Abstract
Moyamoya disease (MMD) causes intracranial arterial stenosis progression. The progression of intracranial arterial stenosis will increase the risk of ischemic cerebrovascular events. This study aims to investigate the relationship between intracranial arterial stenosis progression, vessel wall enhancement (VWE), and the recent neurological symptoms. A total of 39 MMD patients (12 male; 37.6 ± 18.0 years old) were registered in this study analysis between April 2016 and July 2018. All patients received MRI at registration and 6, 12, and 24 months post-registration. The incidence of ischemic cerebrovascular events (transit ischemic attacks or cerebral infarction) was checked until December 2018. We evaluated the relationship between the intensity of VWE, intracranial arterial stenosis, and the recent neurological symptoms. During the mean follow-up period of 13.8 ± 5.5 months, the changes in VWE were observed in 33 hemispheres (42.3%), stenosis progression was observed in 21 hemispheres (26.9%), and recent neurological symptoms occurred in 10 hemispheres (12.8%). Stenosis progression was observed in 11 hemispheres (33.3%) in the VWE(+) group and ten hemispheres (22.2%) in the VWE(−) group (p = 0.310). The recent neurological symptoms were observed in eight hemispheres (21.2%) in the VWE(+) group and two hemispheres (4.44%) in the VWE(−) group (odds ratio 6.88, 95% confidence interval 1.35–34.98, p = 0.015). The intensity of VWE sometimes changes. The changes in VWE were significantly associated with the recent neurological symptoms but not with stenosis progression.
Keywords: moyamoya disease, disease progression, contrast-enhanced MRI, vessel wall imaging
Introduction
Moyamoya disease (MMD) is characterized by an intracranial arterial stenosis progression and the development of a collateral network of fine vessels.1) The disease progression will increase the risk of ischemic or hemorrhagic cerebrovascular events.2,3) Surgical revascularization therapies are beneficial as secondary prevention for symptomatic cases.2) Intracranial arterial stenosis progression occurred in approximately 20%, and an ischemic or hemorrhagic episode was shown in more than half of those patients.4)
This prospective study investigates the relationship between intracranial arterial stenosis progression, vessel wall enhancement (VWE), and the recent neurological symptoms in patients with MMD.
Materials and Methods
This study was approved by the Institutional Ethics Committees (approval number: 2016-0547). All subjects provided written informed consent, or for those considered too young to consent, informed consent was provided by their parent or guardian.
Study design and population
After approval of the hospital research ethics board’s study protocol, MMD patients were registered in this study between April 2016 and July 2018. All patients were diagnosed with MMD according to Tominaga et al.5)
Clinical data collection
All patients received MRI at registration and 6, 12, and 24 months post-registration. During each visit, the presence or absence of recent clinical symptoms was recorded. The incidence of ischemic cerebrovascular events (transit ischemic attacks [TIAs] or cerebral infarction) was checked until December 2018.
Two independent radiologists blinded to the clinical data reviewed consecutive luminal studies and intracranial vessel wall MRI (MR VWI).
MRI protocol
MR VWI can image the vessel wall in three dimensions and detect pathological changes in the vessel wall. This technique was widely used in patients with vasculitis, intracranial dissections, reversible cerebral vasoconstriction syndrome, MMD, intracranial atherosclerotic stenosis, and intracranial aneurysms.6–8)
Patients were scanned using a 3-T Siemens SkyraMR scanner (Siemens Healthcare, Erlangen, Germany). Three-dimensional (3D) HR-MRI has been widely used for vessel wall imaging, offering the advantages of a large coverage volume and multi-planar imaging planes with complete coverage of intracranial arteries. 3D turbo spin-echo (3D TSE) imaging sequences with variable flip angles have been widely used for 3D HR-MRI vessel wall evaluation, with these sequences including SPACE.
The MR VWI protocol included 3D time-of-flight (TOF) MRA (in-plane resolution, 0.5 × 0.5; slice thickness, 0.8 mm; repetition time [TR]/echo time [TE], 23.0/3.81 ms; flip angle, 16°; field of view [FOV], 200 × 200 mm; time, 4:11 min), T1 (0.5 × 0.5 in-plane resolution; slice thickness, 5 mm; TR/TE, 500/11 ms; averages, 4; matrix, 384 × 384 pixels; FOV, 200 × 200 mm; generalized autocalibrating partial parallel acquisition [GRAPPA] factor, 1; turbo factor, 1; time, 1:38 min), pre- and post-contrast HR VWI performed in multiple planes to obtain at least two planes for each lesion, axial and orthogonal 3D T1-weighted HR VWI (in-plane, 0.6 × 0.6; slices, 0.8 mm; FOV, 200 × 200 mm; matrix, 320 × 320; TR/TE, 580/5.6 ms; averages, 1; GRAPPA factor, 2; turbo factor, 40; time, 4:16 min), and 3D T2-weighted sampling perfection with application optimized contrast using different flip angle evolution (SPACE) (0.5 × 0.5 mm in-plane resolution; slice thickness, 1.0 mm; FOV, 200 × 200 ms; TR/TE, 4000/545 ms; slice oversampling, 37.5%; slices, 104; matrix, 384 × 384 mm; turbo factor, 555; time, 3:02 min) sequences.
Evaluation of stenosis progression
We measured the luminal stenosis on the maximum intensity project of 3D TOF MRA images and MR vessel wall images. The concordance of TOF MRA and vessel wall imaging was confirmed by the slice position provided by the digital imaging and communications in medicine (DICOM) tag. We evaluated three arteries’ stenosis (the internal carotid artery terminus, M1 segment of the middle cerebral artery, and P1 segment of the posterior cerebral artery) in each patient. A decrease in the signal intensity on MRA at a distance of ≥5 mm at least in one vessel defined “stenosis progression.” In the case of fetal posterior cerebral arteries, we did not analyze the luminal study.
Evaluation of VWE
T1-weighted MRI sequence without contrast agent (pre-contrast) was compared with the contrast-enhanced sequence (post-contrast) to detect VWE. Gadobutrol (Gadovist, Bayer Health Care, Osaka, Japan) was used as the contrast agent. The injection dose was 0.1 mmol/kg body weight, and MR VWI was done 5 min after contrast agent administration. By comparing T1-weighted pre- and post-contrast imaging, intracranial stenotic lesions were assessed for the presence and intensity of enhancement. Standard protocol TOF MRA was referenced when assessing MR VWI lesions.
Evaluation of the changes in VWE
As in previous reports, we qualitatively graded the intensity of VWE.9) Grade 0, no enhancement; Grade 1, mild enhancement, with the vessel wall’s signal intensity less than that of the pituitary infundibulum; Grade 2, strong enhancement, with the contrast-enhanced vessel wall’s signal intensity similar to or higher than that of the infundibulum. The “change” in the intensity of VWE was defined as the signal increase or decrease with the qualitative evaluation consisting of the three grading systems.
Statistical analysis
We used SPSS for Windows version 20.0 (IBM, Chicago, IL, USA) for all statistical analyses. Continuous variables are presented as the mean ± standard deviation, and categorical variables are described as percentages. The morphological measurements and contrast enhancement intensity changes were compared among different examination dates using the chi-squared test or Mann–Whitney U-test. Binary logistic regression analysis was performed to calculate the odds ratio (OR) and corresponding confidence interval (CI). Inter- and intra-reader agreements for grading intracranial VWE were assessed using Cohen’s Kappa analysis before reader consensus to settle disagreements. Statistical significance was set at p <0.05.
Results
Patients’ characteristics
Between April 2016 and July 2018, a total of 88 patients with intracranial arterial stenosis lesions were consecutively recruited for this study. Forty-nine non-MMD patients were excluded. In total, 39 MMD patients (12 male; 37.6 ± 18.0 years old) were followed up until December 2018. The mean follow-up period was 13.8 ± 5.5 months. The initial neurological symptom was TIAs in 30 hemispheres, cerebral infarction in nine hemispheres, intracranial hemorrhage in two hemispheres, intraventricular hemorrhage in one hemisphere, subarachnoid hemorrhage in two hemispheres, and asymptomatic in 34 hemispheres. The Suzuki stage was 3.25 ± 0.81. The patients’ baseline characteristics are shown in Table 1.
Table 1. Patients’ baseline characteristics.
| All (n = 39), described as n (%) or mean ± SD | |
|---|---|
| Age (years) | 37.6 ± 18.0 |
| Sex (female) | 27 (69.2) |
| Suzuki grade | 3.24 ± 0.81 |
| Initial symptom (hemispheres) | |
| Hemorrhage (ICH, IVH, SAH) | 5 (6.4) |
| Ischemic (CI, TIA) | 39 (50.0) |
| Asymptomatic | 34 (43.6) |
| Changes in VWE (hemispheres) | 33 (42.3) |
| Stenosis progression (hemispheres) | 21 (26.9) |
| New symptom (hemispheres) | 19 (12.8) |
CI: cerebral infarction, ICH: intracranial hemorrhage, IVH: intraventricular hemorrhage, SAH: subarachnoid hemorrhage, SD: standard deviation, TIA: transit ischemic attack, VWE: vessel wall enhancement.
VWE and stenosis progression
The changes in VWE were observed in 33 hemispheres (42.3%), and stenosis progression was observed in 21 hemispheres (26.9%). Stenosis progression was observed in 11 hemispheres (33.3%) in the VWE(+) group and ten hemispheres (22.2%) in the VWE(−) group (p = 0.310). Representative cases are shown in Figs. 1 and 2.
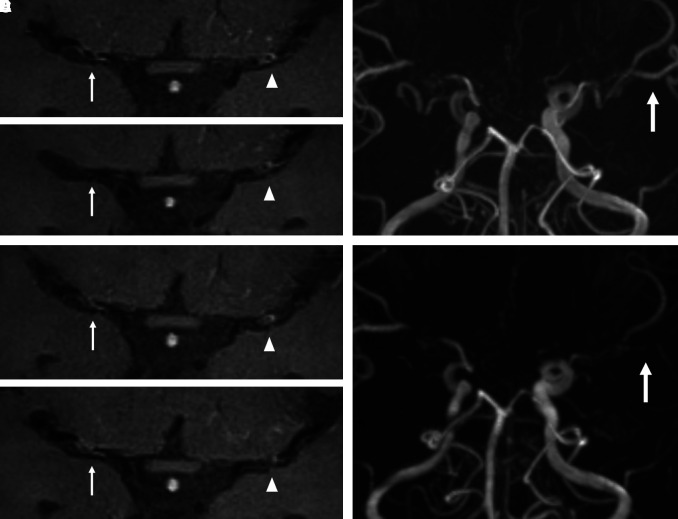
Fig. 1.
Ischemic stroke case. A 19-year-old male without any vascular risk factors presented a right transient ischemic attack. Transverse time-of-flight MRA indicates bilateral middle cerebral artery (MCA) stenosis. The MRA and contrast high-resolution vessel wall MRI obtained at the initial evaluation (A), and 6 (B), 12 (C), and 24 (D) months later are provided. The intensity of vessel wall enhancement (VWE) at right MCA was changed (grade 1→grade 0→grade 1→grade 1; white arrow), but the degree of VWE at left MCA was not changed (grade 1→grade 1→grade 1→grade 1; white arrowhead). The stenosis progression was observed only at left MCA (E and F; white arrow).
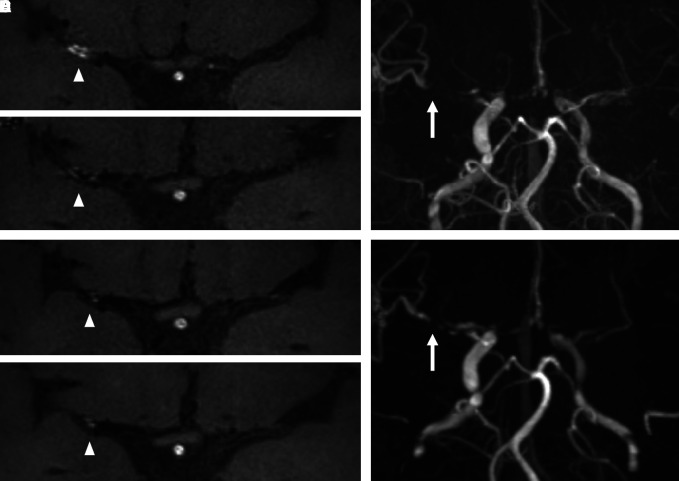
Fig. 2.
Hemorrhagic stroke case. A 50-year-old female presented with an intracranial hemorrhage at the left parietal lobe. Transverse time-of-flight MRA indicates right middle cerebral artery (MCA) stenosis and left internal carotid artery stenosis. The MRA and corresponding contrast high-resolution vessel wall MRI obtained at the initial evaluation (A), and 6 (B), 12 (C), and 24 (D) months later are provided. The intensity of vessel wall enhancement (VWE) at right MCA was changed (grade 2→grade 1→grade 1→grade 1; white arrowhead). The stenosis progression was observed at right MCA (E and F; white arrow).
VWE and recent neurological symptoms
The recent neurological symptom was occurred in 10 hemispheres (12.8%). These symptoms were observed in eight hemispheres (21.2%) in the VWE(+) group and two hemispheres (4.44%) in the VWE(−) group (OR 6.88, 95%CI 1.35–34.98, p = 0.015). Revascularization operation was done with all patients who have new neurological symptoms according to Tominaga et al.5) The patients’ characteristics with the changes in VWE are shown in Table 2.
Table 2. Patients’ characteristics with the changes in VWE.
| No. | Age | Sex | Changes in VWE | Site of stenosis progression | ||
|---|---|---|---|---|---|---|
| L | R | L | R | |||
| 1 | 24 | F | D | I | M1 | M1, P1 |
| 2 | 48 | F | – | I | ICA | |
| 3 | 3 | F | I | I | ICA, M1 | ICA |
| 4 | 40 | F | – | D | ||
| 5 | 44 | F | – | I/D | ||
| 6 | 13 | F | I/D | D | M1 | |
| 7 | 67 | M | I/D | ICA, M1 | M1 | |
| 8 | 46 | F | I | |||
| 9 | 8 | F | I | I | M1 | M1 |
| 10 | 44 | F | D | M1 | ||
| 11 | 52 | F | I/D | D | ||
| 12 | 36 | M | D | |||
| 13 | 39 | M | D | M1 | ||
| 14 | 32 | F | I | |||
| 15 | 27 | M | I | M1 | ||
| 16 | 58 | F | I | |||
| 17 | 37 | F | D | ICA, P1 | ICA | |
| 18 | 46 | F | I/D | M1 | ||
| 19 | 50 | F | I/D | D | M1 | M1 |
| 20 | 19 | M | D | D | M1 | |
| 21 | 8 | F | – | D | ICA | |
| 22 | 37 | F | D | – | ||
| 23 | 33 | M | I | – | ||
| 24 | 12 | F | D | – | ||
| 25 | 41 | M | I | – | ||
| 26 | 7 | M | D | – | ||
D: decrease, F: female, I: increase, ICA: internal carotid artery, M: male, M1, M1: segment of middle cerebral artery, P1: segment of posterior cerebral artery, VWE: vessel wall enhancement.
Reproducibility
The inter-reader agreement between the two readers for the enhancement of the MMD vessel wall and intracranial arterial stenosis progression was good (weighted k = 0.904 and 0.872, respectively).
Discussion
This study investigated the relationship between VWE, stenosis progression, and the recent neurological symptoms in patients with MMD. The changes in VWE were significantly associated with the recent neurological symptoms but not with stenosis progression.
The intensity of VWE closely correlates with the level of inflammatory activity and disease stability in patients with carotid artery stenosis.10–12) Qiao et al. suggested that neovascularization and increased endothelial permeability may affect this contrast enhancement.10) Roder et al. reported that VWE changes sometimes occurred, and these changes may reflect the beginning of angiopathy progression in MMD patients.13) Some studies recently indicated the relationship between genetic background and immunological mediators in patients with MMD and quasi-MMD.14) An active angiogenetic and focal inflammatory response may occur in the enhanced vessel wall in MMD patients.
Contrast enhancement of intracranial atherosclerotic plaques was associated with its likelihood of having caused a recent ischemic event and may serve as a marker of its stability, thereby providing important insight into stroke risk.15,16) Song et al. recently reported that plaque enhancement, positive remodeling, T1 hyperintensity, and surface irregularity were strong imaging factors of symptomatic plaque in patients with ischemic events.17) Unstable aneurysms (symptomatic, growing, or ruptured) had significantly higher odds for aneurysm wall enhancement. Lack of wall enhancement was a strong predictor of aneurysm stability.18–20) Strong enhancement of the intracranial vessel wall was associated with intracranial arterial stenosis progression in patients with MMD, and a lack of enhancement correlated with the stability of intracranial arterial stenosis.9,13,21) In this study, the changes in VWE were associated with the recent neurological symptoms in MMD patients. These changes may reflect disease instability. In clinical practice, CE-HR-MRI of the arterial wall may help identify patients at risk of new strokes, thus suggesting early treatment for future stroke prevention.
Limitations
The present study is associated with some potential limitations. First, our study included a small sample. Larger cohorts are needed to prospectively follow presumably stable intracranial arterial stenosis in patients with MMD to confirm the clinical use of contrast HR MRI VWI to predict clinical instability. Second, the follow-up period was relatively short. We might not detect potential patients who need the revascularization operation. Third, MRA is a well-validated technique used to evaluate intracranial arterial stenosis, and the sensitivity and specificity for the correct diagnosis of intracranial arterial stenosis have been reported to be 70% and 99%, respectively.22) However, complex regional blood-flow disturbances may result in signal loss and subsequent overestimation of both the length and degree of stenosis.23)
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflicts of interest to declare.
References
- 1).Fukui M: Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘Moyamoya’ disease). Clin Neurol Neurosurg 99: s238–240, 1997 [PubMed] [Google Scholar]
- 2).Scott RM, Smith ER: Moyamoya disease and moyamoya syndrome. N Engl J Med 360: 1226–1237, 2009 [DOI] [PubMed] [Google Scholar]
- 3).Wakai K, Tamakoshi A, Ikezaki K, et al. : Epidemiological features of moyamoya disease in Japan-findings from a nationwide survey. Clin Neurol Neurosurg 99: S1–S5, 1997 [DOI] [PubMed] [Google Scholar]
- 4).Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y: Incidence and clinical features of disease progression in adult moyamoya disease. Stroke 36: 2148–2153, 2005 [DOI] [PubMed] [Google Scholar]
- 5).Tominaga T, Suzuki N, Miyamoto S, et al. : Recommendations for the management of moyamoya disease: a statement from research committee on spontaneous occlusion of the circle of Willis (moyamoya disease) [2nd edition]. Surg Cerebral Stroke 46: 1–24, 2018 [Google Scholar]
- 6).Kernan WN, Ovbiagele B, Black HR, et al. : Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 2160–2236, 2014 [DOI] [PubMed] [Google Scholar]
- 7).Hong KS, Bang OY, Kang DW, et al. : Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the Korean stroke society and clinical research center for stroke. J Stroke 15: 2–20, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Johnston SC, Mendis S, Mathers CD: Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 8: 345–354, 2009 [DOI] [PubMed] [Google Scholar]
- 9).Wang M, Yang Y, Zhou F, et al. : The contrast enhancement of intracranial arterial wall on high-resolution MRI and its clinical relevance in patients with moyamoya vasculopathy. Sci Rep 7: 44264, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Qiao Y, Etesami M, Astor BC, Zeiler SR, Trout HH, Wasserman BA: Carotid plaque neovascularization and hemorrhage detected by MR imaging are associated with recent cerebrovascular ischemic events. AJNR Am J Neuroradiol 33: 755–760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C: Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 241: 459–468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hatsukami TS, Yuan C: MRI in the early identification and classification of high-risk atherosclerotic carotid plaques. Imaging Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Roder C, Hauser TK, Ememann U, Tatagiba M, Khan N, Bender B: Arterial wall enhancement in progressive moyamoya disease. J Neurosurg 24: 1–9, 2019 [DOI] [PubMed] [Google Scholar]
- 14).Mikami T, Suzuki H, Komatsu K, Mikuni N: Influence of inflammatory disease on the pathophysiology of moyamoya disease and quasi-moyamoya disease. Neurol Med Chir (Tokyo) 59: 361–370, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Qiao Y, Zeiler SR, Mirbagheri S, et al. : Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 271: 534–542, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Wu F, Song H, Ma Q, et al. : Hyperintense plaque on intracranial vessel wall magnetic resonance imaging as a predictor of artery-to-artery embolic infarction. Stroke 49: 905–911, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Song JW, Pavlou A, Xiao J, Kasner SE, Fan Z, Messé SR: Vessel wall magnetic resonance imaging biomarkers of symptomatic intracranial atherosclerosis: a meta-analysis. Stroke 52: 193–202, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Texakalidis P, Hilditch CA, Lehman V, Lanzino G, Pereira VM, Brinjikji W: Vessel wall imaging of intracranial aneurysms: systematic review and meta-analysis. World Neurosurg 117: 453–458.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 19).Edjlali M, Gentric JC, Régent-Rodriguez C, et al. : Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke 45: 3704–3706, 2014 [DOI] [PubMed] [Google Scholar]
- 20).Matsushige T, Shimonaga K, Ishii D, et al. : Vessel wall imaging of evolving unruptured intracranial aneurysms. Stroke 50: 1891–1894, 2019 [DOI] [PubMed] [Google Scholar]
- 21).Muraoka S, Araki Y, Taoka T, et al. : Prediction of intracranial arterial stenosis progression in patients with moyamoya vasculopathy: contrast-enhanced high-resolution magnetic resonance vessel wall imaging. World Neurosurg 116: e1114–e1121, 2018 [DOI] [PubMed] [Google Scholar]
- 22).Bash S, Villablanca JP, Jahan R, et al. : Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 26: 1012–1021, 2005 [PMC free article] [PubMed] [Google Scholar]
- 23).Nederkoorn PJ, Elgersma OE, Mali WP, Eikelboom BC, Kappelle LJ, van der Graaf Y: Overestimation of carotid artery stenosis with magnetic resonance angiography compared with digital subtraction angiography. J Vasc Surg 36: 806–813, 2002 [PubMed] [Google Scholar]