Abstract
Objective
The aim of this study was to determine the sensitivity and specificity of the MOLES scoring system in differentiating choroidal melanomas from nevi according to Mushroom shape, Orange pigment, Large tumor size, Enlarging tumor, and Subretinal fluid (SRF).
Methods
Color photographs, fundus-autofluorescence images, and optical coherence tomography of 222 melanocytic choroidal tumors were reviewed. Each MOLES feature was retrospectively scored between 0 and 2 and tumors categorized as “common nevus,” “low-risk nevus,” “high-risk nevus,” and “probable melanoma” according to the total score. MOLES scores were compared with the experts' diagnosis of melanoma.
Results
The MOLES scoring system indicated melanoma in all 81 tumors diagnosed as such by ocular oncologists (100% sensitivity) and nevus in 135 of 141 tumors given this diagnosis by these experts (95.7% specificity). Of the 6 tumors with discordant diagnoses, 4 had basal diameters exceeding 6 mm, all with SRF and/or orange pigment, and 2 small tumors showed either significant SRF with traces of orange pigment, or vice versa.
Conclusions
The MOLES system for diagnosing melanocytic choroidal tumors compares well with expert diagnosis but needs to be evaluated when deployed by ophthalmologists and community optometrists in a wide variety of working environments.
Keywords: Choroidal tumor, Melanoma, Choroidal nevus, Ophthalmic oncology, Choroidal moles
Early treatment of choroidal melanoma optimizes opportunities for conserving the eye and vision. There is tentative evidence that treatment of small choroidal melanomas may prevent metastatic spread in some patients [1, 2]. It can be difficult, however, to distinguish choroidal nevi from small melanomas. Patients with a benign lesion may therefore undergo excessive surveillance, experiencing undue anxiety and incurring unnecessary expense. Management of these patients can be burdensome to healthcare services because of the high prevalence of choroidal nevi, especially in the White population (i.e., approx. 6%) [3]. This can delay the care of patients with serious conditions, including choroidal melanoma [4].
Various mnemonics and acronyms have been developed to aid differentiation between choroidal nevi and melanomas [5, 6]; however, these necessitate assessment of internal acoustic reflectivity by ultrasonography. There is scope for aids enabling practitioners to estimate the likelihood of malignancy from widely used imaging, such as color photography, optical coherence tomography (OCT) and fundus autofluorescence imaging (FAF) when ultrasonography is not possible, as in virtual clinics (i.e., tele-ophthalmology) and in optometric and general ophthalmic clinics.
The senior author (B.D.) has devised the acronym MOLES, which represents Mushroom shape, Orange pigment (i.e., lipofuscin), Large tumor size, Enlarging tumor, and Subretinal fluid (SRF). Each of these features is scored between 0 and 2 and tumors are diagnosed according to the total score as “common nevus,” “low-risk nevus,” “high-risk nevus,” or “probable melanoma,” as described previously (Table 1) [7]. The aim of this study was to determine the sensitivity and specificity of the MOLES scoring system in differentiating choroidal melanomas from nevi.
Table 1.
Patient demographics according to the MOLES score and expert diagnosis of choroidal lesions
| Clinical findings/categories | MOLES category |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| common nevus |
low-risk nevus |
high-risk nevus |
probable melanoma |
|||||||
| n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||
| Male | 49 | 69 | 24 | 67 | 17 | 61 | 47 | 54 | 137 | 62 |
| Female | 22 | 31 | 12 | 33 | 11 | 39 | 40 | 46 | 85 | 38 |
|
| ||||||||||
| Eye | ||||||||||
| Left | 35 | 49 | 18 | 50 | 11 | 39 | 39 | 45 | 103 | 46 |
| Right | 36 | 51 | 18 | 50 | 17 | 61 | 48 | 55 | 119 | 54 |
|
| ||||||||||
| Visual acuity | ||||||||||
| 20/20 to 20/30 | 59 | 83 | 31 | 86 | 25 | 93 | 64 | 74 | 179 | 81 |
| 20/40 to 20/60 | 11 | 15 | 4 | 11 | 2 | 7 | 16 | 18 | 33 | 15 |
| 20/80 to 20/200 | 1 | 1 | 1 | 3 | 0 | 0 | 2 | 2 | 4 | 2 |
| CF | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 1 |
| HM-NLP | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 |
|
| ||||||||||
| MOLES score | ||||||||||
| Mushroom shape | ||||||||||
| 0: Absent | 71 | 100 | 36 | 100 | 28 | 100 | 86 | 99 | 222 | 100 |
| 1: Incipient (i.e., erosion of RPE over tumor, possibly with bulging of tumor) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2: Present (i.e., with definite overhang) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Orange pigment | ||||||||||
| 0: Absent | 71 | 100 | 35 | 97 | 27 | 96 | 24 | 28 | 158 | 71 |
| 1: Trace | 0 | 0 | 1 | 3 | 1 | 4 | 24 | 28 | 27 | 12 |
| 2: Confluent (i.e., forming clumps) | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 45 | 37 | 17 |
| Large size | ||||||||||
| 0: Small (i.e., base <3 DD and thickness <1 mm)1 | 71 | 100 | 3 | 8 | 1 | 4 | 1 | 1 | 76 | 34 |
| 1: Borderline (i.e., base 3–4 DD or thickness = 1–2 mm)1 | 0 | 0 | 33 | 92 | 10 | 36 | 29 | 33 | 72 | 32 |
| 2: Large (i.e., base >4 DD or thickness >2 mm)1 | 0 | 0 | 0 | 0 | 17 | 61 | 57 | 66 | 74 | 33 |
| Enlargement | ||||||||||
| 0: None | 71 | 100 | 36 | 100 | 28 | 100 | 74 | 85 | 209 | 94 |
| 1: Uncertain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2: Definite (i.e., with photographic proof of growth >1/3 DD per year) | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 15 | 13 | 6 |
| SRF | ||||||||||
| 0: Absent | 71 | 100 | 34 | 94 | 18 | 64 | 2 | 2 | 125 | 56 |
| 1: Trace (i.e., detected only with OCT)1 | 0 | 0 | 2 | 6 | 9 | 32 | 25 | 29 | 36 | 16 |
| 2: Significant (i.e., visible by color photography or ophthalmoscopy) | 0 | 0 | 0 | 0 | 1 | 4 | 60 | 69 | 61 | 27 |
|
| ||||||||||
| Treatment | ||||||||||
| Nil | 71 | 100 | 36 | 100 | 28 | 100 | 5 | 6 | 140 | 63 |
| PDT | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 8 | 7 | 3 |
| Brachytherapy | 0 | 0 | 0 | 0 | 0 | 0 | 68 | 78 | 68 | 31 |
| Proton beam | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 6 | 5 | 2 |
| Enucleation | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 |
|
| ||||||||||
| Total | 71 | 32 | 36 | 16 | 28 | 13 | 87 | 39 | 222 | 100 |
Tumors are categorized as “common nevus,” “low-risk nevus,” “high-risk nevus,” and “probable melanoma” according to whether the sum total of the scores for the 5 indicators of malignancy is 0, 1, 2 or >2. CF, count fingers; DD, disc diameters; HM, hand motions; NLP, no light perception; PDT, photodynamic therapy; SRF, subretinal fluid.
If ultrasonography is not available, tumor thickness is measured with OCT. If OCT is not possible, thickness is estimated to be >1 mm, 1–2 mm, or >2 mm if the tumor ophthalmoscopically appears flat, minimally thickened, or having a prominent dome or mushroom shape. Cystic intra-retinal edema and retinal pigment epithelial abnormality with cobblestone degeneration or hyper-autofluorescence are not significant if the retina is flat.
Patients and Methods
Patients were included if seen at the Nevus Clinic of Moorfields Eye Hospital between January 2013 and December 2018. They were excluded if diagnosed with melanocytoma or congenital ocular melanocytosis, or if the tumor extended anterior to ora.
Initial assessment was performed by an ocular oncologist and included measurement of Snellen visual acuity, binocular indirect ophthalmoscopy, fundus photography with Optos (Optos, Dumfermline, UK) or Topcon (Topcon fundus camera, Topcon Corporation, Tokyo, Japan), FAF (Optos), OCT (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany), and, in selected cases, B-scan ultrasonography. Patients were monitored or treated with photodynamic therapy, ruthenium plaque radiotherapy, proton beam radiotherapy, or enucleation.
Medical records and digital images were retrospectively scored and tumors categorized according to the MOLES system as “common nevus,” “low-risk nevus,” “high-risk nevus,” and “probable melanoma” [7]. This was done according to clinical features documented at the patient's first visit at our hospital as described in Table 1, except that enlargement was documented if tumor growth was mentioned in the charts or referral letter. The experts' diagnosis was retrospectively categorized as melanoma if this was specified at initial assessment or if the patient underwent radiotherapy or other treatment for this condition. The MOLES scores were compared with the expert diagnosis to compute sensitivity and specificity in differentiating melanomas from nevi (i.e., common, low-risk, and high-risk lesions).
Follow-up was measured from the first clinic date to the November 2, 2019, when this analysis was performed. Data were analyzed with Stata (StataCorp, College Station, TX, USA). Approval from the Audit Committee of Moorfields Eye Hospital was obtained (No. 452). Patient consent was not required. We adhered to the Tenets of Helsinki.
Results
The cohort comprised 222 patients (61.7% male) with a median age of 62.5 years (range 17.3–89.9; Table 1). The tumors had a median basal diameter of 3 disc diameters (DD; range, 0.3–19) and a median thickness of 0.9 mm (range 0–11). One tumor had broken through the retinal pigment epithelium (RPE) but had not developed a mushroom shape. Confluent orange pigment and significant SRF were present in 37 (16.7%) and 61 (27.5%) eyes, respectively. Growth was documented in 13 (5.9%) tumors, all with MOLES scores exceeding 4 and all subsequently having undergone treatment (Table 1). According to MOLES, the tumors were categorized as “common nevus,” “low-risk nevus,” “high-risk nevus,” and “probable melanoma” in 71 (32%), 36 (16.2%), 28 (12.6%), and 87 (39.2%), respectively.
Table 2 itemizes tumor features according to the MOLES score. Table 3 lists the clinical features in 18 tumors whose size category was determined by tumor thickness, resulting in a higher risk category in 6 nevi.
Table 2.
Clinical findings according to the MOLES score
| MOLES score | Size category | Orange pigment | SRF, n (%) |
Total, n (%) | ||
|---|---|---|---|---|---|---|
| absent | trace | significant | ||||
| 0 | Small | Absent | 71 (100) | 0 | 0 | 71 (32) |
| Trace | 0 | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| 1 | Small | Absent | 0 | 2 (6) | 0 | 36 (16) |
| Trace | 1 (3) | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| Borderline | Absent | 33 (92) | 0 | 0 | ||
| Trace | 0 | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| 2 | Small | Absent | 0 | 0 | 1 (4) | 28 (13) |
| Trace | 0 | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| Borderline | Absent | 0 | 9 (32) | 0 | ||
| Trace | 1 (4) | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| Large | Absent | 17 (61) | 0 | 0 | ||
| Trace | 0 | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| 3 | Small | Absent | 0 | 0 | 0 | 12 (5) |
| Trace | 0 | 0 | 1 (8) | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| Borderline | Absent | 0 | 0 | 2 (17) | ||
| Trace | 0 | 2 (17) | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| Large | Absent | 0 | 6 (50) | 0 | ||
| Trace | 1 (8) | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| >3 | Small | Absent | 0 | 0 | 0 | 75 (32) |
| Trace | 0 | 0 | 0 | |||
| Confluent | 0 | 0 | 0 | |||
|
| ||||||
| Borderline2 | Absent | 0 | 0 | 0 | ||
| Trace | 0 | 0 | 7 (9) | |||
| Confluent | 0 | 5 (7) | 13 (17) | |||
|
| ||||||
| Large3 | Absent | 0 | 4 (5)1 | 12 (16) | ||
| Trace | 0 | 5 (7) | 8 (11) | |||
| Confluent | 1 (1) | 3 (4) | 17 (23) | |||
|
| ||||||
| Total | 125 (56) | 36 (16) | 61 (27) | 222 (100) | ||
One case with incipient mushroom shape.
Two cases with documented growth.
Eleven cases with documented growth.
Table 3.
Clinical features of 16 tumors whose size category was determined by thickness
| Case No. | MOLES score | Size category | Mushroom shape | Orange pigment | Tumor diameter, DD | Tumor thickness, mm | Enlargement | SRF | Clinical diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 107 | 1: Low-risk nevus | 1 | A | A | 1.5 | 1.1 | N | A | CN |
| 79 | 1 | A | A | 1 | 1.2 | N | A | CN | |
| 89 | 1 | A | A | 2 | 1.2 | N | A | CN | |
| 99 | 1 | A | A | 2.5 | 1.2 | N | A | CN | |
|
| |||||||||
| 118 | 2: High-risk nevus | 1 | A | T | 2 | 1.1 | N | A | CN |
| 110 | 1 | A | A | 2.5 | 1.1 | N | T | CN | |
|
| |||||||||
| 148 | >3: Probable melanoma | 1 | A | T | 1.5 | 1.2 | N | S | CN |
| 157 | 1 | A | C | 2 | 1.7 | N | T | CM | |
| 149 | 1 | A | T | 2 | 2 | N | S | CM | |
| 164 | 2 | A | A | 3 | 2.2 | N | S | CM | |
| 171 | 2 | A | T | 3 | 2.3 | N | T | CM | |
| 161 | 2 | A | A | 4 | 2.7 | N | S | CM | |
| 183 | 1 | A | C | 2 | 1.1 | N | S | CM | |
| 185 | 1 | A | C | 2 | 1.2 | N | S | CM | |
| 197 | 2 | A | C | 3 | 2.6 | N | T | CM | |
| 204 | 2 | A | C | 4 | 2.3 | N | S | CM | |
SRF, subretinal fluid; A, absent; C, confluent; CM, choroidal melanoma; CN, choroidal nevus; DD, disc diameter; N, nevus; S, significant; T, trace.
The ocular oncologists diagnosed 79 tumors as melanoma and 143 as nevus; however, 2 diagnosed as nevi subsequently received brachytherapy so that the diagnosis was revised to melanoma and nevus in 81 and 141 tumors, respectively. One diagnosed nevus received photodynamic therapy (PDT) to improve vision by reducing SRF. The melanomas were treated with PDT (n = 6), brachytherapy (n = 68), proton beam radiotherapy (n = 5), or enucleation (n = 2) (Table 1).
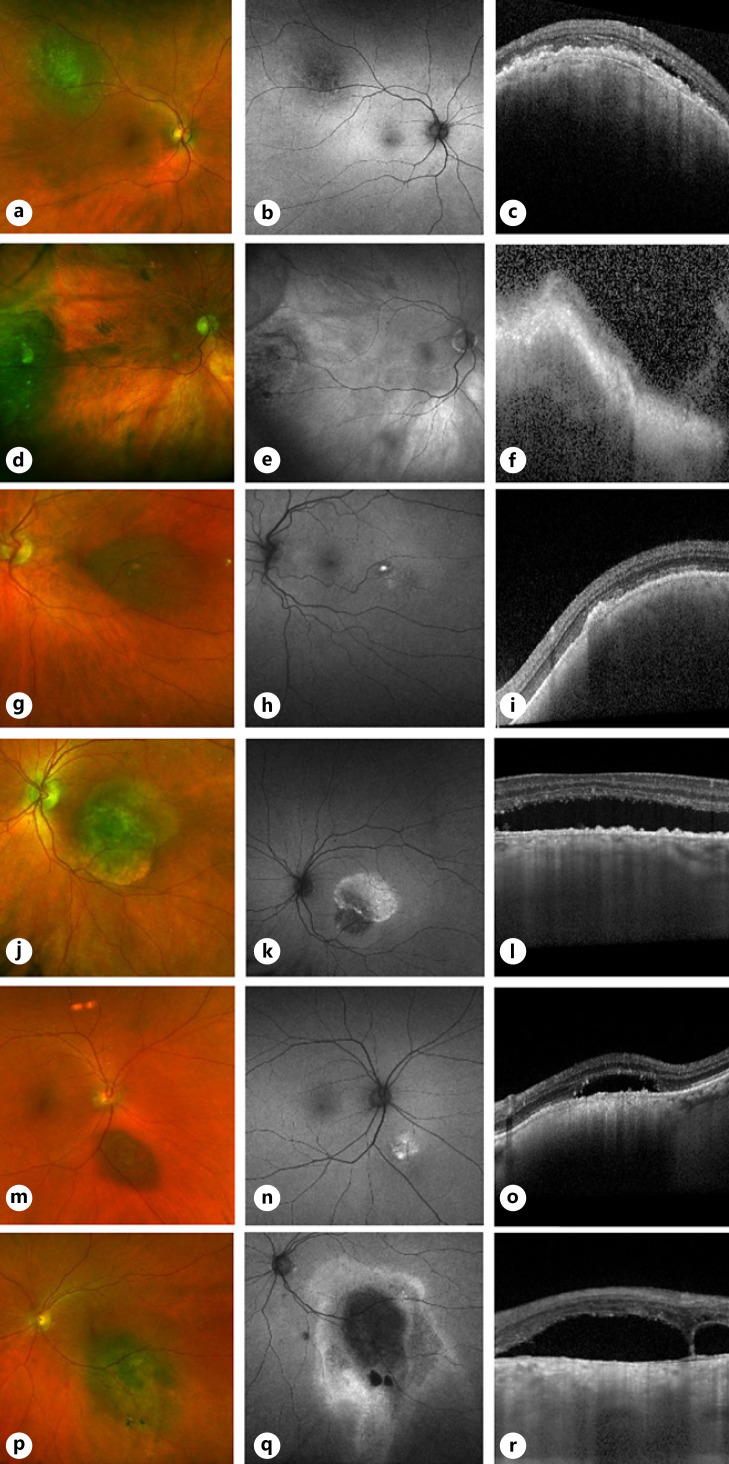
Three (25%) of the 12 tumors with a MOLES score of 3, and 3 out of 75 with higher MOLES scores were not treated because the oncologists had diagnosed nevus (Fig. 1; Table 4). Four of these 6 tumors had basal diameters exceeding 6 mm, all with SRF and/or orange pigment, and 2 small tumors showed either significant SRF with traces of liposfuscin, or vice versa.
Fig. 1.
OCTs and fundus autofluorescence images of 6 tumors with a MOLES score >2 and diagnosed by experts as nevus. a–c Case 141 (MOLES = 00201 = 3), with basal diameter >4 DD and trace SRF. d–f Case 142 (MOLES = 00201 = 3), with basal diameter >4 DD and trace SRF. g–i Case 147 (MOLES = 01200 = 3), with trace orange pigment and basal diameter >4 DD. j–l Case 159 (MOLES = 00202 = 4), with basal diameter >4 mm and significant SRF. m–o Case 148 (MOLES = 01102 = 4), with trace orange pigment, thickness >1 mm and significant SRF. p–r Case 153 (MOLES = 02101 = 4), with confluent orange pigment, basal diameter 3 mm, and trace SRF.
Table 4.
Clinical features of 6 tumors with a MOLES score exceeding 2 and diagnosed by the expert as nevus
| Case No. | Age, years | Sex | Eye | MOLES score | Orange pigment | Basal diameter, DD | Thickness, mm | Enlargement | SRF | Follow-up, years | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 141 | 74 | F | R | 3 | A | 4.5 | 1.4 | N | T | 2.9 | N |
| 142 | 88 | M | R | 3 | A | 8.5 | 1.2 | N | T | 2.7 | N |
| 147 | 70 | F | L | 3 | T | 5 | 1 | N | A | 3.1 | N |
| 148 | 56 | F | L | 4 | T | 1.5 | 1.2 | N | S | 2.7 | PDT |
| 153 | 58 | M | R | 4 | C | 3 | 0.5 | N | T | 3.1 | N |
| 159 | 63 | M | L | 4 | A | 6 | 0.9 | N | S | 2.5 | N |
SRF, subretinal fluid; A, absent; C, confluent; DD, disc diameter; F, female; L, left, M, male; N, nil; PDT, photodynamic therapy; R, right; T, trace.
These results indicate that MOLES correctly identified all 81 melanomas (100% sensitivity) and 135 out of 141 choroidal nevi (95.7% specificity; 95% CI 92.4–99.1).
Discussion
Main Findings
The main finding of this study is that the MOLES score correlated well with the experts' diagnoses of choroidal melanoma and nevus, with sensitivity and specificity levels of 100 and 96%, respectively. Another finding was that ultrasonographic measurement of tumor thickness influenced the MOLES category in only 6/222 (2.7%) tumors.
Strengths and Weaknesses
The strengths of this study are the large number of patients and the multimodality imaging. There are several weaknesses. First, the retrospective data collection from patients' charts prevented us from determining the oncologists' estimate of risk of malignancy, which was not always documented. Second, we do not know how many untreated tumors would have grown with long-term monitoring. We also cannot know how many treated tumors would have remained unchanged if left alone. It would have been unethical to leave diagnosed melanomas untreated, because of the risk of metastasis. This problem is compounded by the lack of consensus amongst ocular oncologists as to which suspicious tumors should be diagnosed as melanoma. Third, MOLES scores were based on images not live examinations; this is an advantage, because it replicates the way images are assessed remotely in virtual clinics. Fourth, the oncologists' diagnosis was not confirmed histologically. It is not conventional practice to biopsy small melanocytic choroidal tumors to confirm malignancy, except in rare, selected cases. This is because with small tumors it is difficult to obtain sufficient tissue for analysis and because of the risk of complications.
Discussion of Methods
We included all consecutive patients irrespective of tumor size, because some large melanocytic choroidal tumors are benign (i.e., “giant nevi”) [8]. We excluded melanocytomas and patients with congenital ocular melanocytosis because the need for monitoring these is accepted and because the MOLES scoring system is not applicable to such lesions.
The follow-up period was computed from the first clinic visit to the date of our study closure because we assumed that all patients with tumor growth would be referred back to our care. We measured the basal tumor diameter in horizontal DD, accepting that this does not always correspond to 1.5 mm; this is because with thin and flat tumors this method is more accurate than ultrasonography. It is also a method that is easy to replicate outside of specialist centers.
The MOLES scoring system is based on clinical signs that are well accepted as features that differentiate choroidal nevi from melanomas. A study of more than 3,000 cases by Shields et al. [6] confirmed orange pigment (p = 0.0004), diameter >5 mm (p = 0.0275), thickness >2 mm (p < 0.0001), and SRF (p < 0.0001) to be statistically associated with tumor growth, which was taken to indicate malignancy. Choroidal nevi only rarely perforate RPE, and when they do, they do not develop a mushroom shape [9]. With such strong evidence in the published literature, further studies to show associations between the MOLES signs and malignancy in melanocytic choroidal tumors would have been redundant.
Some points regarding the MOLES scores require discussion. Although almost pathognomonic for melanoma, mushroom shape is given a MOLES score of only 2 to simplify scoring; this is justified because mushroom-shaped tumors inevitably have a total score exceeding 2 because of thickness. This should ensure that patients are referred for specialist opinion even in the rare event that mushroom shape is the only sign of melanoma. None of the tumors in this study had a mushroom shape and this is because tumors with this sign were triaged directly to our oncology clinic and not the nevus clinic. Orange pigment can develop over hemangiomas and metastases [10, 11]; however, the MOLES system is not designed to differentiate between melanomas and such lesions.
The size categories used by the MOLES system are mostly based on a study by Augsburger et al. [12]. Although there is a size overlap between choroidal nevi and melanomas, the MOLES system adjusts for this by including other risk factors.
Enlargement is included in MOLES because growth is such a strong indicator of malignancy and because in some cases it is the only suspicious feature. Some nevi can enlarge in adulthood; however, such growth is rare and tends to be subtle (i.e., <0.5 mm/year) [13, 14]. Growth before adulthood is not usually a sign of malignancy but nevertheless requires monitoring as choroidal melanoma can develop at a young age [15]. Some patients present with what appears to be a common nevus that was not noted previously. Monitoring of such lesions usually reveals no growth, suggesting that these nevi were missed previously. For this reason, the MOLES system requires enlargement to be confirmed by sequential imaging. SRF can develop over choroidal nevi but is usually minimal; the MOLES scoring system therefore scores this feature as 2 only if it is visible ophthalmoscopically.
The MOLES system omits several features considered to indicate increased risk of malignancy. Statistical studies show that tumor proximity to the optic disc is not an indicator of malignancy [6]. Many nevi do not show drusen on their surface and, conversely, these deposits can develop on melanomas. A recent study has shown lack of haloe to be statistically insignificant as an indicator of malignancy [6]. Assessment of internal acoustic reflectivity requires ultrasonography [6]; however, as mentioned, this is not widely available outside specialist units.
The TFSOM mnemonics categorize signs of malignancy in a binary fashion [6, 16]; however, it may not be possible to decide categorically whether a particular feature is present. The MOLES scoring system therefore includes an intermediate category for borderline and uncertain findings. Tumors with a high MOLES score are categorized as “probable melanomas” and not “melanomas” because non-oncologists may not feel confident or qualified to distinguish melanomas from other tumors.
Discussion of Results
The discrepancy between the MOLES score and the experts' diagnosis in 6 tumors is not surprising considering that there is no consensus as to how patients with indeterminate lesions should be diagnosed [5, 12]. MOLES has a relatively low threshold for indicating malignancy; this is to reduce the risk of misdiagnosing choroidal melanomas as nevi in situations where expertise and equipment are limited and also because it is only designed to guide investigation and monitoring, not treatment.
The ultrasound measurements of tumor thickness increased the MOLES category in 6/222 (2.7%) tumors. This may have been spurious as it is known that ultrasonographic thickness measurements tend to be greater than those obtained by OCT or histology [17, 18].
Previous Studies
Roelofs et al. [7] evaluated the MOLES system in 450 choroidal melanomas treated at our center and found a MOLES score <3 in only 1 patient, whose tumor was located pre-equatorially. This indicates that when used by an expert the risk of misdiagnosing choroidal melanomas as nevi is low. The present study extends the findings of that investigation, also including nevi to investigate the specificity of the MOLES scoring system. Roelofs et al. [19] also evaluated MOLES in a cohort of 99 patients who were treated for choroidal melanoma after a period of surveillance and found that tumor progression was detected without ultrasonography in 98 cases.
Clinical Implications
The MOLES acronym should make it easier for ophthalmologists and optometrists to remember the key signs differentiating melanomas from nevi, which in lay parlance are called “moles.” As mentioned, MOLES should be useful to a wider range of practitioners than mnemonics and acronyms that require ultrasonography to assess internal tumor reflectivity.
MOLES enables practitioners to concisely describe melanocytic choroidal tumors, encouraging more detailed documentation. For example, a MOLES score of “02201 = 5′” succinctly conveys that the tumor is probably a melanoma with a basal diameter >6 mm and/or a thickness >2 mm together with confluent orange pigment and traces of SRF but no mushroom shape and no documented growth.
The scope of MOLES has been increased by the COVID-19 pandemic, which at least in the UK is encouraging practitioners to shift the care of patients with melanocytic choroidal tumors from ocular-oncology centers to ophthalmic units closer to the patients' home and from these clinics to community optometrists [20]. This trend reduces the risk of coronavirus infection to patients and healthcare providers as well as minimizing traveling costs for patients and conserving healthcare resources.
Research Implications
This investigation pertains only to the performance of MOLES when used by ocular oncology experts (e.g., as in virtual clinics); further studies are needed to validate this diagnostic aid when deployed by optometrists and other ophthalmologists, who may lack the expertise and equipment needed to identify the clinical features of malignancy. Spurious MOLES scores may result if practitioners fail to recognize incipient mushroom formation or SRF, or if orange pigment is missed or mistaken for drusen, or if the basal tumor diameter is not measured accurately (e.g., omitting calipers or a ruler). The present investigation suggests that there is scope for such studies, which would take this algorithm for testing in a wider context, also indicating which patients should be referred for specialist opinion and whether expert advice can reliably be based solely on images submitted with the referral documentation, with or without ultrasonography.
To this end, we intend to investigate the ability of optometrists and ophthalmologists to assess tumor diameter and thickness and to detect orange pigment and SRF with and without special imaging. In keeping with the increase in telemedicine during the COVID-19 pandemic, we will perform this investigation online. The planned investigation and other studies may also determine whether tumor thickness can be omitted from the MOLES scoring system, as implied by our finding that this feature only rarely influences tumor categorization.
There is a need for evidence from long-term outcome studies on which to base the categorization of orange pigment and SRF in MOLES scoring. Albertus et al. [21] have developed a method of image analysis that compares autofluorescence of the tumor with that of a control region in the adjacent fundus (i.e., Index of Retinal Autofluorescence); however, a simple system not requiring special software would be more likely to gain acceptance.
The successful deployment of the MOLES system will depend on the efficiency of educational initiatives aimed at improving awareness and detection of clinical indicators of malignancy.
Conclusions
MOLES scores of melanocytic choroidal tumors correlate well with expert diagnosis. Further studies are needed to evaluate this system in general ophthalmic clinics and optometric practice.
Statement of Ethics
Approval from the Audit Committee of Moorfields Eye Hospital was obtained (Ethic Approval No. 452). Patient consent was not required. We adhered to the Tenets of Helsinki.
Conflict of Interest Statement
The authors have no conflicts of interest to report. This manuscript has not previously been submitted for publication. None of the authors have any financial disclosures to declare.
Funding Sources
The Moorfields Eye Charity generously provided a Research Enhancement Award (GR001270) to cover publication costs.
Author Contributions
L.A.H. and B.D.: substantial contribution to acquisition, analysis, and interpretation of data for the work. Drafting the work, revising it for critically important intellectual content. M.S.S., R.O., G.H., A.K.A., P.A.K., and V.M.L.C.: drafting the work, revising it for critically important intellectual content. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Center based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. Furthermore, L.A.H. was generously supported by SEHA Abu Dhabi Scholarships Division. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or SEHA Abu Dhabi.
References
- 1.Damato BE, Heimann H, Kalirai H, Coupland SE. Age, survival predictors, and metastatic death in patients with choroidal melanoma: tentative evidence of a therapeutic effect on survival. JAMA Ophthalmol. 2014;132((5)):605–13. doi: 10.1001/jamaophthalmol.2014.77. [DOI] [PubMed] [Google Scholar]
- 2.Damato B. Ocular treatment of choroidal melanoma in relation to the prevention of metastatic death − a personal view. Prog Retin Eye Res. 2018;66:187–99. doi: 10.1016/j.preteyeres.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Qiu M, Shields CL. Choroidal nevus in the United States adult population: racial disparities and associated factors in the national health and nutrition examination survey. Ophthalmology. 2015;122((10)):2071–83. doi: 10.1016/j.ophtha.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology. 2012;119((8)):1582–9. doi: 10.1016/j.ophtha.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Harbour JW, Paez-Escamilla M, Cai L, Walter SD, Augsburger JJ, Correa ZM. Are risk factors for growth of choroidal nevi associated with malignant transformation? Assessment with a validated genomic biomarker. Am J Ophthalmol. 2019;197:168–79. doi: 10.1016/j.ajo.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK, Jr, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: the 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39((10)):1840–51. doi: 10.1097/IAE.0000000000002440. [DOI] [PubMed] [Google Scholar]
- 7.Roelofs KA, O'Day R, Harby LA, Arora AK, Cohen VML, Sagoo MS, et al. The MOLES system for planning management of melanocytic choroidal tumors: is it safe? Cancers. 2020;12((5)):1311. doi: 10.3390/cancers12051311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HK, Shields CL, Mashayekhi A, Randolph JD, Bailey T, Burnbaum J, et al. Giant choroidal nevus clinical features and natural course in 322 cases. Ophthalmology. 2010;117((2)):324–33. doi: 10.1016/j.ophtha.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Weiss SJ, Stathopoulos C, Shields CL. Choroidal nevus with retinal invasion in 8 cases. Ocul Oncol Pathol. 2019;5((5)):369–78. doi: 10.1159/000495841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramasubramanian A, Shields CL, Harmon SA, Shields JA. Autofluorescence of choroidal hemangioma in 34 consecutive eyes. Retina. 2010;30((1)):16–22. doi: 10.1097/IAE.0b013e3181bceedb. [DOI] [PubMed] [Google Scholar]
- 11.Riechardt AI, Gundlach E, Joussen AM, Willerding GD. The development of orange pigment overlying choroidal metastasis. Ocul Oncol Pathol. 2015;1((2)):93–7. doi: 10.1159/000369823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augsburger JJ, Corrêa ZM, Trichopoulos N, Shaikh A. Size overlap between benign melanocytic choroidal nevi and choroidal malignant melanomas. Invest Ophthalmol Vis Sci. 2008;49((7)):2823–8. doi: 10.1167/iovs.07-1603. [DOI] [PubMed] [Google Scholar]
- 13.Thiagalingam S, Wang JJ, Mitchell P. Absence of change in choroidal nevi across 5 years in an older population. Arch Ophthalmol. 2004;122((1)):89–93. doi: 10.1001/archopht.122.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Mashayekhi A, Siu S, Shields CL, Shields JA. Slow enlargement of choroidal nevi: a long-term follow-up study. Ophthalmology. 2011;118((2)):382–8. doi: 10.1016/j.ophtha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Fry MV, Augsburger JJ, Hall J, Corrêa ZM. Posterior uveal melanoma in adolescents and children: current perspectives. Clin Ophthalmol. 2018;12:2205–12. doi: 10.2147/OPTH.S142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalvin LA, Shields CL, Ancona-Lezama DA, Yu MD, Di Nicola M, Williams BK, Jr., et al. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br J Ophthalmol. 2019;103((10)):1441–7. doi: 10.1136/bjophthalmol-2018-312967. [DOI] [PubMed] [Google Scholar]
- 17.Shah SU, Kaliki S, Shields CL, Ferenczy SR, Harmon SA, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology. 2012;119((5)):1066–72. doi: 10.1016/j.ophtha.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Collaborative Ocular Melanoma Study Group Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study: COMS report No. 21. Arch Ophthalmol. 2003;121((8)):1163–71. doi: 10.1001/archopht.121.8.1163. [DOI] [PubMed] [Google Scholar]
- 19.Roelofs KA, O'Day R, Al Harby L, Hay G, Arora AK, Cohen VML, et al. Detecting progression of melanocytic choroidal tumors by sequential imaging: is ultrasonography necessary? Cancers. 2020;12((7)):1856. doi: 10.3390/cancers12071856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damato B. Managing patients with choroidal melanoma in the COVID-19 era: a personal perspective. Br J Ophthalmol. 2020;104((7)):885–6. doi: 10.1136/bjophthalmol-2020-316686. [DOI] [PubMed] [Google Scholar]
- 21.Albertus DL, Schachar IH, Zahid S, Elner VM, Demirci H, Jayasundera T. Autofluorescence quantification of benign and malignant choroidal nevomelanocytic tumors. JAMA Ophthalmol. 2013;131((8)):1004–8. doi: 10.1001/jamaophthalmol.2013.4007. [DOI] [PubMed] [Google Scholar]