Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, albumin, critical illness, hypoalbuminemia, low serum albumin
OBJECTIVES:
We hypothesized that low serum albumin would contribute to pulmonary edema formation, thereby independently increasing the risk of developing acute respiratory distress syndrome in critically ill patients.
DESIGN:
Retrospective analysis of prospective cohort.
SETTING:
Medical, surgical, and cardiovascular ICUs at Vanderbilt University Medical Center.
PATIENTS:
Patients (n = 993) with serum albumin measured for clinical reasons within 24 hours of study enrollment on ICU day 2 were included.
MEASUREMENTS AND MAIN RESULTS:
The primary outcome was presence of acute respiratory distress syndrome at any time during the first 4 days in the ICU, as defined by the Berlin definition. Secondary outcomes included ventilator-free days and ICU length of stay. In an unadjusted analysis, lower serum albumin levels were associated with a higher occurrence rate of acute respiratory distress syndrome (p < 0.001). In a multivariable analysis controlling for prespecified confounders, lower serum albumin was independently associated with an increased risk of acute respiratory distress syndrome (odds ratio, 1.48 per 1-g/dL decrease in albumin; 95% CI, 1.14–1.94; p = 0.004). Additionally, lower serum albumin was associated with increased mortality (odds ratio, 1.56 per 1-g/dL decrease in albumin; 95% CI, 1.19–2.04; p = 0.001), increased ICU length of stay (incidence rate ratio, 1.19; 95% CI, 1.15–1.23; p < 0.001), higher Sequential Organ Failure Assessment score (p < 0.001), and fewer ventilator-free days (incidence rate ratio, 1.21; 95% CI, 1.19–1.24; p < 0.001).
CONCLUSIONS:
Among adult ICU patients, lower serum albumin was independently associated with increased risk of acute respiratory distress syndrome after controlling for severity of illness and potential confounders. These findings support the hypothesis that low plasma oncotic pressure contributes to pulmonary edema formation in patients at risk for acute respiratory distress syndrome, independent of severity of illness.
The acute respiratory distress syndrome (ARDS) is an inflammatory lung condition that is characterized by increased permeability of the alveolar capillary barrier, resulting in exudation of protein-rich edema fluid into the lung interstitium and airspace (1). Multiple mechanisms contribute to maintaining a dry airspace including high plasma oncotic pressure, low pulmonary microvascular pressure, and relatively impermeable lung endothelial and epithelial barriers. In addition, filtered fluid can be cleared from the airspace by alveolar epithelial ion transport and from the lung interstitium through lymphatic drainage. In the setting of ARDS, disruption of the alveolar capillary barrier and impairment of alveolar fluid clearance (2) lead to accumulation of protein-rich pulmonary edema in the interstitium and alveolar compartment. Edema formation is further compounded in some patients by increased hydrostatic pressure in the lung microvasculature.
Serum albumin accounts for roughly 80% of the colloid oncotic pressure in the circulation (3). Reduced levels of serum albumin (hypoalbuminemia) and the resultant reduced colloid osmotic pressure are key features of critical illness that could contribute to pulmonary edema formation by decreasing the oncotic forces that favor retention of fluid in the microvasculature. The relationship between low serum albumin and ARDS has been well established in the literature (4–10) and is incorporated as an element of the Lung Injury Prediction Score (11). However, the nature of the relationship between ARDS and serum albumin has not been well characterized. We, therefore, hypothesized that lower levels of serum albumin would be independently associated with increased incidence of ARDS among critically ill patients at risk for ARDS. Furthermore, we theorized that the relationship between ARDS and serum albumin would be dose-dependent, with increasingly lower levels of serum albumin being associated with increasingly higher incidence of ARDS.
MATERIALS AND METHODS
Patient Selection and Study Design
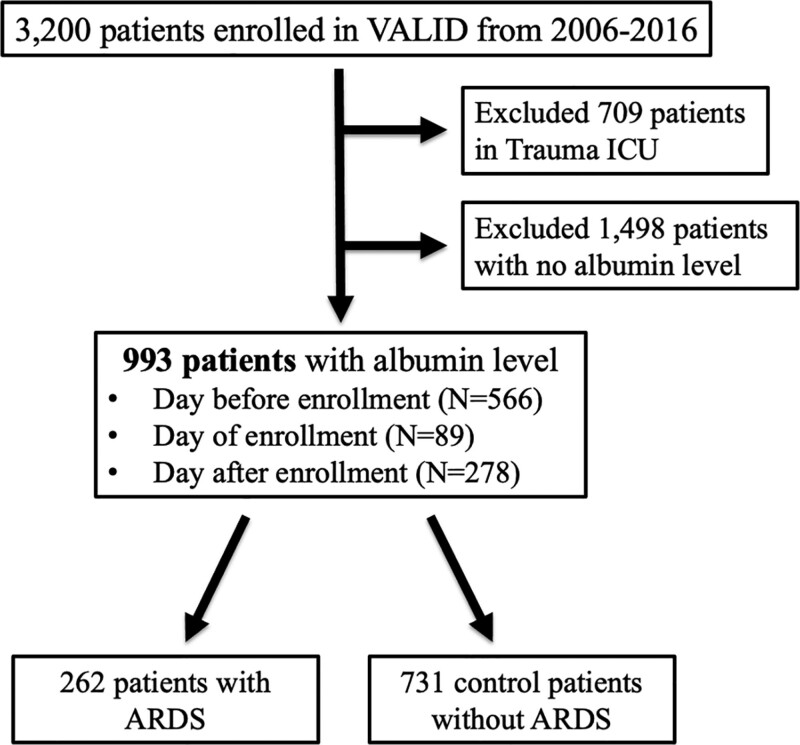
This was a secondary analysis of critically ill patients (age ≥ 18 years) who were enrolled in the Validating Acute Lung Injury markers for Diagnosis (VALID) study (12) from 2006 to 2016. VALID is a single-center prospective observational cohort study, which has enrolled critically ill patients admitted to the Medical, Surgical, Trauma, and Cardiovascular ICUs at Vanderbilt University Medical Center from 2006 to present day. Inclusion criteria for the VALID study are in the Supplemental Information (http://links.lww.com/CCX/A793). The study protocol was approved by the Vanderbilt Institutional Review Board (051065), and patients or their surrogates provided informed consent. A waiver of informed consent was also approved for this minimal risk study in the event that the patient was unable to consent and no surrogate was available. Inclusion and exclusion criteria for VALID have been previously described (13). For the current study, additional exclusions included admission to the Trauma ICU (due to a high percentage of missing albumin data in trauma patients), lack of a serum albumin measured within 1 day before or after enrollment, or failure to remain in any ICU for at least 2 days (Fig. 1).
Figure 1.
Flowchart for inclusion of patients in the current study who were enrolled in VALID during the study period.
Data Collection and Definitions
Patients were enrolled on the morning of ICU day 2. At the time of enrollment, clinical data including demographics, medical history, prehospital medications, admission diagnoses, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) (14) were collected. Laboratory values, hemodynamic variables, ventilator settings, inhospital medications, fluid intake and output, ARDS according to the Berlin definition (by two physician investigator reviews) (15), and evidence of organ failures according to Sequential Organ Failure Assessment scores (SOFA) (16) were recorded daily for the first 4 ICU days. All study patients had daily chest radiographs for evaluation. Clinical outcomes including duration of mechanical ventilation, ventilator-free days (days alive and free of mechanical ventilation over the 28 days after ICU admission), ICU length of stay (LOS), and inhospital mortality were also recorded.
Presence of sepsis (17), pneumonia, and chronic liver disease were determined by systematic chart review. Cumulative fluid balances were calculated as the net positive or negative fluid intake in liters in the 72 hours following ICU admission. Baseline estimated glomerular filtration rate (eGFR) was calculated from creatinine and demographic data (18). Time-stamped clinical laboratory serum albumin levels within 1 day of study enrollment were extracted retrospectively from the electronic medical record. The albumin value for each patient was selected from the earliest qualifying day with available data; if more than one albumin value was measured on that day, the lowest value was selected.
Statistical Analysis
The primary independent variable was the lowest, earliest available serum albumin level measured within 1 day before or after enrollment in VALID. The primary dependent variable was presence of ARDS on at least 2 consecutive ICU days during the first 4 days in the ICU. Patients who had ARDS on only 1 day or only on nonconsecutive days were included in the control group. To control for potentially confounding variables, we performed a multivariable logistic regression analysis with inclusion of age, gender, APACHE II score, presence of sepsis, chronic liver disease, vasopressor use, baseline eGFR, and positive cumulative fluid balance in the first 72 hours in the ICU. For some analyses, serum albumin levels were expressed as quintiles to examine threshold effects. A Mann-Whitney U test was used to compare the lowest serum albumin level in patients who did and did not develop ARDS. For comparison of incidence of ARDS and mortality by quintile of albumin, we used a chi-square test with linear-by-linear association. A univariate Poisson regression was used to examine the association between lower serum albumin and ICU LOS and ventilator-free days. We used Fisher exact tests to examine the associations between categorical variables and ARDS incidence and Mann-Whitney U tests to examine associations between ARDS incidence and continuous variables in Table 1. We also conducted three sensitivity analyses. The first sensitivity analysis excluded all patients without a documented risk factor for ARDS (n = 156 excluded). For the second sensitivity analysis, patients with albumin values from the day before or of enrollment were included, but those with albumin measured the day after enrollment were excluded (n = 153 excluded). The third analysis analyzed only patients without chronic liver disease (n = 195 excluded) due to possibility for reduced albumin synthesis in patients with cirrhosis (19). All statistical analyses were performed using the R Version 3.3.0 software (R Foundation for Statistical Computing, Vienna, Austria) (20). A p value of less than 0.05 was considered statistically significant.
TABLE 1.
Characteristics of 993 Patients From the Medical, Surgical, and Cardiovascular ICU With Serum Albumin Measured Within 1 Day of Enrollment
| Characteristics | No ARDS (n = 731) | ARDS (n = 262) | Overall (n = 993) | p |
|---|---|---|---|---|
| Age, yr, median (IQR) | 56 (45–67) | 56 (47–65) | 56 (47–56) | 0.868 |
| Male sex, n (%) | 394 (54) | 135 (52) | 529 (53) | 0.517 |
| Albumin, g/dL, median (IQR) | 2.7 (2.3–3.2) | 2.5 (2.2–3.0) | 2.8 (2.3–3.1) | < 0.001 |
| ICU location, n (%) | ||||
| Surgical | 79 (11) | 33 (13) | 112 (11) | 0.053 |
| Medical | 622 (85) | 226 (86) | 848 (85) | |
| Cardiovascular | 30 (4) | 3 (1) | 33 (3) | |
| Risk factors for ARDS at enrollment, n (%) | ||||
| Nonpulmonary sepsis | 283 (39) | 108 (41) | 391 (39) | < 0.001 |
| Pneumonia | 98 (13) | 78 (30) | 176 (18) | |
| Multiple transfusions | 93 (13) | 11 (4) | 104 (10) | |
| Aspiration | 44 (6) | 48 (18) | 92 (9) | |
| Pancreatitis | 20 (3) | 2 (1) | 22 (2) | |
| Drug overdose | 32 (4) | 1 (0) | 33 (3) | |
| Other | 8 (1) | 11 (4) | 19 (2) | |
| None | 116 (16) | 2 (1) | 118 (12) | |
| Not available/missing | 37 (5) | 1 (0) | 38 (4) | |
| Acute Physiology and Chronic Health Evaluation Score II score, median (IQR) | 26 (21–32) | 30 (25–36) | 27 (22–33) | < 0.001 |
| 72-hr cumulative fluid balance liters, median (IQR) | +3.0 (0.0–6.1) | +4.1 (1.2–7.7) | +3.2 (0.5–6.6) | 0.001 |
| Inhospital AKI by Kidney Disease Improving Global Outcomes stage, n (%) | ||||
| No AKI (stage 0) | 138 (19) | 24 (9) | 162 (16) | < 0.001 |
| AKI present (stages 1–3) | 584 (80) | 235 (89) | 819 (82) | |
| Insufficient data | 9 (1) | 3 (1) | 12 (1) | |
| Baseline estimated glomerular filtration rate, mL/min/1.73 m2, median (IQR) | 75 (45–103) | 82 (55–108) | 77 (48–105) | 0.013 |
| Inhospital death, n (%) | 163 (22) | 107 (42) | 270 (27) | < 0.001 |
| Days in ICU, median (IQR) | 5 (3–9) | 8 (5–13) | 5 (3–10) | < 0.001 |
| Days on ventilator, median (IQR) | 2 (0–5) | 5 (3–9) | 3 (0–6) | < 0.001 |
| Ventilator-free days, median (IQR) | 25 (11–28) | 13 (0–23) | 23 (5–27) | < 0.001 |
AKI = acute kidney injury, ARDS = acute respiratory distress syndrome, IQR = interquartile range.
p values compare ARDS with non-ARDS group with Fisher exact test for categorical variables and Mann-Whitney U test for continuous variables. Bold font indicates significant p values (p < 0.05).
RESULTS
Patient Characteristics
There were 993 critically ill patients with serum albumin measured included in the study (Fig. 1). The majority of patients (n = 552, 56%) had serum albumin measured on the day prior to enrollment (ICU day 1). The remainder had serum albumin measured on the day of enrollment (n = 331, 33%) or the day following enrollment (n = 216, 22%). Demographic data and clinical outcomes data are listed in Table 1. Among these 993 patients, 835 (84%) had at least one risk factor for ARDS including 391 (39%) with nonpulmonary sepsis, 176 (18%) with pneumonia, and 251 (25%) with other risk factors (Supplementary Table 3, http://links.lww.com/CCX/A773). A full list of “other” risk factors in the VALID study may be found in the Supplementary Information (http://links.lww.com/CCX/A793). Overall, 262 (26%) of the 993 patients had or developed ARDS. Only two patients (1.3%) without a risk factor for ARDS developed ARDS. To determine whether patients who had serum albumin measurements available were systematically different from patients who did not, we compared their clinical characteristics (Supplementary Table 3, http://links.lww.com/CCX/A773). The group without a serum albumin measurement was more likely to be in the surgical ICU and was less likely to have a risk factor for ARDS. In addition, this group had a lower severity of illness and better clinical outcomes. Among patients with a measured serum albumin, higher 72-hour cumulative fluid balance was associated with lower serum albumin levels by quintile (Supplementary Fig. 1, http://links.lww.com/CCX/A767). For this reason, we chose to control for fluid balance in our multivariable models.
Albumin Levels and Risk for ARDS
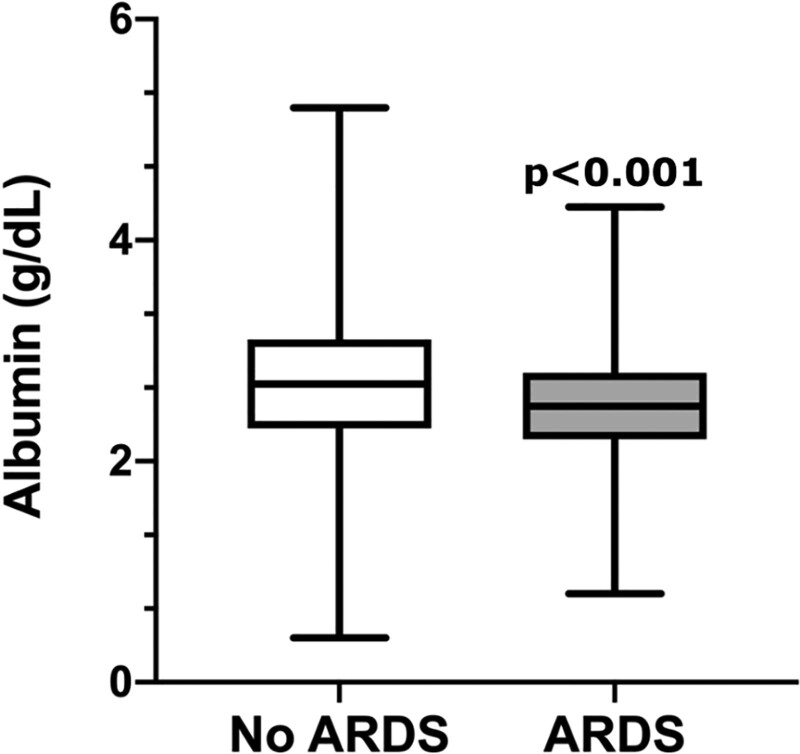
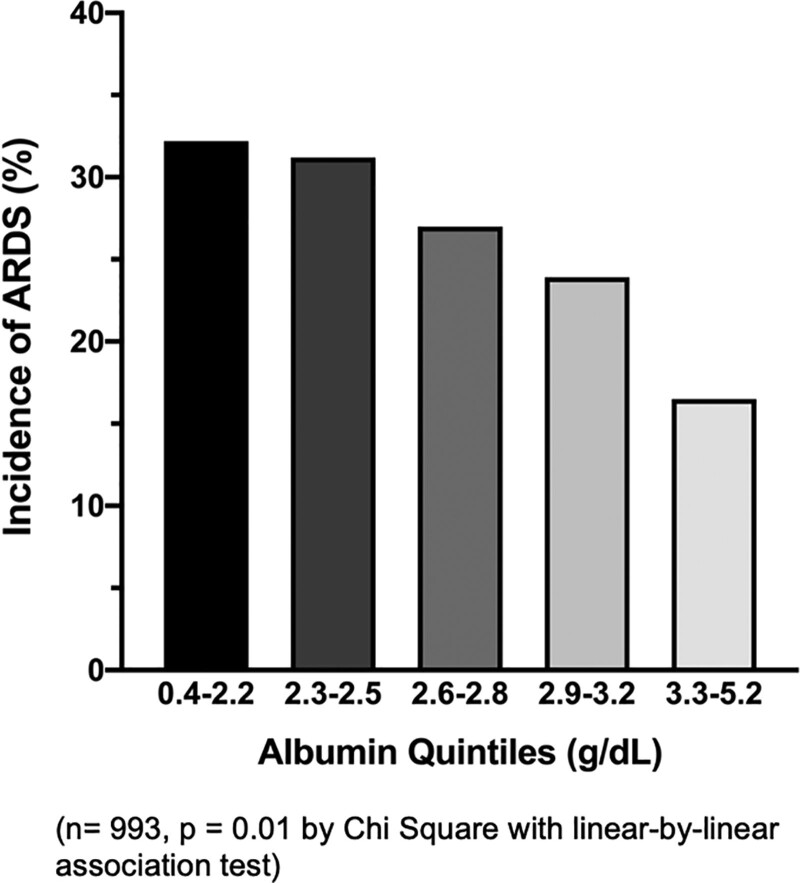
The median serum albumin level was 2.7 g/dL (interquartile range, 2.3–3.1). In an unadjusted analysis, patients who developed ARDS had significantly lower serum albumin levels than those without ARDS (Fig. 2). In a multivariable logistic regression analysis controlling for prespecified confounders (age, gender, APACHE II score, presence of sepsis, chronic liver disease, vasopressor use, baseline eGFR, and positive fluid balance), lower serum albumin remained independently associated with increased risk of ARDS (odds ratio [OR], 1.48 per 1-g/dL decrease in albumin; 95% CI, 1.14–1.93; p = 0.004) (Table 2). When examined by quintile, patients with the lowest albumin levels had the highest incidence of ARDS, with significantly decreasing incidence of ARDS as albumin level increased (p = 0.01) (Fig. 3). This relationship appears linear in nature. When 158 patients without a risk factor for ARDS were excluded from the regression analysis, lower serum albumin was similarly associated with higher incidence of ARDS (OR, 1.38 per 1-g/dL decrease in albumin; 95% CI, 1.06–1.80; p = 0.016) (Supplementary Table 1, http://links.lww.com/CCX/A771).
Figure 2.
Critically ill patients with ARDS had lower levels of serum albumin compared with critically ill patients without ARDS (non-ARDS n = 708, ARDS n = 285, p < 0.001 by Mann-Whitney U test; data represented as median [line], IQR [box], and minimum and maximum values [whiskers]).
TABLE 2.
Logistic Regression of Incidence of Acute Respiratory Distress Syndrome and Minimum Serum Albumin Levels Within 1 Day of Enrollment Controlling for Covariates
| Incidence of Acute Respiratory Distress Syndrome | OR (95% CI) | p |
|---|---|---|
| Variable | ||
| Age (per year) | 0.999 (0.987–1.011) | 0.910 |
| Male sex | 0.789 (0.571–1.089) | 0.149 |
| Acute Physiology and Chronic Health Evaluation II (per 1 point increase) | 1.071 (1.048–1.096) | < 0.001 |
| Sepsis | 3.399 (2.271–5.207) | < 0.001 |
| Chronic liver disease | 0.856 (0.550–1.311) | 0.480 |
| Vasopressor use | 0.774 (0.546–1.091) | 0.146 |
| Baseline eGFR (per mL/min/1.73 m2) | 1.007 (1.002–1.012) | 0.004 |
| Positive 72-hr fluid balance | 1.126 (0.743–1.732) | 0.580 |
| Serum albumin (per 1 g/dL decrease) | 1.48 (1.138–1.934) | 0.004 |
OR = odds ratio.
OR per 1 g/dL decrease in serum albumin. Bold font indicates significant p values (p < 0.05).
Figure 3.
Lower serum albumin levels by quintile are associated with higher incidence of ARDS (n = 993, p = 0.01 by chi-square with linear by linear association test).
Albumin Levels and Clinical Outcomes
In a multivariable logistic regression analysis controlling for prespecified confounders (age, gender, APACHE II score, presence of sepsis, chronic liver disease, vasopressor use, baseline eGFR, and positive fluid balance), lower serum albumin levels were independently associated with higher inhospital mortality (OR, 1.56 per 1-g/dL decrease in albumin; 95% CI, 1.20–2.23; p = 0.001) (Supplementary Table 2, http://links.lww.com/CCX/A772). An analysis by albumin quintiles confirmed that lower serum albumin was associated with significantly higher inhospital mortality (p = 0.006) (Supplementary Fig. 2, http://links.lww.com/CCX/A768); inhospital mortality was more than two-fold higher in the lowest quintile of serum albumin compared with the highest quintile.
ICU LOS was longer (p = 0.007) (Supplementary Fig. 3, http://links.lww.com/CCX/A769) in patients with lower quintiles of serum albumin. In a univariate Poisson regression model comparing serum albumin and ICU LOS, a decrease of 1-g/dL serum albumin was associated with an incidence rate ratio (IRR) of 1.19 (95% CI, 1.15–1.23). This finding suggests that for each 1-g/dL decrease in serum albumin, there is a 19% increase in the probability of a 1-day increase in ICU LOS. Ventilator-free days were fewer (p < 0.001) (Supplementary Fig. 4, http://links.lww.com/CCX/A770) in the lowest quintile of serum albumin. In the univariate model comparing serum albumin and ventilator-free days, a decrease of 1-g/dL serum albumin was associated with an IRR of 0.82 (95% CI, 0.81–0.84), suggesting that for each 1-g/dL decrease in serum albumin, there is a 22% increase in the in the probability of a 1-day decrease in days alive a free of mechanical ventilation. Low serum albumin was also associated with organ failure as measured by SOFA score (21). This analysis is included in the Supplementary Information (http://links.lww.com/CCX/A793). We performed a sensitivity analysis excluding patients who only had serum albumin measured the day after enrollment to demonstrate that hypoalbuminemia increases the risk for ARDS when it precedes or coincides with ARDS onset, lessening the possibility that ARDS may be driving the signal by causing hypoalbuminemia after onset of the syndrome. The final sensitivity analysis excluded those with chronic liver disease as these patients have different basal serum albumin levels. The results of these analyses can be found in the Supplementary Information (http://links.lww.com/CCX/A793).
DISCUSSION
In this retrospective analysis of critically ill patients enrolled in a prospective observational cohort study, hypoalbuminemia was independently associated with risk of ARDS. The importance of this work is underscored by the fact that the prevalence of hypoalbuminemia exceeds 80% in older hospitalized patients (22). In our study, 88% of patients had hypoalbuminemia at enrollment (< 3.5 g/dL). Low serum total protein (< 6 g/dL) has been associated with development of ARDS in patients with sepsis (23), and low levels of serum albumin are strongly associated with mortality across a wide array of cohorts (24–26). The association between hypoalbuminemia and ARDS is described in numerous instances in the literature (5, 8–10), and serum albumin is included as a variable in the Lung Injury Prediction Score (11). However, few reports have systematically characterized the association between hypoalbuminemia and risk of ARDS across the full spectrum of albumin levels, and very few have been adequately powered for a comprehensive multivariable analysis. Jia et al (10) reported an unadjusted association between serum albumin and ARDS incidence but did not perform multivariable regression controlling for potential confounders. Hoeboer et al (8) also reported an association between serum albumin and ARDS in a relatively small cohort of 101 critically ill patients but did not include multivariable analysis. By contrast, the current study includes a relatively large cohort, allowing sufficient power for a multivariable analysis controlling for potential confounders to examine the association between serum albumin and development of ARDS and other organ dysfunction in the ICU.
In our study, we documented for the first time the possible linear relationship of serum albumin levels and ARDS incidence. With each gram decrease of serum albumin, there was an associated OR of 1.56 for development of ARDS. Additionally, serum albumin was associated with hospital mortality, ICU LOS, and ventilator-free days in a linear fashion, as suggested by Supplementary Figure 1 (http://links.lww.com/CCX/A767); Supplementary Figure 2 (http://links.lww.com/CCX/A768); Supplementary Figure 3 (http://links.lww.com/CCX/A769); and Supplementary Figure 4 (http://links.lww.com/CCX/A770).
The equation by Starling (27) stipulates that the net fluid movement out of the vasculature is a function of the hydrostatic pressures of the vasculature and the interstitium as well as the capillary and interstitial colloid oncotic pressures. Under normal conditions, albumin generates roughly 80% of the colloid oncotic pressure in the circulation (28). A decrease in colloid oncotic pressure gradient between the intravascular and interstitial spaces can lead to edema formation even when hydrostatic pressures are low, and vascular permeability is not altered (29, 30). This effect is magnified in the setting of ARDS-induced disruption of lung endothelial and epithelial barriers. Concomitant degradation of the endothelial glycocalyx can also alter the molecular selectivity of the wall of the microvessel and allowing larger solutes to extravasate more quickly (31). Thus, in the setting of a disrupted alveolar capillary barrier, lower serum albumin will contribute to the driving forces that favor edema formation. Considering this information and the pervasiveness of hypoalbuminemia in critically ill patients, several clinical trials have sought to improve ARDS outcomes using albumin therapies.
It remains unclear whether administration of exogenous albumin to patients with ARDS is beneficial. A meta-analysis of three randomized controlled trials comparing albumin with crystalloid fluid therapy in ARDS patients (n = 206) found that albumin improved oxygenation but did not have a significant effect on mortality (32). The Albumin Italian Outcome Sepsis trial (6) randomized 1,818 patients with sepsis to receive 300 mL of 20% albumin and crystalloid or crystalloid alone. Neither 28- nor 90-day mortality improved with albumin infusion. However, the mean arterial pressure was higher, and the net fluid balance was lower in the group that received albumin. These findings suggest that correction of hypoalbuminemia may promote a less positive fluid balance in the setting of critical illness, an effect that might help prevent formation of pulmonary edema in the setting of ARDS.
Serum albumin also has beneficial effects beyond generation of colloid oncotic pressure that may contribute to the observed negative impact of hypoalbuminemia in critical illness. Among these, albumin can bind and transport ligands including vasoactive molecules such as nitric oxide (33). This binding activity can reduce microvascular permeability and inhibit endothelial cell apoptosis (34), which favor retention of fluid in the vasculature in the setting of ARDS. The 17 disulfide bonds and free thiol at Cysteine-34 position on the albumin molecule have antioxidant (35) and anti-inflammatory (36) functions. Albumin can also scavenge free radicals and prevent lipid peroxidation, a process that has been implicated in the pathogenesis of ARDS (37, 38). These noncolloid functions of albumin could also contribute to the benefit of higher serum albumin levels we observed.
Hypoalbuminemia may also be a marker of both acute and chronic severity of illness or inflammation. Hypolbuminemia is commonly observed in acute inflammatory diseases (39) due to contributions of decreased albumin synthesis (40, 41), altered clearance or degradation, and dilutional effects. Hypoalbuminemia is also a marker of chronic malnutrition and inflammation. However, in the current study, serum albumin levels retained their association with development of ARDS in multivariable models controlling for acute and chronic severity of illness (APACHE II score), suggesting that albumin is more than just a marker of severity of illness. Additionally, an increase in permeability of the microvascular barrier in the setting of critical illness can contribute to albumin flux into the extravascular space, potentiating serum albumin depletion and leading to a vicious cycle of hypoalbuminemia-potentiated edema formation.
Our study has several strengths. It was performed in a large cohort of prospectively enrolled heterogeneous critically ill patients, enhancing generalizability. ARDS was phenotyped by review from two experienced physician investigators with strict adherence to Berlin criteria. Serum albumin levels were quantified using clinical laboratory tests as directed by the medical team, increasing the potential clinical applicability of these findings in a variety of cohorts. Furthermore, this study controls for numerous potentially confounding variables, establishing an independent association between hypoalbuminemia and risk of ARDS. The association of low serum albumin with increased mortality and reduced ventilator-free days replicates prior reports (7, 42–46). Although other studies have demonstrated the association between serum albumin and ARDS (5), this study is the first to systematically explore the role of hypoalbuminemia in a large cohort of patients at risk for ARDS while controlling for confounding variables. Furthermore, we showed a possible linear relationship of serum albumin concentration and ARDS incidence.
Our study also has some limitations. Serum albumin levels were only available if measured for clinical purposes; patients enrolled in the VALID cohort who did not have a serum albumin level measured within 1 day of enrollment were excluded. Furthermore, the role of colloid oncotic pressure in pulmonary edema formation is influenced by multiple factors that cannot be easily measured in the current data set. The patient’s blood volume, lymphatic return rate, efflux of albumin to the extravascular space, pulmonary microvascular pressures, and rates of albumin synthesis and catabolism both acutely and chronically may each impact lung fluid balance. These variables were not measurable in this observational trial. The logistic regression analyses performed in this work included a priori confounders with potential for multicollinearity. We report the variance inflation factors for each variable in the Supplemental Information (http://links.lww.com/CCX/A793), and while all are below 10, some collinearity may exist in these models. Finally, this study was observational in nature, and therapeutic administration of albumin, although uncommon in our ICUs, was not captured during the study period, limiting conclusions concerning the effects of possible albumin interventions.
CONCLUSIONS
In conclusion, among a large cohort of adult ICU patients, lower serum albumin was independently associated with increased risk of developing ARDS, even after controlling for severity of illness and other potential confounders such as chronic liver disease. These findings support the hypothesis that low plasma oncotic pressure independently contributes to pulmonary edema formation in patients who are at risk for ARDS.
ACKNOWLEDGMENTS
We thank Dr. Tatsuki Koyama for his advice on statistical approaches to analyses within this article.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Mr. McNeil collected, analyzed, and interpreted patient data and was a major contributor in writing the article. Dr. Jackson analyzed and interpreted patient data and was a major contributor in writing the article. Dr. Wang performed statistical analysis, provided statistical expertise, and helped write the article. Dr. Siew and Mr. Vincz provided renal data and scientific reasoning, and contributed to article writing. Drs. Shaver and Bastarache suggested expanded analytic approaches, improved the article, and contributed to article writing. Dr. Ware directed the project, interpreted patient data, and was a major contributor in writing the article.
Supported, in part, by National Institutes of Health grants HL103836 (L.B.W.), HL135849 (J.A.B., L.B.W.), HL126671, HL150783 (J.A.B.), HL136888 (C.M.S.), and 5P30 D114809 (E.D.S.).
The authors have disclosed that they do not have any potential conflicts of interest.
The study protocol was approved by the Vanderbilt Institutional Review Board (051065).
Patients or their surrogates provided informed consent. A waiver of informed consent was also approved for this minimal risk study in the event that the patient was unable to consent and no surrogate was available.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med. 2000; 342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA: Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001; 163:1376–1383 [DOI] [PubMed] [Google Scholar]
- 3.Fanali G, di Masi A, Trezza V, et al. : Human serum albumin: From bench to bedside. Mol Aspects Med. 2012; 33:209–290 [DOI] [PubMed] [Google Scholar]
- 4.Gong MN, Thompson BT, Williams P, et al. : Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005; 33:1191–1198 [DOI] [PubMed] [Google Scholar]
- 5.Aman J, van der Heijden M, van Lingen A, et al. : Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011; 39:89–97 [DOI] [PubMed] [Google Scholar]
- 6.Caironi P, Tognoni G, Masson S, et al. ; ALBIOS Study Investigators: Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014; 370:1412–1421 [DOI] [PubMed] [Google Scholar]
- 7.Findik O, Aydin U, Baris O, et al. : Preoperative low serum albumin levels increase the requirement of renal replacement therapy after cardiac surgery. Heart Surg Forum. 2016; 19:E123–E127 [DOI] [PubMed] [Google Scholar]
- 8.Hoeboer SH, Oudemans-van Straaten HM, Groeneveld AB: Albumin rather than C-reactive protein may be valuable in predicting and monitoring the severity and course of acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new onset fever. BMC Pulm Med. 2015; 15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson LD, Milberg JA, Anardi D, et al. : Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995; 151:293–301 [DOI] [PubMed] [Google Scholar]
- 10.Jia X, Malhotra A, Saeed M, et al. : Risk factors for ARDS in patients receiving mechanical ventilation for > 48 h. Chest. 2008; 133:853–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, et al. : Acute lung injury prediction score: Derivation and validation in a population-based sample. Eur Respir J. 2011; 37:604–609 [DOI] [PubMed] [Google Scholar]
- 12.Yu WK, McNeil JB, Wickersham NE, et al. : Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit Care. 2019; 23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siew ED, Ware LB, Gebretsadik T, et al. : Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009; 20:1823–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, et al. : APACHE II: A severity of disease classification system. Crit Care Med. 1985; 13:818–829 [PubMed] [Google Scholar]
- 15.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E; ARDS Definition Task Force. et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 16.Jones AE, Trzeciak S, Kline JA: The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009; 37:1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy MM, Fink MP, Marshall JC, et al. ; SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003; 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 18.Siew ED, Ikizler TA, Matheny ME, et al. : Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012; 7:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinella R, Sawhney R, Jalan R: Albumin in chronic liver disease: Structure, functions and therapeutic implications. Hepatol Int. 2016; 10:124–132 [DOI] [PubMed] [Google Scholar]
- 20.RCT: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2018 [Google Scholar]
- 21.Vincent JL, de Mendonça A, Cantraine F, et al. : Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998; 26:1793–1800 [DOI] [PubMed] [Google Scholar]
- 22.Brock F, Bettinelli LA, Dobner T, et al. : Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders. Rev Lat Am Enfermagem. 2016; 24:e2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangialardi RJ, Martin GS, Bernard GR, et al. : Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 2000; 28:3137–3145 [DOI] [PubMed] [Google Scholar]
- 24.Gibbs J, Cull W, Henderson W, et al. : Preoperative serum albumin level as a predictor of operative mortality and morbidity: Results from the National VA Surgical Risk Study. Arch Surg. 1999; 134:36–42 [DOI] [PubMed] [Google Scholar]
- 25.Fulks M, Stout RL, Dolan VF: Albumin and all-cause mortality risk in insurance applicants. J Insur Med. 2010; 42:11–17 [PubMed] [Google Scholar]
- 26.Goldwasser P, Feldman J: Association of serum albumin and mortality risk. J Clin Epidemiol. 1997; 50:693–703 [DOI] [PubMed] [Google Scholar]
- 27.Starling EH: On the absorption of fluids from the connective tissue spaces. J Physiol. 1896; 19:312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Thoracic Society: Evidence-based colloid use in the critically ill: American Thoracic Society Consensus Statement. Am J Respir Crit Care Med. 2004; 170:1247–1259 [DOI] [PubMed] [Google Scholar]
- 29.van der Heijden M, Verheij J, van Nieuw Amerongen GP, et al. : Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and nonseptic critically ill patients with hypovolemia. Crit Care Med. 2009; 37:1275–1281 [DOI] [PubMed] [Google Scholar]
- 30.Verheij J, van Lingen A, Raijmakers PG, et al. : Effect of fluid loading with saline or colloids on pulmonary permeability, oedema and lung injury score after cardiac and major vascular surgery. Br J Anaesth. 2006; 96:21–30 [DOI] [PubMed] [Google Scholar]
- 31.Staverman AJ. The theory of measurement of osmotic pressure. Recueil des Travaux Chimiques des Pays-Bas. 1951; 70:344–352 [Google Scholar]
- 32.Uhlig C, Silva PL, Deckert S, et al. : Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: A systematic review and meta-analysis. Crit Care. 2014; 18:R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margarson MP, Soni N: Serum albumin: Touchstone or totem? Anaesthesia. 1998; 53:789–803 [DOI] [PubMed] [Google Scholar]
- 34.Zoellner H, Siddiqui S, Kelly E, et al. : The anti-apoptotic activity of albumin for endothelium is inhibited by advanced glycation end products restricting intramolecular movement. Cell Mol Biol Lett. 2009; 14:575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakashima F, Shibata T, Kamiya K, et al. : Structural and functional insights into S-thiolation of human serum albumins. Sci Rep. 2018; 8:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedermann CJ: Anti-inflammatory activity of albumin. Crit Care Med. 2007; 35:981–982 [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B: Albumin–an important extracellular antioxidant? Biochem Pharmacol. 1988; 37:569–571 [DOI] [PubMed] [Google Scholar]
- 38.Soriani M, Pietraforte D, Minetti M: Antioxidant potential of anaerobic human plasma: Role of serum albumin and thiols as scavengers of carbon radicals. Arch Biochem Biophys. 1994; 312:180–188 [DOI] [PubMed] [Google Scholar]
- 39.Soeters PB, Wolfe RR, Shenkin A: Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019; 43:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levitt DG, Levitt MD: Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016; 9:229–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner DA, Buck M, Feitelberg SP, et al. : Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J Clin Invest. 1990; 85:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leite HP, Rodrigues da Silva AV, de Oliveira Iglesias SB, et al. : Serum albumin is an independent predictor of clinical outcomes in critically ill children. Pediatr Crit Care Med. 2016; 17:e50–e57 [DOI] [PubMed] [Google Scholar]
- 43.Jellinge ME, Henriksen DP, Hallas P, et al. : Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: A prospective, observational, cohort study. PLoS One. 2014; 9:e105983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akirov A, Masri-Iraqi H, Atamna A, et al. : Low albumin levels are associated with mortality risk in hospitalized patients. Am J Med. 2017; 130:1465.e11–1465.e19 [DOI] [PubMed] [Google Scholar]
- 45.Murat SN, Kurtul A, Yarlioglues M: Impact of serum albumin levels on contrast-induced acute kidney injury in patients with acute coronary syndromes treated with percutaneous coronary intervention. Angiology. 2015; 66:732–737 [DOI] [PubMed] [Google Scholar]
- 46.Lee EH, Baek SH, Chin JH, et al. : Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. 2012; 38:1478–1486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.