Abstract
Background
Metastatic uveal melanoma (UM) has no effective treatment. To date, no publications have reported immunohistochemical evidence of estrogen receptors (ERs) in UM; however, changes in pathologic reporting for ER in breast carcinoma prompted a re-examination of ER in UM, as it could represent a potential therapeutic target.
Objective
To determine if UM tumors express ER by immunohistochemistry (IHC) using current methodology for breast cancer and to evaluate ER gene expression using a publicly available UM database.
Methods
A retrospective IHC analysis with clinical correlation was performed on 2 cohorts: 57 cases from the Cleveland Clinic (CC) and 50 from the Ohio State University Wexner Medical Center (OSUWMC). Analysis of The Cancer Genome Atlas Project (TCGA) UM Dataset of 80 patients was also performed.
Results
Presence of ER was detected by IHC in 20 of 34 (59%) analyzable cases in the CC cohort. Of the 50 patients in the OSU cohort, 52 specimens from 47 patients were sufficient for analysis. Of these 47 cases, 29 (62%) had tumor that was ER positive in ≥1% nuclei. In the second cohort, positivity was classified as positive (≥10% nuclei, 34% cases) or low positive (1–9% nuclei, 28% cases). In 5 patients, there were paired samples, that is, primary tumor and subsequent recurrence or metastasis, with concordance for ER in 4 of 5 cases. In the TCGA database, elevated ESR1 and ESR2 gene expression was identified in a subset of UM tumors with poor genetic prognostic features.
Conclusions and Relevance
Potentially actionable ER expression is present in greater than half of UM cases by IHC. Gene expression of ESR1 and ESR2 was elevated in a subset of UM tumors with poor prognostic features. These data provide a rationale to evaluate ER as a potential target for therapy in UM.
Keywords: Estrogen receptors, Immunohistochemistry, Uveal melanoma, Prognosis, Treatment
Introduction
Uveal melanoma (UM) accounts for about 3% of all melanomas [1]. Singh et al. [1] reviewed the SEER (Surveillance, Epidemiology, and End Results) US database from 1973 to 2013, which showed an unchanged adjusted incidence of 5.2 per million. The mortality rate, close to 50% at 15 years, has remained unchanged for decades [2]. Because metastatic disease is generally incurable, newer therapies are needed. The successful use of selective estrogen receptor modulators (SERMs) in many breast carcinomas raises the possibility of its potential use in UM as a form of targeted therapy, if the estrogen receptor (ER) is present.
To our knowledge, there are no published reports documenting the presence of ER in UM. A PubMed search found 3 reports from the 1990s where testing for ER was performed, but it was not identified [3, 4, 5]. However, contemporary ER testing methodology has not been applied to UM.
Gender-based differences in survival provide additional rationale for evaluating potential hormonal-driven mechanisms in UM. The RARECARE project examining 4,097 cases of UM [6] found that 5-year survival rates were better for women than for men, and another study reported differences in incidence and metastasis-related mortality by gender, with males having a worse prognosis [7]. Additionally, melanocytic lesions have been observed to grow during puberty and pregnancy, suggesting a hormonal role [8].
We evaluated ER expression in UM using current immunohistochemistry (IHC) methodology and gene expression levels from the UM-TCGA database. Association with known prognostic genetic markers and survival was also examined.
Materials and Methods
The study was approved by the Institutional Review Boards (IRB) of the Cleveland Clinic (CCF) and Ohio State Wexner Medical Center (OSUWMC) and adhered to the tenets of the Declaration of Helsinki. Fifty-seven cases of UM from 2004 to 2010 from CCF were identified (initial cohort). Of these, 33 were treated by enucleation or tumor resection, and one had a biopsy-proven hepatic metastasis providing 34 specimens for ER evaluation by IHC. The second independent cohort for validation was collected at OSUWMC and provided samples (n = 55) from 47 patients from 2010 to 2017. There were paired samples (primary plus recurrence or metastasis) from 5 patients. Cases were excluded (n = 3) due to insufficient tissue.
At CCF, IHC was performed on 4-μm sections of formalin-fixed paraffin-embedded tissues using a Discovery XT automated stainer (Ventana Medical Systems, Tucson, AZ, USA). Antigen retrieval consisted of CC1 (Tris/Borate/EDTA buffer, pH 8.0–8.5) (Ventana) for 8 min at 95°C and 28 min at 100°C, with 8-min cool down to 37°C. Slides were incubated with anti-ER rabbit antibody (prediluted, clone ID: SP1, Catalog No. 790–4325, Ventana), 16 min at 37°C. Secondary antibody (UltraMap anti-rabbit AP; Tucson, AZ, USA) was applied for 16 min at 37°C. Chromogenic substrate (ChromoMap Fast Red; Tucson, AZ, USA) was applied for 16 min at 37°C and then 4 min of Activator Red and naphthol. Slides were counterstained with hematoxylin II.
At OSUWMC, 4-micron sections were placed in a 60°C oven for 1 h, cooled, and placed on the Leica BondIII Autostainer. Slides were deparaffinized and rehydrated online with Bond Dewax and 100% alcohol. Antigen retrieval was performed online using Leica's Bond Epitope Retrieval Solution ER for 10 min. Slides were quenched for 5 min in a 3% hydrogen peroxide and then followed by 15-min incubation at room temperature with ER clone SP1 (Spring Biosciences). The detection system used was Leica's Bond Polymer Refine Detection (product code: DS9800). Sections were incubated with DAB mixed online for 10 min and counterstained with Leica hematoxylin for 3 min. Slides were then dehydrated through graded ethanol solutions, xylene, and coverslips applied.
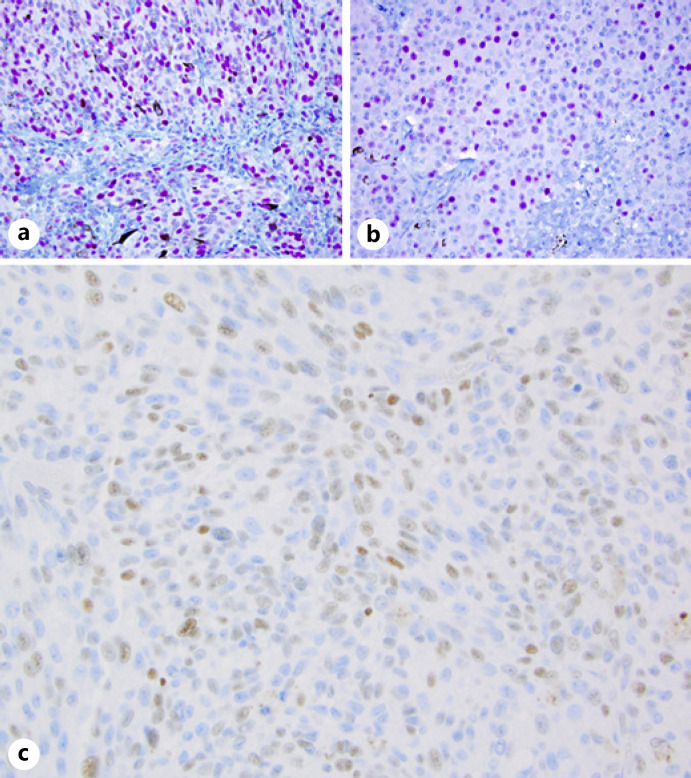
ER expression (shown in Fig. 1) was considered positive if ≥1% of the tumor nuclei were stained, the same cutoff currently used in breast carcinoma. Tumors were further classified as low positive (1–9%) or positive (≥10%). Where available, monosomy 3 or other molecular prognostic indicators, tested by FISH, cytogenetics, SNP arrays, or Castle Bioscience gene expression profile, were incorporated into the study.
Fig. 1.
Immunohistochemical stains for ER in UM: with red chromagen (cohort 1) (a, b); with brown chromagen (cohort 2) (c). ×400. ER, estrogen receptor; UM, uveal melanoma.
Descriptive statistics on both cohorts were performed to evaluate percentages of ER-positive tumors and sex. Survival analysis was performed on the OSUWMC second cohort. The occurrence of metastasis or melanoma-specific death was considered an event. Data were analyzed via a log rank test for ER presence and sex through the use of Kaplan-Meier curves. The interaction between sex and ER presence was also tested via a log rank test. Patients who reached the end of the study but had not progressed were considered censored at the last documented date of contact. Additionally, 2 patients that died from nonmelanoma causes were censored at time of death. A χ2 test of association was also used to examine the relationship between sex and ER presence.
TCGA UM Database Analysis
The TCGA UM dataset was analyzed using the CBioPortal tool [9, 10]. The ESR1 and ESR2 mRNA expression and gene deletion were evaluated and placed in a heat map that included tracks for loss of chromosome 3p and 3q as well as gene expression of BAP1 to identify tumors with poor prognostic markers, including loss of chromosome 3 and loss of BAP1 expression [11].
Results
The results of the 34 cases analyzed in the initial cohort are summarized in Table 1. The majority (59%) of cases were ER(+), most ranging from 1 to 50%, with one case showing 75% positive nuclei. Positivity in males and females was similar (n = 9 and 11, respectively), while most ER(−) tumors were from males (12 males and 2 females). While formal statistics were not performed on this cohort, the following was noted: 5 of 8 males with ER(+) tumors died from disease and/or had metastases compared to 3/12 males with ER(−) tumors. For females, 5/12 ER(+) cases died/had metastases compared to 0/2 ER(−) cases.
Table 1.
ER status in 2 cohorts with clinical and pathologic findings
| OSUWMC cohort | ER status, n (%) |
|
|---|---|---|
| positive (N = 29) | negative (N = 18) | |
| Male | 19 (65.5) | 9 (50) |
| Female | 10 (34.5) | 9 (50) |
| AJCC pT category | ||
| pT1 | 3 (10.3) | 4 (22.2) |
| pT2 | 4 (13.8) | 1 (5.6) |
| pT3 | 10 (34.5) | 6 (33.3) |
| pT4 | 11 (37.9) | 5 (27.8) |
| Biopsy | 0 (0) | 1 (5.6) |
| Liver met | 0 (0) | 1 (5.6) |
| Other | 1 (3.4) | 0 (0.0) |
| Monosomy 3/8q gain and/or class 2 GEP+ | ||
| Positive | 16 (55.2) | 5 (2.8) |
| Negative | 5 (17.2) | 2 (11.1) |
| Cell type | ||
| Epithelioid or mixed | 22 (7.6) | 11 (61.1) |
| Spindle B | 7 (2.4) | 7 (3.9) |
| Cleveland Clinic cohort | ER status, n (%) |
|
|---|---|---|
| positive (N = 20) | negative (N = 14) | |
| Male | 9 (45.0) | 12 (85.7) |
| Female | 11 (55.0) | 2 (14.3) |
| Monosomy 3 (FISH/SNP) | ||
| Positive | 17 (85.0) | 6 (42.9) |
| Negative | 3 (15.0) | 8 (57.1) |
ER, estrogen receptor. + n = 19 were not performed, insufficient for diagnosis, or nonspecific genetic changes.
In the second cohort (Table 1), 55 specimens from 47 patients were evaluated, but 3 cases were excluded due to insufficient tissue. There were 5 matched pairs of primary tumor and recurrent/metastatic tumor. The second cohort had a similar percentage of ER(+) UM cases (62%) as seen in the initial cohort (59%). The quantitative levels of ER expression were categorized as negative (<1%; 38% cases), low positive (1–9%; 28% cases), and positive (≥10%; 34% cases). No case had >50% positive nuclei. In the 5 matched pairs, there was ER concordance in 4/5: ER(+) in both (n = 2) and ER(−) in both (n = 2). The discordant case showed weak staining in 10% of cells in the primary with negative results (<1%) in the metastasis (counted as positive case). ER results were similar in the TNM pT3 and pT4 groups. However, when compared to melanoma cell type, ER-positive tumors were more likely to be epithelioid or mixed epithelioid/spindle cell type. Table 2 lists patient characteristics.
Table 2.
OSUWMC cohort patient characteristics, 47 patients with adequate tissue
| Variable | Level | Total (n = 47) |
|---|---|---|
| ER status | Positive | 29 (62%) |
| Negative | 18 (38%) | |
|
| ||
| Sex | Female | 19 (40%) |
| Male | 28 (60%) | |
|
| ||
| Age, years | Mean (SD) | 60 (16.5) |
| (min, max) | (17, 87) | |
|
| ||
| Metastasis | No | 27 (57%) |
| Yes | 20 (43%) | |
|
| ||
| Death | No | 33 (70%) |
| Yes, due to disease | 12 (26%) | |
| Yes, due to | 2 (4%) | |
| competing cause | ||
|
| ||
| Metastasis or death due to disease | Missing | 1 (2%) |
| Yes | 27 (57%) | |
| No | 19 (40%) | |
ER, estrogen receptor.
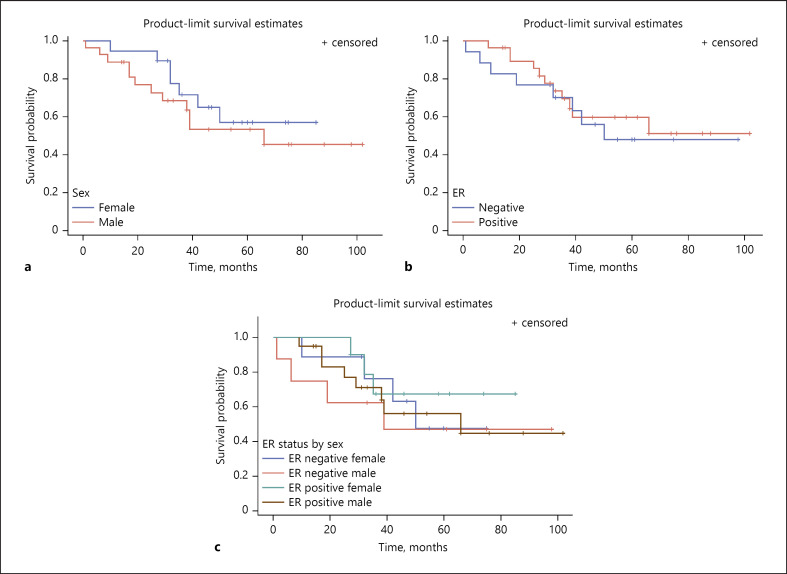
The follow-up time for survival analyses ranged from 1 to 102 months with an average time to death or censoring of 43.9 months (SD = 24.9). Kaplan-Meier survival curves demonstrate no statistically significant difference in probability of surviving without metastasis between ER-positive and -negative UM tumors. We also saw no significant sex differences in survival in the cohort. Neither sex (p = 0.43, Fig. 2a) nor ER (p = 0.68, Fig. 2b) was found to be statistically significant when testing for association with metastasis-free survival via the log rank test. The interaction between sex and ER status was also not statistically significant (p = 0.81, Fig. 2c).
Fig. 2.
Kaplan-Meier curves for sex and UM progression-free survival (a), ER status and UM progression-free survival (b), and interaction of sex and ER status on UM progression-free survival (c). ER, estrogen receptor; UM, uveal melanoma.
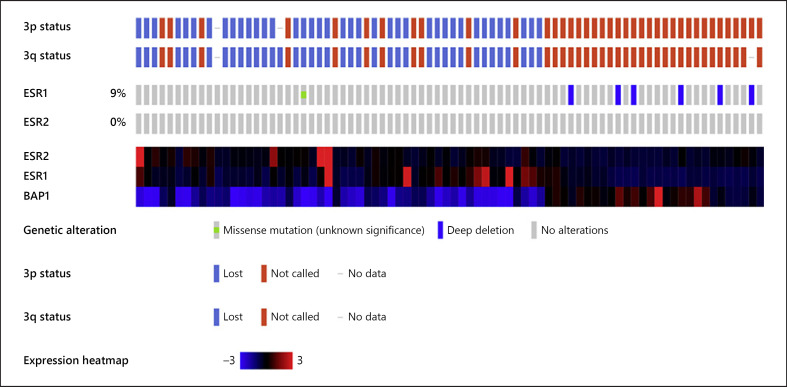
The TCGA database of n = 80 UM tumors was evaluated for mutation in ESR1 and ESR2. These correspond to ERα and ERβ, respectively, the former used in evaluating breast carcinoma and in the current study. No mutations were identified in ESR2 while ESR1 had a deep deletion in 9% of tumors. A subset of UM tumors with elevated ESR1 and/or ESR2 gene expression >3-fold was identified in the tumors that also had loss of chromosome 3p and 3q and BAP1 expression. No tumors lacking chromosome 3 or BAP1 expression had elevated ESR mRNA levels (Fig. 3).
Fig. 3.
TCGA genetic prognostic markers and ESR gene expression in UM primary tumors. The first track shows loss (blue) or retention (red) of chromosome 3p in each UM patient of the TCGA (each brick represents 1 patient, and tracks are aligned so each column shows results from the same patient, n = 80). The second track shows the results for chromosome 3q loss/retention. The tumors with monosomy of chromosome 3 are on the left (blue 3p and 3q tracks). The next tracks show deletion of ESR1 and ESR2 in UM. The final tracks show a heat map of ESR1, ESR2, and BAP1 mRNA gene expression. High ESR1 or ESR2 gene expression (3-fold, bright red) is seen in a subset of patients with UM that have monosomy 3 and loss of BAP1 expression. UM, uveal melanoma.
Discussion/Conclusion
With currently available prognostic tests for UM, allowing for stratification of low versus high-metastatic risk [12, 13], clinical trials for adjuvant treatment are underway. Because of differences in incidence and metastasis-related mortality by gender (males > females) [7], our study looked at both gender and ER expression in UM. We documented ER expression in approximately 60% of cases in 2 independent cohorts. Tumors with epithelioid or mixed morphology (poor prognostic feature) were positive in 71% of ER(+) cases. Our findings from the TCGA database also suggest that these ER(+) tumors correlate with a higher risk for metastasis, with elevated ER gene expression in tumors with loss of BAP1 and chromosome 3.
The discovery that ER(+) breast cancers respond to endocrine therapy has improved survival for many patients. Adjuvant tamoxifen or other SERMs (e.g., aromatase inhibitors) have a favorable safety profile and are generally well tolerated for at least 5 years [14]. All new cases of breast carcinoma undergo ER IHC in order to treat appropriate patients. In addition to its predictive value, ER is a weak prognostic marker with positive tumors conferring a better prognosis, at least early in the disease [15]. Since the 1950s, there has also been interest in hormonal effects in cutaneous melanoma (CM) because of better survival in women [16].
ER, part of the nuclear receptor superfamily of 48 transcription members, is a pro-proliferative and an oncogenic driver in several malignancies, including breast, ovarian, prostate, endometrial, and some hematological cancers [17, 18]. ERα and ERβ (latter poorly understood) mediate their action by ligand-dependent binding in the nucleus leading to transcriptional regulation of target genes. There has been controversy regarding ER expression in CM, but it has been demonstrated in some studies. A 1987 study of 141 patients found ER in 43% of cases, its presence correlating with a more indolent clinical course [19]. A study using RT-PCR and IHC showed both ERα and ERβ in melanocytic lesions and melanomas, and lower levels correlated with increased Breslow thickness [20]. In the 1990s, there were several studies evaluating the utility of tamoxifen in CM. One randomized controlled trial showed improvement in overall survival, but this was confined to women, and the effect size was modest [21]. A meta-analysis of 6 randomized controlled trials, including a total of 912 patients, found no significant improvement in overall survival rate, complete response rate, or survival rate when tamoxifen was administered with combined chemotherapy regimens [22]. These clinical trials did not include tissue evaluation for ER.
In our study, ER was present in 59% of 57 cases in the initial cohort (CCF) and 62% of 47 cases in the second cohort (OSUWMC), raising the possibility of targeted therapy. While the TCGA database suggests that UM tumors with elevated ESR gene expression represent a high risk for metastasis subset, the OSUWMC cohort did not show a statistically significant association between ER and clinical outcome or other prognostic markers, possibly due to small sample size.
While previous literature did not confirm ER in UM, a cell line study demonstrated it as part of the nuclear receptor superfamily of transcription factors [23]. Importantly, the recently published TCGA project, which performed an integrated analysis of genomic expression and epigenetic features of 80 UM tumors, confirmed ER gene expression [11]. Four molecular subsets of UM were identified by the TCGA, with subsets 3 and 4 having a poor prognosis. The regulator and pathway analysis identified elevated ESR1 and ESR2 associated with cluster 4 subset. This correlation of ESR1 and ESR2 with poor prognostic UM and the presence of ER immunostaining in a local recurrence and a metastatic UM sample in our study provide a rationale for targeted endocrine therapy in ER(+) UM.
We suggest that previously reported negativity for ER was due to preanalytic and/or analytic technical factors and/or thresholds for postanalytic interpretation. Testing and reporting for ER has dramatically changed over the past few decades in breast cancer. Before 1990, ER was tested using a ligand-binding assay (LBA), but since the early mid-1990s, laboratories have switched to IHC because of lower cost, ability to test small tumors, lack of requirement for fresh/frozen tissue, and elimination of false positivity from ER in normal adjacent benign breast tissue. It is also superior in predicting treatment response [24]. Many studies worldwide have shown variations in ER results and lack of concordance between local and central laboratories [25]. Law suits have occurred due to false negative results which lead to denial of tamoxifen. Research has elucidated factors that inhibit demonstration of ER. Preanalytic factors include warm and cold ischemia times and time to fixation. There is progressive loss of various labile molecules after interruption of blood flow, leading to ischemia, acidosis, and enzymatic degradation. There are now ASCO/CAP consensus guidelines on ER testing and other prognostic and predictive markers in breast carcinoma. Cold ischemia must be <1 h, and fixation time in 10% neutral buffered formalin should be 6–72 h, prior to processing and embedding [26]. In the previous studies showing lack of ER in UM, the methodology performed was either (1) measurement by LBA, (2) fixation not specified and 1D5 antibody used, or (3) specimen put in saline and 1D5 antibody used, factors not previously recognized to influence ER. In this author's experience (L.S.), the time to fixation was historically in the realm of hours for enucleations and typically multiple days of fixation, both likely to artificially diminish or eliminate ER. An earlier unpublished dataset of 25 archived UM cases at OSUWMC from 2003 to 2013 (L.S.) revealed no ER by IHC. This was subsequently recognized to likely be due to the historical use of fixatives other than 10% neutral buffered formalin and perhaps other preanalytic factors [27].
While several anti-ER antibodies are available, the 3 most sensitive and clinically validated to best correlate with response to tamoxifen are 1D5, 6F11, and SP1. Published studies suggest that SP1 (used in both cohorts here) is a better independent prognostic indicator and 8% more sensitive than 1D5 [28]. Most laboratories today have switched to the SP1 antibody.
ER is in the nucleus of the cell, and as such only nuclear staining is relevant. The cutoff for considering positive expression was ill defined during the 1990s, as methodology was transitioned from LBA to IHC. The level was eventually set in the early 2000s at 1% or more (see ASCO/CAP guidelines) [27, 28].
The importance of confirming ER in UM is that it raises the possibility of considering tamoxifen or other SERMs in the adjuvant setting for treating or preventing UM metastases. While tamoxifen has not been found to be beneficial in CM, UM differs on the molecular level.
Hormone therapy has been used with varying success in other carcinomas. Androgen deprivation therapy has yielded positive clinical responses in salivary duct carcinomas, an aggressive carcinoma found more frequently in men and typically androgen receptor positive [29, 30]. Estrogen has been found to upregulate proliferation and migration in cell cultures of salivary gland adenoid cystic carcinomas [31]. Another study evaluated ER-positive cell lines of salivary adenocarcinomas and found that E2 treatment reduced E-cadherin and increased N-cadherin, vimentin, and inhibitor of differentiation 1 proteins, all associated with epithelial-mesenchymal transition, leading to enhanced cell invasion [32].
The authors conclude that (1) ER is positive in more than half of primary UM tumors as well as 4 of the 5 recurrent or metastatic tumors and (2) increased expression of ER is seen in a subset of tumors with poor prognostic features (genetic and histopathologic). The low case numbers prevent demonstration of significant clinical correlations, but these data set the groundwork for future collaborative studies to investigate the potential of endocrine therapy in ER-positive UM.
Statement of Ethics
The study was approved by the Institutional Review Boards (IRB) of the Cleveland Clinic (CCF) and the Ohio State Wexner Medical Center (OSUWMC). The OSUWMC IRB Reference No. 2016C0098. It was an expedited review with a Waiver of Consent and Full Waiver of HIPAA Research Authorization. There was prior approval by the Comprehensive Cancer Center (CCC) Clinical Scientific Review Committee (CSRC). There were no interventions and no potential harms to the patients, as this was a retrospective study using tissue and data already acquired. All the research, from both institutions, was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
No conflicting relationship exists for any authors.
Funding Sources
This study was funded by CTSA Grant UL1TR002733 and also Ohio State University funds.
Author Contributions
Lynn Schoenfield: data gathering, organization, histologic and immunohistochemistry interpretations, analysis, and manuscript writing and editing. David Kline: statistics. Sarah Janse: statistics and manuscript editing. Mary Beth Aronow: data gathering and analysis. Arun Singh: data gathering and analysis. Caroline Craven: data gathering and analysis. Mohamed Abdel-Rahman: data gathering and analysis. Colleen Cebulla: data gathering and analysis and manuscript editing.
Acknowledgements
We would like to thank the following for their technical help for the immunohistochemistry: Amy Posch HTL (BS) at the Cleveland Clinic and Christina Hopkins MLS (ASCP)CMQIHC at OSUWMC. We would also like to thank the Ohio Lions Eye Research Foundation for their ongoing support for research in uveal melanoma. Some results shown here are based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
References
- 1.Aronow ME, Topham AK, Singh AD. Uveal melanoma: 5-year update on incidence, treatment, and survival (SEER 1973–2013) Ocul Oncol Pathol. 2018;4((3)):145–51. doi: 10.1159/000480640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44((11)):4651–9. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, MacLaughlin DT, Albert DM, Gragoudas ES, Ference M., 3rd Uveal melanomas presenting during pregnancy and the investigation of oestrogen receptors in melanomas. Br J Ophthalmol. 1982;66((11)):695–704. doi: 10.1136/bjo.66.11.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makitie T, Tarkkanen A, Kivela T. Comparative immunohistochemical oestrogen receptor analysis in primary and metastatic uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1998;236((6)):415–9. doi: 10.1007/s004170050099. [DOI] [PubMed] [Google Scholar]
- 5.Foss AJ, Alexander RA, Guille MJ, Hungerford JL, McCartney AC, Lightman S. Estrogen and progesterone receptor analysis in ocular melanomas. Ophthalmology. 1995;102((3)):431–5. doi: 10.1016/s0161-6420(95)31004-4. [DOI] [PubMed] [Google Scholar]
- 6.Mallone S, De Vries E, Guzzo M, Midena E, Verne J, Coebergh JW, et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. 2012;48((8)):1167–75. doi: 10.1016/j.ejca.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Zloto O, Pe'er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54((1)):652–6. doi: 10.1167/iovs.12-10365. [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Shields JA, Eagle RC, Jr, De Potter P, Menduke H. Uveal melanoma and pregnancy. A report of 16 cases. Ophthalmology. 1991;98((11)):1667–73. doi: 10.1016/s0161-6420(91)32060-8. [DOI] [PubMed] [Google Scholar]
- 9.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2((5)):401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6((269)):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer cell. 2017;32((2)):204–20.e15. doi: 10.1016/j.ccell.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AD. Prognostication of uveal melanoma: a work in progress. JAMA Ophthalmol. 2016;134((7)):740–1. doi: 10.1001/jamaophthalmol.2016.1070. [DOI] [PubMed] [Google Scholar]
- 13.Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25((3)):234–9. doi: 10.1097/ICU.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez EA. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol. 2007;18((Suppl 8)):viii26–35. doi: 10.1093/annonc/mdm263. [DOI] [PubMed] [Google Scholar]
- 15.Dowsett M. Estrogen receptor: methodology matters. J Clin Oncol. 2006;24((36)):5626–8. doi: 10.1200/JCO.2006.08.3485. [DOI] [PubMed] [Google Scholar]
- 16.Joosse A, Collette S, Suciu S, Nijsten T, Lejeune F, Kleeberg UR, et al. Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J Clin Oncol. 2012;30((18)):2240–7. doi: 10.1200/JCO.2011.38.0584. [DOI] [PubMed] [Google Scholar]
- 17.Conzen SD. Minireview: nuclear receptors and breast cancer. Mol Endocrinol. 2008;22((10)):2215–28. doi: 10.1210/me.2007-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek SH, Kim KI. Emerging roles of orphan nuclear receptors in cancer. Annu Rev Physiol. 2014;76:177–95. doi: 10.1146/annurev-physiol-030212-183758. [DOI] [PubMed] [Google Scholar]
- 19.Walker MJ, Beattie CW, Patel MK, Ronan SM, Das Gupta TK. Estrogen receptor in malignant melanoma. J Clin Oncol. 1987;5((8)):1256–61. doi: 10.1200/JCO.1987.5.8.1256. [DOI] [PubMed] [Google Scholar]
- 20.de Giorgi V, Mavilia C, Massi D, Gozzini A, Aragona P, Tanini A, et al. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol. 2009;145((1)):30–6. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- 21.Cocconi G, Bella M, Calabresi F, Tonato M, Canaletti R, Boni C, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N Engl J Med. 1992;327((8)):516–23. doi: 10.1056/NEJM199208203270803. [DOI] [PubMed] [Google Scholar]
- 22.Lens MB, Reiman T, Husain AF. Use of tamoxifen in the treatment of malignant melanoma. Cancer. 2003;98((7)):1355–61. doi: 10.1002/cncr.11644. [DOI] [PubMed] [Google Scholar]
- 23.Huffman KE, Carstens R, Martinez ED. A subset of nuclear receptors are uniquely expressed in uveal melanoma cells. Front Endocrinol. 2015;6:93. doi: 10.3389/fendo.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertschuk LP, Feldman JG, Kim YD, Braithwaite L, Schneider F, Braverman AS, et al. Estrogen receptor immunocytochemistry in paraffin embedded tissues with ER1D5 predicts breast cancer endocrine response more accurately than H222Sp gamma in frozen sections or cytosol-based ligand-binding assays. Cancer. 1996;77((12)):2514–9. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2514::AID-CNCR14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.Diaz LK, Sneige N. Estrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracy. Adv Anat Pathol. 2005;12((1)):10–9. doi: 10.1097/00125480-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Yaziji H, Taylor CR, Goldstein NS, Dabbs DJ, Hammond EH, Hewlett B, et al. Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2008;16((6)):513–20. doi: 10.1097/PAI.0b013e31818a9d3a. [DOI] [PubMed] [Google Scholar]
- 27.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134((7)):e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 28.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17((5)):1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 29.Aquino G, Collina F, Sabatino R, Cerrone M, Longo F, Ionna F, et al. Sex hormone receptors in benign and malignant salivary gland tumors: prognostic and predictive role. Int J Mol Sci. 2018;19((2)):399. doi: 10.3390/ijms19020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaspers HC, Verbist BM, Schoffelen R, Mattijssen V, Slootweg PJ, van der Graaf WT, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29((16)):e473–6. doi: 10.1200/JCO.2010.32.8351. [DOI] [PubMed] [Google Scholar]
- 31.Sumida T, Ishikawa A, Kamata YU, Nakano H, Yamada T, Mori Y. Estrogen enhances malignant phenotypes in human salivary adenoid cystic carcinoma cells. Anticancer Res. 2016;36((6)):2793–8. [PubMed] [Google Scholar]
- 32.Sumida T, Ishikawa A, Mori Y. Stimulation of the estrogen axis induces epithelial-mesenchymal transition in human salivary cancer cells. Cancer Genomics Proteomics. 2016;13((4)):305–10. [PubMed] [Google Scholar]