Abstract
Intestinal lymphangiectasia is a rare disease which is causing protein-losing enteropathy. Treatment of intestinal lymphangiectasia can be a challenge for clinicians because of the lack of specific guidelines regarding pharmacological indications. We sought to introduce a diagnostic approach and suggest guidelines for treatment. After exclusion of secondary intestinal lymphangiectasia, magnetic resonance lymphangiography is a promising tool for the assessment of abnormal lymphatic lesions in primary intestinal lymphangiectasia. Determining the extent of the lesion provides direction for treatment options. Focal short-segment intestinal lymphangiectasia can be treated via intestinal resection or radiologic embolization after dietary therapy failure. Diffuse intestinal lymphangiectasia and extensive lymphangiectasia should be treated with several drugs with a full understanding of their mechanisms.
Keywords: Lymphangiectasis, Therapeutic embolization, Octreotide, Sirolimus, Everolimus
INTRODUCTION
Clinical manifestations of protein-losing enteropathy (PLE) arise when the rate of protein loss into the gut exceeds the rate of protein synthesis by liver. PLE can be divided into three categories according to its mechanism: mucosal injury, increased permeability, and lymphatic obstruction [1]. All factors contributing to these lymphatic changes lead to dilatation, rupture of the lymphatic vessels, and opening into intestinal lumen. Intestinal lymphangiectasia is a rare disease causing PLE. Intestinal lymphangiectasia is classified into primary or secondary types. Primary intestinal lymphangiectasia, also called idiopathic lymphangiectasia, occurs congenitally in the absence of causative factors. Secondary intestinal lymphangiectasia is induced by risk factors including heart surgery, chemotherapy, infection, or toxic materials known to trigger lymphatic changes [2].
Clinical symptoms are induced by the loss of lymphatic contents resulting in hypoalbuminemia, which leads to generalized edema. Depending on the location of the abnormal lymphatics, third spaces are often involved causing pleural and pericardial effusion, and ascites [3]. Although it is diagnosed via endoscopy and biopsy, the full extent of the abnormal lesion cannot be determined via endoscopy. Thus, magnetic resonance (MR) lymphangiography has recently been used as an adjunctive diagnostic tool [4]. Treatment of intestinal lymphangiectasia is primarily based on dietary therapy, with high levels of protein and low-fat intake. Many studies reported the effectiveness of surgery and drugs such as octreotide and sirolimus or everolimus for patients who are refractory to dietary therapy [5]. Treatment of intestinal lymphangiectasia can be a challenge for clinicians in the absence of specific guidelines for pharmacologic therapy. Based on our experience, we introduce the diagnostic approach and suggest guidelines for treatment.
DIAGNOSTIC APPROACH
Initial clinical symptoms and laboratory results
The most prominent clinical symptom is generalized edema accompanied by chronic diarrhea. Intestinal lymphangiectasia is suspected and diagnostic examinations can be performed if proteinuria is ruled out by urinalysis and additional clues are obtained via blood tests.
The affected proteins such as albumin, immunoglobulin G, ceruloplasmin, fibrinogen, and transferrin have long half-life. Proteins with a relatively rapid turnover such as complement and insulin are maintained at normal levels [6]. Excessive loss of these proteins by diarrhea results in hypoalbuminemia and hypoglobulinemia, based on laboratory results. Lymphocytopenia is also observed based on the loss of lymphatic contents.
Additional diagnostic approaches are based on stool analysis, and measurement of alpha 1-antitrypsin (A1AT) [7]. A1AT is a glycoprotein synthesized in the liver, with a molecular weight similar to albumin [8]. The A1AT level can be determined accurately based on fecal A1AT clearance from 24-h stool samples. Abnormal fecal A1AT clearance is greater than 56 mL in a 24-h period [9]. However, a one-time measurement of A1AT is more widely used because of the difficulty of collecting all stool samples. The normal range of A1AT is less than 100 mg/mL [10]. Fecal A1AT is also elevated in the bloody stool, suggesting the need for evaluating gastrointestinal bleeding for accurate interpretation of abnormal fecal A1AT.
Exclusion of secondary intestinal lymphangiectasia
Once the intestinal lymphangiectasia is suspected, it is recommended to distinguish whether the disease is of primary or secondary origin (Fig. 1). This investigation is usually performed simultaneously with endoscopy, which is used to validate intestinal lymphangiectasia. Echocardiography and abdominal imaging is generally required since the secondary origins are usually cardiac and associated with tumor conditions such as lymphoma, sarcoma and neuroblastoma [11,12,13]. Central venous pressure is often elevated above the thoracic duct pressure in patients with right-sided heart failure or single-ventricle circulation [14]. It leads to obstructed lymphatic flow, resulting in dilation of thoracic and smaller lymphatic ducts and leakage into the intestine.
Fig. 1. Diagnostic flow of intestinal lymphangiectasia.
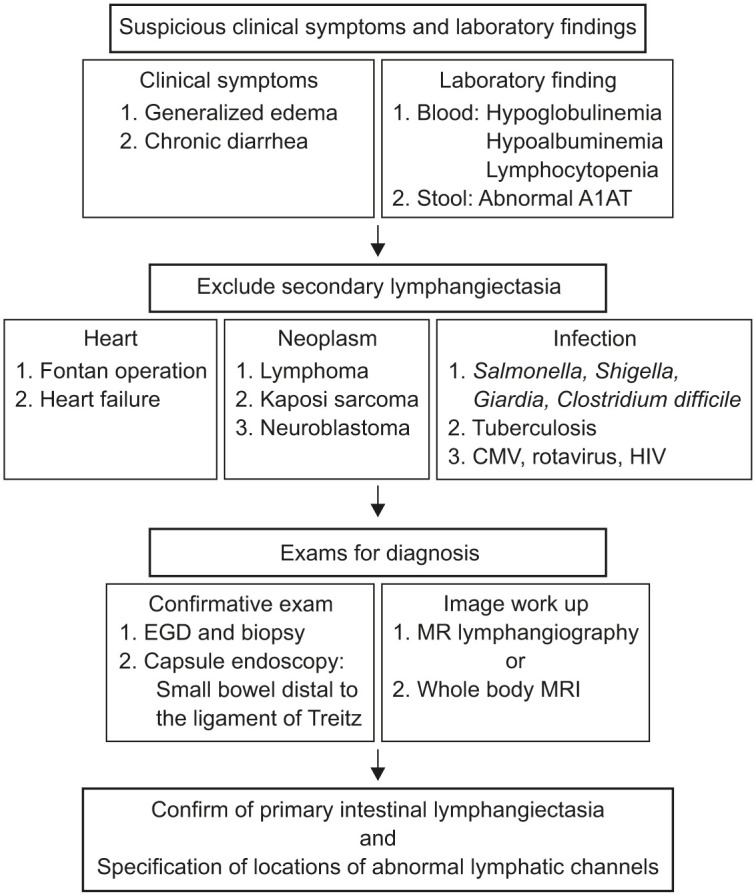
A1AT: alpha 1-antitrypsin, CMV: cytomegalovirus, HIV: human immunodeficiency virus, EGD: esophagogastroduodenoscopy, MR: magnetic resonance, MRI: magnetic resonance imaging.
Infection with toxins also induces lymphangiectasia by altering epithelial permeability, and therefore, infectious conditions should be checked. Bacteria such as Shigella, Salmonella, Giardia and Clostridium difficile and viruses such as cytomegalovirus and rotavirus are common pathogens that cause lymphangiectasia [15,16]. The primary treatment for secondary intestinal lymphangiectasia is correction of underlying disease.
Confirmative tool for diagnosis
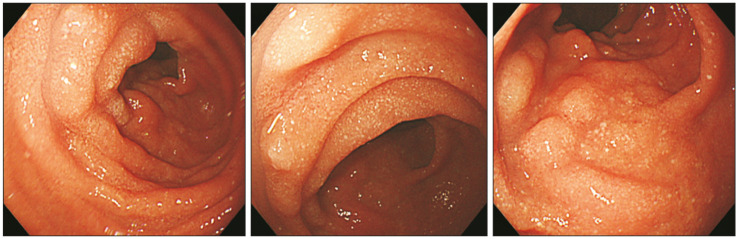
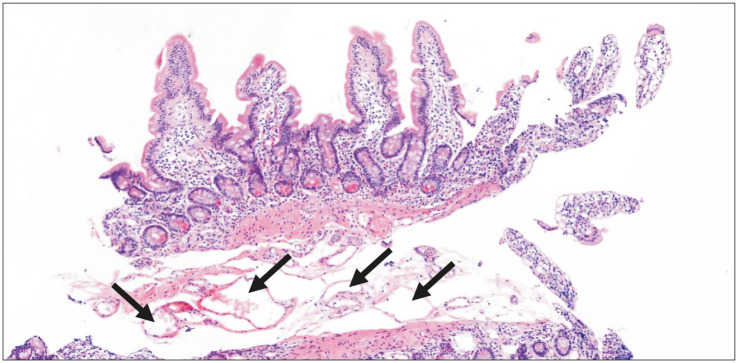
Endoscopy is the confirmative diagnostic approach for intestinal lymphangiectasia. Esophagogastroduodenography reveals snow-flake appearance of the duodenal mucosa [17] (Fig. 2). Pathology of the affected mucosa demonstrates multiple dilated lacteals (Fig. 3). Small bowel distal to the ligament of Treitz can be evaluated via capsule endoscopy [18].
Fig. 2. Image of esophagogastroduodenoscopy shows snow-flake appearance of intestinal lymphangiectasia in duodenum.
Fig. 3. Pathologic findings based on H&E staining (slide ×4) of intestinal lymphangiectasia in duodenum. Arrows indicate dilated lymphatic ducts at the submucosa level.
Imaging modality for assessment of disease extent
Assessing the full extent of the abnormal lymphatic lesions is an important factor in making treatment decisions. MR lymphangiography provides information regarding disease location and even enables the investigation of the pathophysiology of the disease by determining the volume of contrast leakage [19]. However, MR lymphangiography cannot be performed in all hospitals because it requires a skilled doctor and space for the procedure. Whole body MR imaging can be used as a substitute for MR lymphangiography.
Evaluation of the disease extent via imaging techniques entails assessment of lesion localization in the small intestine or widespread dissemination, and determination of lesions in the extremities such as arms and legs. Third spaces such as pleural cavity and retroperitoneal cavity should also be evaluated for disease (Fig. 1).
TREATMENT
Effective treatment strategy
Basic dietary modification is the mainstay of treatment for primary intestinal lymphangiectasia. Dietary therapy consists of high protein (2 g/kg/day) and low-fat content (<25 g/day). Fat component should include more than 90% of medium-chain and short-chain triglycerides (less than 14 carbons in length) [20]. They are transported directly to the portal circulation rather than the lymphatic system, which decreases the intestinal lymphatic flow [21]. However, it is difficult to design a diet strictly under clinical conditions, and it is difficult for the patient to continue to maintain this diet for a long time. In addition, dietary therapy is sometimes not therapeutically effective for lymphangiectasia. Second-line therapies such as surgery, radiologic intervention, and medications are recommended for cases of dietary therapy failure, and selecting the appropriate treatment is a great challenge for clinicians.
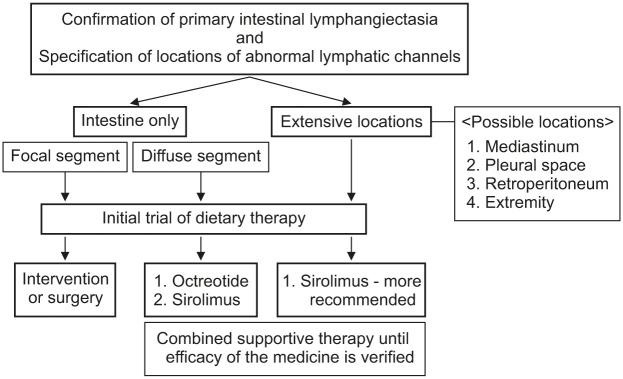
An effective treatment strategy entails diagnostic evaluation of the extent of abnormal lymphatic lesions. First, it is essential to distinguish whether the disease is restricted to the small intestine or disseminated extensively involving the extremities or third space. In the case of intestinal lesions, it is important to determine whether the focal lesion is confined to the small intestine or is diffuse (Fig. 4).
Fig. 4. Individual therapeutic strategy for primary intestinal lymphangiectasia.
Treatment method according to the extent of abnormal lymphatic channels
1. Focal intestinal lymphangiectasia
The presence of abnormal lymphatic channels in focal lesions of the intestine is a mild type; however, large amounts of leakage can cause severe symptoms. Fortunately, in this case, surgery or procedures can be used to rectify the disease due to the small size of the lesion. In the past, surgery was the only method available; however, with the development of interventional radiology, lymphatic embolization is frequently performed currently [22].
The extent of the lesion must be accurately identified prior to surgery. The surgical method also varies depending on the location of the lesion. In the absence of adjacent organs in the vicinity, surgery can be performed using a relatively simple method such as laparoscopic resection. However, when the abnormal lymphatic channels are found in the duodenum, a major surgery known as pylorus-preserving pancreaticoduodenectomy may be necessary because the biliary and pancreatic ducts are connected.
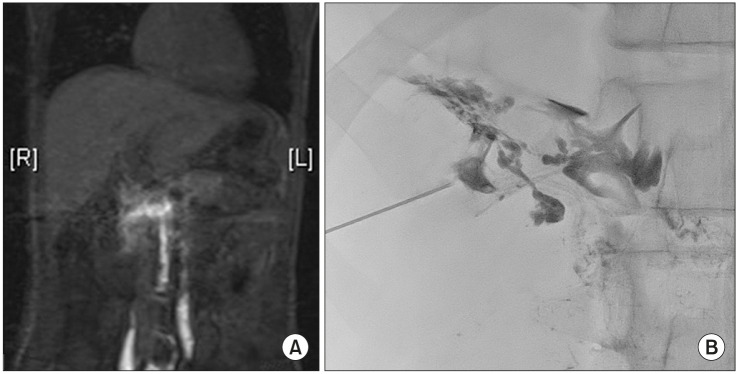
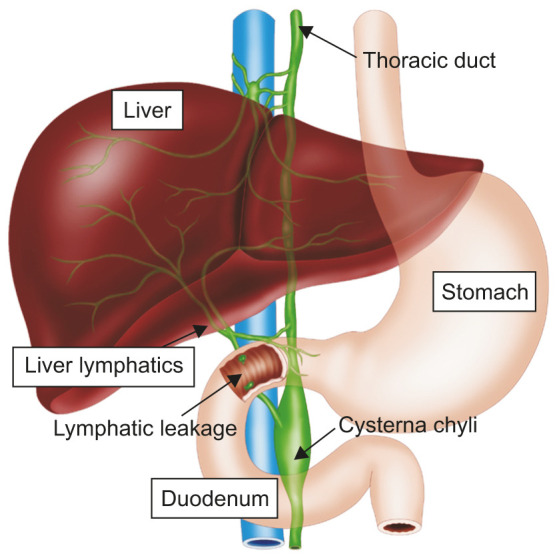
Embolization is routinely performed after Fontan operation or in patients with right heart failure in intestinal lymphangiectasia [23]. Thoracic duct or liver lymphatics are dilated under pressure as the central venous pressure is elevated in these patients. Thus, the embolization of thoracic duct or liver lymphatic has been reported in many studies [24,25]. Recent attempts involved direct blockade of the lymphatic channels leading to the intestine using a microcatheter (Fig. 5). Knowledge of lymphatic anatomy associated with each intestinal segment is essential for the procedure. For example, lymphatic leakage into the duodenum can be caused by liver lymphatics or by lymphatic channels originating in the preaortic gland (Fig. 6). The embolization strategy varies depending on the defective lymphatic channel.
Fig. 5. (A) One example image of contrast-enhanced magnetic resonance lymphangiography which demonstrates abnormal lymphatic leakage into the duodenum. (B) One example image of selective embolization with microcatheter.
Fig. 6. Schematic representation of lymphatic leakage in duodenum. The leakage can be caused by liver lymphatics or by lymphatic channels originating in the preaortic gland inside the body.
2. Diffuse intestinal lymphangiectasia
If the lesion is located only in the intestine but is widely distributed, surgery is seldom indicated because it entails resection of several portions of the intestine, and embolization is also difficult to perform because of the involvement of several lymphatic vessels. Since medication is absorbed systemically, it is an appropriate option for the patient with wide lesions. Appropriate drugs can be used if their mechanism is clearly understood.
Octreotide is a somatostatin analog that decreases splanchnic blood flow, intestinal motility, and triglyceride absorption [26]. Therefore, it may be optimally used by patients with only intestinal involvement of the abnormal lymphatics. A review of cases reporting the effects of octreotide suggested a therapeutic effect only in patients with intestinal lesions, but a suboptimal effect in the case of extensive type lymphangiectasia.
No standardized dosage recommendations are available for treatment with octreotide. As an induction therapy, a subcutaneous injection of 1–10 mcg/kg/dose twice a day for 2 weeks is recommended. After the induction, the same dose injected subcutaneously at 4-week intervals is recommended as maintenance therapy (Table 1) [27,28]. Adverse reactions such as hypertension, sinus bradycardia, and hyperglycemia should be closely monitored during the octreotide treatment.
Table 1. Summary of drugs recommended for patients with primary intestinal lymphangiectasia.
| Medication | Indication | Dose | Time to take effect | Trough level | |
|---|---|---|---|---|---|
| Octreotide | Diffuse intestinal lymphangiectasia | Induction: 1–10 mcg/kg/dose injection twice a day for 2 weeks subcutaneously | Around after 3 to 4 weeks | - | |
| Maintenance: Same dose injection subcutaneously at 4-week intervals | |||||
| Sirolimus | Diffuse intestinal lymphangiectasia | Starting dose | Around after 4 weeks | 5 to 15 ng/mL | |
| Extensive lymphangiectasia | Weight <40 kg: 1–1.6 mg/m2/day P.O. | ||||
| Weight ≥40 kg: 2 mg/day P.O. | |||||
| Adjust dose by monitoring trough level | |||||
| Everolimus | Diffuse intestinal lymphangiectasia | Starting dose | Around after 4 weeks | 5 to 15 ng/mL | |
| Extensive lymphangiectasia | Weight <40 kg: 1–1.6 mg/m2/day P.O. | ||||
| Weight ≥40 kg: 2 mg/day P.O. | |||||
| Adjust dose with monitoring trough level | |||||
| Propranolol | Neonate, infant <7 mo | 1 to 4 mg/kg/day P.O. | Around after 2 weeks | - | |
| Tranexamic acid | Condition of increased fibrinolytic activity (D-dimer elevation) | 25 mg/kg/dose three times a day P.O. (maximum 1,000 mg/dose) for 5 days | Around after 4 weeks | - | |
P.O.: per oral.
3. Extensive intestinal lymphangiectasia
Surgery and embolization are difficult to perform in the case of extensive lymphangiectasia involving the 3rd space and extremities. Considering the mechanism of octreotide described above, it is questionable whether it will act on lymphatic channels other than the intestine.
Sirolimus is has been used to treat lymphangiectasia. Sirolimus acts directly on lymphatic endothelial cells and changes mammalian target of rapamycin signaling, which suppresses lymphatic sprouting and proliferation, and induces apoptosis [29]. Sirolimus acts directly on lymphatic vessels, but not by controlling lymphatic flow unlike octreotide or dietary therapy. Everolimus is a second generation sirolimus derivative with improved pharmacokinetic properties [30]. Its half-life is relatively short and bioavailability is known to be high compared with sirolimus, but its use is limited by the paucity of experience involving everolimus treatment. Further, while sirolimus is indicated for vascular anomalies and lymphangioleiomyomatosis, everolimus is the only available drug for tuberous sclerosis and prophylaxis after transplantation, and thus it is difficult to determine the dose.
There are no standardized recommended doses or duration of treatment with sirolimus. Sirolimus can be started orally at a dose of 1–1.6 mg/m2/day for patients weighing less than 40 kg and at a dose of 2 mg/day for patients weighing more than 40 kg [31,32]. Dosage can be adjusted by monitoring the trough concentration. The optimal therapeutic trough concentration of sirolimus is 5 to 15 ng/mL. Empirically, sirolimus is clinically effective after a 4-week treatment period [3]. Adverse reactions such as cytopenia, tachycardia, hepatotoxicity, hyperglycemia, and electrolyte imbalance should be closely monitored during sirolimus treatment.
There are no standardized recommended doses or duration of treatment with everolimus, but according to a case report suggests a starting dose of 1–1.6 mg/m2/day and adjusting the dose by monitoring the trough concentration in the therapeutic range of 5 to 15 ng/mL [33,34,35].
Other medications for consideration
1. Propranolol
Since it is difficult to use drugs such as sirolimus or everolimus in infants or neonates, it is necessary to consider other drugs. Propranolol, a non-selective beta-blocker, is usually used for treatment of infantile hemangiomas. Propranolol acts on the rapidly accelerated fibrosarcoma kinase and mitogen-activated protein kinase signaling pathway, by reducing the expression of vascular endothelial growth factor levels and direct induction of apoptosis in capillary endothelial cells [36]. It appears to have a therapeutic effect by acting on the endothelium of lymphatic vessels. Propranolol can be started orally at a dose of 1 to 4 mg/kg/day. Some cases reported that propranolol treatment was effective after 12 weeks [37,38].
2. Tranexamic acid
Intestinal protein loss might occur during increased fibrinolytic activity. A subset of patients with intestinal lymphangiectasia manifested increased tissue or plasma fibrinolytic activity [39]. Two case reports involve treatment with tranexamic acid via antiplasmin therapy and normalization of tissue fibrinolytic activity [40,41]. Tranexamic acid 25 mg/kg/dose was used orally (maximum 1,000 mg/dose) for 5 days, three times a day [41].
CONCLUSION
Primary intestinal lymphangiectasia is a rare disease, but the treatment strategy depends on the disease extent based on past clinical experience. Focal short segment of intestinal lymphangiectasia refractory to dietary therapy can be treated via intestinal resection or radiologic embolization. Diffuse intestinal lymphangiectasia and extensive lymphangiectasia require treatment with drugs (octreotide, sirolimus or everolimus) based on a comprehensive understanding of their mechanisms. Propranolol and tranexamic acid may be used in special conditions of primary intestinal lymphangiectasia.
Footnotes
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Wyllie R, Hyams JS, Kay M. Pediatric gastrointestinal and liver disease. 4th ed. Philadelphia: Elsevier/Saunders; 2011. [Google Scholar]
- 2.Wilkinson P, Pinto B, Senior JR. Reversible protein-losing enteropathy with intestinal lymphangiectasia secondary to chronic constrictive pericarditis. N Engl J Med. 1965;273:1178–1181. doi: 10.1056/NEJM196511252732202. [DOI] [PubMed] [Google Scholar]
- 3.Kwon Y, Kim ES, Choe YH, Kim MJ. Individual approach for treatment of primary intestinal lymphangiectasia in children: single-center experience and review of the literature. BMC Pediatr. 2021;21:21. doi: 10.1186/s12887-020-02447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EW, Shin JH, Ko HK, Park J, Kim SH, Sung KB. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol. 2014;15:724–732. doi: 10.3348/kjr.2014.15.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altın Z, Atabay Y, Özer S, Karakoyun M, Ekmekçi S, Yürekli EY, et al. Primary intestinal lymphangiectasia and a review of the current literature. Turk J Gastroenterol. 2018;29:714–716. doi: 10.5152/tjg.2018.18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda H, Ishihama K, Fukui T, Fujishima S, Orii T, Nakazawa Y, et al. Significance of rapid turnover proteins in protein-losing gastroenteropathy. Hepatogastroenterology. 2003;50:1963–1965. [PubMed] [Google Scholar]
- 7.Bernier JJ, Florent C, Desmazures C, Aymes C, L'Hirondel C. Diagnosis of protein-losing enteropathy by gastrointestinal clearance of alpha1-antitrypsin. Lancet. 1978;2:763–764. doi: 10.1016/s0140-6736(78)92650-8. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt PN, Blirup-Jensen S, Svendsen PJ, Wandall JH. Characterization and quantification of plasma proteins excreted in faeces from healthy humans. Scand J Clin Lab Invest. 1995;55:35–45. doi: 10.3109/00365519509075376. [DOI] [PubMed] [Google Scholar]
- 9.Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179–1185. doi: 10.1007/s00431-010-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas DW, Sinatra FR, Merritt RJ. Random fecal alpha-1-antitrypsin concentration in children with gastrointestinal disease. Gastroenterology. 1981;80:776–782. [PubMed] [Google Scholar]
- 11.Crossland DS, Van De Bruaene A, Silversides CK, Hickey EJ, Roche SL. Heart failure in adult congenital heart disease: from advanced therapies to end-of-life care. Can J Cardiol. 2019;35:1723–1739. doi: 10.1016/j.cjca.2019.07.626. [DOI] [PubMed] [Google Scholar]
- 12.Konar A, Brown CB, Hancock BW, Moss S. Protein losing enteropathy as a sole manifestation of non-Hodgkin's lymphoma. Postgrad Med J. 1986;62:399–400. doi: 10.1136/pgmj.62.727.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'amico MA, Weiner M, Ruzal-Shapiro C, DeFelice AR, Brodlie S, Kazlow PG. Protein-losing enteropathy: an unusual presentation of neuroblastoma. Clin Pediatr (Phila) 2003;42:371–373. doi: 10.1177/000992280304200413. [DOI] [PubMed] [Google Scholar]
- 14.Kverneland LS, Kramer P, Ovroutski S. Five decades of the Fontan operation: a systematic review of international reports on outcomes after univentricular palliation. Congenit Heart Dis. 2018;13:181–193. doi: 10.1111/chd.12570. [DOI] [PubMed] [Google Scholar]
- 15.Akkelle BS, Tutar E, Sengul OK, Celikel CA, Ertem D. A rare complication of giardiasis in children: protein-losing enteropathy. Pediatr Infect Dis J. 2018;37:e345–e347. doi: 10.1097/INF.0000000000002025. [DOI] [PubMed] [Google Scholar]
- 16.Blackstone MM, Mittal MK. The edematous toddler: a case of pediatric Ménétrier disease. Pediatr Emerg Care. 2008;24:682–684. doi: 10.1097/PEC.0b013e3181887e89. [DOI] [PubMed] [Google Scholar]
- 17.Asakura H, Miura S, Morishita T, Aiso S, Tanaka T, Kitahora T, et al. Endoscopic and histopathological study on primary and secondary intestinal lymphangiectasia. Dig Dis Sci. 1981;26:312–320. doi: 10.1007/BF01308371. [DOI] [PubMed] [Google Scholar]
- 18.Rivet C, Lapalus MG, Dumortier J, Le Gall C, Budin C, Bouvier R, et al. Use of capsule endoscopy in children with primary intestinal lymphangiectasia. Gastrointest Endosc. 2006;64:649–650. doi: 10.1016/j.gie.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Chavhan GB, Amaral JG, Temple M, Itkin M. MR lymphangiography in children: technique and potential applications. Radiographics. 2017;37:1775–1790. doi: 10.1148/rg.2017170014. [DOI] [PubMed] [Google Scholar]
- 20.Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR. Modern nutrition in health and disease. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 21.Suresh N, Ganesh R, Sankar J, Sathiyasekaran M. Primary intestinal lymphangiectasia. Indian Pediatr. 2009;46:903–906. [PubMed] [Google Scholar]
- 22.Kwon Y, Kim ES, Choe YH, Hyun D, Kim MJ. Therapeutic lymphatic embolization in pediatric primary intestinal lymphangiectasia. Yonsei Med J. 2021;62:470–473. doi: 10.3349/ymj.2021.62.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itkin M, Piccoli DA, Nadolski G, Rychik J, DeWitt A, Pinto E, et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol. 2017;69:2929–2937. doi: 10.1016/j.jacc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol. 2011;28:63–74. doi: 10.1055/s-0031-1273941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maleux G, Storme E, Cools B, Heying R, Boshoff D, Louw JJ, et al. Percutaneous embolization of lymphatic fistulae as treatment for protein-losing enteropathy and plastic bronchitis in patients with failing Fontan circulation. Catheter Cardiovasc Interv. 2019;94:996–1002. doi: 10.1002/ccd.28501. [DOI] [PubMed] [Google Scholar]
- 26.Reichlin S. Secretion of somatostatin and its physiologic function. J Lab Clin Med. 1987;109:320–326. [PubMed] [Google Scholar]
- 27.Suehiro K, Morikage N, Murakami M, Yamashita O, Hamano K. Late-onset primary intestinal lymphangiectasia successfully managed with octreotide: a case report. Ann Vasc Dis. 2012;5:96–99. doi: 10.3400/avd.cr.11.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroiwa G, Takayama T, Sato Y, Takahashi Y, Fujita T, Nobuoka A, et al. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001;36:129–132. doi: 10.1007/s005350170142. [DOI] [PubMed] [Google Scholar]
- 29.Baluk P, Yao LC, Flores JC, Choi D, Hong YK, McDonald DM. Rapamycin reversal of VEGF-C-driven lymphatic anomalies in the respiratory tract. JCI Insight. 2017;2:e90103. doi: 10.1172/jci.insight.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43:83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Ingle GR, Sievers TM, Holt CD. Sirolimus: continuing the evolution of transplant immunosuppression. Ann Pharmacother. 2000;34:1044–1055. doi: 10.1345/aph.19380. [DOI] [PubMed] [Google Scholar]
- 32.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghobrial IM, Gertz M, LaPlant B, Camoriano J, Hayman S, Lacy M, et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenström macroglobulinemia. J Clin Oncol. 2010;28:1408–1414. doi: 10.1200/JCO.2009.24.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollis IB, Reed BN, Moranville MP. Medication management of cardiac allograft vasculopathy after heart transplantation. Pharmacotherapy. 2015;35:489–501. doi: 10.1002/phar.1580. [DOI] [PubMed] [Google Scholar]
- 35.Ozeki M, Hori T, Kanda K, Kawamoto N, Ibuka T, Miyazaki T, et al. Everolimus for primary intestinal lymphangiectasia with protein-losing enteropathy. Pediatrics. 2016;137:e20152562. doi: 10.1542/peds.2015-2562. [DOI] [PubMed] [Google Scholar]
- 36.Wagner MJ, Cranmer LD, Loggers ET, Pollack SM. Propranolol for the treatment of vascular sarcomas. J Exp Pharmacol. 2018;10:51–58. doi: 10.2147/JEP.S146211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liviskie CJ, Brennan CC, McPherson CC, Vesoulis ZA. Propranolol for the treatment of lymphatic malformations in a neonate - a case report and review of literature. J Pediatr Pharmacol Ther. 2020;25:155–162. doi: 10.5863/1551-6776-25.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poralla C, Specht S, Born M, Müller A, Bartmann P, Müller A. Treatment of congenital generalized lymphangiectasia with propranolol in a preterm infant. Pediatrics. 2014;133:e439–e442. doi: 10.1542/peds.2012-2087. [DOI] [PubMed] [Google Scholar]
- 39.Mine K, Matsubayashi S, Nakai Y, Nakagawa T. Intestinal lymphangiectasia markedly improved with antiplasmin therapy. Gastroenterology. 1989;96:1596–1599. doi: 10.1016/0016-5085(89)90532-5. [DOI] [PubMed] [Google Scholar]
- 40.MacLean JE, Cohen E, Weinstein M. Primary intestinal and thoracic lymphangiectasia: a response to antiplasmin therapy. Pediatrics. 2002;109:1177–1180. doi: 10.1542/peds.109.6.1177. [DOI] [PubMed] [Google Scholar]
- 41.Prasad D, Srivastava A, Tambe A, Yachha SK, Sarma MS, Poddar U. Clinical Profile, response to therapy, and outcome of children with primary intestinal lymphangiectasia. Dig Dis. 2019;37:458–466. doi: 10.1159/000499450. [DOI] [PubMed] [Google Scholar]