Abstract
Purpose
The rs641738 C>T in membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) is implicated, along with the rs738409 C>G polymorphism in patatin-like phospholipase domain-containing protein 3 (PNPLA3), in nonalcoholic fatty liver disease (NAFLD). The association of these polymorphisms and NAFLD are investigated in Hispanic children with obesity.
Methods
Obese children with and without NAFLD were enrolled at a pediatric tertiary care health system and genotyped for MBOAT7 rs641738 C>T and PNPLA3 rs738409 C>G. NAFLD was characterized by the ultrasonographic presence of hepatic steatosis along with persistently elevated liver enzymes. Genetic variants and demographic and biochemical data were analyzed for the effects on NAFLD.
Results
Among 126 enrolled subjects, 84 in the case group had NAFLD and 42 in the control group did not. The two groups had similar demographic distribution. NAFLD was associated with abnormal liver enzymes and elevated triglycerides and cholesterol (p<0.05). Children with NAFLD had higher percentage of PNPLA3 GG genotype at 70.2% versus 31.0% in non-NAFLD, and lower MBOAT7 TT genotype at 4.8% versus 16.7% in non-NAFLD (p<0.05). PNPLA3 rs738409 C>G had an additive effect in NAFLD; however, MBOAT7 rs641738 C>T had no effects alone or synergistically with PNPLA3 polymorphism. NAFLD risk increased 3.7-fold in subjects carrying PNPLA3 GG genotype and decreased in MBOAT7 TT genotype.
Conclusion
In Hispanic children with obesity, PNPLA3 rs738409 C>G polymorphism increased the risk for NAFLD. The role of MBOAT7 rs641738 variant in NAFLD is less evident.
Keywords: Polymorphism, genetic; Nonalcoholic fatty liver disease; Lipid metabolism; Pediatric obesity; Hispanic Americans
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has emerged as one of the most challenging health problems in children and adolescents over the last decade. The spectrum of disease varies from asymptomatic fat deposition to nonalcoholic steatohepatitis (NASH) with different degrees of fibrosis leading to cirrhosis and hepatocellular carcinoma. The overall prevalence of fatty liver, an early stage of NAFLD, is approximately 9.6% in the general pediatric population but it is approximately 34% to 38% in children and adolescents with obesity [1,2,3]. Hispanic children have the highest prevalence of fatty liver disease with an estimated rate of 45% and typically present with a more aggressive pattern of disease [4,5,6]. Pathogenesis of NAFLD involves a multiple hit hypothesis starting with hepatic lipid accumulation caused by dysregulation of hepatic lipid metabolism homeostasis, then inflammation, hepatic fibrosis, and cirrhosis. Numerous risk factors for the development of NAFLD have been proposed such as insulin resistance, hormones secreted from adipose tissue, nutritional factors, obesity, dysbiotic gut microbiota, genetics, ethnicity, and epigenetics [7,8,9,10,11,12].
Genome-wide association studies have identified multiple gene polymorphisms that may be implicated in the pathophysiology of NAFLD, with most of these genes responsible for regulation of lipid metabolism [13,14]. The single-nucleotide polymorphism (SNP) rs738409 C>G in patatin-like phospholipase domain-containing 3 (PNPLA3) was the first gene polymorphism reported to be strongly associated with fibrosis or/and steatosis across multiethnic backgrounds [15,16,17,18,19,20]. In addition, the rs641738 C>T polymorphism of the membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) gene was found to be associated with the development of NAFLD [21,22,23,24,25]. In adults and children with Caucasian ethnicity, the rs641738 C>T in MBOAT7 has been associated with elevated liver enzymes, fibrosis, and fat content compared with subjects carrying the wild-type allele [21,22,23,24,25]. A study of Italian children with obesity showed that MBOAT7 rs641738 C>T polymorphism alone had no or only a marginal effect on liver function and development of fibrosis; however, in the presence of PNPLA3 rs738409 C>G polymorphism, a significant synergistic effect was observed [21]. It was noted that PNPLA3 rs738409 C>G polymorphism had the largest impact on the entire spectrum of liver damage from steatosis to fibrosis, while MBOAT7 rs641738 C>T polymorphism has been suggested to influence NAFLD-driven fibrosis but not steatosis in an adult study [25].
The correlation of NAFLD and MBOAT7 rs641738 C>T was not found in Hispanic or African-American cohorts as demonstrated in a limited number of multiethnic studies of adults and pediatric populations [22,23]. Several studies in Asian subjects also failed to establish an association of MBOAT7 rs641738 variants with NAFLD or liver enzymes [26,27,28]. In a genome-wide association study of a Hispanic boy with obesity, in addition to PNPLA3 and transmembrane 6 superfamily member 2 (TM6SF2), novel variants in trafficking protein particle complex 9 and an SNP in a region close to actin-related protein 5 were identified as associating with NAFLD activity score and fibrosis; however, MBOAT7 was not among the genes being identified [29]. Moreover, recent studies show that even in Caucasian adults and children, MBOAT7 rs641738 C>T was neither linked to hepatic fibrosis and hepatic fat nor did it mediate the effect of PNPLA3 rs738409 C>G [30,31,32]. Low levels of MBOAT7 protein expression were observed in the livers of patients with NAFLD but without correlation to rs641738 genotypes [31]. Although several multiethnic and Hispanic-specific cohort studies have illustrated the role of PNPLA3 rs738409 C>G in NAFLD [33,34,35,36], the effects of MBOAT7 rs641738 C>T alone or in combination with PNPLA3 rs738409 C>G polymorphisms on NAFLD are rarely investigated in the Hispanic population.
Available evidence suggests that an association of MBOAT7 rs641738 C>T and NAFLD remains controversial and yet to be validated in ethnicity-specific populations. Given the high risk for fatty liver disease in the Hispanic population, it is imperative to understand if MBOAT7 rs641738 C>T plays a role in the disease. We performed a case-control study in a cohort of Hispanic children with obesity and analyzed the influence of MBOAT7 rs641738 C>T and metabolic factors on the risk of NAFLD characterized by the ultrasonographic presence of hepatic steatosis along with persistently elevated liver enzymes. Additionally, we evaluated whether MBOAT7 rs641738 C>T modifies the effects of the PNPLA3 rs738409 C>G variant on development of NAFLD.
MATERIALS AND METHODS
Study design and study population
Study subjects were identified and enrolled between 2013 and 2018 at a pediatric tertiary care health system via review of electronic medical records. The Nemours Institutional Review Board approved the study protocol (IRB no. 270839).
1. Inclusion criteria
Hispanic subjects with obesity were enrolled into the control group and those with obesity and diagnosed with NAFLD were enrolled into the case group. Obesity was based on a body mass index (BMI) >85th percentile and determined by weight management or gastroenterology ambulatory clinicians.
2. Exclusion criteria
Subjects with other causes of hepatic dysfunction including autoimmune hepatitis, Wilson's disease, alpha-1-antitrypsin deficiency, metabolic liver disease, and hepatitis B or C were excluded. Subjects taking hepatotoxic medications or diagnosed with type 1 or type 2 diabetes mellitus were also excluded.
3. NAFLD diagnosis
NAFLD was based on a retrospective review of pragmatic clinical diagnosis of NAFLD where the ultrasonographic presence of hepatic steatosis was found along with persistently elevated alanine transaminase (ALT) and/or aspartate transaminase (AST) [3,4].
4. Liver steatosis evaluation
Steatosis was determined based on ultrasonographic results. Liver steatosis was assessed as 1) no steatosis: liver and renal cortex of the same echogenicity and 2) steatosis: increased diffuse echogenicity of the liver parenchyma. The final results were reviewed by a board-certified pediatric gastroenterologist.
5. Liver biopsy evaluation
Biopsy-documented steatohepatitis and liver fibrosis were not used to determine NAFLD as only 27% of patients had liver histopathology data available but were evaluated. The NASH activity and fibrosis scores were evaluated according to the NASH clinical research network histological scoring system [37] in those children who had undergone liver biopsy. The NASH score is defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2), and thus ranged from 0 to 8 with scores ≥5 considered a diagnosis of NASH; 3–4 considered not diagnostic, borderline, or positive for NASH; and <3 as none. Fibrosis was graded based on stages 0–4: F0 none, F1 perisinusoidal or periportal, F2 perisinusoidal and portal/periportal, F3 bridging fibrosis, and F4 cirrhosis. The NAFLD patients with a histological liver fibrosis stage of 1–2 were classified as “mild liver fibrosis,” and those with a histological fibrosis stage of 3–4 were classified as “advanced liver fibrosis.”
Subject clinical data collection
Demographics, clinical diagnosis, and anthropometric and biochemical data measurements were collected retrospectively from medical records at the time of either childhood obesity or NAFLD diagnosis after subjects were enrolled. Ethnicity was self-reported. Biochemical data included serum ALT, AST, gamma-glutamyl transferase (GGT), total cholesterol, triglycerides, and hemoglobin A1c (HbA1c). All tests were performed at our clinical laboratory. ALT, AST, GGT, cholesterol, and triglycerides were tested by Vitros Colorimetry System (Ortho Clinical Diagnostics, Raritan, NJ, USA), and HbA1c by Vantage Point of Care Immunoassay (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). Abnormal lab results were defined as values above laboratory-specific normal ranges. Actual test values were not evaluated due to different lab reference values among patients. Biopsy data including NAFLD activity score and fibrosis score were collected for patients in the case group when available. GGT serum values were not available for a subset of patients.
Genotyping
Genomic DNA was extracted from either peripheral whole blood or buccal swabs. Specifically, blood was collected in ethylene diamine tetra-acetic acid tubes, and genomic DNA from peripheral blood leukocytes was obtained using the Qiagen/Gentra Puregene Blood Kit (Valencia, CA, USA) per the manufacturer's protocol. Buccal cells were collected using Whatman Omni Swabs (GE Healthcare, Chicago, IL, USA) per the manufacturer's protocol and genomic DNA was isolated using the Qiagen QIAamp DNA Blood Mini kit following the manufacturer's buccal swab spin protocol. DNA was quantitated on the Qubit (Invitrogen, Carlsbad, CA, USA) using the dsDNA High Sensitivity Assay Kit (ThermoFisher Scientific/Life Technologies, Waltham, MA, USA).
Subjects were genotyped for MBOAT7 rs641738 (C>T) and co-segregates rs626283 (G>C) and PNPLA3 rs738409 (C>G). Briefly, genetic polymorphisms were determined using an ABI 7900HT Real-Time PCR Machine, 384-well plate format (ThermoFisher Scientific/Life Technologies, Grand Island, NY, USA). Genomic DNA was used for direct sequencing using the 40X TaqMan Assays and 2X Universal Master Mix II (ThermoFisher Scientific/Life Technologies) in 5 µL final volume via the manufacturer's recommended cycling conditions. Primers were purchased from Life Technologies: Assay ID C_7241_10 for rs738409, C_8716820_10 for rs641738, and C_2916337_10 for rs626283. SNPs were analyzed with SDS software version 2.4 (ThermoFisher Scientific/Life Technologies) with allelic discrimination and two-cluster enabling.
Statistical analysis
Data were expressed as means±standard deviation for continuous variables, and as a percent of the number of patients for categorical variables. Continuous variables with wide distributions were transformed to Log10 prior to analysis but raw means were calculated and presented in the results. Biochemical data of serum lipid panel and liver function tests were analyzed as categorical variables: either abnormal above lab-specific reference value or normal within the reference per patient, and evaluated as percent of abnormal over total subjects in a group. The genotypes were analyzed as categorical variables and evaluated as percentage of genetic risk variants (none, heterozygous, and homozygous) in a group unless stated otherwise.
The univariable analysis of clinical characteristics and genotype differences between NAFLD and non-NAFLD control patients was performed using t -test for continuous variables or one-way analysis of variance when appropriate, and by chi-square contingency for categorical variables (Table 1). When the genotypes of MBOAT7 (CC, CT, TT) or PNPLA3 (CC, CG, GG) were compared for the differences in NAFLD (%) and other variables, the Kruskal–Wallis H test was used to obtain p-values (Table 2). Hardy–Weinberg equilibrium was assessed by chi-square contingency.
Table 1. Characteristics of non-alcoholic fatty liver disease and control subjects.
| Subjects characteristic | NAFLD (Total n=84) | Control (Total n=42) | p-value | |||
|---|---|---|---|---|---|---|
| Mean±SD | 95% CI | Mean±SD | 95% CI | |||
| Demographics and diagnosis (%=n/total) | ||||||
| Age at diagnosis (yr) | 9.3±3.0 | 8.7–10 | 10.8±3.6 | 9.7–11.9 | 0.018 | |
| BMI z-score at diagnosis | 2.39±0.51 | 2.28–2.51 | 2.33±0.52 | 2.16–2.49 | 0.480 | |
| Sex (male/female) | 45/39 | 0.745–1.220 | 24/18 | 0.668–1.817 | 0.704 | |
| Acanthosis nigricans, % (n) | 66.7 (56) | 0.848–1.456 | 59.5 (25) | 0.497–1.343 | 0.430 | |
| Serum biochemical parameters: abnormal tests/total per cohort (%=n/total) | ||||||
| Hemoglobin A1c, % (n) | 14.3 (12) | 0.603–1.293 | 19.0 (8) | 0.682–2.281 | 0.490 | |
| AST, % (n) | 75.0 (63) | 1.864–3.743 | 9.5 (4) | 0.035–0.244 | <0.001 | |
| ALT, % (n) | 81.0 (68) | 1.975–4.515 | 14.3 (6) | 0.053–0.258 | <0.001 | |
| Triglycerides, % (n) | 54.8 (46) | 1.037–1.709 | 33.3 (14) | 0.321–0.942 | 0.023 | |
| Cholesterol, % (n) | 31.0 (26) | 1.099–1.717 | 11.9 (5) | 0.179–0.96 | 0.019 | |
| Genotype: number of genotypes/total per cohort (%=n/total) | ||||||
| MBOAT7 rs641738 TT genotype, % (n) | 4.8 (4) | 0.237–1.153 | 16.7 (7) | 1.237–3.536 | 0.026 | |
| MBOAT7 rs641738 TT/CT genotype, % (n) | 59.5 (50) | 0.854–1.424 | 52.4 (22) | 0.504–1.35 | 0.445 | |
| PNPLA3 rs738409 GG genotype, % (n) | 70.2 (59) | 1.302–2.406 | 31.0 (13) | 0.194–0.583 | <0.001 | |
NAFLD: nonalcoholic fatty liver disease, SD: standard deviation, CI: confidence interval, BMI: body mass index, AST: aspartate transaminase, ALT: alanine transferase, MBOAT7: membrane-bound O-acyltransferase domain-containing protein 7, PNPLA3: patatin-like phospholipase domain-containing 3.
Table 2. Comparison of phenotypic characteristics of MBOAT7 rs641738 genotypes.
| Characteristic | MBOAT7 rs641738 (Cohort n=126) | p-value | |||
|---|---|---|---|---|---|
| CC (n=54) | CT (n=61) | TT (n=11) | |||
| Demographic and clinical diagnosis | |||||
| Sex (male/female) | 33/21 | 34/27 | 2/9* | 0.033 | |
| Age (yr) | 9.8±3.6 | 9.7±3.0 | 10.7±3.6 | 0.626 | |
| BMI z-score | 2.39±3.6 | 2.35±3.0 | 2.39±3.6 | 0.836 | |
| Acanthosis nigricans | 63.0 (34) | 63.9 (39) | 72.7 (8) | 0.825 | |
| NAFLD | 63.0 (34) | 75.4 (46) | 36.4 (4)* | 0.031 | |
| Serum biochemical parameters: percent of abnormal tests | |||||
| Triglycerides | 44.4 (24) | 47.5 (29) | 63.6 (7) | 0.509 | |
| Cholesterol | 24.1 (13) | 24.6 (15) | 27.3 (3) | 0.975 | |
| AST | 55.6 (30) | 65.6 (40) | 36.4 (4) | 0.159 | |
| ALT | 53.7 (29) | 59.0 (36) | 18.2 (2)* | 0.044 | |
| PNPLA3 rs738409 GG genotype rate | |||||
| rs738409 GG | 63.0 (34) | 55.7 (34) | 36.4 (4) | 0.255 | |
Values are presented as number only, mean±standard deviation, or % (n). %=n/total in group.
MBOAT7: membrane-bound O-acyltransferase domain-containing protein 7, BMI: body mass index, NAFLD: nonalcoholic fatty liver disease, AST: aspartate transaminase, ALT: alanine transferase, PNPLA3: patatin-like phospholipase domain-containing 3.
*p<0.05 when compared with other two groups.
In addition, the genotypes were analyzed with an additive model corresponding to the number of risk alleles carried by each subject [21,23,25]. No risk allele carrier had a risk score of 0, heterozygous risk allele had a score of 1, and homozygous had a score of 2. If more than one SNP was analyzed, the total minor alleles were combined as the sum score of risk alleles carried by each subject, ranging from a score of 0 with no risk allele to a score of 4 if homozygous for both MBOAT7 (TT) and PNPLA3 (GG). The association of the genetic risk score of PNPLA3 and MBOAT7 alone (score 0, 1, 2) or in combination (score 0, 1, 2, 3, 4) with NAFLD (%) were analyzed using a general linear model with risk score as a continuous variable and NAFLD as a categorical variable and adjusted for covariates age, z-score, and sex [21,23,25]. Data were displayed as percent of NAFLD per risk score levels (Fig. 1). Finally, logistic regression analysis was conducted to evaluate the contribution of MBOAT7 and PNPLA3 homozygous genotype on NAFLD along with other factors to obtain an odds ratio and evaluate risk [21,23] (Table 3).
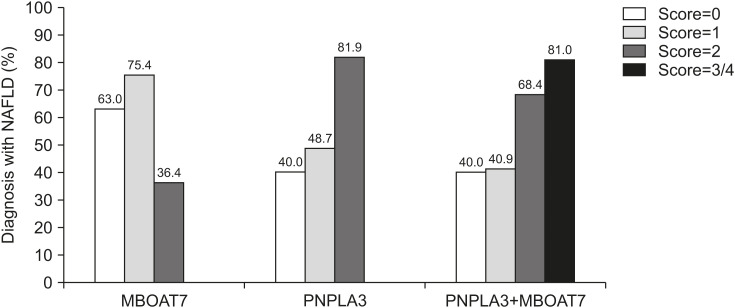
Fig. 1. Association between the genetic risk score and NAFLD diagnosis. Risk score was counted and added for each patient: number of T alleles in MBOAT7 rs641738 (score 0 to 2), number of G alleles in PNPLA3 rs738409 (score 0 to 2), and number of T and G alleles when MBOAT7 rs641738 and PNPLA3 rs738409 were combined (score 0 to 4). Only a few patients had a score of 4, thus were counted as having a score of 3 and together labeled as 3/4. The effect of genetic risk score on NAFLD diagnosis was analyzed in a general linear model with covariates sex, age, and z-score to obtain the p-values. The cohort was grouped based on the total risk scores from 0 to 3/4, and percentage of NAFLD in each risk score group was calculated and presented in the Y-axis of the figure.
Left column grouping: MBOAT7 rs641738 risk score as the dependent variable. Risk score has no correlation with NAFLD (p=0.747) or other covariates (p>0.05). Middle: PNPLA3 rs738409 risk score as the dependent variable. Risk score shows significant correlation with NAFLD p<0.001 and none in other covariates (p>0.05). Right: The combining risk scores of MBOAT7 rs641738 and PNPLA3 rs738409 as the dependent variable. Risk score shows significant correlation with NAFLD (p=0.002), and none in other covariates (p>0.05). p<0.05 was considered statistically significant.
NAFLD: nonalcoholic fatty liver disease, MBOAT7: membrane-bound O-acyltransferase domain-containing protein 7, PNPLA3: patatin-like phospholipase domain-containing protein 3.
Table 3. Comparison of phenotypic characteristics of PNAPL3 rs738409 genotypes in Hispanic cohort.
| Characteristic | PNPLA3 rs738409 (Cohort n=126) | p-value | |||
|---|---|---|---|---|---|
| CC (n=15) | CG (n=39) | GG (n=72) | |||
| Demographic and clinical diagnosis | |||||
| Sex (male/female) | 8/7 | 19/20 | 42/30 | 0.619 | |
| Age (yr) | 9.5±3.3 | 11.3±3.5* | 9.1±3.0* | 0.002 | |
| BMI z-score | 2.39±0.38 | 2.27±0.45 | 2.42±0.56 | 0.335 | |
| Acanthosis nigricans | 46.7 (7) | 53.8 (21) | 73.6 (53)* | 0.037 | |
| NAFLD | 40.0 (6) | 48.7 (19) | 81.9 (59)* | <0.001 | |
| Serum biochemical parameters: percent of abnormal tests | |||||
| Triglycerides | 40.0 (6) | 33.3 (13) | 56.9 (41) | 0.049 | |
| Cholesterol | 13.3 (2) | 15.4 (6) | 31.9 (23) | 0.086 | |
| AST | 33.3 (5) | 41.0 (16) | 73.6 (53)* | <0.001 | |
| ALT | 33.3 (5) | 43.6 (17) | 62.5 (45)* | 0.042 | |
| MBOAT7 rs641738 TT genotype rate | |||||
| rs641738 TT | 20.0 (3) | 10.3 (4) | 5.6 (4) | 0.402 | |
Values are presented as number only, mean±standard deviation, or % (n). %=n/total in group.
PNPLA3: patatin-like phospholipase domain-containing 3, BMI: body mass index, NAFLD: nonalcoholic fatty liver disease, AST: aspartate transaminase, ALT: alanine transferase, MBOAT7: membrane-bound O-acyltransferase domain-containing protein 7.
*p<0.05 when compared with other two groups.
Statistical analysis was performed using the SPSS statistical software system (SPSS version 27; IBM, Armonk, NY, USA). p<0.05 was considered significant.
RESULTS
Demographic, biochemical, and genotype comparison between NAFLD and control subjects
A total of 126 Hispanic children with obesity, 69 boys and 57 girls 5-18 years of age, were included in this study. NAFLD was diagnosed in 84 (67%) patients by the presence of hepatic steatosis on ultrasound with persistently elevated (value>reference) ALT and/or AST (NAFLD group; Table 1). The remaining 42 (33%) subjects comprised the control group without NAFLD (Table 1). No statistically significant differences were found in sex or BMI z-score at diagnosis between the two groups. Children with NAFLD diagnosis were slightly younger than those in the control group (Table 1, Demographics and diagnosis, p=0.018). No difference was found in the diagnosis of acanthosis nigricans or percentage of abnormal HbA1c between the groups (Table 1, Demographics and diagnosis Serum biochemical).
The NAFLD cohort showed a significantly higher percentage of patients with abnormal liver function tests (value>reference) as expected: 75.0% for AST and 81.0% for ALT (100% if abnormal AST and/or ALT), compared with the control group at 9.5% and 14.3%, respectively (p<0.001). Among metabolic markers, percentage of abnormal triglycerides was significantly higher in NAFLD at 54.8% than in the control group at 33.3% (p=0.023), and a similar difference was observed for total cholesterol with 31.0% in case and 11.9% in control (p=0.019).
The genotype distributions of MBOAT7 rs641738 (C>T) and PNPLA3 rs738409 (C>G) polymorphisms were in Hardy–Weinberg equilibrium (p>0.05). MBOAT7 rs641738 homozygous TT genotype rate was significantly lower in NAFLD at 4.8% than the control group at 16.7% (p=0.026), while the TT/TC genotype rates were similar at 59.5% in NAFLD and 52.4% in the control (p=0.445). In contrast, PNPLA3 rs738409 homozygous GG genotype was 70.2% in the NAFLD cohort, two-fold higher than in the control group at 31.0% (p<0.001). MBOAT7 polymorphism rs626283 (G>C) had 100% co-segregation with rs641738 and is not separately reported.
Association of genotypes with demographic, clinical, and biochemical data
No statistical differences were found in demographic characteristics and metabolic traits among MBOAT7 rs641738 genotypes (CC, CT, TT) except greater number of female subjects than male subjects in the TT genotype (Table 2, p=0.033). Furthermore, homozygous TT genotype was associated with a significantly lower percentage of NAFLD at 36.4% versus 75.4% in CT and 63.0% in CC (p=0.031), and lower percentage of abnormal ALT at 18.2% compared with CT at 59.0% and CC at 53.7% (p=0.044). Of interest, MBOAT7 rs641738 TT genotype carried a lower rate for the PNPLA3 rs738409 GG genotype at 36.4%, compared with CT at 55.7% or CC at 63.0%, though not statistically significant (p=0.255).
PNPLA3 rs738409 genotypes (CC, CG, GG) exhibited similar demographic characteristics except that CG genotypes were slightly but significantly older at diagnosis than CC and GG carriers (Table 3, p=0.002). The GG genotype displayed increased percentage of acanthosis nigricans at 73.6% compared with CG at 53.8% and CC at 46.7% (p=0.037), higher diagnosis of NAFLD at 81.9% compared with CG at 48.7% and CC at 40.0% (p<0.001), and increased percentage of abnormal AST and ALT compared with CG and CC (p<0.001; p=0.042; respectively). The percentage of abnormal serum triglycerides and cholesterol was higher in GG genotype of PNPLA3 rs738409 compared with CG and CC (Table 3, p=0.049; 0.086; respectively). PNPLA3 rs738409 homozygous GG genotype had the lowest carrying rate for MBOAT7 rs641738 homozygous TT at only 5.6% while CC genotype had the highest at 20.0%, but the difference was not significant among the three genotypes in the subject population studied (Table 3; p=0.402).
Liver biopsy was only performed in 34 patients who were all diagnosed with NAFLD; none were performed in the control group. The NASH and fibrosis scores of the genotypes of MBOAT7 and PNPLA3 (Table 4) were analyzed. In the 34 patients, 11 had no NASH (score <3), 14 were borderline (score 3–4), and nine had a diagnosis of NASH (score ≥5). Fibrosis data show that 20 patients did not present fibrosis, 10 had mild fibrosis with perisinusoidal or periportal, and only four patients had advanced fibrosis with bridging fibrosis, not cirrhosis. No signficant differences in NASH scores were found among the MBOAT7 rs641738 genotypes. Note that only two cases in the cohort were MBOAT7 rs641738 homozygous TT. There was a trend toward having more cases with a diagnosis of NASH (33.3%) in the GG genotype of PNPLA3 rs738409 compared with CC and CG who had no NASH. Also note there were only two cases with CC and five CG genotypes. MBOAT7 rs641738 genotypes displayed no difference in fibrosis severity, and in fact, no fibrosis was found in the homozygous TT (although there were only two cases). PNPLA3 rs738409 GG genotype displayed more mild (33.3%) and advanced (14.8%) fibrosis compared with GC or CC. No significant differences were discovered in the above analysis even when combining NASH scores of 3–4 and ≥5, or mild and advanced fibrosis, and heterozygous and homozygous genotypes (data not shown).
Table 4. Nonalcoholic steatohepatitis (NASH) and fibrosis scores in MBOAT7 and PNPLA3 genotypes.
| Score | N | MBOAT7 rs641738 | PNPLA3 rs738409 | |||||
|---|---|---|---|---|---|---|---|---|
| CC (11) | CT (21) | TT (2) | CC (2) | CG (5) | GG (27) | |||
| NASH | ||||||||
| <3 | 11 | 5 (45.5) | 6 (28.6) | None | 1 (50.0) | None | 10 (37.0) | |
| 3–4 | 14 | 5 (45.5) | 8 (38.1) | 1 (50.0) | 1 (50.0) | 5 (100) | 8 (29.6) | |
| ≥5 | 9 | 1 (9.1) | 7 (33.3) | 1 (50.0) | None | None | 9 (33.3) | |
| Fibrosis | ||||||||
| F0 | 20 | 7 (63.6) | 11 (52.4) | 2 (100) | 2 (100) | 4 (80.0) | 14 (51.9) | |
| F1 | 10 | 3 (27.3) | 7 (33.3) | None | None | 1 (20.0) | 9 (33.3) | |
| F3 | 4 | 1 (9.1) | 3 (14.3) | None | None | None | 4 (14.8) | |
Values are presented as number only or number (%). % is calculated based on the cases with no NASH (score <3), borderline NASH (score 3–4), or diagnosis of NASH (score ≥5) and no fibrosis (F0), mild fibrosis (F1, perisinusoidal or periportal), or advanced fibrosis (F3, bridging fibrosis) over the total cases in a genotype. Note: no cases with F2 (perisinusoidal or periportal) or F4 (cirrhosis) were in the study cohort.
MBOAT7: membrane-bound O-acyltransferase domain-containing protein 7, PNPLA3: patatin-like phospholipase domain-containing 3.
Association of genetic risk score with NAFLD
The effects of the MBOAT7 rs641738 C>T and PNPLA3 rs738409 C>G polymorphism on NAFLD were evaluated individually or in combination by genetic risk scores under a general linear model (see methods for details). The risk score 2 in the MBOAT7 rs641738 (TT) had the lowest NAFLD at 36.4%, while score 1 (CT) had the highest percentage of NAFLD at 75.4% and score 0 (CC) had 63.0% NAFLD diagnosis, but the difference was not significant when covariates age, z-score, and sex were controlled in the regression model (p=0.747; Fig. 1, left column grouping). In contrast, PNPLA3 rs738409 minor G allele showed an additive effect on NAFLD diagnosis with score 0 (CC) at 40.0%, score 1 (CG) slightly increased to 48.7%, and score 2 (GG) significantly higher at 81.9% even when other covariates were controlled (p<0.001; Fig. 1, middle column grouping). Combining genetic risk scores of MBOAT7 rs641738 and PNPLA3 rs738409 demonstrated a significantly higher NAFLD diagnosis in score 2 and 3/4 subjects compared with scores 0 and 1 (Fig. 1, right column grouping). However, there were no added effects on NAFLD compared with the analysis with individual polymorphism only. Subjects with a combined risk score of 3/4 had an 81.0% NAFLD diagnosis (right column grouping), which is nearly identical to 81.9% found in subjects with a risk score of 2 in the model with PNPLA3 rs738409 risk alleles alone (middle). Furthermore, in the combined model, percent of NAFLD in subjects with a score of 2 was 68.4%, which was lower than in subjects with a score of 2 in the PNPLA3 rs738409 alone model at 81.9%. This is likely due to the lower percent of NAFLD in subjects with a score of 2 in the MBOAT7 rs641738.
Logistic regression analysis was conducted to assess the risk of MBOAT7 rs641738 homozygous TT and PNPLA3 rs738409 homozygous GG on NAFLD when the effects of other factors were controlled. Only variables that showed significant association (p<0.05) with NAFLD or with genotypes in univariable analysis (Tables 1-3) were included in the analysis. Table 5 shows that the PNPLA3 rs738409 GG was significantly associated with an increased risk of NAFLD with an odds ratio of 3.736 (p=0.005) when the influence from other variables were controlled. In contrast, patients carrying the MBOAT7 rs641738 TT genotype exhibited a reduced rate of NAFLD with an odds ratio of 0.185 (p=0.028). None of the other factors showed significant influence on NAFLD in the regression analysis.
Table 5. Logistic regression analysis of factors contributed to NAFLD.
| Independent variable | p-value | Odds ratio (95% CI) |
|---|---|---|
| Sex | 0.200 | 1.83 (0.726–4.611) |
| Age (yr) | 0.192 | 0.914 (0.799–1.046) |
| Acanthosis nigricans | 0.765 | 1.151 (0.459–2.886) |
| Abnormal triglycerides | 0.314 | 1.612 (0.636–4.085) |
| Abnormal cholesterol | 0.089 | 2.921 (0.848–10.062) |
| MBOAT7 rs641738 TT | 0.028 | 0.185 (0.041–0.831) |
| PNPLA3 rs738409 GG | 0.005 | 3.736 (1.488–9.383) |
NAFLD: nonalcoholic fatty liver disease, CI: confidence interval, MBOAT7: membrane-bound O-acyltransferase domain-containing protein 7, PNPLA3: patatin-like phospholipase domain-containing 3.
DISCUSSION
The data in the present study validate the strong correlation between the PNPLA3 rs738409 polymorphism and NAFLD as previously reported in the Hispanic population [15,33,34,35,36]. PNPLA3 rs738409 C>G polymorphism has been implicated in NAFLD across ethnicities and races, though with a higher minor allele frequency in Hispanic ethnicity [15,33,34]. In a limited number of Hispanic-only cohort studies, the minor allele frequency was reported to be 50% to 80% in patients detected with fatty liver [35,36], which was very similar to what we observed in the present study. Together, our data and previous findings support that PNPLA3 expression is important in lipid metabolism pathway and thus NAFLD pathogenesis in a Hispanic population of children with obesity.
However, the present data failed to show that the MBOAT7 rs641738 C>T polymorphism increased the risk of NAFLD. Of note, the MBOAT7 rs641738 genotypic rate of CC, CT, and TT in the present study are similar to that previously reported in Hispanic children with obesity [23]. Lack of correlation between NAFLD and MBOAT7 rs641738 C>T in a Hispanic cohort has only been reported in a limited number of multiethnic studies [22,23]. It is not entirely clear why MBOAT7 rs641738 C>T only correlates with NAFLD in Caucasians and not in Hispanic or African-American populations [22,23]. It is known from epidemiological studies that Caucasian, African-American, and Hispanic populations have different risks for hepatic fat accumulation, likely rooted in genetic differences in lipid metabolism [4,5,6]. Pathogenesis of NAFLD may be less dependent on the lipid metabolism pathway affected by MBOAT7 polymorphism in those of Hispanic ethnicity. The absence of an association of MBOAT7 rs641738 C>T genotypes and NAFLD was also observed in Asian cohort studies [26,27,28], supporting an alternative lipid metabolic pathway in different ethnicities.
A previous study demonstrated the effect of the MBOAT7 rs641738 C>T was on liver fibrosis alone, not on liver enzymes or hepatic steatosis compared with other genetic variants, such as PNPLA3 rs738409, thought to be involved in the full spectrum of NAFLD progression [25]. Therefore, NAFLD based on liver steatosis might not reveal the influence of MBOAT7 rs641738 variants, which might result in the absence of the correlation. However, recent studies in Caucasian subjects seem to dispute this notion [30,31,32]. In a case-control study with a large cohort of European adults, Sookoian et al. [31] found no evidence of correlation between rs641738 variants and NAFLD measured from liver enzymes, hepatic steatosis, NASH, or fibrosis status. In a study of Italian children with obesity, MBOAT7 rs641738 genotypes were found not to associate with NAFLD based on hepatic fat content while PNPLA3 alone or in combination with GCKR (glucokinase regulator) and TM6SF2 risk alleles showed significantly increased risk of NAFLD [32]. This challenges some of the previous publications in Caucasian children [21,23]. These contradictory results imply that the role of MBOAT7 rs641738 polymorphism even in the Caucasian population needs further scrutiny.
The data in the present study show a small but significantly reduced rate of NAFLD in children carrying MBOAT7 rs641738 homozygous TT (Table 1), a finding not previously reported in studies of a Hispanic population. One plausible explanations is that the MBOAT7 rs641738 homozygous TT genotype is accompanied with a low rate of the PNPLA3 rs738409 homozygous GG genotype at 36.4%, compared with CC at 63.0% or CT at 55.7% (Table 2). PNPLA3 rs738409 polymorphism has been known to have the largest genetic impact on liver damage [22,23,25], which was also demonstrated in our study (Table 1). It is possible that the reduced rate of PNPLA3 rs738409 genotype GG in subjects carrying MBOAT7 rs641738 TT could indirectly decrease overall NAFLD risk, thus having a minimal or negative effect as noted in the present data. It is unlikely that the MBOAT7 rs641738 homozygous TT genotype has a direct protective and independent role against NAFLD in Hispanic children. The decreased carrier rate of the PNPLA3 rs738409 GG in MBOAT7 rs641738 TT genotype was not discussed in previous studies [22,23]. If confirmed, the lack of correlation between NAFLD and MBOAT7 rs641738 polymorphism could be explained by this unique relationship between the two polymorphisms in the Hispanic population.
The data in the present study also illustrated that the abnormal results of lipid marker triglycerides and cholesterol were significantly associated with an increased incidence of NAFLD. Several studies in adults illustrate that abnormal serum triglycerides are an independent risk factor for NAFLD [9,10,11,12]. It has also been proposed that excessive free fatty acids delivered to the liver via the circulation from abnormally high serum triglycerides, commonly associated with obesity, may overwhelm the normal capacity of the liver to oxidize or process lipids and may contribute to steatosis [12]. However, the regression analysis in the present study shows that the influence of lipid markers is no longer significant when the influences from other variables were controlled. These data point to a hypothesis that the high prevalence of PNPLA3 rs738409 GG genotype in the NAFLD group at 70% had a dominant effect on NAFLD outcome, overriding the contribution from abnormal lipid markers. Together, it is plausible that the abnormal lipid markers play a less important role in Hispanic pediatric populations with obesity. The driving factor associated with NAFLD is the PNPLA3 rs738409 GG genotype.
Several risk factors have been known to strongly correlate with the development of NAFLD such as obesity, diabetes, insulin resistance, sex, and ethnicity [7,8,9,10,11,12]. Therefore, when interpreting the current data, the influence from these factors needs to be taken into consideration. It is possible that the polymorphisms of MBOAT7 and PNPLA3 might associate with these factors, thus affecting NAFLD outcome. The genetic influence of PNPLA3 and MBOAT7 on NAFLD has been shown to vary according to ethnicity [15,21,23,33,34]. We are examining a Hispanic population, thus the influence from ethnicity is not a concern. Furthermore, we excluded children with diagnosed type 1 diabetes mellitus, which limited the interference on NAFLD.
In our study, acanthosis nigricans, an indicator of insulin resistance, was similar between the NAFLD and non NAFLD groups, although a significant correlation between acanthosis nigricans and PNPLA3 rs738409 GG variant was noted, which in turn could influence the relationship between NAFLD and the variant. However, the regression analysis illustrated that PNPLA3 rs738409 GG variant significantly predicted NAFLD outcome when the influences from other risk factors including acanthosis nigricans were controlled. This result indicates that the independent role of PNPLA3 genetic variant in NAFLD, while acanthosis nigricans, despite showing a correlation with rs738409 GG variant, did not contribute to NAFLD in this Hispanic population with obesity. In a study of Taiwanese children with obesity, PNPLA3 rs738409 genetic variants, along with BMI, male sex, and serum adiponectin, independently and significantly predicted NAFLD but the variants are not correlated with metabolic factors such as serum fasting insulin/glucose, BMI, and sex [19]. Santoro et al. [18] showed that PNPLA3 rs738409 risk allele G, despite having increased hepatic steatosis did not display more insulin resistance than the C homozygote in a multiethnic study of children with obesity. In a case-control study of adults with normal weight and with obesity, the rs641738 of MBOAT7 variants was not linked to glucose metabolism, insulin resistance, or lipid panel [31]. By and large, our findings agree with these previous studies [18,19,31], which suggests that in the population with obesity, the polymorphisms of MBOAT7 and PNPLA3 might independently predict NAFLD, and the influence from insulin resistance plays a less important role.
Furthermore, NAFLD is consistently more prevalent in boys than in girls, which suggests that sex hormones are associated with the predilection for pediatric NAFLD [3,32]. This correlation was consistently perceived in normal, with overweight, and with obesity populations and in both children and adults [19,22,30,32]. However, genetic influence on NAFLD has not been specifically analyzed between males and females. The distribution of MBOAT7 and PNPLA3 genetic variants are reported according to sex, but no difference was reported in MBOAT7 and PNPLA3 polymorphisms [18,19,20,21]. In our cohort, no sex difference was found between NAFLD and the control group. However, our results showed that there were more female subjects in the MBOAT7 rs641738 TT genotype, which could explain the lower NAFLD rate in TT carriers that we observed. However, this is also likely due to the low frequency of TT. Moreover, no association of sex with NAFLD was found in regression analysis. Only MBOAT7 rs641738 TT genotype and PNPLA3 rs738409 GG are significantly associated with NAFLD, even when the influence from sex difference and other variables were controlled in the regression. A larger sample size is needed to further confirm whether or not the lower NAFLD rate in the MBOAT7 rs641738 TT genotype is confounded by the higher percentage of females.
There are limitations to the present study:
1) There is insufficient power to reveal the effects of MBOAT7 rs641738 on NAFLD due to a low frequency of homozygous TT genotype in the studied cohort and less subjects in the control group. However, there was sufficient power for PNPLA3 rs738409 GG genotype to predict NAFLD outcome. Nevertheless, the rate of MBOAT7 rs641738 genotypes in the entire cohort was similar to a previously reported study of Hispanic children with obesity [23]. A larger cohort of patients may generate more robust results required to understand the involvement of the MBOAT7 rs641738 C>T polymorphism in NAFLD and the interaction with other genetic variants. In addition, a selection bias may be introduced in the study due to more cases in the NAFLD group than the control group leading to an error in estimation of the effects of variables on NAFLD.
2) The study did not evaluate the severity of hepatic steatosis by quantitative image analysis. Furthermore, examinations of liver inflammation and fibrosis via biopsy were only obtained for a small number of NAFLD patients with no control patients, thus NAFLD was only determined based on liver steatosis on ultrasound and liver enzyme. Among the 34 patients who had biopsy conducted, the distribution of genotypes, particularly the homozygous, are extremely imbalanced; MBOAT7 rs641738 TT was only 6% (2 of 34) and PNPLA3 rs738409 GG was very high at 80% (27 of 34). Nevertheless, this pattern reflects our reported result that NAFLD is associated with an increased rate of PNPLA3 rs738409 GG and decreased rate of MBOAT7 rs641738 TT in NAFLD. There might be a trend of more NASH diagnoses (score ≥5) and severe fibrosis in patients carrying PNPLA3 rs738409 GG. However, due to the small sample size and uneven genotype distribution, the results on the correlation between the genetic variants and the severity of liver inflammation or fibrosis are inconclusive. Biopsy is infrequently conducted in children in a clinical setting or in research. NAFLD was based on the ultrasonographic presence of hepatic steatosis along with persistently elevated ALT and/or AST. Therefore, the results can only explain the association between this specifically defined NAFLD and genetic variants and cannot predict the development of liver inflammation and fibrosis. Previous studies suggested that elevated liver enzymes along with liver ultrasound can effectively detect NAFLD in children [3,4]. Liver steatosis has been used to investigate the correlation between MBOAT7 variants and NAFLD in several pediatric studies [21,23,24]. We believe that our results are valuable to understand the role of genetic variants in pediatric NAFLD, particularly those with Hispanic ethnicity and obesity.
3) Finally, the study lacks a population without obesity to examine the influence of the two genetic polymorphisms on NAFLD. Therefore, our data are limited to only explaining the relationship in a Hispanic population with obesity. In a study of adults and children, Mangge et al. [20] demonstrated that the rate of PNPLA3 risk variants CG and GG showed no difference between normal weight and with obesity groups, indicating the PNPLA3 polymorphism does not always vary according to weight. In a study of Caucasians with normal weight, the PNPLA3 rs738409, together with age, sex, and BMI was found to be an independent predictor of liver fat [16]. The G allele of PNPLA3 rs738409 showed a strong correlation with NAFLD in a study of a normal weight Japanese adults [17]. These previous studies hint that the genetic variants could influence the development of NAFLD similarly in both normal weight as well as the obese population [16,17,20]. A larger sample size study with a population consisting of an obese and nonobese population will be necessary to confirm our findings, particularly the relationship between MBOAT7 rs641738 risk allele and NAFLD, which has not been widely investigated.
In conclusion, the data support that NAFLD diagnosis is strongly associated with increased rate of PNPLA3 rs738409 GG homozygous genotype in this Hispanic cohort of children with obesity. Conversely, the MBOAT7 rs641738 TT homozygous genotype does not predict the risk of NAFLD or have additive effects when combined with PNPLA3 polymorphism rs738409 risk allele C>G. The core results from our study agree with previous findings that the interplay of genetic predisposition and factors such as hyperlipidemia influence NAFLD. This study is the first to provide valuable insight into the complex relationship between MBOAT7 rs641738 C>T and PNPLA3 rs738409 C>G polymorphisms in this population.
ACKNOWLEDGEMENTS
The authors thank the researchers at the Biomolecular Core Lab and Gastroenterology Research Lab and the Department of Biomedical Research, Nemours/Alfred I. duPont Hospital for Children, for their contribution to this work.
Footnotes
Funding: This research was funded by the Nemours Biomedical Research Department.
Conflict of Interest: The authors have no financial conflicts of interest.
Current Affiliation: Sana Mansoor: Division of Pediatric Gastroenterology, Herman and Walter Samuelson Children's Hospital at Sinai Hospital of Baltimore, Baltimore, MD, USA
Anshu Maheshwari: Division of Pediatric Gastroenterology, University of Illinois College of Medicine in Peoria and Children's Hospital of Illinois, Peoria, IL, USA
Katryn Furuya: University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI, USA
Makala Wang: Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA, USA
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in young adults in the United States. J Clin Gastroenterol. 2018;52:339–346. doi: 10.1097/MCG.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 3.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzuillo P, Miraglia del Giudice E, Santoro N. Pediatric fatty liver disease: role of ethnicity and genetics. World J Gastroenterol. 2014;20:7347–7355. doi: 10.3748/wjg.v20.i23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro N, Feldstein AE, Enoksson E, Pierpont B, Kursawe R, Kim G, et al. The association between hepatic fat content and liver injury in obese children and adolescents: effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care. 2013;36:1353–1360. doi: 10.2337/dc12-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 8.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomizawa M, Kawanabe Y, Shinozaki F, Sato S, Motoyoshi Y, Sugiyama T, et al. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed Rep. 2014;2:633–636. doi: 10.3892/br.2014.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo R, Crespo J, Martínez-Arranz I, Banales JM, Arias M, Mincholé I, et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun. 2018;2:807–820. doi: 10.1002/hep4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallwitz ER, Kumar M, Aggarwal R, Berger R, Layden-Almer J, Gupta N, et al. Ethnicity and nonalcoholic fatty liver disease in an obesity clinic: the impact of triglycerides. Dig Dis Sci. 2008;53:1358–1363. doi: 10.1007/s10620-008-0234-x. [DOI] [PubMed] [Google Scholar]
- 12.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 14.Del Campo JA, Gallego-Durán R, Gallego P, Grande L. Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD) Int J Mol Sci. 2018;19:911. doi: 10.3390/ijms19030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyysalo J, Stojkovic I, Kotronen A, Hakkarainen A, Sevastianova K, Makkonen J, et al. Genetic variation in PNPLA3 but not APOC3 influences liver fat in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2012;27:951–956. doi: 10.1111/j.1440-1746.2011.07045.x. [DOI] [PubMed] [Google Scholar]
- 17.Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, et al. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:172. doi: 10.1186/1471-2350-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoro N, Kursawe R, D'Adamo E, Dykas DJ, Zhang CK, Bale AE, et al. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281–1290. doi: 10.1002/hep.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YC, Chang PF, Hu FC, Yang WS, Chang MH, Ni YH. A common variant in the PNPLA3 gene is a risk factor for non-alcoholic fatty liver disease in obese Taiwanese children. J Pediatr. 2011;158:740–744. doi: 10.1016/j.jpeds.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Mangge H, Baumgartner BG, Zelzer S, Prüller F, Schnedl WJ, Reininghaus EZ, et al. Patatin-like phospholipase 3 (rs738409) gene polymorphism is associated with increased liver enzymes in obese adolescents and metabolic syndrome in all ages. Aliment Pharmacol Ther. 2015;42:99–105. doi: 10.1111/apt.13232. [DOI] [PubMed] [Google Scholar]
- 21.Di Sessa A, Umano GR, Cirillo G, Del Prete A, Iacomino R, Marzuillo P, et al. The membrane-bound O-acyltransferase7 rs641738 variant in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2018;67:69–74. doi: 10.1097/MPG.0000000000001979. [DOI] [PubMed] [Google Scholar]
- 22.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150:1219–30.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umano GR, Caprio S, Di Sessa A, Chalasani N, Dykas DJ, Pierpont B, et al. The rs626283 variant in the MBOAT7 gene is associated with insulin resistance and fatty liver in Caucasian obese youth. Am J Gastroenterol. 2018;113:376–383. doi: 10.1038/ajg.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viitasalo A, Eloranta AM, Atalay M, Romeo S, Pihlajamäki J, Lakka TA. Association of MBOAT7 gene variant with plasma ALT levels in children: the PANIC study. Pediatr Res. 2016;80:651–655. doi: 10.1038/pr.2016.139. [DOI] [PubMed] [Google Scholar]
- 25.Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YC, Chang PF, Chang MH, Ni YH. Genetic determinants of hepatic steatosis and serum cytokeratin-18 fragment levels in Taiwanese children. Liver Int. 2018;38:1300–1307. doi: 10.1111/liv.13689. [DOI] [PubMed] [Google Scholar]
- 27.Koo BK, Joo SK, Kim D, Bae JM, Park JH, Kim JH, et al. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1277–1285. doi: 10.1111/jgh.14056. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi T, Shima T, Mizuno M, Mitsumoto Y, Umemura A, Kanbara Y, et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13:e0185490. doi: 10.1371/journal.pone.0185490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wattacheril J, Lavine JE, Chalasani NP, Guo X, Kwon S, Schwimmer J, et al. Genome-wide associations related to hepatic histology in nonalcoholic fatty liver disease in Hispanic boys. J Pediatr. 2017;190:100–7.e2. doi: 10.1016/j.jpeds.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basyte-Bacevice V, Skieceviciene J, Valantiene I, Sumskiene J, Petrenkiene V, Kondrackiene J, et al. TM6SF2 and MBOAT7 gene variants in liver fibrosis and cirrhosis. Int J Mol Sci. 2019;20:1277. doi: 10.3390/ijms20061277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sookoian S, Flichman D, Garaycoechea ME, Gazzi C, Martino JS, Castaño GO, et al. Lack of evidence supporting a role of TMC4-rs641738 missense variant-MBOAT7- intergenic downstream variant-in the susceptibility to nonalcoholic fatty liver disease. Sci Rep. 2018;8:5097. doi: 10.1038/s41598-018-23453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Costanzo A, Pacifico L, Chiesa C, Perla FM, Ceci F, Angeloni A, et al. Genetic and metabolic predictors of hepatic fat content in a cohort of Italian children with obesity. Pediatr Res. 2019;85:671–677. doi: 10.1038/s41390-019-0303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2013;11:1183–90.e2. doi: 10.1016/j.cgh.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tricò D, Caprio S, Rosaria Umano G, Pierpont B, Nouws J, Galderisi A, et al. Metabolic features of nonalcoholic fatty liver (NAFL) in obese adolescents: findings from a multiethnic cohort. Hepatology. 2018;68:1376–1390. doi: 10.1002/hep.30035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez LA, Larrieta E, Kershenobich D, Torre A. The expression of PNPLA3 polymorphism could be the key for severe liver disease in NAFLD in Hispanic population. Ann Hepatol. 2017;16:909–915. doi: 10.5604/01.3001.0010.5282. [DOI] [PubMed] [Google Scholar]
- 36.Chinchilla-López P, Ramírez-Pérez O, Cruz-Ramón V, Canizales-Quinteros S, Domínguez-López A, Ponciano-Rodríguez G, et al. More evidence for the genetic susceptibility of Mexican population to nonalcoholic fatty liver disease through PNPLA3. Ann Hepatol. 2018;17:250–255. doi: 10.5604/01.3001.0010.8644. [DOI] [PubMed] [Google Scholar]
- 37.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]