Supplemental Digital Content is available in the text.
Keywords: depression, intensive care unit, postintensive care syndrome, posttraumatic stress disorder, virtual reality
OBJECTIVES:
Psychologic sequelae after critical illness, part of the postintensive care syndrome, significantly decrease quality of life. A robustly effective treatment intervention is currently lacking. Virtual reality has beneficial effects on several non-ICU–related psychologic disorders. The aim of this study was to explore patient-related determinants of ICU-specific virtual reality, such as the timing of patients’ self-reported readiness to initiate virtual reality and the number of desired sessions and safety, and to explore the effects of ICU-specific virtual reality on mental health.
DESIGN:
A multicenter, randomized controlled feasibility study.
SETTING:
ICU at a university teaching hospital and a secondary care hospital in Rotterdam, The Netherlands.
PATIENTS:
Consecutive mechanically ventilated patients with sepsis or septic shock.
INTERVENTIONS:
Patients were randomly assigned (1:1) to receive ICU-specific virtual reality (ICU-specific virtual reality group) or exposure to a nature virtual reality environment (control virtual reality group).
MEASUREMENT AND MAIN RESULTS:
Explorative outcomes were feasibility, in terms of patient-related determinants, and safety. The effects of ICU-specific virtual reality on the psychologic components of postintensive care syndrome and quality of life were additionally studied. Fifty patients (median age: 61 yr; 21 [42%] female) were included. Patients in the ICU-specific virtual reality group felt ready to initiate the virtual reality intervention 10 days (median, 95% range, 5–21 d) after ICU discharge, and one session (median, 95% range, 1–6) was desired. ICU-specific virtual reality patients experienced higher immersion, cybersickness scores were low, and no changes in vital signs were observed. They also reported reduced posttraumatic stress disorder and depression scores and better mental health from 2 days until 1 month after initial exposure (Short Form-12 Mental Component Scale: ICU-specific virtual reality, 57 [36–67] vs control virtual reality, 47 [26–63]; p < 0.01). Six months after exposure, this effect was still present for posttraumatic stress disorder and depression, but not for mental quality of life.
CONCLUSIONS:
ICU-specific virtual reality is a feasible and acceptable novel intervention that could be used during recovery from an episode of critical illness in the ICU. A future, adequately powered study should confirm whether virtual reality is able to improve mental health and quality of life.
The increase in survival of critical illnesses with ICU treatment over the last few decades has revealed the effects of these medical states and care on quality of life (1, 2). Up to one-third of all critical illness survivors worldwide suffer from decreased quality of life (1, 3). This poor quality of life is largely attributed to depression, anxiety, and posttraumatic stress disorder (PTSD), part of the postintensive care syndrome (PICS), which comprise long-term mental health impairments (4–6). We recently demonstrated that these patients desire additional information about their ICU stay to better grasp ICU treatment and enhance understanding. As such, they are unsatisfied with the provided information through commonly used and accepted strategies (7).
Prevention and treatment of PICS has been acknowledged as an important theme to improve quality of ICU care in future decades. Despite the increasing awareness of PICS, several interventions, such as ICU diaries, intra-ICU psychologic interventions, and follow-up clinics, have yielded unsatisfactory and ambiguous results for improvements in psychologic well-being and quality of life after ICU treatment (8, 9). Psychologic sequelae after ICU treatment are postulated to reflect a combination of sensory overload and memories of frightening, delirium-related nightmarish, and psychotic experiences (10, 11). Veracious reconstruction of memories to fill in memory gaps and reframe these frightening memories may reduce these psychologic symptoms (12).
Virtual reality (VR) is a relatively new technique that can potentially help to cope with short-term and long-term mental health consequences outside the ICU (13). In several non-ICU–related mental health disorders, VR exposure is accepted as an effective treatment and user-friendly modality (14, 15). Through VR, it is possible to simulate stressful and traumatic experiences in a highly engaging and realistic manner (16–19). VR therefore offers an opportunity for recovering from hospital-related psychologic trauma and stress, and it remains important to expand VR applications toward improving the patient’s experience (20, 21). We designed an ICU-specific VR (ICU-VR) intervention to fill in patients' memory and give context to frightening memories, in which the ICU environment and treatment-related aspects were the main topics (22). In short, in the ICU-VR intervention, several ICU-related “real-life” aspects are shown and explained, that is, the ICU environment, about devices and noises, the necessity of IV catheters, mechanical ventilation, intubation and tracheal tube suction, about the treatment team and ICU workflow, and about sepsis.
Except a recent case report, no studies concerning the clinical implementation of VR after ICU treatment are available (13). Questions for clinical implementation, such as feasibility, in terms of the optimal timing of patient’s self-reported readiness to undergo VR and the number of sessions preferred by patients to effect an improvement, and safety, in terms of cybersickness and changes in vital signs, therefore remain. In addition, the effect of VR on PICS and quality of life remains to be elucidated.
In light of the aforementioned questions, this study sought to contribute to our understanding of the complexity of VR after ICU by first exploring the timing of patients’ self-reported readiness to initiate VR, the number of self-reported desired VR sessions, and the extent of side effects. Second, we studied the effect of ICU-VR on mental health.
MATERIALS AND METHODS
Study Design and Patients
Patients were eligible if they were adults (≥ 18 yr) admitted for sepsis or septic shock and were mechanically ventilated (≥ 24 hr). We stratified this cohort because sepsis and mechanical ventilation are risk factors for poor psychologic recovery after ICU treatment (23, 24). Sepsis and septic shock were defined as hypotension (mean arterial pressure < 65 mm Hg) requiring the administration of a vasopressor (norepinephrine at any dose) or a blood lactate level at or above 3.0 mEq/L during the first 24 hours of ICU admission in patients in whom infection was confirmed during ICU stay (25). Exclusion criteria were as follows: an inability to understand the Dutch language, a Glasgow Coma Scale score less than 15 during inclusion, active delirium or cognitive impairments as determined by a Telephone Interview for Cognitive Status (TICS) score below or equal to 27 during inclusion, preexisting epilepsy, severe psychiatric diseases, or deafness or blindness (26). The Medical Research Ethics Committees United in Nieuwegein and the participating centers’ institutional review boards approved the study protocol (Medical Ethics Committee number NL56741.101.16, February 2, 2017) (Supplement 1, http://links.lww.com/CCX/A791).
Randomization and Masking
After obtaining informed consent, patients were randomly assigned to either the ICU-VR group (intervention group), receiving ICU-VR, or the control VR group (control group), receiving a nature VR environment, at a 1:1 ratio using a centralized internet-based randomization procedure (Castor Electronic Data Capture, Amsterdam, The Netherlands). Patients were randomized in a simple manner without stratification; as such, the investigators were unaware of the assignment sequence. Due to the nature of the intervention, blinding of patients and investigators was not possible.
Interventions
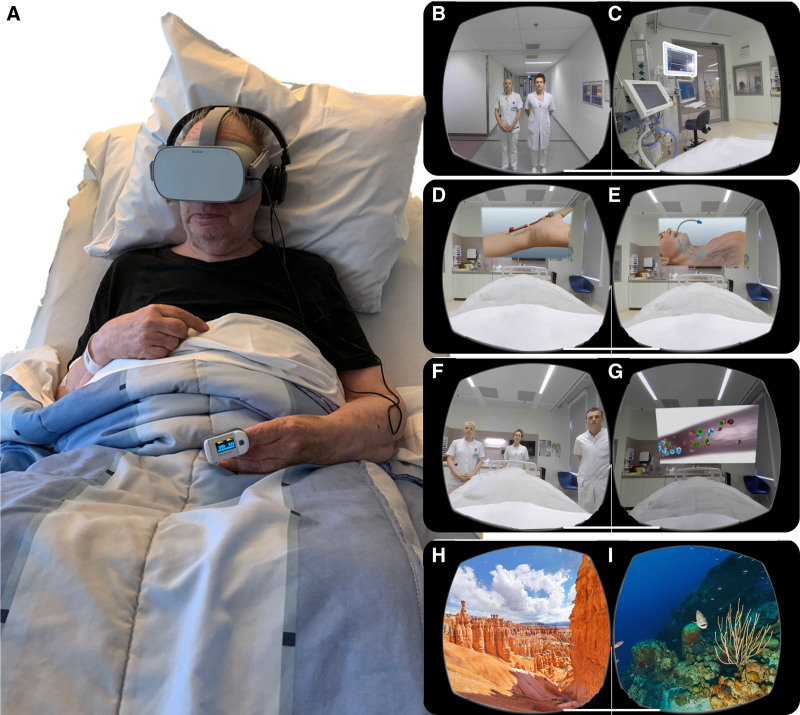
Patients in the ICU-VR group received ICU-VR. ICU-VR content was previously tested in healthy volunteers and was found to be safe and more immersive than 2D exposure (22). In this study, the content is extensively explained, and the script is added as supplementary material (http://links.lww.com/CCX/A577). In short, patients experience different facets of the ICU stay and ICU treatment (Fig. 1B–E).
Figure 1.
Study setup and intervention. A, A patient undergoing the ICU-specific virtual reality (VR) intervention using head-mounted display VR, a controller, and headphones. B–I, Images of the inside of the VR glasses with (B) an introduction by an intensivist and a ICU nurse to welcome the patient to the ICU and VR environment (C) explanation of devices and noises in an ICU room, (D) the necessity of central/peripheral catheters and IV drips, (E) information about mechanical ventilation, intubation, and tracheal tube suction, (F) information and necessity of the treatment team and ICU workflow, (G) an explanation of sepsis, (H and I) nature VR environments (control VR; (H) landscape, (I) water world).
Patients in the control VR group received VR for the same duration as ICU-VR and could choose between different nature environments (Fig. 1F and G). Interventions are described in more details in Supplement 2 (http://links.lww.com/CCX/A791). Nature VR was chosen as an active control to balance the nonspecific conditions associated with a VR intervention (e.g., attention, treatment contact, and social support) between groups, so only the content differed.
Both groups received the assigned intervention shortly after being discharged from the ICU, while still being treated in the hospital ward.
Study Procedures
Within 4 days after ICU discharge, patients were approached, during which their cognitive status was assessed using the TICS (26). Subsequently, patients with a TICS score less than or equal to 27 were excluded. After inclusion and before initiation of VR, we asked patients to self-determine when they found themselves ready to undergo initial VR (Fig. S1, in Supplement 3, http://links.lww.com/CCX/A791). At this point, patients agreed with the statement “I consider my current condition acceptable to start with VR exposure and I understand the nature of VR.” Initial VR exposure was followed by a week of offering VR daily to assess the number of desired sessions. Patients were asked, “Would you like to receive the VR intervention today?” and were free to decide whether to use VR. Patients remained uninformed about which intervention was studied, that is, exposure to ICU-VR or to a nature VR environment, to minimize social desirability bias. Figure 1 represents the study setup and intervention. Directly prior to the first VR session and 2 days, 1 week, and 1 month after, patients were asked to fill out questionnaires. Follow-up was also attempted at 6 months after exposure to determine long-term effects. Procedures are explained in more detail in Supplement 2 (http://links.lww.com/CCX/A791).
Outcomes
Primary outcomes were feasibility in terms of the timing of patients’ self-reported readiness of VR initiation, number of desired sessions, as well as immersiveness, assessed by a measure of presence, and safety, assessed by a measure of cybersickness and changes in vital signs. Prior to the intervention, differences in immersive tendencies, the ability to immerse within virtual environments, were assessed using the Immersive Tendencies Questionnaire (ITQ) (27). To test the immersive nature of the VR content, immersiveness was assessed using the Igroup Presence Questionnaire directly after VR exposure (28). Cybersickness was measured using the Simulator Sickness Questionnaire directly after VR initiation, and changes in vital signs were assessed during the VR intervention using heart rate, respiratory rate, oxygen saturation, and mean arterial pressure (29). Vital signs were measured before the intervention and during the intervention after each of the six modules (ICU-VR group) or after every 1:45 minutes (control VR group), except for blood pressure which was measured only before and after initial VR exposure, as more frequent monitoring of blood pressure could distract participants from the content. In addition, adverse events (AEs) were assessed while patients were still admitted at the hospital ward. During daily visits from the investigator to offer ICU-VR and through daily screening of electronic digital health records, AEs were scored. Mentioning a delirium in the daily status report or a new administration of haloperidol was considered as a new or active delirium.
Psychologic sequelae were expressed as the severity of PTSD- and depression-related symptoms assessed using the Impact of Event Scale-Revised (IES-R) and the Beck Depression Inventory (BDI) II, respectively (30, 31). PTSD-related symptoms were expressed as the IES-R sum score, ranging from 0 to 88, with higher scores indicating more severe symptoms. Depressive symptoms were expressed as the BDI sum score, ranging from 0 to 63, with higher scores indicating more severe depressive symptoms. Continuous outcomes rather than the cutoff values of the psychologic questionnaires were used, given that the IES-R and BDI were not designed as diagnostic tools, but rather to measure a patient’s level of stress- and depression-related symptoms (32, 33).
Health-related quality of life (HRQoL) was assessed using the Mental Component Scale of the Short-Form 12 (MCS-12) and the Physical Component Scale of the Short-Form 12 (PCS-12) scores and the European Quality of Life 5D (EQ-5D) questionnaire (34, 35). The MSC-12 and PCS-12 are the weighted sums of the questions in the section after being transformed to a scale ranging from 0 to 100 and standardized in the general population to have a mean of 50 and a sd of 10. The EQ-5D yields a utility score that ranges from –0.446 (worst quality of life) to 1.00 (best quality of life) (36).
The questionnaires are described in more detail in Supplement 2 (http://links.lww.com/CCX/A791).
Statistical Analysis
Because assessment of feasibility and safety was explorative in nature, and this is the first study of its kind, no formal power calculation could be performed for these outcomes. We therefore decided to power the study on a clinically relevant effect size on PICS-related psychologic sequelae. The required sample size was estimated to identify a clinically meaningful effect size of Cohen’s d equals to 0.8 (0.77–0.87 for skewed distributions of the residuals) on PICS-related psychologic outcomes and quality of life, with a power of 0.8 and a two-sided alpha of 5%, resulting in a total required sample size of 25 patients for each study group.
Continuous outcomes were compared between treatment groups using a Wilcoxon Mann-Whitney U test. Categorical variables were compared between treatment groups using Fisher exact test. Between-group differences in variables of interest throughout the follow-up period were studied using a mixed effect linear regression model. Here, PTSD-related symptoms were expressed as the IES-R sum score, depressive symptoms as the BDI sum score, mental HRQoL as the MCS-12, physical HRQoL as the PCS-12, and overall HRQoL as the EQ-5D utility score. Furthermore, time in days (pTime), randomization (pRandomization), and its interaction (time × randomization; pInteraction) served as dependent variables, and a random intercept for each case was based on model comparisons using the Akaike information criterion. We report the coefficient (β) (95% CI), which implies the estimated mean difference in, for these mixed-effect linear regression models.
A sensitivity analysis was performed to address missing data using both the last observation carried forward (LOCF) method (main analysis) and multiple imputation according to the Markov-Chain Monte Carlo method (all data were assumed missing [completely] at random) (Table S4 in Supplement 3, http://links.lww.com/CCX/A791).
All analyses were performed using R for Statistics (R Foundation for Statistical Computing, Vienna, Austria, 2015). A p value of less than 0.05 was considered statistically significant.
RESULTS
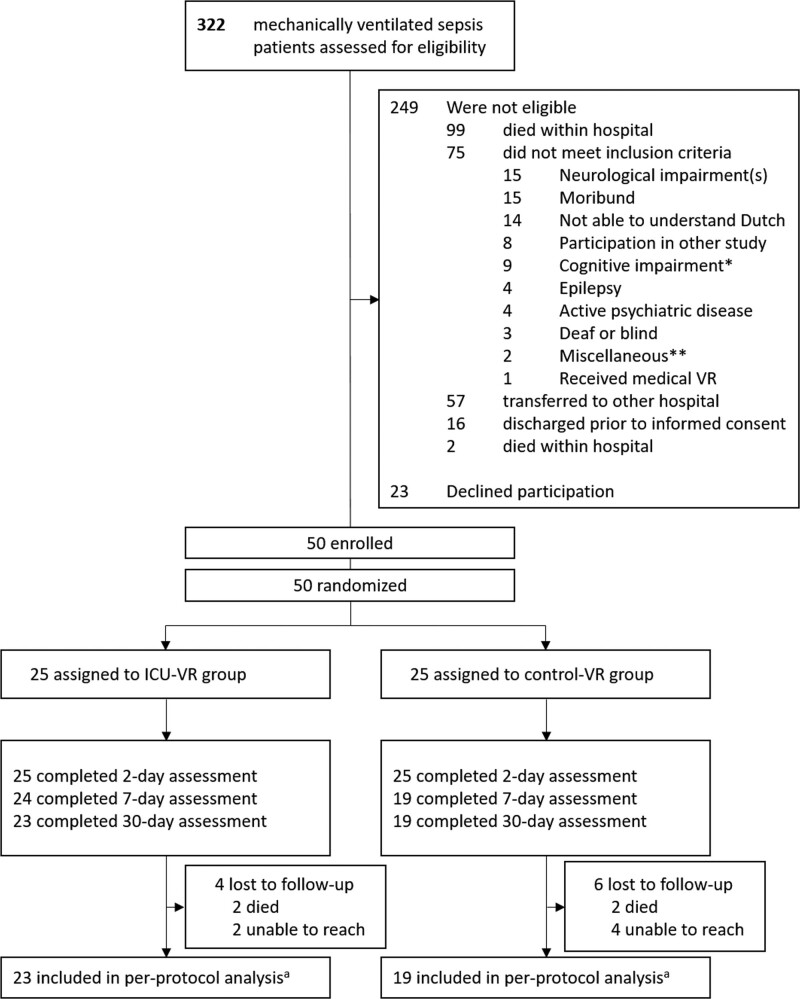
During the study period, 322 patients were admitted with sepsis or septic shock requiring mechanical ventilation. Seventy-three patients met the eligibility criteria of whom 50 were finally enrolled (Fig. 2) (inclusion rate, 68%). Median age was 61 years (95% range, 26–78 yr), and 21 patients (42%) were female. Patients had a median Acute Physiology and Chronic Health Evaluation IV score of 77 (11–130), a median ICU length of stay of 16 (2–75 d), and a median hospital length of stay of 39 days (16–114 d) (Table 1).
Figure 2.
Recruitment and randomization of patients. aBaseline demographics, treatment-related characteristics, and 2-d survey outcomes were available from all patients and were analyzed for all study patients. Outcomes at 6 mo were only imputed in patients from who the 1-mo outcomes were available; as such, per-protocol analyses were conducted with these patients. ICU-VR = ICU-specific virtual reality, VR = virtual reality *As defined by a Telephone Interview for Cognitive Status score below 27. **Claustrophobia (n = 1); Cataract (n = 1)..
TABLE 1.
Study Patients’ Characteristics
| Demographics | ICU-Specific VR (N = 25) | Control VR (N = 25) |
|---|---|---|
| Age, median (95% range), yr | 61 (23–75) | 59 (59–80) |
| Sex, n (%) | ||
| Male | 15 (60) | 14 (56) |
| Female | 10 (40) | 11 (44) |
| Comorbidities, n (%) | ||
| Neurologic disease | 2 (8) | 2 (8) |
| Cardiovascular disease | 3 (12) | 10 (40) |
| Respiratory disease | 7 (28) | 5 (20) |
| Renal disease | 6 (24) | 3 (12) |
| Liver disease | 2 (8) | 5 (20) |
| Psychiatric disease | 3 (12) | 3 (12) |
| Substance abuse | 3 (12) | 2 (8) |
| History of ICU admission, n (%)c | 3 (12) | 6 (24) |
| Telephone Interview for Cognitive Status score, median (95% range)a | 34 (29–40) | 34 (28–38) |
| Immersive Tendencies Questionnaire total score, median (95% range)b | 76 (54–96) | 75 (49–95) |
| Hospital LOS, median (95% range), d | 38 (17–116) | 41 (18–97) |
| ICU LOS, median (95% range), d | 14 (3–81) | 17 (2–79) |
| Hospital stay before ICU admission, median (95% range), d | 1 (0–18) | 1 (0–18) |
| Hospital stay after ICU discharge, median (95% range), d | 19 (7–56) | 18 (8–51) |
| Primary reason for admission, n (%) | ||
| Medical | 15 (60) | 21 (84) |
| Unplanned surgical | 4 (16) | 2 (8) |
| Planned surgical | 6 (24) | 2 (8) |
| Days of mechanical ventilation, median (95% range) | 7 (2–23) | 4 (1–21) |
| Illness severity scores, median (95% range) | ||
| Acute Physiology and Chronic Health Evaluation IVd | 72 (11–124) | 80 (31–130) |
| Simplified Acute Physiology Score IIe | 42 (17–85) | 51 (25–80) |
| Admission Sequential Organ Failure Assessment scoref | 7 (2–12) | 8 (3–16) |
| Health-related quality of life and psychologic distress at inclusion | ||
| Impact of Event Scale—Revised sum scoreg | 23 (4–59) | 24 (3–67) |
| Beck Depression Inventory sum scoreh | 15 (2–39) | 18 (7–31) |
| Mental Component Scale of the Short-Form 12i | 48 (26–59) | 43 (29–60) |
| Physical Component Scale of the Short-Form 12j | 26 (13–39) | 26 (17–38) |
| EQ-5D utility scorek | 0.30 (–0.16 to 0.76) | 0.39 (–0.20 to 0.75) |
| Visual Analogue Scale of the EQ-5Dl | 50 (12–80) | 50 (10–90) |
EQ-5D = European Quality of Life 5D questionnaire, LOS = length of stay, VR = virtual reality.
aTelephone Interview for Cognitive Status score ranges from 0 to 41; a score above 27 is considered as cognitive competent.
bImmersive Tendencies Questionnaire score ranges from 0 to 126; higher scores indicate higher immersive tendencies, indicative for a greater ability to immerse within a virtual environment.
cWithin 3 mo prior to hospital admission.
dAcute Physiology and Chronic Health Evaluation score ranges from 0 to 286; higher scores indicate more severe illness and a higher mortality risk.
eSimplified Acute Physiology Score ranges from 0 to 163; higher scores indicate more severe illness.
fSequential Organ Failure Assessment score ranges from 6 to 24; higher scores indicate worse medical condition.
gImpact of Event Scale—Revised sum score ranges from 0 to 88; higher scores indicate more severe symptoms of posttraumatic stress disorder (PTSD). A score ≥ 24 represents the best cutoff for PTSD.
hBeck Depression Inventory sum score ranges from 0 to 63; higher scores indicate more severe symptoms of depression. A score > 9 indicates mild depression, a score > 18 indicates moderate depression, and a score > 29 indicates severe depression.
iMental Component Scale of the Short-Form 12 ranges from 0 to 100; higher scores indicate a better mental quality of life.
jPhysical Component Scale of the Short-Form 12 ranges from 0 to 100; higher scores indicate a better physical quality of life.
kEQ-5D utility score ranges from –0.446 to 100; higher scores indicate a better health-related quality of life.
lVisual Analogue Scale of the EQ-5D ranges from 0 to 100; higher scores indicate a better perceived health state.
ICU-specific VR is the intervention group; control VR is the control group.
Both in the ICU-VR group and in the control VR group, 8% (2/25) died during 1-month follow-up. At 6 months, the questionnaire response rates were 68% (17/22) in the ICU-VR group and 52% (13/19) in the control VR group.
Feasibility
Feasibility and immersiveness outcomes are presented in Table 2. Patients reported to be ready to initiate VR 8 days (median, 95% range 4–23 d) after ICU discharge; patients in the ICU-VR group received ICU-VR after a median of 10 days (5–21 d), and patients in the control groups received the nature VR environment after a median of 8 days (5–15 d; p = 0.05). The median number of desired sessions was 1 (1–6; 25% of offered sessions) in the ICU-VR group and 1 (1–7; 25% of offered sessions) in the control VR group. All enrolled patients completed at least one VR session.
TABLE 2.
Feasibility, Immersiveness, and Cybersickness Outcomes
| Measure | ICU-Specific VR (N = 25) | Control VR (N = 25) | p |
|---|---|---|---|
| Feasibility | |||
| Completed initial VR, n (%) | 25 (100%) | 25 (100%) | 1.00 |
| Days between ICU discharge and initial VR, median (95% range) | 10 (5–21) | 8 (5–15) | 0.05 |
| Offered number of sessions, median (95% range) | 8 (3–8) | 8 (4–8) | 0.56 |
| Desired number of sessions, median (95% range) | 1 (1–5) | 1 (1–6) | 0.56 |
| Percent of offered sessions, median (95% range) | 25 (13–100) | 25 (13–93) | 0.87 |
| Immersiveness, median (95% range) | |||
| IPQ total scorea | 4.4 (2.4–5.3) | 3.2 (2.5–4.9) | < 0.001 |
| IPQ involvement scorea | 4.5 (2.0–5.6) | 2.1 (1.0–5.5) | < 0.001 |
| IPQ sense of presence scorea | 4.6 (2.2–5.5) | 3.7 (2.2–5.1) | < 0.01 |
| IPQ experienced realism scorea | 4.3 (1.0–5.1) | 3.5 (1.5–4.9) | < 0.01 |
| Cybersickness, median (95% range) | |||
| SSQ total scoreb | 1 (0–10) | 2 (0–7) | 0.55 |
| SSQ nausea scoreb | 0 (0–4) | 0 (0–3) | 0.84 |
| SSQ oculomotor scoreb | 1 (0–7) | 1 (0–4) | 0.87 |
| SSQ disorientation scoreb | 0 (0–4) | 1 (0–3) | 0.41 |
IPQ = Igroup Presence Questionnaire, SSQ = Simulator Sickness Questionnaire, VR = virtual reality.
aThe IPQ measures the feeling of presence within a virtual environment. The total score is the mean of all questions and ranges from 1 to 6; the involvement, sense of presence, and experienced realism scores are the mean of the questions in their section.
bThe SSQ comprises 16 questions representing a cybersickness-related symptom that is scored on a four-point Likert scale (0 = none, 3 = severe). The total SSQ score is the sum of all answers and ranges from 0 to 48. Subscales can be calculated for nausea oculomotor discomfort and disorientation and range from 0 to 21.
The ability to immerse within a virtual environment did not differ between groups prior to the intervention (median ITQ total score: ICU-VR, 76 [95% range, 54–96] vs control VR, 75 [49–95]; p = 0.30). Patients in the ICU-VR group experienced a greater sense of presence, a greater involvement, and a greater experienced realism than patients in the control VR group (Table 2).
Safety
None of the patients, either in the ICU-VR or control group, experienced severe symptoms of cybersickness, and no VR session was interrupted or discontinued due to side effects. Cybersickness scores were low in both groups, and none of the symptoms were still present 15 minutes after taking of the VR headset. In both groups, no changes in vital signs were observed during the assigned VR intervention (Tables S1 and S2 in Supplement 3, http://links.lww.com/CCX/A791). Furthermore, none of our patients reported any AEs, such as a delirium, during in-hospital follow-up.
Psychologic PICS
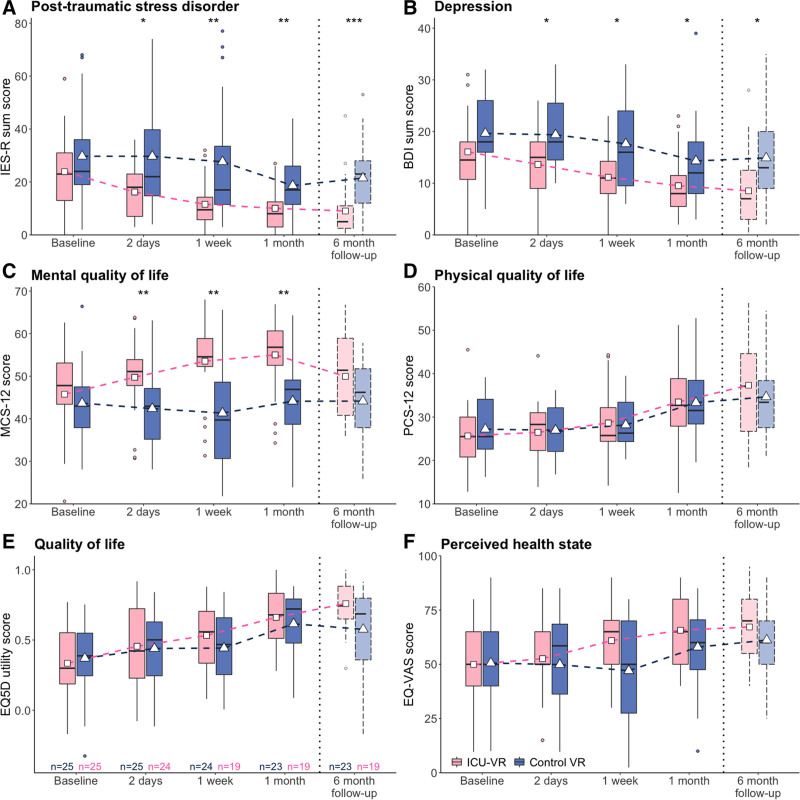
PTSD-related symptoms were less present in the ICU-VR group, already 2 days after initial ICU-VR exposure, than in the control VR group, and this difference persisted throughout follow-up (Fig. 3A). Although PTSD scores decreased over time in all patients (β = –0.04 [95% CI, –0.07 to –0.01]; pTime = 0.02), PTSD decreased more in the ICU-VR group than in the control VR group (β = 10.87 [3.73–18.01; pRandomization< 0.01), and this effect was maintained throughout the study period (β = 0.00 [–0.05 to 0.05]; pInteraction = 0.93). The proportion of patients suffering from PTSD at each follow-up session is depicted in Table S3 in Supplement 3 (http://links.lww.com/CCX/A791).
Figure 3.
Posttraumatic stress disorder (PTSD) (A), depression (B) and quality of life (C–F) throughout follow-up. Box-and-whisker plot show median and interquartile range (IQR) with the 25th and 75th percentiles, respectively; the upper whisker extends from the top of the box to the larger value no greater than 1.5 times the IQR. The bottom whisker extends from the bottom of the box to the smallest value no greater than 1.5 times the IQR; outliers outside the whiskers range are also presented (dots). The triangles and squares indicate the mean score for the control and intervention group, respectively. The black line across the box indicates the median. Boxplots at each time point are staggered to avoid superimposition. *p < 0.05, **p < 0.01, ***p < 0.001. The 6-mo outcomes are made more transparent than the boxplots of the 1- and 3-mo outcomes; these results were analyzed using the last observation carried forward method. PTSD is expressed as the sum score of the Impact of Event Scale-Revised (IES-R sum score), depression as the sum score of the Beck Depression Inventory (BDI sum score), mental quality of life as the Mental Component Scale of the Short-Form 12 (MCS-12), physical quality of life as the Physical Component Scale of the Short-Form 12 (PCS-12), quality of life as the utility score of the European Quality of Life 5D questionnaire (EQ5D), and perceived health state as the Visual Analogue Scale of the EQ-5D (EQ-VAS).
Depressive symptoms were lower in the ICU-VR group, already 2 days after initial ICU-VR exposure, than in the control VR group and persisted over time (Fig. 3B). Although depression scores decreased over time for all patients (β = –0.03 [–0.05 to –0.02]; pTime < 0.001), ICU-VR resulted in lower depression scores in the ICU-VR group than in the control VR group (β = 5.05 [1.31–8.79]; pRandomization = 0.01), and this effect persisted throughout the study period (β = 0.00 [–0.02 to 0.03]; pInteraction = 0.80). The proportion of patients suffering from depression at each follow-up moment is depicted in Table S3 in Supplement 3 (http://links.lww.com/CCX/A791).
Patients in the ICU-VR group experienced improved mental quality of life, already 2 days after ICU-VR exposure up to 1 month afterward, but not at 6 months, than patients in the control VR group (Fig. 3C) (Table S4in Supplement 3, http://links.lww.com/CCX/A791). Although mental quality of life did not improve in all participants over time (β = –0.01 [–0.03 to 0.01]; pTime = 0.37), it was higher in patients in the ICU-VR group than in patients in the control VR group (7.65 [–12.10 to –3.19]; pRandomization < 0.01), and this effect persisted throughout the follow-up period (β = 0.03 [0.00–0.07; pInteraction = 0.07). We did not observe differences in the physical quality of life (PCS-12) (Fig. 3D), overall HRQoL (EQ-5D utility score) (Fig. 3E), or perceived health state (EQ-VAS) (Fig. 3F) between groups.
DISCUSSION
The primary aim of this study was to explore the feasibility and safety of ICU-VR after ICU treatment. Patients found themselves ready to receive VR a median of 8 days after ICU discharge, and they desired only a few VR sessions during recovery in the hospital ward. Second, we observed that ICU-specific content improves mental health up to 6 months after ICU discharge.
To our knowledge, this is the first randomized trial to test the effect of VR on psychologic well-being after ICU stay. We therefore explored the two important issues of feasibility and safety. After ICU treatment, the optimal initiation of VR and the number of desired or needed VR sessions were unknown. In the current study, we found that patients felt themselves ready to initiate 8 days after ICU discharge, the overall median in the study population. These are valuable data that can help us in future studies. Although we found a small difference between groups, that is, patients in the ICU-VR group initiated VR after a median of 10 days, whereas patients in the control group after a median of 8 days, the entire study cohort, irrespective of the randomization allocation, is of importance in determining the optimal timing for patients to feel ready to initiate VR, as both groups received VR. One could argue that the readiness of the patient to initiate a VR intervention may be a more delicate matter and is different for each individual. As such, maybe rather a range of days instead of a prespecified number of days after ICU discharge prior to initiation with VR should be considered in future studies. In addition, patients in the ICU-VR group desired a median of one VR session. This is considerably less than the average number (8–14 sessions) reported in the non-ICU setting (14). This difference may be partially explained by the nature of PTSD in different contexts. Patients with ICU-related PTSD do not necessarily fear the “real” environment of the ICU or events during ICU treatment, but rather fear the frightening experiences they remember; in contrast, patients with non-ICU–related PTSD fear an actual event or situation (10, 37). VR is a new technology with the potential to reframe these frightening critical illness-related memories (38). As such, VR may enable patients to cope with their ICU experience, reframe their memories, and fill in memory gaps to maximize understanding. Also, in the non-ICU setting, randomized studies to determine the optimal number of VR sessions studies are lacking, and the studies have not reported long-term follow-up results, which further limit the ability to justify the number of VR sessions (14, 39). Importantly, the patient-centered approach, that is, giving patients the opportunity to receive the intervention as many times as they want, may improve outcomes. Research investigating this matter must be encouraged.
Previous non-ICU studies demonstrated that VR frequently causes some level of cybersickness (40, 41). Two major points must be addressed. First, although we observed no differences in nausea, oculomotor, or disorientation scores between groups, the ranges indicate that some patients experienced slight levels of arousal. However, none of the sessions required early cessation due to side effects, and cybersickness was mild and uncommon. Second, it is known that the duration of VR exposure is positively correlated with the level of cybersickness and that exposure longer than 20 minutes increases cybersickness (42, 43). We therefore empirically chose a duration of less than 20 minutes.
A global uniform workflow regarding the organization of post-ICU care is still being developed, and as such ICU follow-up guidelines are scarce (44). Because psychologic post-ICU sequelae are considered quite intrusive, ICU-VR mainly focuses on this component (45). Consistent with previous observations, we documented a decline in post-ICU–related sequelae in the control VR group (44). Despite this decline, we still observed effects of ICU-VR on PTSD and depression. A strength of our study is that we were able to show that ICU-VR has beneficial effects on mental health and psychologic well-being. The fact that psychologic well-being was assessed 1 and 6 months after ICU discharge is a strong feature. If most patients establish a new equilibrium after 1 year, it may be helpful to offer an additional VR session, 3 or 6 months after ICU discharge. Future studies should evaluate this issue.
Several limitations should be acknowledged. First, a larger than ideal number of patients were lost to follow-up at 6 months. This may reflect the burden of the questionnaires and might lead to a biased result. To adjust for this, missing data were dealt with using the “LOCF” method for the 6-month time point. To make sure we did not overestimate long-term effectiveness using the LOCF method, we also calculated the long-term effect with the multiple imputation method, which resulted in a comparable effect on patients’ PTSD- and depression-related symptoms. Also, at 1 month, we had a response rate of 84% and observed significant improvements in psychologic well-being and quality of life. In future studies, we should estimate a loss to follow-up of approximately 40% and investigate whether the burden of questionnaires can be reduced and/or employ more vigorous cohort retention techniques (46). Second, as the control group received an active control, daily contact and social support by the investigator might influence findings. Mental health however remained similar in the month after the VR control session. Third, due to our relatively small sample size, we were unable to perform subgroup analyses on patients who had history of anxiety or depression or on patients who had neurologic diseases, despite the knowledge that these subgroups have an increased risk on psychologic sequelae. As such, future studies should unravel whether the effectiveness of ICU-VR differs in certain subgroups. Fourth, although we assessed the desired number of sessions among patients, we cannot entirely be sure that this corresponds with the optimal number of sessions to achieve the highest effectiveness. As the probable working mechanism of ICU-VR mostly relies on reframing of delusional memories or putting frightening experiences into perspective and increasing the understanding of what happened during ICU treatment, one session could suffice to increase understanding or to establish reframing of delusional memories or putting frightening experiences into perspective. One could argue that more sessions would increase the exposure component of ICU-VR and thereby increase effectiveness, although no relation was found between the number of received sessions and the effectiveness in the current study. It is also possible that patients did not desire more sessions because it exaggerated their frightening thoughts or they just found it unhelpful. Also, the long-term effects of only one VR session are unknown. These considerations are rich grounds for future research. Fifth, patients and investigators were not blinded to the randomization allocation, which potentially resulted in some social desirability bias. To minimize this risk of bias, we did not inform patients that ICU-VR was the intervention of interest; the employee delivering the VR followed a strict protocol, and the analyzing investigator was unaware of the group allocations. Last, we included only patients with an episode of sepsis or septic shock, with positive blood cultures, who were mechanically ventilated more than 24 hours, potentially restricting the generalizability of our results. We explicitly chose this group because up to 42% of ICU patients suffer from sepsis, and it is a known independent risk factor for the development of stress disorders among survivors (23, 47).
CONCLUSIONS
In this randomized pilot study, we demonstrated that ICU-VR is a novel immersive intervention that could be used shortly after ICU discharge and is well tolerated among critical illness survivors. Patients randomized to the ICU-VR group reported less psychologic distress and a better mental HRQoL up to 1 month after VR exposure. Future, adequately powered studies should determine the definitive effect of ICU-VR on psychologic distress and should determine the effect of timing, that is, offering ICU-VR shortly after ICU discharge or later in post-ICU care, such as during an ICU follow-up clinic.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
All authors assisted in study design. Dr. Vlake conducted the study, recruited patients, and performed the data gathering. Drs. Korevaar and van Genderen independently verified the data. Drs. Vlake and Korevaar performed the data analyses. Dr. van Genderen wrote the video script. Drs. Vlake and van Genderen wrote the article. Dr. Van Bommel was the principal investigator in the Erasmus Medical Centre. Dr. Wils was the principal investigator in the Franciscus Gasthuis & Vlietland. Drs. Van Bommel, Wils, Korevaar, Bienvenu, Klijn, and Gommers helped to draft the article. All authors reviewed and approved the final article.
Supported, in part, by BeterKeten (foundation), Stichting Coolsingel (foundation), and Franciscus Vriendenfonds (foundation).
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Trial registration: Netherlands Trial Register, identifier: NL6611 (https://www.trialregister.nl/trial/6611).
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Cuthbertson BH, Roughton S, Jenkinson D, et al. : Quality of life in the five years after intensive care: A cohort study. Crit Care. 2010; 14:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lilly CM, Swami S, Liu X, et al. : Five-year trends of critical care practice and outcomes. Chest. 2017; 152:723–735 [DOI] [PubMed] [Google Scholar]
- 3.Prescott HC, Angus DC: Postsepsis morbidity. JAMA. 2018; 319:91. [DOI] [PubMed] [Google Scholar]
- 4.Bienvenu OJ, Friedman LA, Colantuoni E, et al. : Psychiatric symptoms after acute respiratory distress syndrome: A 5-year longitudinal study. Intensive Care Med. 2018; 44:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker AM, Sricharoenchai T, Raparla S, et al. : Posttraumatic stress disorder in critical illness survivors: A metaanalysis. Crit Care Med. 2015; 43:1121–1129 [DOI] [PubMed] [Google Scholar]
- 6.Rabiee A, Nikayin S, Hashem MD, et al. : Depressive symptoms after critical illness: A systematic review and meta-analysis. Crit Care Med. 2016; 44:1744–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlake JH, van Genderen ME, Schut A, et al. : Patients suffering from psychological impairments following critical illness are in need of information. J Intensive Care. 2020; 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schofield-Robinson OJ, Lewis SR, Smith AF, et al. : Follow-up services for improving long-term outcomes in intensive care unit (ICU) survivors. Cochrane Database Syst Rev. 2018; 11:CD012701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geense WW, van den Boogaard M, van der Hoeven JG, et al. : Nonpharmacologic interventions to prevent or mitigate adverse long-term outcomes among ICU survivors: A systematic review and meta-analysis. Crit Care Med. 2019; 47:1607–1618 [DOI] [PubMed] [Google Scholar]
- 10.Jones C, Griffiths RD, Humphris G, et al. : Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001; 29:573–580 [DOI] [PubMed] [Google Scholar]
- 11.Granja C, Gomes E, Amaro A, et al. ; JMIP Study Group: Understanding posttraumatic stress disorder-related symptoms after critical care: The early illness amnesia hypothesis. Crit Care Med. 2008; 36:2801–2809 [DOI] [PubMed] [Google Scholar]
- 12.Peri T, Gofman M: Narrative reconstruction: An integrative intervention module for intrusive symptoms in PTSD patients. Psychol Trauma. 2014;6 :176 [Google Scholar]
- 13.Vlake JH, van Bommel J, Hellemons ME, et al. : Intensive care unit-specific virtual reality for psychological recovery after ICU treatment for COVID-19; A brief case report. Front Med (Lausanne). 2021; 7:629086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kothgassner OD, Goreis A, Kafka JX, et al. : Virtual reality exposure therapy for posttraumatic stress disorder (PTSD): A meta-analysis. Eur J Psychotraumatol. 2019; 10:1654782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechsler TF, Kümpers F, Mühlberger A: Inferiority or even superiority of virtual reality exposure therapy in phobias?-A systematic review and quantitative meta-analysis on randomized controlled trials specifically comparing the efficacy of virtual reality exposure to gold standard in vivo exposure in agoraphobia, specific phobia, and social phobia. Front Psychol. 2019; 10:1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber SM, Jeitziner MM, Knobel SEJ, et al. : Perception and performance on a virtual reality cognitive stimulation for use in the intensive care unit: A non-randomized trial in critically ill patients. Front Med (Lausanne). 2019; 6:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turon M, Fernandez-Gonzalo S, Jodar M, et al. : Feasibility and safety of virtual-reality-based early neurocognitive stimulation in critically ill patients. Ann Intensive Care. 2017; 7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Zhang C, Jia Y, et al. : Development of a virtual reality system for early mobilization of critically ill patients. Stud Health Technol Inform. 2019; 264:1805–1806 [DOI] [PubMed] [Google Scholar]
- 19.Ong TL, Ruppert MM, Akbar M, et al. : Improving the intensive care patient experience with virtual reality-A feasibility study. Crit Care Explor. 2020; 2:e0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits M, Staal JB, van Goor H: Could virtual reality play a role in the rehabilitation after COVID-19 infection? BMJ Open Sport Exerc Med. 2020; 6:e000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dascal J, Reid M, IsHak WW, et al. : Virtual reality and medical inpatients: A systematic review of randomized, controlled trials. Innov Clin Neurosci. 2017; 14:14–21 [PMC free article] [PubMed] [Google Scholar]
- 22.Vlake JH, Wils EJ, van Bommel J, et al. : Virtual reality tailored to the needs of post-ICU patients: A safety and immersiveness study in healthy volunteers. Crit Care Explor. 2021; 3:e0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gestel A, Bakker J, Veraart CP, et al. : Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care. 2004; 8:R153–R162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M, Kang J, Jeong YJ: Risk factors for post-intensive care syndrome: A systematic review and meta-analysis. Aust Crit Care. 2020; 33:287–294 [DOI] [PubMed] [Google Scholar]
- 25.Bone RC, Balk RA, Cerra FB, et al. : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 26.Fong TG, Fearing MA, Jones RN, et al. : Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement. 2009; 5:492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns C, Nunez D, Daya M, et al. : The interaction between individuals’ immersive tendencies and the sensation of presence in a virtual environment. In: Eurographics Workshop on Virtual Environments. Mulder JD, Van Liere R. (Eds). Geneve, The Eurographics Association, 2000, pp 65–74 [Google Scholar]
- 28.Schubert TW: The sense of presence in virtual environments: A three-component scale measuring spatial presence, involvement, and realness. Zeitschrift für Medienpsychologie. 2003; 15:69–71 [Google Scholar]
- 29.Kennedy RS, Lane NE, Berbaum KS, et al. : Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. Int J Aviat Psychol. 1993; 3:203–220 [Google Scholar]
- 30.Beck AT, Steer RA, Brown GK: Beck Depression Inventory (BDI-II). Second Edition. London, Pearson, 1996 [Google Scholar]
- 31.Weiss DS: The impact of event scale: Revised. In: Cross-Cultural Assessment of Psychological Trauma and PTSD. International and Cultural Psychology Series. Wilson JP, Tang CS. (Eds). Boston, MA, Springer, 2007, pp 219–238 [Google Scholar]
- 32.Dozois DJA, Dobson KS, Ahnberg JL: A psychometric evaluation of the Beck depression inventory–II. Psychol Assess. 1998; 10:83 [Google Scholar]
- 33.Creamer M, Bell R, Failla S: Psychometric properties of the impact of event scale - Revised. Behav Res Ther. 2003; 41:1489–1496 [DOI] [PubMed] [Google Scholar]
- 34.The EUroQol Group: EuroQol-A new facility for the measurement of health-related quality of life. Health Policy. 1990; 16:199–208 [DOI] [PubMed] [Google Scholar]
- 35.Ware J, Jr, Kosinski M, Keller SD: A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996; 34:220–233 [DOI] [PubMed] [Google Scholar]
- 36.Versteegh MM, Vermeulen KM, Evers SMAA, et al. : Dutch tariff for the five-level version of EQ-5D. Value Health. 2016; 19:343–352 [DOI] [PubMed] [Google Scholar]
- 37.Askari Hosseini SM, Arab M, Karzari Z, et al. : Post-traumatic stress disorder in critical illness survivors and its relation to memories of ICU. Nurs Crit Care. 2021; 26:102–108 [DOI] [PubMed] [Google Scholar]
- 38.Wiederhold BK, Wiederhold MD: Virtual Reality Therapy for Anxiety Disorders: Advances in Evaluation and Treatment. Second Edition. Washington, D.C., American Psychological Association, 2005 [Google Scholar]
- 39.Difede J, Cukor J, Jayasinghe N, et al. : Virtual reality exposure therapy for the treatment of posttraumatic stress disorder following September 11, 2001. J Clin Psychiatry. 2007; 68:1639–1647 [PubMed] [Google Scholar]
- 40.Barrett J: Side Effects of Virtual Environments: A Review of the Literature. Edinburgh, Australian Government, Department of Defence, Defence Science and Technology Organisation Information Sciences Labarotory, 2004 [Google Scholar]
- 41.Gallagher M, Ferrè ER: Cybersickness: A multisensory integration perspective. Multisens Res. 2018; 31:645–674 [DOI] [PubMed] [Google Scholar]
- 42.Regan C:An investigation into nausea and other side-effects of head-coupled immersive virtual reality. Virtual Reality. 1995; 1:17–31 [Google Scholar]
- 43.Kennedy RS, Stanney KM, Dunlap WP: Duration and exposure to virtual environments: Sickness curves during and across sessions. Presence-Teleop Virt. 2000; 9:463–472 [Google Scholar]
- 44.Hofhuis JG, van Stel HF, Schrijvers AJ, et al. : ICU survivors show no decline in health-related quality of life after 5 years. Intensive Care Med. 2015; 41:495–504 [DOI] [PubMed] [Google Scholar]
- 45.Kerckhoffs MC, Kosasi FFL, Soliman IW, et al. : Determinants of self-reported unacceptable outcome of intensive care treatment 1 year after discharge. Intensive Care Med. 2019; 45:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson KA, Dinglas VD, Sukrithan V, et al. : Updated systematic review identifies substantial number of retention strategies: Using more strategies retains more study participants. J Clin Epidemiol. 2015; 68:1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wintermann GB, Brunkhorst FM, Petrowski K, et al. : Stress disorders following prolonged critical illness in survivors of severe sepsis. Crit Care Med. 2015; 43:1213–1222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.