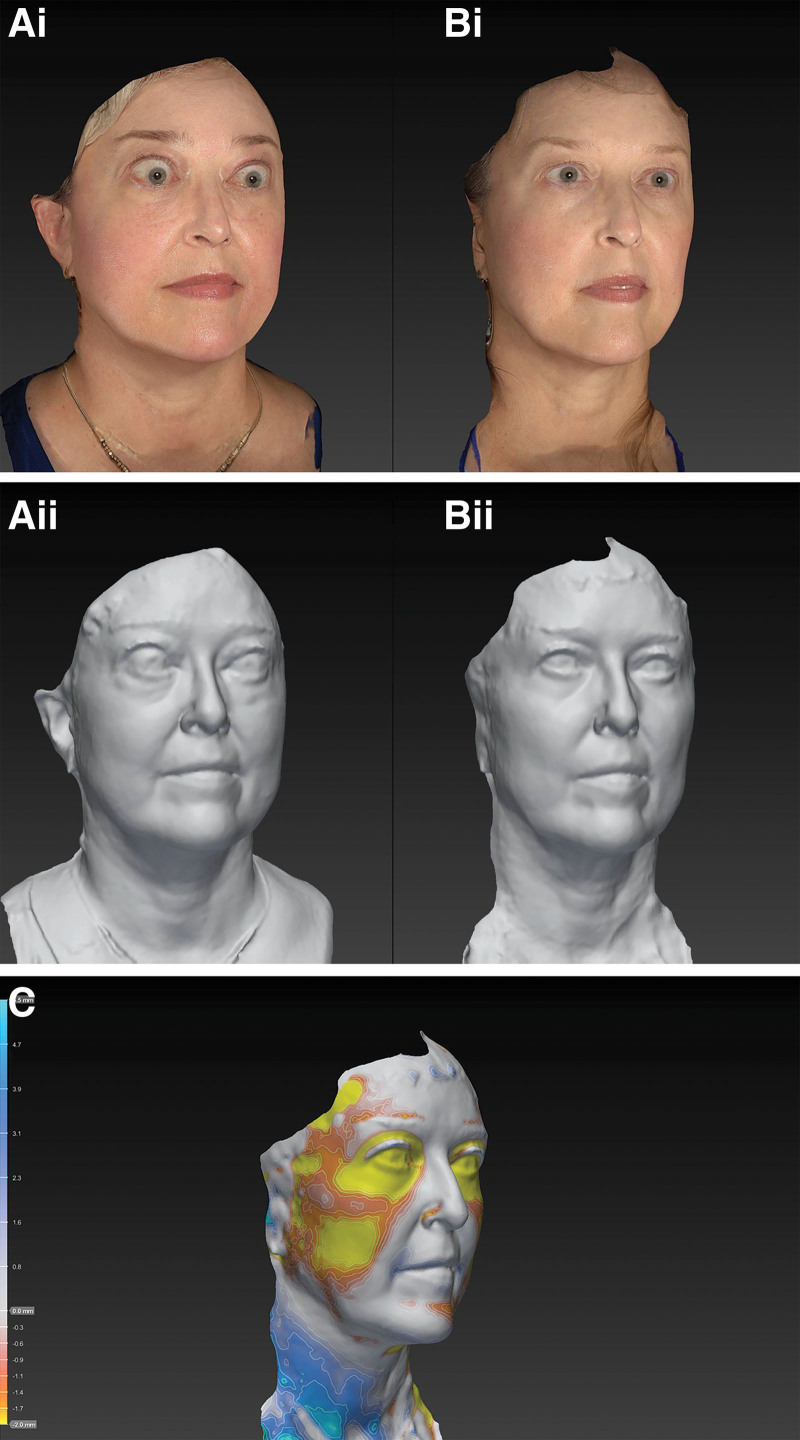
Case 10 is a 59-year-old white woman who presented with a 10-month history of TED. On presentation, she was euthyroid. She had a CAS of 7 OU, with proptosis measurements of 23 mm OD and 22 mm OS. She received six infusions of teprotumumab and following therapy, her CAS reduced to 2 OD and 3 OS. Her proptosis reduced to 18 mm OD and 17 mm OS. Her total body weight reduced from 87 kg before therapy to 82 kg following treatment. The volume across her full face was reduced by 28 mL (Fig. 6).
Fig. 6.
Facial volumetric changes following treatment with Teprotumumab. Ai and Aii, Pretreatment. Bi and Bii, Posttreatment. C, topographical representation of facial volume loss across the face (only regions with the greatest volume loss depicted).
DISCUSSION
There is a growing body of evidence that suggests that the effects of thyroid eye disease on the face are not restricted to the periorbital region. Recent work has demonstrated enlargement of the eyebrow fat and lateral subbrow region3 in patients with TED. A subsequent study revealed an increased expression of the IGF-1R and TSHR within the increased brow fat compartment of patients with TED.1 The same group later found an increase in the temporal fat pads of patients with TED. In summation, these findings suggest that TED may affect other regions of the face outside of the periorbital region.
Teprotumumab, a monoclonal immunoglobulin G1 to IGF-1R, has been found to significantly reduce proptosis through the reduction of orbital soft-tissue volume. Given its inhibition of the downstream processes associated with the overexpressed IGF-1R/TSHR complex, it provides a unique opportunity to study the impact of this pathway on soft-tissue expansion outside of the periorbital region on the face.
In the present study, patients with confirmed TED were treated with teprotumumab. There was a significant reduction in average body weight (mean 2.4 kg, SD 4.7) within the course of a 24-week period. During this time, there was a significant reduction of volume across the full face (mean 8.9 mL, SD 8.7). On closer inspection, the greatest volume decrease was seen over the lower third of the face, followed by the periorbital region and subsequently the midface. The bulk of volume change in the midface and lower face was found in regions that are likely to correspond to the positions of the parotid gland and the buccal fat pad. Furthermore, the decrease in pan facial volume did not correlate with change in body weight, the use of corticosteroids, or duration of TED.
Recent work has shown that the overexpression of the IGF-1R on OFs persists into the chronic stages of TED.22 It is possible that this overexpression may also be present within the extraorbital tissues of patients with chronic TED. Though further work will be required to elucidate this, the present study adds further evidence to the concept that teprotumumab may have efficacy even in patients with chronic TED.
Volume decreases in the periorbital region correlated significantly with reduction in proptosis, supporting the relationship between orbital fat and proptosis. The position of the upper and lower eyelids decreased with teprotumumab therapy, reflected by a decrease in MRD1, MRD2, and the intercanthal distance. It is tempting to relate this to a decrease in orbital volume; however, although there was a significant relationship between the MRD2, intercanthal distance, and proptosis, there was no significant relationship between MRD1 and proptosis. In TED, the upper eyelid may present with retraction or ptosis. The position of the upper eyelid in this context is likely multifactorial and may be related to increases in sympathetic tone in Müller’s muscle, enlargement of levator muscle fibers and contracture/fibrosis of the septum, and anterior lamella.23 Therefore, a linear relationship between the upper eyelid position and proptosis is unlikely.
Patients appraised their own appearance following teprotumumab. The results suggest that the facial changes were positive and that 86% were satisfied with their results, 56% felt their appearance had gone back to their pre-TED status and 74% suggested they would be happy to undergo repeat teprotumumab therapy if required.
The most significant limitations to this study pertain to the number of patients included and the identification of landmarks used to divide regions of the face. The purpose of this article was to review extraorbital changes to the entire face. To that end, the results showed gestalt facial changes following teprotumumab therapy. We divided the face into regions to evaluate where the bulk of the volume changes occur. Though it is accepted that the landmarks used to divide the face are difficult to reliably define between patients, this does not detract from the overall message of this study, in stating that teprotumumab reduces facial soft-tissue expansion caused by TED.
Furthermore, we used 3D volumetric analysis to detect a change in volume between the pretreatment and posttreatment states. The reliability and accuracy of the 3D Vectra system has previously been demonstrated.16,24 In our study, two of the authors were specifically trained by Canfield Sciences on the analysis of 3D images for volumetric analysis. The interobserver and intraobserver variability was low for all measurements. The strength of the study pertains to its prospective longitudinal nature.
Teprotumumab has previously been shown to reduce soft-tissue expansion within the orbit.14 The present study is the first to show a similar, significant reduction of soft tissue in the extraorbital regions of the face. Given the significant impact of disfiguring facial changes on the mental health of patients with TED,25 the potential role of teprotumumab in this group of patients becomes more apparent.
ACKNOWLEDGMENT
The authors thank Val Lambros for kindly providing guidance on facial analysis using the Vectra imaging system.
PATIENT CONSENT
The patient provided written consent for the use of her image.
Footnotes
Published online 15 September 2021.
Disclosure: R.S.D. is a consultant with Horizon Therapeutics and Immunovant. The other authors have no financial interest to declare. This investigator initiated study was funded by Horizon Therapeutics.
REFERENCES
- 1.Hwang CJ, Khadavi NM, Papageorgiou K, et al. Histopathology of brow fat in thyroid-associated orbitopathy. Ophthalmic Plast Reconstr Surg. 2012;28:27–29. [DOI] [PubMed] [Google Scholar]
- 2.Savar LM, Menghani RM, Chong KK, et al. Eyebrow tissue expansion: an underappreciated entity in thyroid-associated orbitopathy. Arch Ophthalmol. 2012;130:1566–1569. [DOI] [PubMed] [Google Scholar]
- 3.Papageorgiou KI, Hwang CJ, Chang SH, et al. Thyroid-associated periorbitopathy: eyebrow fat and soft tissue expansion in patients with thyroid-associated orbitopathy. Arch Ophthalmol. 2012;130:319–328. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary MM, Zhang KR, Johnson S, et al. Temporal fat pad volume in patients with thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2020;36:194–197. [DOI] [PubMed] [Google Scholar]
- 5.Bokman CL, Ugradar S, Rootman DB. Measurement of medial wall bowing and clinical associations in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34:557–559. [DOI] [PubMed] [Google Scholar]
- 6.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62:199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger CC, Neumann S, Place RF, et al. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves’ disease immunoglobins. J Clin Endocrinol Metab. 2015;100:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–5080. [DOI] [PubMed] [Google Scholar]
- 9.Khong JJ, McNab AA, Ebeling PR, et al. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016;100:142–150. [DOI] [PubMed] [Google Scholar]
- 10.Markham A. Teprotumumab: first approval. Drugs. 2020;80:509–512. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Mester T, Raychaudhuri N, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99:E1635–E1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–352. [DOI] [PubMed] [Google Scholar]
- 14.Jain AP, Gellada N, Ugradar S, et al. Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease. Br J Ophthalmol. 2020;137:4611–4618. [DOI] [PubMed] [Google Scholar]
- 15.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camison L, Bykowski M, Lee WW, et al. Validation of the Vectra H1 portable three-dimensional photogrammetry system for facial imaging. Int J Oral Maxillofac Surg. 2018;47:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Menezes M, Rosati R, Ferrario VF, et al. Accuracy and reproducibility of a 3-dimensional stereophotogrammetric imaging system. J Oral Maxillofac Surg. 2010;68:2129–2135. [DOI] [PubMed] [Google Scholar]
- 18.Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4–S9. [DOI] [PubMed] [Google Scholar]
- 19.Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219–2227. [DOI] [PubMed] [Google Scholar]
- 20.Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ugradar S, Wang Y, Mester T, et al. Improvement of asymmetric thyroid eye disease with teprotumumab. Br J Ophthalmol. 2021:bjophthalmol-2020-318314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugradar S, Shi L, Wang Y, et al. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye. 2020;42:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grove AS, Jr. Upper eyelid retraction and Graves’ disease. Ophthalmology. 1981;88:499–506. [DOI] [PubMed] [Google Scholar]
- 24.Hyer JN, Murta F, Juniat VAR, et al. Validating three-dimensional imaging for volumetric assessment of periorbital soft tissue. Orbit. 2021;40:9–17. [DOI] [PubMed] [Google Scholar]
- 25.Farid M, Roch-Levecq AC, Levi L, et al. Psychological disturbance in graves ophthalmopathy. Arch Ophthalmol. 2005;123:491–496. [DOI] [PubMed] [Google Scholar]